Abstract
Background
Andrographis paniculata (Burm.f.) Nees has been well-researched for its immunomodulatory effects.
Objective(s)
To investigate the immunomodulatory effects of standardized A. paniculata extract (SAPE) in healthy adults.
Materials and methods
The study was an open-label, single-centre study conducted for 30 days. Thirty participants with absolute lymphocyte counts of 1000–4000 cells/mm3 were enrolled and were instructed to ingest 200 mg of SAPE daily for 30 days. The participants visited the clinic at baseline, and days 3, 7, and 30. Immune cells such as NK cell (CD3-CD16+CD56+), T cells (CD3+), T helper cells (CD3+CD4+), T cytotoxic cells (CD3+CD8+) were measured using flow cytometry. Serum cytokines that include interferon gamma (IFN-γ), interleukin-4 (IL-4), interleukin-2 (IL-2), interleukin-12 (IL-12), and tumor necrosis factor alpha (TNF-α) were measured using ELISA. The SAPE used in this study was a standardized proprietary extract (AP-Bio®/KalmCold®) developed from the leaf extracts of A. paniculata.
Results
SAPE increased T cells, T helper cells and significantly increased IFN-γ, IL-4, and decreased IL-2 at day 30. A subgroup analysis of participants with absolute lymphocyte counts of 1000–3000 cells/mm3 indicated that there is a significant increase in the T cells, T helper cells at day 7 and 30 and significant increase in IFN-γ, IL-4 and decrease in IL-2 at day 30. There was no treatment related adverse effects following SAPE intake for 30 days.
Conclusion
Supplementation of SAPE resulted in immunomodulatory effects evidenced by its effects on immune cells and cytokines and it was found to be safe and tolerable.
Keywords: Andrographis paniculata, Interferon gamma, Immunity, Interleukin-4, Interleukin-2, T cells, T helper cells
1. Introduction
Andrographis paniculata (Burm.f.) Nees is an herbaceous plant belonging to Acanthaceae found throughout tropical and sub-tropical Asia, Southeast Asia, and India. A. paniculata is popularly known as ‘king of bitters’ and is widely used in traditional medicine in India, China, Thailand, Bangladesh, Pakistan, Hong Kong, Philippines, Malaysia, and Indonesia for various ailments such as common cold, respiratory infections, pharyngitis, pharyngolaryngitis, pneumonia, lung infections, urinary tract infections, indigestion, liver diseases, skin infections, encephalitis B, Herpes Zoster, etc. [1,2]. The ‘king of bitters’ and its major constituents viz., diterpenoids, flavonoids, and polyphenols have been reported in the published literature for anti-bacterial, anti-malarial, filaricidal, anti-diarrhoeal, anti-ulcerogenic, anti-hyperglycemic, hepatoprotective, anti-inflammatory, immunostimulatory, and anti-allergic properties. The published clinical studies on A. paniculata reported prophylactic and therapeutic benefits for upper respiratory tract infections, increase in immune cell counts in HIV infected patients, and anti-cancer effects [3,4]. There are thirty-three randomized controlled trials involving 7175 patients that support the effectiveness of A. paniculata (as a monotherapy and as an herbal mixture) in respiratory tract infections typically caused by seasonal viruses [5]. Also, several animal and cell-based studies are available to indicate broad spectrum anti-viral activity of the plant against viruses such as influenza A virus, dengue virus, chikungunya virus, human immunodeficiency virus, hepatitis B virus, hepatitis C virus, herpes simplex virus 1, Epstein–Barr virus, and human papillomavirus [6]. The effects on the seasonal cold and flu virus and other viruses are suggestive that A. paniculata may be a modifiable factor in impacting immune function.
Immune system protects the host from pathogenic organisms (virus, bacteria etc.) and helps in cell cycle homeostasis. Humans possess innate and adaptive immune responses that work synergistically and function in a highly orchestrated manner. The innate arm involves NK cells, phagocytes as a quick non-specific defense while the adaptive arm involves T and B lymphocytes. Another important component of the immune system is the cytokines that regulate the immune cells and has many other functions such as anti-viral activity. The immune responses are compromised due to stress, age, and lifestyle choices that render the host vulnerable to infections [7,8]. Hence, herbs/phytoactives that are biological response modifiers known to modulate immune function aids in protecting the host from infections.
A. paniculata has been widely researched for its immunomodulatory activities in vitro and in vivo including lymphocyte proliferation, promoting cell mediated lytic activity through NK cells, antibody-dependent cellular cytotoxicity, and phagocytic activity [9]. While there is a plethora of evidence available for immunomodulatory effects of A. paniculata in cell and animal systems, there is a paucity of clinical evidence on the effects of A. paniculata on immune system in healthy individuals. Hence, the current pilot open-label, single-arm clinical study was conducted to evaluate whether a standardized A. paniculata leaf extract (SAPE) would modulate the immune functions in healthy adults.
2. Materials and methods
2.1. Participant recruitment
The participants were recruited from May to June 2020 at the trial site, Bangalore, India. All the participants signed the informed consent form before undergoing any clinical trial related procedures. Participants recruited were male and female aged between 18 and 60 years with a body mass index of 18–30 kg/m2 who are healthy as determined by medical history, physical examination, and clinical judgement by the investigators.
The exclusion criteria of the study were as follows:
1) pregnant/lactating or plans to become pregnant during the trial period, 2) had evidence of any clinically significant acute or chronic cardiovascular, endocrine, immune, respiratory, liver biliary, kidney and urinary, neuropsychiatry, musculoskeletal, inflammatory and hematologic diseases/disorders, 3) had history of allergy or undergone surgery during last one year, 4) had received organ transplant, 5) chronic smokers, 6) has received influenza vaccination within 3 months before the screening examination, 7) were on any medication or health function food or herbal products within 1 month prior to the screening examination, 8) were on anti-psychotic medication within 3 months prior to the screening examination, 9) had experienced alcohol or drug abuse, and 10) have participated in other clinical trials within 3 months prior to the screening examination.
2.2. Study design
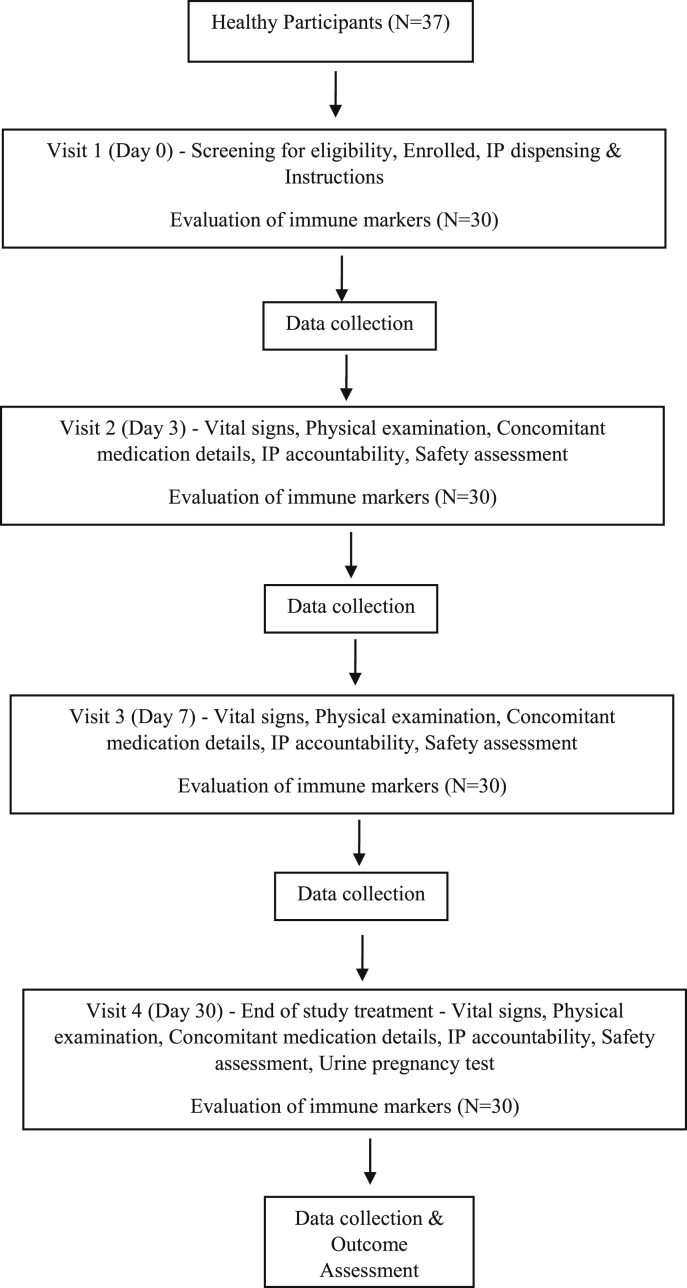
An open-label, single-arm, single centre study was conducted for 30 days. Based on the inclusion and exclusion criteria 30 participants were recruited who took investigational product (IP) for 30 days. Participants were invited to the clinic before starting the treatment (day 0) and thrice at day 3, 7, and 30 after IP ingestion. The study flow chart is shown in Fig. 1.
Fig. 1.
Flowchart.
2.3. Intervention
The investigational product SAPE is developed and registered by the sponsor based in Bangalore, India. AP-Bio or KalmCold is a standardized extract of A. paniculata. It contains diterpene lactones (>33%) with andrographolide (>30.0% w/w) as analysed by HPLC. The content of 14-deoxy-11,12-didehydroandrographolide is not more than 15% of the total diterpene lactones as per the United States pharmacopeia [11].
The composition adheres to the international quality requirements which include analysis of solvent residue, heavy metals residue, mycotoxin residue, pesticide residue evaluation and microbial contamination. The results indicated that investigational product meets the requirements specified by the US Pharmacopoeia and the British Pharmacopoeia.
The investigational product was filled in 0 size hydroxypropyl methylcellulose (HPMC) capsules. Each capsule contained 100 mg of SAPE and excipient microcrystalline cellulose. The capsules were packed in a container of sample size 60 capsules/container. Participants were instructed to take one capsule in the morning and one in the evening every day for 30 days. The participants were instructed to maintain their regular lifestyle until 30 days including the dietary and other lifestyle habits.
2.4. Outcome measurements
The primary outcome measures of the study were the change in the NK cell (CD3−CD16+CD56+) counts. The secondary outcome measures included the following immune parameters: change in the total T cells (CD3+ cells), CD4+ T cells, CD8+ T cells counts, the changes in the cytokines such as interferon gamma (IFN-γ), interleukin-4 (IL-4), interleukin-2 (IL-2), interleukin-12 (IL-12), and tumour necrosis factor alpha (TNF α). In addition, secondary outcome measures also included treatment emergent adverse events, frequency and severity.
2.5. Immune cells determination
In brief, 50 μl of EDTA blood was incubated with 10 μl of antibody CD4-PE-Cy7/CD8-AOC-Cy7/CD3-FITC/CD45-PerCP-Cy5.5/CD19-APC/CD 16-56-PE Cocktail or CD4-PE-Cy7/CD8-APC-Cy7/CD3-FITC/CD45-PerCP-Cy5.5 (BD, Biosciences, USA) followed by addition of FACS lysing solution (BD, Biosciences, USA). The lymphocytes were gated using side scatter (SSC) and fluorescein isothiocyanate (FITC) fluorescence intensity and flow cytometric dot plots were obtained. The acquisition and analysis were performed using FACS canto clinical software calibur (BD, Biosciences, USA).
2.6. Cytokine quantification
Serum cytokine concentrations were measured using the Fine test cytokine assay kit (Wuhan Fine Biotech Co., Ltd., China) according to the manufacturer's protocol.
2.7. Statistical analysis
Baseline characteristics were summarized using means and standard deviations for continuous variables and counts. The primary and secondary parameters were analysed using ANOVA for changes from baseline to end of the treatment using SAS 9.4 (SAS Institute, Cary, North Carolina, US). All the analyses were conducted at the significance level of 0.05.
3. Result
Of the thirty-seven screened, thirty participants were enrolled and all the thirty participants completed the trial. There were no dropouts or lost to follow-up during the study period (Fig. 1). All the participants ingested greater than 80% capsules (with an actual compliance of 95.11%; lowest compliance rate - 93.33% (47% of participants) and highest compliance rate - 96.67% (53% of participants)). During the study period the participants did not eat or drink much more than they were already used to and maintained with the regular lifestyle without any severe changes to diet and lifestyle. The baseline characteristics of the participants are presented in Table 1.
Table 1.
Baseline characteristics of participants taking SAPE.
| SAPE (N = 30) | |
|---|---|
| Age (yrs.) | |
| Mean ± SD | 34.4 ± 10.3 |
| Range (Min-max) | 19–58 |
| Gender n (%) | |
| Male | 17 (56.67) |
| Female | 13 (43.33) |
| Body weight (Kg) | |
| Mean ± SD | 69.15 ± 8.33 |
| Range (Min-max) | 49.8–83.6 |
| Height (cm) | |
| Mean ± SD | 163.98 ± 6.83 |
| Range (Min-max) | 155.4–185.0 |
| BMI (Kg/m2) | |
| Mean ± SD | 25.71 ± 2.73 |
| Range (Min-max) | 19.8–29.6 |
SD: Standard deviation; Min-Minimum; Max-Maximum; BMI: Body mass index.
3.1. Effects of SAPE on immune cells & cytokines
Effects of SAPE on the absolute number of total lymphocytes and lymphocyte sub-populations that include NK cells (CD3-CD16+CD56+), T cells (CD3+), T helper cells (CD3+CD4+), T cytotoxic cells (CD3+CD8+) along with CD4+/CD8+ ratio are presented in Table 2. The data demonstrated a trend in the increase of absolute number of total lymphocytes, T cells (CD3+), and T helper cells (CD3+CD4+) while the trend is not evident in T cytotoxic cells and NK cells (Table 2).
Table 2.
Outcome measurements of participants (N = 30) with lymphocytes range between 1000 and 4000 cells/mm3 before and after taking SAPE.
| Parameter | Normal Range | Day 0 | Day 3 | P-value | Day 7 | P-value | Day 30 | P-value |
|---|---|---|---|---|---|---|---|---|
| Absolute lymphocytes/mm3 | 1000–4000 | 2616.5 ± 786.5 | 2615.2 ± 726.7 | 1 | 2757.8 ± 629.1 | 0.15 | 2852.7 ± 632.2 | 0.14 |
| CD3+ (cells/μL) | 655–2823 | 1915.2 ± 591.2 | 1891.7 ± 561.4 | 0.71 | 1975.3 ± 480.2 | 0.38 | 2134.4 ± 460.9 | 0.06 |
| T cells/μL (CD3+CD4+) | 449–1372 | 1058.4 ± 317.0 | 1049.4 ± 313.7 | 0.82 | 1118.7 ± 305.1 | 0.14 | 1199.8 ± 370.1 | 0.087 |
| T cells/μL (CD3+CD8+) | 223–1103 | 744.5 ± 320.1 | 742.4 ± 306.3 | 0.92 | 744.1 ± 237.3 | 1 | 773.8 ± 284.9 | 0.433 |
| CD4/CD8 ratio | 0.82–3.21 | 1.6 | 1.58 | 0.71 | 1.6 | 1 | 1.62 | 0.69 |
| NK cells/μL (CD3-CD16+CD56+) | 60–900 | 292.2 ± 159.5 | 294.0 ± 149.5 | 0.8 | 332.0 ± 222.1 | 0.09 | 269.6 ± 147.4 | 0.45 |
| IFN- gamma (pg/mL) | 7.39 ± 10.93 | 4.71 ± 6.85 | 0.28 | 6.27 ± 8.26 | 0.65 | 20.82 ± 12.01 | 0.0001 | |
| IL-2 (pg/mL) | 0–12 | 2.7 ± 1.6 | 3.1 ± 1.5 | 0.43 | 3.8 ± 1.2 | 0.0007 | 0.9 ± 1.4 | <0.0001 |
| IL-4 (pg/mL) | 0–5 | 1.55 ± 1.87 | 1.59 ± 2.38 | 1 | 0.71 ± 0.04 | 0.018 | 3.46 ± 2.08 | 0.0007 |
| IL-12 (pg/mL) | 65–483 | 313.22 ± 143.58 | 351.78 ± 174.73 | 0.2 | 351.72 ± 163.67 | 0.21 | 338.76 ± 150.79 | 0.46 |
| TNF – alpha (pg/mL) | 46.52 ± 23.68 | 42.16 ± 20.21 | 0.44 | 46.25 ± 23.30 | 1 | 54.68 ± 30.37 | 0.22 |
Values shown are mean ± standard deviation.
P-values are differences between the baseline (day 0), and day 3/7/30 determined using ANOVA.
SAPE administration significantly increased the IFN- γ, IL-4 levels while significantly decreased IL-2 levels at day 30 when compared to baseline (Table 2).
A subgroup analysis on the 21 participants whose lymphocyte counts ranged between 1000 and 3000 cells/mm3 was performed. The effects of SAPE in these subsets of individuals are presented in Table 3. The absolute number of total lymphocytes, T cells (CD3+) and T helper cells (CD3+CD4+) increased significantly at day 7 and the effects continued till day 30 when compared with baseline. SAPE administration significantly increased the IFN- γ, IL-4 levels while significantly decreased IL-2 levels at day 30 when compared to the baseline (Table 3).
Table 3.
Outcome measurements of participants (N = 21) with lymphocytes range between 1000 and 3000 cells/mm3 before and after taking SAPE.
| Parameter | Normal Range | Day 0 | Day 3 | P-value | Day 7 | P-value | Day 30 | P-value |
|---|---|---|---|---|---|---|---|---|
| Absolute lymphocytes/mm3 | 1000–4000 | 2198.7 ± 462.6 | 2253.6 ± 499.0 | 0.64 | 2482.3 ± 440.2 | 0.013 | 2714.0 ± 582.3 | 0.0035 |
| CD3+ (cells/μL) | 655–2823 | 1619.3 ± 359.1 | 1634.3 ± 404.8 | 0.86 | 1777.5 ± 304.4 | 0.03 | 2024.4 ± 431.2 | 0.0018 |
| T cells (CD3+CD4+) (cells/μL) | 449–1372 | 912.9 ± 217.7 | 930 ± 277.8 | 0.75 | 1014.8 ± 206.1 | 0.019 | 1185 ± 345.8 | 0.001 |
| T cells (CD3+CD8+) (cells/μL) | 223–1103 | 601.5 ± 225.4 | 612.3 ± 225.0 | 0.74 | 653.5 ± 198.6 | 0.07 | 663.9 ± 245.4 | 0.2 |
| CD4/CD8 ratio | 0.82–3.21 | 1.71 | 1.69 | 0.84 | 1.67 | 0.54 | 1.78 | 0.23 |
| NK cells/μL (CD3-CD16+CD56+) | 60–900 | 256.6 ± 130.0 | 266.4 ± 127.1 | 0.29 | 320.8 ± 217 | 0.05 | 263.4 ± 159.2 | 0.78 |
| IFN- gamma (pg/mL) | 5.5 ± 8.7 | 5.2 ± 7.6 | 0.92 | 5.8 ± 7.7 | 0.92 | 20.8 ± 14.4 | 0.0005 | |
| IL-12 (pg/mL) | 65–483 | 302.26 ± 136.72 | 338.63 ± 174.28 | 0.26 | 313.99 ± 108.29 | 0.69 | 363.90 ± 169.51 | 0.14 |
| IL-4 (pg/mL) | 0–5 | 1.57 ± 1.98 | 1.98 ± 2.78 | 0.62 | 0.71 ± 0.05 | 0.05 | 3.37 ± 2.27 | 0.01 |
| IL-2 (pg/mL) | 0–12 | 2.92 ± 1.62 | 2.91 ± 1.66 | 1 | 3.84 ± 1.29 | 0.01 | 1.12 ± 1.7 | 0.001 |
| TNF – alpha (pg/mL) | 41.72 ± 25.16 | 40.55 ± 16.99 | 0.86 | 51.02 ± 24.24 | 0.14 | 50.45 ± 31.67 | 0.32 |
Values shown are mean ± standard deviation.
P-values are differences between the baseline (day 0), and day 3/7/30 determined using ANOVA.
3.2. Safety assessment
The participants treated with SAPE did not exhibit any treatment related adverse events during the intervention period.
4. Discussion
The immune system is intrinsic and is well integrated into all physiological systems protecting the body against infections every second of our lives, every day and night as the body is attacked constantly by pathogens. However, a wide range of factors can perturb the human immune system – age related changes that are compounded by certain lifestyle factors including diet, environmental factors (pollution, temperature changes), sleeping habits, crowded places, commuting, frequent travelling, sedentary lifestyle, stress, excessive exercise, alcohol abuse, smoking, etc. influence and modify, suppressing the immune functions [12,13]. It remains imperative to maintain optimal healthy immune system to protect from constant pathogenic challenges for a good quality life. Herbs and bioactive metabolites are believed to be effective in balancing and proper functionality of immune system through various modules of immune modification involving stimulation and suppression [14].
The present study is the first open-label pilot study conducted to investigate the immunomodulatory effects of the SAPE in healthy adults. The study revealed that SAPE supplementation increased adaptive immune responses evident from the increase in total lymphocytes, T cells, CD4+ T helper cells, and cytokines such as IFN-γ, IL-4 and a decrease in IL-2 but SAPE supplementation did not increase NK cell, cytotoxic T lymphocytes counts, IL-12, and TNFα. Importantly, SAPE supplementation did not cause any significant treatment related adverse effects during the study period.
In the current study, we did not observe significant increase in the NK cell counts. It is worthy to note that the researchers in clinical trials investigating immune modulation of prominent herbs such as Ashwagandha, ginseng, cordyceps, polyherbal tea containing Withania somnifera, Glycyrrhzia glabra, Zingiber officinale, Ocimum sanctum and Elettaria cardamomum have investigated the NK cell activity than looking simply at the NK counts [[15], [16], [17], [18]]. Probably A. paniculata may be effective in increasing the NK cell activity as reported by Sheeja and Kuttan [10]. The study demonstrated that administration of A. paniculata and andrographolide, the key component of A. paniculata significantly enhanced the NK cell activity in normal as well as tumor-bearing animals. Maximum NK cell cytotoxicity in A. paniculata and andrographolide treated normal animals after 5 days was 46.82% and 40.79% respectively [10].
The present study suggests that SAPE administration increased the CD3+ and CD4+ T lymphocytes. The increase is significant in individuals with lymphocytes counts ranging from 1000 to 3000 cells/mm3. The T cells form an important component of cell-mediated immunity that regulate the activation and proliferation of other immune system cells such as macrophages, B cells and other T cells. The T helper cells (CD4+) perform crucial functions in the immune response by recognizing foreign antigens and secreting cytokines that activate T and B cells and thus make these T lymphocytes critical elements of the immune system. Their dysfunction and destruction in HIV-1 infection seriously impairs the ability to respond to diverse pathogens [19]. The current study findings are in concurrence with the study findings on the intake of andrographolide in HIV participants with low CD4+ lymphocytes count. The authors reported a significant increase in CD4+ counts after 6 weeks of andrographolide intake. In the HIV study, effect on CD4+ counts were comparable to the increases seen in many clinical trials on lamivudine, zidovudine, didanosine, zidanosine or ritonavir as monotherapies or in combination therapies in HIV but did not appear to result primarily from an anti-viral effect [20]. In another published case study, the use of combination of anti-viral drugs and andrographolide derived natural product Restomune in HIV patient resulted in significant decrease of viral load, increase in CD4+ T Lymphocyte and CD8+ T cell counts, suggesting an enhanced immune condition and slowdown of viral replication. The possible anti-HIV property of Restomune, a product made from the whole plant A. paniculata was attributed to its anti-hepatotoxic and immunostimulant properties A. paniculata [21].
The present study also investigated the effects of SAPE on the endogenously produced cytokines such as IFN-γ, IL-4, IL-2, IL-12 and TNF α. The extract significantly increased the IFN-γ and IL-4 cytokines. These two cytokines play a critical role in early differentiation of human Th1 and Th2 cells. The three to four-fold increase in IFN-γ indicates initial polarization of Th1 response followed by Th2 response due to a two-fold increase in IL-4 levels. The cytokine patterns depict effects of SAPE on Th1 and Th2 responses. In general, IFN-γ acts as one of the inhibitors of Th2 type response due to IL-4 and IL-4 secretion in turn limits over-activation of Th1 by inhibiting the actions of INF-γ [22]. The fold increase in IFN-γ and IL-4 are not the same and such similar cytokine patterns i.e., increase in IFN-γ and IL-4 was also observed in healthy volunteers following ethanolic O. sactum extract intake for a month [23].
There was a significant decrease in IL-2 at day 30 post SAPE administration in healthy adults. This cytokine plays a central role in the generation and maintenance of an effective T cell response and homeostasis of Treg cells. Blocking IL-2 signals weaken Treg cells activity and promotes immune responses. It is also reported that it is sufficient to transiently inhibit IL-2 to bias Th and Treg cell balance towards immunity [24]. Probably this could be the reason for the transient significant increase in IL-2 followed by a significant decrease to modulate the immune responses. This phenomenon is also observed in healthy volunteers who took rice bran fermented with Lentinus edodes (RBEP). Although not significant, but treatment with RBEP decreased the IL-2 levels at the end of the treatment period [25].
The limitations of the present study are – the study employed an open label group design without placebo. However, the major advantage of this approach is that since the human immune system is highly variable between the individuals [26] the effects of SAPE in the same individuals pre- and post-treatment will be clearer. Also as reported by White and Ernst [27], uncontrolled trials serve several crucial purposes, including establishing firmly that there is a clinical effect worth investigating and providing information on how large the effect might be. Another limitation of the study is lack of data on further phenotyping of the T cell (mainly T helper cells to Th1, Th2 and Treg cells) which would have helped in meaningful corroborations with the IL-2 levels.
This was the first pilot study investigating the immunomodulatory effects of SAPE in healthy adults. The data presented here suggest that SAPE supplementation increases immune cells such as T cells, T helper cells and IFN-γ, IL-4 production, but does not increase the NK cell counts. Furthermore, SAPE supplementation is not associated with any significant adverse effects.
5. Conclusion
SAPE administration for 30 days increased the lymphocyte counts and modulated the cytokine production which suggests that it modulates the immune function in healthy people. In addition, it is found to be safe and tolerable.
Ethical approval
Ethics committee approval was obtained from Shetty's Hospital ethics committee, Bangalore, India. Individuals who chose to participate signed an informed consent voluntarily for participation in the study.
Source(s) of funding
The trial was funded by Natural Remedies Pvt. Ltd. (Bangalore).
Conflict of interest
Bharathi B, Deepak M and Prashanth D'Souza are employed with Natural Remedies Pvt. Ltd.
Author contributions
Bharathi B, Deepak M, and Prashanth D Souza conceptualized and designed the study. Dr. Rajanna M and Shivakumar B R screened and enrolled the participants and evaluated the adverse events. Vijayabhaskar T involved in laboratory investigations and coordination. Dr. Prabakaran D and Arun Bhuvanendran compiled the data and analysed the data. All authors participated in data interpretation. Bharathi B was involved in the preparation of the manuscript. All authors critically reviewed and edited the manuscript and approved the final version.
Acknowledgement
Our sincere thanks to Surya Medical Centre & In vitro Research solutions Pvt. Ltd. for successfully carrying out the trial. We personally thank all the staff and principal investigator for their support and co-ordination during the trial.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
References
- 1.Jayakumar T., Hsieh C.Y., Lee J.J., Sheu J.R. Experimental and clinical pharmacology of Andrographis paniculata and its major bioactive phytoconstituent Andrographolide. Evid base Compl Alternative Med. 2013;2013:846740. doi: 10.1155/2013/846740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hossain M.S., Urbi Z., Sule A., Hafizur Rahman K.M. Andrographis paniculata (Burm. f.) Wall. ex Nees: a review of ethnobotany, phytochemistry, and pharmacology. Sci World J. 2014;2014:274905. doi: 10.1155/2014/274905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Samy R., Thwin P., Gopalakrishnakone P M.M. Phytochemistry, pharmacology and clinical use of Andrographis paniculata. Nat Prod Commun. 2007 doi: 10.1177/1934578X0700200519. [DOI] [Google Scholar]
- 4.Okhuarobo A., Falodun J.E., Erharuyi O., Imieje V., Falodun A., Langer P. Harnessing the medicinal properties of Andrographis paniculata for diseases and beyond: a review of its photochemistry and pharmacology. Asian Pac J Trop Dis. 2014;4:213–222. [Google Scholar]
- 5.Hu X.Y., Wu R.H., Logue M., Blondel C., Lai L.Y.W., Stuart B. Andrographis paniculata (Chuān Xīn Lián) for symptomatic relief of acute respiratory tract infections in adults and children: a systematic review and meta-analysis [published correction appears in PLoS One. 2018 Nov 14;13(11):e0207713] PLoS One. 2017;12(8):e0181780. doi: 10.1371/journal.pone.0181780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta S., Mishra K.P., Ganju L. Broad-spectrum antiviral properties of Andrographolide. Arch Virol. 2017;162:611–623. doi: 10.1007/s00705-016-3166-3. [DOI] [PubMed] [Google Scholar]
- 7.Davison G., Kehaya C., Wyn Jones A. Nutritional and physical activity interventions to improve immunity. Am J Lifestyle Med. 2014;10:152–169. doi: 10.1177/1559827614557773. [Accessed 25 November 2014] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arango Duque G., Descoteaux A. Macrophage cytokines: involvement in immunity and infectious diseases. Front Immunol. 2014;5:491. doi: 10.3389/fimmu.2014.00491. [Accessed 7 October 2014] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Puri A., Saxena R., Saxena R.P., Saxena K.C., Srivastava V., Tandon J.S. Immunostimulant agents from Andrographis paniculata. J Nat Prod. 1993;56:995–999. doi: 10.1021/np50097a002. [DOI] [PubMed] [Google Scholar]
- 10.Sheeja K., Kuttan G. Modulation of natural killer cell activity, antibody-dependent cellular cytotoxicity, and antibody-dependent complement-mediated cytotoxicity by Andrographolide in normal and ehrlich ascites carcinoma-bearing mice. Integr Canc Ther. 2007;6:66–73. doi: 10.1177/1534735406298975. [DOI] [PubMed] [Google Scholar]
- 11.Saxena R.C., Singh R., Kumar P., Yadav S.C., Negi M.P.S., Saxena V.S. A randomized double blind placebo controlled clinical evaluation of extract of Andrographis paniculata (KalmCold) in patients with uncomplicated upper respiratory tract infection. Phytomedicine. 2010;17:178–185. doi: 10.1016/j.phymed.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Maggini S., Maldonado P., Cardim P., Fernandez Newball C., Sota Latino E.R. Vitamins C, D and Zinc: synergistic roles in immune function and infections. Vitam Miner. 2017;6:167. doi: 10.4172/2376-1318.1000167. [DOI] [Google Scholar]
- 13.Maggini S., Pierre A., Calder P.C. Immune function and micronutrient requirements change over the life course. Nutrients. 2018;10:1531. doi: 10.3390/nu10101531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sultan M., T Buttxs M., Qayyum S., M M N., Suleria H.A.R. Immunity: plants as effective mediators. Crit Rev Food Sci Nutr. 2014;54:1298–1308. doi: 10.1080/10408398.2011.633249. [DOI] [PubMed] [Google Scholar]
- 15.Mikolai J., Erlandsen A., Murison A., Brown K.A., Gregory W.L., Raman-Caplan P. In vivo effects of Ashwagandha (Withania somnifera) extract on the activation of lymphocytes. J Alternative Compl Med. 2009;15:423–430. doi: 10.1089/acm.2008.0215. [DOI] [PubMed] [Google Scholar]
- 16.Bhat J., Damle A., Vaishnav P.P., Albers R., Joshi M., Banerjee G. In vivo enhancement of natural killer cell activity through tea fortified with ayurvedic herbs. Phytother Res. 2010;24:129–135. doi: 10.1002/ptr.2889. [DOI] [PubMed] [Google Scholar]
- 17.Cho Y.J., Son H.J., Kim K.S. A 14-week randomized, placebo-controlled, double-blind clinical trial to evaluate the efficacy and safety of Ginseng polysaccharide (Y-75) J Transl Med. 2014;12:283. doi: 10.1186/s12967-014-0283-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jung S., Jung E., Choi E., Sin H.S., Ha K.C., Chae S.W. Immunomodulatory effects of a mycelium extract of Cordyceps (Paecilomyces hepiali; CBG-CS-2): a randomized and double-blind clinical trial. BMC Compl Alternative Med. 2019;19:77. doi: 10.1186/s12906-019-2483-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ali M., Lurwan M., Halliru S.N., Salihi A.M. Role of T-helper cells (CD4+ T Cells) in human immune system against some microbial infection: a mini review. Int J Clin Microbiol Biochem Technol. 2020;3:26–29. [Google Scholar]
- 20.Calabrese C., Berman S.H., Babish J.G., Ma X., Shinto L., Dorr M. A phase I trial of Andrographolide in HIV positive patients and normal volunteers. Phytother Res. 2000;14:333–338. doi: 10.1002/1099-1573(200008)14:5<333::aid-ptr584>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 21.Basak Ajoy, Li Suiyang, Banik Upendra K. A new combination drugs using Andrographolide derived natural product restomune for management of HIV Case. Rep Clin Pract Rev. 2003;4:223–233. [Google Scholar]
- 22.Jiang H., Chess L. Integrated view of suppressor T cell subsets in immunoregulation. J Clin Invest. 2004;114:1198–1208. doi: 10.1172/JCI23411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mondal S., Varma S., Bamola V.D., Naik S.N., Mirdha B.R., Padhi M.M. Double-blinded randomized controlled trial for immunomodulatory effects of Tulsi (Ocimum sanctum Linn) leaf extract on healthy volunteers. J Ethnopharmacol. 2011;136:452–456. doi: 10.1016/j.jep.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 24.Carmenate T., Ortíz Y., Enamorado M., García-Martínez K., Avellanet J., Moreno E. Blocking IL-2 signal in vivo with an IL-2 antagonist reduces tumor growth through the control of regulatory T cells. J Immunol. 2018;200:3475–3484. doi: 10.4049/jimmunol.1700433. [DOI] [PubMed] [Google Scholar]
- 25.Choi J.Y., Paik D.J., Kwon D.Y., Park Y. Dietary supplementation with rice bran fermented with lentinus edodes increases interferon-γ activity without causing adverse effects: a randomized, double-blind, placebo-controlled, parallel-group study. Nutr J. 2014;13:35. doi: 10.1186/1475-2891-13-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brodin P., Davis M.M. Human immune system variation. Nat Rev Immunol. 2017;17:21–29. doi: 10.1038/nri.2016.125. White A, Ernst E. The case for uncontrolled clinical trials: A starting point for the evidence base for CAM. Complementary Therapies in Medicine 2001;9:111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.White A., Ernst E. The case for uncontrolled clinical trials: a starting point for the evidence base for CAM. Compl Ther Med. 2001;9:111–116. doi: 10.1054/ctim.2001.0441. [DOI] [PubMed] [Google Scholar]