Key points.
-
•
Divers breathe gases at high partial pressures, which have detrimental physiological effects.
-
•
Knowledge of the gas laws is essential to understand diving medicine.
-
•
Rapid ascent from a dive may cause barotrauma.
-
•
Rapid ascents can also cause decompression sickness (DCS), but most cases of DCS follow theoretically safe dives and are caused by paradoxical gas embolism across a right-to-left shunt.
-
•
DCS has many manifestations and can be hard to diagnose.
Learning objectives.
By reading this article you should be able to:
-
•
Explain the physiological and pathophysiological effects of working at increased ambient pressure.
-
•
Detail the effects on the human body of gases under pressure and their toxic effects.
-
•
Identify the effects that expansion of gas may have on the human body.
-
•
Distinguish the signs and symptoms of decompression sickness and arterial gas embolism secondary to pulmonary barotrauma, which can be difficult to distinguish and, if so, they are called decompression illness.
-
•
Describe the principles of treatment of decompression illness.
This article explains the physiological effects of breathing gases at altered partial pressures and the pathophysiology of common diving-related diseases. In a second linked article we describe some of the medical conditions that affect the ability to dive safely.1
Few people, besides divers and astronauts, deliberately enter an environment that does not support respiration. The changes in ambient pressures when diving can cause pulmonary barotrauma. At high pressure, the increased tissue uptake of gases that are usually innocuous exposes a diver to their toxic effects, such as nitrogen narcosis and acute oxygen toxicity. Reduction in pressure during ascent may cause decompression sickness (DCS). Safe diving requires understanding the behaviour of gases under pressure and their effects on physiology.
Divers are also at risk of immersion pulmonary oedema. The high thermal conductivity of water and some breathing gases can cause hypothermia.
Effects of descent: gas compression and increased gas uptake
Gases are very compressible, but body tissues vary in their degree of compressibility. Nerve tissue starts to exhibit altered function at a depth of approximately 150 m of seawater (16 bar, 1.62 MPa, 1.216 × 104 mmHg). Bone only deforms at much higher pressures.
Body cavities that contain gas are affected by pressure according to Boyle's law. Air in the lungs of a breath-holding diver at the surface (1 bar) compresses to half the surface volume at a depth of 10 m (2 bar) and to one third at a depth of 20 m (3 bar). The lungs typically reach residual volume at a depth of approximately 30 m. Further descent causes blood to be drawn into the chest from the limbs, spleen, and abdomen, and the diaphragm and abdominal viscera are pushed up high into the ribcage to compensate for further reductions in lung volume. Thus, the record breath-hold dive to 214 m was possible despite the amount of gas in the lungs and rigid air spaces being reduced to <1/22nd of the surface total lung capacity. On ascent back to the surface, the gas in the diver's lungs expands to occupy the original volume at the surface.
If the volume of gas in a space is compressed by increased pressure and further gas cannot be drawn in, part of the space will be filled by body tissues which may cause barotrauma of descent or a ‘squeeze’. An example is pain when a diver fails to equalise the pressure of the gas in the middle ear with the pressure of the water outside the tympanic membrane. When the pressure differential is great the tympanic membrane will rupture, which leads to entry of cold water, vertigo, and potentially drowning. If a diver descends with an obstructed sinus the walls of the sinus may implode, usually by rupture of vessels, filling the sinus with blood and causing pain.
Scuba divers (and divers whose gas is supplied by an ‘umbilical’ hose) breathe gas supplied at the ambient pressure of the surrounding water, which enables maintenance of relatively normal lung volumes during the respiratory cycle. Nevertheless, those divers can suffer a squeeze if there is failure to equalise pressures in gas-containing spaces, such as the middle ear.
Also, during descent, the supplied gas becomes denser in proportion to the pressure and the work of breathing becomes harder. Maximum voluntary ventilation decreases in proportion to the square root of gas density: at 30 m (4 bar) maximum voluntary ventilation is half that at the surface (1 bar) with the same breathing gas. This may cause carbon dioxide retention.
There is free exchange of gases between gases in the alveoli and the dissolved gases in the bloodstream. On descent, the partial pressures of the gases in the alveoli increase and, by Henry's law, the number of molecules of gases dissolved in the blood and in the body tissues increases.
Nitrogen
Increased Pn2 leads to nitrogen narcosis, which causes impaired cognition and predisposes to accidents. Nitrogen is poorly soluble in water and blood, but is much more soluble in lipids and hence cell membranes, and importantly, neurological tissues. The uptake half-life of nitrogen is shortest in well-perfused tissues. Therefore the brain, which is well perfused and lipid-rich, takes up large amounts of nitrogen very rapidly as a diver breathing a gas with a fixed percentage of nitrogen, such as air, descends and the pressure increases. In a diver breathing air, some narcotic effects are present at 20 m and at depths >50 m, the diver's cognitive function is very likely to be severely impaired. At depth, nitrogen is thought to act in a similar manner to an anaesthetic and may alter the equilibrium between open and closed states of various neurotransmitter receptors, such as the gamma-aminobutyric acid type A (GABAA) receptor.2 Nitrogen narcosis is cured by ascent. It is avoided by replacing some or all of the nitrogen with a less narcotic gas, usually helium.
Oxygen
High pressure oxygen has several effects. Maximum oxygen loading of haemoglobin reduces its carbon dioxide carriage, and hence increases central venous Pco2 with a concomitant decrease in blood pH, which stimulates ventilation and counteracts inhibition caused by excess Po2 diminishing carotid body stimulation. There is also vagally mediated bradycardia, vasoconstriction of the intracranial and peripheral vessels, and a small decrease in cardiac output.
Paul Bert showed in 1878 that oxygen is lethal at high pressures. Air at 15–20 bar caused convulsions in larks: oxygen at 5 bar produced the same effect. Humans can experience CNS oxygen toxicity at a Po2 as low as 1.6 bar. CNS oxygen toxicity is reported in about 0.5% of treatments of DCS in a recompression chamber using a high Po2 (typically 2.8 bar).3 The most dramatic manifestation is an unheralded grand mal convulsion. Underwater, the diver may lose his mouthpiece and drown. Exercise, increased Pco2 and immersion lower the threshold for CNS toxicity. To allow a margin of safety, divers usually keep the Po2 below the 1.6 bar threshold, typically by selecting a maximum Po2 of 1.3 bar.
Divers can be exposed to dangerously high Po2 in their breathing gas if there is equipment failure or they breathe a gas with an inappropriately high Fo2 for their depth resulting in a dangerously high Po2. CNS oxygen toxicity may occur when diving with rebreather sets (see later). It can also occur if divers use high Po2 in order to speed elimination of nitrogen to decrease decompression time for a dive.
Pulmonary oxygen toxicity can be a problem for saturation divers who may be living for up to 4 weeks in a chamber pressurised to 10 bar or greater. They experience high partial pressures of oxygen despite breathing oxygen percentages of 3.5% or lower. There are no pathognomonic chest X-ray changes. Reductions in vital capacity can be monitored, although this is less sensitive than the clinical symptoms and signs such as tracheobronchitis, acute respiratory distress syndrome, chest pain, and dyspnoea. Reduction in vital capacity is progressive throughout oxygen exposure and can continue for several hours afterwards. It may take up to 12 days to return to normal.
Carbon dioxide
The normal partial pressure of carbon dioxide in the alveoli is approximately 5.6 kPa (42 mmHg, 0.055 bar). Ideally when diving, the arterial and alveolar carbon dioxide tensions should be maintained at approximately this level. In a diver breathing air, the alveolar pressures of nitrogen and oxygen increase in proportion to depth but, because alveolar carbon dioxide is a product of metabolism and is constant at fixed workloads, the alveolar pressure of carbon dioxide remains virtually unchanged while its percentage decreases. However, a combination of high levels of exercise and increased work of breathing is a potent provocation for hyperventilation followed by CO2 retention secondary to perturbed respiratory control if the CO2 concentration increases to high levels. Hypercapnia may also occur in divers using rebreather sets (if the CO2 absorber fails) or where there is inadequate ventilation of a commercial diver's helmet because of increased dead space.
The sequential clinical features of progressively increasing levels of hypercapnia are: increasing dyspnoea; increases in BP and HR; mental confusion and lack of coordination; loss of consciousness; and death. Although CO2 is a respiratory stimulant, most of its effects are caused by the acidosis it produces in the CSF, which is a neurological depressant.
Adaptation to higher than normal inspired levels of carbon dioxide can occur and is characterised by increased tidal volume and reduced ventilatory frequency. This adaptation is important in saturation diving, in submersibles, and during extended submarine patrols.
Carbon monoxide
By law, breathing gas must contain <5 parts per million of carbon monoxide. If breathing gas contains increased concentrations, the partial pressure of carbon monoxide may increase to lethal levels during compression. The usual reason for carbon monoxide contamination of gas in diving cylinders is when a compressor's air inlet is close to the exhaust of the compressor's engine so that exhaust fumes are drawn into the gas cylinder. Carbon monoxide has high affinity for haemoglobin (200 times that of oxygen) and thus reduces oxygen carriage of blood. It is also a respiratory poison of mitochondrial cytochrome c oxidase.
Exotic diving gas mixtures
Air is not used as a breathing gas for deep dives because of the risks of CNS oxygen toxicity, nitrogen narcosis, and the effort of breathing the dense gas. For dives deeper than 50 m, some of the nitrogen, and depending on the depth, some of the oxygen is replaced by helium. Helium is not narcotic even at depths of 600 m, but has disadvantages, including high thermal conductivity (which necessitates heating breathing gas and diving suits during long exposures, such as commercial saturation diving), voice distortion, and high cost. In addition, very rapid compression to depth using helium/oxygen mixtures (‘heliox’) may cause high-pressure nervous syndrome (HPNS). HPNS causes decreased motor and intellectual performance, dizziness, nausea, vomiting, and tremor. The incidence of HPNS is decreased by slowing the rate of compression to depth and by adding to the helium/oxygen mixture a small amount of an anaesthetic gas, usually nitrogen. This helium/oxygen/nitrogen mixture is known as ‘trimix’.
Originally, only divers working offshore in the oil and gas industry used trimix and heliox. Since the 1970s, recreational and scientific divers have increasingly used trimix to dive to greater depths.
Hydrogen has also been used in breathing mixtures by professional divers. Hydrogen has a lower density than helium but greater narcotic potential. When present in gas mixtures containing >4% oxygen, there is a high risk of explosion.
Liquid breathing
Ventilation of the lungs with an oxygenated fluid instead of a compressible gas mixture has been considered to avoid DCS.4 Liquid-filled lungs mean that the partial pressure of respiratory gases hardly changes with pressure and inert gas pressures in the blood and tissues remain roughly constant during a dive. Saline and fluorocarbons (FC-80) have been tested. FC-80 is inert in the lung, but a persistent acidosis in animal models, possibly related to increased pulmonary vascular resistance, and difficulty in transitions from and back to air breathing have prevented the adoption of liquid breathing as a diving tool in humans.
Long-term effects of working under pressure
Some professional divers and compressed air workers, who have long-term exposure to pressure or repeated decompressions, develop dysbaric osteonecrosis, a form of aseptic necrosis of long bones, but a case has been reported in a sport diver.5 The pathogenesis is not understood.
Effects of ascent: gas expansion and gas elimination
Pressure change can cause barotrauma on ascent, as it can on the diver's descent. Therefore if a sinus has become obstructed whilst at depth, during ascent the walls of the sinus may explode painfully resulting in the entry of gas and infected mucus into blood vessels and surrounding tissues. If a diver swallows air during a dive, he may experience abdominal pain or even gastric rupture during ascent, particularly if there is a history of gastro-oesophageal surgery.
The most serious forms of barotrauma of ascent are pulmonary. If the lung is obstructed on ascent (e.g. by a scuba diver holding his/her breath), the gas in the lung expands until the lung reaches its bursting pressure (roughly 70 mmHg and at about 115% of voluntary total lung capacity) when it ruptures. Gas may escape from the lungs into other tissues in three ways to cause:
-
(i)
Arterial gas embolism if gas invades the pulmonary veins to cause systemic gas embolism and typically neurological effects.
-
(ii)
Pneumothorax, which can become a tension pneumothorax during continued ascent because gas in the pleura expands as pressure is reduced.
-
(iii)
Pneumomediastinum.
Decompression sickness
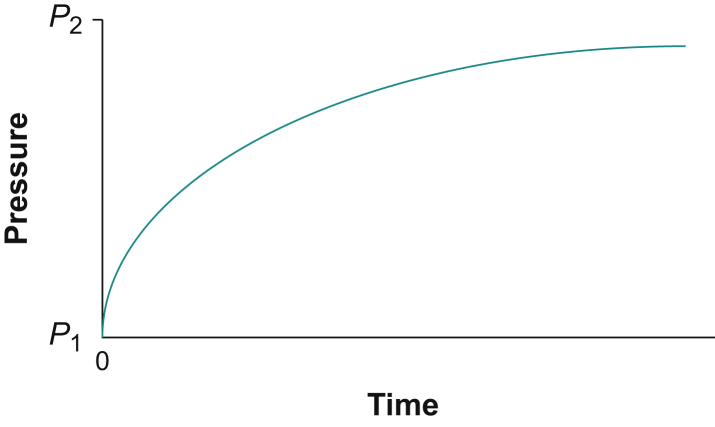
During a dive, the increased partial pressure of an inert gas breathed causes tissues to take up greater amounts of the dissolved gas than at the surface. If the ambient pressure is suddenly increased from P1 to P2, the gas tensions within that tissue will increase exponentially towards P2 (Fig. 1).
Fig 1.
The exponential time course for inert gas absorption into a hypothetical tissue when the partial pressure of the gas is increased rapidly from P1 to P2.
During decompression, the tissues contain excess numbers of dissolved gas molecules taken up during compression, which means that the tissues are supersaturated (the sum of dissolved gas pressures in a tissue exceeds the ambient pressure). The excess dissolved gas must leave the tissues and return to the lungs. If a diver ascends slowly, so that ambient pressure is reduced gradually, the partial pressure of gases in the alveoli and hence in arterial and capillary blood decrease proportionately. The tissues have higher partial pressures of dissolved inert gas (usually nitrogen) than capillary blood and the dissolved inert gas diffuses out of the tissues into capillary blood down the concentration gradient and is carried in venous blood back to the lungs to diffuse into the alveoli. Gases that are largely biochemically inert in humans (e.g. nitrogen, helium) are sparingly soluble in blood. Therefore if decompression is more rapid, the gas that has dissolved in the tissues will come out of solution to form bubbles in the tissues and in venous blood. Echocardiography shows that venous bubbles are transported to the lungs where, in most cases, the gas passes out of the bubbles into the alveoli down the concentration gradient as the bubbles pass through the pulmonary capillaries. Usually this decompression process does not result in illness. If the rate of decompression is too rapid, so that the number of bubbles or the site where they lodge causes injury, the diver suffers DCS.
Robert Boyle in 1670 was the first to observe the effects of decompression in animals. In the late 19th century, Paul Bert described many of the manifestations of DCS and showed that the more serious forms of DCS were provoked by the presence of large volumes of free gas (mainly nitrogen), as opposed to dissolved gas.
Early observations of DCS were on compressed air workers during civil engineering at a time when safety regulations for workers were poor. When they were subjected to a rapid reduction in pressure, their blood literally frothed, to cause cardiopulmonary DCS (dyspnoea, chest pain, and hypotension, which they called ‘the chokes’). It may be rapidly fatal unless immediate recompression occurs. If decompression was slower, but still rapid by current safety standards, vestibulocochlear DCS (‘the staggers’ causing ataxia, vertigo, and vomiting) or spinal DCS might occur and result in severe neurological impairment or delayed death. The commonest form of DCS in these workers (but not in divers) was musculoskeletal DCS—also called ‘the bends’.
The term ‘the bends’ was coined by caisson workers building the supports under the riverbed for the bridge across the Mississippi at St. Louis. It referred to the affected ‘stiff-legged’ gait of fashionable young ladies, which resembled the behaviour of workmen who contracted this form of DCS causing pain in or around a joint.
Navies realised that underwater operations would become part of modern warfare, and became interested in preventing DCS, because it was found that military and commercial divers suffered clinical manifestations of DCS similar to caisson workers but musculoskeletal DCS is less frequent and neurological and cutaneous manifestations are much more frequent in divers (Table 1).
Table 1.
Classification of the signs and symptoms of DCS. The most frequent cause for all types of DCS, other than musculoskeletal DCS, is paradoxical gas embolism across a right-to-left shunt
| Cerebral | Impairment of consciousness and higher cerebral function, hemiplegia, hemisensory abnormalities, visual disturbance, isolated neurological abnormalities |
| Spinal | Paraplegia, sensory level, girdle abdominal discomfort, urinary retention, isolated neurological abnormalities in limbs |
| Vestibular | Ataxia, vertigo and vomiting |
| Cardiorespiratory | Chest discomfort, dyspnoea, shock |
| Cutaneous | Rash typically on trunk, buttocks, or thighs, which maybe pruritic, maybe mottled or confluent, and maybe pink, red or purple |
| Lymphatic | Lymphatic or breast swelling, which may be tender |
| Musculoskeletal | Pain usually in large joints |
| Constitutional | Severe fatigue (this may be a variant of neurological DCS) |
In the early 20th century Professor J.S. Haldane was asked to devise regulations for the safe conduct of underwater work by divers.6 He showed that if goats breathing air were exposed to a raised pressure (P2) for a ‘prolonged period’ when it was assumed that all the tissues were saturated (in about 3 h) and then the pressure was rapidly reduced to a new level (P1), the goats would exhibit signs of DCS if the pressure reduction was >50%. If the pressure drop was less than half, the goats did not show signs of DCS. This ratio P1/P2 ≥ 0.5 was assumed to be valid for all decompressions over a wide range of values for P1 and P2 and applicable to all tissues.
It was assumed that the gas elimination curve is the inverted image of the uptake curve in Figure 1. This elimination curve is the rationale behind nearly all subsequent mathematical models for treatments of dissolved gas exchange in tissues.
Later, it became clear that some of Haldane's assumptions are not quite correct. Subsequent sets of decompression tables have been published, most notably by the Swiss physiologist Bühlmann.7 They rely on dividing the body into many theoretical ‘tissues’, each with its own half-time for gas elimination. Such ‘tissues’ do not correspond to any anatomical structure or structures. These mathematical models have been incorporated into small computers that integrate pressure (depth) and time and can be worn on the diver's wrist. They allow multilevel diving rather than restricting the diver to a single maximum depth before return to the surface, and control ascent rates to theoretically reduce the risk of DCS. After a dive, a computer can be interrogated to ascertain the dive profiles undertaken, for example if a diver presents to a recompression chamber with DCS.
Analysis of the dives by divers who had DCS showed that many had followed dive profiles that were within the limits of conservative decompression algorithms without missed decompression stops or rapid ascents to the surface. The profiles would have liberated venous bubbles, but in numbers considered safe. We now know that most of these divers have right-to-left shunts—usually a large persistent foramen ovale (PFO), but in 5–10% of cases a pulmonary shunt.8, 9, 10 Their shunts allowed venous bubbles to bypass the pulmonary capillaries, so that the bubbles reached the systemic circulation and were carried to the tissues to cause paradoxical gas embolism.
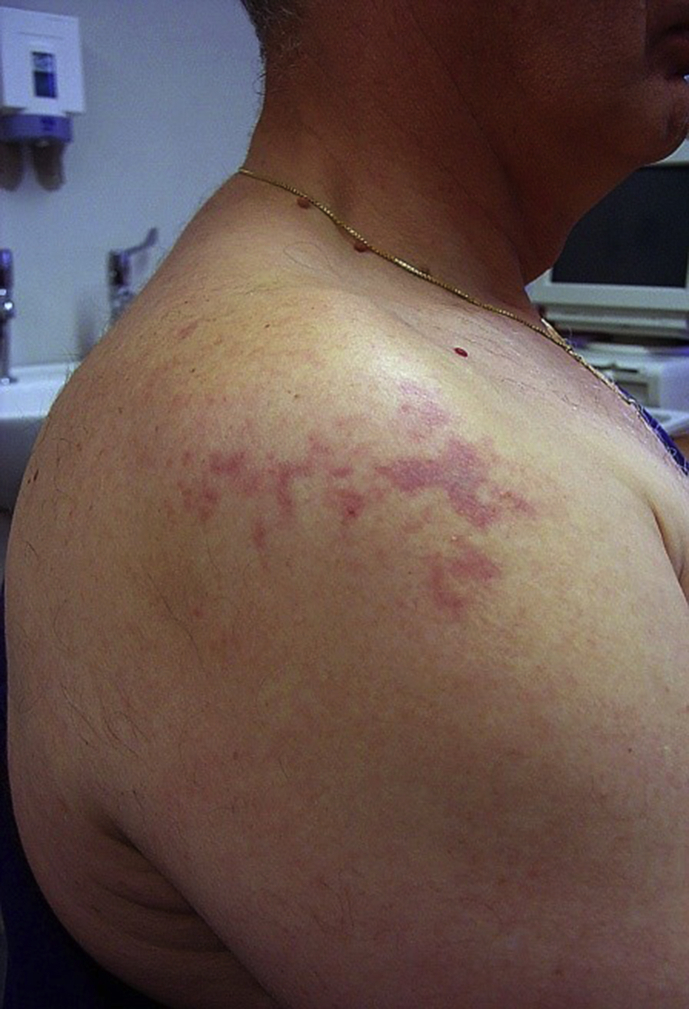
In a person not exposed recently to high ambient pressure, small numbers of bubble emboli do no harm—a fact important for the bubble contrast echocardiogram test for persistent foramen ovale. Because the tissues have a lower partial pressure of nitrogen than the bubble emboli, gas passes down the concentration from the bubble to the tissue and the gas dissolves. However, soon after a dive, tissues are supersaturated with dissolved inert gas (nitrogen if diving breathing air) and embolic bubbles enlarge as dissolved gas passes from supersaturated tissues into the bubbles.11 The amplified bubbles cause local ischaemia and pressure effects. The manifestations of paradoxical gas embolism depend on which tissues are supersaturated at the time when the bubbles traverse the shunt.11 The greater a tissue's blood flow and hence the faster its nitrogen elimination half-life, the shorter its susceptibility to paradoxical gas embolism. However, if there are paradoxical bubble emboli, the greater a tissue's blood flow the greater its chance of being embolised. Therefore timing of venous gas nucleation is important and there is often a delay. Another factor is the total inert gas content of the tissue. Nitrogen is relatively lipid soluble. Lipid-rich tissues have the greatest ability to amplify embolic bubbles and are at greatest risk of injury. Of lipid-rich tissues, the spinal cord has a slower blood flow and hence nitrogen elimination half-life than the brain. The spinal cord remains susceptible to paradoxical gas embolism for longer after surfacing than the brain. Subcutaneous tissue also has high lipid content, but slow blood flow, so cutaneous DCS (Fig. 2) can manifest later after a diver surfaces.
Fig 2.
A mottled, pruritic rash in a diver as a result of cutaneous DCS.
It can be difficult to distinguish some types of DCS from arterial gas embolism secondary to pulmonary barotrauma, particularly when manifestations are neurological. Therefore the collective clinical term decompression illness (DCI) is used.12
Shunt-mediated DCS accounts for the majority of neurological (cerebral, spinal and vestibular), cutaneous (Fig. 2), and cardiorespiratory DCI in amateur divers.8,9,13, 14, 15 Provocative dive profiles, when the diver had missed decompression stops required by their decompression algorithm (dive table or computer) or had made a rapid ascent to the surface, cause a minority of cases.8,9,11,14,15 Arterial gas embolism secondary to pulmonary barotrauma also causes a minority of cases of neurological and cardiorespiratory DCI soon after divers surface.8,11,14,15 Musculoskeletal DCS is generally caused by a provocative dive profile or very deep dives, even without obvious deviation from the decompression algorithm.14,15
About 50% of cases of DCS have signs and symptoms within 1 h of surfacing and 90% present within 6 h. Some 85% of neurological DCS manifests within 1 h, but cutaneous and joint DCS are often delayed.16
A diver with cerebral DCS may fail to notice he is unwell and fail to seek help. Delayed diagnosis by colleagues and doctors is common. Sensory abnormalities and painless neurogenic urinary retention secondary to spinal DCS may be missed. A diver (or caisson worker) with cerebral DCS may be misdiagnosed as under the influence of alcohol or drugs.
Treatment of DCI
Rapid diagnosis of DCI is important, because treatment is expeditious recompression in a recompression chamber, except for some mild forms of DCS. Distinguishing DCS from arterial gas embolism secondary to pulmonary barotrauma does not usually affect emergency management, but it often affects subsequent advice about return to diving. During transportation to a recompression chamber the diver should breathe 100% oxygen even if his arterial oxyhaemoglobin saturation is normal. The primary purpose is not to increase oxygenation, but to eliminate nitrogen from the diver's lungs to increase the nitrogen elimination gradient.
Recompression within minutes of symptom onset is very effective, but less predictable outcomes follow longer delays. Nevertheless, it is generally considered that recompression should be performed as quickly as practicable, especially in cases of serious neurological symptoms or signs. Published algorithms show the depths, times, and treatment gases to be used depending on the manifestations of DCS. Treatment aims to compress the bubbles of gas that cause the problem, create a gradient for elimination of inert gas, and to supply oxygen to the hypoxic tissues. Fluid is given orally or i.v., because dehydration is common.
Divers who have had DCI need careful assessment before return to diving to exclude significant cardiac shunts or lung disease that may predispose to recurrence.17 Transcatheter closure of a PFO is sometimes performed to permit return to diving.18,19
The second article describes how the physiological effects of diving interact with some medical conditions to affect fitness to dive.1
Declaration of interests
The authors declare that they have no conflicts of interest.
Biographies
Chris Edge PhD AFOM FRCA CChem Cert GStat is a consultant anaesthetist at the Royal Berkshire Hospital and an honorary senior lecturer at the Department of Life Sciences, Imperial College, London. He is past chair of the UK Diving Medical Committee. He is statistical editor of Diving and Hyperbaric Medicine. His major clinical interests are anaesthesia for robotic surgery and research in diabetes and diving medicine. His non-clinical interests include biostatistics.
Peter Wilmshurst BSc MB ChB FRCP FISM FFSEM is a consultant cardiologist at the Royal Stoke University Hospital and a past chair of the UK Diving Medical Committee. He has conducted research in diving medicine for more than 40 years. He was the first to report the role of right-to-left shunts in decompression sickness and the first to describe immersion pulmonary oedema in divers and swimmers.
Matrix codes: 1A01, 2H01, 3I00
MCQs
The associated MCQs (to support CME/CPD activity) will be accessible at www.bjaed.org/cme/home by subscribers to BJA Education.
References
- 1.Edge C.J., Wilmshurst P.T. Medical conditions that affect the risk of diving. BJA Educ. 2021;21:349–354. doi: 10.1016/j.bjae.2021.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Franks N.P., Lieb W.R. Molecular and cellular mechanisms of general anaesthesia. Nature. 1994;367:607–614. doi: 10.1038/367607a0. [DOI] [PubMed] [Google Scholar]
- 3.Banham N.D.G. Oxygen toxicity seizures: 20 years’ experience from a single hyperbaric unit. Diving Hyperb Med. 2011;41:202–210. [PubMed] [Google Scholar]
- 4.Ongaro F., Daniotti A., Putinati S., Guerrini P., Potena A. What is liquid breathing and ventilation? Monaldi Arch Chest Dis. 2000;55:394–397. [PubMed] [Google Scholar]
- 5.Wilmshurst P., Ross K. Dysbaric osteonecrosis of the shoulder in a sport scuba diver. Br J Sports Med. 1998;32:344–345. doi: 10.1136/bjsm.32.4.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boycott A.E., Damant G.C., Haldane J.S. The prevention of compressed-air illness. J Hyg (Lond) 1908;8:342–443. doi: 10.1017/s0022172400003399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bühlmann A.A. Springer-Verlag; Berlin: 1984. Decompression—decompression sickness. [Google Scholar]
- 8.Wilmshurst P., Bryson P. Relationship between the clinical features of neurological decompression illness and its causes. Clin Sci. 2000;99:65–75. [PubMed] [Google Scholar]
- 9.Wilmshurst P.T., Pearson M.J., Walsh K.P., Morrison W.L., Bryson P. Relationship between right-to-left shunts and cutaneous decompression illness. Clin Sci. 2001;100:539–542. [PubMed] [Google Scholar]
- 10.Wilmshurst P.T. The role of persistent foramen ovale and other shunts in decompression illness. Diving Hyperbar Med. 2015;45:98–104. [PubMed] [Google Scholar]
- 11.Wilmshurst P., Davidson C., O’Connell G., Byrne C. Role of cardiorespiratory abnormalities, smoking and dive characteristics on the manifestations of neurological decompression illness. Clin Sci. 1994;86:297–303. doi: 10.1042/cs0860297. [DOI] [PubMed] [Google Scholar]
- 12.Francis T.J.R., Smith D.J. Proceedings of the 42nd workshop of the undersea and hyperbaric medical society. Undersea and Hyperbaric Medical Society Inc; Bethesda, MD, USA: 1991. Describing decompression illness. [Google Scholar]
- 13.Cantais E., Louge P., Suppini A., Foster P., Palmier B. Right-to-left shunt and risk of decompression illness with cochleovestibular and cerebral symptoms in divers: case control study in 101 consecutive dive accidents. Crit Care Med. 2003;31:84–88. doi: 10.1097/00003246-200301000-00013. [DOI] [PubMed] [Google Scholar]
- 14.Wilmshurst P.T., Byrne J.C., Webb-Peploe M.M. Relation between interatrial shunts and decompression sickness in divers. Lancet. 1989;334:1302–1306. doi: 10.1016/s0140-6736(89)91911-9. [DOI] [PubMed] [Google Scholar]
- 15.Wilmshurst P.T., Byrne J.C., Webb-Peploe M.M. Relation between interatrial shunts and decompression sickness in divers. In: Sterk W., Geeraedts L., editors. Proceedings of the European Undersea Biomedical Society Symposium 1990. EUBS; Amsterdam: 1990. pp. 147–153. [Google Scholar]
- 16.Francis T.J.R., Pearson R.R., Robertson A.G., Hodgson M., Dutka A.J., Flynn E.T. Central nervous system decompression sickness: latency of 1070 human cases. Undersea Biomed Res. 1989;15:403–417. [PubMed] [Google Scholar]
- 17.Smart D., Mitchell S., Wilmshurst P., Turner M., Banham N. Joint position statement on persistent foramen ovale (PFO) and diving. South Pacific Underwater Medicine Society (SPUMS) and United Kingdom Sports Diving Medical Committee (UKSDMC) Diving Hyperb Med. 2015;45:129–131. [PubMed] [Google Scholar]
- 18.Wilmshurst P., Walsh K., Morrison L. Transcatheter occlusion of foramen ovale with a button device after neurological decompression illness in professional divers. Lancet. 1996;348:752–753. doi: 10.1016/S0140-6736(05)65638-3. [DOI] [PubMed] [Google Scholar]
- 19.Wilmshurst P.T., Morrison W.L., Walsh K.P., Pearson M.J., Nightingale S. Comparison of the size of persistent foramen ovale and atrial septal defects in divers with shunt-related decompression illness and in the general population. Diving Hyperb Med. 2015;45:89–93. [PubMed] [Google Scholar]