Learning objectives.
By reading this article you should be able to:
-
•
Discuss theories accounting for the aetiology of fat embolism syndrome (FES).
-
•
Describe the presenting clinical features and investigations undertaken in patients with suspected FES.
-
•
Explain the management of FES, including the importance of early surgical fixation, and recognising the limited evidence for drug treatments.
Key points.
-
•
Patients with multiple long bone fractures are at highest risk of fat embolism syndrome (FES).
-
•
FES classically presents with respiratory, neurological, and dermatological signs.
-
•
Patients with FES should be referred for review and admission to critical care.
-
•
Early fixation of long bone fractures is thought to reduce the risk of FES.
-
•
The role of corticosteroids in the prophylaxis and treatment of FES is unclear.
Fat embolism (FE) is defined as the presence of fat globules within the circulation.1 Fat embolism is extremely common after trauma and occurs, to a variable extent, in the majority of patients suffering long bone or pelvic fractures.2 In the majority of patients, fat emboli appear to have minimal physiological effects and most patients display no signs or symptoms. Fat embolism syndrome (FES) is a rare but potentially fatal consequence of FE resulting in a spectrum of end organ damage. Fat embolism syndrome was originally described by the symptomatic triad of respiratory distress, neurological dysfunction, and petechial rash.1 Although first reported in humans by Zenker in 1862, the pathophysiology of FES remains incompletely understood. Despite an accumulating body of literature describing the occurrence of FES, there remains significant clinical uncertainty regarding its diagnosis, prevention, and management.
Aetiology and epidemiology
Fat embolism syndrome can be traumatic or non-traumatic in origin.2,3 Non-traumatic cases have been described during acute illness (pancreatitis, sickle cell disease, osteomyelitis, diabetes mellitus, fatty liver disease) or after iatrogenic intervention (extended corticosteroid therapy, liposuction, fat translocation during cosmetic augmentation), but the most common presentation of FES is after major traumatic injury.2,4 Although FE can occur after severe soft tissue trauma in the absence of bony fracture, FE, and therefore FES, are most common after blunt force trauma with long bone injury.4, 5 After femoral shaft fracture, 98% of patients have evidence of fat globules in their blood on admission, with the highest concentration in the venous circulation draining the fracture site.6 During medullary reaming for long bone fixation, 88% of patients have intraoperative echocardiographic evidence of circulating FE.7
The incidence of FES is difficult to determine with precision but is much lower than the incidence of FE.2,8 Retrospective cohort studies report an incidence of FES between 1 in 111 to 1 in 385 after isolated long bone injury, increasing to 1 in 78 in patients with multiple closed fractures.4,9 Prospective examination of individual cases can yield a much higher incidence of FES, with one study reporting FES in one in nine patients after long bone and pelvic injuries.10 Fat embolism syndrome occurs most frequently in young men, perhaps as a confounder of the greater incidence of high-velocity trauma sustained in this group. Fat embolism syndrome has only rarely been reported in children. In the absence of large-scale registry data, the mortality of FES is difficult to estimate accurately, but is reported to be between 7 and 36%.2,4,9,10 Nevertheless, even in cases with severe presenting features of FES, the condition can be self-limiting and such patients can make a complete neurological and cardiovascular recovery.
Clinical presentation
Fulminant FES, resulting from sudden large emboli and leading to immediate coma and cardiopulmonary arrest, rarely occurs.11 More typically, FES presents insidiously 24–72 h after injury, with a reported median presentation time of 48.5 h after long bone injury.2,4 In contrast to bone cement implantation syndrome, FES rarely presents during surgery. The classically described triad of concurrent respiratory distress, neurological dysfunction, and petechial rash does not occur in all patients. Hypoxia is the most common clinical finding (present in 96% of patients with FES), followed by mental status changes (59%) and petechiae (33%).9 The typical clinical progression of FES is shown in Fig. 1 and the documented sequelae of the syndrome are listed in Table 1.
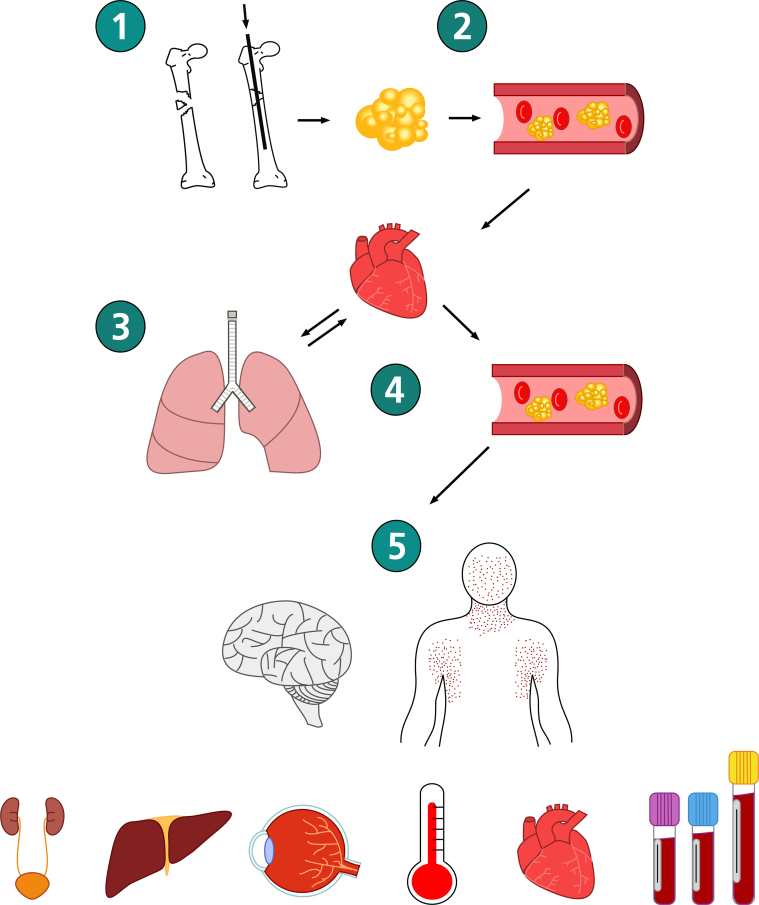
Fig 1.
Typical clinical progression of fat embolism syndrome. (1) Long bone fracture or intramedullary instrumentation causes (2) bone marrow fat to enter the venous circulation. (3) Fat embolises to the pulmonary capillary bed causing alveolar damage and dysfunction. (4) Fat may enter the systemic circulation via a patent foreman ovale, arteriovenous shunts, the pulmonary capillary bed, or all three. (5) Fat subsequently embolises and damages other organs including neurological, dermatological, renal, hepatic, ophthalmic, cardiovascular, and haematological systems (see Table 1 for organ-specific signs and symptoms).
Table 1.
Documented sequelae of fat embolism syndrome. ARDS, acute respiratory distress syndrome.
| Respiratory | Tachypnoea Hypoxaemia ARDS |
| Neurological | Confusion Seizures Altered level of consciousness Focal neurological deficits |
| Dermatological | Petechial rash |
| Systemic | Fever |
| Cardiovascular | Tachycardia Hypotension Arrhythmia Myocardial ischaemia Pulmonary hypertension Right-sided heart failure |
| Ophthalmic | Purtscher's retinopathy (cotton wool exudates, macular oedema and haemorrhage) |
| Renal | Oliguria Proteinuria Lipiduria Haematuria |
| Hepatic | Jaundice |
| Haematological | Anaemia Thrombocytopenia Coagulopathy Fat macroglobulinaemia |
Respiratory symptoms
In addition to occurring most commonly, respiratory symptoms often provide the earliest evidence of FES, because the pulmonary capillary bed is the first site where emboli are deposited.12 A biphasic respiratory response to FE has been described with an initial reflex tachypnea, caused by direct irritation of the lung parenchyma and increased alveolar dead space, followed by a second hypoxemic phase arising from impaired gas exchange from pulmonary hyperpermeability, oedema, and haemorrhage.12 This can progress to respiratory failure and acute respiratory distress syndrome (ARDS). It has been reported that 44% of patients with FES require mechanical ventilation.9
Neurological symptoms
A spectrum of neurological disturbance can occur in association with FES, including acute confusion, altered level of consciousness, tonic-clonic seizures, and focal neurological deficits.11 Cerebral oedema resulting from intracranial FE has been reported as a cause of death in patients after trauma. Neurological sequelae usually closely follow the onset of respiratory signs, but are non-specific and require evaluation in the context of other causes of altered consciousness after trauma, surgery, or both.
Dermatological manifestations
The petechial rash has a characteristic distribution typically on the non-dependent aspect of the axillae, neck, face, oral mucosa, and conjunctivae. This distribution may reflect the low density of fat and therefore its tendency to accumulate in non-dependant areas.12
Other documented sequelae
These are often non-specific and are listed in Table 1.
Diagnosis
No single diagnostic test is sufficiently sensitive or specific for FES to be useful in clinical practice. After presentation with one or more of the above signs or symptoms and after biochemical and radiological investigation, the diagnosis is typically made as one of exclusion in a patient with known risk factors.
Diagnostic criteria
Various criteria have been proposed to formalise the diagnosis of FES. The most well-known are Gurd's criteria (Table 2).1 Other scoring systems (e.g. those proposed by Lindeque or Schonfeld) have also been described for disease recognition and diagnosis.8 All the diagnostic criteria for FES may be criticised as they have been based on small sample sizes and lack validation.
Table 2.
Gurd's diagnostic criteria. The presence of one major and four minor criteria were proposed as sufficient for a diagnosis of fat embolism syndrome.
| Major criteria | Axillary or subconjunctival petechiae Hypoxaemia with bilateral radiographic changes Cerebral signs unrelated to head injury or any other condition |
| Minor criteria | Tachycardia Pyrexia Emboli present in the retina on fundoscopy Fat present in urine A sudden decrease in haematocrit or platelet concentrations Increasing erythrocyte sedimentation rate Fat globules present in the sputum |
Haematology and biochemistry investigations
Such investigations are non-specific but may assist in the diagnosis of FES and are a routine component of the clinical management of the patient with traumatic injuries who is deteriorating. Anaemia, thrombocytopenia, and increased concentrations of inflammatory markers are commonly reported. Increased serum lipase and free fatty acid concentrations (which are drawn in a serum separating tube and a fluoride oxalate tube, respectively) can lead to hypocalcaemia and hypoalbuminaemia.2 Fat globules may also be detected in blood, urine, sputum, and bronchoalveolar lavage samples. However, these are also not specific to FES and may be found in other clinical contexts such as sepsis.11 Arterial blood gas analysis most commonly shows hypoxaemia with an increased oxygen alveolar-arterial (A-a) gradient because of ventilation–perfusion mismatch from increased dead space. Such a finding is highly suspicious of FES in a patient at risk, although other causes of increased A-a gradient in a patient who is hypoxic (high Fio2, right-to-left shunt, alveolar capillary diffusion defect, increased oxygen extraction ratio) must be considered. An ECG is usually normal but may show signs of right heart strain in severe cases.11
Imaging
Plain film chest radiography may show bilateral non-specific patchy diffuse infiltrates in the lung fields. CT of the chest is the preferred imaging technique for examining the lungs in FES. Patchy ground-glass opacification associated with smooth interlobar septal thickening has been described as a ‘crazy paving’ pattern, but this appearance is shared with many other possible pathologies, such as bacterial pneumonia, ARDS, pulmonary oedema, and pulmonary haemorrhage.13 Variations in lung perfusion at the time of embolisation can result in distinct lobular sparing. Small centrilobular nodules in the periphery of the upper lobes may represent resolution of early mechanical obstruction from FE.13
In patients with cerebral evidence of suspected FES, MRI is the radiological modality of choice. Diffusion-weighted MRI detects a ‘starfield’ appearance of fat microembolism as early as 1 h after symptom onset.13 Approximately 4 h after symptoms, onset T2-weighted MRI will show multiple non-confluent hyperintense lesions scattered throughout the grey and white matter, with the number of lesions broadly correlating to the severity of neurological symptoms. The main differential diagnoses for this MRI appearance are vascular causes of thromboembolism and diffuse axonal injury. Although FE can be seen by transoesophageal ultrasound, transcranial Doppler, and peripheral venous duplex scanning, their presence does not necessarily correlate with the development of FES.7
Differential diagnoses
Because of the non-specific nature of the clinical presentation, it is vital to consider potential differential diagnoses including pulmonary emboli, bacterial pneumonia, sepsis, ARDS, and COVID-19 infection.
Pathogenesis
The pathogenesis of FES has not been fully elucidated. Three theories have been proposed and widely discussed in the literature (Fig. 2). The contributory role of each theory to the overall syndrome in individual patients is unclear.
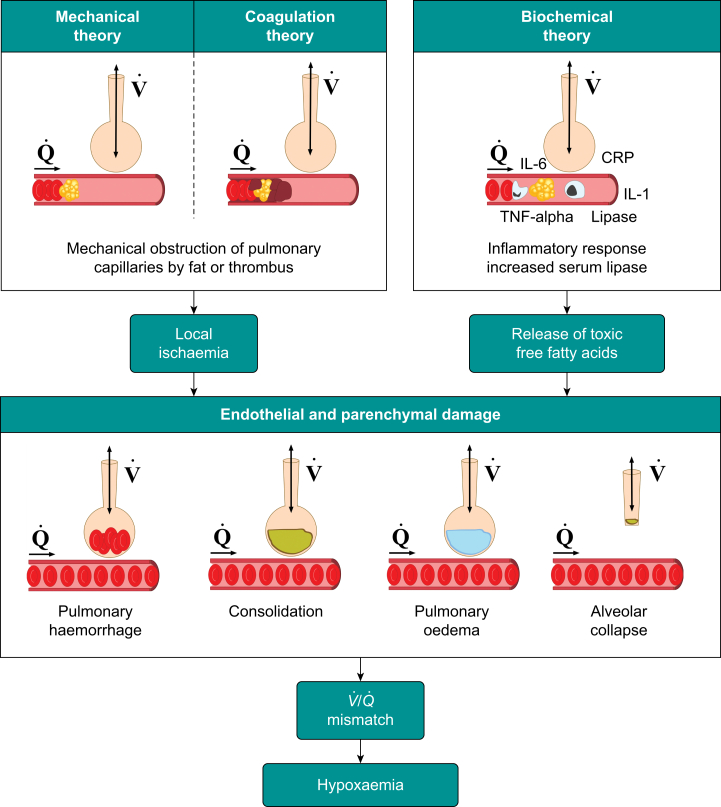
Fig 2.
Pathogenesis of fat embolism. Three theories have been proposed: mechanical, coagulation, and biochemical. Fat embolisation causes local parenchymal damage after vascular occlusion (by fat or thrombus), an exaggerated inflammatory response, or both. Subsequently, pulmonary haemorrhage, consolidation, pulmonary oedema and/or alveolar collapse result in a ventilation ()/perfusion () mismatch and hypoxaemia. CRP, C-reactive protein; IL-1, interleukin-1; IL-6, interleukin-6; TNF-alpha, tumour necrosis factor-alpha.
Mechanical theory
This theory was first proposed by Gauss in 1924.8 After traumatic disruption to the architecture of the medullary canal, increases in intramedullary pressure that exceed neighbouring venous pressure result in extrusion of marrow adipose tissue into the circulation. Fat embolises to the lung, but may reach the arterial circulation in the presence of a patent foreman ovale, an arteriovenous shunt, or simply by passing through the pulmonary alveolar capillary bed under the influence of a markedly raised pulmonary artery pressure. Macroemboli and microemboli cause mechanical obstruction within capillary beds, resulting in local ischaemia and organ dysfunction.2,12 However, the mechanical theory alone does not explain the temporal separation of FE events and subsequent FES, which typically manifests 24–72 h after injury.
Biochemical theory
Free fatty acids may play a significant role in the pathogenesis of FES and explain, in part, its delayed presentation.2 Bone marrow contains neutral fat which does not cause direct endothelial damage. However, after trauma, serum lipase levels increase. Lipase is believed to catalyse the breakdown of embolised fat globules into free fatty acids, which are toxic to endothelial cells. Endothelial damage provokes an inflammatory response, exacerbating endothelial dysfunction and promotes agglutination of microemboli into larger fat globules, increasing the risk of mechanical obstruction.2
The role of inflammation in a host's response to trauma and more specifically FES has gained traction in recent years.12,14,15 An initial traumatic event stimulates a proinflammatory state involving a network of inflammatory mediators, combined with an innate cellular response. The aim of this response is to promote repair and prevent secondary injury such as infection. However, the ‘second hit’ theory postulates that some patients may then mount an exaggerated response to any subsequent traumatic insults, such as fat emboli or surgery. An excessive response may then cause additional endothelial cell injury and worsening end organ dysfunction. The strength of this response is thought to correlate with the morbidity and mortality from multiorgan failure seen in some patients after major trauma.14 Increased concentrations of proinflammatory markers such as interleukin-6 have been suggested as indicating an increased risk of developing FES after long bone or pelvic trauma.15 Whether or not a patient develops FES after FE may depend on the strength of the inflammatory response provoked.
Coagulation theory
Circulating bone marrow fat triggers an inflammatory response. Combined with the relative hypovolaemia and endothelial damage observed after trauma, this inflammation causes a prothrombotic state. Activation of the clotting cascade potentially leads to an increase in the size of FE leading to a greater degree of physical obstruction within vascular beds. This may explain the observed thrombocytopenia and disseminated intravascular coagulation observed in a minority of FES cases.2,12
Management
Definitive strategies for the management of FES remain elusive. Early surgical fixation of high-risk fractures should reduce the likelihood of FES, although definitive data to support this supposition are lacking. Patients with suspected FES often warrant admission to a critical care environment, where they benefit from detailed physiological observation and early institution of invasive treatment should deterioration occur. Clinical teams most involved in the management of high-risk patients, particularly trauma and orthopaedic services, must be familiar with the condition and its differential diagnosis to ensure prompt clinical management and liaison with local critical care services.
Hypoxaemia and respiratory failure
Pulse oximetry correlates well with unrecognised hypoxaemia and patients developing pulmonary complications of FES may be identified using standardised early warning systems such as the NHS National Early Warning Score 2 (NEWS-2).16 Hypoxaemia should initially be managed with supplemental oxygen therapy, anticipating that non-invasive or invasive ventilatory support is required in 10–44% of patients.2,9 No specific ventilatory strategies have been developed for FES beyond the lung-protective ventilation techniques used in a wide range of patients with or without ARDS.17 Advanced ventilation strategies including prone positioning, airway pressure release ventilation, and extracorporeal membrane oxygenation have been described in the management of FES.18 Such strategies may be difficult to implement in patients who have sustained multiple traumatic injuries, and concurrent cerebral monitoring should be considered if prone positioning or permissive hypercapnia are undertaken.
Neurological management and protection
There are no specific guidelines for the management of cerebral fat emboli. General principles for the management of patients with brain injury should be followed, aiming to reduce secondary brain injury. Seizure prophylaxis may be considered. In cases of severe neurological impairment, regular neurological surveillance (including consideration of ICP monitoring) should be instituted to identify cerebral oedema and optimise cerebral perfusion pressure.18 Cerebral complications are usually managed conservatively, often with complete neurological recovery. However, cases requiring emergency decompressive neurosurgery are described.19
Cardiovascular
Patients should be adequately resuscitated on initial presentation after trauma. Some authors have advocated the use of i.v. albumin in FES because, in animal models, albumin lowers the circulating concentration of free fatty acids and potentially attenuates their toxic effect.11,18 The increased mortality observed in patients suffering traumatic brain injuries when resuscitated with albumin20 means its role in the management of FES remains controversial. Cardiovascular instability in FES is commonly managed with a combination of resuscitation with i.v. fluids, pulmonary vasodilators, peripheral vasoconstrictors, and inotropic drugs.18,21 The use of extracorporeal circulatory support has also been reported.
Pharmacological management
Many drug treatments have been studied over the last 50 yrs, including heparin, corticosteroids, hypertonic glucose, aspirin, N-acetylcysteine, and aliskiren.2,11,22 None have found universal acceptance in the specific prophylaxis or treatment of patients with FES. Producing definitive clinical evidence of effectiveness for any single intervention in FES is extremely challenging, given the relatively low incidence of the clinically manifested condition. The agents most commonly advocated are anticoagulants and corticosteroids.
Anticoagulants
Heparin was identified as a potential therapy for patients with FES >60 yrs ago.2 Heparin increases lipase enzyme activity, clearing lipaemic plasma, but at the potential cost of increased free fatty acid concentrations, which could accelerate local tissue damage. A lack of mortality benefit in animal models of FES, together with the challenge of anticoagulating patients at risk of systemic haemorrhage after major trauma, means ‘treatment dose’ heparin therapy is not commonly used. For the same reason, aspirin, although supported by small-scale clinical trials, is not routinely recommended in FES, but is often advocated in the event of embolic phenomena causing a neurological deficit.2
Corticosteroids
Corticosteroids are the most extensively studied group of agents for the prophylaxis of FES.23 With respect to FES, reaching evidence-based conclusions on the efficacy of prophylactic steroids is hampered by non-standardised outcome reporting, methodological heterogeneity, and the overall low incidence of the condition. The long recruitment periods necessary to obtain adequate samples of patients with FES, mean study data risks being outdated by the time of publication because wider clinical practice (e.g. the increasingly early fixation of long bone fractures) has independently evolved since the trials were conceived. A meta-analysis in 2009 of seven RCTs pooled data from 389 patients and reported a relative risk reduction of 78% (95% confidence interval 43–92%) using i.v. steroids as prophylaxis against FES. This equates to a number needed to treat of eight patients for the prevention of one episode of FES.23 Despite this apparently advantageous effect, the authors cautioned against the routine use of steroids to prevent FES, pointing to the significant potential negative effects of such therapy including secondary infection, delayed wound healing, and osteonecrosis. Such concerns also serve to discourage most clinicians from using steroids for the treatment of established FES, although steroids have had some success in other critical pulmonary pathologies. Low-dose steroid regimes have shown some benefit on cardiopulmonary recovery in patients with severe ARDS and COVID-19 pneumonitis.24 Inhaled steroids have also been examined for prophylactic effect in FES. Theoretically, inhalation delivery selectively targets affected lung parenchyma, thereby limiting systemic complications, but a 2015 study that randomised patients with femoral shaft fractures to either the inhaled ciclesonide or control, reported no significant between-group differences in subsequent occurrence of FES at 72 h.25
Surgical management
Circulatory discharge of FE from a long bone fracture site is likely to be highest during three distinct phases of injury: at the time of initial trauma, during subsequent closed manipulation, splinting, or both, and finally during definitive surgical fixation. Minimising physical movement at the fracture site during and between these episodes, and expediting definitive fixation as early as practical, may reduce FE and therefore the risk of subsequent FES.
Definitive long bone fracture fixation
External fixation and internal plate fixation cause less non-FES lung injury than intramedullary nailing, which causes marked increases in intramedullary canal pressure and extrusion of medullary fat to the circulation. Minimising this pressure should be one of the aims of definitive surgical treatment. The contributory role of reaming compared with non-reaming, hollow or solid nails, cavity venting compared with non-venting in the quantified prevention of FE or FES during surgery remains unclear.
Future research
Recent studies have focused on the mechanisms that underlie FES, including the role of the renin angiotensin system (RAS), and RAS inhibition has been shown to reduce lung parenchymal pathology in a rodent model of FE-induced lung injury.22 A more detailed understanding of the pathogenesis of this rare syndrome will facilitate targeted strategies for prevention and treatment. Further prospective high-quality clinical trials are required to clarify the role of steroids in the clinical management of FES.
Declaration of interests
The authors declare that they have no conflicts of interest.
Biographies
David Hewson BSc (Hons) PGCert FHEA FRCA PhD is a consultant anaesthetist at Queen's Medical Centre, Nottingham University Hospitals NHS Trust, one of the largest major trauma centres in the UK. He is an honorary clinical associate professor in the school of medicine at the University of Nottingham. He undertakes regular clinical sessions in trauma and orthopaedic surgery and is the morbidity and mortality lead for his department.
Delme Luff BSc (Hons) FRCA is a specialty registrar in anaesthesia at Nottingham University Hospitals NHS Trust. He has recently completed a 12-month advanced fellowship in regional anaesthesia including regular sessions in complex orthopaedic surgery and major trauma.
Matrix codes: 1A01, 2A06, 3A10
MCQs
The associated MCQs (to support CME/CPD activity) will be accessible at www.bjaed.org/cme/home by subscribers to BJA Education.
References
- 1.Gurd A.R. Fat embolism: an aid to diagnosis. J Bone Jt Surg Br. 1970;52:732–737. [PubMed] [Google Scholar]
- 2.Mellor A., Soni N. Fat embolism. Anaesthesia. 2001;56:145–154. doi: 10.1046/j.1365-2044.2001.01724.x. [DOI] [PubMed] [Google Scholar]
- 3.Meng Y., Zhang M., Ling H. Nontraumatic multiple-organ fat embolism: an autopsy case and review of literature. Am J Forensic Med Pathol. 2020;41:131–134. doi: 10.1097/PAF.0000000000000544. [DOI] [PubMed] [Google Scholar]
- 4.Stein P.D., Yaekoub A.Y., Matta F., Kleerekoper M. Fat embolism syndrome. Am J Med Sci. 2008;336:472–477. doi: 10.1097/MAJ.0b013e318172f5d2. [DOI] [PubMed] [Google Scholar]
- 5.Hiss J., Kahana T., Kugel C. Beaten to death: why do they die? J Trauma. 1996;40:27–30. doi: 10.1097/00005373-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Allardyce D.B., Meek R.N., Woodruff B., Cassim M.M., Ellis D. Increasing our knowledge of the pathogenesis of fat embolism: a prospective study of 43 patients with fractured femoral shafts. J Trauma. 1974;14:955–962. [PubMed] [Google Scholar]
- 7.Christie J., Robinson C.M., Pell A.C.H., McBirnie J., Burnett R. Transcardiac echocardiography during invasive intramedullary procedures. J Bone Jt Surg Br. 1995;77:450–455. [PubMed] [Google Scholar]
- 8.Talbot M., Schemitsch E.H. Fat embolism syndrome: history, definition, epidemiology. Injury. 2006;37:S3. doi: 10.1016/j.injury.2006.08.035. [DOI] [PubMed] [Google Scholar]
- 9.Bulger E.M., Smith D.G., Maier R.V., Jurkovich G.J. Fat embolism syndrome: a 10-year review. Arch Surg. 1997;132:435–439. doi: 10.1001/archsurg.1997.01430280109019. [DOI] [PubMed] [Google Scholar]
- 10.Fabian T.C., Hoots A.V., Stanford D.S., Patterson C.R., Mangiante E.C. Fat embolism syndrome: prospective evaluation in 92 fracture patients. Crit Care Med. 1990;18:42–46. [PubMed] [Google Scholar]
- 11.Tzioupis C.C., Giannoudis P.V. Fat embolism syndrome: what have we learned over the years? Trauma. 2011;13:259–281. [Google Scholar]
- 12.Husebye E.E., Lyberg T., Røise O. Bone marrow fat in the circulation: clinical entities and pathophysiological mechanisms. Injury. 2006;37:S8. doi: 10.1016/j.injury.2006.08.036. [DOI] [PubMed] [Google Scholar]
- 13.Newbigin K., Souza C.A., Torres C. Fat embolism syndrome: state-of-the-art review focused on pulmonary imaging findings. Respir Med. 2016;113:93–100. doi: 10.1016/j.rmed.2016.01.018. [DOI] [PubMed] [Google Scholar]
- 14.Giannoudis P.V. Current concepts of the inflammatory response after major trauma: an update. Injury. 2003;34:397–404. doi: 10.1016/s0020-1383(02)00416-3. [DOI] [PubMed] [Google Scholar]
- 15.Prakash S., Sen R.K., Tripathy S.K., Sen I.M., Sharma R.R., Sharma S. Role of interleukin-6 as an early marker of fat embolism syndrome: a clinical study trauma. Clin Orthop Relat Res. 2013;471:2340–2346. doi: 10.1007/s11999-013-2869-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Early Warning Score (NEWS) 2. Royal College of Physicians London. Available from https://www.rcplondon.ac.uk/projects/outputs/national-early-warning-score-news-2 (18 December 2020).
- 17.Serpa Neto A., Simonis F.D., Barbas C.S.V. Association between tidal volume size, duration of ventilation, and sedation needs in patients without acute respiratory distress syndrome: an individual patient data meta-analysis. Intensive Care Med. 2014;40:950–957. doi: 10.1007/s00134-014-3318-4. [DOI] [PubMed] [Google Scholar]
- 18.Habashi N.M., Andrews P.L., Scalea T.M. Therapeutic aspects of fat embolism syndrome. Injury. 2006;37:S68–S73. doi: 10.1016/j.injury.2006.08.042. [DOI] [PubMed] [Google Scholar]
- 19.Couturier C., Dupont G., Vassal F., Boutet C., Morel J. Case report effectiveness of decompressive hemicraniectomy to treat a life-threatening cerebral fat embolism. Case Rep Crit Care. 2019;2019:2708734. doi: 10.1155/2019/2708734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Myburgh J., James Cooper D., Finfer S. Saline or albumin for fluid resuscitation in patients with traumatic brain injury. N Engl J Med. 2007;357:874–884. doi: 10.1056/NEJMoa067514. [DOI] [PubMed] [Google Scholar]
- 21.Konstam M.A., Kiernan M.S., Bernstein D. Evaluation and management of right-sided heart failure: a scientific statement from the American Heart Association. Circulation. 2018;137:578–622. doi: 10.1161/CIR.0000000000000560. [DOI] [PubMed] [Google Scholar]
- 22.Fletcher A.N., Molteni A., Ponnapureddy R., Patel C., Pluym M., Poisner A.M. The renin inhibitor aliskiren protects rat lungs from the histopathologic effects of fat embolism. J Trauma Acute Care Surg. 2017;82:338–344. doi: 10.1097/TA.0000000000001278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bederman S.S., Bhandari M., McKee M.D., Schemitsch E.H. Do corticosteroids reduce the risk of fat embolism syndrome in patients with long-bone fractures? A meta-analysis. Can J Surg. 2009;52:386–393. [PMC free article] [PubMed] [Google Scholar]
- 24.Steinberg K.P., Hudson L.D., Goodman R.B. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med. 2006;354:1671–1684. doi: 10.1056/NEJMoa051693. [DOI] [PubMed] [Google Scholar]
- 25.Agarwal A.K., Sen R., Tripathy S.K., Aggarwal S., Nirmalraj G., Gupta D. Is there any role of inhalational corticosteroids in the prophylaxis of post-traumatic fat embolism syndrome? Cureus. 2015;7:e332. doi: 10.7759/cureus.332. [DOI] [PMC free article] [PubMed] [Google Scholar]