Abstract
Background
Food is the basic requirement and an essential part of all living beings which sustains life. A lot of emphasize has been given to food in the classics of Ayurveda and a detailed description of food which are both wholesome and unwholesome have been described. Viruddhahara (incompatible food) is a unique concept explained in Ayurveda which, in long run, may be harmful to the body.
Objective(s)
Intake of Kadaliphala (Musa paradisiaca. Linn, a variety of banana) and cow milk is an example of Samyoga Viruddha (incompatibility with reference to combination of things). This combination is routinely consumed by people. The present study was planned to evaluate the toxicological implication of their combination on wistar rats.
Materials and methods
A subacute toxicity study was conducted on Wistar rats following the repeated dose 28-day oral toxicity study in rodents, 407 - OECD guidelines. Different haematological and biochemical parameters along with histopathology of important organs were carried out to assess the toxicological implication of the combination.
Results
Repeated administration of the combination of Kadaliphala (Banana) and cow milk showed statistically significant increase in SGOT & urea and statistically significant decrease in creatinine. Significant decrease was observed in the food intake, faecal wet weight and faecal water in the 7th day of study, food conversion ratio in the 14th day of study, in the food intake, faecal wet weight and faecal water in the 21st day of study and significant decrease in the food conversion ratio in the 28th day of study. In histopathological examination, the test drug administered group showed mild to moderate myocarditis in the sections from heart. In sections from liver of two rats of test group, diffused micro fatty changes were observed. In sections from spleen; mild to moderate increase in the white pulp portion was observed.
Conclusion
Marked variation in SGOT, urea and creatinine levels and alteration in sections of heart, liver and spleen are indicative of mild toxicological implications of the combination of banana and milk. Continuous intake of this incompatible combination may hence prove harmful to the body.
Keywords: Banana, Cow milk, Incompatible combination, Viruddha, Toxicological implication
1. Introduction
Ayurveda, the science of life is a time tested treasure of knowledge that aims for total positive health which not only emphasizes on the treatment of illness but also gives equal importance to the maintenance of physical, mental and spiritual wellbeing of a person. Ahara (food), Nidra (sleep) and Brahmacarya (abstinence) are the three sub pillars described in Ayurveda for total positive health [1, trisraishaneeya adhyaya: ch 11/35]. Ahara (food) has been enumerated first and a lot of importance has been given to it. Food is necessary for the growth and maintenance of health of an individual. It has its own role to play in both health and diseased state. Ahara or food is said to enhance the complexion (Varna), graciousness (Prasada), good voice (Sauswarya), longevity (Jeevitam), happiness (Sukham), satisfaction (Santushti), nourishment (Pushti), strength (Bala) & intellect (Medha) [1, annapana vidhi adhyaya: ch 27/349]. But all this can be achieved only if one indulges in wholesome diet, resulting in good health. On the contrary, intake of unwholesome foods and drinks causes vitiation of bodily Dosha (regulatory functional factors of the body) and Dhatu (major structural components of the body), resulting in a state of diseases. Acharya Charaka has laid down several laws in dietetics, which are directly responsible for a state of health or disease in a person [1, annapana vidhi adhyaya: ch27/3]. Even the present day dietetics emphasises the need for planning of nutrition both for the normal and sick persons [2]. Ayurveda describes certain codes of dietetics to be essentially followed in order to achieve the fullest benefits of the food one eats. Any aberration in diets and even in their style of preparation leads to ill health.
There has been a tremendous change in the lifestyle of human beings in the past few decades, especially with regards to his food and routine activity. Dietary habits of an individual have been noticing a drastic change and unfortunately, in order to cut short the time period or effort involved in the process of cooking, or to manage the workload, a lot of harmful food and food habits have entered the routine life of human beings. Viruddhahara (incompatible food) is one of the novel concepts given by the science of Ayurveda. It includes the incompatible foods which hampers the normalcy of the Dosha and remain intimidating to the bodily tissues [1, atreya bhadrakaapeeya adhyaya: ch 26/85]. Indulgence in incompatible diets dislodges the morbid humors, do not eliminate them from the body, are unwholesome [3] tend to disagree with the body system and acts like a poison [4]. Several disease entities may be caused as a result of Viruddhahara including the eight Maharoga, genetic disturbances and even sometimes the death of the person [1, hitaahiteeyam adhyayam: ch 20/13]. Acharya Sushruta has mentioned the combination of Kadaliphala (Musa paradisiaca. Linn) and cow milk as an example for Samyoga Viruddha [5]. Many people consume this combination frequently nowadays. Individually both Kadaliphala (M. paradisiaca. Linn) and cow milk are not harmful, rather they are considered as nutritious food [6,7]. But we do not know what happens actually when both of them are combined and how their combination (Samyoga) makes them a Viruddhahara to the body. Moreover, there is no precise explanation regarding the variety of banana which when used along with milk is Viruddha. There are many varieties present in banana and it varies from region to region. Also, banana is used both as a fruit and as a vegetable in ripe and unripe form respectively. In the locality where the study was conducted, M. paradisiaca. Linn (Puttubale) was considered as a good variety of banana fruit and in combination with cow milk, was used for the preparation of different dishes like ‘rasayana’, which is served as prasadam at temples and also consumed regularly by people in the form of a desert or as breakfast. As this variety is considered to be the best one, we wanted to find out whether any viruddhata was present by using this variety of banana fruit when combined with milk which was most commonly consumed by people.
Thus, a need was felt to empirically prove the efficacy of the concept of Samyoga Viruddha with special reference to the combination of Kadaliphala and cow milk. This paper discusses in detail the toxicological implication of combination of Kadaliphala (M. paradisiaca Linn) and cow milk w.s.r to the concept of Samyoga Viruddha when administered in wistar rats.
2. Materials and methods
2.1. Collection of test drugs
The test drugs used in the study were Kadaliphala and cow milk. Kadaliphala (M. paradisiaca. Linn) which was locally called as Kadaliphala or Puttubale, was purchased from the local market of Udupi, Karnataka. A dozen of unripe banana was purchased a week prior to the beginning of the study and was ripened naturally. It was identified by the Department of Dravyaguna, SDM College of Ayurveda, Udupi, Karnataka based on its physical appearance and morphological features. Cow milk was procured from a reliable milkman who resided just beside the campus. The milk procured was from malnad gidda, a desi variety of cow breed whose milk is considered to have rich medicinal value. The milk was supplied within 15 min of milking, everyday, throughout the course of the study. The milk was immediately boiled in a gas stove, on a high flame, until it started bubbling and the foam started to raise. Then, the flame was simmered and the milk continuously stirred for 5 min in low flame. It was then allowed to self cool completely and used as and when required for the study. On an average, the milk was used to prepare the test drug 2 h after it was boiled, without adding any water.
2.2. Rationale behind fixation of the dose of the combination
As both the test drugs used in the combination are Ahara Dravyas, (items related to food) a precise dose cannot be fixed as such. But then normally the minimum quantity of banana that a man can consume at a time is one. Hence that dose for human was finalized. About a dozen of same sized Kadaliphala were weighed separately to finalise on the weight to be selected. Approximately the weight ranged from 45 g to 55 g So 50 g of Kadaliphala was finalised as the hypothetical human dose for the study. The quantity of milk taken was equal to the weight of one banana. Though these two items have different units of measurements, approximation of units was done with equal quantity of cow milk in mL, that is 50 mL of milk was taken. The combination of 50 g of banana and 50 mL of milk was prepared by mixing the two manually, until it formed a smooth paste. Thus 100 g was considered as the notional human dose. The dose for the rat was calculated on the basis of body surface area ratio by referring to the standard table of Paget and Barnes (Pagets et and Barnes, 1964).
2.3. Experimental animals and sample size
Wistar strain albino rats of either sex weighing between 120 and 230 g and an average of 5 months of age were used for the experimental study. The animals were obtained from animal house attached to the Pharmacology Laboratory of S.D.M Centre for Research in Ayurveda and Allied Sciences, Udupi, Karnataka. IAEC clearance was obtained for the study and experiments were conducted as per the guidelines of Institutional Animal Ethical Committee, according to Government of India accepted principles for laboratory animals’ use and care. (Reg.No. IAEC NO: SDMCAU/IAEC/2013–14–15). The animals received humane care, in compliance with the host institutional animal ethics guidelines. Animals were exposed to natural day and night cycles with ideal laboratory condition in terms of ambient temperature, humidity. They were fed with rat pellets supplied by Saidurga feeds, Bengaluru and tap water ad libitum.
2.4. Experimental study design
The rats which were found appropriate to the study based on their weight and age were marked from 1 to 12 and using lottery method were randomly divided into two groups. With 6 rats in each group. No inclusion or exclusion criteria was fixed. Group I rats were administered with 0.5% gum acacia and considered as vehicle control. The Group II rats were given Kadaliphala (a variety of banana) and cow milk in a dose of 1.8 mL/200 g. Animals were kept on acclimatization for 7days. Group specific drugs were administered orally once a day for 28 consecutive days using oral catheter fit to a syringe. On 28th day all animals were kept for overnight fasting. Next day blood was collected by retro orbital plexus with the help of micro capillary tubes under mild ether anaesthesia for estimation of serum biochemical and haematological parameters followed by sacrifice with over dose of ether anaesthesia. The abdomen was opened through midline incision followed by dissecting out the important organs as mentioned below and extraneous tissues was removed and weighed. The tissue was transferred to bottles containing 10% formalin for the purpose of histopathological study.
2.5. Outcome measures: estimation of haematological and biochemical parameters
Blood samples were taken from the orbital plexuses, using capillary tubes, under anesthesia at the end of the period or collected just prior to or as part of the procedure of humane sacrifice. The haematological parameters like haematocrit, hemoglobin concentration, erythrocyte count, total and differential leucocyte count, platelet count etc were measured using ‘Aggape mindra’ auto cell counter. Determinations of biochemical parameters in plasma or serum were glucose, urea, creatinine, total protein, SGOT, SGPT, and ALP. In all these procedures requisite quantity of serum was fed to the ‘Erba 360′ fully automatic biochemical analyser to obtain the results. The person conducting these parameters was blinded and had no knowledge of the treatment allocation to both the groups of the study.
2.6. Histopathological study
Brain, heart, liver, spleen, lungs, kidney, stomach, jejunum, testis, uterus were the organs subjected to histopathological study. After noting the weight of the organ they were transferred to 10% formaldehyde solution for fixation and sent to a commercial laboratory for preparation of slides. The slides with sections obtained were scanned in Trinocular Carl Zeiss’s microscope (Germany) under different magnifications. Changes, if any in the cytoarchitecture were noted down.
2.7. Statistical analysis
The results were expressed as mean ± SD where each value represents a minimum of 6 rats (n = 6). The data for relative organ weights, haematology and serum biochemistry were tested using unpaired student t test in which the results were compared with that of control rats. The results were considered statistically significant at p ≤ 0.05 level. GraphPad Prism©version 3.5 for Windows (GraphPad Software, USA) was used for all statistical analysis.
3. Observations and results
3.1. Effect on haematological parameters, LFT & RFT in control and test drug groups
The haematological parameters such as haemoglobin, WBC, RBC, PCV, MCV, MCH, MCHC, RDWSD, RDWCV and platelet were estimated to analyse the haematological toxicity caused by the combination of test drugs in Wistar albino rats. Among these parameters we could observe increase in the haemoglobin level, total count level, red blood cell count level, packed cell volume level and mean corpuscular volume level in the test drug administered group as compared to the control group. However, the observed increase was found to be statistically non-significant. Statistically significant increase was noticed in the packed cell volume level in the test drug group as compared to the control group. A marginal and statistically non-significant increase was noticed in MCH and RDWSD level in the test drug group. Also, a marginal decrease in mean cell haemoglobin concentration level and RDW was noticed in the test drug administered group as compared to the control group which was statistically non significant. The values of platelet count were comparable with that of normal control group.
Repeated administration of combination of Kadali phala and cow milk caused increase in the total and direct bilirubin level. However, both the increase was statistically non-significant. The test drug exhibited statistically significant increase in the liver enzyme SGOT (∗p < 0.05), a marginal and statistically non-significant decrease in SGPT level and a marginal but statistically non-significant increase in alkaline phosphatase level as compared to the control group.
Repeated administration of the test drug caused statistically significant increase in the urea level and statistically significant decrease in creatinine level as compared to the control group (∗p < 0.05). There was increase in uric acid level in the test drug group when compared with control group. However the observed increase was found to be statistically non-significant (Table 1).
Table 1.
Changes in haematological values, LFT & RFT in control and test drug groups.
| S.No | Hematological value | Control | Banana and milk (9 ml/kg) |
|---|---|---|---|
| 1. | Haemoglobin (g/dL) | 14.76 ± 0.17 | 15.35 ± 0.22 |
| 2. | TWBCC (103./µL) | 10987.5 ± 1565.4 | 12800 ± 1254.1 |
| 3. | RBC (106/µL) | 7.62 ± 0.13 | 7.90 ± 0.18 |
| 4. | PCV (%) | 41.57 ± 0.70 | 43.45 ± 0.81* |
| 5. | MCV (fL) | 54.61 ± 0.55 | 55.08 ± 0.48 |
| 6. | MCH (pg) | 19.32 ± 0.16 | 19.40 ± 0.26 |
| 7. | MCHC (g/dL) | 35.47 ± 0.23 | 35.31 ± 0.37 |
| 8. | RDW CV (%) | 13.70 ± 0.18 | 13.67 ± 0.19 |
| 9. | RDWSD (FL) | 26.73 ± 0.55 | 26.85 ± 0.57 |
| 10. | PLT (103/µ l) | 6.00 ± 0.78 | 6.00 ± 0.39 |
| S.No | LFT value | ||
| 1. | Bilrubin-T (mg/dL) | 0.15 ± 0.01 | 0.17 ± 0.01 |
| 2. | Bilirubin-D (mg/dL) | 0.07 ± 0.007 | 0.09 ± 0.01 |
| 3. | SGOT (U/L) | 119.95 ± 2.42 | 132.75 ± 2.58** |
| 4. | SGPT (U/L) | 60.35 ± 2.32 | 59.91 ± 2.13 |
| 5. | ALPase (U/L) | 427.75 ± 55.39 | 442.00 ± 48.48 |
| 6. | TP (g/dL) | 6.82 ± 0.24 | 6.73 ± 0.18 |
| 7. | Albumin (g/dL) | 3.11 ± 0.12 | 2.77 ± 0.08 |
| S.No | RFT value | ||
| 1. | Urea (mg/dL) | 44.03 ± 3.88 | 52.90 ± 2.39* |
| 2. | Creatinine (mg/dL) | 0.66 ± 0.02 | 0.54 ± 0.03* |
| 3. | Serum Uric acid (mg/dL) | 2.57 ± 0.20 | 2.78 ± 0.16 |
Data expressed in MEAN ± SEM, *P < 0.05, **P < 0.01.
TWBCC – Total White blood cells Count, RBC – Red blood cells, PCV – packed cell volume, MCV – Mean cell volume, MCH – Mean cell haemoglobin, MCHC – Mean cell haemoglobin concentration, RDWCV – Red cell distribution width coefficient variation, RDWSD – Red cell distribution width standard deviation, PLT – Platelet count. Bilrubin –T – Bilirubin total, Bilirubin–D – Bilirubin direct, SGOT – Serum glutamate oxaloacetate transaminase, SGPT – Serum glutamate pyrovet transaminase, ALPase – Alkaline Phosphatase, TP – total protein.
3.2. Effect on serum cholesterol level
Repeated administration of the test drug caused marginal increase in cholesterol level, triglyceride level, high density lipoprotein level and decrease in low density lipoprotein level when compared with control group. However, all the observed change were found to be statistically non-significant (Table 2).
Table 2.
Effect on serum lipid profile: Data expressed in MEAN ± SEM.
| S.No | Serum cholesterol | Control | Banana and milk (9 mL/kg) |
|---|---|---|---|
| 1. | Cholesterol (mg/dL) | 55.37 ± 3.98 | 57.00 ± 4.62 |
| 2. | Triglyceride level (mg/dL) | 92.37 ± 12.03 | 121.72 ± 13.49 |
| 3. | HDL- Cholesterol (mg/dL) | 28.51 ± 2.38 | 34.48 ± 3.33 |
| 4. | LDL Cholesterol (mg/dL) | 11.44 ± 0.84 | 11.04 ± 0.72 |
3.3. Effecton serum sugar level
Repeated administration of the test drug caused decrease in sugar level in Kadaliphala and cow milk group when compared with control group. However, the observed decrease was found to be statistically non-significant (Table 3).
Table 3.
Effect of combination of Banana and milk on Serum sugar level.
| S.No | Serum sugar | Control | Banana and milk (9 mL/kg) |
|---|---|---|---|
| 1. | Serum Sugar (mg/dL) | 131.05 ± 13.30 | 121.37 ± 4.94 |
Data expressed in MEAN ± SEM.
3.4. Effect on body weight changes
Repeated administration of the test drug resulted in a statistically significant increase in body weight on the 7th 14th, 21st and 28th day when compared with initial body weight. However, this increase was noticed in both the groups. Anyhow, there was no statistically significant difference in weight gain pattern between the groups (Table 4).
Table 4.
Effect of Banana and milk on body weight changes.
| Criteria | Groups | Initial | 7th day | 14th day | 21st day | 28th day |
|---|---|---|---|---|---|---|
| Body weight changes in grams (Data expressed is in comparision with original weight) | Control | 173.37 ± 14.12 | 186.62 ± 14.87∗ | 198.75 ± 17.61∗∗ | 207.00 ± 18.87∗∗ | 213.25 ± 20.09∗∗ |
| Banana and milk (9 ml/kg) | 192.37 ± 12.80 | 199.5 ± 12.58 | 217.75 ± 15.34∗∗ | 224.25 ± 16.90∗∗ | 230.12 ± 18.96∗∗ |
Data expressed in MEAN ± SEM, ∗P < 0.05, ∗∗P < 0.01.
3.5. Effect on food related parameters
The test drug caused decrease in percentage changes in body weight of 7th, 14th, 21st and 28th day when compared with percentage changes in body weight of control group respectively. However, the difference was found to be statistically non-significant. The relative value of food intake of rats showed a statistically significant decrease in food intake of 7th and 21st day of Kadaliphala and cow milk group when compared with food intake of control group. Though there was decrease in this parameter on 14th and 28th day, difference was found to be statistically non significant. A statistically very significant decrease was noticed in faecal wet weight of 7th and 21st day of test drug group when compared with control group. However, on the 14th and 28th day, the data did not show any difference between the control and treated groups. With regards to the faecal dry weight there was a statistically non-significant decrease on day 7 and 21 and increase on 14th and 28th day in the test drug group. There was a statistically very significant decrease in faecal water on 7th and 21st day of Kadaliphala & cow milk group when compared with control group. There was a statistically non-significant decrease in food conversion ratio on 7th and 21st day and statistically significant decrease on 14th and 28th day of Kadaliphala and cow milk group when compared with food conversion ratio of control group (Table 5).
Table 5.
Effect of combination of Banana and milk on food related parameters.
| Criteria | Groups | 7th day | 14th day | 21st day | 28th day |
|---|---|---|---|---|---|
| Percentage changes in Body weight | Control | 7.84 ± 1.34 | 14.26 ± 1.79 | 18.86 ± 2.02 | 22.18 ± 2.11 |
| Banana and milk | 3.91 ± 1.55 | 13.04 ± 2.18 | 16.11 ± 2.17 | 18.78 ± 2.70 | |
| Food intake in g/100 g body weight | Control | 7.67 ± 0.54 | 6.80 ± 0.27 | 6.61 ± 0.19 | 6.00 ± 0.15 |
| Banana and milk | 5.41 ± 0.32 ∗ | 6.26 ± 0.20 | 5.66 ± 0.18 ∗ | 5.44 ± 0.18 | |
| Faecal wet weight in g/100 g body weight | Control | 1.66 ± 0.13 | 2.08 ± 0.04 | 2.60 ± 0.16 | 2.51 ± 0.12 |
| Banana and milk | 1.35 ± 0.09 ∗∗ | 2.08 ± 0.20 | 1.97 ± 0.07 ∗∗ | 2.48 ± 0.08 | |
| Faecal dry weight in g/100 g body weight | Control | 0.96 ± 0.04 | 1.11 ± 0.04 | 1.29 ± 0.05 | 1.31 ± 0.04 |
| Banana and milk | 0.90 ± 0.03 | 1.16 ± 0.12 | 1.12 ± 0.04 | 1.33 ± 0.05 | |
| Faecal water in g/100 g body weight | Control | 0.69 ± 0.08 | 1.00 ± 0.05 | 1.30 ± 0.11 | 1.19 ± 0.09 |
| Banana and milk | 0.45 ± 0.05 ∗∗ | 0.91 ± 0.08 | 0.84 ± 0.06∗∗ | 1.15 ± 0.07 | |
| Food conversion ratio | Control | 8.19 ± 0.90 | 6.21 ± 0.41 | 5.16 ± 0.24 | 4.71 ± 0.11 |
| Banana and milk | 5.98 ± 0.13 | 5.77 ± 0.53∗∗ | 5.03 ± 0.12 | 4.16 ± 0.10 ∗ |
Data expressed in MEAN ± SEM, ∗P < 0.05, ∗∗P < 0.01.
3.6. Ponderal changes
Repeated administration of combination of Kadaliphala and cow milk lead to decrease in brain, heart and jejunum weight when compared with control group, all of which were statistically non-significant. The combination caused increase in lung, liver, kidney, spleen, testis and uterus. However, the increase was statistically non-significant. The repeated administration of combination of Kadaliphala and cow milk lead to statistically significant increase in stomach weight when compared with the control group (Table 6).
Table 6.
Combination of Banana and milk induced Ponderal changes.
| Organs | Control | Banana and milk (9 mL/kg) |
|---|---|---|
| Brain weight (g) | 1.36 ± 0.08 | 1.28 ± 0.11 |
| Lungs weight (g) | 1.44 ± 0.11 | 1.50 ± 0.15 |
| Heart weight (g) | 0.67 ± 0.04 | 0.69 ± 0.06 |
| Liver weight (g) | 7.08 ± 0.87 | 7.18 ± 0.79 |
| Stomach weight (g) | 1.20 ± 0.12 | 1.54 ± 0.08∗ |
| Kidney weight (g) | 1.42 ± 0.11 | 1.66 ± 0.17 |
| Spleen weight (g) | 0.81 ± 0.13 | 1.13 ± 0.32 |
| Testis weight (g) | 2.57 ± 0.14 | 2.82 ± 0.19 |
| Uterus weight (g) | 0.75 ± 0.01 | 1.23 ± 0.16 |
| Jejunum weight (g) | 0.67 ± 0.06 | 0.66 ± 0.06 |
Data: MEAN ± SEM, ∗P < 0.05.
3.7. Histopathological examination
The histopathological examination of brain, stomach, colon, jejunum, spleen, liver, heart, lungs, kidney, uterus, and testis were carried out in the present study. No difference could be observed between control and test groups and normal cytoarchitecture was observed in sections of brain, stomach, lungs, kidneys, uterus and testis.
3.7.1. Heart
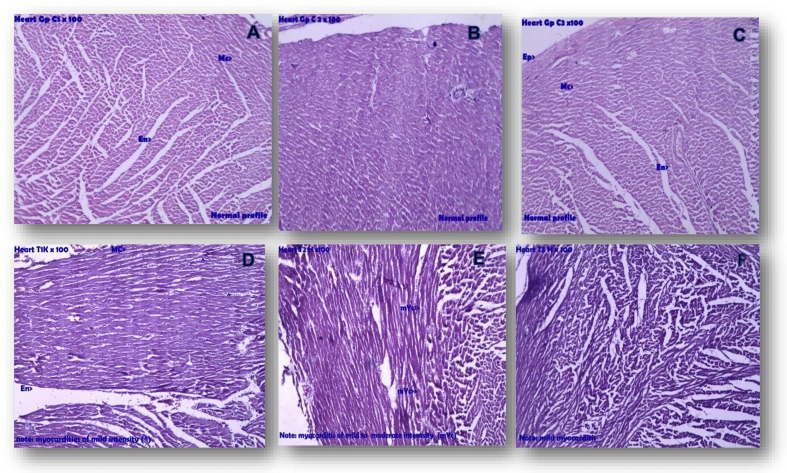
Microscopic examination of the heart of rats belonging to both control and test drug administered group was carried out. In test drug administered group mild to moderate myocarditis was observed in sections from three rats. Photographs of representative sections can be found in Fig. 1.
Fig. 1.
Photomicrograph of representative sections of heart. A,B,C – Heart control; D,E,F – Heart test; In Heart T1, T2, T3 Mild myocarditis. Note: Mc- Myocardium, Myc- Myocarditis, Ep- Epicardium.
3.7.2. Liver
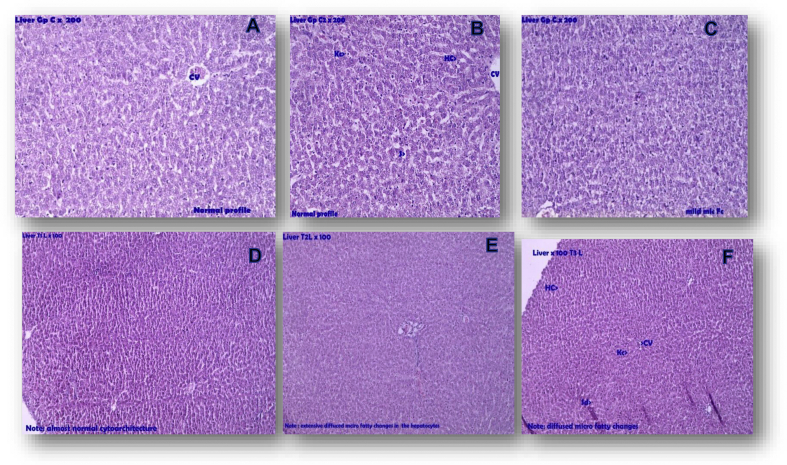
In sections of liver from two rats, diffused micro fatty changes were observed. The sections from remaining rat exhibited normal cytoarchitecture. Photographs of representative sections can be found in Fig. 2.
Fig. 2.
Photomicrograph of representative sections of liver. A,B,C – Liver control; D,E,F – Liver test; In Liver T2, T3 Mild fatty changes. Note: Hc- Hepatic cell, Kc- Kupffer cell, S- Sinusoid, Cv- Central vein.
3.7.3. Spleen
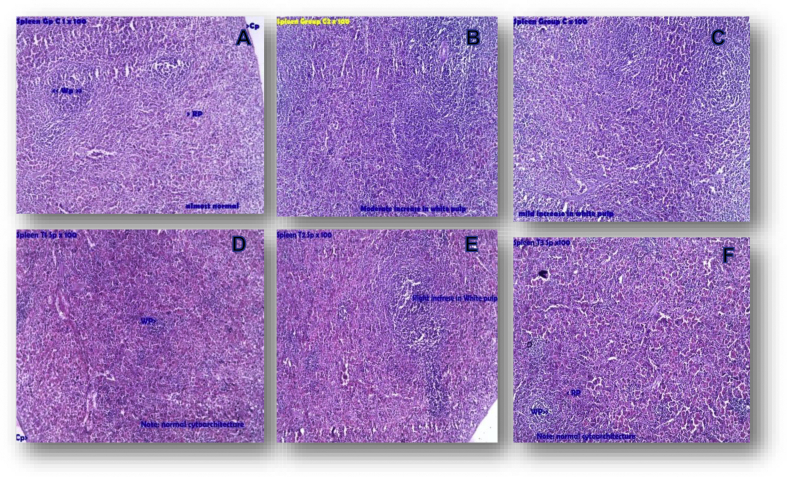
Microscopic examination of sections of spleen from the test drug administered group showed normal cytoarchitecture with mild increase in white pulp proportion in sections from 2 rats; in sections from one rat moderate increase in white pulp proportion was observed. Photomicrographs of representative sections can be found in Fig. 3.
Fig. 3.
Photomicrograph of representative sections of spleen. A,B,C – Spleen control; D,E,F – Spleen test; In Spleen T2, T3 – Mild increase of white pulp. Note: Wp- White pulp, Rp- Red pulp, Cp- Capsule.
4. Discussion
Samyoga Viruddha, one among the different types of Viruddha is one such variety where in the combination of foods makes them toxic to the body. In the present scenario, a wide range of newer food combinations are being practiced; but the importance and attention given towards the qualities of individual food items in the combination, interaction between the foods in the combination and status of digestion after its intake are less and they are most neglected. Many examples have been quoted for Samyoga Viruddha in the classics. Intake of banana with cow milk is one such example told by Acharya Sushruta and a common type of incompatible combination which is commonly consumed by people. But details of the specific ill effect of the same are not mentioned in the classics.
Out of 10 haematological parameters studied, there is a significant increase in packed cell volume% in the test group. There is also an increase in the Hb% & RBC in the test group. Though it is statistically non-significant, it might be due to the fact that banana fruit, especially M. paradisiaca. Linn regenerates the RBC by stimulating the production of haemoglobin [8,9]. The increase in packed cell volume% may be corresponding to the increased Hb% and RBC counts.
It is observed that among the LFT, SGOT shows an increase which is statistically significant indicating some damage process of biliary cells [10]. In spite of the damage to the liver cells, a decrease is observed in SGPT, which might be because of the decreased synthesis of liver enzyme. SGPT and SGOT both use pyridoxine as a coenzyme. When there is a deficiency in pyridoxine, it inhibits the synthesis of SGPT [11]. SGOT is a mitochondrial enzyme whereas SGPT is a cytosolic enzyme [12]. Injury to the hepatocytes results in the leakage of these enzymes into the circulation. Hence this significant increase in SGOT might be indicating the damage to the hepatocytes. Anyhow to substantiate this more precisely we need a chronic study. SGOT also gets elevated during cardiac injury [13]. In this context it is pertinent to mention that histopathological examination showed mild to moderate changes in both liver and heart. The elevation observed in this enzyme may be due to these changes.
Among renal function tests, it was observed that two parameters show significant changes. There was an increase in the serum urea level in the test group when compared to the control group which was statistically significant. Usually the increase in serum urea level is seen due to some renal pathology [14]. It is also affected by hydration, hepatic metabolism of protein and reduced GFR (glomerular filtration rate) [15]. But as there is no corresponding increase in the serum creatinine level, which is more specific to renal diseases, renal pathology cannot be suspected here. Conditions like catabolic states of tissue breakdown, high protein intake etc may lead to high serum urea with normal creatinine levels [16]. Here the significant elevation in the serum urea level might be the result of some muscle tissue breakdown or excess catabolism of bodily proteins.
A decrease was observed in the serum creatinine level in the test group when compared to the control group which is statistically significant. Serum creatinine is an important indicator of renal health because creatinine is a non-protein waste product of creatine phosphate metabolism by skeletal muscle tissue. Creatinine production is continuous and is always proportional to muscle mass [21]. Hence the respective significant increase and decrease in the blood urea and creatinine level might be because of the catabolism of the muscle tissue. As a result of this, a decrease might have been observed in the increasing gradient of the body weight among the test group animals which is discussed in food and faecal matter analysis part. It may be indicative of decreased muscle activity also. However these results should be confirmed by a chronic study.
Among all the lipid profile parameters, it is observed that there is an increase in cholesterol, triglycerides and HDL level and a decrease in the LDL level in the test group when compared to the control group but they are statistically not significant.
5. Conclusion
Food is the basic requirement in a human life and the ability to eat healthy and right things are important aspects of good quality of life. One should take wholesome diet which may result in a good state of health. Consumption of Viruddhahara may give rise to various disorders. In the study conducted, though specific organ toxicity cannot be pinpointed, there are several observations suggestive of the abnormalities induced by the combination in long run. Statistically significant increase in PCV, SGOT & Urea, decrease in Creatinine, significant decrease in the food intake, faecal wet weight, faecal water, food conversion ratio, mild to moderate myocarditis in the sections from heart, diffused micro fatty changes in sections from liver and mild to moderate increase in the white pulp in sections from spleen are suggestive of some toxicological implications of the combination. A chronic study with more precise parameters, enzymatic tests and histopathological examinations may be essential to gather accurate results which are organ specific. Anyhow, based on the findings, it is evident that this combination may be harmful, if consumed daily, for a long period of time. In many parts of our country, this combination is used commonly. Hence a survey may be done as to in which part of the country this combination is regularly used and awareness regarding the harmfulness of it, on long term may be emphasized.
6. Limitations of the study and future recommendations
-
a)
The study was conducted with a hypothetical dose. A comparatively higher dose groups could also have been included which would give a clearer picture.
-
b)
No analysis was done for the test drugs, either individually or in combination which may be included in further studies.
-
c)
Though the study is conducted as per “The repeated dose 28-Day Oral Toxicity Study in Rodents” 407 OECD guidelines, certain variations have been made - like only 6 animals inclusive of either sex were used in each group and not 10. Moreover only one test group was studied at a notional dose and not 3. These changes were done as the combination was a routine food substance and not a chemical and moreover it was a preliminary study with no prior data available for a study of such a kind. Based on the data obtained, a well planned, several test dose groups study strictly adhering to the OECD repeated dose toxicity guidelines may be planned.
-
d)
The euthanasia method adopted was overdose of ether which may lead to artifacts. Hence this may be avoided in future studies.
-
e)
The parameters included were not inclusive of the enzymatic studies. A sub chronic study including the liver enzymes and digestive enzymes would be more informative.
Source(s) of funding
None.
Conflict of interest
None.
Acknowledgement
The authors highly acknowledge SDM Ayurveda College and SDM Research centre, Udupi for the infrastructure provided for the work. The authors also thank Dr. B. Ravishankar, Ex. Director, SDM Research Centre, Udupi and Dr. Ravi M, Assistant Professor, Department of Pharmacology, WIMS, Wayanad, Kerala, for his their guidance and support.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
References
- 1.Acharya Jadavaji Trikamji., editor. Commentary Ayurveda Dipika of Chakrapanidatta on Charaka Samhita of Agnivesa, Sootra Sthana; Trisraishaneeya Adhyaya: Chapter 11, verse 35. 1st ed. Chaukhamba Surbharati Prakashan; Varanasi: 2000. p. 74. [Google Scholar]
- 2.Manore Melinda M., Myers Esther F. Research and the dietetics profession: making a bigger impact. J Acad Nutr Diet. 2003;103(1):p108–p112. doi: 10.1053/jada.2003.50021. Jadavaji Trikamji Acharya, editor, (1st ed.). Commentary Nibandha Sangraha of Dalhanacharya and Nyayachandrika of Gayadasa, Sushruta Samhita of Sushruta, Sootra Sthana; Hitaahiteeyam adhyayam: [Chapter 20], Verse 20. Varanasi: Chaukhamba Orientalia, 1997; p.97. [DOI] [PubMed] [Google Scholar]
- 3.Paradkar Vaidya Bhisagacharya Harishastri., editor. Commentary Sarvangasundara of Arunadatta and Ayurveda Rasayanam of Hemadri on Astangahrdayam of Acharya Vagbhata, Sootra Sthana; Annaraksha Adhyaya: Chapter 7, verse 29. 1st ed. Krishnadas Academy; Varanasi: 2000. p. 133. [Google Scholar]
- 4.Sharma Shivprasad., editor. Commentary Shashileka of Indu on Ashtanga Sangraha of Vagbhata, Sootra Sthana; Viruddhanna Vijnaneeyo Adhyaya: Chapter 9, verse 21. 1st ed. Chaukhamba Sanskrit Series office; Varanasi: 2006. p. 90. [Google Scholar]
- 5.Oguntibeju Oluwafemi Omoniyi. Antidiabetic, anti-Inflammatory, antibacterial, anti-helminthic, antioxidant and nutritional potential of Musa paradisiaca. Asian J Pharmaceut Clin Res. 2019;12(10):9–13. doi: 10.22159/ajpcr.2019.v12i10.34239. [DOI] [Google Scholar]
- 6.Marangoni Franca, Pellegrino Luisa, Verduci Elvira, Ghiselli Andrea, Bernabei Roberto, Calvani Riccardo. Cow’s milk consumption and health: a health professional’s guide. J Am Coll Nutr. 2018;38(3):197–208. doi: 10.1080/07315724.2018.1491016. [DOI] [PubMed] [Google Scholar]
- 7.Nadakarni’s K.M. vol. 2. Popular Prakashan; Mumbai: 2010. p. 824. (Indian Materia Medica, Revised and Enlarged by A. K. NadaKarni). reprinted. [Google Scholar]
- 8.Garcı´a Olga P., Martı´nez Mara, Romano Diana, Camacho Mariela, de Moura Fabiana F., Abrams Steve A. Iron absorption in raw and cooked bananas: a field study using stable isotopes in women. Food Nutr Res. 2015;59:25976. doi: 10.3402/fnr.v59.25976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Babb R.R. The clinical significance of the SGOT test. Calif Med. 1973 May;118(5):89–91. [PMC free article] [PubMed] [Google Scholar]
- 10.Johnston David E. Special considerations in interpreting liver function tests. Am Fam Physician. 1999 Apr 15;59(8):2223–2230. [PubMed] [Google Scholar]
- 11.Salvaggio A., Periti M., Miano L., Tavanelli M., Marzorati D. Body mass index and liver enzyme activity in serum. Clin Chem. 1991;37:720–723. [PubMed] [Google Scholar]
- 12.Manolio T.A., Burke G.L., Savage P.J., Jacobs D.R., Jr., Sidney S., Wagenknecht L.E. Sex- and race-related differences in liver-associated serum chemistry tests in young adults in the CARDIA study. Clin Chem. 1992;38:1853–1859. [PubMed] [Google Scholar]
- 13.Nisha R., Srinivasa Kannan S.R., Thanga Mariappan K., Jagatha P. Biochemical evaluation of creatinine and urea in patients with renal failure undergoing hemodialysis. J Clin Path Lab Med. 2017;1(2):1–5. [Google Scholar]
- 14.Noor ul A., Raja Tahir M., Javaid Asad M., Zafar Mudassar, Mehmood Raja Asad. Evaluating urea and creatinine levels in chronic renal failure pre and post dialysis: a prospective study. J Cardiovasc Dis. 2014;2:1–5. [Google Scholar]
- 15.Calles-Escandon J., Cunningham J.J., Snyder P., Jacob R., Huszar G., Felig P. Influence of exercise on urea, creatinine, and 3-methylhistidine excretion in normal human subjects. Am J Physiol. 1984;246(4):E334–E338. doi: 10.1152/ajpendo.1984.246.4.E334. [DOI] [PubMed] [Google Scholar]
- 16.Baxmann Alessandra Calábria, Ahmed Marion Souza, Marques Natália Cristina, Menon Viviane Barcellos, Pereira Aparecido Bernardo, Kirsztajn Gianna Mastroianni. Influence of muscle mass and physical activity on serum and urinary creatinine and serum cystatin C. Clin J Am Soc Nephrol. 2008 Mar;3(2):348–354. doi: 10.2215/CJN.02870707. [DOI] [PMC free article] [PubMed] [Google Scholar]