Abstract
Benign paroxysmal positional vertigo (BPPV) is the most common cause of positional vertigo. Vitamin D deficiency may be one of the causes of its development. To assess the relation between recurrent attacks BPPV and Vitamin D deficiency. A case control study in which 40 patients were clinically diagnosed as posterior canal BPPV, Serum 25(OH) D was measured at 1st visit. Patients were divided into two groups; group A (20 patients) received Vitamin D supplementation in addition to canal repositioning maneuver and group B (20 patients) treated by canal repositioning maneuver only. Follow up of all patients for 6 months, neuro-otological assessment was repeated and recurrent attacks were recorded. Serum vitamin D was repeated after 6 month. This study included 14 males and 26 females age ranged from 35 to 61 years, Average serum of 25 (OH) D at the first visit was (12.4 ± 2 ng/ml) for group A, and (12.2 ± 1.7 ng/ml) for group B, all patients had low serum level of 25(OH) D (below 20 ng/ml). Recurrent BPPV episodes, were significantly lower in group A than that of group B. There is a relation between BPPV recurrence and low serum Vitamin D.
Subject terms: Diseases, Medical research
Introduction
Positional vertigo is the vertigo induced by head motion1. Dizziness (including vertigo) affects about 15 to 20% of adults and vestibular vertigo accounts about a quarter of dizziness complaints2. BPPV is defined as recurrent positional vertigo attacks1 and is considered as the commonest vestibular disease3–5 its incidence rate among population is approximately 10%6. It is more common around age of sixty7. Dislodgement of calcium carbonate crystals (otoconia) from the utricle into the semicircular canals (most commonly the posterior canal) is one of the accepted theories of pathogenesis of BPPV. Vitamin D plays a major role in Calcium metabolism which may affect the calcium carbonate crystals (otoconia) density and matrix8.
The otoconia crystals consist of two parts; central core and peripheral zone. The core is mainly organic (which is predominantly glycoprotein) with a lower level of Ca2+, and the peripheral zone is mainly inorganic (which is predominantly a polymorph of calcium carbonate CaCO3) with a higher level of Ca2+9,10. The core, periphery and external surface of the crystals all have inter-connecting fibrous material with varied diameters and organization. The crystals of otoconia are formed by the active calcium metabolic process of the vestibular organ. Otoconia crystals are partially embedded in a fibrous matrix and are connected to hair cells with protein fiber11.
Calcium absorption from intestine and kidney increased and affected by vitamin D. The maintenance of blood calcium is controlled by parathyroid hormone. Vitamin D has direct effect on the mechanism of deposition of calcium and phosphate in the bone, teeth and otoconial particle formation in the vestibular system12. There are common features between bone and otoconia biomineralization. As the matrix organization and protein constituents are similar between the two tissues. Biomineralization in otoconia involves tight regulation of the formation of an organic matrix at specific sites and the deposition of mineral crystallites in an ordered manner similar to that in bone and teeth8,13,14.
In osteoporosis, there is a disturbance in the metabolism of both vitamin D and calcium and this is probably the key element of the pathogenesis of BPPV. Vitamin D level and deposition of calcium crystals affect the otoconia matrix and density similar to its effect on bone structures8. Vitamin D insufficiency correlated with the severity of BPPV and its recurrence15–17. In fact, the recurrence attacks of BPPV may decreased with vitamin D supplementation. Interestingly, a large number of cases was accomplished a complete remission after trials of vitamin D supplementation18,19.
The association between BPPV and osteoporosis was postulated in some studies20. This relationship originate from the essential role of calcium metabolism in the homeostasis of otoconia metabolism which regulate the synthesis and absorption of otoconia, which mainly composed of calcium carbonate21. So that any disturbance in calcium metabolism, as in osteoporosis and osteopenia, may contribute to the development of BPPV22. BPPV was related to a 1.28 times higher odds of osteoporosis. In addition, osteoporosis was associated with a 1.34 times higher odds of BPPV23.
Vitamin D are maintained in its adequate level through its cutaneous photosynthesis and oral ingestion. By some estimates, one billion people worldwide have vitamin D deficiency or insufficiency. Photosynthesis and bioavailability of vitamin D influenced by many factors and these factors may contribute to risk of impaired vitamin D status. These factors include variation in sun exposure due to geographic latitude, time of day, solar radiation exposure, season, weather condition, air pollution, clothing, sunscreen use and skin pigmentation, as well as age, obesity and the incidence of several chronic illnesses24.
Although BPPV may result secondary to migraines, head trauma, vestibular neuritis, prolonged bed rest and otologic surgery, 80% of all cases are idiopathic25. Canal repositioning maneuver (CRM) is rapid and effective in which the dislodged crystals are returned to the utricle where they may be absorbed by the body. However, BPPV often recurs despite the effectiveness of this treatment. Many researches have confirmed that vitamin D receptors are founded on calcium channel transport systems of the labyrinth and act to regulate proper calcium balance. This mechanism may help to explain the role of vitamin D in maintaining proper auditory function25. Deficiency of vitamin D has been attributed to cochlear demineralization and cochlear deafness. The deficient vitamin D may exert its effect by disturbed calcium metabolism as calcium ions play an important role in membrane permeability. Ionized calcium is necessary for normal function of the nerve and its deficiency may affect the action potential generation in cochlea. Low level of vitamin D and calcium may lead to demineralization of otic capsule, degenerative changes in the spiral ligament, stria vascularis, and cochlear hair cells26. Brooks et al. has reported improvement in the degree of hearing after restoration of serum vitamin D level27.
Aim of the work
The aim of this study is to assess Vitamin D serum level in BPPV patients and to assess the relation between recurrent attacks of BPPV and Vitamin D deficiency, Also to evaluate the effect of Vitamin D supplementation in decreasing number of recurrent attacks of BPPV.
Patients and methods
Ethics approval and consent to participate
The study was approved by Qena Faculty of Medicine Ethics Committee. The reference number of the committee is 54/4/7/2020. We confirm that all research was performed in accordance with relevant guidelines/regulations; informed consent was obtained from all participants and/or their legal guardians in accordance with the Declaration of Helsinki.
Verbal informed consent to participate in the study was obtained from parents or legal guardians of all cases. The consent was verbal as most of our cases parents or legal guardians were not educated. The Qena Faculty of Medicine Ethics Committee approved the verbal consent in our study.
Forty patients suffering from BPPV presented at the Qena university hospital and private clinic from January 2019 to June 2020. These patients were included in the study after obtaining written and oral consent. These patients include 26 female and 14 male their average age was 48 years.
Inclusion criteria
Diagnosis of canalithiaisis or cupulothiasis BPPV of posterior semicircular canal which was made based on history (Recurrent episodes of positional vertigo or positional dizziness induced by turning over or lying down in the supine position and the attacks ends < 1 min), clinical examination (Positional nystagmus appears after a latency period of few seconds in canalithiaisis or elicited after a brief or no latency in cupulothiasis by the Dix-Hallpike maneuver. The nystagmus is a mix of torsional nystagmus with the upper pole of the eyes beating toward the lower ear combined with vertical nystagmus beating upward typically lasting < 1 min in canalithiaisis and > 1 min in cupulothiasis and not caused by another disorder28.
Exclusion criteria
History of head and ear trauma, surgery or infectious disease of the ear during the preceding month of BPPV attack to exclude secondary BPPV. Also patients with chronic renal diseases, pulmonary, hematologic, gastrointestinal and cardiovascular diseases. Also patients taking Supplementary calcium and/or Vitamin D or taking medication that alter Vitamin D metabolism in the last year, Patients with history or active case of inner ear disease. Patients with a typical history of BPPV without exhibit nystagmus on clinical tests and Patients whose data is incomplete or denied for consent may be excluded29.
Patients were subjected to a detailed history taking, clinical examination for spontaneous nystagmus, headshaking nystagmus, positional and positioning nystagmus using frenzel google and laboratory investigation of Vitamin D by measuring serum 25 hydroxy Vitamin D level at first visit. All patients should had history of previous visiting the clinic with the diagnosis of BPPV of at least 2 or more attacks of BPPV over 6 months prior to inclusion in the previous 2 years.
Vitamin D status was classified according to measured 25(OH) D concentration: less than 10 ng/mL: deficient; between 11 and 20: insufficient; higher than 20 ng/ml: optimal30 For patients with insufficiency and deficiency serum level and no history of nephrolithiasis, Vitamin D supplement was given in regimen of cholecalciferol 8000 IU daily for 2 weeks followed by 4000 IU daily for 2 weeks then 8000 IU single dose weekly for 3 months31.
CRM was done for all patients at the first visit once and the patients were instructed to visit the clinic 2–3 days later. The disappearance of symptoms and disappearance of nystagmus in Dix-Hallpike test was considered as criteria for complete cure and successful maneuver. Instead occurrence of vertigo 1 month or more after complete recovery which led to identification of nystagmus with frenzel glasses and consequently to diagnose BPPV was considered as criteria for recurrence.
Patients were divided randomly into two groups group A (received supplementary Vitamin D and CRM was done to them) and group B (CRM only was done to them). Follow up period was 6 months. During this period the clinical assessment was repeated and serum Vitamin D level was repeated for both groups after 6 months. Patients assessed and reassessed at first visit, after 1 month, 2 months, 3 months and 6 months. The number of BPPV attacks in each group was recorded over 6 months follow up period in both groups.
Statistical analysis
Data were analyzed using Statistical Program for Social Science (SPSS) version 24. Quantitative data were expressed as mean ± standard deviation (SD). Qualitative data were expressed as frequency and percentage. Mean (average): the central value of a discrete set of numbers, specifically the sum of values divided by the number of values. Standard deviation (SD): is the measure of dispersion of a set of values. A low SD indicates that the values tend to be close to the mean of the set, while a high SD indicate that the values are spread out over a wider range.
The following tests were done:
Independent-samples t-test of significance: was used when comparing between two means. Chi-square test: was used when comparing between non-parametric data. Probability (P-value) P-value < 0.05 was considered significant.
Results
In this study 40 patients diagnosed with posterior canal BPPV were included. Successful epley maneuver was performed and serum Vitamin D levels were calculated in the first visit. Besides we select only patients with vitamin D less than 20 ng/ml.
Patients were divided randomly into two groups, group A with mean age (49.9 ± 7.4) and group B with mean age (50.2 ± 9.6) with no significant difference (Table 1). 20 patients in Group A; 6 males (30%) and 14 females (70%), also in group B: 20 patients were included; 8 males (40%) and 12 females (60%) with no significant difference (Table 1). As regard serum Vitamin D level of the first visit, group A mean serum level was (12.4 ± 2) versus (12.2 ± 1.7) for group B also with no significant difference between both groups (Table 1).
Table 1.
Comparison between demographic data of both groups.
| Variable | Vit D and epily (group A 20 patients) | Epily only (group B 20 patients) | P value |
|---|---|---|---|
| Age |
49.9 ± 7.4 Average = 49.9 |
50.2 ± 9.6 Average = 50.2 |
0.9 |
| Sex | |||
| Males | 6 (30%) | 8 (40%) | 0.6 |
| Females | 14 (70%) | 12 (60%) | |
| Vitamin D level in the first visit |
12.4 ± 2 Average = 12.44 |
12.2 ± 1.7 Average = 12.23 |
0.8 |
After 6 months of treatment and Vitamin D therapy in group A; Vitamin D serum levels were increased with mean level (26.3 ± 4.1) but in group B vitamin D serum level didn’t show any change with mean level (12.2 ± 1.7) with highly significant difference between group A and group B (Table 2). The mean value of recurrence in 6 months follow up duration was (0.2 ± 0.4) in group A in comparison to (1.5 ± 0.7) in group B with highly significant p value (Table 2).
Table 2.
Comparison between vitamin D level and recurrence rate after 6 months in group A and group B.
| Variable | Vit D and Eply | Eply only | P value |
|---|---|---|---|
| Vitamin D level after 6 months | 26.3 ± 4.1 | 12.2 ± 1.7 | 0.000* |
| Recurrence during 6 months period | 0.2 ± 0.4 | 1.5 ± 0.7 | 0.000* |
∗highly significant.
In comparison between Vitamin D serum level 6 months of Vitamin D therapy there were significant increase in the mean serum Vitamin D level (26.3 ± 4.1) versus (12.4 ± 2) in the first visit with highly significant p value (Table 3).
Table 3.
Comparison between vitamin D level in group A in pre and post vitamin D therapy.
| Vit D in groupA first visit | Vit D in group A pos V D therapy | T value | P value | |
|---|---|---|---|---|
| Vitamin D | 12.4 ± 2 | 26.3 ± 4.1 | 18.2 | 0.000 |
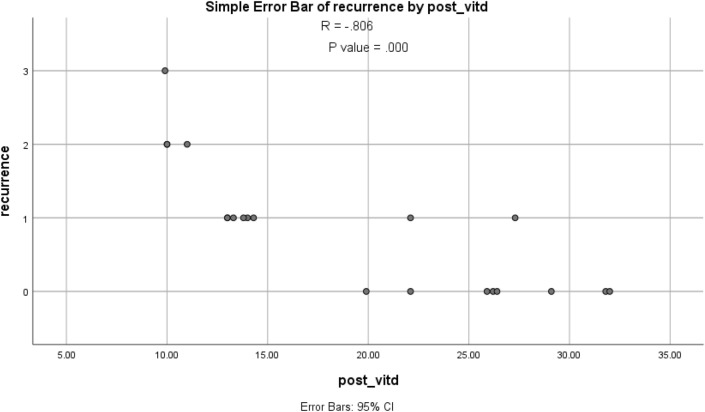
As regard correlation there were negative correlation between recurrence rate of BPPV episodes and Vitamin D deficiency with R = − 0.806; which means that Vitamin D deficiency may have a role in recurrence of BPPV (Table 4, Fig. 1).
Table 4.
Correlation between VIT D and recurrence.
| R | P value | |
|---|---|---|
| Vit D and recurrence | − 0.806 | 0.000* |
∗highly significant.
Figure 1.
Correlation between Vitamin D and BPPV recurrence.
The number of episodes during 6 months of follow up: in group A who received Vitamin D therapy in addition to Epley maneuver 4 patients out of 20 patients developed one episode in 6 months follow up duration, and the recurrence was near the 1 month in 4 patients but in group B who received Epley maneuver only: 12 patients (60%) developed one episode, 6 patients (30%) developed 2 episodes, and 2 patients (10%) developed three episode and the recurrence was in different times in the follow up month but almost of them had the 1st recurrence in 1st month. There were statistically significant difference between group A and group B as regard number of recurrent episodes with p value = 0.003 (Table 5).
Table 5.
Comparison of number of recurrent episodes during 6 months follow up in group A and group B.
| Recurrence in 6 months duration | Group A | Group B | P value |
|---|---|---|---|
| 0 episode | 16 (80%) | 0 (0%) | 0.003* |
| 1 episode | 4 (20%) | 12 (60%) | |
| 2 episode | 0 (0%) | 6 (30%) | |
| 3 episodes | 0 (0%) | 2 (10%) |
∗highly significant.
Discussion
Egyptian people have a high incidence of Vitamin D deficiency, which may be related to different factors influencing vitamin D as clothing, variation in sun exposure, age, obesity, several chronic illnesses also there is seasonal variation as vitamin D deficiency especially in season there is less exposure to sun light. A seasonal variation of patients presenting with BPPV has been observed in the United States and Iraq. In Boston, the number of BPPV clinic visits was greatest during March to May, months where serum vitamin D levels are at their lowest16,32. Populations living in sunny, neighboring regions of the Equator, have high serum vitamin D levels due to higher radiation incidence from the sun with fairly short wavelengths, ultraviolet B (UVB)33.
BPPV Patients were found to have low serum Vitamin D level less than20 ng/ml. This is almost near that of Austrian population who has average 20.9 mg/ml17. Patients in this study had significantly low serum level of 25(OH) D, Mean level of 25(OH) Din group A was (12.4 ± 2) and in group B was (12.2 ± 1.7). The number of women was higher in the study groups, but with no statistically significant difference. Which is similar results of Study done by Rhim, the number of women was higher in the study group (P = 0.051), but this difference was not statistically significant, and this difference is explained with differences in sex hormones34, another study done by Mithal et al. who reported that the incidence of Vitamin D deficiency was also more among females to35. The mean age of our study groups was 48 years which is also near to results of Rhim34 in which the median age of subjects was 50 years.
Our study showed that the mean of recurrent attacks of BPPV in group A (0.2 ± 0.4) was significantly lower than that in group B (1.5 ± 0.7). There was a negative correlation between recurrence rate of BPPV episodes and Vitamin D deficiency which means that Vitamin D deficiency may have a role in recurrence of BPPV. These results are similar to other studies. Recurrence rates of BPPV are known to be 30–50% between 3 and 5 years36,37, and usually associated with female sex, old age, ear diseases, lateral canal, chronic diseases, and Vitamin D deficiency36–39. Rahim et al. assessed the recurrence rates of BPPV episodes for a long period of time without limiting the age, sex or locations of semicircular canals, Patients had history of BPPV recurrent episodes before therapy 3–4 episodes/year. After Vitamin D therapy, there was significant decrease in the recurrence rate during 6 months of follow up and he concluded that serum Vitamin D concentrations significantly affect the recurrence of BPPV34.
Büki et al. found that his patients with idiopathic Benign Positional Vertigo had low Vitamin D serum levels (23 ng/ml)16. He recorded 4 patients having recurrent episodes of BPPV for a longer period before examination with a frequency of 4–6 episodes/year for several years. These patients had statistically lower serum Vitamin D level than patients with the first episode. After Vitamin D therapy, BPPV patients have not reported recurrent episodes in the follow up period for 8 months of follow up16.
In another meta-analysis that investigated the difference between the recurrence and non recurrence of BPPV, there was a significant difference in the Vitamin D levels between the two groups, which indicated that Vitamin D play, a role in the recurrent nature of BPPV. Consistently, it reported that the BPPV recurrence was frequent among the patients with osteoporosis20. Mohsin et al. reported also that patients with BPPV had low serum level of Vitamin D (11.6 ng/ml). He recorded 9 patients had recurrent attacks of BPPV for several years with a frequency of 3–4 relapses/year. These patients were found to have statistically lower serum level of Vitamin D than patients with first episode. After Vitamin D therapy patients with BPPV reported no episodes in 10 months follow up duration6.
In 2003 Vibert et al. found that the recurrence rate of BPPV was 27% and most patients relapse in the first 6 months. He also reported a relation between BPPV and osteoporosis, since bone metabolism may have a role in pathogenesis of BPPV40. Yamnaka et al. found that the incidence of osteoporosis in BPPV patients was 26.2% and the incidence of recurrent episodes of BPPV was 56.3%. These rates were statistically higher than that recorded in patients with normal bone density (16.1%)41. Jeong et al. found that the levels of serum 25(OH)D in 100 patients with idiopathic BPPV was lower than that in control group42, and the incidence of 25 (OH)D insufficiency (< 20 ng/ml) in patients with BPPV was significantly higher than that in control group. Xiang et al., proved that both the 25(OH)D insufficiency (10–20 ng/ml) and deficiency (< 10 ng/ml) are associated with BPPV, and suggest that low serum 25(OH)D may have a role in the pathogenesis of BPPV1. Xiang also reported that in cases with chronically recurrent severe BPPV episodes, low levels of serum 25 (OH) D could be measured and, BPPV did not recur after supplementation with Vitamin D1.
Hae and his colleges in 2013 found serum vitamin D lower in patients with BPPV than healthy controls. Patients with recurrent BPPV had lower Vitamin D serum than other patients. They recommended that serum Vitamin D should be above 30 ng/ml with safe sun exposure for up to 30 min per day to decrease recurrence of BPPV43. In another study vitamin D supplementation was given to patients with posterior semicircular canal BPPV and vitamin D deficiency for 3 months. In follow up of those patients the recurrence rate was significantly lower than patients did not receive Vitamin D supplementation which is similar to this study18.
Conclusion
Our data suggest that most patients with BPPV in Egypt have low serum Vitamin D and Vitamin D supplement may have a role in decreasing recurrent attacks of BPPV. Further studies must be done to assess role of Vitamin D therapy in treatment of BPPV.
Recommendation
Larger sample size will give more information about both vitamin D deficiency and BPPV and their relation.
Limitation
Vestibular Migraine is highly associated with occurrence of BPPV and its a cause of positional vertigo which may confused with recurrent BPPV and it is also a possible cause of BPPV recurrence.
Acknowledgements
To members of Ear, Nose and Throat Department, Tropical Medicine and Gastroenterology Department, Clinical Pathology Department, Faculty of Medicine, South Valley University, Egypt and Audiovestibular medicine (ENT) Faculty of Medicine Elminia University, Egypt.
Author contributions
A.A.A. have made substantial contributions to the conception, design of the work; the acquisition, analysis, interpretation of data, have drafted the work and substantively revised it. AND also, approved the submitted version (and any substantially modified version that involves the author's contribution to the study) AND to have agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature. A.A.A. have read and approved the final manuscript file. D.F.M.F. have made substantial contributions to the conception, design of the work; the acquisition, analysis, interpretation of data, and substantively revised it. AND also, approved the submitted version (and any substantially modified version that involves the author's contribution to the study) AND to have agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature. D.F.M.F. have read and approved the final manuscript file. S.E.S.B. has made substantial contributions to the conception, design of the work, analysis, interpretation of data, has drafted the work and substantively revised it. AND also, approved the submitted version (and any substantially modified version that involves the author's contribution to the study) AND to have agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature. S.E.S.B. have read and approved the final manuscript file. M.F.A. have made substantial contributions to the conception, design of the work; the acquisition, analysis, interpretation of data, and substantively revised it. AND also, approved the submitted version (and any substantially modified version that involves the author's contribution to the study) AND to have agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature. M.F.A. have read and approved the final manuscript file. Z.F.A. has made substantial contributions to the conception, design of the work, analysis, interpretation of data, has drafted the work and substantively revised it. AND also, approved the submitted version (and any substantially modified version that involves the author's contribution to the study) AND to have agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature. Z.F.A. have read and approved the final manuscript file. All authors have read and approved the manuscript.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gu X, Dong F, Gu J. Analysis of effect of 1α-hydroxyvitamin D3 on benign paroxysmal positional vertigo and risk factors. Exp. Ther. Med. 2018;15:2321–2326. doi: 10.3892/etm.2018.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neuhauser HK. The epidemiology of dizziness and vertigo. Handb. Clin. Neurol. 2016;137:67–82. doi: 10.1016/B978-0-444-63437-5.00005-4. [DOI] [PubMed] [Google Scholar]
- 3.Neuhauser HK, Lempert T. Vertigo: Epidemiologic aspects. Semin. Neurol. 2009;29(05):473–481. doi: 10.1055/s-0029-1241043. [DOI] [PubMed] [Google Scholar]
- 4.Parnes LS, Agrawal SK, Atlas J. Diagnosis and management of benign paroxysmal positional vertigo (BPPV) Can. Med. Assoc. J. 2003;169(7):681–693. [PMC free article] [PubMed] [Google Scholar]
- 5.Nedzelski JM, Barber HO, McIlmoyl L. Diagnoses in a dizziness unit. J. Otolaryngol. 1986;15(2):101–104. [PubMed] [Google Scholar]
- 6.Mohsin FD, Alharbawi FA, Alraho ST. Benign paroxysmal positional vertigo and vitamin D deficiency. Pharma Innov. J. 2019;8(3):49–52. [Google Scholar]
- 7.Bhattacharyya N, Gubbels PS, Schwartz RS, Edlow EH, Fife T, Holmberg MJ, et al. Clinical practice guideline: Benign paroxysmal positional vertigo (update) executive summary. Otolaryngol. Head Neck Surg. 2017;156(3):403–416. doi: 10.1177/0194599816689660. [DOI] [PubMed] [Google Scholar]
- 8.Yang H, Zhao X, Xu Y, Wang L, He Q, Lundberg YW. Matrix recruitment and calcium sequestration for spatial specific otoconia development. PLoS ONE. 2011;6:e20498. doi: 10.1371/journal.pone.0020498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lundberg YW, Zhao X, Yamoah EN. Assembly of the otoconia complex to the macular sensory epithelium of the vestibule. Brain Res. 2006;1091(1):47–57. doi: 10.1016/j.brainres.2006.02.083. [DOI] [PubMed] [Google Scholar]
- 10.Lins U, Farina M, Kurc M, et al. The otoconia of the guinea pig utricle: Internal structure, surface exposure, and interactions with the filament matrix. J. Struct. Biol. 2000;131:67–78. doi: 10.1006/jsbi.2000.4260. [DOI] [PubMed] [Google Scholar]
- 11.Lundberg YW, Zhao X, Yamoah EN. Assembly of the otoconia complex to the macular sensory epithelium of the vestibule. Brain Res. 2006;1091:47–57. doi: 10.1016/j.brainres.2006.02.083. [DOI] [PubMed] [Google Scholar]
- 12.Pillai NG, Gopinath I. A prospective analysis of vitamin D and recurrent benign paroxysmal positional vertigo. Int. J. Otorhinolaryngol. Head Neck Surg. 2019;5(6):1548–1551. doi: 10.18203/issn.2454-5929.ijohns20194924. [DOI] [Google Scholar]
- 13.Zhao X, Yang H, Yamoah EN, Lundberg YW. Gene targeting reveals the role of Oc90 as the essential organizer of the otoconial organic matrix. Dev. Biol. 2007;304:508–524. doi: 10.1016/j.ydbio.2007.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu Y, Zhang H, Yang H, Zhao X, Lovas S, Lundberg YW. Expression, functional, and structural analysis of proteins critical for otoconia development. Dev. Dyn. 2010;239:2659–2673. doi: 10.1002/dvdy.22405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Talaat HS, Abuhadied G, Talaat AS, Abdelaal MS. Low bone mineral density and vitamin D deficiency in patients with benign positional paroxysmal vertigo. Eur. Arch. Otorhinolaryngol. 2015;272(9):2249–2253. doi: 10.1007/s00405-014-3175-3. [DOI] [PubMed] [Google Scholar]
- 16.Buki B, Ecker M, Junger H, Lundberg YW. Vitamin D deficiency and benign paroxysmal positioning vertigo. Med. Hypotheses. 2013;80(2):201–204. doi: 10.1016/j.mehy.2012.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kudlacek S, Schneider B, Peterlik M, et al. Assessment of vitamin D and calcium status in healthyadult Austrians. Eur. J. Clin. Investig. 2003;33:323–331. doi: 10.1046/j.1365-2362.2003.01127.x. [DOI] [PubMed] [Google Scholar]
- 18.Talaat HS, Kabel AM, Khaliel LH, Abuhadied G, El-Naga HA, Talaat AS. Reduction of recurrence rate of benign paroxysmal positional vertigo by treatment of severe vitamin D deficiency. Auris Nasus Larynx. 2016;43(3):237–241. doi: 10.1016/j.anl.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 19.Sheikhzadeh M, Lotfi Y, Mousavi A, Heidari B, Monadi M, Bakhshi E. Influence of supplemental vitamin D on intensity of benign paroxysmal positional vertigo: A longitudinal clinical study. Casp. J. Intern. Med. 2016;7(2):93. [PMC free article] [PubMed] [Google Scholar]
- 20.Yu S, Liu F, Cheng Z, Wang Q. Association between osteoporosis and benign paroxysmal positional vertigo: A systematic review. BMC Neurol. 2014;14(1):110. doi: 10.1186/1471-2377-14-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hughes I, Thalmann I, Thalmann R, Ornitz DM. Mixing model systems: Using zebrafish and mouse inner ear mutants and other organ systems to unravel the mystery of otoconial development. Brain Res. 2006;1091(1):58–74. doi: 10.1016/j.brainres.2006.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bruintjes TD, Van der Zaag-Loonen HJ, Eggelmeijer F, Van Leeuwen RB. The prevelance of benign positional vertigo in patients with osteoprosis. Eur. Arch. Oto-Rhino-Laryngol. 2018 doi: 10.1007/s00405-018-5164-4. [DOI] [PubMed] [Google Scholar]
- 23.Kim SY, Kim HJ, Min C, Choi HG. Association between benign paroxysmal positional vertigo and osteoporosis: Two nested case-control studies. Osteoporos. Int. 2020;31:2017–2024. doi: 10.1007/s00198-020-05478-x. [DOI] [PubMed] [Google Scholar]
- 24.Tsiaras WG, Weinstock MA. Factors influencing vitamin D status. Acta Derm. Venereol. 2011;91:115–124. doi: 10.2340/00015555-0980. [DOI] [PubMed] [Google Scholar]
- 25.Sturges, M. & Canell, J Treating vitamin D deficiency may help reduce the recurrence rate of vertigo, according to study Posted on (2015).
- 26.Weir N. Sensorineural deafness associated with recessive hypophosphataemic rickets. J. Laryngol. Otol. 1977;91:717–722. doi: 10.1017/S0022215100084255. [DOI] [PubMed] [Google Scholar]
- 27.Brookes EB, Morrison AW. Vitamin D deficiency and deafness. Br. Med. J. (Clin. Res. Ed.) 1981;283:273–274. doi: 10.1136/bmj.283.6286.273-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Von Brevern M, Bertholon P, Brandt T, Fife T, Imai T, Nuti D, Newman-Toker D. Benign paroxysmal positional vertigo: Diagnostic criteria Consensus document of the Committee for the Classification of Vestibular Disorders of the Bárány Society. J. Vestib. Res. 2015;25:105–117. doi: 10.3233/VES-150553. [DOI] [PubMed] [Google Scholar]
- 29.Dhameliya JD, Chandra UK, Vishwakarma SK, Ganganpalli D, Verma A. Investigating the association between serum vitamin D deficiency and idiopathic benign paroxysmal positional vertigo. Int. Clin. Neurosci. J. 2020;7(3):122–126. doi: 10.34172/icnj.2020.12. [DOI] [Google Scholar]
- 30.Souberbielle JC, Body JJ, Lappe JM, et al. Vitamin D and musculoskeletal health, cardiovasculardisease, autoimmunity and cancer: Recommendations for clinical practice. Autoimmun. Rev. 2010;9:709–715. doi: 10.1016/j.autrev.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 31.Thacher TD, Clarke BL. Vitamin D insufficiency. Mayo Clin. Proc. 2011;86:50–60. doi: 10.4065/mcp.2010.0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saeed BMN, Omari AF. Climatic variations and benign paroxysmal positional vertigo. J. Otol. 2016;11:33–37. doi: 10.1016/j.joto.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zuma E Maia FC, de Fraga RB, Ramos BF, Cal RV, Mangabeira Albernaz PL. Seasonality and solar radiation variation level in benign paroxysmal positional vertigo. J. Otol. 2019;139(6):497–499. doi: 10.1080/00016489.2019.1590636. [DOI] [PubMed] [Google Scholar]
- 34.Rhim LG. Serum vitamin D and long-term outcomes of benign paroxysmal positional vertigo. Clin. Exp. Otorhinolaryngol. 2019;12(3):273–278. doi: 10.21053/ceo.2018.00381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mithal A, Wahl DA, Bonjour JP, Burckhardt P, Dawson-Hughes B, Eisman JA, et al. Global vitamin D status and determinants of hypovitaminosis D. Osteoporos. Int. 2009;20:1807–1820. doi: 10.1007/s00198-009-0954-6. [DOI] [PubMed] [Google Scholar]
- 36.Sakaida M, Takeuchi K, Ishinaga H, Adachi M, Majima Y. Long-term outcome of benign paroxysmal positional vertigo. Neurology. 2003;60(9):1532–1534. doi: 10.1212/01.WNL.0000061477.03862.4D. [DOI] [PubMed] [Google Scholar]
- 37.Brandt T, Huppert D, Hecht J, Karch C, Strupp M. Benign paroxysmal positioning vertigo: A long-term follow-up (6–17 years) of 125 patients. Acta Otolaryngol. 2006;126(2):160–163. doi: 10.1080/00016480500280140. [DOI] [PubMed] [Google Scholar]
- 38.Tanimoto H, Doi K, Nishikawa T, Nibu K. Risk factors for recurrence of benign paroxysmal positional vertigo. J. Otolaryngol. Head Neck Surg. 2008;37(6):832–835. [PubMed] [Google Scholar]
- 39.Rhim GI. Serum vitamin D and recurrent benign paroxysmal positional vertigo. Laryngosc. Investig. Otolaryngol. 2016;1(6):150–153. doi: 10.1002/lio2.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vibert D, Kompis M, Hausler R. Benign paroxysmal positional vertigo in older women may be related to osteoporosis and osteopenia. Ann. Otol. Rhinol. Laryngol. 2003;112:885–889. doi: 10.1177/000348940311201010. [DOI] [PubMed] [Google Scholar]
- 41.Yamanaka T, Shirota S, Sawai Y, Murai T, Fujita N, Hosoi H. Osteoporosis as a risk factor for the recurrence of benign paroxysmal positional vertigo. Laryngoscope. 2013;123(11):2813–2816. doi: 10.1002/lary.24099. [DOI] [PubMed] [Google Scholar]
- 42.Jeong SH, Kim JS, Shin JW, Kim S, Lee H, Lee AY, et al. Decreased serum vitamin D in idiopathic benign paroxysmal positional vertigo. J. Neurol. 2013;260(3):832–838. doi: 10.1007/s00415-012-6712-2. [DOI] [PubMed] [Google Scholar]
- 43.Jeong SH, Kim JS, Shin JW, Kim S, Lee H, Lee AY, et al. Decreased serum vitamin D in idiopathic benign paroxysmal positional vertigo. J. Neurol. 2013;260:832–838. doi: 10.1007/s00415-012-6712-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.