Highlights
-
•
Proteoglycans (PGs) and glycosaminoglycans (GAGs) play vital roles in key signaling pathways to regulate bone homeostasis.
-
•
The highly negatively charged GAGs are crucial in retaining bound water and modulating mechanical properties of bone.
-
•
Age-related changes of PGs, GAGs, and bound water contribute to deterioration of bone quality during aging.
Keywords: Proteoglycans, Glycosaminoglycans, Bound water, Bone toughness, Aging
Abstract
Proteoglycans (PGs) contain long unbranched glycosaminoglycan (GAG) chains attached to core proteins. In the bone extracellular matrix, PGs represent a class of non-collagenous proteins, and have high affinity to minerals and collagen. Considering the highly negatively charged character of GAGs and their interfibrillar positioning interconnecting with collagen fibrils, PGs and GAGs play pivotal roles in maintaining hydrostatic and osmotic pressure in the matrix. In this review, we will discuss the role of PGs, especially the small leucine-rich proteoglycans, in regulating the bioactivity of multiple cytokines and growth factors, and the bone turnover process. In addition, we focus on the coupling effects of PGs and GAGs in the hydration status of bone extracellular matrix, thus modulating bone biomechanical properties under physiological and pathological conditions.
Introduction
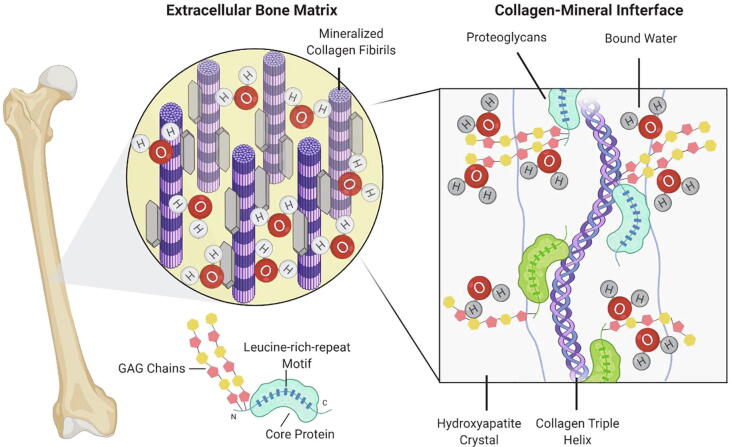
Osteoporosis and age-related bone fragility fractures are one of major health concerns for our rapidly growing elderly population. Bone mineral density (BMD) is the standard measure for the prognosis of fragility fractures. However, the prediction of bone fracture by using BMD alone lacks sensitivity to accurately evaluate the biomechanical performance of bone [1], [2]. There have been reports showing the fracture risk for elderly people is much higher than that of young adults with the same BMD [3], [4]. This has raised the necessity of elucidating the ultrastructural and molecular origins of bone fragility to better understand and prevent bone fragility fractures. Bone has a highly hierarchical structure, which is comprised of mineralized collagen fibrils embedded in an extrafibrillar matrix. The composition of bone includes 60% by weight mineral phase (mainly hydroxyapatite), 30% organic phase, including type I collagen fibrils and non-collagenous proteins, and 10% water [5]. Thus, the biomechanical properties of bone are dependent on the quality and spatial arrangement of these constituents.
Previously, extensive efforts have been made to illustrate the contribution of the mineral and collagen phases to bone mechanical competence [6], [7], [8], [9], [10]. In addition, the non-collagenous proteins are structural proteins dispersed throughout the bone extracellular matrix, which have been found to be directly involved in the deformation and failure of bone [11], [12], [13]. As a major part of non-collagenous proteins, proteoglycans (PGs) have strong affinity to collagen and mineral crystals [14], [15]. PGs are composed of core proteins with covalently attached glycosaminoglycan (GAG) chains, which are long unbranched polysaccharides [16]. These molecules are polar with a high negative charge, forming interfibrillar supramolecular assemblies that could absorb water into the bone extracellular matrix, while water has been reported to function as a critical plasticizer of bone [17], [18]. Moreover, PGs and GAGs interact with a multitude of growth factors and cytokines, regulating their activity and bioavailability [19].
It has been well documented that PGs and GAGs play an important role in modulating mechanical behavior of tendon, articular cartilage, and intervertebral disks [20], [21], [22]. In this review, we mainly focus on the contribution of PGs and GAGs to the physiological and biomechanical properties of bone. We will address the function of PGs and GAGs in mediating molecular signaling pathways and bone remodeling processes involving bone formation and bone resorption. We also focus on the coupling effects of PGs and water in regulating bone biomechanical properties. Lastly, age-related changes of PGs and GAGs, and their capability in water retention will be discussed with regards to the mechanisms contributing to deterioration of bone quality during aging.
The roles of small leucine-rich proteoglycans in bone physiology
The GAG chains of PGs consist of a series of repeating disaccharide units, which contain one of two modified hexosamines, N-acetylgalactosamine (GalNAc) or N-acetylglucosamine (GlcNAc), and uronic acid (i.e., glucuronate or iduronate) with the exception of keratan sulfate that contains galactose instead of uronic acid [23]. According to the disaccharide unit type, GAGs can be classified as chondroitin sulfate (CS), dermatan sulfate (DS), keratan sulfate (KS), heparin and heparan sulfate (HS) and can carry sulfate at various positions. In addition, HA is the only GAG that is synthesized as single chains not bound on a core protein and is not modified by sulfate groups. The sulfated GAGs are anchored to their core proteins by unique linkages. The CS, DS and HS are attached via serine residues of core proteins by way of xylose. While KS is N-glycan linked to core protein through an asparagine residue or O-glycan linked via serine or threonine [23], [24]. GAGs identity relays structural and functional properties to PGs. In the bone extracellular matrix, core protein and GAG chains of PGs directly bind to collagen fibrils and regulate collagen fibrillar diameter and formation [25], [26]. Additionally, PGs bind to cytokines, growth factors, and cell surface receptors, which regulate cell proliferation, matrix deposition and bone remodeling [27], [28].
The most abundant type of PGs in bone matrix are the small leucine-rich proteoglycans (SLRPs), which are characterized by relatively small protein cores (36–42 kDa) harboring 10–12 tandem leucine rich repeats. Based primarily on the nucleotide and protein sequence conservation, functional properties, and intron–exon organization, the SLRP gene family has expanded to 18 members classified into five distinct classes, including canonical (classes I–III) and non-canonical (classes IV and V) [16], [19]. The core proteins of SLRPs are proposed to adopt a horseshoe shaped structure, which may promote interaction with other matrix components. Biglycan (Bgn), a class I SLRPs, is distributed abundantly throughout the bone matrix [29]. It has two Ser-Gly sites in the N-terminal region and these two sites are used to attach two CS or DS chains. Pioneering work demonstrating the direct evidence of the biomechanical contribution of PGs/GAGs in bone has been described by Xu et al, using the Bgn deficient mouse model [30]. Mice with targeted disruption of Bgn exhibits age-dependent osteoporosis, with less trabecular volume and thinner cortices compared to WT counterparts, due to a decrease in bone formation. At 6 months of age, the four-point bending test of Bgn knockout (KO) mouse bone shows reduced values of failure load and yield energy, indicating impaired bone strength and ductility, respectively. These mice also exhibit structural abnormalities in bone collagen fibrils, with a marked variation in diameter [31]. The studies of the underlying cellular and molecular mechanisms reveal that Bgn KO mice have significantly diminished capacity to produce bone marrow stromal cells, and these cells are metabolically defective, with reduced response to exogenous transforming growth factor-β (TGF-β), reduced collagen synthesis and increased apoptosis compared to wildtype (WT) littermates [32]. In addition, Bgn deficiency blunts bone morphogenetic protein-4 (BMP-4)-induced osteoblast differentiation in vitro [33]. The absence of Bgn decreases BMP-4 sensitivity caused by less BMP-4 binding and is completely rescued by viral transfection of Bgn. Another study highlights the importance of GAG chains of Bgn in modulating osteoblast differentiation, showing the expression of a mutant Bgn lacking GAGs binding sites fail to rescue the differentiation deficiency [34]. Furthermore, Bgn has been found to directly bind to BMP-2 and regulate BMP-2 induced osteoblast differentiation [35]. Apart from the TGF-β/BMP signaling, Bgn is shown to trigger the Wnt pathway by binding to the canonical Wnt ligand Wnt3a as well as the Wnt receptor low density lipoprotein receptor-related protein 6 (LRP6). Both glycosylated and non-glycosylated forms of Bgn are shown to activate this signaling pathway [36], [37]. Bgn also plays a crucial role in ERK phosphorylation and signal transduction through the transcription factor Runx2 [38]. This activation is mediated through the GAG chains, as phosphorylation of ERK is not observed when only core protein of Bgn is presented. Decorin (Dcn) also belongs to class I SLRP, carrying a single DS or CS GAG chain. Dcn was named because of its ability to decorate collagen fibrils, and is considered as Bgn’s closest related PG [39]. Bgn and Dcn are major PGs in the mineralized bone matrix. They share 57% identity at the amino acid level and are co-expressed in bone [40], [41]. In agreement with the high level of expression of Dcn in the dermis, mice harboring a targeted disruption of the Dcn gene have fragile skin with markedly reduced tensile strength [42]. Although Dcn deficiency also introduces changes in collagen fibril size and shape in bone, no major changes in bone mass or architecture are observed in these mice [31]. Due to the structural similarity of Bgn and Dcn, a compensatory mechanism is likely triggered when either is ablated, leading to an increased expression of the other, which could partially be explained by clustering of SLRP family members genes on their respective chromosomes [33], [37], [43]. Consistently, Dcn/Bgn double KO mice exhibit more severe skin and bone phenotypes than either single Bgn or Dcn KO models [31]. Compared to WT controls, skin tissue in Dcn/Bgn double KO mice is much thinner and fragile, and bone abnormalities develop earlier and affect both the cortical and trabecular bone mass as well as cranial suture fusion. These data imply a synergistic function between Bgn and Dcn. Bgn and Dcn are critical TGF-β-binding PGs [44], [45]. The absence of Bgn and Dcn prevents TGF-β from appropriate sequestration within the extracellular matrix, leading to increased TGF-β signaling in bone marrow stromal cells of Dcn/Bgn double KO mice. This is followed by a ‘switch in fate’ from growth to apoptosis, resulting in decreased osteoprogenitor cell numbers and reduced bone formation [46]. Thus, Bgn and Dcn appear to be essential for maintaining a proper number of mature osteoblasts by modulating the proliferation and survival of bone marrow stromal cells. Moreover, a recent report indicates that Bgn and another SLRP, fibromodulin, are coupling components and regulate osteoclastogenesis [47]. Fibromodulin is a class II SLRP with KS side chains, and is expressed in cartilage and bone cells during fetal endochondral and intramembranous ossification [48]. Single fibromodulin deficient mice develop ectopic bone in tendon, while the effect is exaggerated when both Bgn and fibromodulin are deleted, further suggesting the synergistic activities of SLRPs [49]. Bgn/Fibromodulin double KO mice have markedly low bone mass, which is explained by the direct binding of both Bgn and fibromodulin to tumor necrosis factor-alpha (TNF-α) as well as receptor activator of NF-κB ligand (RANKL) [47]. The lack of these two SLRPs leads to elevated TNF-α and RANKL in the cellular microenvironment, resulting in enhanced osteoclastogenesis.
In addition to the SLRPs, heparan sulfate proteoglycans (HSPGs) can also participate in bone resorption regulation. Osteoprotegerin (OPG) is a decoy receptor for RANKL and is known to be a high-affinity HS-binding protein [50], [51]. An in vivo study using knock-in mice with mutated OPG, which is incapable of binding to HS but binds RANKL normally, shows a severe osteoporotic phenotype similar to OPG-null mice [52]. The interaction of HS and secreted OPG immobilizes OPG at the cell surface and facilitates OPG binding to membrane anchored RANKL. In summary, the PGs in bone may regulate bioactivity and availability of multiple cytokines and growth factors, modulate bone formation and resorption, and ultimately influence bone turnover process (Fig. 1).
Fig. 1.
Schematic summary of the roles of small leucine-rich proteoglycans (SLRPs) in bone homeostasis. SLRPs interact with multiple cytokines, growth factors, and cell surface receptors, which are involved in key signaling pathways in regulating bone homeostasis. Decorin and biglycan are canonical class I SLRPs, with one or two chondroitin sulfate/dermatan sulfate chains attached to horseshoe-shaped core proteins, respectively. Fibromodulin belongs to class II SLRPs, with five binding sites for keratan sulfate chain. Biglycan promotes osteoblast differentiation through ERK phosphorylation and transcription factor Runx2. Decorin and biglycan bind TGF-β and regulate the downstream SMAD2/3 signaling to control cell proliferation and apoptosis. Alternatively, biglycan binds to BMP and activates SMAD1/5/8 phosphorylation to promote osteogenesis. In addition, biglycan promotes Wnt signaling and its downstream β-catenin to facilitate bone formation. TNF-α induces the expression of Dickkopf-1 (DKK-1) and sclerostin (SOST), which are inhibitors of Wnt signaling. Biglycan and fibromodulin bind TNF-α and RANKL, adjusting their bioavailability for bone resorption regulation by sequestering them in the cellular microenvironment. Figure created using BioRender (https://biorender.com/).
Coupling effects of proteoglycans and water in bone biomechanical properties
The negative charge of GAGs is conferred by acidic sugar residues and/or sulphate groups [24], [53]. Some types of GAGs, such as chondroitin-6-sulphate, DS and KS could form homo- and hetero-aggregates [54]. These complex assemblies are stabilized primarily by electrostatic forces, hydrogen bonds and hydrophobic interactions. As a consequence, the charged matrix functions as a ‘semi-permeable’ membrane that attracts water to provide charge equilibrium, which makes them occupy an enormous hydrodynamic volume in solution [20], [55], [56]. It has been estimated that the large CS PG aggrecan may decrease 80% of their volume under dry conditions, highlighting the contributions of PGs and GAGs in absorbing water, and maintaining hydrostatic and osmotic pressure of the tissue [57]. Evidence is emerging that hydration status significantly affects bone strength and toughness [43], [58], [59]. Water comprises 15%-25% of the volume of bone and exists in three different compartments, including free water (also referred to as pore, or mobile water), bound water, and structural water [60], [61]. Free water, which resides in the vascular space and the lacuno-canalicular network, can move according to pressure gradients that develop during movement of the skeleton. The amount of free water primarily reflects intracortical porosity, which is inversely proportional to the mechanical properties of bone [62], [63]. Structural solid-like water is a part of the mineral lattice or is integrated into the tropocollagen ultrastructure [61]. Bound water may account for as much as 40%-60% of the total water in bone, arising from hydrogen bonding (collagen) and electrostatic attractions (mineral) with varying degrees of affinity, ranging from loosely to tightly bound to the matrix [64], [65]. Tightly bound water is found within the collagen triple helix, which helps stabilize collagen. Loosely bound water localizes at the collagen-mineral interface. It has been suggested that water bound to the crystal surface helps orientate apatite crystals during biomineralization [66]. More importantly, loosely bound water allows load transfer between collagen and mineral, dissipating energy and reducing shear stresses at the interface, thus conferring ductility and plasticity to bone at bulk and ultrastructural levels [18], [67]. Synchrotron X-ray scattering and other studies show that bound water residing in small ultrastructural spaces (<4.0 Å) dictates the mechanical characteristics of bone, and the loss of bound water correlates strongly with bone toughness deterioration [18], [60], [63], [68].
Previous studies have used enzymatic digestion for GAG removal to investigate the influence of PGs and GAGs on the mechanical response of dental tissues. Chondroitinase ABC specifically degrades CS and DS by catalyzing the eliminative degradation of polysaccharides containing (1-4)-β-d-hexosaminyl and (1-3)-β-d-glucuronosyl or (1-3)-α-l-iduronosyl linkages to disaccharides containing 4-deoxy-β-d-gluc-4-enuronosyl groups. After GAG removal from the cementum-dentin junction using Chondroitinase ABC, the atomic force microscope (AFM)-based nanoindentation properties of the enzymatically treated specimens were compared to those from untreated tissues. Results suggest that the digestion of GAGs significantly decreases the hardness and elastic modulus of cementum-dentin junction [56], [69]. A later study further verified the contributions of PGs and GAGs to the nanoindentation creep behavior of human dentin, showing their importance in the durability of dentin and likely other mineralized tissue [70]. Our previous novel findings suggest that PGs play a crucial role in toughening bone through their ability to retain water molecules in the matrix [71]. Cortical bone specimens were prepared from the posterior mid-diaphyseal femur region of male human donors around 50 years old. The human cadaveric bone was treated with or without peptide-N-glycosidase F (PNGase F). The PNGase F enzyme with cleavage of N-glycosylated oligosaccharides can only enzymatically remove N-glycan of a minor subset of keratin sulfate PGs present in bone matrix. These PGs include fibromodulin, PRELP, and osteoadherin [48], [72], [73], [74]. The nanoscratch test developed by Islam et al was utilized to measure the tissue-level toughness of bone specimens under both wet and dehydrated conditions [75]. The results show that removal of N-glycan linked GAGs has significant effects on the tissue-level toughness of wet bone samples. In contrast, the toughness of dry bone is not affected by GAGs removal, indicating that GAGs in bone play a pivotal role in sustaining the toughness of the tissue only when water is present. Since Bgn is a major subtype of PGs and is highly expressed in bone tissues [76], [77], [78], using the Bgn-deficient mouse model, we recently set out to determine the specific role of Bgn in retaining bound water in bone matrix and maintaining bone toughness [79]. The amount of total GAGs and bound water determined by low-field nuclear magnetic resonance (NMR) are reduced significantly in the Bgn KO mice bone matrix, along with the reduction of bone toughness. However, such differences are diminished once the bound water is removed from bone matrix. Consistent with previous findings of the positive correlation between bound water and bone mechanical properties, a significant reduction of tissue-level toughness of bone in WT is observed after removal of bound water. These changes are significantly reduced in Bgn KO mice. These results suggest that the coupling effect of water and PGs in sustaining the toughness of bone is disrupted once Bgn is removed from the matrix.
Classically, CS is predominantly attached to Bgn and Dcn within mineralized tissues, such as dentine and bone, whereas DS is mainly associated with soft connective tissues, such as skin and ligament. It has been reported that the GAG chains of Bgn and Dcn change from DS to CS during the differentiation process of the cell proliferation phase to the mineralization phase [80]. Interestingly, treating human bone specimens with chondroitinase ABC or a protein deglycosylation mix, which could remove any type of GAGs from core proteins, results in comparable levels of GAG removal [81]. Moreover, agarose electrophoresis reveals that only CS band is identified in the mineralized compartment of human and mouse bone matrix, without noticeable presence of HS and DS [79], [81]. These observations imply that CS is the major GAG in mouse bone matrix. It has been shown that CS intradermally injected in vivo could be incorporated into mouse tissues [82]. We have showed that supplementation of CS to WT mice increases the total amount of GAGs and bound water in bone matrix, the whole-body BMD, and the tissue-level toughness of bone [79]. However, CS administration does not improve the biomechanical properties of bone from Bgn KO mice. Additionally, bone histomophormetry studies suggest a decreased catabolic response in CS injected WT mice revealed by inhibited osteoclastogenesis. In fact, several previous reports have indicated the anti-catabolic function of CS by upregulating OPG production while decreasing RANKL expression, thus exerting a positive outcome for bone [83], [84], [85]. CS also has direct effects on inhibition of osteoclast differentiation, expression of TRAP and cathepsin K, and protects type I collagen from degradation by cathepsin K [86], [87], [88]. Lastly, a recent in vivo study using CS treatment for diabetic osteoporosis reveals an increased BMD, repaired bone morphology and decreased femoral osteoclasts [89].
Taken together, PGs and GAGs, especially Bgn and CS play pivotal roles in retaining bound water in bone matrix, thus imparting toughness to bone (Fig. 2). The administration of CS, possibly locally, could serves as a viable treatment strategy for improving bone quality.
Fig. 2.
The schematic representation of proteoglycans (PGs) in attracting bound water in the extracellular bone matrix. Structurally, bone is comprised of mineralized collagen fibrils embedded in bone extrafibrillar matrix. PGs have high affinity to minerals and collagen. The glycosaminoglycan (GAG) side chains of PGs are highly negatively charged, thus play a pivotal role in retaining bound water at the collagen-mineral interface and maintaining hydrostatic and osmotic pressure of bone. The bound water allows mechanical load transfer between collagen and mineral, dissipating energy and reducing shear stresses at the interface, thus conferring ductility and plasticity to bone. Figure created using BioRender (https://biorender.com/).
Changes of proteoglycans and water retention during aging
During the aging process, the loss of the delicate balance of bone homeostasis toward more bone resorption coupled with less bone formation leads to significant bone loss and changes in bone microarchitecture, including bone size and geometry. In addition, changes in the bone extracellular matrix material properties also occurs with aging. The loss of bone quantity and altered bone quality may weaken bones and culminate in osteoporosis with an increased risk of fractures [90].
The age-related changes in bone matrix affect different components in different ways, however, the effects in one component can have profound implications for changes of other components within the matrix, and ultimately for the deterioration of mechanical properties associated with aging [7], [64]. For example, the changes of secretion and glycanation states of PGs may influence their binding to collagen. In human intervertebral discs and articular cartilage tissues, the glycanated forms of both Bgn and Dcn represent a greater proportion of the total proteoglycan population in juveniles, but the GAG-free forms become predominant with age [91], [92]. A similar reduction in Dcn glycanation was further confirmed in cortical bone from aged WT mice [93]. Consistently, the GAG chains of fibromodulin are shortened with aging until the non-glycanated form becomes the predominant one [94]. These age-related changes of PGs can be a result of an increased rate of degradation of their glycanated precursors, or changes in growth factor/cytokine synthesis with aging [37], [95]. In addition to PGs, the ratio of bound water and free water in bone matrix also changes during aging. The water that is bound to collagen and mineral declines with age, while free water increases with age as bone mass is lost [68], [96]. There is also a re-distribution of water from the loosely bound fraction to the more tightly bound fraction [97]. These changes contribute to skeletal fragility by reducing the amount that bone can deform before fracturing, leading to age-related deterioration of bone toughness [64]. Interestingly, it has been found that loss of water in bone can initiate glycation reactions which cause the accumulation of advanced glycation end-products (AGEs). AGEs are intrafibrillar collagen crosslink-like bonds formed through a series of posttranslational modifications involving the condensation of arginine, lysine, and free sugars. AGEs increase naturally with age, and modify the physical properties of collagen fibers, including an increase in fiber diameter and stiffness, resulting in a loss of solubility and flexibility [98]. Alternatively, increasing the stability of the collagen network by cross-linking could prevent the collagen from binding water [99]. Our recent findings indicate that the loss of water and reduction in toughness with age, may partly depend on changes of the PGs/GAGs in bone [81]. Human cadaveric bone specimens from male donors in three different age groups: young (aged 26 ± 6 years), mid-aged (aged 52 ± 5 years), and elderly (aged 73 ± 5 years) were used in this study. There is a significant reduction (17%) of matrix GAGs in the elderly group compared to young human bone samples, accompanied by a 40% decrease in the overall bound water fraction and 25%-30% reduction in bone toughness. Correspondingly, we observed a decline of CS and Bgn in bone mineralized matrix with aging. Pearson correlation analysis suggests that the amount of bound water, GAGs, and the tissue-level toughness of bone are significantly correlated. This demonstrates that loss of GAGs has the potential to be one of the molecular origins of age-related deterioration of bone quality via reducing the amount of bound water. Existing evidence suggests that the receptor for AGEs (RAGE) functions as a receptor for CS GAGs, which is the major subtype of GAGs in bone [100]. Furthermore, the age-related accumulation of AGEs may negatively modulate the synthesis of PGs [101], [102], acting as a possible mechanism responsible for the loss of PGs/GAGs in the bone extracellular matrix during aging process. To summarize, the deterioration of bone toughness during aging could be through a deterministic combination of factors that include decreased GAGs/PGs, increased AGE accumulation, and the reduction of bound water, especially in the loosely bound fraction in bone matrix.
Conclusions
Bone is a dynamic organ undergoing constant modeling and remodeling and has a highly hierarchical nature comprised of type I collagen fibrils embedded in a matrix of non-collagenous proteins, water, and a hydroxyapatite mineral phase. PGs are a group of structural non-collagenous proteins in the bone extracellular matrix. PGs and their GAG side chains form supramolecular aggregates that interconnect the collagenous network and maintain the hydrostatic and osmotic pressure of tissue. However, the participation of PGs and GAGs in biomechanics of mineralized tissues has been overlooked. The SLRPs are a family of the most abundant PGs in the bone matrix, including Bgn and Dcn. They adopt specific structures for collagen fibril binding, and play vital roles in key signaling pathways, such as the TGF-β, BMP-2/-4, TNF-α, and Wnt signaling, and modulating bone development and homeostasis. In addition, since GAGs are highly negatively charged, they are ideal candidates to attract water into the bone extracellular matrix. There is a strong correlation of hydration status with bone strength and toughness, and ablation or removal of GAGs from PGs results in loss of bound water. This is associated with reduction of bone biomechanical properties. Supplementation of CS, the major subtype of GAGs in bone matrix, increases the total amount of GAGs and bound water in the bone matrix, accompanied by improved bone toughness. Moreover, the reduction of GAGs and bound water also correlates with the deterioration of bone biomechanical characteristics during aging. In conclusion, PGs and GAGs, especially SLRPs, have molecular signaling functions that regulate bone remodeling, and are critical to the hydration status of bone, thus they contribute significantly to the biomechanical properties of bone.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors thank Eduardo R. Cardenas for critical reading of the manuscript. The work was supported by NIH grants, AR076190 to J.X.J and Welch Foundation grant AQ-1507 to J.X.J.
References
- 1.Schuit S.C., van der Klift M., Weel A.E., de Laet C.E., Burger H., Seeman E., Hofman A., Uitterlinden A.G., van Leeuwen J.P., Pols H.A. Fracture incidence and association with bone mineral density in elderly men and women: the rotterdam study. Bone. 2004;34(1):195–202. doi: 10.1016/j.bone.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Aspray T.J., Prentice A., Cole T.J., Sawo Y., Reeve J., Francis R.M. Low bone mineral content is common but osteoporotic fractures are rare in elderly rural Gambian women. J Bone Miner Res. 1996;11(7):1019–1025. doi: 10.1002/jbmr.5650110720. [DOI] [PubMed] [Google Scholar]
- 3.Hui S.L., Slemenda C.W., Johnston C.C. Age and bone mass as predictors of fracture in a prospective study. J Clin Investig. 1988;81(6):1804–1809. doi: 10.1172/JCI113523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kanis J.A., Johnell O., Oden A., Dawson A., De Laet C., Jonsson B. Ten year probabilities of osteoporotic fractures according to BMD and diagnostic thresholds. Osteopor Int. 2001;12(12):989–995. doi: 10.1007/s001980170006. [DOI] [PubMed] [Google Scholar]
- 5.Weiner S., Traub W. Bone structure: from angstroms to microns. FASEB J. 1992;6(3):879–885. [PubMed] [Google Scholar]
- 6.Nalla R.K., Kinney J.H., Ritchie R.O. Effect of orientation on the in vitro fracture toughness of dentin: the role of toughening mechanisms. Biomaterials. 2003;24(22):3955–3968. doi: 10.1016/s0142-9612(03)00278-3. [DOI] [PubMed] [Google Scholar]
- 7.Wang X., Shen X., Li X., Mauli Agrawal C. Age-related changes in the collagen network and toughness of bone. Bone. 2002;31(1):1–7. doi: 10.1016/s8756-3282(01)00697-4. [DOI] [PubMed] [Google Scholar]
- 8.Burr D.B. Bone material properties and mineral matrix contributions to fracture risk or age in women and men. J Musculoskelet Neuronal Interact. 2002;2(3):201–204. [PubMed] [Google Scholar]
- 9.Wang X., Bank R.A., TeKoppele J.M., Agrawal C.M. The role of collagen in determining bone mechanical properties. J Orthopaed Res. 2001;19(6):1021–1026. doi: 10.1016/S0736-0266(01)00047-X. [DOI] [PubMed] [Google Scholar]
- 10.Currey J.D. Effects of differences in mineralization on the mechanical properties of bone. Philos Trans R Soc Lon Ser B Biol Sci. 1984;304(1121):509–518. doi: 10.1098/rstb.1984.0042. [DOI] [PubMed] [Google Scholar]
- 11.Morgan S., Poundarik A.A., Vashishth D. Do non-collagenous proteins affect skeletal mechanical properties? Calcif Tissue Int. 2015;97(3):281–291. doi: 10.1007/s00223-015-0016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poundarik A.A., Diab T., Sroga G.E., Ural A., Boskey A.L., Gundberg C.M., Vashishth D. Dilatational band formation in bone. Proc Natl Acad Sci USA. 2012;109(47):19178–19183. doi: 10.1073/pnas.1201513109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sroga G.E., Vashishth D. Effects of bone matrix proteins on fracture and fragility in osteoporosis. Curr Osteopor Rep. 2012;10(2):141–150. doi: 10.1007/s11914-012-0103-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Best S.M., Duer M.J., Reid D.G., Wise E.R., Zou S. Towards a model of the mineral-organic interface in bone: NMR of the structure of synthetic glycosaminoglycan- and polyaspartate-calcium phosphate composites. Magn Reson Chem MRC. 2008;46(4):323–329. doi: 10.1002/mrc.2168. [DOI] [PubMed] [Google Scholar]
- 15.Hashimoto Y., Lester G.E., Caterson B., Yamauchi M. EDTA-insoluble, calcium-binding proteoglycan in bovine bone. Calcif Tissue Int. 1995;56(5):398–402. doi: 10.1007/BF00301609. [DOI] [PubMed] [Google Scholar]
- 16.Iozzo R.V., Schaefer L. Proteoglycan form and function: a comprehensive nomenclature of proteoglycans. Matrix Biol. 2015;42:11–55. doi: 10.1016/j.matbio.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nyman J.S., Roy A., Shen X., Acuna R.L., Tyler J.H., Wang X. The influence of water removal on the strength and toughness of cortical bone. J Biomech. 2006;39(5):931–938. doi: 10.1016/j.jbiomech.2005.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Samuel J., Sinha D., Zhao J.-G., Wang X. Water residing in small ultrastructural spaces plays a critical role in the mechanical behavior of bone. Bone. 2014;59:199–206. doi: 10.1016/j.bone.2013.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nikitovic D., Aggelidakis J., Young M.F., Iozzo R.V., Karamanos N.K., Tzanakakis G.N. The biology of small leucine-rich proteoglycans in bone pathophysiology. J Biol Chem. 2012;287(41):33926–33933. doi: 10.1074/jbc.R112.379602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han EunHee, Chen S., Klisch S., Sah R. Contribution of proteoglycan osmotic swelling pressure to the compressive properties of articular cartilage. Biophys J. 2011;101(4):916–924. doi: 10.1016/j.bpj.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robinson K.A., Sun M., Barnum C.E., Weiss S.N., Huegel J., Shetye S.S., Lin L., Saez D., Adams S.M., Iozzo R.V., Soslowsky L.J., Birk D.E. Decorin and biglycan are necessary for maintaining collagen fibril structure, fiber realignment, and mechanical properties of mature tendons. Matrix Biol. 2017;64:81–93. doi: 10.1016/j.matbio.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sivan S., Merkher Y., Wachtel E., Ehrlich S., Maroudas A. Correlation of swelling pressure and intrafibrillar water in young and aged human intervertebral discs. J Orthopaedic Res. 2006;24(6):1292–1298. doi: 10.1002/jor.20144. [DOI] [PubMed] [Google Scholar]
- 23.U. Lindahl, J. Couchman, K. Kimata, J.D. Esko, Proteoglycans and Sulfated Glycosaminoglycans, in: rd, A. Varki, R.D. Cummings, J.D. Esko, P. Stanley, G.W. Hart, M. Aebi, A.G. Darvill, T. Kinoshita, N.H. Packer, J.H. Prestegard, R.L. Schnaar, P.H. Seeberger (Eds.), Essentials of Glycobiology, Cold Spring Harbor (NY), 2015, pp. 207-221. [PubMed]
- 24.Prydz K. Determinants of Glycosaminoglycan (GAG) Structure. Biomolecules. 2015;5(3):2003–2022. doi: 10.3390/biom5032003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raspanti Mario, Viola Manuela, Forlino Antonella, Tenni Ruggero, Gruppi Cristian, Tira Maria Enrica. Glycosaminoglycans show a specific periodic interaction with type I collagen fibrils. J Struct Biol. 2008;164(1):134–139. doi: 10.1016/j.jsb.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 26.Hedlund H., Mengarelli-Widholm S., Heinegard D., Reinholt F.P., Svensson O. Fibromodulin distribution and association with collagen. Matrix Biol. 1994;14(3):227–232. doi: 10.1016/0945-053x(94)90186-4. [DOI] [PubMed] [Google Scholar]
- 27.Schaefer L., Iozzo R.V. Biological functions of the small leucine-rich proteoglycans: from genetics to signal transduction. J Biol Chem. 2008;283(31):21305–21309. doi: 10.1074/jbc.R800020200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lamoureux F., Baud'huin M., Duplomb L., Heymann D., Redini F. Proteoglycans: key partners in bone cell biology. BioEssays. 2007;29(8):758–771. doi: 10.1002/bies.20612. [DOI] [PubMed] [Google Scholar]
- 29.Ingram R.T., Clarke B.L., Fisher L.W., Fitzpatrick L.A. Distribution of noncollagenous proteins in the matrix of adult human bone: evidence of anatomic and functional heterogeneity. J Bone Mineral Res. 1993;8(9):1019–1029. doi: 10.1002/jbmr.5650080902. [DOI] [PubMed] [Google Scholar]
- 30.Xu Tianshun, Bianco Paolo, Fisher Larry W., Longenecker Glenn, Smith Erica, Goldstein Steven, Bonadio Jeffrey, Boskey Adele, Heegaard Anne-Marie, Sommer Beatrice, Satomura Kazuhito, Dominguez Pedro, Zhao Chengyan, Kulkarni Ashok B., Robey Pamela Gehron, Young Marian F. Targeted disruption of the biglycan gene leads to an osteoporosis-like phenotype in mice. Nat Genet. 1998;20(1):78–82. doi: 10.1038/1746. [DOI] [PubMed] [Google Scholar]
- 31.Corsi A., Xu T., Chen X.D., Boyde A., Liang J., Mankani M., Sommer B., Iozzo R.V., Eichstetter I., Robey P.G., Bianco P., Young M.F. Phenotypic effects of biglycan deficiency are linked to collagen fibril abnormalities, are synergized by decorin deficiency, and mimic Ehlers-Danlos-like changes in bone and other connective tissues. J Bone Mineral Res. 2002;17(7):1180–1189. doi: 10.1359/jbmr.2002.17.7.1180. [DOI] [PubMed] [Google Scholar]
- 32.Chen X.D., Shi S., Xu T., Robey P.G., Young M.F. Age-related osteoporosis in biglycan-deficient mice is related to defects in bone marrow stromal cells. J Bone Mineral Res. 2002;17(2):331–340. doi: 10.1359/jbmr.2002.17.2.331. [DOI] [PubMed] [Google Scholar]
- 33.Chen X.D., Fisher L.W., Robey P.G., Young M.F. The small leucine-rich proteoglycan biglycan modulates BMP-4-induced osteoblast differentiation. FASEB J. 2004;18(9):948–958. doi: 10.1096/fj.03-0899com. [DOI] [PubMed] [Google Scholar]
- 34.Ye Y., Hu W., Guo F., Zhang W., Wang J., Chen A. Glycosaminoglycan chains of biglycan promote bone morphogenetic protein-4-induced osteoblast differentiation. Int J Mol Med. 2012;30(5):1075–1080. doi: 10.3892/ijmm.2012.1091. [DOI] [PubMed] [Google Scholar]
- 35.Mochida Y., Parisuthiman D., Yamauchi M. Biglycan is a positive modulator of BMP-2 induced osteoblast differentiation. Adv Exp Med Biol. 2006;585:101–113. doi: 10.1007/978-0-387-34133-0_7. [DOI] [PubMed] [Google Scholar]
- 36.Berendsen A.D., Fisher L.W., Kilts T.M., Owens R.T., Robey P.G., Gutkind J.S., Young M.F. Modulation of canonical Wnt signaling by the extracellular matrix component biglycan. PNAS. 2011;108(41):17022–17027. doi: 10.1073/pnas.1110629108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kram V., Shainer R., Jani P., Meester J.A.N., Loeys B., Young M.F. Biglycan in the Skeleton. J Histochem Cytochemistry. 2020;68(11):747–762. doi: 10.1369/0022155420937371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Xiaoyan, Harimoto Kenichi, Xie Sijia, Cheng Hao, Liu Jing, Wang Zhao. Matrix protein biglycan induces osteoblast differentiation through extracellular signal-regulated kinase and Smad pathways. Biol Pharm Bull. 2010;33(11):1891–1897. doi: 10.1248/bpb.33.1891. [DOI] [PubMed] [Google Scholar]
- 39.Ruoslahti Erkki. Structure and biology of proteoglycans. Annu Rev Cell Biol. 1988;4(1):229–255. doi: 10.1146/annurev.cb.04.110188.001305. [DOI] [PubMed] [Google Scholar]
- 40.Iozzo R.V. The family of the small leucine-rich proteoglycans: key regulators of matrix assembly and cellular growth. Crit Rev Biochem Mol Biol. 1997;32(2):141–174. doi: 10.3109/10409239709108551. [DOI] [PubMed] [Google Scholar]
- 41.Iozzo Renato V. The biology of the small leucine-rich proteoglycans. Funct Netw Interact Proteins J Biol Chem. 1999;274(27):18843–18846. doi: 10.1074/jbc.274.27.18843. [DOI] [PubMed] [Google Scholar]
- 42.Danielson K.G., Baribault H., Holmes D.F., Graham H., Kadler K.E., Iozzo R.V. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J Cell Biol. 1997;136(3):729–743. doi: 10.1083/jcb.136.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Young M.F., Bi Y., Ameye L., Chen X.D. Biglycan knockout mice: new models for musculoskeletal diseases. Glycoconj J. 2002;19(4–5):257–262. doi: 10.1023/A:1025336114352. [DOI] [PubMed] [Google Scholar]
- 44.Hildebrand A., Romaris M., Rasmussen L.M., Heinegard D., Twardzik D.R., Border W.A., Ruoslahti E. Interaction of the small interstitial proteoglycans biglycan, decorin and fibromodulin with transforming growth factor beta. Biochem J. 1994;302(Pt 2):527–534. doi: 10.1042/bj3020527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kolb M., Margetts P.J., Sime P.J., Gauldie J. Proteoglycans decorin and biglycan differentially modulate TGF-beta-mediated fibrotic responses in the lung, American journal of physiology. Lung Cell Mol Physiol. 2001;280(6):L1327–L1334. doi: 10.1152/ajplung.2001.280.6.L1327. [DOI] [PubMed] [Google Scholar]
- 46.Bi Yanming, Stuelten Christina H., Kilts Tina, Wadhwa Sunil, Iozzo Renato V., Robey Pamela G., Chen Xiao-Dong, Young Marian F. Extracellular matrix proteoglycans control the fate of bone marrow stromal cells. The J Biol Chem. 2005;280(34):30481–30489. doi: 10.1074/jbc.M500573200. [DOI] [PubMed] [Google Scholar]
- 47.Kram V., Kilts T.M., Bhattacharyya N., Li L., Young M.F. Small leucine rich proteoglycans, a novel link to osteoclastogenesis. Sci Rep. 2017;7(1):12627. doi: 10.1038/s41598-017-12651-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gori Francesca, Schipani Ernestina, Demay Marie B. Fibromodulin is expressed by both chondrocytes and osteoblasts during fetal bone development. J Cell Biochem. 2001;82(1):46–57. doi: 10.1002/jcb.1115. [DOI] [PubMed] [Google Scholar]
- 49.Ameye L., Aria D., Jepsen K., Oldberg A., Xu T., Young M.F. Abnormal collagen fibrils in tendons of biglycan/fibromodulin-deficient mice lead to gait impairment, ectopic ossification, and osteoarthritis. FASEB J Ofi Federation Am Soc Exp Biol. 2002;16(7):673–680. doi: 10.1096/fj.01-0848com. [DOI] [PubMed] [Google Scholar]
- 50.Li Miaomiao, Yang Shuying, Xu Ding. Heparan sulfate regulates the structure and function of osteoprotegerin in osteoclastogenesis. J Biol Chem. 2016;291(46):24160–24171. doi: 10.1074/jbc.M116.751974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Theoleyre S., Kwan Tat S., Vusio P., Blanchard F., Gallagher J., Ricard-Blum S., Fortun Y., Padrines M., Redini F., Heymann D. Characterization of osteoprotegerin binding to glycosaminoglycans by surface plasmon resonance: role in the interactions with receptor activator of nuclear factor kappaB ligand (RANKL) and RANK. Biochem Biophys Res Commun. 2006;347(2):460–467. doi: 10.1016/j.bbrc.2006.06.120. [DOI] [PubMed] [Google Scholar]
- 52.Li Miaomiao, Xu Ding. Antiresorptive activity of osteoprotegerin requires an intact heparan sulfate-binding site. PNAS. 2020;117(29):17187–17194. doi: 10.1073/pnas.2005859117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Prydz K., Dalen K.T. Synthesis and sorting of proteoglycans. J Cell Sci. 2000;113(Pt 2):193–205. doi: 10.1242/jcs.113.2.193. [DOI] [PubMed] [Google Scholar]
- 54.Scott J.E. Elasticity in extracellular matrix 'shape modules' of tendon, cartilage, etc A sliding proteoglycan-filament model. J Physiol. 2003;553(2):335–343. doi: 10.1113/jphysiol.2003.050179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gandhi N.S., Mancera R.L. The structure of glycosaminoglycans and their interactions with proteins. Chem Biol Drug Des. 2008;72(6):455–482. doi: 10.1111/j.1747-0285.2008.00741.x. [DOI] [PubMed] [Google Scholar]
- 56.Bertassoni Luiz E., Swain Michael V. The contribution of proteoglycans to the mechanical behavior of mineralized tissues. J Mech Behav Biomed Mater. 2014;38:91–104. doi: 10.1016/j.jmbbm.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 57.Myers E.R., Lai W.M., Mow V.C. A continuum theory and an experiment for the ion-induced swelling behavior of articular cartilage. J Biomech Eng. 1984;106(2):151–158. doi: 10.1115/1.3138473. [DOI] [PubMed] [Google Scholar]
- 58.Broz J.J., Simske S.J., Greenberg A.R., Luttges M.W. Effects of rehydration state on the flexural properties of whole mouse long bones. J Biomech Eng. 1993;115(4A):447–449. doi: 10.1115/1.2895510. [DOI] [PubMed] [Google Scholar]
- 59.Young M.F., Bi Y., Ameye L., Xu T., Wadhwa S., Heegaard A., Kilts T., Chen X.D. Small leucine-rich proteoglycans in the aging skeleton. J Musculoskelet Neuronal Interact. 2006;6(4):364–365. [PubMed] [Google Scholar]
- 60.Granke Mathilde, Does Mark D., Nyman Jeffry S. The role of water compartments in the material properties of cortical bone. Calcif Tissue Int. 2015;97(3):292–307. doi: 10.1007/s00223-015-9977-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Unal Mustafa, Creecy Amy, Nyman Jeffry S. The role of matrix composition in the mechanical behavior of bone. Curr Osteopor Rep. 2018;16(3):205–215. doi: 10.1007/s11914-018-0433-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bae W.C., Chen P.C., Chung C.B., Masuda K., D'Lima D., Du J. Quantitative ultrashort echo time (UTE) MRI of human cortical bone: correlation with porosity and biomechanical properties. J Bone Miner Res. 2012;27(4):848–857. doi: 10.1002/jbmr.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Granke M., Makowski A.J., Uppuganti S., Does M.D., Nyman J.S. Identifying novel clinical surrogates to assess human bone fracture toughness. J Bone Mineral Res. 2015;30(7):1290–1300. doi: 10.1002/jbmr.2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Burr David B. Changes in bone matrix properties with aging. Bone. 2019;120:85–93. doi: 10.1016/j.bone.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 65.Ong H.H., Wright A.C., Wehrli F.W. Deuterium nuclear magnetic resonance unambiguously quantifies pore and collagen-bound water in cortical bone. J Bone Mineral Res. 2012;27(12):2573–2581. doi: 10.1002/jbmr.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang Yan, Von Euw Stanislas, Fernandes Francisco M., Cassaignon Sophie, Selmane Mohamed, Laurent Guillaume, Pehau-Arnaudet Gérard, Coelho Cristina, Bonhomme-Coury Laure, Giraud-Guille Marie-Madeleine, Babonneau Florence, Azaïs Thierry, Nassif Nadine. Water-mediated structuring of bone apatite. Nat Mater. 2013;12(12):1144–1153. doi: 10.1038/nmat3787. [DOI] [PubMed] [Google Scholar]
- 67.Samuel Jitin, Park Jun-Sang, Almer Jonathan, Wang Xiaodu. Effect of water on nanomechanics of bone is different between tension and compression. J Mech Behav Biomed Mater. 2016;57:128–138. doi: 10.1016/j.jmbbm.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nyman Jeffry S., Ni Qingwen, Nicolella Daniel P., Wang Xiaodu. Measurements of mobile and bound water by nuclear magnetic resonance correlate with mechanical properties of bone. Bone. 2008;42(1):193–199. doi: 10.1016/j.bone.2007.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ho Sunita P., Sulyanto Rosalyn M., Marshall Sally J., Marshall Grayson W. The cementum-dentin junction also contains glycosaminoglycans and collagen fibrils. J Struct Biol. 2005;151(1):69–78. doi: 10.1016/j.jsb.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 70.Bertassoni Luiz E., Kury Matheus, Rathsam Catherine, Little Christopher B., Swain Michael V. The role of proteoglycans in the nanoindentation creep behavior of human dentin. J Mech Behav Biomed Mater. 2016;55:264–270. doi: 10.1016/j.jmbbm.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 71.Wang X., Xu H., Huang Y., Gu S., Jiang J.X. Coupling Effect of Water and Proteoglycans on the In Situ Toughness of Bone. J Bone Mineral Res. 2016;31(5):1026–1029. doi: 10.1002/jbmr.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Funderburgh J.L. Keratan sulfate: structure, biosynthesis, and function. Glycobiology. 2000;10(10):951–958. doi: 10.1093/glycob/10.10.951. [DOI] [PubMed] [Google Scholar]
- 73.Li H., Cui Y., Luan J., Zhang X., Li C., Zhou X., Shi L., Wang H., Han J. PRELP (proline/arginine-rich end leucine-rich repeat protein) promotes osteoblastic differentiation of preosteoblastic MC3T3-E1 cells by regulating the beta-catenin pathway. Biochem Biophys Res Commun. 2016;470(3):558–562. doi: 10.1016/j.bbrc.2016.01.106. [DOI] [PubMed] [Google Scholar]
- 74.Sommarin Yngve, Wendel Mikael, Shen Zhenxin, Hellman Ulf, Heinegård Dick. Osteoadherin, a cell-binding keratan sulfate proteoglycan in bone, belongs to the family of leucine-rich repeat proteins of the extracellular matrix. J Biol Chem. 1998;273(27):16723–16729. doi: 10.1074/jbc.273.27.16723. [DOI] [PubMed] [Google Scholar]
- 75.Islam Anowarul, Neil Dong X., Wang Xiaodu. Mechanistic modeling of a nanoscratch test for determination of in situ toughness of bone. J Mech Behav Biomed Mater. 2012;5(1):156–164. doi: 10.1016/j.jmbbm.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 76.Fisher L.W., Termine J.D., Dejter S.W., Whitson S.W., Yanagishita M., Kimura J.H., Hascall V.C., Kleinman H.K., Hassell J.R., Nilsson B. Proteoglycans of developing bone. J Biol Chem. 1983;258(10):6588–6594. [PubMed] [Google Scholar]
- 77.Fisher L.W., Termine J.D., Young M.F. Deduced protein sequence of bone small proteoglycan I (biglycan) shows homology with proteoglycan II (decorin) and several nonconnective tissue proteins in a variety of species. J Biol Chem. 1989;264(8):4571–4576. [PubMed] [Google Scholar]
- 78.Bianco P., Fisher L.W., Young M.F., Termine J.D., Robey P.G. Expression and localization of the two small proteoglycans biglycan and decorin in developing human skeletal and non-skeletal tissues. J Histochem Cytochem. 1990;38(11):1549–1563. doi: 10.1177/38.11.2212616. [DOI] [PubMed] [Google Scholar]
- 79.Hua Rui, Ni Qingwen, Eliason Travis D., Han Yan, Gu Sumin, Nicolella Daniel P., Wang Xiaodu, Jiang Jean X. Biglycan and chondroitin sulfate play pivotal roles in bone toughness via retaining bound water in bone mineral matrix. Matrix Boil J Int Soc Matrix Biol. 2020;94:95–109. doi: 10.1016/j.matbio.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Waddington RJ, Roberts HC, Sugars RV, Schönherr E. Differential roles for small leucine-rich proteoglycans in bone formation. Eur Cells Mater. 2003;6:12–21. doi: 10.22203/ecm.v006a02. [DOI] [PubMed] [Google Scholar]
- 81.Wang Xiaodu, Hua Rui, Ahsan Abu, Ni Qingwen, Huang Yehong, Gu Sumin, Jiang Jean X. Age-related deterioration of bone toughness is related to diminishing amount of matrix glycosaminoglycans (Gags) JBMR Plus. 2018;2(3):164–173. doi: 10.1002/jbm4.10030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang J.Y., Roehrl M.H. Glycosaminoglycans are a potential cause of rheumatoid arthritis. PNAS. 2002;99(22):14362–14367. doi: 10.1073/pnas.222536599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pecchi E., Priam S., Mladenovic Z., Gosset M., Saurel A.S., Aguilar L., Berenbaum F., Jacques C. A potential role of chondroitin sulfate on bone in osteoarthritis: inhibition of prostaglandin E(2) and matrix metalloproteinases synthesis in interleukin-1beta-stimulated osteoblasts. Osteoarthr Cartilage. 2012;20(2):127–135. doi: 10.1016/j.joca.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 84.Tat Steeve, Pelletier Jean-Pierre, Vergés Josep, Lajeunesse Daniel, Montell Eulàlia, Fahmi Hassan, Lavigne Martin, Martel-Pelletier Johanne. Chondroitin and glucosamine sulfate in combination decrease the pro-resorptive properties of human osteoarthritis subchondral bone osteoblasts: a basic science study. Arthritis Res Ther. 2007;9(6):R117. doi: 10.1186/ar2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Monfort J., Pelletier J.P., Garcia-Giralt N., Martel-Pelletier J. Biochemical basis of the effect of chondroitin sulphate on osteoarthritis articular tissues. Ann Rheum Dis. 2008;67(6):735–740. doi: 10.1136/ard.2006.068882. [DOI] [PubMed] [Google Scholar]
- 86.Salbach Juliane, Kliemt Stefanie, Rauner Martina, Rachner Tilman D., Goettsch Claudia, Kalkhof Stefan, von Bergen Martin, Möller Stephanie, Schnabelrauch Matthias, Hintze Vera, Scharnweber Dieter, Hofbauer Lorenz C. The effect of the degree of sulfation of glycosaminoglycans on osteoclast function and signaling pathways. Biomaterials. 2012;33(33):8418–8429. doi: 10.1016/j.biomaterials.2012.08.028. [DOI] [PubMed] [Google Scholar]
- 87.Miyazaki T., Miyauchi S., Anada T., Tawada A., Suzuki O. Chondroitin sulfate-E binds to both osteoactivin and integrin alphaVbeta3 and inhibits osteoclast differentiation. J Cell Biochem. 2015;116(10):2247–2257. doi: 10.1002/jcb.25175. [DOI] [PubMed] [Google Scholar]
- 88.Tatara Y., Suto S., Itoh K. Novel roles of glycosaminoglycans in the degradation of type I collagen by cathepsin K. Glycobiology. 2017;27(12):1089–1098. doi: 10.1093/glycob/cwx083. [DOI] [PubMed] [Google Scholar]
- 89.Zheng Hong Xing, Chen De Jing, Zu Yue Xin, Wang En Zhu, Qi Shan Shan. Chondroitin sulfate prevents STZ induced diabetic osteoporosis through decreasing blood glucose, antioxidative stress, anti-inflammation and OPG/RANKL expression regulation. Int J Mol Sci. 2020;21(15):5303. doi: 10.3390/ijms21155303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Goltzman D. The aging skeleton. Adv Exp Med Biol. 2019;1164:153–160. doi: 10.1007/978-3-030-22254-3_12. [DOI] [PubMed] [Google Scholar]
- 91.Johnstone B., Markopoulos M., Neame P., Caterson B. Identification and characterization of glycanated and non-glycanated forms of biglycan and decorin in the human intervertebral disc. Biochem J. 1993;292(Pt 3):661–666. doi: 10.1042/bj2920661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Roughley P.J., White R.J., Magny M.C., Liu J., Pearce R.H., Mort J.S. Non-proteoglycan forms of biglycan increase with age in human articular cartilage. Biochem J. 1993;295(Pt 2):421–426. doi: 10.1042/bj2950421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chan W.L., Steiner M., Witkos T., Egerer J., Busse B., Mizumoto S., Pestka J.M., Zhang H., Hausser I., Khayal L.A., Ott C.E., Kolanczyk M., Willie B., Schinke T., Paganini C., Rossi A., Sugahara K., Amling M., Knaus P., Chan D., Lowe M., Mundlos S., Kornak U. Impaired proteoglycan glycosylation, elevated TGF-beta signaling, and abnormal osteoblast differentiation as the basis for bone fragility in a mouse model for gerodermia osteodysplastica. PLoS Genet. 2018;14(3) doi: 10.1371/journal.pgen.1007242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Roughley Peter J., White Robert J., Cs-Szabó Gabriella, Mort John S. Changes with age in the structure of fibromodulin in human articular cartilage. Osteoarthritis Cartilage. 1996;4(3):153–161. doi: 10.1016/s1063-4584(96)80011-2. [DOI] [PubMed] [Google Scholar]
- 95.Melching L.I., Roughley P.J. Modulation of keratan sulfate synthesis on lumican by the action of cytokines on human articular chondrocytes. Matrix Boil. 1999;18(4):381–390. doi: 10.1016/s0945-053x(99)00033-5. [DOI] [PubMed] [Google Scholar]
- 96.Uppuganti Sasidhar, Granke Mathilde, Makowski Alexander J., Does Mark D., Nyman Jeffry S. Age-related changes in the fracture resistance of male Fischer F344 rat bone. Bone. 2016;83:220–232. doi: 10.1016/j.bone.2015.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nyman Jeffry S., Gorochow Lacey E., Adam Horch R., Uppuganti Sasidhar, Zein-Sabatto Ahbid, Manhard Mary Katherine, Does Mark D. Partial removal of pore and loosely bound water by low-energy drying decreases cortical bone toughness in young and old donors. J Mech Behav Biomed Mater. 2013;22:136–145. doi: 10.1016/j.jmbbm.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yamamoto Masahiro, Sugimoto Toshitsugu. Advanced glycation end products. Diabetes Bone Strength Curr Osteoporosis Rep. 2016;14(6):320–326. doi: 10.1007/s11914-016-0332-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kopp J., Bonnet Madeleine, Renou J.P. Effect of collagen crosslinking on collagen-water interactions (a DSC investigation) Matrix. 1990;9(6):443–450. doi: 10.1016/s0934-8832(11)80013-2. [DOI] [PubMed] [Google Scholar]
- 100.Mizumoto Shuji, Takahashi Jun, Sugahara Kazuyuki. Receptor for advanced glycation end products (RAGE) functions as receptor for specific sulfated glycosaminoglycans, and anti-RAGE antibody or sulfated glycosaminoglycans delivered in vivo inhibit pulmonary metastasis of tumor cells. J Biol Chem. 2012;287(23):18985–18994. doi: 10.1074/jbc.M111.313437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Borrebaek J., Prydz K., Fjeldstad K., Vuong T.T., Berg T.J., Holkov C., Kolset S.O. The AGE product N epsilon-(carboxymethyl)lysine serum albumin is a modulator of proteoglycan expression in polarized cultured kidney epithelial cells. Diabetologia. 2001;44(4):488–494. doi: 10.1007/s001250051647. [DOI] [PubMed] [Google Scholar]
- 102.DeGroot J., Verzijl N., Bank R.A., Lafeber F.P., Bijlsma J.W., TeKoppele J.M. Age-related decrease in proteoglycan synthesis of human articular chondrocytes: the role of nonenzymatic glycation. Arthritis Rheum. 1999;42(5):1003–1009. doi: 10.1002/1529-0131(199905)42:5<1003::AID-ANR20>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]