Abstract
Background
COVID-19 has affected global communities with multiple neurological complications in addition to other critical medical issues. COVID-19 binds to the host’s angiotensin-converting enzyme 2 (ACE2) receptors, which are expressed in the neurons and glial cells, acting as an entry port to the central nervous system (CNS). ACE2 receptors are abundantly expressed on dopamine neurons, which may worsen the prognosis of motor symptoms in Parkinson’s disease (PD). SARS-CoV-2 may lead to an indirect response via immune-mediated cytokine storms and propagate through the CNS leading to damage. In this systematic review, we aim to provide thorough analyses of associations between COVID-19 and neurological outcomes for patients with PD.
Methods
Using PRISMA statement 2020, a systematic review was conducted to isolate confirmed COVID-19 patients and analyze the PD-associated neurological outcomes using the following databases: PubMed, Science Direct, Google Scholar, and Cochrane databases. The following keywords were used “COVID19, SARS-CoV-2, Parkinson’s disease, Pandemic, Mortality.” A modified Delphi process was employed.
Results
Of the 355 studies located during the initial round of screening, 16 were included in the final synthesis. Of PD patients who tested positive for SARS-CoV-2, worsening motor symptoms and other viral-associated symptoms were reported. These symptoms included bradykinesia, tremors, gait disturbances, delirium and dementia, and severe spasms of arms and legs. Encephalopathy was presented in 2 of the included studies. Increased mortality rates were identified for hospitalized patients due to COVID-19 and PD as compared to other patient groups.
Conclusion
Patients with PD may experience substantial worsening of symptoms due to COVID 19. Given the novelty of neurological-viral associations, clinical studies in the future ought to explore the disease severity and neurological outcomes in COVID-19 positive patients with PD as compared to non-PD patients, in addition to understanding the role of ACE2 in increased vulnerability to contracting the infection and as a treatment modality.
Keywords: COVID-19, Parkinson’s disease, SARS-CoV-2, neurological, ACE2
Introduction
At the onset of the coronavirus disease (2019) pandemic on 31 December 2019, many reports found that SARS-CoV-2 primarily caused pneumonia-like symptoms.1 This virus emerged as a global threat and a public health emergency of imminent concern across countries worldwide, with exponential transmission capability. As of May 25, 2021, there have been 167 million confirmed cases of COVID-19, with reported deaths up to 3.47 million globally.2 COVID-19 belongs to a novel member of the coronaviridae family named SARS-CoV-2 (Severe Acute Respiratory Syndrome Coronavirus 2).3 Coronaviruses are enveloped positive single-stranded RNA viruses, and the 3′ terminal contains structural proteins. The Spike (S) protein allows the virus to fuse to host cell membranes in proximity to infected and uninfected cells.4 A surge in the levels of cytokines (TNF-α, IL-6, IL-8, and IL-10) have been reported, leading to a suppression of T-cell response.5 To date, a multitude of studies have assessed the role of angiotensin-converting enzyme 2 (ACE2) receptors in the increasing vulnerability of hosts, however, there is a dearth of analysis on the role of ACE inhibitors in treating Parkinson’s disease (PD) patients. A meta-analytical study found that PD is a common comorbidity in older patients with COVID-19, which is also associated with poor in-hospital outcomes.6 The results of the meta-regression further consolidated that mortality from COVID-19 was statistically associated with age, and not with gender or dementia, which is key in monitoring PD to minimize infection risks and prevent adverse events.6 In this systematic review, we aim to provide an updated, thorough analysis of associations between COVID-19 and neurological outcomes for patients with PD.
Coronavirus disease (2019) has prompted many challenges in global healthcare systems due to its unpredictability and unique manifestations in (1) pulmonary, (2) neurological, (3) cardiovascular, (4) gastrointestinal, and (5) hematological systems. The scientific literature confirms the presence of SARS-CoV-2 in CSF, in addition to respiratory, fecal, and blood samples. In about 36.4% of cases, neurological findings have been identified, ranging from dizziness, headache, hyposmia, hypogeusia, dysphagia, muscle pain, seizures, and loss of consciousness.7 COVID-19 also influences socializing factors such as the surge in isolation-induced neurological effects, limited social activities possibly leading to short or long-term neuropsychiatric disorders.
We hypothesize that COVID-19 induced lockdowns, self-isolation, and the fears associated with contracting disease accentuate neuro-psychiatric problems such as depression and cognition. Figure 1 illustrates motor and non-motor manifestations in addition to autonomic manifestations that are pertinent in the assessment of the clinical outcomes of confirmed positive PD patients. Given the dearth of data, we aim to provide a comprehensive and updated systematic review to guide clinical care and practice in understanding the effects of COVID-19 on Parkinson’s disease.
Figure 1.
Clinical manifestations of Parkinson’s disease.
Methodology
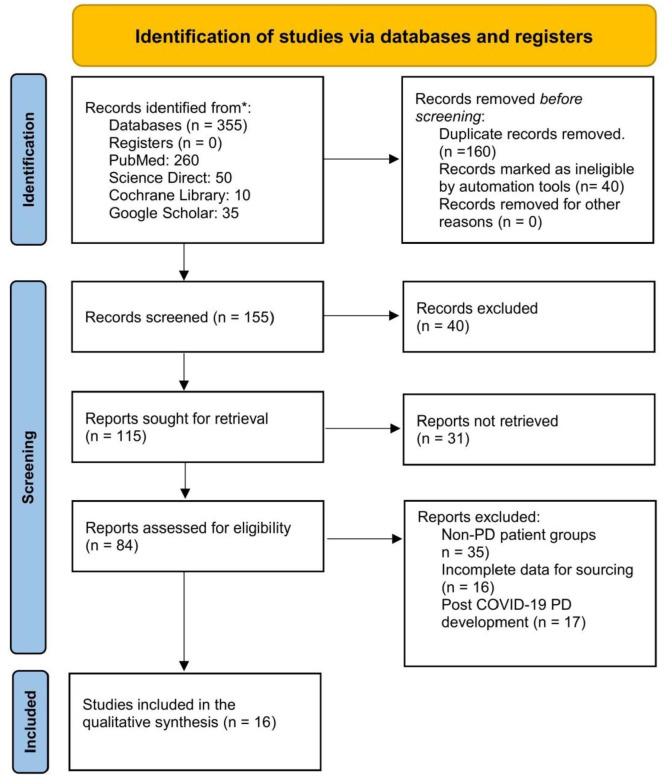
Using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 statement, a systematic literature search was performed from December 2019 through May 2021.8 The following set of keywords were used, employing the Boolean (and/or) logic: “COVID19,” “SARS-CoV-2,” “Parkinson’s disease,” “Pandemic,” “Mortality.” We searched the following databases, namely, PubMed, Cochrane, Science Direct, EMBASE, and Google Scholar. A modified Delphi approach was utilized to include studies and ensure that the clinical outcome measures are identified in a systematic and unanimous order in the included studies.9 The a-priori Delphi process for systematically analyzing the available data involved 2 rounds of screening with a final panel feedback round.9 During round 1, 10 questions pertaining to PD and clinical outcome measures were identified using a pre-determined panel of experts, which was graded on a Likert scale (1-5). In round 2, the top 5 questions were sent to the panel and they were individually scored by all members. In the panel feedback round, the prioritized research questions with the final list and scores were sent to the panel for information sharing. The PRISMA flowchart is illustrated in Figure 2.
Figure 2.
PRISMA Flowchart.
Study Selection
We included studies with confirmed COVID-19 disease who had a history of Parkinson’s disease. Studies published with open access availability were included. In total, 355 studies were located during the initial round of screening. During this round of screening, all authors scanned the abstracts and titles independently, which led to the location of 155 studies. In the second screening round, full-text studies were screened, and 125 articles that met the exclusion criteria were removed. During the third screening round, 84 studies were screened for eligibility. In total, 35 studies were excluded as non-PD patient groups were present, 16 studies had incomplete data for sourcing, and 16 of the full-text studies present with post-COVID-19 PD development, which met the exclusion criteria. Finally, 16 studies were included in the qualitative synthesis.
Eligibility Criteria
The inclusion criteria for studies and the target population consisted of (1) COVID-19 patients with Parkinson’s disease, (2) patients diagnosed with Parkinson’s disease before COVID-19 infection, (3) age group >21 years old, (4) male and/or female, and (5) available articles in English language only. Studies were excluded if they met 1 or more of the following criteria (1) COVID-19 negative PD patients, (2) studies on pregnant women, (3) the pediatric population, (4) crossover study design, and (5) commentaries/perspective pieces.
Data Extraction and Synthesis
The following data were extracted from eligible studies: serial number, author and year, study design, sample size, gender, age, comorbidities, disease duration, PD outcomes, COVID-19 associated symptoms. A shared spreadsheet was used to input data, which was extracted independently by 3 authors for analysis and cumulative result interpretation (V.J., D.A., Z.S.). The fourth reviewer (A.S.) solved any discrepancies in data extraction and reach a consensus in case of any disagreements.
Quality assessment was conducted by 2 authors (V.J. and Z.S.), who independently assessed the (1) criteria of diagnosis of COVID-19 patients, (2) confirmed diagnosis of PD before the COVID-19 pandemic, (3) PD sequelae in the included studies. A descriptive analysis was conducted and presented for the included studies. Percentages and means were presented. Frequent outcomes were reported if they have a frequency of at least 5% or more in the included studies. The term PD complications were based on the motor, non-motor, and autonomic complications mentioned in Figure 1.
No funding was obtained for this study.
Results
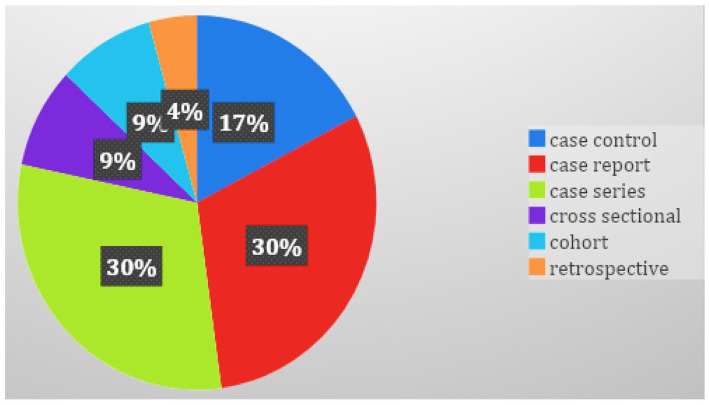
Of the 355 studies identified during the first round of screening, 16 studies comprising, 7 (43.8%) were case reports and case series, 4 (25%) case-control studies, 2 (12.5%) cross-sectional, 2 (12.5%) cohort, and 1 (6.25%) retrospective study were added (Figure 3).10-25 In total, a synthesis of 1290 PD patients with COVID-19 and the characteristics of all included studies are presented in Table 1.
Figure 3.
Types of studies included in the systematic review.
Table 1.
Characteristics of Included Studies.
| No | Authors | Study design | Sample (N) PD | Males, % | Age, Mean | Comorbidities % | Disease duration, mean | Outcomes | PD related symptoms with COVID % |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Cilia et al10 | Case-control | 12 cases 36 control |
41.7% cases 41.7% controls |
65.5 years cases 66.3 years control |
8.3% COPD 33.3 HTN 8.3% obesity 8.3% cardiopathy 16.7% cancer 8.3% immuno-compromised |
6.3 years cases 6.1 years control |
50% affected by COVID-19 (cases group); 0% affected by COVID-19 in control group, 8.3% hospitalized | Worsening of motor symptoms |
| 2 | Del Prete et al11 | Case-control | 7 cases 14 control |
57.2% cases 57.2% control |
75.7 years cases vs 75 years control | Cases: 71.5% HTN 42.8% diabetes 28.5% cardiomyopathy 14.2% malignancies |
9.3 years cases 8.9 years control |
13% mortality 14% case fatality |
30% worsening |
| 3 | Zhai et al12 | Case report | 2 | 50% | 77.5 | HTN | 22 years | 100% | 100% worsening |
| 4 | Lo Monaco et al13 | Case series | 5 | 66.6% | 74.2 | 20% chronic Renal failure 20% diabetes hypertension |
NA | 20% case fatality | 60% worsening |
| 5 | Fasano et al14 | Case-control survey | 105 | 52.4% | 70.5 | Obesity 18.1% HTN 41.9% COPD 5.6% Diabetes 7.6% Cancer .9% |
9.9 years | 17.1% hospitalized 5.7% mortality |
|
| 6 | Filatov et al15 | Case report | 1 | 74 | Atrial fibrillation Cardioembolic stroke COPD |
NA | ICU hospitalization Poor prognosis |
Encephalopathy | |
| 7 | Brown et al16 | Cross sectional | 51 | 47% | 65 | Immunocompromised 22% Heart Disease 20% HTN 25% Lung disease 14% |
0–3 years, 21 3–6 years, 12 6–9 years, 9 >9 years, 9 |
9.8% hospitalized 3.9% ICU 2%ventilator |
55% worsening 18% new PD symptoms |
| 8 | Kobylecki et al17 | Cohort, observational | 58 | 62.5% | 78.3 | Diabetes 8% HTN 38% |
9.5 | 22.4% Mortality rate | 69% Delirium 54% Dementia |
| 9 | Lo Monaco et al18 | Case report | 1 | 0% | 58 | NA | 8 years | NA | Severe spasms of arms and legs. |
| 10 | Antonini et al19 | Case series | 10 | 60% | 78.3 | Diabetes Orthostatic hypotension CHF COPD Asthma IHD, CKD, dementia, osteoporosis anxiety disorder |
12.7 years | 40% mortality rate | Worsening of mobility with fall Worsening of motor symptoms Worsening of anxiety |
| 11 | Vignatelli et al20 | Cohort | 696 PD 184 parkinsonism control 8590 |
58.8 and PD 57.1 parkinsonism 58.2 control |
75 PD 80.5 PS Control 76.0 |
PD and PS 22.6% cardiomyopathies 14.6% Cerebrovascular disease 8.5% Chronic pulmonary disease 0.3% Liver disease 5%Renal disease 10% DM 8.5% malignancies |
NA | Hospitalization 0.6% PD 3.3% PS 0.7% control Mortality rate 35.1% in all group |
PD: 72.8%Tremor 80.4% Bradykinesia 81.6% Clinical features at onset unilateral PS: 52.5% Tremor 89.2% Bradykinesia 52.5% Clinical features at onset unilateral |
| 12 | Artusi et al21 | Multiple case reports | 8 | 62.5% | 74 | HTN diabetes, depression Lung neoplasm Atrial fibrillation |
12.2 years | 75% mortality rate | 50% worsening of PD symptoms |
| 13 | Santos-García et al22 | Cross-sectional | 15 | 47.1% | 76.3 | 26.7% HTN 20% DM 64.3% dyslipidemia 13.3% cardiomyopathy 6.7% valvular cardiomyopathy 20% cardiac arrhythmia 6.7% cardiac insufficiency 14.3% pulmonary disease 6.7% smoking |
NA | 33.3% Hospitalized | 65.7% worsening of symptoms 47.7% bradykinesia 41.4% sleep problems 40.7% rigidity 34.5% gait disturbances 31.3% anxiety 28.5% pain 28.3% fatigue 27.6% Depression 20.8% tremor |
| 14 | Sainz-Amo et al23 | Case control | Case: 33 Control:172 |
5 59 | Case:75.9 Control: 73.9 |
36% Dementia | Case: 8.9 Control8.5 |
54% hospitalization 21% mortality rate |
NA |
| 15 | Li et al24 | Case report | 1 | Female | 85 | HTN Stroke |
6 years | Worsening motor symptoms. Encephalopathy | |
| 16 | de Marcaida et al25 | Retrospective | 36, 22 PD | 64% | 74.5 | HTN cardiovascular disease, renal disease, diabetes, chronic lung disease, immunosuppression | 16.2 years | 67% hospitalization 36% mortality |
75% Alteration mental status 19% worsening of their Movement abnormality 31% worsening mobility |
Male gender was predominant in 10 studies,10-19 and female gender was the predominant 1 in 6 studies.18,20-24 Of the available patient data, the cumulative mean of age in years of included patients was 76.9 years. The average disease duration spanned 11 years before the incidence of COVID-19 disease. Majority of the included patients presented with comorbid conditions, comprising of hypertension,10,14,16,17,21,22,24,25 diabetes,11,13,17,19-22 obesity,10 dyslipidemia,22 cardiovascular disease,10,11,15,16,20-22,25 immunocompromised,10,16,25 COPD,10,14,15,19 asthma,19 chronic renal, and liver diseases.19,20,25 Of the PD patients that were tested and positive infection with SARS-CoV-2, worsening of motor symptoms10-12,15-17,19,20,22,24 including bradykinesia, tremors, gait disturbances, delirium, and dementia14,20 were noted; severe spasms of the arms and legs23 were found, with individual study percentages of motor symptoms ranging from 19% to 100%, along with other COVD-19 symptoms. Encephalopathy was also one of the major symptoms, which presented in 2 (12.5%) of the 16 studies.20,24 Mortality rates for PD patients with COVID-19 who were hospitalized ranged from 5.7% to 100%.10-17,19-23,25
Discussion
To our best understanding, this is the first systematic review to assess clinical outcomes of Parkinson’s disease (PD) in confirmed COVID-19 patients with a pre-pandemic PD diagnosis. Wide varieties of neurological consequences have been reported in scientific literature among COVID-19 positive patients.26-34 Neurological symptoms comprised of the following include those associated with core dysfunction (fatigue, headache, confusion, stroke,35 dizziness, syncope,36 seizure, anorexia, and insomnia),37-39 central-peripheral mixture (Guillain Barre syndrome),40 enteric, or peripheral nervous systems dysfunction (anosmia, ageusia, myoclonus,41 neuropathic pain, and myalgia).42 The increase in hospital admission and mortality rates in PD and other chronic neurological diseases during the COVID-19 pandemic is of imminent concern as the long-term sequelae are currently undetermined. Most neurological diseases, including PD, are dose-dependent on prescribed medications for symptomatic management. With the compounded neuropsychiatric symptoms due to social isolation and outpatient clinics, temporarily being suspended and irregular visits, these are causative in worsening PD symptoms among COVID-19 patients.43
In this systematic review, we included studies with COVID-19 positive patients with a prior history of Parkinson’s disease. The most common manifestations due to COVID-19 in PD patients were found to be motor dysfunction. The majority of the studies showed a common motor deficit domain in the range of moderate to severe. Some of the patients presented with delirium, dementia, and encephalopathy among other COVID-19 complications. We posit that this may be related to the ACE2 mechanism in the nervous system. It is postulated that SARS-CoV-2 enters the cell, increases the activity of T cells, causing vasodilation, thrombosis, and hypoxemia, which may then lead to stroke and seizures. One theory supports the effect of the virus by stating that SARS-COV-2 attacks ACE 2 receptors in a multi-organ manner, targeting the brain. A study found that ACE2 receptors act locks on cells and the SARS-CoV-2 spike proteins act rapidly multiply on entering the cells. These ACE2 receptors control tissues in the eye, reproductive system, renal-excretory, digestive, and respiratory system, and 21 different regions of the brain, which require further deliberation in PD patients.44
We found that the average age of all participants was 76.9 years with a male predominance, in addition to a prior disease duration of 11 years. The majority of the patients reported the presence of other comorbidities, of which the following were the most common: hypertension, diabetes, obesity, dyslipidemia, cardiovascular disease, immunocompromised, COPD, asthma, chronic renal, and chronic liver diseases. Documented COVID-19 neurological complications include stroke, especially in younger patients, reflecting the hypercoagulable state, leukoencephalopathy, and hemorrhagic leukoencephalopathy. Although direct encephalitis was documented, it is rare but does occur. Further, cranial neuropathies are also infrequent but include the loss of sense of smell (cranial nerve 1) and Bell’s palsy (cranial nerve 7).45 There have been neurocognitive signs, particularly in older age groups that warrant further associations to PD symptoms, clinical care, and management in the short and long term.
The COVID-19 pandemic has led to adaptive changes to acute PD care. A majority of multidisciplinary care is performed remotely, and the reallocation of resources has taken place in an ad hoc fashion across many centers.46 The acute care of PD has been facilitated using telemedicine, albeit with certain limitations such as remotely assessing mood and anxiety, cognition, postural reflexes, and rigidity.46 Other adaptive strategies in treating acute PD during the COVID-19 pandemic include remote deep brain stimulation (DBS) programming-based tools that may evaluate patient symptoms online, which have shown to improve the motor symptoms among PD patients during quarantine.46 The signs and symptoms of COVID-19 in patients with PD may appear 2 to 14 days post-exposure, and without any cure, the several FDA approved vaccines may protect from hospitalization or severe illness. In the United States, BioNTech/Pfizer, Johnson & Johnson’s, and Moderna have been widely distributed in the event of viral exposure.47 Data also shows that the vaccines are safe and beneficial to patients with Parkinson’s disease. While patients with PD may experience side effects such as temporary worsening of pre-existing Parkinson’s symptoms, vaccines are believed to not cause any long-term changes to PD signs and symptoms.47 To improve compliance to life post-COVID-19, it is essential that adapting to life with PD and viral infection incorporates daily hygiene measures. Symptoms such as altered speech, poor balance, restlessness, and fatigue may lead to poor adherence to daily hygiene. Through PD tailored home equipment, adjustments, and routine habit integration, patients may be able to manage the disease well during and post the COVID-19 pandemic.
Limitations
We had certain limitations. The first included the presence of confounder factors. These related to the finding that multiple studies of patients with elderly PD patients had other comorbidities, possibly leading to an overrepresentation of findings. Secondly, the duration of PD and the severity of the pre-pandemic symptoms possibly influenced the synthesis of clinical outcomes due to COVID-19 infection. Thirdly, pertinent information, including prior and current medications and compliance to treatment was lacking, leading to a lack of generalized recommendations for PD and COVID-19 clinical care. Albeit, our findings are pertinent for long-term PD patients with acquired COVID-19 infection.
Conclusion
COVID-19 has a peaked mortality rate in older age groups, compounded with comorbid conditions. The CNS is increasingly susceptible to SARS-CoV-2, deteriorating neurological findings, particularly in patients with PD. We find that motor dysfunction, delirium, dementia, severe spasms of arms and legs, and encephalopathy are pertinent clinical findings in PD patients and ought to be addressed in current care and practice. The susceptible groups of COVID-19 positive patients with a history of PD are elderly males with 10 years or longer duration of illness and other comorbid conditions. It is still unclear how SARS-CoV2 affects the long-term health of the nervous system in PD patients. It is recommended that healthcare practitioners address diminished neurological outcomes, changes in-hospital stay, and raised mortality in PD patients.
Acknowledgments
All authors are thankful to Jack Michel, MD and the Larkin Health System, South Miami, FL, USA in boosting scholarly research pursuits. We would like to acknowledge (1) Samia Jahan, (2) Madiha Zaidi, (3) Wanessa Matos, (4) Sana Javed, (5) Asma Mohammadi, and (6) Sujan poudel affiliated with Larkin Community Hospital, South Miami, FL, USA, for their early contributions. We would like to thank Akash Jaiswal (All India Institute of Medical Science New Delhi, India) and Shehar Bano (Fatima Jinnah Medical University, Lahore, Pakistan) for their input in the final review.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Zouina Sarfraz  https://orcid.org/0000-0002-5132-7455
https://orcid.org/0000-0002-5132-7455
Azza Sarfraz  https://orcid.org/0000-0001-8206-5745
https://orcid.org/0000-0001-8206-5745
Diana F. Sánchez Velazco  https://orcid.org/0000-0002-7529-4109
https://orcid.org/0000-0002-7529-4109
References
- 1.Wu YC, Chen CS, Chan YJ.The outbreak of COVID-19: an overview. J Chin Med Assoc. 2020;83:217-220. doi: 10.1097/jcma.0000000000000270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO. WHO Coronavirus Disease Dashboard. 2021. WHO.int. [Google Scholar]
- 3.Favalli EG, Ingegnoli F, De Lucia O, Cincinelli G, Cimaz R, Caporali R.COVID-19 infection and rheumatoid arthritis: faraway, so close! Autoimmun Rev. 2020;19:102523. doi: 10.1016/j.autrev.2020.102523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Umakanthan S, Sahu P, Ranade AV, et al. Origin, transmission, diagnosis and management of coronavirus disease 2019 (COVID-19). Postgrad Med J. 2020;96:753-758. doi: 10.1136/postgradmedj-2020-138234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Costela-Ruiz VJ, Illescas-Montes R, Puerta-Puerta JM, Ruiz C, Melguizo-Rodríguez L.SARS-CoV-2 infection: the role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev. 2020;54:62-75. doi: 10.1016/j.cytogfr.2020.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Putri C, Hariyanto TI, Hananto JE, Christian K, Situmeang RFV, Kurniawan A.Parkinson’s disease may worsen outcomes from coronavirus disease 2019 (COVID-19) pneumonia in hospitalized patients: a systematic review, meta-analysis, and meta-regression. Parkinsonism Relat Disord. 2021;87:155-161. doi: 10.1016/j.parkreldis.2021.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhidayasiri R, Virameteekul S, Kim JM, Pal PK, Chung SJ.COVID-19: an early review of its global impact and considerations for Parkinson’s disease patient care. J Mov Disord. 2020;13:105-114. doi: 10.14802/jmd.20042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg. 2021;88:105906. doi: 10.1016/j.ijsu.2021.105906 [DOI] [PubMed] [Google Scholar]
- 9.Sarfraz A, Sarfraz Z, Sanchez-Gonzalez M, et al. Randomized controlled trials of remdesivir in hospitalized coronavirus disease 2019 patients: a meta-analysis. Turk J Emerg Med. 2021;21:43. doi: 10.4103/2452-2473.309139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cilia R, Bonvegna S, Straccia G, et al. Effects of COVID-19 on Parkinson’s disease clinical features: a community-based case-control study. Mov Disord. 2020;35:1287-1292. doi: 10.1002/mds.28170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Del Prete E, Francesconi A, Palermo G, et al. Prevalence and impact of COVID-19 in Parkinson’s disease: evidence from a multi-center survey in Tuscany region. J Neurol. 2021;268:1179-1187. doi: 10.1007/s00415-020-10002-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhai H, Lv Y, Xu Y, et al. Characteristic of Parkinson’s disease with severe COVID-19: a study of 10 cases from Wuhan. J Neural Transm. 2021;128:37-48. doi: 10.1007/s00702-020-02283-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lo Monaco MR, Colacicco G, Marotta J, Bentivoglio AR.An educational case series of Parkinson’s disease during the COVID-19 pandemic. Rev Neurol. 2021;177:134-136. doi: 10.1016/j.neurol.2020.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fasano A, Elia AE, Dallocchio C, et al. Predictors of COVID-19 outcome in Parkinson’s disease. Parkinsonism Relat Disord. 2020;78:134-137. doi: 10.1016/j.parkreldis.2020.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Filatov A, Sharma P, Hindi F, Espinosa PS.Neurological complications of coronavirus disease (COVID-19): encephalopathy. Cureus. 2020;12:e7352. doi: 10.7759/cureus.7352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown EG, Chahine LM, Goldman SM, et al. The effect of the COVID-19 pandemic on people with Parkinson’s disease. J Parkinsons Dis. 2020;10:1365-1377. doi: 10.3233/JPD-202249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kobylecki C, Jones T, Lim CK, Miller C, Thomson AM.Phenomenology and outcomes of In-patients with Parkinson’s disease during the Coronavirus disease 2019 pandemic. Mov Disord. 2020;35:1295-1296. doi: 10.1002/mds.28205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lo Monaco MR, Bentivoglio AR, Fusco D, Calabresi P, Piano C.Subacute onset dystonia in a woman affected by Parkinson’s disease following SARS-COV-2 infection. Clin Park Relat Disord. 2021;4:100082. doi: 10.1016/j.prdoa.2020.100082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Antonini A, Leta V, Teo J, Chaudhuri KR.Outcome of Parkinson’s disease patients affected by COVID-19. Mov disord. 2020;35:905-908. doi: 10.1002/mds.28104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vignatelli L, Zenesini C, Belotti LMB, et al. Risk of hospitalization and death for COVID-19 in people with Parkinson’s disease or Parkinsonism. Mov Disord. 2021;36:1-10. doi: 10.1002/mds.28408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Artusi CA, Romagnolo A, Imbalzano G, et al. COVID-19 in Parkinson’s disease: report on prevalence and outcome. Parkinsonism Relat Disord. 2020;80:7-9. doi: 10.1016/j.parkreldis.2020.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Santos-García D, Oreiro M, Pérez P, et al. Impact of coronavirus disease 2019 pandemic on Parkinson’s disease: a cross-sectional survey of 568 Spanish patients. Mov Disord. 2020;35:1712-1716. doi: 10.1002/mds.28261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sainz-Amo R, Baena-álvarez B, Pareés I, et al. COVID-19 in Parkinson’s disease: what holds the key? J Neurol. 2021;268:2666-2670. doi: 10.1007/s00415-020-10272-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li J, Long X, Zhu C, et al. Management of a Parkinson’s disease patient with severe COVID-19 pneumonia. Ther Adv Chronic Dis. 2020;11:2040622320949423. doi: 10.1177/2040622320949423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Marcaida JA, Lahrmann J, Machado D, et al. Clinical characteristics of coronavirus disease 2019 (COVID-19) among patients at a movement disorders center. Geriatrics. 2020;5:54. doi: 10.3390/geriatrics5030054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prasad S, Holla VV, Neeraja K, et al. Parkinson’s disease and COVID-19: perceptions and implications in patients and caregivers. Mov Disord. 2020;35:912-914. doi: 10.1002/mds.28088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Helmich RC, Bloem BR.The impact of the COVID-19 pandemic on Parkinson’s disease: hidden sorrows and emerging opportunities. J Parkinsons Dis. 2020;10:351-354. doi: 10.3233/JPD-202038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stoessl AJ, Bhatia KP, Merello M.Movement disorders in the world of COVID-19. Mov Disord Clin Pract. 2020;7:355-356. doi: 10.1002/mdc3.12952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Papa SM, Brundin P, Fung VSC, et al. Impact of the COVID-19 pandemic on Parkinson’s disease and movement disorders. Mov Disord Clin Pract. 2020;7:357-360. doi: 10.1002/mdc3.12953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:683-690. doi: 10.1001/jamaneurol.2020.1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Esposito G, Pesce M, Seguella L, Sanseverino W, Lu J, Sarnelli G.Can the enteric nervous system be an alternative entrance door in SARS-CoV2 neuroinvasion? Brain Behav Immun. 2020;87:93-94. doi: 10.1016/j.bbi.2020.04.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li YC, Bai WZ, Hashikawa T.The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol. 2020;92:552-555. doi: 10.1002/jmv.25728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moriguchi T, Harii N, Goto J, et al. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Internet J Infect Dis. 2020;94:55-58. doi: 10.1016/j.ijid.2020.03.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poyiadji N, Shahin G, Noujaim D, Stone M, Patel S, Griffith B.COVID-19-associated acute hemorrhagic necrotizing encephalopathy: imaging features. Radiology. 2020;296:E119-E120. doi: 10.1148/radiol.2020201187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beyrouti R, Adams ME, Benjamin L, et al. Characteristics of ischaemic stroke associated with COVID-19. J Neurol Neurosurg Psychiatry. 2020;91:889-891. doi: 10.1136/jnnp-2020-323586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ebrille E, Lucciola MT, Amellone C, Ballocca F, Orlando F, Giammaria M.Syncope as the presenting symptom of COVID-19 infection. HeartRhythm Case Rep. 2020;6:363-366. doi: 10.1016/j.hrcr.2020.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lechien JR, Chiesa-Estomba CM, Place S, et al. Clinical and epidemiological characteristics of 1420 European patients with mild-to-moderate coronavirus disease 2019. J Intern Med. 2020;288:335-344. doi: 10.1111/joim.13089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zanin L, Saraceno G, Panciani PP, et al. SARS-CoV-2 can induce brain and spine demyelinating lesions. Acta Neurochir. 2020;162:1491-1494. doi: 10.1007/s00701-020-04374-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rogers JP, Chesney E, Oliver D, et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020;7:611-627. doi: 10.1016/S2215-0366(20)30203-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Toscano G, Palmerini F, Ravaglia S, et al. Guillain-Barré syndrome associated with SARS-CoV-2. New Engl J Med. 2020;382:2574-2576. doi: 10.1056/NEJMc2009191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rábano-Suárez P, Bermejo-Guerrero L, Méndez-Guerrero A, et al. Generalized myoclonus in COVID-19. Neurology. 2020;95:e767-e772. doi: 10.1212/WNL.0000000000009829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323:1061. doi: 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guo D, Han B, Lu Y, et al. Influence of the COVID-19 pandemic on quality of life of patients with Parkinson’s disease. Parkinsons Dis. 2020;2020:1216568. doi: 10.1155/2020/1216568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lukiw WJ, Pogue A, Hill JM.SARS-CoV-2 infectivity and neurological targets in the brain. Cell Mol Neurobiol. Published online August 25, 2020. doi: 10.1007/s10571-020-00947-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Costello F, Dalakas MC.Cranial neuropathies and COVID-19: neurotropism and autoimmunity. Neurology. 2020;95:195-196. doi: 10.1212/WNL.0000000000009921 [DOI] [PubMed] [Google Scholar]
- 46.Fearon C, Fasano A.Parkinson’s disease and the COVID-19 pandemic. J Parkinsons Dis. 2021;11(2):431-444. doi: 10.3233/JPD-202320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Parkinson’s Foundation. COVID-19 & Parkinson’s | Parkinson’s Foundation. 2021. Accessed July 3, 2021. https://www.parkinson.org/understanding-parkinsons/covid-19