Abstract
Background
Percutaneous patent foramen ovale (PFO) closure has been well established in the secondary prevention of cryptogenic stroke with overall low rates of procedural complications. One such complication is PFO closure device thrombus formation which is now rarely reported with newer generation devices.
Case summary
We present the unusual case of a 59-year-old woman with myelofibrosis who developed late-onset recurrent embolic strokes related to Amplatzer PFO closure device thrombus whilst therapeutically anticoagulated on Warfarin. Surgical management was deemed too high risk and our patient was conservatively managed with enoxaparin. Serial transthoracic echocardiography demonstrated a reduction in thrombus size and the patient had no further neurological events.
Discussion
Overall, the risk of serious complications following percutaneous PFO closure, such as device-associated thrombus, remains low. The risk of thrombus formation in patients with hypercoagulable states is not well characterized. Despite good evidence for the efficacy in preventing recurrent cryptogenic stroke, the role of PFO closure in addition to anticoagulation is unclear. Given this uncertain benefit of PFO closure in anticoagulated patients and the unclear risk profile, patient selection, and thorough pre-procedural evaluation are vital when assessing the appropriateness of percutaneous PFO closure.
Keywords: Thrombus, Patent foramen ovale, PFO closure device, Stroke, Myelofibrosis, Case report
Learning points
Late patent foramen ovale (PFO) closure device-associated thrombus is a rare but serious complication of percutaneous PFO closure
The risk of device-associated thrombus in patients with a procoagulant state is not well established
Patient selection is of utmost importance in evaluating the appropriateness of PFO closure
Left atrial disc separation from the interatrial septum is an uncommon finding after PFO closure and may represent a nidus for thrombus formation
Introduction
Multiple large randomized controlled trials have demonstrated the effectiveness of percutaneous patent foramen ovale (PFO) closure for secondary prevention of cryptogenic stroke when compared with medical therapy.1–6 Thrombus formation on percutaneous PFO closure devices is a well-recognized, albeit uncommon, post-procedural complication.7 Here, we present the unusual case of late thrombus development on a PFO closure device resulting in recurrent strokes in a patient on dual anti-thrombotic therapy.
Timeline
| 2003 | Diagnosed with CALR positive myelofibrosis and commenced on hydroxyurea. |
| July 2018 | Aortic thrombus with embolization resulting in hepatic and splenic infarcts. No precipitating cause found. Transthoracic echocardiography did not demonstrate intra-cardiac thrombus. Patient commenced on Warfarin. |
| August 2018 | Unprovoked proximal right upper limb deep venous thrombosis with clot extending into right sub-clavian and right internal jugular veins. Warfarin was changed to long-term Enoxaparin. |
| September 2018 | Right occipital lobe infarct. Regular Enoxaparin anticoagulation had been withheld for 2 days prior to presentation for a dental procedure. Patent foramen ovale (PFO) identified on transoesophageal echo with a left-to-right shunt and negative bubble study. |
| January 2020 | Unprovoked pulmonary embolism while on Apixaban. Patient was transitioned onto warfarin. |
| April 2020 | Underwent percutaneous PFO closure with a 35 mm Amplatzer PFO occluder (Abbott Laboratories). |
September 2020
|
Patient presented with occipital headache and visual changes and was found to have bilateral occipital and right fronto-parietal strokes. Routine post-stroke transthoracic echocardiography identified thrombus on PFO device. |
| October 2020 | Thrombus on PFO device reduced in size. |
Case presentation
A 59-year-old woman presented to the emergency department after 5 days of occipital headache with associated visual floaters. Initial neurological examination was unremarkable with preserved visual fields and acuity and no limb weakness. Cardiovascular examination was unremarkable with no signs of heart failure or cardiac murmurs. Electrocardiogram demonstrated sinus rhythm.
Non-contrast computed tomography did not demonstrate any acute intracranial pathology. Magnetic resonance imaging (MRI) brain identified two areas of diffusion restriction consistent with recent bilateral occipital lobe infarcts.
Background medical history included myelofibrosis with recurrent arterial and venous thromboembolism. Two years prior to presentation the patient suffered a multifocal right occipital lobe infarct consistent with a thrombo-embolic source after her regular anticoagulation was withheld for a dental procedure. Transoesophageal echocardiogram (TOE) at the time demonstrated a patent foramen ovale, although with a left-to-right shunt and negative bubble study. There was no left atrial thrombus. Following this likely thromboembolic stroke and despite the negative bubble study, 6 months prior to presentation, the patient underwent percutaneous PFO closure at another institution with a 35 mm Amplatzer PFO occluder (Abbott Laboratories). During the PFO closure procedure, a 25 mm Amplatzer device was initially trialled; however, intraprocedural TOE demonstrated a malpositioning of the left atrial disc against the inter-atrial septum and the device was exchanged for a larger 35 mm device with improved positioning on TOE.
Baseline medications included warfarin, aspirin 100 mg daily, and hydroxyurea 500 mg daily. International normalized ratio was sub-therapeutic at 1.5 (target 2.0–3.0) on presentation. The patient denied non-compliance with warfarin or aspirin. Haemoglobin level was low at 99 g/L (normal range: 115–165 g/L). Platelet count was elevated at 780 × 109/L (normal range: 150–450 × 109/L). White cell count was normal at 7.30 × 109/L (3.50–11.00 × 109/L).
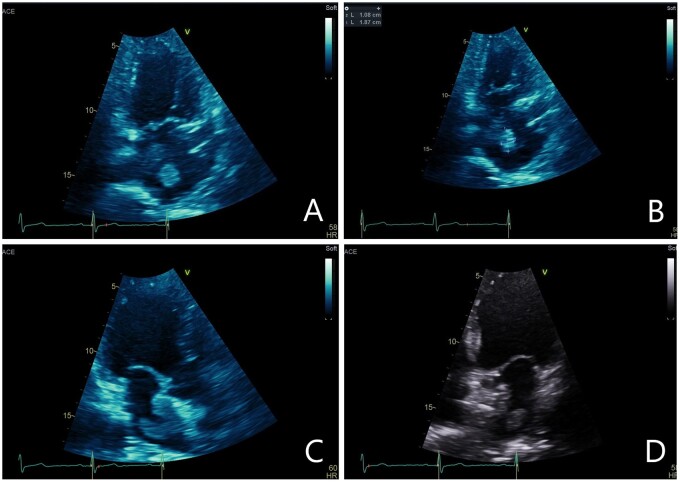
Resting transthoracic echocardiography (TTE) demonstrated a mobile thrombus (1.8 cm by 1.0 cm) in the left atrium attached to the PFO closure device (Figure 1). There was no residual inter-atrial shunt. There was separation of the left atrial disc of the 35 mm Amplatzer PFO device from the inter-atrial septum adjacent to the thrombus (Figure 2). The patient was commenced on intravenous heparin anticoagulation.
Figure 1.
(A) Zoomed apical two-chamber view demonstrating 1.0 cm × 1.8 cm left atrial thrombus. (B) Apical two-chamber view demonstrating left atrial thrombus. (C) Apical three-chamber view demonstrating large left atrial thrombus adherent to patent foramen ovale-closure device. (D) Apical four-chamber view demonstrating mobile left atrial thrombus adjacent to patent foramen ovale-closure device.
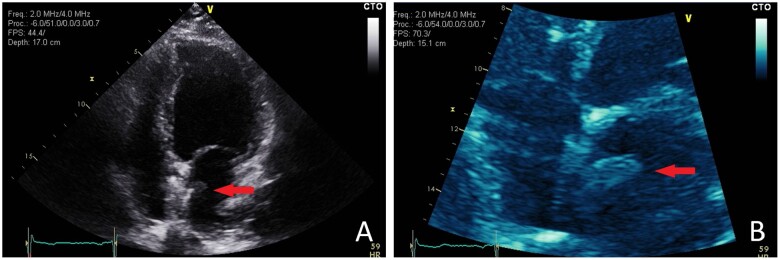
Figure 2.

Apical four-chamber view demonstrating significant separation of the left atrial disc from the inter-atrial septum which represents a potential nucleation point for the development of thrombus. Mobile left atrial thrombus attached to the patent foramen ovale-closure device is also demonstrated.
During her admission, the patient subsequently developed left upper limb weakness and paraesthesia. Repeat MRI demonstrated further multifocal embolic infarcts in the right parietal and frontal lobes.
Multi-disciplinary discussion between cardiology, neurology, cardiothoracic surgery, and haematology teams was undertaken. Given the patient’s history of myelofibrosis and hypercoagulable state, surgical device explantation and subsequent repair were deemed inappropriately high risk. Given the sub-therapeutic INR, the patient was transitioned to subcutaneous enoxaparin anti-thrombotic therapy long-term. Repeat TTE 6 days following the initial TTE demonstrated a reduction in thrombus size to 0.7 cm by 0.7 cm and the patient was discharged home (Figure 3).
Figure 3.
(A) Post-anticoagulation apical four-chamber view showing reduced thrombus (red arrow) size 6 days following initiation of anticoagulation. (B) Post-anticoagulation zoomed apical four-chamber view showing residual left atrial thrombus adjacent to patent foramen ovale-closure device (red arrow).
Transoesophageal echocardiogram 5 weeks after initial presentation demonstrated residual device-associated thrombus with no significant change in size since discharge. Factor Xa assay at this time showed the patient’s enoxaparin anticoagulation was at a therapeutic level. On follow-up at 6 months, she had not suffered any further neurological events, repeat echocardiography was not performed at this time.
Discussion
In this report, we present the case of a late-onset PFO closure device-associated thrombus in a patient on warfarin and aspirin anti-thrombotic therapy. The use of newer generation PFO closure devices has made thrombus formation uncommon, with a reported rate between 0.4 and 1.1%.1–4 The recent PC and DEFENSE-PFO trials utilized the Amplatzer PFO occluder device and did not report any device-associated thrombi in their total of 264 patients.5,6 Device-associated thrombi typically occur early after implantation, usually within the first 3 months.7,8 There are, however, rare reports of thrombus formation as late as 8 years after device implantation, highlighting the importance of regular follow-up of implanted devices.9
To date, there is little data describing risk factors for device thrombus formation. There is an association between device-associated thrombi and atrial fibrillation.7 Prolonged periods of monitoring did not identify atrial fibrillation in our patient. Persistent atrial septal aneurysm, not present in our patient, has also been associated with thrombus development.7 There is little data on the risk of device thrombus formation in the setting of haematological malignancy and subsequent procoagulant state. Gastmann et al.10 describe the case of a device-associated thrombus in the setting of factor XII deficiency. Other cardiovascular disease risk factors, such as age, diabetes, or hypertension do not appear to increase the risk of device-associated thrombus.7,8 There is no data as to whether anatomical factors, such as a long tunnel PFO, increase the risk of device thrombus formation.
Implantation of larger devices, such as the 35 mm Amplatzer device in our case, has not been associated with thrombus formation. Larger devices have been associated with increased rates of residual shunt, which itself is associated with an increased risk of recurrent stroke.11–14 Significant left atrial disc separation from the inter-atrial septum, as in our case, is an uncommon finding post-PFO closure device deployment and represents a blind pouch which may provide a nidus for thrombus formation. Such disc separation should prompt consideration of anticoagulation. Any blind pouch formation would be less probable and smaller in size with a smaller left atrial disc as exists in the 25 mm Amplatzer PFO device.
PFO closure device-associated thrombus is rare in the setting of therapeutic anticoagulation. Divchev et al.15 describe a case of device thrombus after percutaneous atrial septal defect closure with a StarFLEX occluder whilst the patient was anticoagulated with phenprocoumon. There are no randomized trials comparing the efficacy of antiplatelet vs. anticoagulation therapy or anticoagulation in addition to antiplatelet agents in preventing device-associated thrombus. In their cohort, Krumsdorf et al.7 found no significant difference between single-agent aspirin, dual antiplatelet, or warfarin anticoagulation regimes although their series was significantly underpowered.
No randomized trials have examined the optimal management of device-associated thrombi. In a series of 12 patients with device-associated thrombus, 10 resolved with therapeutic anticoagulation.8 Krumdorf et al.7 found 17 of 20 thrombi resolved with unfractionated heparin or warfarin within 6 months, 11 of these resolving within 4 weeks. The remaining three patients required surgical thrombectomy.7 To date there is no evidence regarding the use of direct oral anticoagulants (DOAC) or low-molecular-weight heparin in the management of PFO closure device thrombus. In our case, given the patient’s sub-therapeutic INR on admission, enoxaparin was chosen for its stable therapeutic profile and reduced need for monitoring. This is the first reported instance of the use of a low-molecular-weight heparin in the management of PFO closure device thrombus. The patient had a reasonable response to enoxaparin with objective reduction in thrombus size and no further neurological events.
Conclusion
Device-associated thrombus is an uncommon late complication of percutaneous PFO closure, as such, evidence as to the optimal management is lacking. Our case is unique, due to device-associated thrombus formation in the setting of both anticoagulant and antiplatelet therapy in a patient with prior recurrent thrombotic events and with blind pouch formation between the left atrial disc and septum post-PFO device deployment. This case highlights the importance of patient selection when evaluating the appropriateness of percutaneous PFO closure and consideration of initial 25 mm Amplatzer PFO device selection in higher-risk patient subsets.
Lead author biography

Lennox Jerzyna graduated from The University of Sydney in 2018. He is currently in his first year as a Basic Physician Trainee at Wollongong Hospital, Wollongong, Australia.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing these cases and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for submission and publication of this case report, including images and associated text, has been obtained from the patient in line with COPE guidance.
Conflict of interest: None declared.
Funding: None declared.
Supplementary Material
References
- 1.Furlan AJ, Reisman M, Massaro J, Mauri L, Adams H, Albers GW, et al. ; CLOSURE I Investigators. Closure or medical therapy for cryptogenic stroke with patent foramen ovale. N Engl J Med 2012;366:991–999. [DOI] [PubMed] [Google Scholar]
- 2.Carroll JD, Saver JL, Thaler DE, Smalling RW, MacDonald LA, Marks DS, et al. ; RESPECT Investigators. Closure of patent foramen ovale versus medical therapy after cryptogenic stroke. N Engl J Med 2013;368:1092–1100. [DOI] [PubMed] [Google Scholar]
- 3.Mas JL, Derumeaux G, Guillon B, Massardier E, Hosseini H, Mechtouff L. et al. Patent foramen ovale closure or anticoagulation vs. antiplatelets after stroke. N Engl J Med 2017;377:1011–1021. [DOI] [PubMed] [Google Scholar]
- 4.Søndergaard L, Kasner SE, Rhodes JF, Andersen G, Iversen HK, Nielsen-Kudsk JE, et al. ; Gore REDUCE Clinical Study Investigators. Patent foramen ovale closure or antiplatelet therapy for cryptogenic stroke. N Engl J Med 2017;377:1033–1042. [DOI] [PubMed] [Google Scholar]
- 5.Meier B, Kalesan B, Mattle HP, Khattab AA, Hildick-Smith D, Dudek D, et al. ; PC Trial Investigators. Percutaneous closure of patent foramen ovale in cryptogenic embolism. N Engl J Med 2013;368:1083–1091. [DOI] [PubMed] [Google Scholar]
- 6.Lee PH, Song JK, Kim JS, Heo R, Lee S, Kim DH. et al. cryptogenic stroke and high-risk patent foramen ovale: the DEFENSE-PFO trial. J Am Coll Cardiol 2018;71:2335–2342. [DOI] [PubMed] [Google Scholar]
- 7.Krumsdorf U, Ostermayer S, Billinger K, Trepels T, Zadan E, Horvath K. et al. Incidence and clinical course of thrombus formation on atrial septal defect and patient foramen ovale closure devices in 1,000 consecutive patients. J Am Coll Cardiol 2004;43:302–309. [DOI] [PubMed] [Google Scholar]
- 8.Hornung M, Franke J, Bertog S, Taaffe M, Vaskelyte L, Pittl U. et al. Long-term results of a comparison of three patent foramen ovale closure devices in a randomized trial (Amplatzer versus CardioSEAL-STARflex versus Helex occluder). J Am Coll Cardiol 2012;60:B224–B225. [DOI] [PubMed] [Google Scholar]
- 9.Kodankandath TV, Mishra S, Libman RB, Wright P.. Recurrent stroke due to patent foramen ovale closure device thrombus eight years after implantation. J Stroke Cerebrovasc Dis 2016;25:161–162 [DOI] [PubMed] [Google Scholar]
- 10.Gastmann O, Werner GS, Babic UU, Figulla HR.. Thrombus formation on transcatheter ASD occluder device in a patient with coagulation factor XII deficiency. Cathet Cardiovasc Diagn 1998;43:81–83. [DOI] [PubMed] [Google Scholar]
- 11.Greutmann M, Greutmann-Yantiri M, Kretschmar O, Senn O, Roffi M, Jenni R. et al. Percutaneous PFO closure with Amplatzer PFO occluder: predictors of residual shunts at 6 months follow-up. Congenit Heart Dis 2009;4:252–257. [DOI] [PubMed] [Google Scholar]
- 12.Wahl A, Tai T, Praz F, Schwerzmann M, Seiler C, Nedeltchev K. et al. Late results after percutaneous closure of patent foramen ovale for secondary prevention of paradoxical embolism using the amplatzer PFO occluder without intraprocedural echocardiography: effect of device size. JACC Cardiovasc Interv 2009;2:116–123. [DOI] [PubMed] [Google Scholar]
- 13.Wahl A, Meier B, Haxel B, Nedeltchev K, Arnold M, Eicher E. et al. Prognosis after percutaneous closure of patent foramen ovale for paradoxical embolism. Neurology 2001;57:1330–1332. [DOI] [PubMed] [Google Scholar]
- 14.Windecker S, Wahl A, Chatterjee T, Garachemani A, Eberli FR, Seiler C. et al. Percutaneous closure of patent foramen ovale in patients with paradoxical embolism: long-term risk of recurrent thromboembolic events. Circulation 2000;101:893–898. [DOI] [PubMed] [Google Scholar]
- 15.Divchev D, Schaefer A, Fuchs M, Breymann T, Drexler H, Meyer GP.. Thrombus formation on an atrial septal defect closure device: a case report and review of the literature. Eur J Echocardiogr 2007;8:53–56. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.