Abstract
Numerous retrospective studies have demonstrated that the density of intra-tumoral immune cell infiltration is prognostic in epithelial ovarian cancer (OC). These observations together with reports of programmed death ligand-1 (PD-L1) expression in advanced OC provided the rationale for investigating the benefit of programmed death-1 (PD1) or PD-L1 inhibition in OC. Unfortunately clinical trials to date evaluating PD1/PD-L1 inhibition in patients with relapsed OC have been disappointing. In this review we will discuss early results from single agent PD1/PD-L1 inhibitors and the strategies to enhance benefit from immune-oncology agents in OC, including proposing anti-PD-L1 in combination with other agents (cytotoxics, anti-angiogenics, poly(ADP-ribose) polymerase. (PARP) inhibitors, targeted therapies or other immunotherapies), as well as evaluating these agents earlier in the disease course, or in biomarker selected patients.
Keywords: anti-angiogenic, combinations, immune checkpoint inhibitors, ovarian cancer, PARP inhibitors, predictive biomarkers
Background and rationale for targeting immune checkpoints in epithelial ovarian cancer
A number of studies over the past two decades have suggested that ovarian cancers (OCs) are immunogenic and capable of stimulating host anti-tumor immune responses. Tumor infiltrating lymphocytes (TILs) can be detected in half of OC tumors at diagnosis.1 These oligoclonal TILs recognize tumor-associated antigens (TAAs) and can generate autologous tumor cell-specific cytotoxicity in vitro.2,3 Humoral or cellular immunity against TAAs has been demonstrated in patients with OC including antibodies or T-cell subsets against oncogenic p53, NY-ESO-1 or LAGE-1.4–7 Most importantly, high TILs have been consistently and reproducibly associated with survival.8,9 In particular, the most recent and largest meta-analysis including 21 studies and almost 3000 patients with OC confirmed that high levels of intra-epithelial CD3+ or CD8+ T cells were most strongly associated with both improved progression-free survival (PFS) and overall survival (OS).9 Taken together these data suggest that the OC microenvironment could have an impact on prognosis and/or response to treatment. However, to be effective, an anti-tumor immune response requires a sequence of tightly orchestrated interactions. A functional antigen processing machinery has been shown to influence CD8+ and or CD3+ immune cell recruitment and activation. Not surprisingly, down-regulation in certain major histocompatibility complexes (MHC) results in decreased TIL density and reduced survival in advanced OC.10,11 In addition, a vast network of immune inhibitory processes balance and counteract the cytotoxic function of T cells. For example, regulatory T cells (Tregs), identified by their combined expression of CD4, CD25 and intracellular forkhead box P3 (FOXP3), have the capacity to inhibit the activity of immune cells through cytokines such as transforming growth factor beta (TGF-β) and interleukin (IL)-10 and induce an immunosuppressive phenotype in other cells such as macrophages thereby limiting anti-tumor immunity and favoring malignant cell growth.12–14 In addition, the recruitment of immunosuppressive M2 polarized tumor-associated macrophages or immature dendritic cells (DCs) further contribute to an immunosuppressive tumor microenvironement.15 Finally, upregulation of inhibitory receptors in tumor or immune cells may further promote immune tolerance in OC. For example, OC cells frequently over-express CD47, a ‘don’t eat me’ signal that allows them to escape phagocytosis by innate immune cells,16 while lymphocytes or myeloid DCs in ovarian tumor tissue and draining lymph nodes often express the inhibitory molecule programmed cell death ligand-1 (PD-L1).17 In fact programmed death-1 (PD1) and its ligand PD-L1 are probably the best described immune co-inhibitory molecules. The PD1 receptor can be expressed on CD8+ and CD4+ T cells (including Tregs), whereas PD-L1 is expressed on activated T cells, tumor-infiltrating macrophages or fibroblasts and cancer cells. An early study found that almost two thirds of ovarian tumors demonstrated low level PD-L1 expression, mainly on immune cells rather than tumor cells, and that expression of PD-L1 was associated with significantly worst prognosis.18 The authors also found that the density of intraepithelial CD8+ T cells was inversely correlated to the expression of PD-L1, suggesting that the expression of PD-L1 on tumor cells may result in CD8+ T-cell exclusion.
Overall, the immune TME (tumor microenvironment) may be relevant in OC, a high infiltration by effector CD8 T cells may contribute to survival in some patients with OC. Manipulating the immune environment with immune checkpoint inhibitors (ICIs) could therefore represent an attractive strategy. However, results from clinical trials to date have been somewhat disappointing and suggest that harnessing an efficient anti-tumor immune response in OC may require thinking beyond PD1/PD-L1 inhibition alone in unselected patients with OC. In this review we will discuss early results from single agent PD1/PD-L1 inhibitors and the strategies to enhance benefit from immune-oncology agents in OC. Response to PD1/PD-L1 inhibition may be improved by combining them with other agents [cytotoxics, anti-angiogenics, PARP inhibitors (PARPis), or other ICIs]), as well as proposing them earlier in the disease course, or in biomarker selected patients.
Early clinical data on PD-L1/PD1 inhibition in OC
Early phase trials have evaluated the benefit of various PD-L1/PD1 inhibitors, nivolumab, pembrolizumab and avelumab, in platinum-resistant ovarian cancer (PROC) and reported response rates (RRs) of 10–15%.19–21 Patient numbers were small and most patients included were heavily pre-treated. KEYNOTE-100 was the largest published phase II trial of a PD1 inhibitor alone in relapsed mainly PROC. Unfortunately, the RR was disappointing at less than 10%, with little difference according to degree of PD-L1 expression.22
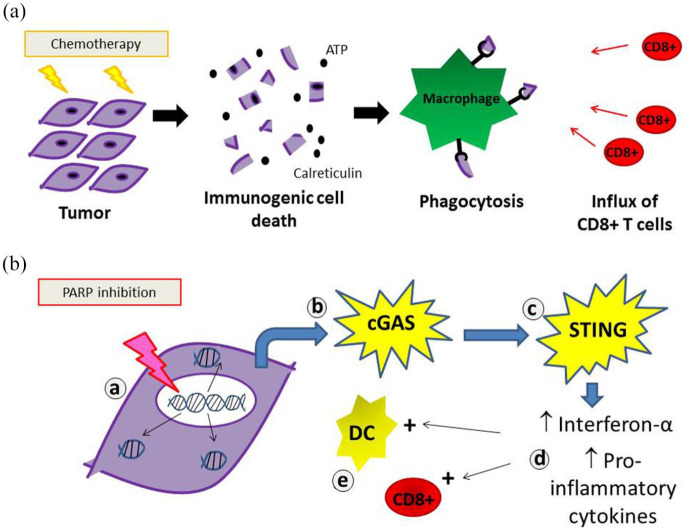
Chemotherapy remains the cornerstone of medical management of OC and pre-clinical data attribute immunogenic properties to certain cytotoxics. In addition, a recent study looking at paired OC samples before and after treatment with chemotherapy showed increased natural killer (NK) cell infiltration and oligoclonal expansion of T cells.23 Other studies have reported that neoadjuvant chemotherapy was associated with increased stromal tumor infiltrating lymphocytes, increased PD-L1 expression and a shift in the balance of cytotoxic to suppressor immune cells in favor of an anti-tumor response.24,25 These studies suggest that chemotherapy can potentiate the immunogenicity of OC and multiple studies exploring the role of chemotherapy plus ICIs are currently underway.26 In vitro studies have demonstrated that oxaliplatin or doxorubicin induce the release of danger signals (calreticulin, adenosine triphosphate (ATP)...) from dying cells resulting in an immunogenic cell death (ICD) capable of triggering an immune response (Figure 1).27 As such ICD-inducing chemotherapies could enhance ICI efficacy. Pegylated liposomal doxorubicin (PLD) is standard treatment for PROC, and thus could provide an attractive partner for PD-L1/PD1 inhibitors. This hypothesis was tested in the JAVELIN-200 trial, which recruited 566 PROC patients randomly assigned 1:1:1 to PLD versus the anti-PD-L1 avelumab, versus avelumab + PLD. Once again, the results fell short of expectation, median PFS was no different across arms (1.9 versus 3.5 versus 3.7months, respectively; Table 1).28 There was, however, a trend for improved PFS with avelumab + PLD compared with PLD alone among patients with PD-L1+ tumors. In parallel, a phase III trial evaluating avelumab in first line with carboplatin and paclitaxel (JAVELIN-100) closed early after an interim futility analysis showed that the combination with chemotherapy would not meet the first endpoint of superiority compared with chemotherapy alone.29
Figure 1.
Proposed immune-stimulatory properties of certain cytotoxic agents (a) capable of inducing an immunogenic cell death, such as anthracyclines or (b) with immunogenic activity, such as PARP (Poly(ADP-ribose) polymerase) inhibitors: PARP inhibition results in (a) accumulation of cytosolic double strand DNA, which (b) activates cytosolic DNA sensor cyclic GMP-AMP (guanosine monophosphate-adenosine monophosphate) synthetase (cGAS) and (c) the stimulator of interferon genes (STING) pathway. This upregulates (d) type I interferons and pro-inflammatory cytokines. This in turn results in (e) increased antigen presenting capacity of dendritic cells (DCs) and CD8+ mediated anti-tumor immune response.
Table 1.
Activity of PD1/PD-L1 inhibitors alone or in combination with chemotherapy in OC.
| ICI | Target | Phase | N | Indication | RR (%) | PFS (median) | Ref |
|---|---|---|---|---|---|---|---|
| Single agent | |||||||
| Pembrolizumab | PD1 | Ib | 26 | PD-L1 + recurrent OC | 11 | 1.9 mo | Varga et al.20 |
| Avelumab | PD-L1 | Ib | 125 | Recurrent OC | 10 | 1 yr PFS 10% | Disis et al.21 |
| Nivolumab | PD1 | II | 20 | PROC | 15 | 3.5 mo | Hamanishi et al.18 |
| Pembrolizumab | PD1 | II | |||||
| Cohort A | 285 | 1–3 lines TFI 3–12 mo | 7 | 2.1 | |||
| Cohort B | 91 | 4–6 lines TFI > 3 mo | 10 | 2.1 | Matulonis et al.22 | ||
| ICI + chemotherapy | |||||||
| Avelumab versus PLD versus AVE + PLD | PD-L1 | III randomized 1 :1 :1 | 188 | PROC | 4 | 1.9 mo | Pujade-Lauraine et al.28 |
| 190 | 4 | 3.5 mo | |||||
| 188 | 13 | 3.7 mo |
AVE, avelumab; ICI, immune checkpoint inhibitor; OC, ovarian cancer; PD1, Programmed cell death protein 1; PD-L1, programmed death ligand 1; PFS, progression-free survival; PLD, pegylated liposomal doxorubicin; PROC, platinum resistant ovarian cancer; RR, response rate; TFI, treatment free interval.
Rationale for IO combinations to improve benefit in OC
As described by Chen and Mellman, an effective anticancer immune response requires a series of stepwise events.30 These are referred to as the cancer-immunity cycle. Briefly, tumor associated neoantigens are released from dying cancer cells and processed by DCs for presentation to T cells. This results in the priming and activation of antigen presenting cells and effector T cells. Activated effector T cells traffic to and infiltrate the tumor bed, where they specifically recognize and bind to cancer cells through the interaction between its T-cell receptor (TCR) and its cognate antigen bound to MHC, and kill their target cancer cell. Cancer cell lysis releases additional TAAs to increase the breadth and depth of the anti-tumor immune response.
In most patients, the cancer-immunity cycle does not perform optimally and immune strategies have been developed to boost the immune response at various steps along the cycle. For example, PD-L1 inhibitors restore the last step, T-cell mediated cytotoxicity, and have proved an effective strategy against melanoma or non-small cell lung cancer (NSCLC). However in OC, re-activating anti-tumor immunity may require targeting more than one step along the immunity cycle.31 Possible approaches include increasing TAA release with cytotoxics or targeted therapies, promoting T-cell infiltration to the tumor bed using vascular endothelial growth factor inhibitors or increasing T-cell priming and activation using CTLA4 antibodies. Whether combining PD1/PD-L1 inhibition with one or more of these strategies could enhance the immune response in patients with OC is currently being explored in a number of trials.
Combining PD-L1/PD1 inhibition with PARPis
Approximately 25% of high-grade ovarian cancers (HGOCs) are associated with a BRCA1 or BRCA2 mutation, and these tumors frequently demonstrate greater CD8+ lymphocyte infiltration. In addition, some studies have suggested that BRCA1/2 mutated OC harbors higher levels of neoantigens, greater PD-L1 expression and demonstrates interferon-gamma immune signatures associated with T-cell mediated cytotoxicity.32,33 Studies have pointed to crucial interactions between genomic aberration patterns and immune microenvironment in HGOC. For example, tumors harboring homologous recombination deficient (HRD) signatures display high levels of intra-epithelial CD8+ lymphocytes suggestive of target engagement. In contrast, HGOC tumors demonstrating a very distinct pattern of fold-back inversions (mutually exclusive of HRD tumors) show lower T-cell infiltration and antigen presenting capacity.34 In line with this, murine models recapitulating various HGOC genotypes have been shown to shape the tumor immune composition and modulate responsiveness to ICI/targeted therapy combinations.35 Whether HRD-associated immunogenic genomic alterations also make BRCA1/2 mutated OC more sensitive to IO strategies has not been established. In fact, a subgroup analysis of the JAVELIN 100 trial failed to demonstrate any trend in improvement in survival among patients with BRCA mutations treated with avelumab.22 Nevertheless, the possibility of an interaction between BRCA mutations and benefit of IO compounds has led to trials evaluating the combination of PARPis and IO in BRCA mutated OC. The MEDIOLA trial evaluated the association of the PARPi, olaparib with the PD-L1 inhibitor, durvalumab in molecularly defined cohorts. In the BRCA1/2 mutated OC cohort, the reported RR was impressive at 72%, but should be interpreted with caution in light of the inclusion criteria (platinum-sensitive relapsed BRCA mutated OC not previously exposed to a PARPi), a patient population, which would be expected to have a RR to olaparib alone close to 70%36 (Table 2). Thus it is difficult to draw conclusions regarding the added benefit of the IO in this particular setting.
Table 2.
Activity of PD1/PD-L1 inhibitors in combination with targeted therapies (PARP inhibitors, anti-angiogenic or other ICIs) in relapsed OC.
| ICI combo | Phase | N | Indication | RR (%) | PFS | Ref |
|---|---|---|---|---|---|---|
| ICI + PARP inhibitor | ||||||
| Durvalumab + olaparib | I/II | 32 | PSOC BRCAm | 72 | 11 mo | Drew et al.36 |
| 32 | PSOC BRCAwt | 34 | NA | Liu et al.44 | ||
| Pembrolizumab + niraparib | I/II | 62 | PROC or platinum ineligible 80% BRCAwt | 18 | NA | Konstantinopoulos et al.39 |
| ICI + VEGF inhibitor | ||||||
| Durvalumab + cediranib | I | 12 | Recurrent OC | 50 | NA | Lee et al.43 |
| Nivolumab + bevacizumab | II | 38 | Recurrent OC | 29 | 8 mo | Liu et al.44 |
| - PROC (N = 18) | 40 | |||||
| - PSOC (N = 20) | 17 | |||||
| Pembrolizumab + lenvatinib | II | 31 | Recurrent OC | 32 | NA | Lwin et al.46 |
| Carbo/Pac + bevacizumab + atezolizumab versus placebo | III | 1301 | 1st line advanced OC | NA | 19.5 mo versus 18.4 p:NS | Moore et al.49 |
| ICI + PARP inhibitor + VEGF inhibitor | ||||||
| Durvalumab + olaparib + bevacizumab | I/II | 32 | PSOC BRCAwt | 87 | NA | Liu et al.44 |
| ICI + ICI | ||||||
| Nivolumab + ipilimumab versus placebo | II | 100 | Recurrent OC PFI < 12 mo | 31 versus 12 | 3.9 mo versus 2 mo | Zamarin et al.53 |
ICI, immune checkpoint inhibitor; OC, ovarian cancer; PARP, poly(ADP-ribose) polymerase; PD1, Programmed cell death protein 1; PD-L1, Programmed death ligand 1; PFS, progression-free survival; PLD, pegylated liposomal doxorubicin; PROC, platinum resistant ovarian cancer; PSOC, platinum sensitive ovarian cancer; RR, response rate; VEGF, vascular endothelial growth factor.
However, intriguing preclinical data suggest that PARPi-induced cell tumor death may be in part mediated via immunogenic mechanisms [Figure 1(b)].37 PARPi treatment has been shown to result in the accumulation of cytosolic double strand DNA, which results in activation of cytosolic DNA sensor cyclic GMP-AMP synthetase (cGAS) and of the stimulator of interferon genes (STING) pathway. This immunogenic signaling in turn upregulates the expression of interferon genes and increases both DC antigen presenting capacity as well as the CD8+ mediated anti-tumor immune response.38 The hypothesis that PARPi could enhance benefit from PD-L1/PD1 inhibition even in BRCA wild-type OC has been tested. The TOPACIO trial evaluated the combination of the PARPi, niraparib with pembrolizumab in women with recurrent OC, irrespective of BRCA mutation status or platinum sensitivity. In the pooled ovarian carcinoma cohort of 62 patients, 60 patients were evaluable for efficacy and the objective response rate (oRR) was 18%39 (Table 2). While clinical activity was modest, prolonged responses were observed in some patients with platinum-resistant BRCA wild-type tumors which would not be expected to respond to either agent alone. Further studies will be required to determine whether the combination is actually synergistic and if so, in which molecularly defined subsets.39 In this regard, the ongoing ANITA trial may provide some answers (NCT03598270). This phase III trial is evaluating the benefit of platinum-based chemotherapy with or without atezolizumab followed by niraparib maintenance with or without atezolizumab in patients with platinum-sensitive recurrent OC.
Combining PD1/PD-L1 inhibition with anti-angiogenic agents
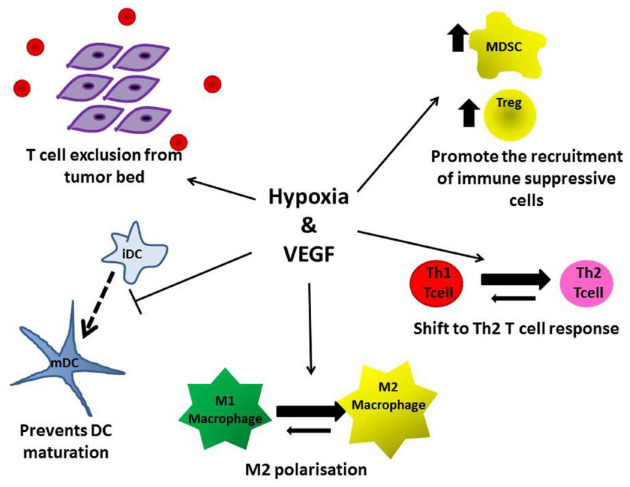
Under hypoxic conditions in the TME, there is a general shift from an anti-tumoral T helper (Th)1-type response to a protumoral Th2-type and recruitment of Tregs leading to the induction of tumor tolerance and neoangiogenesis (Figure 2).40 In addition, DC maturation is inhibited leading to impaired antigen presentation and activation of tumor-specific CD8+ T cells (Figure 2). This is also accompanied by infiltration of tissue associated macrophages that are subsequently co-opted to promote tumor progression via the upregulation of growth factors such as fibroblast growth factor 2 (FGF2), platelet-derived growth factor (PDGF), and vascular endothelial growth factor (VEGF).41 In addition, VEGF itself has potent immunosuppressive properties. Thus VEGF blockade could synergize with ICI by enabling the normalization of the tumor vasculature thus increasing the infiltration of immune effector cells into tumors and promoting the switch from an immunosuppressive to a pro-inflammatory anti-tumor TME.42
Figure 2.
Both hypoxia and vascular endothelial growth factor (VEGF) promote immune tolerance via a number of mechanisms. This provides the rationale for immune checkpoint inhibitor (ICI) + VEGF inhibitor combinations in ovarian cancer (OC).
In a small study which included 12 patients with relapsed OC, Lee et al.43 reported an impressive 50% (6/12) RR to the combination of durvalumab and the oral VEGF tyrosine kinase inhibitor cediranib43 (Table 2). More recently, another study evaluated nivolumab and bevacizumab in 38 patients with relapsed OC.44 The RR was 40% among patients with platinum-sensitive disease and 17% among those with platinum-resistant OC. Importantly, in both these trials activity was seen even in patients with PD-L1 negative tumors. Recent data have shown that the combination of lenvatinib [a multiple kinase inhibitor that inhibits vascular endothelial growth factor receptor (VEGFR) 1, 2 and 3, fibroblast growth factor receptor (FGFR) 1, 2, 3 and 4, and platelet-derived growth factor receptor (PDGFR) alpha] and pembrolizumab resulted in RRs of ~40% in recurrent endometrial cancer, and 30% in recurrent OC.45,46 A study by Zsiros et al.47 reported an oRR of 48% among 40 patients with recurrent OC treated with the combination of pembrolizumab, oral cyclophosphamide and bevacizumab. Finally, the most striking activity was observed with the triple combination of olaparib, bevacizumab and durvalumab, which resulted in an impressive 87% RR in a small cohort of 30 patients with platinum-sensitive, BRCA wild-type recurrent OC.48 Although patient numbers are small, these provocative data provide a hint of additive, possibly synergistic, benefit for the combination of PD1/PD-L1 and VEGF inhibition.
There was therefore significant optimism regarding the results of the large randomized phase III IMAGYN050 trial testing the benefit of adding the PD-L1 antibody atezolizumab to first line carboplatin, paclitaxel and bevacizumab in women with newly diagnosed advanced stage OC. The trial enrolled 1301 women with advanced stage III or IV OC who were either planned for neoadjuvant chemotherapy, or had residual disease after primary debulking. Co-primary endpoints were PFS and OS. Unfortunately, at the data cut-off after a median of 20 months follow-up, the trial failed to meet its PFS endpoint. The addition of atezolizumab did not significantly improve PFS in either the whole population, or the PD-L1+ subset compared with standard chemotherapy and bevacizumab alone.49 These disappointing results are in stark contrast to the activity reported in the previously mentioned small phase II trials combining anti-angiogenic and ICI in patients with OC. Differences in trial settings (first line versus recurrent) and endpoints (RR versus PFS) could account for these diverging results. In addition, further follow-up may be required to identify whether a small subset might benefit – the so-called ‘long tail’ of the survival curve. Experience from ICI trials in other solid tumors suggests that certain considerations in trial design and statistical analyses may be required to capture the true benefit of ICIs. Indeed as these agents do not target the cancer cell directly, but rather stimulate the host immune system, responses may be delayed. In addition, prolonged responses and survival induced by immune activation will be observed in some but not all patients. Longer-term follow-up may be required, novel endpoints such as durable response rates (DRRs), or PFS/OS landmark analyses beyond the medians may also prove useful.50,51 The question arises whether, with a median follow-up of 20 months and median PFS of 19 months in the IMAGYN050 trial, the cut-off for data analysis may have been too early.
Combined PD-L1/PD1 and CTLA4 blockade
Preclinical studies in OC have demonstrated that up to half of TILs may be positive for both CTLA4 and PD-1, and dual blockade of both immune checkpoints resulted in doubling of responses compared with either agent given alone.52 A recent phase II study of combination the CTLA4 antibody, ipilimumab with nivolumab versus nivolumab alone in recurrent OC revealed a significantly improved RR in the nivolumab plus ipilimumab group (31% versus 12%, p = 0.034) with a near doubling of the median PFS (4 versus 2 months, respectively).53 However, grade ⩾3 related adverse events were higher in the combination group compared with the nivolumab only group (49% versus 33%). PD-L1 expression was not significantly associated with response in either treatment group.
A number of trials are ongoing exploring combined PD-L1 and CTLA4 inhibition. Given the non-negligible risk of increased toxicity with these combinations, questions remain regarding the dosage and schedule of the CTLA4 inhibitor. Interestingly, at least two of these randomized trials are evaluating this combination in the neoadjuvant setting (NCT03899610, NCT03249142). Paired tumor samples are obtained before and after treatment with anti-PD-L1 +/− anti-CTLA4 and will provide a unique opportunity to elucidate the biological effects of ICI on tumor cells and the immune microenvironment in newly diagnosed OC.
Overcoming ICI resistance in OC: targeting the phosphatidylinositol 3-kinase (PI3K)/Akt or Wnt pathways
Activation of the phosphatidylinositol 3-kinase (PI3K)-Akt pathway and/or loss of phosphatase and tensin homolog (PTEN) protein have been shown to suppresses T-cell infiltration and cytotoxicity.54,55 Alterations in the phosphatidylinositol 3-kinase (PI3K)/PTEN pathway are frequently observed in OC56 and PTEN loss has been correlated with a significant reduction of CD8+ T-cell infiltration in melanomas.57 BKM120, a pan-PI3K inhibitor, has been shown significantly to inhibit the growth of a human bladder cancer cell line bearing a PI3KCA mutation, with associated increased immune cell infiltration (hCD45+) and expression of chemokines and immune genes.58 Moreover, the addition of BKM120 rendered PI3KCA-mutated tumors sensitive to PD-1 blockade.58
Tumor infiltrating Tregs, which mediate a pro-tumorigenic anti-inflammatory tumor microenvironment, have been correlated with worse outcomes in OC,59 and the depletion of Tregs enhances anti-tumor immunity and promotes tumor regression.55 Inhibitors of PI3K and Akt have been shown selectively to inhibit the proliferation of human and murine Tregs when compared with conventional T cells, leading to enhanced anti-tumor therapeutic efficacy in a Treg dependent manner.55 Inhibition of the PI3Kα/δ isoforms of PI3K with a PI3Kα/δ specific inhibitor, AZD 8835, has been associated with dynamic suppression of Tregs, improved CD8+ T-cell activation and memory in mouse syngeneic tumor models.60 The afore-mentioned data thus provide a rationale for combination strategies of PI3K/Akt inhibitors to overcome resistance to ICIs. Several studies exploring this combination are underway including a phase I study of the Akt inhibitor capivasertib (AZD5363) combined with olaparib and durvalumab in patients with solid malignancies with ongoing expansion in patients with gynecological malignancies (NCT03772561).
Immune cell exclusion in treatment-naïve high grade serous OC was recently found to be associated with functional mutations in negative regulators of the Wnt pathway leading to increased Wnt signaling and amplification of MYC target genes.23 Wnt mediated immune exclusion has previously been described in melanoma as well.61 There are several therapeutic approaches being explored to downregulate the Wnt pathway in tumors, including via inhibition of porcupine acyltransferase (PORCN). There are several PORCN inhibitors now being studied in clinical trials including LGK974 (NCT01351103) and ETC159 (NCT02521844). The phase I study of ETC159 also includes a phase I dose escalation with the PD-1 inhibitor pembrolizumab, and will be recruiting a cohort of patients with endometrial and OC in the dose expansion phase (NCT02521844).
Predictive biomarkers
High tumor mutation burden (TMB) and PD-L1 expression levels are useful (albeit imperfect) predictive biomarkers in patients with NSCLC or melanoma.62 Unfortunately neither TMB, nor PD-L1 expression has proved to be as useful in OC. While most OCs display some degree of PD-L1 expression, it is usually only modest; in the IMAGYN050 trial, less than 25% of patients demonstrated >5% PD-L1+ immune cells.49 In addition, in OC, PD-L1 is mostly expressed on immune cells rather than tumor cells themselves. This is in contrast with NSCLC, a classic immune responsive tumor, PD-L1 is expressed on tumor cells and at much higher levels. Finally there is no standard cut-off to define a PD-L1+ OC tumor. Some studies considered a tumor positive if >1%, >5% or >10% of cells stained positive for PD-L1. In addition, studies varied according to which type of cell was actually considered: some counted percentage stained immune cells, others percentage stained tumor cells. Finally, some recent trials used a combined positive score (CPS) of tumor and immune cells PD-L1 staining.63
With regard to TMB, most epithelial ovarian tumors have low TMB. The most common high grade serous OC is a disease of copy number alterations, not point mutations. One notable exception may be ovarian tumors associated with mismatch repair defects (MMRds), which result in the accumulation of point mutations, high neoantigen levels and remarkable sensitivity to ICIs.64 While mutations or hypermethylation of MMR genes are most frequent in endometrial cancers, 10–20% of clear cell or endometrioid OC may also be MMRd.65,66 Forty to 50% of patients with non-colorectal MMRd tumors respond to single agent PD-L1 blockade and MMRd status can be easily determined using routine technologies. In the USA, the US Food and Drug Administration (FDA) has even approved one ICI, pembrolizumab, for any MMRd relapsed solid tumor. Although this indication is not yet available in Europe, MMR status should probably be sought for all patients with relapsed endometrioid or clear cell OCs in order to orient them towards trials of ICIs.
In the case of clear cell OC, data from KEYNOTE-100 demonstrated that this histological subtype of OC was associated with the highest RR to pembrolizumab (15.8%)22 and the recently presented randomized study of nivolumab versus gemcitabine or pegylated liposomal doxorubicin for patients with platinum-resistant ovarian cancer67 also revealed a numerically longer overall survival for clear cell patients treated with nivolumab compared with chemotherapy. This may be related to the higher incidence of dMMR status in these tumors but there are also unique tumor microenvironmental features in ovarian clear cell carcinoma (OCCC) that are suggestive of the potential for increased efficacy with ICIs. These include immunosuppression caused by increased expression of lymphocyte activation gene 3 (LAG3), T-cell immunoglobulin mucin-3 (TIM-3), and PD-1, as well as activating PIK3CA and ARID1A loss of function mutations. In addition, other factors may contribute such as: HNF1-β (hepatocyte nuclear factor 1 beta) signaling or activation of signal transducer and activator of transcription 3 (STAT3) and nuclear factor kappa light-chain-enhancer of activated B cells (NF-κB) upregulation leading to increased IL-6 and IL-8 expression.68 There are several studies currently evaluating the role of ICIs in ovarian clear cell carcinoma such as the MOCCA trial (NCT03405454 – multicenter phase II randomized trial of durvalumab versus physician’s choice chemotherapy in recurrent ovarian clear cell adenocarcinoma) that has completed recruitment, the PEACOCC trial (NCT 03425565 – pembrolizumab in the population of gynecological clear cell carcinoma), and the LARA trial (NCT04699071 – phase II trial of lenvatinib + pembrolizumab in recurrent gynecological clear cell carcinomas).
Another rare histological subtype which could prove particularly suited to immune therapies is small cell carcinoma of the ovary, hypercalcemic type (SCCOHT). SCCOHT is a highly aggressive monogenic cancer driven by SMARCA4 mutations and characterized by very low mutation rates.69 Unexpectedly for a low TMB cancer, Jelinic et al.70 reported durable responses to PD-L1 inhibition in four patients and described for the first time the TME of SCCOHTs. The majority of the tumors (eight of 11 cases) demonstrated PD-L1 expression with strong associated T-cell infiltration. Transcriptional profiling revealed increased expression of genes related to Th1 and cytotoxic cell function in PD-L1-high tumors, suggesting that PD-L1 acts as a pathway of adaptive immune resistance in SCCOHT. These data have provided the rationale for a trial evaluating the benefit of adding pembrolizumab to first line chemotherapy in newly diagnosed SCCOHT in an effort to improve outcomes from this devastating disease (NCT04602377).
As mentioned previously, BRCA1/2 mutated OC, or tumors with other defects leading to HRD have been associated with higher levels of TILs and PD-L1 expression, whether this also confers greater sensitivity to ICIs requires further investigation.71 In this regard, intriguing data have been generated by correlative analyses performed on tumors from patients enrolled in the TOPACIO trial of an association of PARP and PD1 inhibition. This study included mainly BRCA wild-type platinum-resistant patients and investigators demonstrated that a homologous recombination deficient signature as well as the presence of interferon-primed exhausted T cells in the tumor microenvironment predicted benefit.72
Immunogenic gene expression signatures in OC
Analysis of gene expression signatures in high grade serous endometrioid OC have consistently defined four main molecular subsets – C1 (mesenchymal), C2 (immunoreactive or Epi B), C4 (differentiated), C5 (proliferative or Stem A) – with distinct clinical outcomes.73–75 Genes and signaling pathways associated with immune cells are found to be enriched in immunoreactive subtype (C2/Epi B) tumors, with genes related to the adaptive immune response, including markers of T-cell activation (CD8A) and T-cell trafficking (CXCL9), found to be significantly overexpressed.75 The immunoreactive/C2 subtype is not only immunogenic but is also associated with defects in the homologous recombination (HR) DNA repair pathway, including BRCA1/2 mutations.76,77 The C2 molecular subtype has also been associated with a better clinical outcome compared with other subtypes (C1, C4, C5).73–75 A recent study of gene expression signatures in OCCC revealed two distinct subtypes of OCCC – epithelial clear cell ovarian cancer (EpiCC) and mesenchymal (MesCC).78 EpiCCs are associated with a lower epithelial mesenchymal transition (EMT) score, lower disease stage and lower risk of progression while MesCCs are associated with a higher EMT score, more advanced disease, and a greater propensity to progress.78 EpiCCs showed enriched expression of genes associated with Tregs and activated DCs whereas MesCCs showed enriched expression of genes associated with TILs including CD4 memory and γδ T cells.78 These studies appear to indicate that an immunogenic tumor microenvironment exists in subsets of OC and should be explored as predictive biomarkers in samples collected for translational research in phase III studies of ICIs in OC.
Conclusions
Despite strong biological rationale, epithelial OC has not proved to be the ideal candidate for ICIs. Resoundingly negative clinical data from phase I, II and III trials confirm the lack of benefit for single agent PD-L1/PD1 inhibition in PROC. Whether this rules out all immune-modulatory strategies in PROC remains to be determined. Typically ‘immune cold’ OC may require combinatorial approaches to improve benefit. Preclinical and early phase clinical studies support combining PD-L1 inhibition with conventional cytotoxics, PARPis or anti-angiogenics. A huge number of patients are being treated within ongoing phase III randomized trials evaluating the benefit of ICI +/− PARPi +/− anti-angiogenic in first line. These studies are enrolling all comers, with some exceptions, often stratified on BRCA1/2 status and PD-L1 expression. The questions will be regarding tolerance, magnitude of benefit in the whole population, in defined subsets, cost, and the true value of putting all our molecules in frontline, leaving relapsed disease an area of unmet medical need. The PD1/PD-L1 axis may not be the most relevant immune checkpoint in OC. There is a huge number of other actionable immune co-regulatory molecules (IDO, LAG3, TIGIT, OX40, TIM3, NKG2A, CD47, Sirp1α, etc....) and many can already be targeted by drugs in early development. It will be crucial to gain more insight into the unique molecular and immune features of the ovarian TME in order to optimize benefit from ICIs for patients with OC.
Footnotes
Conflict of interest statement: AL: paid fees to the institution or to myself for ad boards: AZ, Clovis, MSD, MERCK serono, Ability Pharma, Biocad, Zentalis, Tesaro, GSK. Travel to congress from AZ, Roche, Clovis, Tesaro, GSK CI, PI or coInv on trials sponsored and or funded by AZ, Clovis, Ability, Roche, MSD, BMS, GSK, Agenus, AZ, Iovance.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Jonathan Ledermann  https://orcid.org/0000-0003-3799-3539
https://orcid.org/0000-0003-3799-3539
Contributor Information
Alexandra Leary, Institut Gustave Roussy, 114 rue Edouard Vaillant, Villejuif 94805, France, Université Paris-Saclay, INSERM U981, Villejuif, France.
David Tan, Department of Haematology–Oncology, National University Cancer Institute, Singapore, Cancer Science Institute, National University of Singapore, Singapore.
Jonathan Ledermann, UCL Cancer Institute, Cancer Research UK and UCL Trials Centre, London, UK.
References
- 1.Zhang L, Conejo-Garcia JR, Katsaros D, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med 2003; 348: 203–213. [DOI] [PubMed] [Google Scholar]
- 2.Dadmarz RD, Ordoubadi A, Mixon A, et al. Tumor-infiltrating lymphocytes from human ovarian cancer patients recognize autologous tumor in an MHC class II-restricted fashion. Cancer J Sci Am 1996; 2: 263–272. [PubMed] [Google Scholar]
- 3.Kooi S, Freedman RS, Rodriguez-Villanueva J, et al. Cytokine production by T-cell lines derived from tumor-infiltrating lymphocytes from patients with ovarian carcinoma: tumor-specific immune responses and inhibition of antigen-independent cytokine production by ovarian tumor cells. Lymphokine Cytokine Res 1993; 12: 429–437. [PubMed] [Google Scholar]
- 4.Goodell V, Salazar LG, Urban N, et al. Antibody immunity to the p53 oncogenic protein is a prognostic indicator in ovarian cancer. J Clin Oncol 2006; 24: 762–768. [DOI] [PubMed] [Google Scholar]
- 5.Odunsi K, Jungbluth AA, Stockert E, et al. NY-ESO-1 and LAGE-1 cancer-testis antigens are potential targets for immunotherapy in epithelial ovarian cancer. Cancer Res 2003; 63: 6076–6083. [PubMed] [Google Scholar]
- 6.Schlienger K, Chu CS, Woo EY, et al. TRANCE- and CD40 ligand-matured dendritic cells reveal MHC class I-restricted T cells specific for autologous tumor in late-stage ovarian cancer patients. Clin Cancer Res 2003; 9: 1517–1527. [PubMed] [Google Scholar]
- 7.Deniger DC, Pasetto A, Robbins PF, et al. T-cell responses to TP53 “hotspot” mutations and unique neoantigens expressed by human ovarian cancers. Clin Cancer Res 2018; 24: 5562–5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hwang WT, Adams SF, Tahirovic E, et al. Prognostic significance of tumor-infiltrating T cells in ovarian cancer: a meta-analysis. Gynecol Oncol 2012; 124: 192–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li J, Wang J, Chen R, et al. The prognostic value of tumor-infiltrating T lymphocytes in ovarian cancer. Oncotarget 2017; 8: 15621–15631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han LY, Fletcher MS, Urbauer DL, et al. HLA class I antigen processing machinery component expression and intratumoral T-cell infiltrate as independent prognostic markers in ovarian carcinoma. Clin Cancer Res 2008; 14: 3372–3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Callahan MJ, Nagymanyoki Z, Bonome T, et al. Increased HLA-DMB expression in the tumor epithelium is associated with increased CTL infiltration and improved prognosis in advanced-stage serous ovarian cancer. Clin Cancer Res 2008; 14: 7667–7673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bu M, Shen Y, Seeger WL, et al. Ovarian carcinoma-infiltrating regulatory T cells were more potent suppressors of CD8+ T cell inflammation than their peripheral counterparts, a function dependent on TIM3 expression. Tumour Biol 2016; 37: 3949–3956. [DOI] [PubMed] [Google Scholar]
- 13.Landskron J, Helland O, Torgersen KM, et al. Activated regulatory and memory T-cells accumulate in malignant ascites from ovarian carcinoma patients. Cancer Immunol Immunother 2015; 64: 337–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Redjimi N, Raffin C, Raimbaud I, et al. CXCR3+ T regulatory cells selectively accumulate in human ovarian carcinomas to limit type I immunity. Cancer Res 2012; 72: 4351–4360. [DOI] [PubMed] [Google Scholar]
- 15.Yuan X, Zhang J, Li D, et al. Prognostic significance of tumor-associated macrophages in ovarian cancer: a meta-analysis. Gynecol Oncol 2017; 147: 181–187. [DOI] [PubMed] [Google Scholar]
- 16.Brightwell RM, Grzankowski KS, Lele S, et al. The CD47 “don’t eat me signal” is highly expressed in human ovarian cancer. Gynecol Oncol 2016; 143: 393–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Curiel TJ, Wei S, Dong H, et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med 2003; 9: 562–567. [DOI] [PubMed] [Google Scholar]
- 18.Hamanishi J, Mandai M, Iwasaki M, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A 2007; 104: 3360–3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamanishi J, Mandai M, Ikeda T, et al. Safety and antitumor activity of anti-PD-1 antibody, nivolumab, in patients with platinum-resistant ovarian cancer. J Clin Oncol 2015; 33: 4015–4022. [DOI] [PubMed] [Google Scholar]
- 20.Varga A, Piha-Paul S, Ott PA, et al. Pembrolizumab in patients with programmed death ligand 1-positive advanced ovarian cancer: analysis of KEYNOTE-028. Gynecol Oncol 2019; 152: 243–250. [DOI] [PubMed] [Google Scholar]
- 21.Disis ML, Taylor MH, Kelly K, et al. Efficacy and safety of avelumab for patients with recurrent or refractory ovarian cancer: phase 1b results from the JAVELIN solid tumor trial. JAMA Oncol 2019; 5: 393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matulonis UA, Shapira-Frommer R, Santin AD, et al. Antitumor activity and safety of pembrolizumab in patients with advanced recurrent ovarian cancer: results from the phase II KEYNOTE-100 study. Ann Oncol 2019; 30: 1080–1087. [DOI] [PubMed] [Google Scholar]
- 23.Jimenez-Sanchez A, Cybulska P, Mager KL, et al. Unraveling tumor-immune heterogeneity in advanced ovarian cancer uncovers immunogenic effect of chemotherapy. Nat Genet 2020; 52: 582–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leary A, Genestie C, Blanc-Durand F, et al. Neoadjuvant chemotherapy alters the balance of effector to suppressor immune cells in advanced ovarian cancer. Cancer Immunol Immunother 2021; 70: 519–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mesnage SJL, Auguste A, Genestie C, et al. Neoadjuvant chemotherapy (NACT) increases immune infiltration and programmed death-ligand 1 (PD-L1) expression in epithelial ovarian cancer (EOC). Ann Oncol 2017; 28: 651–657. [DOI] [PubMed] [Google Scholar]
- 26.Ghisoni E, Imbimbo M, Zimmermann S, et al. Ovarian cancer immunotherapy: turning up the heat. Int J Mol Sci 2019; 20: 2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vanmeerbeek I, Sprooten J, De Ruysscher D, et al. Trial watch: chemotherapy-induced immunogenic cell death in immuno-oncology. Oncoimmunology 2020; 9: 1703449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pujade-Lauraine E, Fujiwara K, Ledermann JA, et al. Avelumab alone or in combination with pegylated liposomal doxorubicin versus pegylated liposomal doxorubicin alone in platinum-resistant or refractory epithelial ovarian cancer: primary and biomarker analysis of the phase III JAVELIN ovarian 200 trial. Gynecol Oncol 2019; 154: 21–22. [Google Scholar]
- 29.Ledermann JA, Colombo N, Oza AM, et al. Avelumab in combination with and/or following chemotherapy vs chemotherapy alone in patients with previously untreated epithelial ovarian cancer: results from the phase 3 javelin ovarian 100 trial. J Gynecol Oncol 2020; 159: 13–14. [Google Scholar]
- 30.Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity 2013; 39: 1–10. [DOI] [PubMed] [Google Scholar]
- 31.Gao D. Compound-therapy based on cancer-immunity cycle: promising prospects for antitumor regimens. Am J Cancer Res 2019; 9: 212–218. [PMC free article] [PubMed] [Google Scholar]
- 32.Strickland KC, Howitt BE, Shukla SA, et al. Association and prognostic significance of BRCA1/2-mutation status with neoantigen load, number of tumor-infiltrating lymphocytes and expression of PD-1/PD-L1 in high grade serous ovarian cancer. Oncotarget 2016; 7: 13587–13598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hao D, Liu J, Chen M, et al. Immunogenomic analyses of advanced serous ovarian cancer reveal immune score is a strong prognostic factor and an indicator of chemosensitivity. Clin Cancer Res 2018; 24: 3560–3571. [DOI] [PubMed] [Google Scholar]
- 34.Zhang AW, McPherson A, Milne K, et al. Interfaces of malignant and immunologic clonal dynamics in ovarian cancer. Cell 2018; 173: 1755–1769.e22. [DOI] [PubMed] [Google Scholar]
- 35.Iyer S, Zhang S, Yucel S, et al. Genetically defined syngeneic mouse models of ovarian cancer as tools for the discovery of combination immunotherapy. Cancer Discov 2021; 11: 384–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Drew Y, Kaufman B, Banerjee S, et al. Phase II study of olaparib + durvalumab (MEDIOLA): updated results in germline BRCA-mutated platinum-sensitive relapsed (PSR) ovarian cancer (OC). Ann Oncol 2019; 30 (Suppl. 5): v475–v532. [Google Scholar]
- 37.Shen J, Zhao W, Ju Z, et al. PARPi triggers the STING-dependent immune response and enhances the therapeutic efficacy of immune checkpoint blockade independent of BRCAness. Cancer Res 2019; 79: 311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chabanon RM, Muirhead G, Krastev DB, et al. PARP inhibition enhances tumor cell-intrinsic immunity in ERCC1-deficient non-small cell lung cancer. J Clin Invest 2019; 129: 1211–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Konstantinopoulos PA, Waggoner S, Vidal GA, et al. Single-arm phases 1 and 2 trial of niraparib in combination with pembrolizumab in patients with recurrent platinum-resistant ovarian carcinoma. JAMA Oncol 2019; 5: 1141–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McNamee EN, Korns Johnson D, Homann D, et al. Hypoxia and hypoxia-inducible factors as regulators of T cell development, differentiation, and function. Immunol Res 2013; 55: 58–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Henze AT, Mazzone M. The impact of hypoxia on tumor-associated macrophages. J Clin Invest 2016; 126: 3672–3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ott PA, Hodi FS, Buchbinder EI. Inhibition of immune checkpoints and vascular endothelial growth factor as combination therapy for metastatic melanoma: an overview of rationale, preclinical evidence, and initial clinical data. Front Oncol 2015; 5: 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee JM, Cimino-Mathews A, Peer CJ, et al. Safety and clinical activity of the programmed death-ligand 1 inhibitor durvalumab in combination with poly (ADP-Ribose) polymerase inhibitor olaparib or vascular endothelial growth factor receptor 1-3 inhibitor cediranib in women’s cancers: a dose-escalation, phase I study. J Clin Oncol 2017; 35: 2193–2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu JF, Herold C, Gray KP, et al. Assessment of combined nivolumab and bevacizumab in relapsed ovarian cancer: a phase 2 clinical trial. JAMA Oncol 2019; 5: 1731–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Makker V, Taylor MH, Aghajanian C, et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer. J Clin Oncol 2020; 38: 2981–2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lwin Z, Gomez-Roca C, Saada-Bouzid E, et al. LEAP-005: phase 2 study of lenvatinib plus pembrolizumab in patients with previously treated advanced solid tumors. Ann Oncol 2020; 31 (Suppl. 4): S1170. [Google Scholar]
- 47.Zsiros E, Lynam S, Attwood KM, et al. Efficacy and safety of pembrolizumab in combination with bevacizumab and oral metronomic cyclophosphamide in the treatment of recurrent ovarian cancer: a phase 2 nonrandomized clinical trial. JAMA Oncol 2021; 7: 78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Drew Y, Penson RT, O’Malley DM. Phase II study of olaparib (O) plus durvalumab (D) and bevacizumab (B) (MEDIOLA): initial results in patients (pts) with non-germline BRCA-mutated (non-gBRCAm) platinum sensitive relapsed (PSR) ovarian cancer (OC). Ann Oncol 2020; 31: S615–S616. [Google Scholar]
- 49.Moore KN, Okamoto A, Wu F, et al. IMagyn050/GOG3015/ENGOT-ov39: a randomized, double-blind, phase III study of atezolizumab vs placebo combined with chemotherapy + bevacizumab in stage III-IV ovarian, fallopian tube & peritoneal cancers (OC). Ann Oncol 2017; 28 (Suppl. 5): v330–v354. [Google Scholar]
- 50.Ascierto PA, Long GV. Progression-free survival landmark analysis: a critical endpoint in melanoma clinical trials. Lancet Oncol 2016; 17: 1037–1039. [DOI] [PubMed] [Google Scholar]
- 51.Branchoux S, Bellera C, Italiano A, et al. Immune-checkpoint inhibitors and candidate surrogate endpoints for overall survival across tumour types: a systematic literature review. Crit Rev Oncol Hematol 2019; 137: 35–42. [DOI] [PubMed] [Google Scholar]
- 52.Duraiswamy J, Kaluza KM, Freeman GJ, et al. Dual blockade of PD-1 and CTLA-4 combined with tumor vaccine effectively restores T-cell rejection function in tumors. Cancer Res 2013; 73: 3591–3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zamarin D, Burger RA, Sill MW, et al. Randomized phase II trial of nivolumab versus nivolumab and ipilimumab for recurrent or persistent ovarian cancer: an NRG oncology study. J Clin Oncol 2020; 38: 1814–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sai J, Owens P, Novitskiy SV, et al. PI3K inhibition reduces mammary tumor growth and facilitates antitumor immunity and anti-PD1 responses. Clin Cancer Res 2017; 23: 3371–3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Abu-Eid R, Samara RN, Ozbun L, et al. Selective inhibition of regulatory T cells by targeting the PI3K-Akt pathway. Cancer Immunol Res 2014; 2: 1080–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang J, Zhang L, Greshock J, et al. Frequent genetic abnormalities of the PI3K/AKT pathway in primary ovarian cancer predict patient outcome. Genes Chromosomes Cancer 2011; 50: 606–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Peng W, Chen JQ, Liu C, et al. Loss of PTEN promotes resistance to T cell-mediated immunotherapy. Cancer Discov 2016; 6: 202–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Borcoman E, De La Rochere P, Richer W, et al. Inhibition of PI3K pathway increases immune infiltrate in muscle-invasive bladder cancer. Oncoimmunology 2019; 8: e1581556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med 2004; 10: 942–949. [DOI] [PubMed] [Google Scholar]
- 60.Carnevalli LS, Sinclair C, Taylor MA, et al. PI3Kα/δ inhibition promotes anti-tumor immunity through direct enhancement of effector CD8+ T-cell activity. J Immunother Cancer 2018; 6: 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Spranger S, Bao R, Gajewski TF. Melanoma-intrinsic beta-catenin signalling prevents anti-tumour immunity. Nature 2015; 523: 231–235. [DOI] [PubMed] [Google Scholar]
- 62.Yarchoan M, Albacker LA, Hopkins AC, et al. PD-L1 expression and tumor mutational burden are independent biomarkers in most cancers. JCI Insight 2019; 4: e126908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gaillard SL, Coleman RL. Identifying markers of immune response in ovarian cancer: does PD-L1 expression meet the mark? Ann Oncol 2019; 30: 1025–1028. [DOI] [PubMed] [Google Scholar]
- 64.Marabelle A, Le DT, Ascierto PA, et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol 2020; 38: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Willis BC, Sloan EA, Atkins KA, et al. Mismatch repair status and PD-L1 expression in clear cell carcinomas of the ovary and endometrium. Mod Pathol 2017; 30: 1622–1632. [DOI] [PubMed] [Google Scholar]
- 66.Fraune C, Rosebrock J, Simon R, et al. High homogeneity of MMR deficiency in ovarian cancer. Gynecol Oncol 2020; 156: 669–675. [DOI] [PubMed] [Google Scholar]
- 67.Omatsu K, Hamanishi J, Katsumata N. Nivolumab versus gemcitabine or pegylated liposomal doxorubicin for patients with platinum-resistant (advanced or recurrent) ovarian cancer: open-label, randomized trial in Japan (NINJA trial). Ann Oncol 2020; 31 (Suppl. 4): S611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Oda K, Hamanishi J, Matsuo K, et al. Genomics to immunotherapy of ovarian clear cell carcinoma: unique opportunities for management. Gynecol Oncol 2018; 151: 381–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Auguste A, Blanc-Durand F, Deloger M, et al. Small cell carcinoma of the ovary, hypercalcemic type (SCCOHT) beyond SMARCA4 mutations: a comprehensive genomic analysis. Cells 2020; 9: 1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jelinic P, Ricca J, Van Oudenhove E, et al. Immune-active microenvironment in small cell carcinoma of the ovary, hypercalcemic type: rationale for immune checkpoint blockade. J Natl Cancer Inst 2018; 110: 787–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Morse CB, Toukatly MN, Kilgore MR, et al. Tumor infiltrating lymphocytes and homologous recombination deficiency are independently associated with improved survival in ovarian carcinoma. Gynecol Oncol 2019; 153: 217–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Farkkila A, Gulhan DC, Casado J, et al. Immunogenomic profiling determines responses to combined PARP and PD-1 inhibition in ovarian cancer. Nat Commun 2020; 11: 1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature 2011; 474: 609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tan TZ, Miow QH, Huang RY, et al. Functional genomics identifies five distinct molecular subtypes with clinical relevance and pathways for growth control in epithelial ovarian cancer. EMBO Mol Med 2013; 5: 1051–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tothill RW, Tinker AV, George J, et al. Novel molecular subtypes of serous and endometrioid ovarian cancer linked to clinical outcome. Clin Cancer Res 2008; 14: 5198–5208. [DOI] [PubMed] [Google Scholar]
- 76.George J, Alsop K, Etemadmoghadam D, et al. Nonequivalent gene expression and copy number alterations in high-grade serous ovarian cancers with BRCA1 and BRCA2 mutations. Clin Cancer Res 2013; 19: 3474–3484. [DOI] [PubMed] [Google Scholar]
- 77.Patch AM, Christie EL, Etemadmoghadam D, et al. Whole-genome characterization of chemoresistant ovarian cancer. Nature 2015; 521: 489–494. [DOI] [PubMed] [Google Scholar]
- 78.Tan TZ, Ye J, Yee CV, et al. Analysis of gene expression signatures identifies prognostic and functionally distinct ovarian clear cell carcinoma subtypes. EBioMedicine 2019; 50: 203–210. [DOI] [PMC free article] [PubMed] [Google Scholar]