Abstract
Objectives
To evaluate choroidal arterial watershed zones (CWZ) in highly myopic patients. The relationships between CWZ location and myopic maculopathy location and classification were also examined.
Methods
This retrospective study included 102 consecutive patients who had been diagnosed with myopic maculopathy. Indocyanine green videoangiography was used to evaluate CWZ presence, location, and configuration. Maculopathy signs were used to examine the relationship between CWZ and myopic maculopathy.
Results
Various CWZ types were identified in 102 of 158 eyes. The CWZ patterns were classified as vertical optic nerve head (vertical-ONH) in 30 eyes, stellate in 29 eyes, vertical-ONH extending to the macula in 28 eyes, horizontal fovea in eight eyes, and vertical parafovea in seven eyes. Choroidal neovascularization occurred within CWZs in 35 of 42 eyes, and macular atrophy was located within foveal CWZs in 20 of 23 eyes. The CWZ type was significantly correlated with mCNV presence (OR = 5.652, P = 0.014).
Conclusions
Variations in CWZ topography are associated with myopic maculopathy, particularly in eyes with myopic choroidal neovascularization (mCNV) and macular atrophy, and CWZ is a risk factor for mCNV. This suggests that eyes with macular CWZs are vulnerable to developing myopic maculopathy and are predisposed to mCNV because of ischaemic hypoxia.
Subject terms: Eye manifestations, Outcomes research
Introduction
Myopic maculopathy is a major cause of irreversible visual impairment and blindness globally and particularly in East Asia. The Beijing Eye Study [1] and Shihpai Eye Study [2] have shown that it is the second most frequent cause of low vision and blindness in China. Signs of myopic maculopathy include chorioretinal atrophy, myopic choroidal neovascularization (mCNV), Fuch’s spots (Fs), lacquer cracks (Lc) and posterior staphyloma [3]. Myopic chorioretinal atrophy can be classified as either diffuse atrophy or patchy atrophy. Diffuse chorioretinal atrophy is an ill-defined, yellowish white atrophic area in the posterior pole and does not severely impair vision. In contrast, patchy atrophy presents as several well-defined, greyish-white lesions in the macula or around the optic disc, while progressive patch enlargement ultimately leads to significant vision loss, particularly in older, highly myopic patients.
Macular choroidal neovascularization occurs in 5–10% of eyes with pathological myopia [4] and is the most common cause of vision loss in eyes with high myopia [5]. Unfortunately, the pathogenesis of mCNV remains unclear. Photodynamic therapy and intravitreal bevacizumab are currently the most common treatment options for mCNV [6, 7]. However, late stage chorioretinal macular atrophy is progressive, and long-term treatment outcomes are not favourable. Therefore, it is important to elucidate mCNV pathogenesis so that additional prevention strategies can be developed.
Choroidal circulatory disturbances that occur in highly myopic eyes have been investigated in previous histopathologic and angiographic studies [8, 9]. Choroidal watershed zones (CWZ) have a relatively poor blood flow supply. These zones are located at the border between two or more perfused areas that are each supplied by an anatomic end-artery. Therefore, CWZs are particularly vulnerable to ischaemia and hypoxia [10, 11]. Although optical coherence tomography angiography is an advanced method of studying chorioretinal vasculature, it is not suitable for studying CWZ because the velocity of flow in the deep choroid is significantly high and the provided field of view is significantly small. In contrast, CWZ location and CWZ pattern can be identified using indocyanine green angiography (ICGA) and fluorescein angiography [12]. As the locations of CWZs, CNV and atrophy have been shown to coincide, CNV is thought to develop via an ischaemic mechanism [13–15]. However, the relationship between CWZs and maculopathy has not been extensively investigated in highly myopic eyes.
The aims of the current study were to investigate the CWZs in highly myopic patients and to determine the topographical relationship between CWZs and myopic maculopathy to suggest a pathologic mechanism for myopic maculopathy.
Materials and methods
Study participants
This observational, cross-sectional, retrospective study was reviewed and approved by the Ethics Committee of the Beijing Friendship Hospital (Beijing, China). The study protocol adhered to the tenets of the Declaration of Helsinki, and written informed consent was obtained from all patients whose medical records were examined.
This case series included consecutive patients who were diagnosed with myopic maculopathy at the Beijing Friendship Hospital between January 2015 and December 2018 and underwent ICGA (HRA2, Heidelberg Engineering GmbH, Heidelberg, Germany) for macular diseases associated with high myopia. All subjects had a refractive spherical equivalent exceeding −6.0 dioptres (D) or an axial length longer than 26.5 mm. Subjects suspected of having age-related macular degeneration, angioid streaks, multifocal choroiditis or other choroidal abnormalities related to CNV or atrophy were excluded. Eyes with chorioretinal atrophy that allowed scleral visibility, a history of surgery or vitreoretinal abnormality, clinical evidence suggestive of a vitreoretinal condition, elevated intraocular pressure (>21 mmHg) or visual field defects were also excluded.
Fluorescein and indocyanine green angiography
Fluorescein angiography was performed with intravenous injection of 5 ml of 10% sodium fluorescein. ICGA was performed with intravenous injection of ICG dye (25 mg in 2.5 ml aqueous solvent). We injected the ICG dye at 0.6 ml/s. The angular measurement is 55° centred on the macula. Early choroidal vessel filling was captured using high-speed digital videography for 1 min after ICG infusion to facilitate CWZ detection. The first eye that filled with choroidal vascular dye according to ICG videoangiography was chosen as the study eye. Late-phase single ICG angiography images were also obtained at 5, 10 and 20 min. A region was considered to have a filling delay if a distinct area with a prolonged circulatory defect was identified in the fovea at 15–25 s (i.e. no filling in the arterial and arteriovenous phases and gradual filling in the venous phase). Two blinded readers analysed all ICGA findings. When discrepancies in analyses occurred, the two readers discussed these to achieve a consensus.
Optical coherence tomography
Optical coherence tomography (SD-OCT; Heidelberg Engineering GmbH, Heidelberg, Germany) images were obtained using a high-definition spectral-domain OCT. The raster and macular scans were used to examine retinal and choroidal integrity and to determine the presence/absence of mCNV and patchy atrophy. Choroidal thickness, defined as the distance between the retinal pigment epithelium (RPE) and the scleral interface (reflective line beneath the fovea), was manually measured by two masked observers using enhanced-depth imaging OCT images [16] and Heidelberg Eye Explorer software. The final choroidal thickness was recorded as the average of the two independent measurements. The presence of mCNV was confirmed using fluorescein angiography and OCT images and was determined by two authors (JLW, JS). Cases of disagreement were discussed to reach a consensus.
Myopic maculopathy and choroidal watershed zone classification
The definition of myopic maculopathy has not been consistent across previous studies [3]. This study used the simplified META-PM classification system [3], which categorises myopic maculopathy lesions into the following five categories: no myopic retinal lesions (C0), tessellated fundus only (C1), diffuse chorioretinal atrophy (C2), patchy chorioretinal atrophy (C3) and macular atrophy (C4). These categories were defined based on long-term clinical observations that revealed progression patterns and the mCNV development risk for each stage. Three additional features were later added to these categories, considered as ‘positive signs’: they included Lc, mCNV and Fs, which were determined using fluorescein angiography and ICGA.
CWZs were classified using only ICGA image findings. As with mCNV status, final CWZ characteristics were determined by consensus between the two blinded readers when discrepancies arose. Chorioretinal atrophy, which presents as hypofluorescence; and CWZs, were differentiated by the large choroidal vessels that traversed atrophic regions during the venous phase of ICGA. Two established systems were used to create a novel CWZ classification system for eyes with myopic maculopathy [17, 18].
Statistical analyses
Data are presented as means ± standard deviations and n (%) as applicable. Characteristics were compared using non-parametrical tests. Categorical variables were compared using the chi-square or Fisher’s exact test. Multivariate logistic regression analyses were conducted to identify the odds ratio (OR) for factors identified as significant in stepwise regression analysis. Ordinal logistic regression analyses were also performed to identify factors that influenced CWZ and mCNV classifications. Statistical analyses were performed using SPSS statistical software (version 24.0, SPSS, Inc., Chicago, IL, USA), and statistical significance was defined as P < 0.05.
Results
Patient characteristics
Characteristics of patients with and without mCNV are summarised in Table 1. Briefly, subjects with mCNV were significantly older (62.3 ± 9.7 years vs. 55.9 ± 13.7 years, P = 0.031), more likely to be female (78.6% vs. 55.0%, P = 0.020), and more likely to have Fs (31.0% vs. 0%, P < 0.001) than subjects without mCNV. Furthermore, choroidal thickness (P = 0.018) and the proportion of patients with Lc (P = 0.003) and Fs (P = 0.001) varied significantly with the mCNV category (Table 2).
Table 1.
Characteristics of myopic maculopathy patients with and without myopic choroidal neovascularization.
| mCNV | Myopic maculopathy category | |||||||
|---|---|---|---|---|---|---|---|---|
| Yes | No | P | C1 | C2 | C3 | C4 | P | |
| n, eyes | 42 | 60 | – | 27 | 19 | 33 | 23 | – |
| Age, years | 62.3 ± 9.7 | 55.9 ± 13.7 | 0.031* | 54.7 ± 13.6 | 56.5 ± 11.3 | 60.6 ± 13.7 | 61.5 ± 9.5 | 0.670 |
| Male | 9 (21.4%) | 27 (45.0%) | 0.020* | 11 (40.7%) | 9 (47.4%) | 13 (39.4%) | 3 (13.0%) | 0.065 |
| Female | 33 (78.6%) | 33 (55.0%) | 16 (59.3%) | 10 (52.6%) | 20 (60.6%) | 20 (87.0%) | ||
| CT (μm) | 54.47 ± 40.51 | 77.33 ± 57.59 | 0.100 | 104.63 ± 60.11 | 48.17 ± 27.49 | 53.96 ± 37.46 | 50.95 ± 47.94 | <0.001* |
| Lc | 27 (64.3%) | 40 (66.7%) | 1.000 | 13 (48.1%) | 16 (84.2%) | 25 (75.8%) | 13 (56.5%) | 0.014* |
| Fs | 13 (30.1%) | 0 (0%) | <0.001* | 0 (0%) | 2 (10.5%) | 5 (15.2%) | 6 (26.1%) | 0.027* |
Data are presented as mean ± standard deviation or n (%) as applicable.
mCNV myopic choroidal neovascularization, C1 tessellated fundus only, C2 diffuse chorioretinal atrophy, C3 patchy chorioretinal atrophy, C4 macular atrophy, CT choroidal thickness, Lc lacquer cracks, Fs Fuchs spots.
*Statistically significant value.
Table 2.
Choroidal watershed zone classification in patients with myopic maculopathy.
| mCNV | P | Myopic maculopathy classification | ||||||
|---|---|---|---|---|---|---|---|---|
| Yes | No | C1 | C2 | C3 | C4 | P | ||
| n, eyes | 42 | 60 | – | 27 | 19 | 33 | 23 | – |
| CWZ type | 0.001* | 0.007* | ||||||
| I | 4 (9.5%) | 26 (43.3%) | 15 (55.6%) | 5 (26.3%) | 9 (27.3%) | 1 (4.3%) | ||
| II | 13 (31.0%) | 15 (25.0%) | 8 (29.6%) | 6 (31.6%) | 9 (27.3%) | 5 (21.7%) | ||
| III | 3 (7.1%) | 4 (6.7%) | 1 (3.7%) | 2 (10.5%) | 2 (6.1%) | 2 (8.7%) | ||
| IV | 17 (40.5%) | 12 (20.0%) | 2 (7.4%) | 5 (26.3%) | 11 (33.3%) | 11 (47.8%) | ||
| V | 5 (11.9%) | 3 (5.0%) | 1 (3.7%) | 1 (5.3%) | 2 (6.1%) | 4 (17.4%) | ||
Data are presented as n (%).
mCNV myopic choroidal neovascularization, CWZ choroidal watershed zone, Type I vertical pattern coursing through the optic nerve head (ONH), Type II vertical pattern through the ONH that extends to the fovea. Type III vertical pattern coursing through the parafovea, Type IV stellate pattern that involves the ONH, macula, and vessel arcade, Type V horizontal pattern coursing through the fovea (no ONH involvement), C1 tessellated fundus only, C2 diffuse chorioretinal atrophy, C3 patchy chorioretinal atrophy, C4 macular atrophy.
*Statistically significant value.
Novel choroidal watershed zone classification system
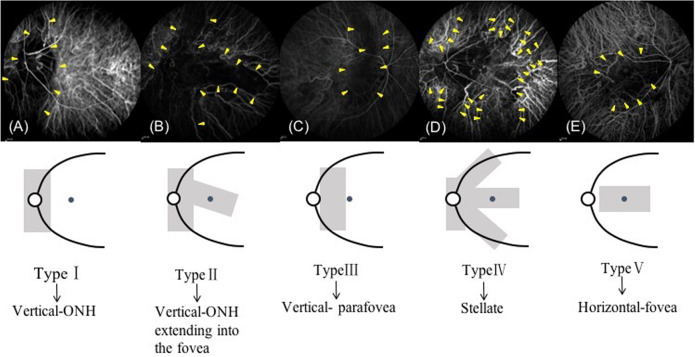
We designed our novel CWZ classification system to assign CWZ type (Fig. 1). Briefly, Type I involved vertically oriented CWZ located at the optic nerve head (ONH). Type II was similar to Type I, but the CWZ extended horizontally to the fovea. Type III involved vertically oriented CWZ located in the parafovea. Type IV involved CWZ with a stellate pattern and the ONH and fovea. Type V involved horizontally oriented CWZ located in the fovea. Representative images of eyes with Type II CWZ are shown in Fig. 2.
Fig. 1. Schematic illustrations of choroidal watershed zone (CWZ) patterns used for CWZ classification.
Type I (A): vertical pattern coursing through the optic nerve head (ONH) without extending to the fovea. Type II (B): vertical pattern coursing through the ONH and extending into the fovea. Type III (C): vertical pattern coursing through the parafovea without extending to the ONH. Type IV (D): stellate pattern that involves the ONH and macula. Type V (E): horizontal pattern coursing through the fovea without extending to the ONH.
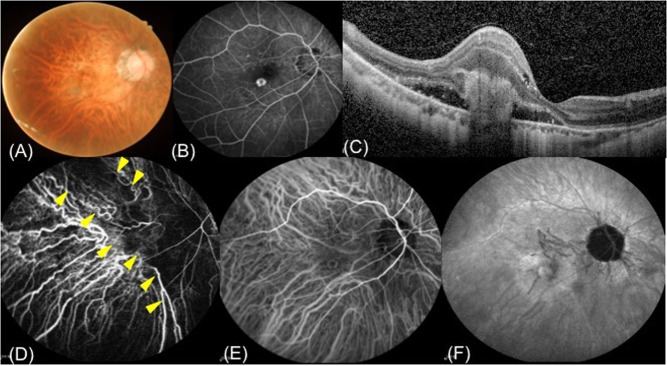
Fig. 2. Myopic choroidal neovascularization (mCNV) in a 55-year-old man with myopic maculopathy.
A colour fundus photograph (A) is presented for reference. Late-phase fluorescein angiography (B) and optical coherence tomography (C) images show the mCNV lesion. Early-phase indocyanine green angiography images (D) also clearly show mCNV location within a Type II (stellate) choroidal watershed zone (CWZ) (arrowheads). The CWZ is no longer visible in the late-phase indocyanine green angiography image (E, F).
Choroidal watershed zone and myopic maculopathy topography
CWZ was detected in 102 examined eyes; Table 2 summarises CWZ classifications (based on ICGA findings). The distribution of CWZ types was significantly different between patients with and without mCNV (P = 0.001). For example, a CWZ that involved the fovea (Types II, IV and V) was observed more often in patients with mCNV (35 [83.3%] of 42 eyes) than in those without mCNV (30 [50.0%] of 60 eyes, P = 0.001). Additionally, CWZ type distribution differed significantly among the maculopathy categories (P = 0.007). Type IV CWZ was most commonly present in C4 eyes (11 eyes [47.8%]), but Type I CWZ was most commonly present in C1 eyes (15 eyes [55.6%]). Five eyes were not included in CWZ analyses as they could not be categorised.
Several risk factors were identified for developing mCNV (Table 3) including having a CWZ that involved the fovea (Types II, IV and V). An ordinal logistic regression analysis was then performed to analyse the risk factors for the myopic maculopathy category associated with the CWZ type (Supplementary Table S1). This revealed that a thinner choroidal thickness (P = 0.004) and CWZ type (P = 0.043) were both significantly associated with the myopic maculopathy category. Further analyses revealed that choroidal thickness was also associated with the presence/absence of Lc (P = 0.017, Supplementary Table S2).
Table 3.
Factors associated with the presence of myopic choroidal neovascularization.
| Significant Factors | OR | B | Standard Error | 95% CI for B | P | |
|---|---|---|---|---|---|---|
| Model 1 | Foveal CWZ | 8.195 | 2.104 | 0.666 | 2.222–30.232 | 0.002* |
| Model 2 | Foveal CWZ | 6.510 | 1.873 | 0.681 | 1.713–24.740 | 0.006* |
| Model 3 | Foveal CWZ | 5.652 | 1.732 | 0.702 | 1.428–22.367 | 0.014* |
Foveal CWZ presence of a choroidal watershed zone that involved the fovea (Types II, IV and V), CWZ choroidal watershed zone, OR odds ratio, B regression correlation coefficient, CI confidence interval, Model 1 crude model, Model 2 further adjustment for sex and age, Model 3 further adjustment for choroidal thickness and lacquer cracks, CT choroidal thickness, Lc lacquer crack.
*Statistically significant value. Findings based on logistic regression analyses.
Discussion
Our study revealed that myopic maculopathy had a topographical relationship with CWZ. This finding suggests that eyes with macular CWZs are vulnerable to developing myopic maculopathy and are predisposed to mCNV because of ischaemic hypoxia.
Proper choroidal circulation function is crucial for maintaining the outer retinal cell function [19]. An in vivo study of the choroidal vascular bed showed that both the posterior ciliary arteries (PCAs) and the choriocapillaris are segmentally structured end-arterial systems [17]. However, a corrosion cast study also demonstrated that submacular choroidal vessels have anastomoses to capillaries, arterioles and venules at various levels [20].
Choroidal watershed zones are located at the border between two or more areas perfused by anatomic end-arteries. These zones are of great clinical importance because they have relatively poor blood flow and are consequently vulnerable to ischaemia and hypoxia [19]. Multiple CWZs meet in the submacular choroid; therefore, the macula is more vulnerable to ischaemia than any other part of the posterior choroid. Hence, it is not surprising that CWZs play a role in choroidal ischaemic infarctions in ONH ischaemic disorders, including nonarteritic anterior ischaemic optic neuropathy [21]. The current study showed that CWZs are also likely to play a role in mCNV development in eyes with myopic maculopathy. It should be noted that CWZ can be classified in most patients, but that severe atrophy makes CWZ visualisation difficult in some cases. Giuffre et al. [22] examined the fluorescein angiography images of 800 healthy subjects and observed a well-demarcated CWZ in 44.6% of the subjects. In agreement with this, we were able to detect and classify CWZs in 64.6% of our highly myopic patients. Similarly, Takahashi et al. [23] found that CWZs are more easily observed in myopic eyes because of optics. In agreement, Wakabayashi and Ikuno [24] concluded that choroidal filling delays are present in highly myopic eyes; these are different from watershed zones, and, at least in part, originate in a CWZ.
The current study categorised CWZs with a new classification system that was based on two previously established CWZ classification systems for eyes with age-related macular degeneration [17, 18]. Mendrinos and Pournaras classified CWZs into the following three categories: stellate, vertical and angled. Hayashi and de Laey classified CWZ into the following five categories: nasal filling (I), temporal filling (II-A), temporal filling with delayed foveal fluorescence (II-B), upper or lower temporal quadrant filling (III) and scattered dye filling (IV). Unlike these prior reports, we identified five types of CWZs and examined how CWZ type affected myopic maculopathy. In our study, types I, II, IV and V were similar to the patterns found by Hayashi, and type III was also observed by Mendrinos and Pournaras. Segmental distribution of the human choroid by various PCAs can explain the CWZ location [25], along with Hayrah’s theory [26]. The posterior choroid is supplied by the lateral and medial PCAs in ~90% of eyes [27]; therefore, a CWZ between the medial and lateral PCA is vertically oriented between the macula and ONH in 60% of the eyes [17]. A horizontal watershed zone can also be present at the macula when there are multiple lateral PCAs. However, physiological CWZ variation is prevalent.
The location of multiple submacular CWZs plays an important role in the pathogenesis of macular ischaemic lesions, including those associated with myopic maculopathy. All temporal SPCAs pierce the sclera, join the choroid in the macular region, and subsequently run radially toward the equator. These areas where multiple watershed zones meet have poor vascularity and are most vulnerable to ischaemic disorders. However, the literature describes the macular choroid as the most vascularises choroidal region as it has an increased arterial supply, maximum choriocapillaris density and high arterial pressure [26]. Our study demonstrated a topographical relationship between CWZ and myopic maculopathy and, more specifically, between mCNV and macular atrophy. Table 2 shows the proportion of CWZ types in eyes with and without mCNV and in eyes belonging to different maculopathy categories. Type IV CWZs (stellate pattern) were observed most often in eyes with mCNV, followed by CWZ types II and V. The locations of mCNV and CWZ were correlated in 35 (83.3%) of 42 eyes. In contrast, Type I CWZ was observed most frequently in eyes without mCNV. Therefore, our findings indicate that Type IV (stellate) CWZs are the most common pathological type. This proximity of mCNV to CWZs supports the concept that ischaemia plays an important role in mCNV development. Our multivariate logistic regression analysis also showed a correlation between CWZ and mCNV location. As in prior studies [24, 28], we found that a more advanced stage of myopic maculopathy, the presence of Lc was associated with mCNV development.
Our study has several strengths and limitations. The analyses of ICGA images allowed us to develop a novel CWZ classification system for highly myopic eyes. This system may be useful in both clinical practice and future studies. To the best of our knowledge, the relationship between CWZs and myopic maculopathy has not been investigated previously. This study was limited by its retrospective design and relatively small sample size. Additionally, a control group of healthy volunteers was not included because ICGA is an invasive test.
In conclusion, topographic CWZ variation is associated with myopic maculopathy, particularly in eyes with mCNV and macular atrophy. Therefore, our findings may provide insight into myopic maculopathy pathogenesis, which may subsequently lead to preventative therapies.
Summary
What was known before
Myopic maculopathy is a major cause of irreversible visual impairment and blindness globally.
What this study adds
Variations in choroidal watershed zone (CWZ) topography are associated with myopic maculopathy, particularly in eyes with myopic choroidal neovascularization (mCNV) and macular atrophy.
Supplementary information
Funding
Research Foundation of Beijing Friendship Hospital, Capital Medical University (No. yyqdkt2019-29).
Author contributions
YW contributed to the acquisition and analysis of data. JW designed the current study and revised the manuscript. JS designed the study, analysed data and drafted the manuscript. All authors read and approved the final manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Yanling Wang, Jialin Wang
Contributor Information
Yanling Wang, Email: wangyanl@ccmu.edu.cn.
Jialin Wang, Email: wangjialin@bjmu.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41433-021-01427-y.
References
- 1.Xu L, Wang Y, Li Y, Wang Y, Cui T, Li J, et al. Causes of blindness and visual impairment in urban and rural areas in Beijing: the Beijing eye study. Ophthalmology. 2006;113:1134.e1–11. doi: 10.1016/j.ophtha.2006.01.035. [DOI] [PubMed] [Google Scholar]
- 2.Hsu WM, Cheng CY, Liu JH, Tsai SY, Chou P. Prevalence and causes of visual impairment in an elderly Chinese population in Taiwan: the Shihpai eye study. Ophthalmology. 2004;111:62–69. doi: 10.1016/j.ophtha.2003.05.011. [DOI] [PubMed] [Google Scholar]
- 3.Ohno-Matsui K, Lai TY, Lai CC, Cheung CM. Updates of pathologic myopia. Prog Retin Eye Res. 2016;52:156–87. doi: 10.1016/j.preteyeres.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Tano Y. Pathologic myopia: where are we now? Am J Ophthalmol. 2002;134:645–60. doi: 10.1016/S0002-9394(02)01883-4. [DOI] [PubMed] [Google Scholar]
- 5.Neelam K, Cheung CM, Ohno-Matsui K, Lai TY, Wong TY. Choroidal neovascularization in pathological myopia. Prog Retin Eye Res. 2012;31:495–525. doi: 10.1016/j.preteyeres.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Ikuno Y, Ohno-Matsui K, Wong TY, Korobelnik JF, Vitti R, Li T, et al. Intravitreal aflibercept injection in patients with myopic choroidal neovascularization: The MYRROR study. Ophthalmology. 2015;122:1220–7. doi: 10.1016/j.ophtha.2015.01.025. [DOI] [PubMed] [Google Scholar]
- 7.Wolf S, Balciuniene VJ, Laganovska G, Menchini U, Ohno-Matsui K, Sharma T, et al. RADIANCE: a randomized controlled study of ranibizumab in patients with choroidal neovascularization secondary to pathologic myopia. Ophthalmology. 2014;121:682–.e2. doi: 10.1016/j.ophtha.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 8.Ferrara D, Waheed NK, Duker JS. Investigating the choriocapillaris and choroidal vasculature with new optical coherence tomography technologies. Prog Retin Eye Res. 2016;52:130–55. doi: 10.1016/j.preteyeres.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Ikuno Y, Fujimoto S, Jo Y, Asai T, Nishida K. Choroidal thinning in high myopia measured by optical coherence tomography. Clin Ophthalmol. 2013;7:889–93. doi: 10.2147/OPTH.S44138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gewaily DY, Grunwald JE, Pistilli M, Ying GS, Maguire MG, Daniel E, et al. Delayed patchy choroidal filling in the comparison of age-related macular degeneration treatments trials (CATT) Am J Ophthalmol. 2014;158:525–.e2. doi: 10.1016/j.ajo.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ciulla TA, Harris A, Danis RP. Presumed macular choroidal watershed vascular filling, choroidal neovascularization, and systemic vascular disease in patients with age-related macular degeneration. Am J Ophthalmol. 1998;126:153–5. doi: 10.1016/S0002-9394(98)00233-5. [DOI] [PubMed] [Google Scholar]
- 12.Bei L, Lee I, Lee MS, Van Stavern GP, McClelland CM. Acute vision loss and choroidal filling delay in the absence of giant-cell arteritis. Clin Ophthalmol. 2016;10:1573–8. doi: 10.2147/OPTH.S112196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feigl B, Brown B, Lovie-Kitchin J, Swann P. Functional loss in early age-related maculopathy: the ischaemia postreceptoral hypothesis. Eye. 2006;21:689–96. doi: 10.1038/sj.eye.6702389. [DOI] [PubMed] [Google Scholar]
- 14.Hanyuda N, Akiyama H, Shimoda Y, Mukai R, Sano M, Shinohara Y, et al. Different filling patterns of the choriocapillaris in fluorescein and indocyanine green angiography in primate eyes under elevated intraocular pressure. Invest Ophthalmol Vis Sci. 2017;58:5856–61. doi: 10.1167/iovs.17-22223. [DOI] [PubMed] [Google Scholar]
- 15.Burggraaff MC, Trieu J, de Vries-Knoppert WA, Balk L, Petzold A. The clinical spectrum of microcystic macular edema. Invest Opthalmol Vis Sci. 2014;55:952–61. doi: 10.1167/iovs.13-12912. [DOI] [PubMed] [Google Scholar]
- 16.Spaide RF, Koizumi H, Pozzoni MC. Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol. 2008;146:496–500. doi: 10.1016/j.ajo.2008.05.032. [DOI] [PubMed] [Google Scholar]
- 17.Mendrinos E, Pournaras CJ. Topographic variation of the choroidal watershed zone and its relationship to neovascularization in patients with age-related macular degeneration. Acta Ophthalmol. 2009;87:290–6. doi: 10.1111/j.1755-3768.2008.01247.x. [DOI] [PubMed] [Google Scholar]
- 18.Hayashi K, de Laey JJ. Indocyanine green angiography of submacular choroidal vessels in the human eye. Ophthalmologica. 1985;190:20–29. doi: 10.1159/000309488. [DOI] [PubMed] [Google Scholar]
- 19.Linsenmeier RA, Zhang HF. Retinal oxygen: from animals to humans. Prog Retin Eye Res. 2017;58:115–51. doi: 10.1016/j.preteyeres.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee JE, Ahn KS, Park KH, Pak KY, Kim HJ, Byon IS, et al. Functional end-arterial circulation of the choroid assessed by using fat embolism and electric circuit simulation. Sci Rep. 2017;7:2490. doi: 10.1038/s41598-017-02695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim MK, Kim US. Analysis of fundus photography and fluorescein angiography in nonarteritic anterior ischemic optic neuropathy and optic neuritis. Korean J Ophthalmol. 2016;30:289–94. doi: 10.3341/kjo.2016.30.4.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giuffrè G. Main posterior watershed zone of the choroid. Variations of its position in normal subjects. Doc Opthtalmol. 1989;72:175–80. doi: 10.1007/BF00156707. [DOI] [PubMed] [Google Scholar]
- 23.Takahashi K, Muraoka K, Kishi S, Shimizu K. Watershed zone in the human peripheral choroid. Ophthalmology. 1996;103:336–42. doi: 10.1016/S0161-6420(96)30695-7. [DOI] [PubMed] [Google Scholar]
- 24.Wakabayashi T, Ikuno Y. Choroidal filling delay in choroidal neovascularisation due to pathological myopia. Br J Ophthalmol. 2010;94:611–5. doi: 10.1136/bjo.2009.163535. [DOI] [PubMed] [Google Scholar]
- 25.Hayashi K, de Laey JJ. Indocyanine green angiography of choroidal neovascular membranes. Ophthalmologica. 2010;190:30–39. doi: 10.1159/000309489. [DOI] [PubMed] [Google Scholar]
- 26.Hayreh SS. In vivo choroidal circulation and its watershed zones. Eye. 1990;4:273–89. doi: 10.1038/eye.1990.39. [DOI] [PubMed] [Google Scholar]
- 27.Hayreh SS. The ophthalmic artery: III. Branches. Br J Ophthalmol. 1962;46:212–47. doi: 10.1136/bjo.46.4.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim YM, Yoon JU, Koh HJ. The analysis of lacquer crack in the assessment of myopic choroidal neovascularization. Eye. 2011;25:937–46. doi: 10.1038/eye.2011.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.