Abstract
This study aimed to review the obstetric complications during subsequent pregnancies after uterine artery embolization (UAE) for postpartum hemorrhage (PPH) by exploring the relationship between prior UAE and obstetric complications through a meta-analysis. We conducted a systematic literature review through March 31, 2021, using PubMed, Scopus, and the Cochrane Central Register of Controlled Trials in compliance with the PRISMA guidelines and determined the effect of prior UAE for PPH on the rate of placenta accreta spectrum (PAS), PPH, placenta previa, hysterectomy, fetal growth restriction (FGR), and preterm birth (PTB). Twenty-three retrospective studies (2003–2021) met the inclusion criteria. They included 483 pregnancies with prior UAE and 320,703 pregnancies without prior UAE. The cumulative results of all women with prior UAE indicated that the rates of obstetric complications PAS, hysterectomy, and PPH were 16.3% (34/208), 6.5% (28/432), and 24.0% (115/480), respectively. According to the patient background-matched analysis based on the presence of prior PPH, women with prior UAE were associated with higher rates of PAS (odds ratio [OR] 20.82; 95% confidence interval [CI] 3.27–132.41) and PPH (OR 5.32, 95% CI 1.40–20.16) but not with higher rates of hysterectomy (OR 8.93, 95% CI 0.43–187.06), placenta previa (OR 2.31, 95% CI 0.35–15.22), FGR (OR 7.22, 95% CI 0.28–188.69), or PTB (OR 3.00, 95% CI 0.74–12.14), compared with those who did not undergo prior UAE. Prior UAE for PPH may be a significant risk factor for PAS and PPH during subsequent pregnancies. Therefore, at the time of delivery, clinicians should be more attentive to PAS and PPH when women have undergone prior UAE. Since the number of women included in the patient background-matched study was limited, further investigations are warranted to confirm the results of this study.
Subject terms: Outcomes research, Risk factors
Introduction
Postpartum hemorrhage (PPH) occurs in approximately 5% of deliveries, and severe PPH has led to approximately 140,000 annual maternal deaths worldwide1–5. According to the American College of Obstetricians and Gynecologists, PPH results in a cumulative blood loss of ≥ 1000 mL or is characterized by the presence of symptoms of hypovolemia related to blood loss within 24 h after vaginal or cesarean delivery6. First-line treatment for PPH includes pharmacological measures, intrauterine tamponade, uterine artery ligation, and uterine compression sutures; uterine artery embolization (UAE) is performed for women with treatment-refractory severe PPH6–12. If these procedures cannot achieve homeostasis, then hysterectomy is performed.
UAE is a useful alternative to hysterectomy for managing severe PPH6. It is an effective and minimally invasive procedure with feasible side effects and a consistent success rate of more than 90% for achieving hemostasis. Therefore, UAE is an essential procedure for treating severe PPH. Moreover, UAE for PPH has feasible short-term and long-term adverse effects13–16. According to a systematic review, the fertility rate after UAE for patients attempting another pregnancy is 70–80%17.
Prior UAE appears to be associated with an increased rate of various obstetric complications, such as placenta accreta spectrum (PAS), placenta previa, and PPH, during subsequent pregnancies18–21. Nevertheless, the rates of fetal growth restriction (FGR) and preterm birth (PTB) have not been sufficiently studied. Knowing the risks of maternal outcomes and obstetric outcomes of subsequent pregnancies after UAE may be helpful for its antenatal diagnosis and treatment involving multidisciplinary care22,23. Notably, recent systematic reviews have reported that the antenatal diagnosis of PAS is associated with improved maternal outcomes24,25. This study aimed to determine the effect of prior UAE on obstetric complications, including PAS, and maternal outcomes of subsequent pregnancies.
Materials and methods
Systematic literature review approach
A systematic review was performed to review the effect of prior UAE on subsequent pregnancies. The outcomes of interest were the rates of PAS, hysterectomy, PPH, placenta previa, FGR, PTB, and UAE, and the maternal outcomes (rates of urinary tract injury, infection, and transfusion). In compliance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, 2020 edition26, a systematic search was performed using PubMed (sorting by most recent), Scopus, and the Cochrane Central Register of Controlled Trials (CENTRAL) from inception to March 31, 2021, using MeSH terms (if applicable) and text words for the concepts “Uterine Artery Embolization” and “pregnancy” (see Supplemental Table S1 for complete search strategies). There were no date, language, or other restrictions.
Eligibility criteria, information sources, and search strategy
The concepts “Uterine Artery Embolization” and “pregnancy” were searched using the text words listed in Supplemental Table S1. These key words were entered in PubMed, Scopus, and CENTRAL to identify studies that examined the association between prior UAE and the outcomes of interest (MeSH terms were used for the PubMed and CENTRAL searches).
Study selection
The inclusion criteria, which were based on the Patient/Population, Intervention, Comparator, Outcome, Study (PICOS) process are shown in Supplemental Table S2. Studies were selected according to the following inclusion criteria: (1) the effect of prior UAE on the risk of the outcome of interest was examined; (2) PPH was controlled by UAE during prior pregnancy; (3) a comparative study of the outcome of interest was also included (UAE versus non-UAE); and (4) at least four subsequent pregnancies were included.
The exclusion criteria were as follows: (1) insufficient information about the outcomes of interest; (2) included > 10% of women who underwent UAE for uterine myoma; (3) included > 10% of women who underwent UAE for early pregnancy; (4) articles were not written in English; and (5) conference abstracts, case reports, case series, and reviews.
Studies were identified by screening the titles, abstracts, and full texts of the relevant articles. All titles, abstracts, and full texts were independently screened by the authors (Sh.M. and L.M.).
Data extraction
Two authors (Sh.M. and L.N.) independently extracted the data and recorded the following variables: UAE type; year of study; first author’s name; study location; number of included cases; PAS and PPH definitions; obstetric outcomes (rates of PAS, hysterectomy, PPH, placenta previa, FGR, and PTB); and maternal outcomes (rates of urinary tract injury, infection, and transfusion). Information regarding embolic agents for UAE was also collected. Since the preparation of agents may affect the quantity of embolic agents, information regarding the agent preparation was also collected27.
Outcome measure analysis and assessment of the risk of bias
Our primary objective was to assess the effect of prior UAE on the rates of PAS and hysterectomy during subsequent pregnancies. One secondary objective was divided into two sub-objectives, namely examining the effect of prior UAE on the rate of PPH and examining the effect of prior UAE on other obstetric complications such as placenta previa, FGR, and PTB. Another secondary objective was the assessment of the effect of prior UAE on maternal outcomes such as the rates of urinary tract injury, infection, and transfusion. The risk of bias was assessed using the Risk Of Bias in Nonrandomized Studies of Interventions (ROBINS-I) tool28–30.
Meta-analysis plan
Using the eligible study data, the risks of the outcomes of interest (PAS, hysterectomy, PPH, placenta previa, FGR, PTB, and UAE during subsequent pregnancies after UAE) were computed using the 95% confidence intervals (CIs) of the reported values to estimate the odds ratios (ORs) for the rate of these outcomes. The heterogeneity of the studies was examined using I2 statistics to measure the percentage of total variation across these studies. According to the Cochrane Handbook for Systematic Reviews of Interventions (version 6.0), heterogeneity was assessed based on the I2 value with the following modifications: 0% to < 30%, low heterogeneity; 30–60%, moderate heterogeneity; 50–90%, substantial heterogeneity; and 75–100%, considerable heterogeneity31.
We conducted the meta-analysis and created all graphics using RevMan ver. 5.4.1 software (Cochrane Collaboration, Copenhagen, Denmark). For consistency, data regarding all outcomes (continuous and bivariate) were entered into the software so that negative effect sizes or relative risks < 1 favored active intervention. During the pooled analysis, a fixed-effect analysis was performed if the heterogeneity of the studies was considered low; a random-effect analysis was performed if the heterogeneity of the studies was considered moderate to considerable.
Statistical analysis
Differences in baseline demographics between the two groups were assessed with the Fisher exact test or chi-square as appropriate32. All statistical analyses were based on two-sided hypotheses, and P < 0.05 was considered statistically significant. Statistical Package for Social Sciences (IBM SPSS, version 27.0, Armonk, NY, USA) was used for the analysis.
Ethical approval
The approval of Institutional Review Board exempted the use of publicly available data.
Results
Study selection
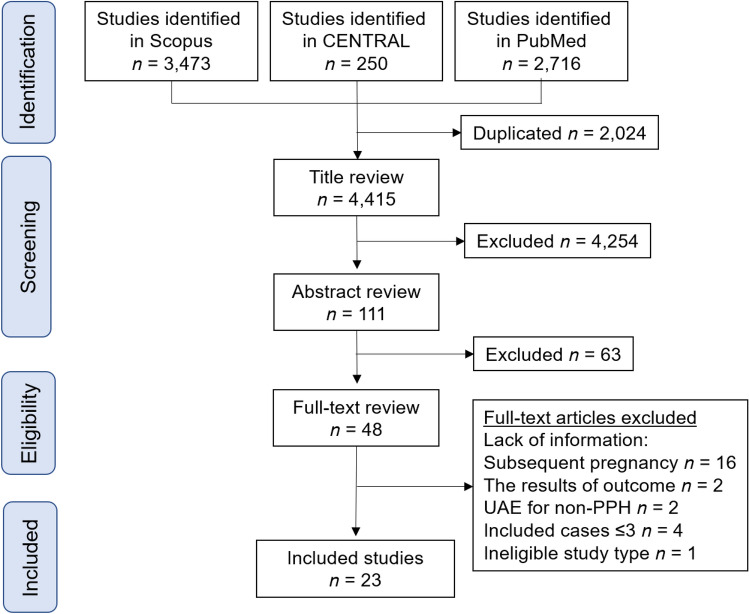
Figure 1 illustrates the study selection scheme. Overall, 6,439 studies were examined; among these, 23 studies13,16,18–21,33–49 including 483 pregnancies with prior UAE and 320,703 pregnancies without prior UAE met the inclusion criteria for the descriptive analysis.
Figure 1.
Study selection scheme used for the systematic review of the literature. PPH postpartum hemorrhage, UAE uterine artery embolization, CENTRAL Cochrane Central Register of Controlled Trials.
Study characteristics
Supplemental Tables S3 and S4 summarize the metadata of the evaluated studies. Of the 23 included studies, 18 were non-comparator studies13,16,21,34–48 and five were comparator studies18–20,33,49. Among these five comparator studies, the patient’s background was matched by including only women with previous PPH in two studies19,20. In these two studies19,20, we determined the prevalence of obstetric complications for women with previous PPH with or without UAE. In another study, cases were matched in a 1:3 ratio by maternal age, parity, ethnicity, year and mode of delivery, birth weight, and gestational age33. The two remaining studies did not match the patients' backgrounds; therefore all pregnancies were included and divided into prior UAE and non-UAE groups18,49. Of the 18 non-comparator studies, one was a population-based observational study and the others were single-institution or multi-institution retrospective studies.
The included studies were published between 2003 and 2021. Nearly half of them were from Europe (n = 11, 47.8%), followed by Japan (n = 6, 26.1%), Korea (n = 3, 13.0%), the United States (n = 2, 8.7%), and Taiwan (n = 1, 4.3%). No studies examined the effect of prior UAE on the risk of PAS with a matched obstetric background.
Risk of bias in the included studies
Among the 23 studies, which were all retrospective, five had a non-randomized comparative design. No prospective studies were identified. The risk of bias assessment for the comparative studies demonstrated a possible moderate publication bias (moderate quality) in two studies19,20 and severe publication bias (low quality) in the other three studies (Supplemental Table S5)18,33,49.
Definitions of PAS and PPH
Among the 23 studies, six defined PAS. Specifically, four studies defined PAS as histopathologically confirmed PAS and two defined PAS as histopathologically and clinically confirmed PAS. Ten studies defined PPH based on the following transfusion requirements at delivery: > 2000 mL (one study), > 1000 mL (one study), > 1000 mL (cesarean delivery) (two studies) or > 500 mL (vaginal delivery) (one study), and > 500 mL (five studies).
Meta-analysis
Risk of PAS and hysterectomy
Seventeen studies examined the rate of PAS after UAE for PPH during subsequent pregnancies, and 19 studies determined the rate of hysterectomy. The cumulative results of all studies indicated that the rates of PAS and hysterectomy were 16.3% (34/208) and 6.5% (28/432), respectively (Table 1).
Table 1.
Summary of the rate of PAS and hysterectomy in subsequent pregnancies after UAE.
| Author | Year | No | Age | PAS | Hyst | Def_PAS |
|---|---|---|---|---|---|---|
| Comparator study | ||||||
| Jitsumori18 | 2020 | 16 | 35 (4.3) | 6 (37.5%) | 6 (37.5%)† | Path |
| Control | 3139a, b | 33.7 (5.4) | 37 (1.2%) | 55 (1.8%) | ||
| Imafuku19 | 2020 | 14 | 30.5 (26–38) | 7 (50%) | – | Path/clin |
| Control | 32a,b | 32.0 (21–41) | 1 (3.1%) | – | ||
| Cho49 | 2017 | 217 | 31.1 (3.5) | – | 11 (5.1%) | – |
| Control | 317,453a,b | 32.5 (3.1) | – | 204 (0.1%) | – | |
| Poggi20 | 2015 | 17 | 30.5 (5.5) | 4 (23.5%) | 3 (17.6%) | Path |
| Control | 18a | 29.0 (6.0) | 0 | 0 | ||
| Non-comparator study | ||||||
| Ono34 | 2020 | 6 | – | 0 | 0 | – |
| Toguchi35 | 2020 | 10b | – | 4 (40.0%) | – | – |
| Cheng16 | 2017 | 14 | – | – | 0 | – |
| Inoue21 | 2014 | 30 | – | 5 (16.7%) | 5 (16.7%) | Path |
| Takeda36 | 2014 | 8 | – | 0 | 0 | – |
| Lee37 | 2013 | 13b | – | 0 | 0 | – |
| Hardeman38 | 2010 | 11b | – | – | 0 | – |
| Sentilhes13 | 2009 | 19b | – | 2 (10.5%) | 1 (5.3%) | Path/clin |
| Fiori39 | 2009 | 11b | 33 (20–43) | 0 | 0 | – |
| Gaia40 | 2009 | 18 | – | 3 (16.7%) | 0 | – |
| Chauleur41 | 2008 | 16b | – | 0 | 0 | – |
| Eriksson42 | 2007 | 6b | – | – | 0 | – |
| Shim43 | 2006 | 6 | – | 0 | 0 | – |
| Descargues44 | 2004 | 6 | – | 0 | 0 | – |
| Salomon45 | 2003 | 4 | 34.5 (34–36) | 2 (50.0%) | 2 (50.0%) | Path |
| Ornan46 | 2003 | 6 | – | 0 | 0 | – |
| Picone47 | 2003 | 8 | – | 1 (12.5%) | 0 | – |
| Group | No | PAS | Hyst | |||
|---|---|---|---|---|---|---|
| Effect of UAE on the rate of PAS and hysterectomy (all studies) | ||||||
| UAE | – | 456 | – | 34/208 (16.3%) | 28/432 (6.5%) | |
The median (range) or mean (standard deviation) or number (percentage per column) is shown. aWomen without prior UAE. bSome patients had multiple deliveries. Some values listed above might be slightly different from the original values, as estimated by the authors.
UAE uterine artery embolization, No. number of prior uterine embolization cases, PAS placenta accreta spectrum, Hyst hysterectomy, Def_PAS definition of placenta accreta spectrum, Path pathology, clin clinical diagnosis.
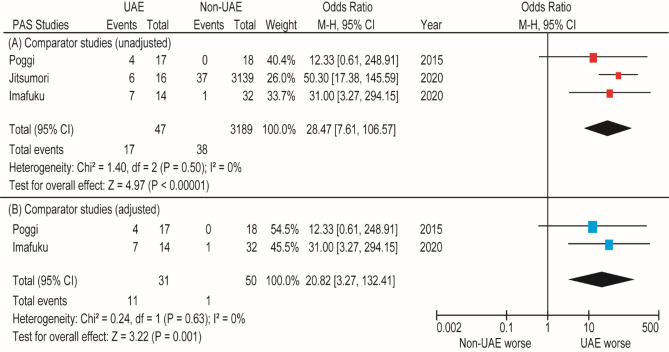
We found three comparator studies that compared the rate of PAS between women who did and did not undergo prior UAE. In these three studies, 47 women underwent prior UAE and 3189 women did not undergo prior UAE. Considering the lack of heterogeneity, we conducted a fixed-effects analysis. The unadjusted pooled analysis (n = 3) demonstrated that women with prior UAE had a higher rate of PAS (OR 28.47, 95% CI 7.61–106.57) than those who did not undergo prior UAE (Fig. 2). In the adjusted pooled analysis (n = 2, all women had PPH during their previous delivery), prior UAE was associated with PAS (OR 20.82, 95% CI 3.27–132.41).
Figure 2.
Results of the meta-analysis of the effect of prior UAE on the prevalence of PAS. The pooled odds ratios of (A) PAS and (B) PAS with previous PPH for women who did and did not undergo prior UAE. Some values listed might be slightly different from the original values because the calculation was performed using Revman ver. 5.4.1. PAS placenta accreta spectrum, UAE uterine artery embolization, CI confidence interval, df degrees of freedom.
Twenty-one of 23 studies did not report the timing of the PAS diagnosis; therefore, the relationship between the timing of the PAS diagnosis and maternal outcomes could not be examined. Among the remaining two studies, the timing of the PAS diagnosis was mentioned for six women; all were diagnosed with PAS intrapartum. The rates of emergent cesarean deliveries and of multidisciplinary care and interventional radiology procedures were not reported.
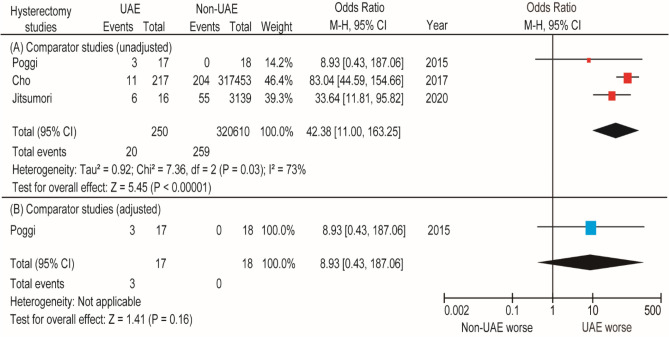
We also found three comparator studies that compared the rates of hysterectomy between women who did and did not undergo prior UAE. In these three studies, 250 women underwent prior UAE and 320,610 women did not undergo prior UAE. According to the unadjusted pooled analysis (n = 3), women who underwent prior UAE had a higher rate of hysterectomy (OR 42.38, 95% CI 11.00–163.25; heterogeneity: P = 0.03, I2 = 73%) than women who did not undergo prior UAE (Fig. 3). Because of the small number of studies examined, the risk of publication bias could not be calculated. According to the adjusted pooled analysis (n = 1), the rate of hysterectomy was higher for women who underwent prior UAE than for women who did not undergo prior UAE (17.6% [3/17] versus 0% [0/18]); however, the difference was not statistically significant (P = 0.16).
Figure 3.
Results of the meta-analysis of the effect of prior UAE on the rate of hysterectomy. The pooled odds ratios of (A) hysterectomy and (B) hysterectomy with previous PPH for women who did and did not undergo prior UAE. Some values listed might be slightly different from the original values because the calculation was performed using Revman ver. 5.4.1. UAE uterine artery embolization, CI confidence interval, df degrees of freedom.
To examine maternal outcomes after delivery, rates of urinary tract injury and infection were reviewed. As shown in Supplemental Table S4, the rate of urinary tract injury was reported in one comparator study and three non-comparator studies. No urinary tract injuries were reported for women after UAE (n = 58). The rate of infection was not reported in the included studies.
Risk of PPH
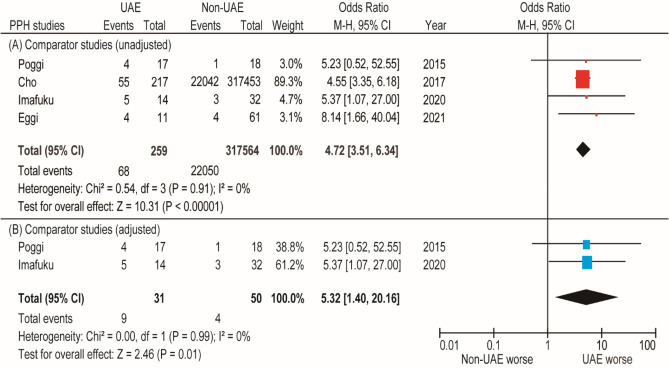
Four comparator studies (three with low quality and one with moderate quality) examined the effect of prior UAE on the rate of PPH during subsequent pregnancies, and 19 non-comparator studies reported the rate of PPH for women who underwent prior UAE (Table 2). Because of the lack of heterogeneity, a fixed-effects analysis was performed. We found three comparator studies investigating 259 women who underwent prior UAE and 317,564 women who did not undergo prior UAE. According to the unadjusted pooled analysis (n = 4), women who underwent prior UAE were more likely to have PPH (OR 4.72, 95% CI 3.51–6.34; P < 0.01; heterogeneity: P = 0.91 and I2 = 0%) than women who did not undergo prior UAE (Fig. 4). However, the risk of publication bias could not be calculated because of the small number of included studies.
Table 2.
Summary of the rate of PPH in subsequent pregnancies after UAE.
| Author | Year | No | Age | PPH | UAE | Def_PPH |
|---|---|---|---|---|---|---|
| Comparator study | ||||||
| Eggi33 | 2021 | 11 | – | 4 (36.4%) | – | > 500 ml |
| Control | 61 | – | 4 (6.6%) | – | ||
| Imafuku19 | 2020 | 14 | 30.5 (26–38) | 5 (35.7%) | – | > 2000 ml |
| Control | 32a,c | 32.0 (21–41) | 3 (9.4%) | – | ||
| Cho49 | 2017 | 217 | 31.1 (3.5) | 55 (25.3%) | 13 (6.0) | – |
| Control | 317,453a,c | 32.5 (3.1) | 22,042 (6.9%) | 328 (0.1) | – | |
| Poggi20 | 2015 | 17 | 30.5 (5.5) | 4 (23.5%) | 1 (5.9%) | Transfusion |
| Control | 18a | 29.0 (6.0) | 1 (5.6%) | 0 | ||
| Non-comparator study | ||||||
| Grönvall48 | 2021 | 16c,d | – | 3 (23.1%)d | – | – |
| Jitsumori18 | 2020 | 16 | – | 9 (56.3%)b | 0 | > 1000 ml |
| Ono34 | 2020 | 6 | – | 0 | 0 | – |
| Toguchi35 | 2020 | 10c | – | 1 (10.0%) | – | – |
| Cheng16 | 2017 | 14 | – | 2 (14.3%)‡ | 1 (7.1%) | > 500 ml |
| Inoue21 | 2014 | 8 | – | 0 | 0 |
> 500 ml (VD) > 1000 ml (CD) |
| Takeda36 | 2013 | 13c | – | 0 | 0 | – |
| Lee37 | 2014 | 30 | – | 7 (23.3%) | 0 | > 500 ml |
| Hardeman38 | 2010 | 11c | 2 (18.2%) | 0 | – | |
| Sentilhes13 | 2009 | 19c | – | 6 (31.6%) | 1 (5.3%) | – |
| Fiori39 | 2009 | 11c | 33 (20–43) | 1 (9.1%) | 0 | > 1000 ml |
| Gaia40 | 2009 | 18 | – | 3 (16.7%) | 3 (16.7%) | > 500 ml |
| Chauleur41 | 2008 | 16c | – | 1 (6.3%) | 0 | > 500 ml |
| Eriksson42 | 2007 | 6c | 0 | 0 | – | |
| Shim43 | 2006 | 6 | – | 1 (16.7%) | 0 | – |
| Descargues44 | 2004 | 6 | – | 0 | 0 | – |
| Salomon45 | 2003 | 4 | 34.5 (34–36) | 4 (100%) | 0 | – |
| Ornan46 | 2003 | 6 | – | 0 | 0 | – |
| Picone47 | 2003 | 8 | – | 7 (87.5%) | 1 (12.5%) | – |
| Group | No | Age | PPH | UAE | ||
|---|---|---|---|---|---|---|
| Effect of UAE on the rate of PPH (all studies) | ||||||
| UAE | – | 483 | – | 115/480 (24.0%) | 20/432 (4.6%) | |
Median (range) or mean (standard deviation) or number (percentage per column) are shown.
UAE uterine artery embolization, VD vaginal delivery, CD cesarean delivery, No. number of prior uterine embolization cases, Indi indication, PPH postpartum hemorrhage, Def_PPH definition of postpartum hemorrhage, Type type of uterine artery embolization.
aWomen without prior UAE. bUnpublished data. cSome patients had multiple deliveries. dPPH recurred in three of the 13 women (23.1%). Some values listed above might be slightly different from the original ones due to the estimation procedure used by the authors.
Figure 4.
Results of the meta-analysis of the effect of prior UAE on the rate of PPH. The pooled odds ratio of (A) PPH and (B) PPH with previous PPH for women who did and did not undergo prior UAE. Some values listed might be slightly different from the original values because the calculation was performed using Revman ver. 5.4.1. PPH postpartum hemorrhage, UAE uterine artery embolization, CI confidence interval, df degrees of freedom.
In the patient background-matched comparator analysis (all cases had prior PPH), the prevalence rates of recurrent PPH for women who did and did not undergo prior UAE were examined using a fixed analysis (n = 2). Women who underwent prior UAE were more likely to have recurrent PPH (OR 5.32, 95% CI 1.40–20.16, P < 0.01; heterogeneity: P = 0.99 and I2 = 0%) than women who did not undergo prior UAE (Fig. 4).
To estimate the severity of PPH, the rates of fresh-frozen plasma (FFP) and platelet transfusions and the prevalence of disseminated intravascular coagulation were explored. However, most studies did not report these data (Supplemental Table S4).
Two comparator studies reported the rates of UAE treatment. In one study (in which the patient background was not matched), the women who were treated with UAE during a previous pregnancy were more likely to have UAE than women in the control group (6.0% [13/217] versus 0.1% [328/317,453], P < 0.01)48. In another study (the patient background was matched with previous PPH), the rate of UAE for women treated with UAE during a previous pregnancy was similar to that of the control group (5.9% [1/17] versus 0% [0/18]; P = 0.47)20.
Risks of placenta previa, FGR, and PTB
To investigate the effect of prior UAE on placenta previa, FGR, and PTB during subsequent pregnancies, the individual rates of these complications were examined in four, two, and three comparator studies, respectively (Table 3). Furthermore, 11, 12, and 16 non-comparator studies reported the rates of placenta previa, FGR, and PTB, respectively.
Table 3.
Summary of the rate of PP, FGR and PTB in subsequent pregnancies after UAE.
| Author | Year | No | Age | PP | FGR | PTB |
|---|---|---|---|---|---|---|
| Comparator study | ||||||
| Jitsumori18 | 2020 | 16 | 35 (4.3) | 2 (12.5%) | 1 (6.3%) | 2 (12.5%) |
| Control | 3139a,b | 33.7 (5.4) | 123 (3.9%) | 313 (10.0%) | 446 (14.2%) | |
| Imafuku19 | 2020 | 14 | 30.5 (26–38) | 1 (7.1%) | 1 (7.1%) | 3 (21.4%) |
| Control | 32a,b | 32.0 (21–41) | 1 (3.1%) | 0 | 3 (9.4) | |
| Cho49 | 2017 | 217 | 31.1 (3.5) | 20 (9.2%) | – | – |
| Control | 317,453a,b | 32.5 (3.1) | 2070 (0.7%) | – | – | |
| Poggi20 | 2015 | 17 | 30.5 (5.5) | 2 (11.8%) | – | 3 (17.6%) |
| Control | 18a | 29.0 (6.0) | 1 (5.6%) | – | 1 (5.6%) | |
| Non-comparator study | ||||||
| Grönvall48 | 2021 | 16b | – | – | 0 | 0 |
| Ono34 | 2020 | 6 | – | 0 | 0 | 0 |
| Toguchi35 | 2020 | 10b | – | 0 | – | – |
| Cheng16 | 2017 | 14 | – | – | 1 (7.1%) | 3 (21.4%) |
| Inoue21 | 2014 | 30 | – | – | – | 4 (13.3%) |
| Takeda36 | 2014 | 8 | – | – | – | 0 |
| Lee37 | 2013 | 13b | – | – | – | 2 (15.4%) |
| Hardeman38 | 2010 | 11b | – | 0 | 1 (9.1%) | – |
| Sentilhes13 | 2009 | 19b | – | 1 (5.3%) | 0 | 0 |
| Fiori39 | 2009 | 11b | 33 (20–43) | 0 | 0 | 1 (9.1%) |
| Gaia40 | 2009 | 18 | – | 0 | – | 0 |
| Chauleur41 | 2008 | 16b | – | 0 | 1 (6.3%) | 1 (6.3%) |
| Eriksson42 | 2007 | 6b | – | – | – | 2 (33.3%) |
| Shim43 | 2006 | 6 | – | 0 | 0 | 0 |
| Descargues44 | 2004 | 6 | – | 0 | 0 | 0 |
| Salomon45 | 2003 | 4 | 34.5 (34–36) | 0 | 0 | 0 |
| Ornan46 | 2003 | 6 | – | 0 | 0 | 0 |
| Picone47 | 2003 | 8 | – | – | 0 | 2 (25.0%) |
| Group | – | No | – | PP | FGR | PTB |
|---|---|---|---|---|---|---|
| Effect of UAE on the rate of complication | ||||||
| UAE | – | 472 | – | 26/377(6.9%) | 5/153 (3.3%) | 23/234 (9.8%) |
Median (range) or mean (standard deviation) or number (percentage per column) are shown. aWomen without prior UAE. b Some patients had multiple deliveries. Some values listed above might be slightly different from the original ones due to the estimation procedure used by the authors.
– not applicable, PP placenta previa, FGR fetal growth restriction, PTB preterm birth, UAE uterine artery embolization, No. number of prior uterine embolization cases.
The analysis that included both comparator and non-comparator studies indicated that the rates of placenta previa, FGR, and PTB were 6.9% (26/377), 3.3% (5/153), and 9.8% (23/234), respectively. According to the unadjusted pooled analysis, women who underwent prior UAE had higher rates of placenta previa (n = 3; OR 5.62, 95% CI 1.48–21.34; heterogeneity: P = 0.04 and I2 = 65%) (Supplemental Figure S1) than women who did not undergo UAE. Furthermore, the rates of FGR (n = 3; OR 1.48, 95% CI 0.14–15.39; heterogeneity: P = 0.20 and I2 = 38%) (Supplemental Figure S2) and PTB (n = 3; OR 1.63, 95% CI 0.64–4.17; heterogeneity: P = 0.49 and I2 = 0%) (Supplemental Figure S3) were similar between the two groups. There were few included studies; hence, the risk of publication bias could not be calculated.
According to the patient background-matched pooled analysis, women who underwent prior UAE did not have higher rates of placenta previa (OR 2.31, 95% CI 0.35–15.22), FGR (OR 7.22, 95% CI 0.28–188.69), and PTB (OR 3.00, 95% CI 0.74–12.14) than women who did not undergo prior UAE.
Embolic agents, particle sizes, and quantity of materials
The effects of embolic agents and the corresponding particle size on obstetric outcomes were examined. Among the 23 included studies, nine reported the embolic agents used. Most women with prior UAE were treated with a gelatin sponge. To estimate the particle size, the preparation of embolic agents was examined. Of 23 studies, five reported the following preparations of embolic agents: pumping (two studies); slurry (one study); cube (one study); and cutting (one study). No studies specified the quantity of materials used to treat PPH.
Since most cases were treated with a gelatin sponge, and because the information regarding agent preparation and the quantity of materials was limited, we did not investigate the association between obstetric outcomes and embolic agents.
Discussion
Key findings
There were two key findings during this study. First, prior UAE is a significant risk factor for PAS during subsequent pregnancies. Second, women who underwent prior UAE had a higher rate of PPH during subsequent pregnancies than women who did not undergo prior UAE; however, they did not have higher rates of other obstetric complications such as placenta previa, FGR, and PTB.
Comparison with existing literature
Of the conditions associated with PPH, PAS has the highest risk. Furthermore, PAS is associated with increased maternal morbidity and mortality rates caused by massive hemorrhage during delivery50–54. For women with PAS, the mean blood loss during cesarean delivery is approximately 3000 mL, and the hysterectomy rate is approximately 40–70%50–54. The main risk factor for PAS is placenta previa, with an approximate OR of 50–10055–57. However, PAS has been linked to other risk factors, including a history of cesarean delivery (OR 5–9), uterine surgery (OR 2–3), multiparity (OR 3), advanced maternal age (OR 2.1)56,58, and in vitro fertilization embryo transfer (OR 3–14)59–63. Although the rate of PAS could be high for women who underwent prior UAE for PPH, this has not been determined by meta-analyses.
During our study, the OR of prior UAE was 28.47 in the unadjusted analysis and 20.82 in the adjusted analysis. Therefore, prior UAE may be a substantial risk factor for PAS. Since women with PAS often need to undergo hysterectomy because of severe PPH, the high rate of hysterectomy may have been caused by the increasing rate of PAS50,51.
Our study had several possible biases. As shown in Table 4, the estimated recurrence rates of PAS64–67, placenta previa64,68,69, PPH70,71, FGR72,73, and PTB74,75 have been widely reported as high during subsequent pregnancy. For instance, nearly half of PAS cases involve PPH, and homeostasis is often achieved by UAE64–67,76,77. Therefore, it should be noted that our study and previous studies could not exclude the effect of the presence of PAS during previous pregnancies. A previous report indicated that the rate of recurrent PAS (including clinical PAS) was 19.9%65. Therefore, if PPH is caused by PAS during the first pregnancy, then the risk of PAS during the subsequent pregnancy may be high. Similarly, women who had PPH, FGR, and PTB during the previous pregnancy had a high rate of recurrence of these complications (Table 4). Therefore, a patient background-matched study with a larger sample size is warranted to examine the effect of PAS on obstetric complications.
Table 4.
The estimated prevalence of obstetric complications and the recurrence rate of each complication.
| Disease | Prevalence (%) | Recurrence rate (%) |
|---|---|---|
| PAS64–66 | 0.1–3a | ~ 20.0 |
| Hysterectomy78 | 0.10 | – |
| PPH70,71 | 1–5 | 20–25 |
| Placenta previa64,68,69 | 0.3–1.0 | 2–8 |
| FGR72,73 | 4–6 | 20–25 |
| PTB74,75 | 4–6 | 20–30 |
PAS placenta accreta spectrum, PPH postpartum hemorrhage, FGR fetal growth restriction, PTB preterm birth.
aIncluding pathological and clinical diagnosis of placenta accreta spectrum.
An antenatal diagnosis of PAS helps reduce hemorrhagic morbidity and improves the prognosis, possibly because of the comprehensive multidisciplinary care received by patients, which includes planned cesarean hysterectomy, transfusion preparation, and treatment administered by skilled physicians79–82. Therefore, the timing of diagnosis of PAS is an important factor to examine, especially because undiagnosed PAS is associated with adverse maternal outcomes22,79,83. Moreover, while multidisciplinary care and/or interventional radiology procedures have the potential to improve the maternal outcomes of PAS, these data were not available; this lack of data was a limitation of this study23,51.
Although limited, the available data (n = 6) indicated that all women with PAS after prior UAE were diagnosed intrapartum. We believe that an understanding of the effect of prior UAE on the risks of PAS and hysterectomy during subsequent pregnancies would be helpful to making an antenatal diagnosis of PAS. Notably, prior UAE may be a strong risk factor for PAS, and women who underwent prior UAE may have PAS even without placenta previa.
A recent study that compared PAS in women without placenta previa (n = 106) and PAS in women with placenta previa (n = 245) revealed that PAS without placenta previa is less likely to be diagnosed antepartum (OR 0.1, 95% CI 0.05–016)50. Considering that placenta previa is a significant risk factor for PAS, antepartum evaluations might be performed more carefully for women with placenta previa than for women without placenta previa. This step may lead to a lower diagnosis rate for women without placenta previa50. Additionally, a low diagnosis rate can potentially lead to the missed opportunity for multidisciplinary team management (OR 0.11, 95% CI 0.07–019)50. Despite the absence of placenta previa and less placental invasion, severe maternal morbidity during delivery was similar between groups (18.9% versus 19.6%, OR 0.88, 95% CI 0.49–1.59)50.
The current study results demonstrated that knowledge of prior UAE as a high-risk factor for PAS, PPH, and high hysterectomy rates are useful for clinicians. Additionally, pregnant women with prior UAE need to undergo careful antepartum evaluations for PAS and prepare for PPH during delivery.
Some studies have discussed the relationship between prior UAE and a high rate of PAS. Uterine necrosis is a complication of UAE performed for PPH, and reduced blood flow to the uterus and damage to the endometrium may lead to uterine necrosis84. We hypothesized that endometrial damage occurs even in women without complications who are treated with UAE. Endometrial damage is a risk factor for PPH and may be one of the causes of PAS.
Previous studies have suggested that embolic agents, particle sizes, and the quantity of materials could potentially affect the short-term or long-term complications of UAE, including endometrial damage. A previous animal study involving renal artery embolization was performed with different embolic agents for dogs and examined recanalization of embolic vessels according to the type of embolic agent85. Complete recanalization was observed with an absorbable gelatin sponge and no recanalization was observed with non-absorbable materials. Cases of uterine necrosis after UAE involving non-absorbable materials have been reported as well86,87.
Although a gelatin sponge is an absorbable embolic agent, the smaller size of the gelatin sponge is associated with a higher rate of complications such as uterine necrosis and intrauterine synechia40,84,88. Therefore, the association between the complication rate and the size of the embolic agent for UAE is relevant. However, a clinical study that compared the incidence of complications based on the size of embolic agent for UAE was underpowered88. Moreover, this study revealed that the effect of the size of embolic agent for UAE on obstetric outcomes during subsequent pregnancy is unclear.
Large volumes of embolic agents for UAE or repeated UAE for severe PPH may be associated with higher complication rates89–91. A possible reason for this association is that large-volume embolic agents may simultaneously block the upper and lower anastomotic uterine blood supplies, thus leading to a high probability of necrosis or damage of the uterus92.
We hypothesized that non-absorbable embolic agents, small particles, and the use of large volumes of embolic agents could potentially increase the rate of long-term complications and worsen the obstetric outcomes during a subsequent pregnancy. However, we could not examine the effects of these factors on obstetric outcomes; this was another limitation of this study. Further studies should examine the effects of type, size, and quantity of embolic agents used during a prior UAE on the obstetric outcomes during subsequent pregnancies.
Strengths and limitations
One strength of this study is that it is likely the first systematic review to focus on the effect of prior UAE for PPH on PAS during subsequent pregnancies. Our study revealed that prior UAE performed to treat PPH is associated with high rates of PAS and PPH. However, as mentioned, this study had additional limitations. First, bias was not measured because all the included studies were retrospective. Potential sources of confounding variables in the study included the varying definitions of PAS and PPH across studies, unmatched patient backgrounds, and the lack of data regarding UAE indications during previous pregnancies. In particular, different definitions of PAS and PPH among studies may have caused severe bias; thus, we should note this as a strong limitation of this study. Another important limitation was that no studies matched the obstetric patient backgrounds to examine the effect of prior UAE for PPH on the rate of obstetric complications. Second, we only found two comparator studies that examined the rate of obstetric complications of women with previous PPH who did and did not undergo prior UAE. Because PPH and its causes (e.g., PAS and placenta previa) are highly recurrent, future studies of background-matched patient cohorts are necessary. Third, the embolic agent used for prior UAE, the severity of PPH, the timing of the PAS diagnosis, the transfusion rates (FFP and platelet), the prevalence of disseminated intravascular coagulation, and the presence of PAS were not identified in most studies. These factors might have influenced the results of this study; hence, the lack of such data is a notable limitation of this study. Fourth, publication bias is a matter of concern because the negative relationship between prior UAE for PPH and PAS might not have been reported in the original articles. To confirm the results of this study, a more robust study should be conducted. Considering that a randomized control study is difficult to conduct because of the rarity of women who underwent prior UAE for PPH, a prospective study seems appropriate. Fifth, the sample size was limited in most studies; thus, the possibility of type II error needs to be recognized, especially in the interpretation of the results of the adjusted pooled analysis. Sixth, the type of embolic agent, particle size, and quantity of agents may have affected the obstetric outcomes of women with prior UAE. However, only limited data were available in our included studies; therefore, we could not examine such associations. Further investigations are warranted to improve obstetric outcomes after UAE. Finally, the protocol of the systematic review has not been registered. Without preregistration, it is unknown whether the main outcomes, such as PAS, were predefined as primary outcomes. Therefore, this could cause bias of the systematic review and should be noted as a limitation of this study.
Conclusions
Prior UAE for PPH may be a significant risk factor for PAS. Moreover, PPH frequently recurs. Therefore, we should note that pregnant women who underwent prior UAE are at high risk for PPH during subsequent pregnancies. To confirm the results of this study, a patient background-matched study or prospective study exploring the effect of prior UAE is warranted.
Supplementary Information
Author contributions
Conceptualization: Sh.M., Y.N., Ts.T., A.K., K.M., Sa.M., M.J.; data curation: Sh.M., Y.N., L.M., Sa.M.; formal analysis: Sh.M., Y.N., Y.U.; investigation: all authors; methodology: Sh.M., Y.N., L.M.; project administration: Sh.M., Ta.T., M.E., T.K.; resources: all; software: Sh.M.; supervision: Ta.T., M.E., T.K.; validation: Sh.M., Y.N.; visualization: Sh.M.; writing—original draft: Sh.M., Y.N., Ts.T.; revised the manuscript: Sh.M., L.M., M.M.; writing—review and editing: all authors.
Funding
This research did not receive any specific Grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
All the studies used in this study are published in the literature.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Shinya Matsuzaki and Misooja Lee.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-96273-z.
References
- 1.Adnan N, Conlan-Trant R, McCormick C, Boland F, Murphy DJ. Intramuscular versus intravenous oxytocin to prevent postpartum haemorrhage at vaginal delivery: Randomised controlled trial. BMJ. 2018;362:k3546. doi: 10.1136/bmj.k3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bishanga DR, Charles J, Tibaijuka G, Mutayoba R, Drake M, Kim YM, et al. Improvement in the active management of the third stage of labor for the prevention of postpartum hemorrhage in Tanzania: A cross-sectional study. BMC Pregnancy Childbirth. 2018;18:223. doi: 10.1186/s12884-018-1873-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pacheco LD, Saade GR, Costantine MM, Clark SL, Hankins GD. An update on the use of massive transfusion protocols in obstetrics. Am. J. Obstet. Gynecol. 2016;214:340–344. doi: 10.1016/j.ajog.2015.08.068. [DOI] [PubMed] [Google Scholar]
- 4.Matsuzaki S, Endo M, Ueda Y, Mimura K, Kakigano A, Egawa-Takata T, et al. A case of acute Sheehan's syndrome and literature review: A rare but life-threatening complication of postpartum hemorrhage. BMC Pregnancy Childbirth. 2017;17:188. doi: 10.1186/s12884-017-1380-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kearney L, Kynn M, Reed R, Davenport L, Young J, Schafer K. Identifying the risk: A prospective cohort study examining postpartum haemorrhage in a regional Australian health service. BMC Pregnancy Childbirth. 2018;18:214. doi: 10.1186/s12884-018-1852-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Committee on Practice B-O Practice bulletin No. 183: Postpartum hemorrhage. Obstet. Gynecol. 2017;130:e168–e186. doi: 10.1097/AOG.0000000000002351. [DOI] [PubMed] [Google Scholar]
- 7.Dashtinejad E, Abedi P, Afshari P. Comparison of the effect of breast pump stimulation and oxytocin administration on the length of the third stage of labor, postpartum hemorrhage, and anemia: A randomized controlled trial. BMC Pregnancy Childbirth. 2018;18:293. doi: 10.1186/s12884-018-1832-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kong CW, To WW. Prognostic factors for the use of intrauterine balloon tamponade in the management of severe postpartum hemorrhage. Int. J. Gynaecol. Obstet. 2018;142:48–53. doi: 10.1002/ijgo.12498. [DOI] [PubMed] [Google Scholar]
- 9.Ishii T, Sawada K, Koyama S, Isobe A, Wakabayashi A, Takiuchi T, et al. Balloon tamponade during cesarean section is useful for severe post-partum hemorrhage due to placenta previa. J. Obstet. Gynaecol. Res. 2012;38:102–107. doi: 10.1111/j.1447-0756.2011.01625.x. [DOI] [PubMed] [Google Scholar]
- 10.Aoki M, Tokue H, Miyazaki M, Shibuya K, Hirasawa S, Oshima K. Primary postpartum hemorrhage: Outcome of uterine artery embolization. Br. J. Radiol. 2018;91:20180132. doi: 10.1259/bjr.20180132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nishikawa A, Matsuzaki S, Mimura K, Kanagawa T, Kimura T. Short interpregnancy interval after B-Lynch uterine compression suture: A case report. Clin. Exp. Obstet. Gynecol. 2016;43:434–436. [PubMed] [Google Scholar]
- 12.Suzuki Y, Matsuzaki S, Mimura K, Kumasawa K, Tomimatsu T, Endo M, et al. Investigation of perioperative complications associated with use of uterine compression sutures. Int. J. Gynaecol. Obstet. 2017;139:28–33. doi: 10.1002/ijgo.12249. [DOI] [PubMed] [Google Scholar]
- 13.Sentilhes L, Gromez A, Clavier E, Resch B, Verspyck E, Marpeau L. Fertility and pregnancy following pelvic arterial embolisation for postpartum haemorrhage. BJOG. 2010;117:84–93. doi: 10.1111/j.1471-0528.2009.02381.x. [DOI] [PubMed] [Google Scholar]
- 14.Ruiz Labarta FJ, Pintado Recarte MP, Alvarez Luque A, Joigneau Prieto L, Perez Martín L, Gonzalez Leyte M, et al. Outcomes of pelvic arterial embolization in the management of postpartum haemorrhage: A case series study and systematic review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016;206:12–21. doi: 10.1016/j.ejogrb.2016.07.510. [DOI] [PubMed] [Google Scholar]
- 15.Aguilar-Crespo A, Morales-Roselló J, Sánchez-Ajenjo C, Valle-Tejero A, García-Marcos R, Perales-Marín A. Postpartum hemorrhage with pelvic arterial embolization, study of 33 cases. J. Matern. Fetal neonatal Med. 2019;32:573–578. doi: 10.1080/14767058.2017.1387527. [DOI] [PubMed] [Google Scholar]
- 16.Cheng HH, Tsang LL, Hsu TY, Kung CT, Ou CY, Chang CD, et al. Transcatheter arterial embolization as first-line rescue in intractable primary postpartum hemorrhage: Assessment, outcome, and subsequent fertility. J. Formosan Med. Assoc. 2017;116:380–387. doi: 10.1016/j.jfma.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 17.Soro MP, Denys A, de Rham M, Baud D. Short & long term adverse outcomes after arterial embolisation for the treatment of postpartum haemorrhage: A systematic review. Eur. Radiol. 2017;27:749–762. doi: 10.1007/s00330-016-4395-2. [DOI] [PubMed] [Google Scholar]
- 18.Jitsumori M, Matsuzaki S, Endo M, Hara T, Tomimatsu T, Matsuzaki S, et al. Obstetric outcomes of pregnancy after uterine artery embolization. Int. J. Women's Health. 2020;12:151–158. doi: 10.2147/IJWH.S236443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Imafuku H, Yamada H, Morizane M, Tanimura K. Recurrence of post-partum hemorrhage in women with a history of uterine artery embolization. J. Obstet. Gynaecol. Res. 2020;46:119–123. doi: 10.1111/jog.14129. [DOI] [PubMed] [Google Scholar]
- 20.Poggi SH, Yaeger A, Wahdan Y, Ghidini A. Outcome of pregnancies after pelvic artery embolization for postpartum hemorrhage: Retrospective cohort study. Am. J. Obstet. Gynecol. 2015;213(576):e1–5. doi: 10.1016/j.ajog.2015.06.063. [DOI] [PubMed] [Google Scholar]
- 21.Inoue S, Masuyama H, Hiramatsu Y. Efficacy of transarterial embolisation in the management of post-partum haemorrhage and its impact on subsequent pregnancies. Aust. N. Z. J. Obstet. Gynaecol. 2014;54:541–545. doi: 10.1111/ajo.12228. [DOI] [PubMed] [Google Scholar]
- 22.Buca D, Liberati M, Cali G, Forlani F, Caisutti C, Flacco ME, et al. Influence of prenatal diagnosis of abnormally invasive placenta on maternal outcome: Systematic review and meta-analysis. Ultrasound Obstetr. Gynecol. 2018;52:304–309. doi: 10.1002/uog.19070. [DOI] [PubMed] [Google Scholar]
- 23.D'Antonio F, Iacovelli A, Liberati M, Leombroni M, Murgano D, Cali G, et al. Role of interventional radiology in pregnancy complicated by placenta accreta spectrum disorder: Systematic review and meta-analysis. Ultrasound Obstetr. Gynecolo. 2019;53:743–751. doi: 10.1002/uog.20131. [DOI] [PubMed] [Google Scholar]
- 24.Iacovelli A, Liberati M, Khalil A, Timor-Trisch I, Leombroni M, Buca D, et al. Risk factors for abnormally invasive placenta: A systematic review and meta-analysis. J. Matern. Fetal Neonatal Med. 2020;33:471–481. doi: 10.1080/14767058.2018.1493453. [DOI] [PubMed] [Google Scholar]
- 25.Palacios-Jaraquemada JM, D'Antonio F, Buca D, Fiorillo A, Larraza P. Systematic review on near miss cases of placenta accreta spectrum disorders: Correlation with invasion topography, prenatal imaging, and surgical outcome. J. Matern. Fetal Neonatal Med. 2020;33:3377–3384. doi: 10.1080/14767058.2019.1570494. [DOI] [PubMed] [Google Scholar]
- 26.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. Updating guidance for reporting systematic reviews: Development of the PRISMA 2020 statement. J. Clin. Epidemiol. 2021;134:103–112. doi: 10.1016/j.jclinepi.2021.02.003. [DOI] [PubMed] [Google Scholar]
- 27.Katsumori T, Kasahara T. The size of gelatin sponge particles: Differences with preparation method. Cardiovasc. Intervent. Radiol. 2006;29:1077–1083. doi: 10.1007/s00270-006-0059-y. [DOI] [PubMed] [Google Scholar]
- 28.Sterne JA, Hernan MA, Reeves BC, Savovic J, Berkman ND, Viswanathan M, et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Danna SM, Graham E, Burns RJ, Deschenes SS, Schmitz N. Association between depressive symptoms and cognitive function in persons with diabetes mellitus: A systematic review. PLoS One. 2016;11:e0160809. doi: 10.1371/journal.pone.0160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.ROBINS-I detailed guidance (2016). https://www.riskofbias.info/welcome/home/current-version-of-robins-i/robins-i-detailed-guidance-2016. Accessed 20 Sep 2020.
- 31.Cochrane Handbook for Systematic Reviews of Interventions. Version 6.1, 2020. Chapter 10: Analysing Data and Undertaking Meta-analyses. https://training.cochrane.org/handbook/current/chapter-10. Accessed 18 Dec 2020.
- 32.Matsuzaki S, Enomoto T, Serada S, Yoshino K, Nagamori S, Morimoto A, et al. Annexin A4-conferred platinum resistance is mediated by the copper transporter ATP7A. Int. J. Cancer. 2014;134:1796–1809. doi: 10.1002/ijc.28526. [DOI] [PubMed] [Google Scholar]
- 33.Eggel B, Bernasconi M, Quibel T, Horsch A, Vial Y, Denys A, et al. Gynecological, reproductive and sexual outcomes after uterine artery embolization for post-partum haemorrage. Sci. Rep. 2021;11:833. doi: 10.1038/s41598-020-80821-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ono Y, Kariya S, Nakatani M, Ueno Y, Yoshida A, Maruyama T, et al. Clinical results of transarterial embolization for post-partum hemorrhage in 62 patients. J. Obstetr. Gynaecol. Res. 2020;20:20. doi: 10.1111/jog.14476. [DOI] [PubMed] [Google Scholar]
- 35.Toguchi M, Iraha Y, Ito J, Makino W, Azama K, Heianna J, et al. Uterine artery embolization for postpartum and postabortion hemorrhage: A retrospective analysis of complications, subsequent fertility and pregnancy outcomes. Jpn. J. Radiol. 2020;38:240–247. doi: 10.1007/s11604-019-00907-2. [DOI] [PubMed] [Google Scholar]
- 36.Takeda A, Koike W, Imoto S, Nakamura H. Three-dimensional computerized tomographic angiography for diagnosis and management of intractable postpartum hemorrhage. Eur. J. Obstetr. Gynecol. Reprod. Biol. 2014;176:104–111. doi: 10.1016/j.ejogrb.2014.02.026. [DOI] [PubMed] [Google Scholar]
- 37.Lee HJ, Jeon GS, Kim MD, Kim SH, Lee JT, Choi MJ. Usefulness of pelvic artery embolization in cesarean section compared with vaginal delivery in 176 patients. J. Vasc. Interv. Radiol. 2013;24:103–109. doi: 10.1016/j.jvir.2012.09.029. [DOI] [PubMed] [Google Scholar]
- 38.Hardeman S, Decroisette E, Marin B, Vincelot A, Aubard Y, Pouquet M, et al. Fertility after embolization of the uterine arteries to treat obstetrical hemorrhage: A review of 53 cases. Fertil Steril. 2010;94:2574–2579. doi: 10.1016/j.fertnstert.2010.02.052. [DOI] [PubMed] [Google Scholar]
- 39.Fiori O, Deux JF, Kambale JC, Uzan S, Bougdhene F, Berkane N. Impact of pelvic arterial embolization for intractable postpartum hemorrhage on fertility. Am. J. Obstetr. Gynecol. 2009;200(384):e1–4. doi: 10.1016/j.ajog.2008.11.029. [DOI] [PubMed] [Google Scholar]
- 40.Gaia G, Chabrot P, Cassagnes L, Calcagno A, Gallot D, Botchorishvili R, et al. Menses recovery and fertility after artery embolization for PPH: A single-center retrospective observational study. Eur. Radiol. 2009;19:481–487. doi: 10.1007/s00330-008-1140-5. [DOI] [PubMed] [Google Scholar]
- 41.Chauleur C, Fanget C, Tourne G, Levy R, Larchez C, Seffert P. Serious primary post-partum hemorrhage, arterial embolization and future fertility: A retrospective study of 46 cases. Hum. Reprod. (Oxf., Engl.) 2008;23:1553–1559. doi: 10.1093/humrep/den122. [DOI] [PubMed] [Google Scholar]
- 42.Eriksson LG, Mulic-Lutvica A, Jangland L, Nyman R. Massive postpartum hemorrhage treated with transcatheter arterial embolization: Technical aspects and long-term effects on fertility and menstrual cycle. Acta Radiol. (Stockholm, Sweden: 1987) 2007;48:635–642. doi: 10.1080/02841850701370683. [DOI] [PubMed] [Google Scholar]
- 43.Shim JY, Yoon HK, Won HS, Kim SK, Lee PR, Kim A. Angiographic embolization for obstetrical hemorrhage: Effectiveness and follow-up outcome of fertility. Acta Obstetr. Gynecol. Scand. 2006;85:815–820. doi: 10.1080/00016340500438652. [DOI] [PubMed] [Google Scholar]
- 44.Descargues G, Mauger Tinlot F, Douvrin F, Clavier E, Lemoine JP, Marpeau L. Menses, fertility and pregnancy after arterial embolization for the control of postpartum haemorrhage. Hum. Reprod. (Oxf., Engl.) 2004;19:339–343. doi: 10.1093/humrep/deh082. [DOI] [PubMed] [Google Scholar]
- 45.Salomon LJ, deTayrac R, Castaigne-Meary V, Audibert F, Musset D, Ciorascu R, et al. Fertility and pregnancy outcome following pelvic arterial embolization for severe post-partum haemorrhage. A cohort study. Hum. Reprod. (Oxf., Engl.) 2003;18:849–852. doi: 10.1093/humrep/deg168. [DOI] [PubMed] [Google Scholar]
- 46.Ornan D, White R, Pollak J, Tal M. Pelvic embolization for intractable postpartum hemorrhage: Long-term follow-up and implications for fertility. Obstetr. Gynecol. 2003;102:904–910. doi: 10.1016/s0029-7844(03)00769-5. [DOI] [PubMed] [Google Scholar]
- 47.Picone O, Salomon LJ, Ville Y, Kadoch J, Frydman R, Fernandez H. Fetal growth and Doppler assessment in patients with a history of bilateral internal iliac artery embolization. J. Matern. Fetal Neonatal Med. 2003;13:305–308. doi: 10.1080/jmf.13.5.305.308. [DOI] [PubMed] [Google Scholar]
- 48.Gronvall M, Tikkanen M, Paavonen J, Loukovaara M, Stefanovic V. Is there an association between postpartum hemorrhage, interventional radiology procedures, and psychological sequelae? J. Matern. Fetal Neonatal Med. 2021;34:1792–1796. doi: 10.1080/14767058.2019.1649389. [DOI] [PubMed] [Google Scholar]
- 49.Cho GJ, Shim JY, Ouh YT, Kim LY, Lee TS, Ahn KH, et al. Previous uterine artery embolization increases the rate of repeat embolization in a subsequent pregnancy. PLoS One. 2017;12:e0185467. doi: 10.1371/journal.pone.0185467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carusi DA, Fox KA, Lyell DJ, Perlman NC, Aalipour S, Einerson BD, et al. Placenta accreta spectrum without placenta previa. Obstetr. Gynecol. 2020;136:458–465. doi: 10.1097/AOG.0000000000003970. [DOI] [PubMed] [Google Scholar]
- 51.Kingdom JC, Hobson SR, Murji A, Allen L, Windrim RC, Lockhart E, et al. Minimizing surgical blood loss at cesarean hysterectomy for placenta previa with evidence of placenta increta or placenta percreta: The state of play in 2020. Am. J. Obstetr. Gynecol. 2020;223:322–329. doi: 10.1016/j.ajog.2020.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jauniaux E, Bunce C, Gronbeck L, Langhoff-Roos J. Prevalence and main outcomes of placenta accreta spectrum: A systematic review and meta-analysis. Am. J. Obstetr. Gynecol. 2019;221:208–218. doi: 10.1016/j.ajog.2019.01.233. [DOI] [PubMed] [Google Scholar]
- 53.Shamshirsaz AA, Fox KA, Erfani H, Clark SL, Shamshirsaz AA, Nassr AA, et al. Outcomes of planned compared with urgent deliveries using a multidisciplinary team approach for morbidly adherent placenta. Obstetr. Gynecol. 2018;131:234–241. doi: 10.1097/AOG.0000000000002442. [DOI] [PubMed] [Google Scholar]
- 54.Matsuzaki, S., Mandelbaum, R. S., Sangara, R. N., McCarthy, L. E., Vestal, N. L., Klar, M., et al. Trends, characteristics, and outcomes of placenta accreta spectrum: A national study in the United States. Am. J. Obstetr. Gynecol. 2021 (in press). [DOI] [PubMed]
- 55.Silver RM, Landon MB, Rouse DJ, Leveno KJ, Spong CY, Thom EA, et al. Maternal morbidity associated with multiple repeat cesarean deliveries. Obstetr. Gynecol. 2006;107:1226–1232. doi: 10.1097/01.AOG.0000219750.79480.84. [DOI] [PubMed] [Google Scholar]
- 56.Wu S, Kocherginsky M, Hibbard JU. Abnormal placentation: Twenty-year analysis. Am. J. Obstetr. Gynecol. 2005;192:1458–1461. doi: 10.1016/j.ajog.2004.12.074. [DOI] [PubMed] [Google Scholar]
- 57.Eshkoli T, Weintraub AY, Sergienko R, Sheiner E. Placenta accreta: Risk factors, perinatal outcomes, and consequences for subsequent births. Am. J. Obstetr. Gynecol. 2013;208(219):e1–7. doi: 10.1016/j.ajog.2012.12.037. [DOI] [PubMed] [Google Scholar]
- 58.Baldwin HJ, Patterson JA, Nippita TA, Torvaldsen S, Ibiebele I, Simpson JM, et al. Antecedents of abnormally invasive placenta in primiparous women: Risk associated with gynecologic procedures. Obstetr. Gynecol. 2018;131:227–233. doi: 10.1097/AOG.0000000000002434. [DOI] [PubMed] [Google Scholar]
- 59.Nagata C, Yang L, Yamamoto-Hanada K, Mezawa H, Ayabe T, Ishizuka K, et al. Complications and adverse outcomes in pregnancy and childbirth among women who conceived by assisted reproductive technologies: A nationwide birth cohort study of Japan environment and children's study. BMC Pregnancy Childbirth. 2019;19:77. doi: 10.1186/s12884-019-2213-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kaser DJ, Melamed A, Bormann CL, Myers DE, Missmer SA, Walsh BW, et al. Cryopreserved embryo transfer is an independent risk factor for placenta accreta. Fertil Steril. 2015;103:1176–84 e2. doi: 10.1016/j.fertnstert.2015.01.021. [DOI] [PubMed] [Google Scholar]
- 61.Esh-Broder E, Ariel I, Abas-Bashir N, Bdolah Y, Celnikier DH. Placenta accreta is associated with IVF pregnancies: A retrospective chart review. BJOG. 2011;118:1084–1089. doi: 10.1111/j.1471-0528.2011.02976.x. [DOI] [PubMed] [Google Scholar]
- 62.Ishihara O, Araki R, Kuwahara A, Itakura A, Saito H, Adamson GD. Impact of frozen-thawed single-blastocyst transfer on maternal and neonatal outcome: An analysis of 277,042 single-embryo transfer cycles from 2008 to 2010 in Japan. Fertil. Steril. 2014;101:128–133. doi: 10.1016/j.fertnstert.2013.09.025. [DOI] [PubMed] [Google Scholar]
- 63.Matsuzaki S, Nagase Y, Takiuchi T, Kakigano A, Mimura K, Lee M, et al. Antenatal diagnosis of placenta accreta spectrum after in vitro fertilization-embryo transfer: A systematic review and meta-analysis. Sci. Rep. 2021;11:9205. doi: 10.1038/s41598-021-88551-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jauniaux E, Grønbeck L, Bunce C, Langhoff-Roos J, Collins SL. Epidemiology of placenta previa accreta: A systematic review and meta-analysis. BMJ Open. 2019;9:e031193. doi: 10.1136/bmjopen-2019-031193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cunningham KM, Anwar A, Lindow SW. The recurrence risk of placenta accreta following uterine conserving management. J. Neonatal-perinatal Med. 2015;8:293–296. doi: 10.3233/NPM-15915028. [DOI] [PubMed] [Google Scholar]
- 66.Sentilhes L, Kayem G, Chandraharan E, Palacios-Jaraquemada J, Jauniaux E. FIGO consensus guidelines on placenta accreta spectrum disorders: Conservative management. Int. J. Gynaecol. Obstetr. 2018;140:291–298. doi: 10.1002/ijgo.12410. [DOI] [PubMed] [Google Scholar]
- 67.Zhu L, Zhang Y, Liu Y, Zhang R, Wu Y, Huang Y, et al. Maternal and live-birth outcomes of pregnancies following assisted reproductive technology: A retrospective cohort study. Sci. Rep. 2016;6:35141. doi: 10.1038/srep35141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rasmussen S, Albrechtsen S, Dalaker K. Obstetric history and the risk of placenta previa. Acta Obstetr. Gynecol. Scand. 2000;79:502–507. [PubMed] [Google Scholar]
- 69.Lavery JP. Placenta previa. Clin. Obstetr. Gynecol. 1990;33:414–421. doi: 10.1097/00003081-199009000-00005. [DOI] [PubMed] [Google Scholar]
- 70.Oberg AS, Hernandez-Diaz S, Palmsten K, Almqvist C, Bateman BT. Patterns of recurrence of postpartum hemorrhage in a large population-based cohort. Am. J. Obstetr. Gynecol. 2014;210(229):e1–8. doi: 10.1016/j.ajog.2013.10.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ruiter L, Kazemier BM, Mol BWJ, Pajkrt E. Incidence and recurrence rate of postpartum hemorrhage and manual removal of the placenta: A longitudinal linked national cohort study in The Netherlands. Eur. J. Obstetr. Gynecol. Reprod. Biol. 2019;238:114–119. doi: 10.1016/j.ejogrb.2019.05.022. [DOI] [PubMed] [Google Scholar]
- 72.Berghella V. Prevention of recurrent fetal growth restriction. Obstetr. Gynecol. 2007;110:904–912. doi: 10.1097/01.AOG.0000267203.55718.aa. [DOI] [PubMed] [Google Scholar]
- 73.Rotshenker-Olshinka K, Michaeli J, Srebnik N, Terlezky S, Schreiber L, Farkash R, et al. Recurrent intrauterine growth restriction: Characteristic placental histopathological features and association with prenatal vascular Doppler. Arch. Gynecol. Obstetr. 2019;300:1583–1589. doi: 10.1007/s00404-019-05339-x. [DOI] [PubMed] [Google Scholar]
- 74.Phillips C, Velji Z, Hanly C, Metcalfe A. Risk of recurrent spontaneous preterm birth: A systematic review and meta-analysis. BMJ Open. 2017;7:e015402. doi: 10.1136/bmjopen-2016-015402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Saccone G, Khalifeh A, Elimian A, Bahrami E, Chaman-Ara K, Bahrami MA, et al. Vaginal progesterone vs intramuscular 17α-hydroxyprogesterone caproate for prevention of recurrent spontaneous preterm birth in singleton gestations: Systematic review and meta-analysis of randomized controlled trials. Ultrasound Obstetr. Gynecol. 2017;49:315–321. doi: 10.1002/uog.17245. [DOI] [PubMed] [Google Scholar]
- 76.Izbizky G, Meller C, Grasso M, Velazco A, Peralta O, Otano L, et al. Feasibility and safety of prophylactic uterine artery catheterization and embolization in the management of placenta accreta. J. Vasc. Interv. Radiol. 2015;26:162–169. doi: 10.1016/j.jvir.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 77.Jung HN, Shin SW, Choi SJ, Cho SK, Park KB, Park HS, et al. Uterine artery embolization for emergent management of postpartum hemorrhage associated with placenta accreta. Acta Radiol. (Stockholm, Sweden: 1987) 2011;52:638–642. doi: 10.1258/ar.2011.100514. [DOI] [PubMed] [Google Scholar]
- 78.van den Akker T, Brobbel C, Dekkers OM, Bloemenkamp KW. Prevalence, indications, risk indicators, and outcomes of emergency peripartum hysterectomy worldwide: A systematic review and meta-analysis. Obstetr. Gynecol. 2016;128:1281–1294. doi: 10.1097/AOG.0000000000001736. [DOI] [PubMed] [Google Scholar]
- 79.Tikkanen M, Paavonen J, Loukovaara M, Stefanovic V. Antenatal diagnosis of placenta accreta leads to reduced blood loss. Acta Obstetr. Gynecol. Scand. 2011;90:1140–1146. doi: 10.1111/j.1600-0412.2011.01147.x. [DOI] [PubMed] [Google Scholar]
- 80.Warshak CR, Ramos GA, Eskander R, Benirschke K, Saenz CC, Kelly TF, et al. Effect of predelivery diagnosis in 99 consecutive cases of placenta accreta. Obstetr. Gynecol. 2010;115:65–69. doi: 10.1097/AOG.0b013e3181c4f12a. [DOI] [PubMed] [Google Scholar]
- 81.Bouvier A, Sentilhes L, Thouveny F, Bouet PE, Gillard P, Willoteaux S, et al. Planned caesarean in the interventional radiology cath lab to enable immediate uterine artery embolization for the conservative treatment of placenta accreta. Clin. Radiol. 2012;67:1089–1094. doi: 10.1016/j.crad.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 82.Matsuzaki S, Okada A, Endo M, Nagase Y, Nakagawa S, Hiramatsu K, et al. Horizontal cervix as a novel sign for predicting adhesions on the posterior extrauterine wall in cases of placenta previa. J. Clin. Med. 2019;8:20. doi: 10.3390/jcm8122141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Erfani H, Fox KA, Clark SL, Rac M, Rocky Hui SK, Rezaei A, et al. Maternal outcomes in unexpected placenta accreta spectrum disorders: Single-center experience with a multidisciplinary team. Am. J. Obstetr. Gynecol. 2019;221(337):e1–e5. doi: 10.1016/j.ajog.2019.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Poujade O, Ceccaldi PF, Davitian C, Amate P, Chatel P, Khater C, et al. Uterine necrosis following pelvic arterial embolization for post-partum hemorrhage: Review of the literature. Eur. J. Obstetr. Gynecol. Reprod. Biol. 2013;170:309–314. doi: 10.1016/j.ejogrb.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 85.Vlahos L, Benakis V, Dimakakos P, Dimopoulos C, Pontifex G. A comparative study of the degree of arterial recanalization in kidneys of dogs following transcatheter embolization with eight different materials. Eur. Urol. 1980;6:180–185. doi: 10.1159/000473322. [DOI] [PubMed] [Google Scholar]
- 86.Pirard C, Squifflet J, Gilles A, Donnez J. Uterine necrosis and sepsis after vascular embolization and surgical ligation in a patient with postpartum hemorrhage. Fertil. Steril. 2002;78:412–413. doi: 10.1016/S0015-0282(02)03229-6. [DOI] [PubMed] [Google Scholar]
- 87.Cottier JP, Fignon A, Tranquart F, Herbreteau D. Uterine necrosis after arterial embolization for postpartum hemorrhage. Obstetr. Gynecol. 2002;100:1074–1077. doi: 10.1016/s0029-7844(02)02050-1. [DOI] [PubMed] [Google Scholar]
- 88.Saiga A, Yokota H, Higashide T, Takishima H, Omoto A, Kubota Y, et al. The relationship between gelatin sponge preparation methods and the incidence of intrauterine synechia following uterine artery embolization for postpartum hemorrhage. Cardiovasc. Interv. Radiol. 2019;42:195–204. doi: 10.1007/s00270-018-2078-x. [DOI] [PubMed] [Google Scholar]
- 89.Kim MJ, Kim IJ, Kim S, Park IY. Postpartum hemorrhage with uterine artery embolization: The risk of complications of uterine artery embolization. Minim. Invasive Ther. Allied Technol. 2020;2020:1–8. doi: 10.1080/13645706.2020.1789662. [DOI] [PubMed] [Google Scholar]
- 90.Kwon JH. Uterine necrosis and hysterectomy in a postpartum hemorrhage patient who underwent repeated uterine artery embolization. Taiwan J. Obstetr. Gynecol. 2015;54:791–792. doi: 10.1016/j.tjog.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 91.Coulange L, Butori N, Loffroy R, Filipuzzi L, Cercueil JP, Douvier S, et al. Uterine necrosis following selective embolization for postpartum hemorrhage using absorbable material. Acta Obstetr. Gynecol. Scand. 2009;88:238–240. doi: 10.1080/00016340802596041. [DOI] [PubMed] [Google Scholar]
- 92.Palacios Jaraquemada JM, Garcia Monaco R, Barbosa NE, Ferle L, Iriarte H, Conesa HA. Lower uterine blood supply: Extrauterine anastomotic system and its application in surgical devascularization techniques. Acta Obstetr. Gynecol. Scand. 2007;86:228–234. doi: 10.1080/00016340601089875. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the studies used in this study are published in the literature.