Introduction
Cardiac device oversensing can result in significant morbidity, and potentially mortality, for affected patients. Consequences include underpacing and inappropriate shocks, and are typically noted shortly after implantation. Here we describe an unusual case of P-wave oversensing identified serendipitously many years after initial implant.
Case report
A 71-year-old man was referred for query pacemaker malfunction. He had a dual-chamber pacemaker implanted 19 years prior for complete atrioventricular block, and had 2 pulse generator changes in the interim (most recently nearly 2 years prior to presentation; current device Medtronic Advisa A3DR01, Medtronic 4092 RV lead, Medtronic 4592 RA lead). Pre- and postoperatively on the day of an elective orthopedic surgery, abnormalities were noted on telemetry and electrocardiogram (Figure 1). A magnet was applied to the device for the duration of the surgery, which proceeded without issue, and the patient was referred for pacemaker assessment.
Figure 1.
Left: Perioperative telemetry recording. Right: Perioperative electrocardiogram.
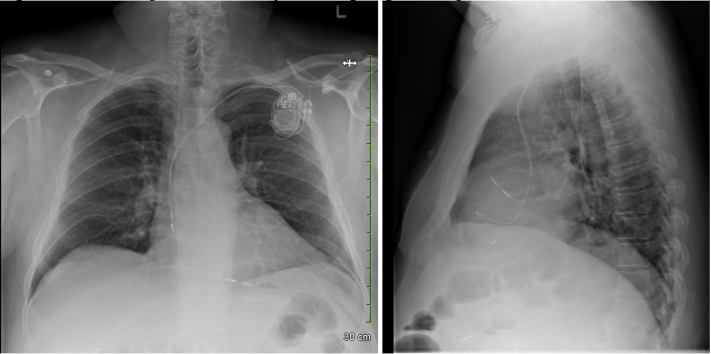
When seen in our assessment unit, the patient described having episodes of unexplained dizziness over the last few months, occurring several times per week. He denied syncope. There had been no chest wall trauma, and no intervening procedures that could have resulted in device manipulation. Review of systems was otherwise unremarkable. Chest radiograph showed unchanged (in comparison to imaging from 10 years prior) lead positions in the right atrial appendage and right ventricular (RV) apex (Figure 2).
Figure 2.
Posteroanterior and lateral chest radiography views at presentation.
Electrogram (EGM) recording on interrogation of his device is shown in Figure 3 (left) and demonstrated intermittent P-wave oversensing on the ventricular channel corresponding to the early surface lead P wave (representative of right atrial depolarization). Device programming was noted as DDD 60–130. Sensing and pacing for both leads was in bipolar configuration. Lead impedance (atrial: 494 ohms; ventricular 437 ohms) and threshold were stable for both leads in bipolar and unipolar configurations. P wave measured 4.5 mV, R wave measured 1.1 mV (ventricular sensitivity 0.9 mV); notably, R wave measurements over years prior ranged from 7 to 14 mV, and at interrogations the patient was frequently noted to have an intrinsic rhythm in the 30s. R-wave amplitude trend (Figure 3, right) showed a previous high-amplitude recording with a change to a wide range of R-wave values and sudden change to a low R-wave amplitude more recently. Review of EGM recordings at prior visits showed similar appearance but without evidence of oversensing. Notably, bipolar ventricular sensitivity threshold was 2 mV prior to last pulse generator change; nominal programming for the current device was left unchanged at 0.9 mV.
Figure 3.
Results from device interrogation. Left: Electrogram (EGM) recording from pacemaker interrogation. EGM 2 (top) is summed; EGM 1 (middle) is atrial; EGM 3 (bottom) is ventricular. Right: R-wave amplitude trend, demonstrating R waves in the 10–16 mV range until July 2019; more recent wide range in R-wave amplitude April–October 2020; and a sudden change to ∼1 mV from October 2020 onward.
The bipolar sensitivity of the ventricular lead was adjusted to a higher threshold (0.9 mv to >5.6 mV) and ventricular pacing changed to unipolar. He was assessed in follow-up 1 month, 3 months, and 6 months later and reported resolution of symptoms. Lead parameters remained stable and without evidence of fracture.
This case demonstrates the rare phenomenon of P-wave oversensing (also called “far-field P-wave sensing”) by the ventricular lead causing inhibition of ventricular pacing. Initial management included placement of a magnet over the device to convert to asynchronous pacing, which avoided underpacing in this ventricular pacing–dependent patient. Lead dislodgement and lead fracture/insulation breach were investigated by imaging and device interrogation, neither of which demonstrated suggestive features. Evaluation of EGMs demonstrated clear intermittent oversensing of the P wave by the ventricular lead, which inhibited pacing. The R-wave amplitude trend and assessment of prior EGMs showed similar far-field P wave appearance, though without evidence of oversensing. Review of EGMs prior to most recent pulse generator change demonstrated lower-amplitude far-field signals, though notably a different pulse generator was in place and gain settings may have been different. Likely, symptomatic P-wave oversensing did not occur previously owing to a combination of (1) higher sensitivity setting with the prior pulse generator precluding oversensing and (2) presence of escape rhythm, which would limit prolonged asystole.
Owing to initial concern for the possibility of impending lead fracture given the age of the lead and sudden change in far-field sensing, we changed the pacing polarity to unipolar (tip-to-can). As the ring pacing conductor is external to the tip pacing conductor, in the event of progressive lead fracture bipolar pacing would be anticipated to fail prior to unipolar pacing. As such, changing to unipolar pacing offers an additional measure of protection and bipolar threshold testing can act as an early warning sign of progressive lead failure prior to impacting pacing function. Stability of lead parameters at serial visits provided reassurance, but ongoing vigilance is necessary to ensure early intervention can be performed to avert pacemaker failure owing to lead fracture.
Discussion
P-wave oversensing, or far-field P-wave sensing, has been rarely described previously in several contexts. A case of VVI pacemaker oversensing due to insulation breach and interaction with an abandoned lead was described by van Gelder and colleagues.1 Barold and colleagues2 describe 2 cases of P-wave oversensing in a dual-chamber pacemaker, both following a reduction in ventricular sensitivity setting in order to promote PVC identification (notably, 1 case with oversensing of sinus P waves, and the second with oversensing of ectopic P waves). Van Gelder and colleagues3 describe a single-chamber RV pacemaker with P-wave oversensing when the sensitivity threshold was reduced. Mond and colleagues4 discuss many types of pacemaker malfunction including P-wave oversensing owing to RV lead position close to the atrium (specific cases included epicardial and coronary sinus lead positions and retraction of an RV apical lead to the tricuspid annulus). Mulder and colleagues5 report a case of a subcutaneous-lead implantable cardioverter-defibrillator delivering an inappropriate shock owing to P-wave and T-wave oversensing.
A unique feature of our case is the occurrence of P-wave oversensing in a dual-chamber device, with nominal settings, remote from the time of implant, and with apical RV lead positioning. In pacemaker-dependent patients like ours, this can be a devastating event, potentially leading to syncope or sudden cardiac death owing to prolonged asystole. Fortunately, pacemaker malfunction was noted serendipitously, emphasizing the importance of knowledge of pacemaker function and malfunction in the general medical community. This case also highlights the importance of awareness for nominal device programming and consideration of adjustments in appropriate contexts. In the case of a pacemaker-dependent patient with a nondefibrillating device, there is limited value to a low-sensitivity setting and adjustment to higher setting on implant or early in follow-up could be considered to limit risk of oversensing, given the minimal risk of undersensing in such cases.
The occurrence of the sensed event early during the surface P wave suggests it is a right atrial signal detected. One explanation for the phenomenon in our case is an insulation breach in the atrial portion of the ventricular lead allowing for current leak, similar to the case described by van Gelder and colleagues.1 The age of the lead certainly raises the concern for insulation breach and/or impending fracture. However, the stable lead parameters and lack of progression over a >6-month follow-up period argue against this etiology. A noninvasive test to assess for insulation break involving chest wall stimulation has been described by Ferek and colleagues,6 though this has not been validated. Another potential mechanism for P-wave oversensing is the presence of a large muscular right atrial appendage allowing far-field sensing of adequate amplitude to produce oversensing. However, it is unclear why this would not have presented earlier, other than small changes in relative position that produced the oversensing. There did not appear to be a positional or respiratory association. One explanation is the loss of a ventricular escape rhythm, the presence of which would limit prolonged asystole owing to P-wave oversensing. This is supported by the temporal association of symptoms with the R-wave trend changes.
Options for managing this condition include elevating the sensitivity threshold above the far-field P-wave amplitude (as was done in this case); programming to DDT pacing mode, if available; programming to asynchronous pacing mode (use of a magnet is an alternative if a programmer is unavailable); and replacing the lead. We also have taken the additional precaution of increased frequency of clinic visits and programming to unipolar pacing in case lead compromise were to develop between assessments.
P-wave oversensing is a rare but potentially serious problem for many cardiac devices and can present both early and far after lead implantation. Awareness of this situation, its potential causes, and how it can be managed are important for all clinicians managing patients with cardiac devices.
Key Teaching Points.
-
•
Consider potential causes of pacemaker malfunction. Underpacing should raise consideration for oversensing.
-
•
Be aware of variability in nominal device settings (especially when changing models at pulse generator change) and consider patient-tailored adjustments.
-
•
Low sensitivity settings are of low clinical utility in pacing devices. Consideration should be given to raising the sensitivity threshold for pacemaker-dependent patients to minimize risk of oversensing.
-
•
Consider adjustment to unipolar pacing if concern for impending lead fracture.
Acknowledgments
Special thank-you to the nurses of the UOHI Cardiac Device Clinic (especially Sherry Coulas and Cathy Iob) for their assistance.
Footnotes
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Conflict of Interest Statement: All authors have no conflicts to disclose.
References
- 1.van Gelder B.M., Bracke F.A., El Gamal M.I. P wave oversensing in a unipolar VVI pacemaker. Pacing Clin Electrophysiol. 1995;18:370–373. doi: 10.1111/j.1540-8159.1995.tb02529.x. [DOI] [PubMed] [Google Scholar]
- 2.Barold S.S., Garrigue S., Clémenty J. Far-field P-wave sensing by the right ventricular lead of conventional dual chamber pacemakers. J Interv Card Electrophysiol. 2002;6:77–80. doi: 10.1023/a:1014132408411. [DOI] [PubMed] [Google Scholar]
- 3.van Gelder L.M., el Gamal M.I., Tielen C.H. P-wave sensing in VVI pacemakers: useful or a problem? Pacing Clin Electrophysiol. 1988;11:1413–1418. doi: 10.1111/j.1540-8159.1988.tb04989.x. [DOI] [PubMed] [Google Scholar]
- 4.Mond H.G., Sloman J.G. The malfunctioning pacemaker system. Part II. Pacing Clin Electrophysiol. 1981;4:168–181. doi: 10.1111/j.1540-8159.1981.tb06540.x. [DOI] [PubMed] [Google Scholar]
- 5.Mulder B.A., Maass A.H., Blaauw Y. Inappropriate shock caused by P wave oversensing in an entirely subcutaneous ICD. Neth Heart J. 2018;26:411–412. doi: 10.1007/s12471-018-1099-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferek B., Pasini M., Pustisek S., Jursić M., Tonković S. Noninvasive detection of insulation break. Pacing Clin Electrophysiol. 1984;7:1063–1068. doi: 10.1111/j.1540-8159.1984.tb05658.x. [DOI] [PubMed] [Google Scholar]