Abstract
Noncommunicable diseases, the leading cause of mortality around the world, are responsible for approximately 75% of premature adult deaths (ages 30–69). To tackle this issue, a healthy diet based on functional foods, including cocoa and its derivatives, has been increasingly promoted. The polyphenols present in cocoa have been of interest due to their antioxidant potential and their possible protective role in the context of noncommunicable diseases, such as diabetes and cardiovascular conditions. However, during cocoa postharvest and industrialization, the concentration of these bioactive compounds is reduced, possibly affecting their health-promoting properties. Therefore, this paper reviews in the literature in this field to find the total polyphenol content in cocoa during the postharvest and industrialization processes in order to define concentration ranges as a reference point for future research. In addition, it discusses in vitro and in vivo studies into the biological antioxidant potential of cocoa and its derivatives. This review covers publications in indexed databases from 2010 to 2020, their data were processed and presented here using box plots. As a result, we identified the concentration ranges of polyphenols depending on the type of matrix, treatment and country, as well as their relationship with the main bioactive compounds present in cocoa that are associated with their possible antioxidant biological potential and health-related benefits.
Keywords: Polyphenols, Theobroma cacao L., Noncommunicable diseases, Biological antioxidant potential, Chocolate, Cocoa postharvest, Cocoa industrialization
polyphenols; Theobroma cacao L.; noncommunicable diseases; biological antioxidant potential; chocolate; cocoa postharvest, cocoa industrialization
1. Introduction
According to the World Health Organization (WHO), noncommunicable diseases (NCDs), the leading cause of mortality worldwide, were responsible for approximately 41 million deaths in 2016. Importantly, the most common NCDs behind these deaths were cardiovascular disease, cancer, chronic respiratory diseases and diabetes [1].
Therefore, new strategies should be designed and implemented in order to prevent, suppress, or mitigate the progression of NCDs. In this context, plant-based compounds have been presented as an attractive strategy to fight some of the mechanisms involved in disease onset and development because they contain a large number of phytochemicals and micronutrients, which have demonstrated different biological activities, such as oxidative stress and inflammatory response regulation [2, 3].
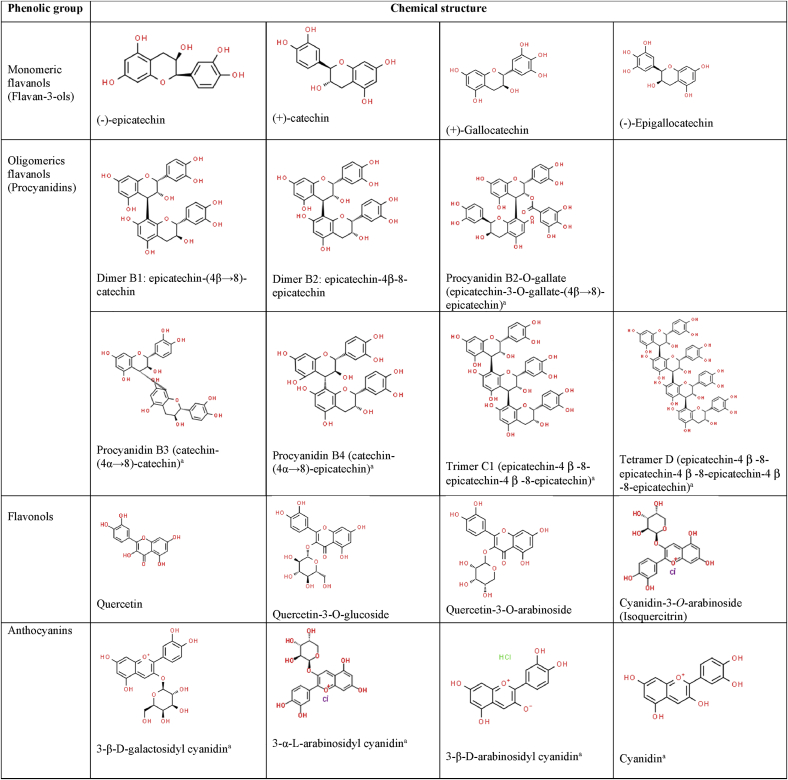
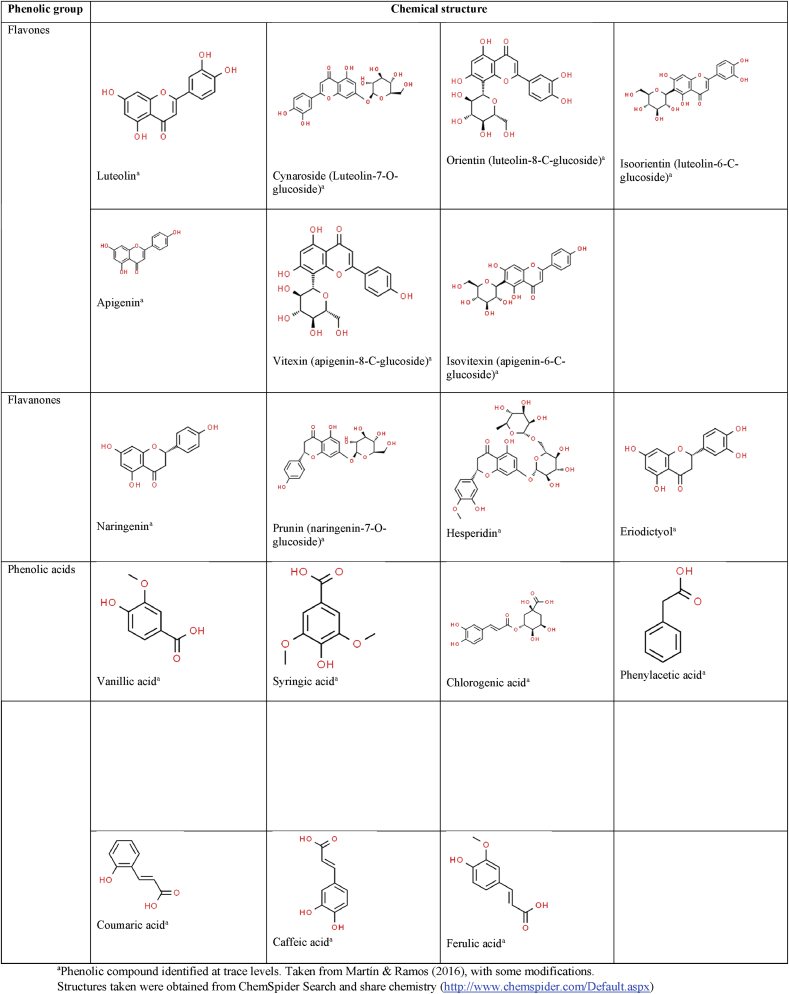
A good example of a plant-based material with exceptional potential for antioxidant capacity intervention is Theobroma cacao L. due to the large group of phenolic compounds reported in cocoa. Although most studies agree that they are flavanols (mainly epicatechin and catechin), as well as flavonols (such as quercetin and some of its derivates), the other compounds in the polyphenols group (such as anthocyanins, flavanones, flavones and phenolic acids) are reported in trace concentrations or not reported in most papers [4, 5, 6, 7]. Figure 1 shows the chemical structure of the main phenolic compounds present in cocoa and its derivatives reported in previous studies.
Figure 1.
Chemical structure of the main phenolic compounds present in cocoa and derivatives reported in previous studies [4, 8].
Nonetheless, polyphenol content is greatly affected by factors such as cocoa variety, postharvest treatments, industrialization steps and different types of cocoa derivatives (chocolate, powder and cocoa liquor), among others. Varieties such as Forastero are associated with a greater concentration of polyphenols compared to the Criollo variety, which presents a low content of anthocyanins. Further, some studies [9, 10] have reported that less catechins are found in Criollo than in Upper Amazon Forastero, Lower Amazon Forastero, Nacional and Trinitario varieties.
Postharvest treatments such as fermentation (where pH levels and temperature are modified) affect polyphenols’ stability, while also promoting α-glucosidase activity. Glucosidase acts on the glycosidic bond present in flavonoids, hydrolyzing them to aglycones and producing sugars (such as arabinose and galactose), which are reactions that take place primarily during embryo death [11, 12]. Further, (-)-epicatechin has been reported to be one of the catechins most affected, after pre-conditioning and fermentation, by oxidation and polymerization reactions [13]. Simple flavonols suffer from degradation during drying processes due to the action of polyphenol oxidase and migration during water removal [14, 15].
Industrialization steps such as alkalinization of the nibs, liquor or cocoa powder might promote polyphenol oxidization, resulting in a lower flavanol content, where (-)-epicatechin, (+)-catechin, and catechin dimers are the most sensitive to degradation [10]. On the other hand, it has been reported that quercetin is the flavonol that undergoes the greatest decrease during alkanization. Roasting is also a critical step that affects polyphenols, specially flavonoids and catechins; its high temperatures for long periods translate into a bitter taste that results from the formation of insoluble compounds between flavonoids and proteins, peptides, polysaccharides and products of the Maillard reaction [16, 17, 18]. Further, roasting also promotes the epimerization of (-)-epicatechin to (+)-catechin [19].
Although the factors mentioned above decrease the polyphenol content in cocoa and its derivatives, other studies have demonstrated that, even under these circumstances, with a low content of total polyphenols, they have a protective effect due to their functional property of antioxidant capacity [20, 21, 22]. In addition, food synergy may be responsible for the biological potential of cocoa derivatives, despite being subjected to chemical and physical transformations that cause said decrease in polyphenols [23].
However, demonstrating the effectiveness of the antioxidant capacity of some foods or raw materials (such as cocoa and its derivates) on the health of those who consume them requires more advanced studies that go beyond reporting the total polyphenol content in the food. This is because one of the biggest limitations of plant-based bioactive compounds (e.g., polyphenols) to produce health-related benefits is the complex mechanism that regulates their bioaccessibility and bioavailability [24, 25]. Evidence has shown that the interaction of cocoa and its derivatives with other xenobiotics and the food matrix, host-related factors (such as genetic polymorphisms in xenobiotic-metabolizing enzymes), and the interplay with the intestinal microbiota are directly involved in their bioaccessibility and bioavailability. Likewise, it has been determined that polyphenols present low bioaccessibility and bioavailability as approximately 5–10% are absorbed in the small intestine and the remaining portion is subjected to biotransformation by gut microbiota, producing bioactive metabolites [25].
Therefore, this literature review covers the last decade of findings about the in vitro and in vivo effects of polyphenols found in cocoa and its derivatives in the context of their biological antioxidant potential. Further, we report the range of concentration of total polyphenols and antioxidant capacity in postharvest and industrialization steps, which serves as a framework for future studies and the development of cocoa and its derivatives with functional traits.
2. Methods
This literature review was conducted using the most comprehensive databases in the fields of biomedicine and chemistry: Science Direct, Springer, Taylor & Francis, Scopus and MDPI. We restricted the search to articles published between 2010 and 2020 and used the following search equation to find studies that included these terms in the title and the abstract: cocoa AND antioxidant AND health AND polyphenols.
These criteria were selected to find studies about different concentrations of polyphenols in cocoa and its derivatives caused by postharvest and transformation processes, and how polyphenols influence health. This search resulted in a list of research papers concerning the antioxidant capacity of polyphenols, both in vitro and in vivo. The papers on combinations of different extracts and purified compounds from cocoa, as well as mixtures with other plants, were outside the scope of this review.
Subsequently, we conducted a descriptive analysis to define the ranges of concentration of polyphenols after each postharvest and transformation stage using box plots and performed a data analysis in R software version 2.9.2 (2009-08-24).
3. Results and discussion
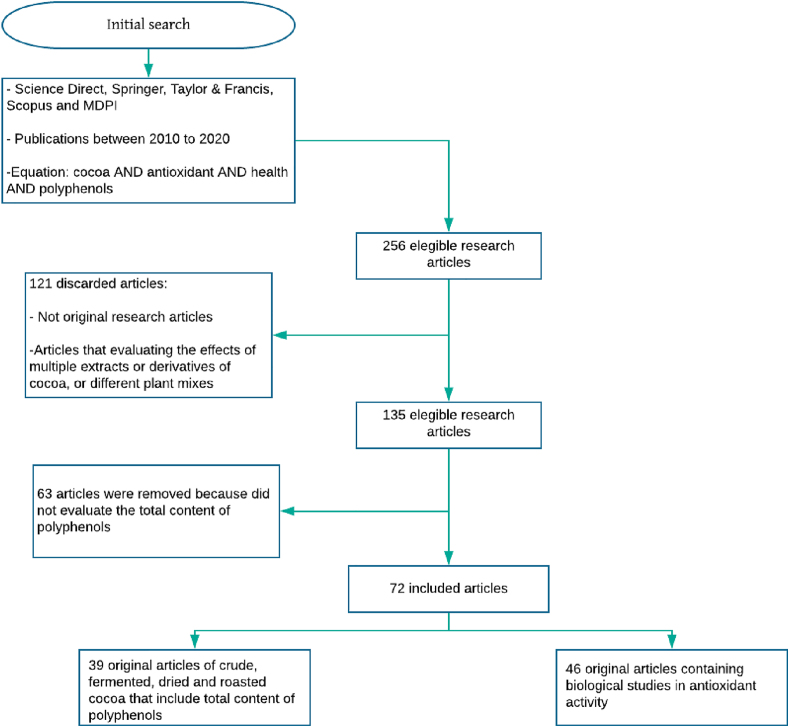
The search equation was adjusted to relate the traceability of the concentration of phenolic compounds in cocoa and its derivatives during post-harvest and pre-industrialization with their antioxidant potential. This resulted in a preliminary list of articles reporting total polyphenols, epidemiological studies, and in vitro and in vivo analyses that have evaluated the effectiveness of their antioxidant capacity. A total of 256 research articles matched the search terms we used, but 121 of them were discarded because they were not original research articles or they evaluated the effects of multiple extracts or derivatives of cocoa or different plant mixtures. A group of 72 research articles met all the criteria applied here, as shown in Figure 2.
Figure 2.
Filters used in the search equation to select the articles for the literature review.
Table 1 summarizes the information we extracted from the studies, such as country, matrix, type of postharvest treatment and transformation process. It also reports the polyphenol concentrations, classified into subcategories, and their antioxidant capacity.
Table 1.
Articles selected for this literature review and the data they report about the total content of polyphenols, concentrations of protocatechuic acid and procyanidins, and antioxidant capacity.
| Country | Matrix | Total Polyphenols Content (mg GAE/g) | Protocatechuic acid | Procyanidins | Antioxidant capacity | Ref. |
|---|---|---|---|---|---|---|
| Malaysia | The mixed cocoa clones (PBM 123, BR 25, MCBC 1 and MCBC 8) | 21.8 | 1.68 mg/g catechin | NR | NR | [26] |
| Costa Rica Madagascar | Bean and husk (Two varieties of cocoa) | 71.1 | NR | NR | NR | [27] |
| Venezuela | Seed without mucilage | 62.0 | (+)-catechin 0.96 ± 0.08 mg/g (-)-epicatechin 33.4 ± 0.95 mg/g |
NR | NR | [5] |
| Seed with mucilage | 80.0 | protocathetic acid: 2.85 ± 0.1 mg/g (+)-catechin 1.21 ± 0.06 mg/g (-)-epicatechin 32.15 ± 2.54 mg/g |
NR | NR | ||
| Malaysia | Fresh unfermented cocoa beans - Fermented cocoa beans | 181.7 | NR | NR | NR | [28] |
| Mexico | Fruits of 45 cacao accessions and one Pataste (T. bicolor) accession and Criollo variety cacao crude FHSA03 | 6.85 | NR | NR | 10.98 mmol TEAC/g db) | [29] |
| Nicaragua | Cacao beans of the same group (Trinitario) nonfermented | 115 | NR | NR | 709 ± 17 μM Trolox | [30] |
| Spain | Raw material: cocoa beans (cocoa butter) | 0.0332 | NR | NR | 5.82 ± 0.4 AA, mg Trolox/100 g dm DPPH |
[31] |
| Mexico | Raw material: cocoa beans (husk and cotyledon) | 135.92 | 132,88 ± 0,245 mg/g epicatechin, 4,62 ± 0,047 mg/g catechin | NR | 0,0124 ± 0,0017 DPPH mg/mL | [32] |
| Croatia | Raw materials: cocoa nibs | 3.1318 | 56.82 ± 1.93 mg GAE/L | 6.62 ± 0.20 proanthocyanidins mg CyE/L | 9 mM Trolox ABTS | [33] |
| Raw materials: cocoa beans | 0.7364 | 172.27 ± 7.78 mg GAE/L | 58.73 ± 1.96 proanthocyanidins mg CyE/L | 9 mM Trolox ABTS | ||
| Madagascar | Pure cocoa | 32.5 | NR | NR | 0.30 ± 0.10 TE (μmol/g) DPPH | [34] |
| Colombia - Bajo Cauca | Mix of cocoa beans (FLE-2, FEC-2, ICS-1, CCN-51) | 168.4 | 2.5 ± 0.1 epicatechin (mg/g) 0.23 ± 0.1 catechin (mg/g) |
122460.80 ± 13945.60 ORAC (TE/100g) | [35] | |
| Colombia - Uraba | Mix of cocoa beans (FLE-2, FEC-2, ICS-1, CCN-51) | 598.5 | 0.7 ± 0.03 epicatechin (% mg/g) | 299486.33 ± 19243.35 ORAC (TE/100g) | ||
| Colombia - Magdalena Medio | Mix of cocoa beans (FLE-2, FEC-2, ICS-1, CCN-51) | 311.6 | 0.8 ± 0.02 epicatechin (mg/g) | NR | 160941.87 ± 675.59 ORAC (TE/100g) | |
| Malaysia | The mixed cocoa clones (PBM 123, BR 25, MCBC 1 and MCBC 8) fermented with starter culture and dried. | 69.81 | 6.33 mg/g catechin | NR | NR | [26] |
| Malaysia | Fermented cocoa beans were obtained from Malaysian Cocoa Board |
86.3 | NR | NR | NR | [28] |
| Malaysia | Varieties of clones - fermented cocoa beans | 3.4 | NR | NR | NR | [36] |
| Malaysia | Varieties of clones - fermented cocoa beans. | 67.1 | NR | NR | NR | |
| Malaysia | Fresh and fermented cocoa beans | 69.9 | NR | NR | 97.9 DPPH inhibition (%) | [37] |
| Malaysia | Fresh and fermented cocoa beans | 65.4 | NR | NR | 65.2 DPPH inhibition (%) | |
| Nicaragua | Cacao beans of the same group (Trinitario) fermented | 43 | NR | NR | 124 ± 4 μM Trolox | [30] |
| Nicaragua | Partially Fermented - cacao beans of the same group (Trinitario) poorly fermented | 56 | NR | NR | 155 ± 8 μM Trolox | |
| Colombia - Bajo Cauca | Mix of cocoa beans (FLE-2, FEC-2, ICS-1, CCN-51) | 139.7 | 0.6 ± 0.03 (mg/g) epicatechin | NR | 95308.53 ORAC (TE/100g) | [12] |
| Colombia - Uraba | Mix of cocoa beans (FLE-2, FEC-2, ICS-1, CCN-51) | 162.9 | 0.6 ± 0.09 (mg/g) epicatechin | NR | 40616.43 ORAC (TE/100g) | |
| Colombia - Magdalena Medio | Mix of cocoa beans (FLE-2, FEC-2, ICS-1, CCN-51) | 226.6 | 0.5 ± 0.0 (mg/g) epicatechin | NR | 129193.47 ORAC (TE/100g) | |
| Ecuador | Fermented and dried cocoa - Unroasted cocoa beans | 25.42 | NR | NR | NR | [38] |
| Colombia | 37.66 | NR | NR | NR | ||
| Ghana | 25.21 | NR | NR | NR | ||
| Dominican Republic | 19.65 | NR | NR | NR | ||
| Venezuela | 10.34 | NR | NR | NR | ||
| Peru | 27.78 | NR | NR | NR | ||
| Mbanga, Littoral, Cameroon | Fresh cocoa beans fermented and dried control (DCB) | 84 | NR | NR | NR | [39] |
| Pahang, Malaysia | Mixed clone varieties of unfermented and dried cocoa beans by adsorption drying | 104 | NR | NR | NR | [40] |
| Pahang, Malaysia | Mixed clone varieties of unfermented and dried cocoa beans by vacuum oven | 112 | NR | NR | NR | |
| Pahang, Malaysia | Mixed clone varieties of unfermented and dried cocoa beans by freeze drying | 126 | NR | NR | NR | |
| Pahang, Malaysia | Mixed clone varieties of unfermented and dried cocoa beans by oven | 94 | NR | NR | NR | |
| Malaysia | Fermented and dried cocoa beans were obtained from Malaysian Cocoa Board |
15 | NR | NR | NR | [28] |
| Ecuador | Fermented and dried cocoa - variety Arriba Nacional | 20 | Epicatechin 1 mg/g, catechin 0.055 mg/g | NR | 58 mg TE/g FRAP | [41] |
| Ecuador | Mature pods (the fruit of Theobroma cacao L.) from the variety Arriba Nacional | 60 | Epicatechin 4.2 mg/g, catechin 0.08 mg/g | NR | 30 mg TE/g FRAP | |
| Colombia | Dried cocoa beans | 70.1 | 3.562 ± 0.016 epicatechin (mg/g) 1.297 ± 0.031 catechin (mg/g) | NR | 61812 ± 5042 (μmol TE/100 g). ORAC | [42] |
| Trinidad and Tobago | Fermented, dried beans | 0.081 | NR | NR | NR | [43] |
| Indonesia | Unfermented and dried | 0.052 | NR | NR | 1160.7 ppm DPPH | |
| Indonesia | Partially fermented and dried cocoa beans | 0.162 | NR | NR | (250 ppm) DPPH | |
| Colombia | Unfermented dried cocoa | 37.31 | 0.215 ± 0.018 catechin mg/g 8.256 ± 0.015 epicatechin mg/g | NR | 351.41 ± 10.98 DPPH μmol/g 44.00 ± 0.08 FRAP mg/g 19.00 ± 1.73 mg/g O2 2873.58 ± 141.40 ORAC μmol/g |
[44] |
| Colombia | Fermented and dried cocoa | 50.2 | 0.023 ± 0.004 catechin mg/g 3.336 ± 0.031 epicatechin mg/g | NR | 325.48 ± 16.72 DPPH μmol/g 42.37 ± 0.52 FRAP mg/g 24.37 ± 0.42 mg/g O2 2096.56 ± 84.51 ORAC μmol/g |
|
| Ecuador | Roasted cocoa beans | 22.92 | NR | NR | NR | [38] |
| Colombia | Roasted cocoa beans | 37.81 | NR | NR | NR | |
| Ghana | Roasted cocoa beans | 18.43 | NR | NR | NR | |
| Dominican Republic | Roasted cocoa beans | 18.23 | NR | NR | NR | |
| Venezuela | Roasted cocoa beans | 9.96 | NR | NR | NR | |
| Mbanga, Littoral, Cameroon | Fresh cocoa beans fermented and dried and 5 min roasted in oven (DORCB 5) | 82 | NR | NR | NR | [39] |
| Mbanga, Littoral, Cameroon | Fresh cocoa beans fermented and dried and 5 min roasted traditional (DTRCB 5) | 78 | NR | NR | NR | |
| Mbanga, Littoral, Cameroon | Fresh cocoa beans fermented and dried and 10 min roasted in oven (DORCB 10) | 83 | NR | NR | NR | |
| Mbanga, Littoral, Cameroon | Fresh cocoa beans fermented and dried and 10 min roasted traditional (DTRCB 10) | 63 | NR | NR | NR | |
| Brazil | Cocoa liquor | 28.5 | NR | NR | 7957 ± 589 (μmol TE/100 g dm) DPPH, 45193 ± 272 (μmol TE/100 g dm) ORAC. | [45] |
| Dominican Republic, Sao Tome | Cocoa liquors from Criollo and Forastero with a different proportion of: A: Raw (unroasted) B: Roasted cocoa beans - 0% of cocoa liquor A and 100% of cocoa liquor B (cocoa liquor for the production of the CH0 chocolate) |
8.4 | (+)-catechin 0.0215 ± 0.0002 mg/g (-)-epicatechin: 1.9231 ± 0.0201 mg/g |
procyanidin B2 1.9104 ± 0.0805 mg/g procyanidin C1 1.1420 ± 0.012 mg/g |
537 ± 15 μM TE/g | [46] |
| Cocoa liquors with a different proportion of: A: Raw (unroasted) B: Roasted cocoa beans - 25% of cocoa liquor A and 75% of cocoa liquor B (the mixture of cocoa liquors intended for the production of the CH25 chocolate) |
13.6 | (+)-catechin 0.0233 ± 0.0003 mg/g (-)-epicatechin 4.1371 ± 0.0503 mg/g |
procyanidin B2 2.8056 ± 0.0613 mg/g procyanidin C1 1.6265 ± 0.0415 mg/g |
961 ± 28 μM TE/g | ||
| Cocoa liquors with a different proportion of: A: Raw (unroasted) B: Roasted cocoa beans - 50% of cocoa liquor A and 50% of cocoa liquor B (the mixture of cocoa liquors intended for the production of the CH50 chocolate), |
13.6 | (+)-catechin 0.0254 ± 0.0001 mg/g (-)-epicatechin 5.3563 ± 0.0197 mg/g |
procyanidin B2 2.8818 ± 0.0343 mg/g procyanidin C1 1.8439 ± 0.0202 mg/g |
735 ± 11 μM TE/g | ||
| Cocoa liquors with a different proportion of: A: Raw (unroasted) B: Roasted cocoa beans - 75% of cocoa liquor A and 25% of cocoa liquor B (the mixture of cocoa liquors intended for the production of the CH75 chocolate), |
13.5 | (+)-catechin 0.0273 ± 0.0003 mg/g (-)-epicatechin 5.5374 ± 0.0478 mg/g |
procyanidin B2 3.2236 ± 0.0301 mg/g procyanidin C1 1.9145 ± 0.0278 mg/g |
719 ± 10 μM TE/g | ||
| Cocoa liquors with a different proportion of: A: Raw (unroasted) B: Roasted cocoa beans - 100% of cocoa liquor A and 0% of cocoa liquor B (cocoa liquor intended for the production of the CH100 chocolate). |
10.7 | (+)-catechin 0.0187 ± 0.0002 mg/g (-)-epicatechin 3.6170 ± 0.0522 mg/g |
procyanidin B2 2.1014 ± 0.0106 mg/g procyanidin C1 1.2587 ± 0.0199 mg/g |
590 ± 14 μM TE/g | ||
| Turkey | Cocoa beans and alkalized | 8.8 | catechin 2.595 ± 0.0032 mg/g epicatechin 1.9782 ± 0.1562 mg/g | 1.1102 ± 0.1241 Dimer B2 (mg/g), 0.2567 ± 0.0154 Trimer C1 (mg/g) | 143.51 ± 20.80 H-ORAC (μmol TE/g), 5.50 ± 0.65 L-ORAC (μmol TE/g) 149.01 TAC (μmolTE/g) |
[47] |
| Cocoa beans and alkalized | 2.9 | catechin 0.1264 ± 0.0067 mg/g epicatechin 1.46 ± 0.1021 mg/g | 0.9890 ± 0.1052 Dimer B2 (mg/g), 0.1358 ± 0.0125 Trimer C1 (mg/g) | 191.0 ± 16.01H-ORAC (μmol TE/g), 4.95 ± 1.10 L-ORAC (μmol TE/g) 195.95 TAC (μmol TE/g) | ||
| Croatia | Cocoa products: cocoa liquor | 2.3 | (-)-epicatechin: 0.998 ± 0.140 mg/g | procyanidin B2: 0.413 ± 0.022 mg/g | NE | [48] |
| Serbia | Eleven different commercial cocoa powders, 6 non-alkalized (natural) and 5 alkalized | 41.55 mg GAE/g in natural cocoa powder | Total flavonoids 27.64 ± 0.47 (mg CE/g) in natural cocoa powder |
Total procyanidins (PA) 8.51 ± 0,95 (mg CyE/g) in natural cocoa powder |
8.51 ± 0.95 PA (mg CyE/g) 352.8 ± 9.9 DPPH (μM TE/g) 454.7 ± 2.1 FRAP (μM TE/g) 262.3 ± 9.03 ABTS (μM TE/g) |
[49] |
| Serbian | Twelve different commercial dark, milk chocolates and dark chocolate samples with added raspberries | 35.4 | 63.3 ± 5.2 TFC (μmol CE/g) 1.217 ± 0.008 epicatechin (mg/g) 0.666 ± 0.018 catechin (mg/g) |
7.07 ± 0.44 PAC (mg CyE/g) | NR | [50] |
| Spain | Cocoa product rich in fiber (containing 33 · 9% of total dietary fiber (TDF) and 13·9 mg/g of soluble polyphenols) in milk. | 13.9 | NR | NR | NR | [51] |
| Spain | A cocoa product rich in cocoa fiber (CP) has been produced from cocoa husks | 12.4 | (+)-catechin 0.26025 mg/g (-)-epicatechin 1.152 mg/g |
procyanidin B2 0.9135 mg/g Total pro-anthocyanidins 10.6425 mg/g |
NR | [52] |
| Spain | 40g of cocoa powder with 500 mL of skimmed milk | 9.4 | NR | NR | NR | [53] |
| Italy | Commercial cocoa powder | 9.3 | NR | NR | NR | [54] |
| Italy | After production (t0) and stored in their own packages in an air-conditioned room at 21+/-2 °C and 65% Relative Humidity (RH) for eighteen months | 2.1 | NR | NR | NR | [55] |
| Chocolate milk (M) | 0.6 | NR | NR | NR | ||
| Chocolate gianduja (G) | 1.0 | NR | NR | NR | ||
| Chocolate with cocoa mass in solid form (CM) | 2.1 | NR | NR | NR | ||
| Chocolate with cocoa powder 22–24 (°C) | 7.3 | NR | NR | NR | ||
| Chocolate with hazelnut paste (HP) | 0.3 | NR | NR | NR | ||
| Ecuador | Roasted cocoa beans came from the 2014 harvest and were submitted for testing by one of the chocolate producers in Poland | 36.09 | NR | NR | NR | [38] |
| Colombia | 40.55 | NR | NR | NR | ||
| Ghana | 24.56 | NR | NR | NR | ||
| Dominican Republic | 25.31 | NR | NR | NR | ||
| Venezuela | 9.10 | NR | NR | NR | [38] | |
| Peru | 32.13 | NR | NR | NR | ||
| Pahang, Malaysia | Commercially available dark chocolate in tablet (cocoa solids >85%, cocoa mass, fat-reduced cocoa, cocoa butter, sugar, and vanilla) | 5.79 | NR | NR | NR | [56] |
| Italy | Chocolate bars with different concentrations of stevia | 31.5 | NR | NR | 329 ± 29 μMol TE/g | [57] |
| Italy | Commercial dark | 8.1 | 0.34 ± 0.08 mg QE/g total flavonoid content | NR | 3.62 ± 0.09 DPPH (mgTEAC/g) | [58] |
| Commercial dark | 16.4 | 0.74 ± 0.02 mg QE/g total flavonoid content | NR | 3.58 ± 0.03 DPPH (mgTEAC/g) | ||
| Serbian | Commercial dark | 7.2 | 11.3 ± 0.9 TFC (μmol CE/g) 0.181 ± 0.018 epicatechin (mg/g) 0.057 ± 0.012 catechin (mg/g) | 0.181 ± 0.018 PAC (mg CyE/g) | NR | [50] |
| Commercial dark | 12.7 | 24.4 ± 0.6 TFC (μmol CE/g) 0.229 ± 0.037 epicatechin (mg/g) 0.151 ± 0.039 catechin (mg/g) | 3.68 ± 0.03 PAC (mg CyE/g) | NR | ||
| Croatia | Chocolate samples were produced in semi-industry conditions - chocolate 1 | 11.3 | 0.62 ± 0.04 mg/g (-)-epicatechin 0.06 ± 0.00 mg/g (+)-catechin | 0.74 ± 0.06 procyanidin B2, | 19 μmol Trolox/g DPPH 80 μmol Trolox/g ABTS |
[59] |
| Chocolate samples were produced in semi-industry conditions - chocolate 4 | 11.3 | 0.54 ± 0.03 (-)-epicatechin <LOD (+)-catechin |
0.52 ± 0.03 procyanidin B2, | 10 μmol Trolox/g DPPH 60 μmol Trolox/g ABTS |
||
| Chocolate samples were produced in semi-industry conditions - chocolate 2 | 8.3 | 0.28 ± 0.01 mg/g (-)-epicatechin < LOD (+)-catechin | 0.43 ± 0.02 procyanidin B2, | 21 μmol Trolox/g DPPH 80 μmol Trolox/g ABTS |
||
| Venezuela | Dark chocolate (45 %) | 101.5 | NR | NR | 3.06 ± 2.25 μmol Trolox/g∗ | [60] |
| Dark chocolate (70 %) | 152.3 | NR | NR | 3.95 ± 2.80 μmol Trolox/g∗ | ||
| Milk chocolate | 89.7 | NR | NR | 1.93 ± 0.51 μmol Trolox/g∗ | ||
| White chocolate | 4.4 | NR | NR | 0.023 ± 1.62 μmol Trolox/g∗ | ||
| Italy | Dark chocolate | 0.8 | epicathechin 0.59 μg/mL cathechin 0.32 μg/mL |
NR | NR | [61] |
| Milk chocolate | 0.3 | epicathechin 0.16 μg/mL cathechin 0.13 μg/mL |
NR | NR | ||
| Korea | Commercial cocoa: dark chocolate (DC) | 4.3 | 4.269 ± 1.761 mg/g | NR | NR | [62] |
| Commercial cocoa: milk chocolate (MC) | 1.1 | 1.108 ± 0.539 mg/g | NR | NR | ||
| Commercial cocoa: choco-syrup (CS) | 1.0 | 1.025 ± 0.798 mg/g | NR | NR | ||
| Croatia | Cocoa products: dark chocolate | 1.8 | 0.752 + 0.081 mg/g | 1.548 + 0.001 mg/g | NR | [48] |
| Cocoa products: semi- sweet chocolate | 1.7 | 0.416 + 0.011 mg/g | 1.384 + 0.001 mg/g | NR | ||
| Cocoa products: milk chocolate | 1.3 | 0.242 + 0.027 mg/g | 1.163 + 0.070 mg/g | NR |
NR: Not reported; CyE: Cyanidin chloride equivalents; CE: Catechin equivalents; LOD: Limit of detection; GAE: Gallic acid equivalents; TFC: Total flavonoid content; QE: Quercetin equivalents; TE: Trolox equivalent, DM: dried matter, H-ORAC: hydrophilic Oxygen Radical Absorbance Capacity; L-ORAC: lipophilic Oxygen Radical Absorbance Capacity, FRAP: ferric reducing antioxidant power, DPPH: 2,2-diphenyl-1-picrylhydrazyl.
Cocoa liquors sample preparation: it was prepared four types of cocoa liquors and then they were mixed in different ratios. 1- cocoa liquor from unroasted Criollo beans, 2- cocoa liquor from unroasted Forastero beans, 3- cocoa liquor from roasted Criollo beans, 4- cocoa liquor from roasted Forastero beans. Mixes: 1- cocoa liquor A (cocoa liquor 1 and 2 in a ratio 1:1), 2- cocoa liquor B (cocoa liquor 3 and 4 in a ratio 1:1). Then, cocoa liquor A and B were combined in various proportions with each other and directly used to obtain chocolates with different content of cocoa liquor. Chocolates: CH0 (100 % B), CH25 (25 % A: 75 % B), CH50 (50 % A: 50 % B), CH75 (75 % A: 25 % B); CH100 (100 % A).
The results indicate that factors such as fermentation, drying, roasting and industrialization processes affect the total polyphenol content (TPC) in cocoa. Specifically, these processes reduce said content, which might be caused by high processing temperatures and/or long processing times that induce the degradation of polyphenolic compounds available in cocoa components.
To highlight the features identified in different cocoa matrices, Table 1 details their total quantity of polyphenols and antioxidant capacity, as well as the treatments they were subjected to. Such matrices are raw, fermented, dry and industrialized cocoa (which includes cocoa powder and chocolate).
3.1. Postharvest
3.1.1. Unfermented cocoa beans
The content of polyphenols in cocoa beans depends on the geographical location, even though the same varieties are cultivated in different regions [10]. For instance, a study in Colombia employed a mixture of clones (CCN-51, ICS-1, FLE-2 and FEC-2) harvested and fermented in Bajo Cauca, Urabá and Magdalena Medio (which are subregions in the same department). In that case, the unfermented cocoa mixture with the highest polyphenol content was reported in Urabá (598.5 mg GAE/g), followed by Magdalena Medio (311.6 mg GAE/g) and Bajo Cauca (168.4 mg GAE/g) [12].
Polyphenol content is mostly influenced by cocoa intraspecies genetic variability, growing region, level of maturity, weather conditions during growth, harvest date and storage time after harvest; nevertheless, the latter two were the same in the Colombian study mentioned above [63]. Sukha, Bharath, Ali, and Umaharan (2014) evaluated 20 clones of the ICS variety in Trinidad and Tobago and found that harvest date has an effect ten times that of clone type (harvested between 2006 and 2009) on antioxidant capacity [43].
Polyphenols in cocoa beans are stored in the pigment cells of the cotyledons, and, depending on the number of anthocyanins found on those pigment cells also called polyphenol-storage cells their color varies from white to deep purpure. It is known that polyphenols in cocoa beans consist of catechins (33–42%), leucocyanidins (23–25%) and anthocyanins (5%). Anthocyanins are responsible for the typical purplish color of unfermented beans, and, during bean fermentation, anthocyanins hydrolyze into a sugar and cyanidin, which results in reduced anthocyanin content [64]. Studies into unfermented cocoa beans have identified two main types of polyphenols: catechins and cinnamic acids (and their derivatives). Caffeic acid and p-coumaric acid have been detected in the tegument, given that this tissue exhibits a strong presence of cells with lignified walls, and these acids are involved in lignin synthesis. On the other hand, catechin and epicatechin are mainly found in the cotyledon, specifically in big vacuolated polyphenolic cells [5, 65].
In addition, the storage process has an impact on the content of phenolic compounds in raw cocoa beans. Pod storage time, i.e., the time the pods are stored after harvesting but before splitting them, is usually maximum 2 weeks. Pulp preconditioning causes reductions in the content of polyphenolic compounds; however, a study reported that 5, 10 and 15 days of pod storage did not have a significant effect on (+)-catechin [66]. Other authors suggest that cocoa pods should not be stored for more than 7 days, since it could affect the fermentation process and degrade polyphenols [67].
3.1.2. Fermented matrix
The content of several polyphenols in cocoa beans is associated with the degree of fermentation [68]. In the postharvest processes, oxidation reactions, both enzymatic and not enzymatic, have the biggest impact on polyphenol reduction [10]. During the fermentation period, polyphenol compounds such as anthocyanins are hydrolyzed to anthocyanidins and sugars such as arabinose and galactose. In turn, the sugars polymerize with catechins to form complex tannins. Anthocyanins usually disappear during the fermentation process; therefore, anthocyanin content is employed as an indicator of the degree of cocoa bean fermentation [10, 66]. Further, the color change of cocoa beans is a sign of fermentation, caused by polyphenol oxidase, which converts o-dihydroxyphenols to o-benzoquinones; this results in browning, which affects both the flavor and the color of the product [10, 66].
Given that raw cocoa beans are astringent due to the presence of polyphenols and tannins, loss of bitterness and astringency occurs during fermentation since polyphenols migrate out of the cotyledon and are then oxidized [69].
Reduced polyphenol content during fermentation of raw cocoa beans has been evidenced in the study by Prayoga, Murwani, and Anwar (2013), where the polyphenol content and antioxidant properties of low-quality cocoa beans were higher in unfermented (0.162 g GAE/g) than in partially fermented (0.052 g GAE/g) samples [64].
Several mechanisms are commonly used to limit the effects of fermentation on polyphenol content reduction, including techniques such as water blanching, which inactivates polyphenol oxidase, thus increasing polyphenol retention during fermentation [64]. Menon et al. (2015) studied the effect of water blanching on polyphenol content using two temperatures (80 °C and 90 °C) and three treatment times (5, 10 and 15 min). In their case, polyphenol retention was the highest (119.4 mg GAE/g) when the blanching was performed at 90 °C for 5 min on a fresh sample. Similarly, when they treated fermented beans, the highest polyphenol retention occurred with blanching at 90 °C for 5 min (69.9 mg GAE/g), followed by the treatment at 90 °C for 10 min (65.4 mg GAE/g) [37].
Another study evaluated the effects of fermentation on the total polyphenol content and antioxidant activity of Trinitario cocoa beans from Nicaragua [30]. Three degrees of fermentation were evaluated: nonfermented, poorly fermented and fermented. As expected, the polyphenol content was lower in fermented (43 mg GAE/g) than in nonfermented samples (115 mg GAE/g). In addition, according to the studies that reported the epicatechin content (shown in Table 1), the fermentation process decreases the concentration of this monomer with respect to raw cocoa, reducing it from maximum 132.15 mg/g to 0.6 mg/g. As stated before, this polyphenol content loss is associated with oxidation and condensation reactions of single polyphenols to insoluble complex tannins that interact with proteins. Furthermore, nonenzymatic processes such as Maillard reactions take place during fermentation and browning, which involve the reduction of sugars and the amino groups of proteins to produce brown polymeric compounds [30].
Finally, other authors have reported that, after 60 h of fermentation, the total polyphenol content decreases up to 24%; and, after eight days, this content is further reduced by up to 58% [70]. Specifically, epicatechins and soluble polyphenols are reduced by 10% and 20%, respectively [71].
3.1.3. Dried matrix
Drying is a decisive step to guarantee the quality of cocoa in terms of sensorial, chemical and microbiologic properties. The main objective of the drying process is to reduce the moisture content down to 7–8%. If moisture is higher than that, it could result in microbial contamination (e.g., fungi); if it is lower, sensory qualities are reduced because the product is more susceptible to fissures, compounds affecting aroma and flavor are lost, and its commercial value is reduced given its dependence on grain weight [72].
Upon harvesting, cocoa farmers use the sun and hot air to dry cocoa beans and achieve the desired degree of moisture in the final product [28, 36]. The drying process ensures various chemical and biochemical changes that are necessary to complete the oxidative stage of fermentation and thereby reduce astringency, bitterness, and acidity, while enhancing flavor development and the brown color associated with well-fermented beans. Likewise, flavor and aroma precursors are also produced in the subsequent roasting process [36, 69].
Sun drying is known to be the best method for flavor development, but it has several drawbacks, such as long drying time. Additionally, the high temperatures reached with this method greatly affect aroma profiling, since most aroma precursor compounds—that must follow specific chemical reactions—are sensitive to temperature. Further, sun drying results in heterogeneous quality during the rainy season [73].
During the drying process, polyphenols in cocoa beans degrade due to complex reactions (known as browning) and thermal effects. However, although polyphenols do degrade during drying, the remaining traces still give cocoa products an astringent taste after processing [36]. While drying reduces polyphenol oxidase activity (only 2% of polyphenol degradation is attributable to enzymatic processes), oxidation and polymerization reactions still occur, confirming the presence of non-enzymatic oxidation reactions of phenolic compounds. The loss of polyphenols is also attributed to the migration of these compounds due to water evaporation [10].
Abhay et al. (2016) studied the effects of high temperature and long exposure times on cocoa polyphenols during drying. They found that the highest retention of polyphenols occurred at 70 °C, in contrast with the other temperatures they evaluated (60 °C and 80 °C). Moreover, the loss of polyphenols at high temperatures was caused by thermal degradation of volatile phenolic constituents, whereas degradation at low temperatures was mainly attributed to enzymatic degradation mechanisms [36]. In a similar study, Teh et al. (2016) evaluated polyphenol degradation kinetics. They proposed a first-order reaction kinetics model to describe the polyphenol degradation process, in which the activation energy (9 kJ/mol) is much lower than the energy barrier for moisture diffusion (11.8 kJ/mol). In addition, they compared the degree of retention of polyphenols in a hot air drying system with sun drying and found that—with respect to fresh samples—retention in the former system was in the 8.3–23.2% range, whereas in the latter it was 11.4%–23.2% [28].
Finally, epicatechin the most abundant compound in the catechins family, is found in high quantities in fresh cocoa seeds, and, by the end of the cocoa fermentation and drying processes, epicatechin concentration is reduced by ~75% [41]. The studies reviewed here have reported concentrations of epicatechin in countries such as Colombia and Ecuador, where its values have been between 8,256 mg/g and 1 mg/g in unfermented dry samples and in fermented dry cocoa, respectively, which highlights the effect of fermentation on the loss of this compound. The same behavior has been observed regarding anthocyanins, which are affected by the fermentation and drying process, as verified in two studies carried out in Colombia. In the first one, the authors used the ICS-1 clone, and the total anthocyanin content decrease from 1.26 ± 0.045 mg/g to 1.05 ± 0.021 mg/g. The second study investigated a mixture of clones in three regions and reported an average decrease of 67.2 ± 0.01%; in that case, the greatest change occurred from 2.63 ± 0.12 to 0.84 ± 0.04 [44,74]. Monitoring the anthocyanin concentration in the clone mixture, it was observed that between 72 and 96 h of fermentation and optimal conditions (45 °C and a pH between 3.8 and 4.5) produced the greatest advance in hydrolysis of anthocyanins. This was probably due to the action of glucosidase enzymes causing the breaking of the glycosidic bond of the two main fractions of anthocyanins present in cocoa (i.e., cyanidin-3-O-galactoside and cyanidin-3-O-arabinoside), which are hydrolyzed to anthocyanidins. This decrease in anthocyanins, is associated with a color change (decrease in violet) throughout postharvest, is a practical indicator for producers, allowing them to visually differentiate the stage of fermentation.
3.2. Industrialization
3.2.1. Roasted matrix
Cocoa roasting is a decisive step in aroma development due to the interaction of flavor precursors resulting from fermentation. Good roasting prevents sensorial defects such as bitter, acidic, astringent and nutty flavors. Nonetheless, in this process, nutrients and functional compounds responsible for health benefits (e.g., polyphenols) are lost, and the thermic contaminants such as acrylamide are generated [58]. Moreover, roasting can promote lipid oxidation and non-enzymatic browning, which greatly depend on the operation parameters used during this step and the composition of cocoa, both dried and fermented [37].
Urbánska and Kowalska (2019) reported polyphenol contents in chocolate prepared with roasted cocoa beans from five countries. The Colombian sample had the highest polyphenol content (37.81 mg/g product), whereas the lowest value was reported in Venezuelan cocoa (9.96 mg/g product) [38]. These differences might be attributed to high genetic variability because Colombian varieties present intercrossing (e.g., Forastero/Amazonian and Trinitario clones) [40], while Venezuelan cocoa comes from varieties such as Criollo and Arriba, characterized by a low polyphenol content [59]. Polyphenol presence in roasted and unroasted beans employed for chocolate production varies from region to region. These chocolates are characterized by their great ability to scavenge stable DPPH radicals. Although unroasted cocoa from Ghana showed the best DPPH results, the effects of roasting change in different regions. Venezuelan cocoa was the least affected by roasting, since its ability to scavenge stable DPPH radicals diminished only 1% from unroasted to roasted beans; in turn, the DPPH scavenging ability of Colombian cocoa was reduced by 1.7%. Overall, these author showed a correlation between polyphenol concentration and DPPH scavenging ability (r = 0,86) by evaluating roasted cocoa employed in chocolate production.
Gültekin-Özgüven et al., (2016) evaluated the effects of roasting temperature on total polyphenol content. They found that an increase from 115 °C to 135 °C in roasting temperature decreased the polyphenol content by ~14% and reduced the flavonoid content as well. Both polyphenols and flavonoids have a strong correlation with antioxidant capacity (R2 = 0.83 and R2 = 0.80, respectively), confirmed by a reduced antioxidant capacity by both ORAC and DPPH [45]. Similarly, Jolic et al., (2011) reported a reduction in total polyphenols of up to 10% during roasting. Furthermore, roasting also reduces the presence of catechin, epicatechin, dimer B2 and Trimer C1; by contrast, the concentration of (-)-catechin, increases, possibly due to the epimerization of (-)-epicatechin [75]. However, in the articles retrieved in this study using the search equation detailed above do not report catechin concentration individually, which could confirm the effect of roasting on the reduction of its content.
Tonfack et al. (2018) evaluated the effects of cocoa roasting time and method on polyphenol concentration. Interestingly, cocoa roasting time affects total polyphenol concentration depending on the method. For instance, when traditional roasting was used (cooking pot at 200–220 °C) and the time was increased from 5 to 10 min, the polyphenol concentration was reduced by 19.2%, possibly due to the volatilization of low molecular weight polyphenols. Conversely, the same time increase in a controlled roasting (oven at 180 °C) reduced the polyphenol content by only 1.2% [39].
3.2.2. Cocoa liquor matrix
Cocoa liquor is the most important intermediate product in the cocoa industry. Such liquor is made of selected grains, which are husked, roasted, ground and refined. This implies physical disintegration for size reduction, sieving for separation of particular solids, and final tempering before packing. These tasks affect the composition and, according to the studies reported in Table 1, the total polyphenol content in cocoa liquor.
Żyżelewicz et al., (2018) reported a relationship between antioxidant capacity, total polyphenol quantity and the concentration of some of the compounds (e.g., catechins, procyanidins, and flavonol glycosides) in a mixture of Criollo and Forastero cocoa liquor prepared at different ratios with and without roasting (see below). As stated above, there is a significant reduction in total polyphenol content when the grains are roasted, (e.g., in cocoa liquor), which is caused by processes such as polyphenol precipitation after interaction with proteins, polymerization or hydrolysis. Nevertheless, in their study, polyphenol content was not significantly affected by the different roasted:unroasted cocoa ratios (i.e., 75:25, 50:50 and 25:75); instead, there was a higher polyphenol content when they used unroasted cocoa only, as expected [46]. Particular compounds were individually affected, however, the concentration of compounds such as flavan-3-ols [(+)-catechin and (-)-epicatechin] decreased when the proportion of roasted cocoa increased, possibly due to the non-enzymatic oxidation of these compounds to o-quinones, followed by the condensation with other polyphenols and polymerized substances, as well as epicatechin epimerization to catechin. As result, the content of epicatechin ranged between 1.9231 and 5.5374 mg/g and that of catechin, between 0.0187 and 0.0273 mg/g. However, the procyanidins they evaluated showed the highest concentrations in the roasted/unroasted mixtures. With respect to flavonols, the maximum concentration was obtained with a 25:75 roasted:unroasted ratio, whereas the lowest concentration was achieved using roasted cocoa only. Finally, in terms of antioxidant capacity, they suggested a 25:75 roasted:unroasted mixture, which showed the highest ORAC value, for future formulations that maximize their functional properties.
The impact of the industrialization process on the flavanol content is evident in studies on cocoa at different postharvest and transformation stages. Komes et al. (2011) reported fermented cocoa beans with a value of flavan-3-ols of 113.12 ± 5.76 mg/L, and cocoa nibs obtained by a drying process with a value of 11.92 ± 0.82 mg/L [33]. In another study, dried beans from different cocoa-growing areas in Colombia presented a quantity of flavan-3-ols equal to 11.240 ± 0.825 mg/g [42]. Other authors have compared the flavanol content in processed products such as chocolate (quercitin-3-O-arabinosid 0.0704 mg/g and quercitin-3-O-glucoside 0.0757 mg/g), revealing a significant degradation of flavanol during roasting [46].
Belscak-Cvitanovic et al. (2012) studied different cocoa matrices and chocolate from Croatia. They found a strong correlation between polyphenol content, specifically of flavan-3-ols (epicatechin and procyanidin B2), and antioxidant capacity in cocoa products that have a high content of non-fat cocoa solids (NFCS). Cocoa liquor and dark chocolate, which have a high content of NFCS, exhibited the most potent cytotoxic effects, as well as antioxidant properties at higher concentrations; nevertheless, these values are low compared to those in the study mentioned in the previous paragraph [48].
3.2.3. Cocoa powder matrix
Cocoa powder can be employed as raw material, and, interestingly, it can be submitted to an alkaline treatment (also known as Dutching). This alkaline treatment is applied until a neutral or basic pH is achieved in order to reduce the acidity and bitterness of cocoa, while also increasing its solubility. Although said treatment also improves some sensory descriptors (such as the intense chocolate flavor and color in the final products), it has been shown to reduce the total polyphenol content, which is why natural cocoa powder is more commonly employed in research for commercial purposes. Todorovic et al. (2017) evaluated 11 cocoa powder samples (6 alkalinized and 5 natural) and established that on average there was a 45.5% reduction in total polyphenol content in the alkalinized powder versus its natural counterpart. Furthermore, they determined a relative antioxidant capacity index using ABTS, DPPH and FRAP, finding the highest correlation with polyphenol total content among the studies in this review (0.929 ≤ r ≤ 0.957, P < 0.01) [49].
Three studies conducted in Spain examined the effect of total polyphenols present in cocoa powder on the reduction of risk of some cardiovascular pathologies, such as hypercholesterolemia, high HDL cholesterol, and low LDL levels [49, 50, 60]. Khan et al. (2012) investigated compounds such as protocatechuic acid, procyanidins, and cinnamic acids and their derivatives in cocoa powder, where they found a high content of pro-anthocyanidins and vanillin compared to other matrices described in Table 1 [52].
3.2.4. Chocolate
Torri et al. (2017) compared seven chocolate samples processed in Italy and sweetened with various extracts. Some samples were sweetened with crude Stevia rebaudiana (Bertoni) extract, which contains polyphenols (76.6 mg/g product); while others were sweetened with Stevioside, which contains a low concentration of polyphenols (0.8 mg/g product) and maltitol. Among the seven chocolate samples analyzed in their study, no significant differences were observed in terms of phenol and flavonoid content, although some contradictory values were reported regarding ORAC (see Table 1). This apparent contradiction might be explained by the occurrence of interactions among phenolic compounds, carbohydrate-sweeteners, proteins and other bioactive compounds, which may influence the antioxidant capacity of the final product [57].
By contrast with the results reported by Belščak-Cvitanović et al. (2012) indicate that the total polyphenol content was 1.7 mg GAE/g [48]. However, and similar to the study mentioned before, the sweetener did not significantly affect the polyphenol content, at least with respect to dark chocolate. In dark chocolate, the non-fat cocoa solids content was increased by ~40 %. This is reflected in the (-)-epicatechin and procyanidin B2 concentrations, which increased 44.6% and 16%, respectively, and is significantly different from cocoa in a dairy matrix. Such difference between chocolate matrices might be associated with the aqueous extraction. The nature of the matrix could affect the recovery of polyphenolic, flavan-3-ol and procyanidin compounds since the dairy matrix has the strongest catechin-protein interaction and hinders optimal extraction [61]. A more recent study by Belščak-Cvitanović et al. (2015) evaluated various sweetener mixtures that guarantee a 20% lower caloric value than chocolate prepared with sucrose. The sweeteners they evaluated, such as stevia and peppermint leaves, presented a bioactive profile with phenolic acids, flavones (e.g., luteolin and apigenin) and flavonols (quercetin) derivatives. Nevertheless, these derivatives were not identified in the control samples; hence, the chocolate with the sweeteners mentioned above had the same polyphenolic content as the chocolate with sucrose, but a better antioxidant capacity measured by the FRAP method [59].
Fernández et al. (2014) [60], Loffredo et al. (2014) [61] and Lee et al. (2010) [76] compared dark chocolate, white milk chocolate and derivatives such as chocolate syrup. Overall, dark chocolate had a higher polyphenol content (up to 63%) than milk chocolate, which was represented in a higher proportion of epicatechin than catechin, as shown in [56]. The same tendency was reported by Fernández et al. (2011) [55]. Furthermore, these TPC values are higher than in fruits such as grapes, acai, guava, strawberry, pineapples, soursop and passionfruit [63]. The difference in TPC between dark and white chocolate was in the 95–97% range, which they infer might be associated with raw materials, in addition to the interactions between flavonoids and milk peptides during chocolate preparation. According to Lee et al. (2012) [62] milk chocolate and syrup have 74.4% and 76.7% less TPC than dark chocolate, respectively.
Todorovic et al. (2015) analyzed, not only the differences between chocolate types (dark, milk and dark with raspberries), but also the chocolate profile of Serbian chocolates in terms of their antioxidant capacity. Their results show that dark chocolate samples had up to 77.5% more polyphenol and flavonoid content than milk chocolate; while the total polyphenol and flavonoid content of dark chocolate (with or without raspberries) was similar, with overall ranges of 7.21–12.65 mg GAE/g of polyphenols and 11.3–24.4 mmol CE/g of flavonoids. The content of proanthocyanidins in chocolate/cocoa extracts ranged between 0.69 mg CyE/g in milk chocolates and 3.68 mg CyE/g in dark chocolates; thus, TPC depends primarily on the type of cocoa used to produce the chocolate. Regarding antioxidant capacity, they evaluated the chocolates implementing the most commonly used methods: ABTS, FRAP, DPPH and ORAC. In their case, the dark chocolate with raspberries presented the best antioxidant capacity, despite showing no significant differences in TPC with respect to dark chocolate. This might be due to the presence of vitamins and the polyphenol profile of the raspberries. Furthermore, the authors found a correlation (R2 = 0.798, p < 0.05) between TPC and ACI, thus confirming that their results serve to explain the antioxidant properties of Serbian chocolates [29].
Overall, the variation in TPC among chocolates depends, among others, on eight factors: (i) the amount of cocoa employed from the non-fat fraction, given that this fraction has the highest polyphenol content; (ii) the use of various cocoa ecotypes with bioactive profiles and flavors that are classified as high quality beans; (iii) the steps of chocolate production, given that roasting, alkalinization, and cold pressing are the most critical steps in polyphenol reduction (due to polymerization and hydrolysis, as well as protein interactions and Maillard reactions); (iv) the amount of cocoa present in dark chocolate or milk chocolate (low cocoa concentrations) formulations; (v) the lack of standardization of the extraction procedures of phenolic compounds, as well as use of solvents with polarities that cannot recover the maximum content of polyphenols; (vi) the use of different phenolic compounds to calibrate the standard curve in colorimetric procedures, and the presence of reducing compounds that interfere with the test; (vii) colorimetric method interferences, which also translate into an underestimation of the real TPC values; and (viii) a low concentration of certain phenolic compounds and their flavonoid-protein-sugar complexes that hinder the total appraisal of polyphenols.
The variability in antioxidant capacity results, which obstructs comparisons and classifications to make decisions regarding their functional properties, might have three causes: (i) a large number of heterogeneous tests that make antioxidant activity comparisons problematic; (ii) the use of unique tests to determine antioxidant capacity that do not provide a global understanding of the action mechanism; and (iii) tests of correlations between TPC and antioxidant activity that show a wide range of “good” or “strong” correlations which, despite being useful for the categorization of the raw material or final product, require a validation using other methods.
Finally, in the studies reviewed here, we identified that the transformation from unfermented cocoa beans to different types of chocolate produces a decrease in cinnamic acids. For instance, the chlorogenic acid content can range from 10.52 ± 0.84 mg/g in unfermented cocoa from Venezuela to an average of 2.32 mg/g in chocolates produced in Croatia by different methods. Similarly, a decrease in caffeic acid (91.3% on average) was identified in the same samples [5, 61].
3.3. Effect of postharvest treatments and industrialization processes on polyphenol content
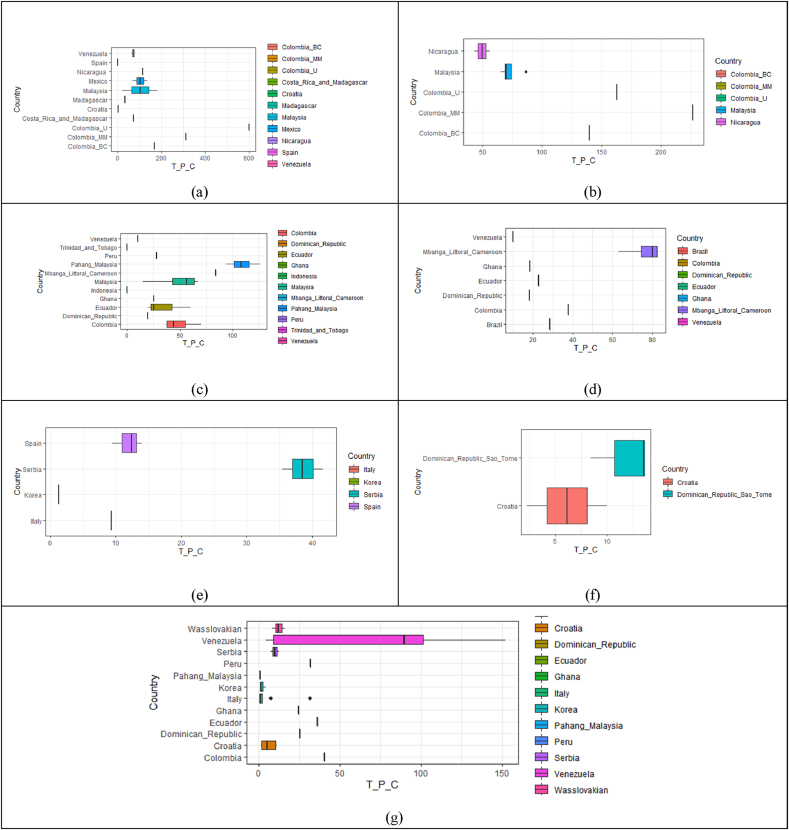
Based on a descriptive statistical analysis, we defined a range of total polyphenol concentration according to the type of matrix, i.e., unfermented, fermented, dry, roasted, cocoa liquor, cocoa powder and chocolate (as shown in Figure 3). The values reported for the different matrices evaluated in this review constitute a reference for consultation, consolidating relevant data for the cocoa industry, and present concentration ranges according to the transformation stages to which this raw material is subjected. Likewise, this classification is a starting point for the development and standardization of methods to characterize and compare cocoa and its derivatives with respect to expected TPC.
Figure 3.
Box plots of ranges of total polyphenols present in the matrices, (a) unfermented, (b) fermented, (c) dried, (d) roasted, (e) cocoa liquor, (f) cocoa powder and (g) chocolate matrices classified by the location where the study was conducted.
As shown in Figure 3, the total polyphenol content diminishes as postharvest treatments and industrialization advance. In fact, its maximum value in unfermented cocoa can be up to 600 mg GAE/g. The Colombian raw material presents the highest TPC value, while studies in other countries have found values under 200 mg GAE/g. Once fermentation takes place, the polyphenol concentration is reduced to 250 mg GAE/g, regardless of the initial quantity of grains employed. During the drying process, i.e., the key step in postharvest, polyphenol content decreases to 150 mg GAE/g [35]. Examining at the industrialization process, it can be observed that one of the key steps is obtaining the roasted grains, where the TPC drops below 100 mg GAE/g [39, 40]. After obtaining one of the main subproducts available in the market (i.e., cocoa liquor), polyphenol values are well below 50 mg GAE/g [77]; while cocoa powder shows values under 15 mg GAE/g [53, 78]. Finally, the range of polyphenol content in chocolate preparations is wide due to the different available preparations of this cocoa derivative. Polyphenol content in chocolate depends on the concentration of the cocoa liquor employed for the preparation, treatment and the factors mentioned above. More specifically, the polyphenol content in chocolate can be almost 150 mg GAE/g, but in most cases it is well below 50 mg GAE/g [57, 58, 59, 60, 61, 62].
3.4. Health-related benefits promoted by the antioxidant potential of cacao and its derivatives
Despite that the postharvest and industrialization of cocoa reduces its TPC content, the final concentration of these compounds is high enough to have positive effects on human health, as shown below for cocoa and its derivatives.
These observations could be related to the biological antioxidant potential of cocoa, which are mainly mediated by the modulation of the Keap1-Nrf2 (Kelch-like ECH-associated protein 1 - Nuclear factor erythroid 2-related factor 2) pathway [79, 80, 81], considered the master regulator of the defense against oxidative stress by inducing cytoprotective genes expression [82].
This pathway is activated when Keap1-thiol residues are modified by free radicals or electrophiles (such us phytochemical electrophiles), which allows Nrf2 release and translocation to the nucleus, where it binds to antioxidant element response (ARE) or electrophile-response element (EpRE) at the promoters of Nrf2-induced genes, including γ-glutamyl cysteine synthetase (γ-GCS), heme oxygenase 1 (HO-1), glutathione reductase (GR), glutathione peroxidase (GPx), thioredoxin-1, thioredoxin reductase, catalase (CAT), NAD(P)H:quinone oxidoreductase-1 (NQO1), superoxide dismutase (SOD) and peroxiredoxins [83]. The protein products of these genes are involved in the elimination of reactive species and drug detoxification [84]; additionally, it is important to note that recent studies have revealed new targets genes of Nrf2 and for instance has pointing out new functions of the Keap1-Nrf2 pathway, such us metabolic reprogramming, unfolded protein response and proteostasis, autophagy, mitochondrial physiology and biogenesis and, inflammation and immunity [85].
3.4.1. Epidemiological studies
In the period selected for this literature review, few epidemiological studies evaluated the effects of the consumption of T. cacao and its derivatives on markers of oxidative stress. Recently, Mehrabani et al. (2020) performed a meta-analysis in order to evaluate different target populations with diverse health status (i.e. healthy subjects, cardiovascular diseases, and metabolic conditions). They found that chocolate consumption significantly reduced malondialdehyde (p < 0.048) and 8-iso-prostaglanding F2α (p < 0.008), which are markers of oxidative stress [86]. These observations could be related to the beneficial effects of chocolate and cocoa products on endothelial function [87] and the reduction of heart failure risk [88], suggesting the potential of cocoa for disease intervention.
3.4.2. In vitro studies
Some studies have evaluated the health benefits of cocoa in different in vitro models. Cocoa ethanolic and methanolic extracts have been shown to reduce the viability of tumor-derived cell lines from lung, breast, liver and cervical tissues and also to up-regulate genes related to the cellular defense against oxidative stress, such as epoxide hydrolase 2 (EPHX2) and cytochrome B-245 beta chain (CYBB) [23, 89]. Similar results have been obtained with water-chocolate extracts, cocoa liquor and cocoa phenolic extracts (CPEs) in Hep2, HepG2, RLE and SH-SY5Y cells [[49], [92], [93], [94], [95]], due to the stimulation of Nrf2, the subsequent increase in cytoprotective genes, such as GPx and GR, and the decrease in reactive oxygen species (ROS) formation. Interestingly, these results were observed even in high-glucose-induced oxidative stress conditions [91] or in the presence of pro-oxidant agents, such as tert-butyl-hydroperoxide [90] and hydrogen peroxide [92]. Additionally, CPEs have shown potential to induce the expression of genes involved in stress response and detoxifying pathways (e.g., cytochrome P450 family 1 subfamily A member) [94], and also to modulate the activation of mitogen-activated protein kinases (MAPKs) [90, 92, 95], which is important taking into account that the MAPK signaling pathway is involved in the regulation of key cellular processes, such as proliferation, differentiation, apoptosis and stress responses [96].
Other studies have evaluated the anti-inflammatory potential of CPEs and purified molecules from cocoa. It was showed that CPEs down-regulate the activation of the vascular endothelial growth factor (VEGF) expression by the modulation of the tumor necrosis factor α (TNF-α) in JB6 P+ mouse epidermal cells [97], the reduction of prostaglandin E2 secretion in Caco-2 cells stimulated with interleukin-1β [98], and the induction of anti-inflammatory cytokines in THP-1-derived macrophages [99]. This suggests that CPEs might have anti-inflammatory properties.
In the case of purified molecules from cocoa, procyanidins have been of great interest due to their beneficial properties to manage acute and chronic diseases. For example, in models of colonic inflammation, cocoa extracts and high-molecular-weight polymeric procyanidins were the most effective in reducing the secretion of interleukin-8 in response to inflammatory stimuli [100]. Additionally, procyanidins have shown interesting biological activities, such as the induction of glutathione s-transferase pi 1 (GSTP1) expression and activity in colonic cells [101]; metalloproteinase 2 (MMP2) downregulation and induction of apoptosis through ROS-mediated mechanism in ovarian carcinoma cell lines [100]; and the prevention of acrylamide induced apoptosis [102] and deoxycholic acid induced oxidant production [103] in colon cancer models through the modulation of protein kinase B (Akt), mitogen-activated protein kinases ERK1/2 and p38 activation.
In the context of pathological conditions, e.g., cardiovascular disease and metabolic syndrome, in vitro models have been used in order to evaluate the protective effect of cocoa. CPEs modulates oxidative stress in endothelial cells challenged with the pro-oxidants tert-butyl-hydroperoxide and hydrogen peroxide, by limiting ROS production and inducing antioxidant enzymes activity [27, 95]. On the other hand, cocoa extracts and cocoa flavonols have been shown to reduce the expression of pro-inflammatory molecules and ROS production, and also to restore glutathione levels and mitochondrial-membrane potential and function in RAW264.7 macrophages, 3T3-L1 adypocytes, liver-derived HepG2 cells and in pancreatic beta-derived β-TC3 and INS-1 832/13 cells, which results in the attenuation of adipogenesis, lipid accumulation and counteraction of insulin resistance [104, 105, 106]. These effects have also been observed in HepG2 and Ins-1E pancreatic beta cells, even in the presence of pro-oxidant agents, such as tert-butyl-hydroperoxide [107, 108].
3.4.3. Interventional and in vivo studies
Interventional studies have explored some of the possible mechanisms of action of cocoa extracts and its derivatives (Table 2). Spadafranca et al. (2010) showed the positive effect of dark chocolate (containing 860 mg of total polyphenols, including 58 mg of epicatechin), after two weeks of consumption, on twenty healthy participants, rising plasma epicatechin levels and significantly decreasing (p < 0.05) DNA damage on mononuclear blood cells exposed to hydrogen peroxide [109]. Similar results were observed in a randomized, placebo-controlled, double-blind study which includes 84 subjects who presented at least 3 of the following 5 risk factors: (i) glucose greater than 100 mg/dL, (ii) triglycerides levels greater than 160 mg/dL, (iii) LDL greater than 130 mg/dL, (iv) HDL lower than 45 mg/dL, and (v) body mass index (BMI) greater than 29. Participants receive a daily dose of 2 g of dark chocolate with high phenolic and flavonoid content. After six months of consumption, a significantly reduction in DNA damage in buccal epithelial cells was observed [110].
Table 2.
Interventional and in vivo studies evaluating the antioxidant biological activity of cocoa and its derivatives.
| Substances | Subjects | Study type | Dose | Outcome | Authors |
|---|---|---|---|---|---|
| Dark chocolate | 20 healthy subjects | Interventional | 40g/day for two weeks | ↑ Epicatechin levels | [109] |
| ↓ DNA damage | |||||
| Dark chocolate | 84 subjects with risk factors of metabolic syndrome | Interventional | 2g/day for 6 months | ↓ Genotoxicity in buccal epithelial cells | [110] |
| Cocoa powder | 18 healthy subjects | Interventional | 40g/day for 1 week cocoa powder in water | ↓ NFκβ activation | [111] |
| Dark chocolate | 20 healthy subjects | Interventional | 40g/day | ↑ Serum epicatechin level | [112] |
| 20 smokers | ↓ ROS production | ||||
| ↓ 8-iso-PGF2α and NADPH oxidase activation in smokers | |||||
| Flavanol-rich cocoa | 6 Healthy subjects | Interventional | 1–4g/day for 4 weeks cocoa | ↑ Plasma epicatechin and GSH levels | [113] |
| ↓ Reduction of F2 isoprostanes levels | |||||
| Modulation of PUFA metabolism | |||||
| Cocoa extract | 24 overweight/obese middle-aged subjects | Interventional | 1.4g cocoa extract/daily for 4 weeks | ↑ Cocoa-derived metabolites in plasma | [116] |
| ↓ Systolic blood pressure | |||||
| Cocoa powder | 42 high-risk volunteers | Interventional | 40g/day for 4 weeks | ↑ HDL | [52] |
| ↓ Oxidized LDL | |||||
| Cocoa powder | 100 subjects with type 2 diabetes | Interventional | 20g/day for 6 weeks | ↓ Blood cholesterol, triglyceride, LDL | [115] |
| ↓ TNF-α, high sensitive C-reactive protein, IL-6 | |||||
| Inhibition of lipid peroxidation | |||||
| Cocoa product rich in fiber | 24 healthy subjects | Interventional | 30g/day cocoa product rich in fiber | ↑ Serum HDL | [114] |
| 20 hypercholesterolaemic subjects | ↓ Glucose, IL-1β and IL-10 | ||||
| Cocoa product rich in fiber | 21 volunteers with moderate hypercholesterolaemia | Interventional | 30g/daily for 8 weeks | ↓ Systolic and diastolic blood pressure | [114] |
| ↓ MDA values | |||||
| Dark chocolate | Sprague-dawley rats (40–60 g) | in vivo | 10% of bodies weight for 3 weeks | ↓ COX-2 expression levels | [120] |
| Regulation of proliferation index | |||||
| ↓ Lower ACF formation induced by AOM | |||||
| Cocoa extract | BALB/c mice | in vivo | 34.5 mg/kg for 2 weeks | Suppression of hepatotoxicity and inflammation | [119] |
| Early stage antifibrotic and anticarcinogenic effects | |||||
| Cocoa liquor | Streptozotocin (STZ)-induced diabetic rats | in vivo | 3.6 g/kg to 7.2 g/kg body weight by day for 40 days of cocoa liquor in water | ↓ Plasma triacylglycerol levels | [45] |
| ↑ HDL and plasma antioxidant capacity | |||||
| ↓ Lipid peroxidation in plasma and tissues | |||||
| Cocoa-rich diet | Type 2 diabetic Zucker diabetic fatty rats | in vivo | All experimental groups were provided with food and water ad libitum | Improved glucose tolerance and insulin resistance | |
| ↓ ROS levels and carbonyl content | [121] | ||||
| Polyphenol enriched cocoa powder | Prediabetic model triggered by a sucrose rich diet | in vivo | 200 mg/kg body weight by day for 3 weeks | ↓ Insulin resistance | [122] |
| ↓ Hepatic carbohydrate and lipid dysmetabolism | |||||
| ↓ Oxidative stress and inflammation | |||||
| Cocoa fiber product | Spontaneously hypertensive rats | in vivo | 200, 400, and 800 mg/kg/day by 10 weeks | ↓ Systolic blood pressure | [123] |
| ↓ Glucose, cholesterol, and triglyceride levels | |||||
| ↓ Liver and plasma MDA levels | |||||
| Cocoa-rich diet | Spontaneously hypertensive rats | in vivo | 300 mg/kg of cocoa-rich extract | ↓ Systolic blood pressure | [124] |
| Phenolic-rich extract from Cocoa liquor | Wistar rats with high-fat diet | in vivo | 2.1 g of liquor/kg to 7.2 g of liquor/kg body weight by day for 28 days | ↓ Lipid peroxidation | [77] |
| ↑ Plasma and tissue antioxidant capacities | |||||
| Cocoa powder | Sprague Dawley rats with high-fat diet | in vivo | 52–108 days with 12.5% cocoa supplementation regimes | ↓ NADPH oxidase protein levels | [125] |
| ↓ Inflammation and fibrosis | |||||
| Cocoa bean extracts | Wistar rats with high-fat diet | in vivo | 2.25–2.45% of the total diet | Improvement of physiological parameters | [105] |
| Enhancement of antioxidant status |
DNA: deoxyribonucleic acid; NFκβ: nuclear factor kappa β; ROS: reactive oxygen species; 8-iso-PGF2α: 8-iso-prostaglandin F2α; NADPH: nicotinamide adenine dinucleotide phosphate; GSH: glutathione; PUFA: polyunsaturated fatty acids; HDL: high-density lipoprotein; LDL: low-density lipoprotein; TNF-α: tumor necrosis factor alpha; IL-6: interleukin 6; IL-1β: interleukin 1β; IL-10: interleukin 10; MDA: malondialdehyde; COX-2: cyclooxygenase-2; ACF: aberrant crypt foci; AOM: azoxymethane.
In another interventional study, subjects with an acute consumption (40g of cocoa powder in water for one week) presented significantly reduced levels of active subunit of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) phosphorylation compared to baseline conditions (p < 0.05), which suggests that cocoa has anti-inflammatory effects [111]. In an interesting crossover single-blind study published in 2011, Carnevale et al. (2011) examined 20 healthy subjects and 20 smokers and found that ingestion of dark chocolate increased serum epicatechin levels in both groups. Additionally, impairment of platelet activation by the significant down-regulation of ROS production and also reduction of 8-iso-prostaglanding F2α and NADPH oxidase activation were observed in smokers, supporting the evidence that suggests that cocoa extracts have antioxidant and anti-inflammatory potential, even in highly oxidative contexts [112]. Recently, similar results were obtained with a daily intervention (4 weeks in total) in healthy subjects with a flavonol-rich cocoa supplement. In that case, the authors observed increased epicatechin (p < 0.05) and glutathione levels (p < 0.0001), a reduction in F2-isoprostane levels (p < 0.0001), and also an improvement in the lipid profile and modulation of metabolism of polyunsaturated fatty acids, thus decreasing arachidonic acid/eicosapentaenoic acid levels (p < 0.001) [113].
These observations are relevant because oxidative stress and inflammation are important features in the pathogenesis of metabolic and cardiovascular diseases. In this regard, cocoa consumption from different matrices (e.g. chocolate bars, cocoa fiber, cocoa powder and cocoa extracts) has been shown to modulate total blood cholesterol, triglycerides, LDL and HDL levels, lipid peroxidation, insulin resistance, fasting plasma glucose, blood pressure, malondialdehyde levels and inflammatory markers, in subjects with cardiovascular and metabolic conditions [52, 53, 110, 114, 115, 116].
Importantly, a clinical trial is currently being carried out in the United States called Cocoa Supplement and Multivitamin Outcomes Study or COSMOS (https://clinicaltrials.gov/ct2/show/NCT02422745). With an enrollment of more than 20,000 men and women 60 and older, COSMOS is designed to investigate the potential of cocoa flavanols (2 capsules each day containing a total of 600 mg cocoa flavanols, including 80 mg of (-)-epicatechin, and 50 mg of theobromine) to reduce the risk of developing heart disease, stroke and cancer.
Animal models have also been employed to test the antioxidant potential of cocoa (Table 2). For example, it has been observed that cocoa extracts or purified molecules (such as epicatechin) enhance the antioxidant defense and the modulation of inflammation in highly pro-oxidant conditions induced by chemical agents, e.g. azoxymethane [117], dextran sulfate sodium [118] and carbon tetrachloride [119], thus suppressing aberrant crypt foci formation in the colon, acute intestinal injury and hepatotoxicity, respectively. Additionally, it has been shown that cocoa extracts improved glucose metabolism after an imbalance induced by carbon tetrachloride [119], which is an interesting observation, taking into account the protective effect of cocoa in the context of pathological conditions (such as metabolic syndrome and cardiovascular disease), as previously discussed.
For these reasons, several animal models have been used in order to evaluate the cocoa antioxidant and anti-inflammatory properties of cocoa (Table 2). Cocoa liquor with a high polyphenol content has been found to increase plasma antioxidant capacity and modulates lipid peroxidation levels in streptozotocin-induced diabetic rats [45]. Likewise, a cocoa rich-diet has improved glucose tolerance, alleviates insulin resistance and enhance the hepatic antioxidant/detoxifying defenses in diabetic fatty rats [126], and CPEs have increased glutathione levels and reduced insulin resistance, as well as COX-2 and iNOS levels in a rat model of prediabetes [122]. Similarly, soluble cocoa fiber products and cocoa powder rich in polyphenols have been shown to possess antihypertensive properties [123, 124], apparently mediated by the up-regulation of nitric oxide and the subsequent reduction in blood pressure [124].
Finally, it has been reported that, in high-fat diet models, cocoa enhances plasma and tissue antioxidant status and inhibits lipid peroxidation [77, 125, 127].
4. Conclusion
This literature review showed that the total content of polyphenols present in cocoa decreases as the latter undergoes transformations to obtain its derivatives. In particular, fermentation and the process of obtaining cocoa powder were found to be the stages that most reduce TPC. The findings reported here can be a useful reference point for different actors in the cocoa and chocolate industry because they provide data on the expected limits of polyphenol content depending on the type of matrix, origin of the raw material, type of clone and concentration of bioactive compounds.
In addition, the traceability of the results of the studies that have evaluated the biological activity of cocoa polyphenols in different models suggest that these compounds have the potential to modulate key pathophysiological processes through the regulation of plasma and tissue antioxidant/detoxifying systems, which produces the attenuation of mechanisms that are directly related to disease onset and progression. However, more clinical trials should be conducted in order to validate these observations.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This work was supported by Instituto Tecnológico Metropolitano and Colciencias Colfuturo, and the Universidad Nacional de Colombia (647-2015).
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.World Health Organization . 2018. Noncommunicable Diseases Country Profiles 2018, Geneva. [Google Scholar]
- 2.Zhang Y.J., Gan R.Y., Li S., Zhou Y., Li A.N., Xu D.P., Bin Li H., Kitts D.D. Antioxidant phytochemicals for the prevention and treatment of chronic diseases. Molecules. 2015;20:21138–21156. doi: 10.3390/molecules201219753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wootton-Beard P.C., Ryan L. Improving public health?: the role of antioxidant-rich fruit and vegetable beverages. Food Res. Int. 2011;44:3135–3148. [Google Scholar]
- 4.Martín M.A., Ramos S. Cocoa polyphenols in oxidative stress : potential health implications. J. Funct. Foods. 2016;27:570–588. [Google Scholar]
- 5.Cerri M., Reale L., Zadra C. Metabolite storage in theobroma cacao L. Seed: cyto-histological and phytochemical analyses. Front. Plant Sci. 2019;10:1–11. doi: 10.3389/fpls.2019.01599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rojo-Poveda O., Barbosa-Pereira L., Mateus-Reguengo L., Bertolino M., Stévigny C., Zeppa G. Effects of particle size and extraction methods on cocoa bean shell functional beverage. Nutrients. 2019;11:1–19. doi: 10.3390/nu11040867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dorota Ż., Oracz J., Antolak H., Kr D., Kaczmarska M. The e ff ect on bioactive components and characteristics of chocolate by functionalization with raw cocoa beans. 2018;113:234–244. doi: 10.1016/j.foodres.2018.07.017. [DOI] [PubMed] [Google Scholar]
- 8.Royal Society of Chemistry ChemSpider search and share chemistry. Chem. Struct. Data Base. 2021 [Google Scholar]
- 9.Elwers S., Zambrano A., Rohsius C., Lieberei R. Differences between the content of phenolic compounds in Criollo, Forastero and Trinitario cocoa seed (Theobroma cacao L.) Eur. Food Res. Technol. 2009;229:937–948. [Google Scholar]
- 10.Oracz J., Zyzelewicz D., Nebesny E. The content of polyphenolic compounds in cocoa beans (theobroma cacao L.), depending on variety, growing region, and processing operations: a review. Crit. Rev. Food Sci. Nutr. 2015;55:1176–1192. doi: 10.1080/10408398.2012.686934. [DOI] [PubMed] [Google Scholar]
- 11.Afoakwa E.O., Quao J., Takrama F.S., Budu a.S., Saalia F.K. Changes in total polyphenols, o-diphenols and anthocyanin concentrations during fermentation of pulp pre-conditioned cocoa (Theobroma cacao) beans. Int. Food Res. J. 2012;19:1071–1077. [Google Scholar]
- 12.Gil M., Jaramillo Y., Bedoya C., Llano S.M., Gallego V., Quijano J., Londono-Londono J. Chemometric approaches for postharvest quality tracing of cocoa: an efficient method to distinguish plant material origin. Heliyon. 2019;5 doi: 10.1016/j.heliyon.2019.e01650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hii C., Law C., Suzannah S., Cloke M. Asian journal of food and agro-industry polyphenols in cocoa (theobroma cacao L.) As. J. Food Ag-Ind. 2009;2:702–722. [Google Scholar]
- 14.Barrientos L.D.P., Oquendo J.D.T., Garzón M.A.G., Álvarez O.L.M. Effect of the solar drying process on the sensory and chemical quality of cocoa (Theobroma cacao L.) cultivated in Antioquia, Colombia. Food Res. Int. 2018;115:259–267. doi: 10.1016/j.foodres.2018.08.084. [DOI] [PubMed] [Google Scholar]
- 15.Di Mattia C., Martuscelli M., Sacchetti G., Scheirlinck I., Beheydt B., Mastrocola D., Pittia P. Effect of fermentation and drying on procyanidins, antiradical activity and reducing properties of cocoa beans. Food Bioprocess Technol. 2013;6:3420–3432. [Google Scholar]
- 16.Ioannone F., Di Mattia C.D., De Gregorio M., Sergi M., Serafini M., Sacchetti G. Flavanols, proanthocyanidins and antioxidant activity changes during cocoa (Theobroma cacao L.) roasting as affected by temperature and time of processing. Food Chem. 2015;174:256–262. doi: 10.1016/j.foodchem.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 17.Sacchetti G., Ioannone F., De Gregorio M., Di Mattia C., Serafini M., Mastrocola D. Non enzymatic browning during cocoa roasting as affected by processing time and temperature. J. Food Eng. 2016;169:44–52. [Google Scholar]
- 18.Hii C.L., Menon A.S., Chiang C.L., Sharif S. Kinetics of hot air roasting of cocoa nibs and product quality. J. Food Process. Eng. 2017;40:1–6. [Google Scholar]
- 19.Payne M.J., Hurst W.J., Miller K.B., Rank C., Stuart D.A. Impact of fermentation, drying, roasting, and Dutch processing on epicatechin and catechin content of cacao beans and cocoa ingredients. J. Agric. Food Chem. 2010;58:10518–10527. doi: 10.1021/jf102391q. [DOI] [PubMed] [Google Scholar]
- 20.Álvarez-Cilleros D., López-Oliva M.E., Morales-Cano D., Barreira B., Pérez-Vizcaíno F., Goya L., Ramos S., Martín M.Á. Dietary cocoa prevents aortic remodeling and vascular oxidative stress in diabetic rats. Mol. Nutr. Food Res. 2019;63:1–9. doi: 10.1002/mnfr.201900044. [DOI] [PubMed] [Google Scholar]
- 21.Kerimi A., Williamson G. The cardiovascular benefits of dark chocolate. Vascul. Pharmacol. 2015 doi: 10.1016/j.vph.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 22.Rimbach G., Melchin M., Moehring J., Wagner A.E. Polyphenols from cocoa and vascular health - a critical review. Int. J. Mol. Sci. 2009;10:4290–4309. doi: 10.3390/ijms10104290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baranowska M., Suliborska K., Todorovic V., Kusznierewicz B., Chrzanowski W., Sobajic S., Bartoszek A. Interactions between bioactive components determine antioxidant, cytotoxic and nutrigenomic activity of cocoa powder extract. Free Radic. Biol. Med. 2020;154:48–61. doi: 10.1016/j.freeradbiomed.2020.04.022. [DOI] [PubMed] [Google Scholar]
- 24.Teng H., Chen L. Polyphenols and bioavailability: an update. Crit. Rev. Food Sci. Nutr. 2019;59:2040–2051. doi: 10.1080/10408398.2018.1437023. [DOI] [PubMed] [Google Scholar]
- 25.Sorrenti V., Ali S., Mancin L., Dvinelli S., Paoli A., Scapagnini G. Cocoa polyphenols and gut microbiota interplay: bioavailability, prebiotic effect, and impact on human health. Nutrients. 2020;12:3–8. doi: 10.3390/nu12071908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ooi T.S., Ting A.S.Y., Siow L.F. Influence of selected native yeast starter cultures on the antioxidant activities, fermentation index and total soluble solids of Malaysia cocoa beans: a simulation study. LWT. 2020;122:108977. [Google Scholar]
- 27.Felice F., Fabiano A., De Leo M., Piras A.M., Beconcini D., Cesare M.M., Braca A., Zambito Y., Di Stefano R. Antioxidant effect of cocoa by-product and cherry polyphenol extracts: a comparative study. Antioxidants. 2020;9:1–14. doi: 10.3390/antiox9020132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Teh Q.T.M., Tan G.L.Y., Loo S.M., Azhar F.Z., Menon A.S., Hii C.L. The drying kinetics and polyphenol degradation of cocoa beans. J. Food Process. Eng. 2016;39:484–491. [Google Scholar]
- 29.Vázquez-Ovando A., Molina-Freaner F., Nuñez-Farfán J., Betancur-Ancona D., Salvador-Figueroa M. Classification of cacao beans (Theobroma cacao L.) of southern Mexico based on chemometric analysis with multivariate approach. Eur. Food Res. Technol. 2015;240:1117–1128. [Google Scholar]
- 30.Suazo Y., Davidov-Pardo G., Arozarena I. Effect of fermentation and roasting on the phenolic concentration and antioxidant activity of cocoa from Nicaragua. J. Food Qual. 2014;37:50–56. [Google Scholar]
- 31.Rodríguez Ó., Ortuño C., Simal S., Benedito J., Femenia A., Rosselló C. Acoustically assisted supercritical CO2 extraction of cocoa butter: effects on kinetics and quality. J. Supercrit. Fluids. 2014;94:30–37. [Google Scholar]
- 32.Quiroz-Reyes C., Aguilar-Mendez M., Ramirez-Ortiz M., Ronquillo E. Comparative study of ultrasound and maceration techniques for the extraction of polyphenols from cocoa beans (Theobroma cacao L.) Rev. Mex. Ing. Química. 2013;12:11–18. [Google Scholar]
- 33.Komes D., Belščak-Cvitanović A., Horžić D., Drmić H., Škrabal S., Miličević B. Bioactive and sensory properties of herbal spirit enriched with cocoa (theobroma cacao L.) polyphenolics. Food Bioprocess Technol. 2012;5:2908–2920. [Google Scholar]
- 34.Bubonja-Sonje M., Giacometti J., Abram M. Antioxidant and antilisterial activity of olive oil, cocoa and rosemary extract polyphenols. Food Chem. 2011;127:1821–1827. [Google Scholar]
- 35.Gil M., Jaramillo Y., Bedoya C., Llano S.M., Gallego V., Quijano J., Londono-Londono J. Chemometric approaches for postharvest quality tracing of cocoa: an efficient method to distinguish plant material origin. Heliyon. 2019;5 doi: 10.1016/j.heliyon.2019.e01650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abhay S.M., Hii C.L., Law C.L., Suzannah S., Djaeni M. Effect of hot-air drying temperature on the polyphenol content and the sensory properties of cocoa beans. Int. Food Res. J. 2016;23:1479–1484. [Google Scholar]
- 37.Menon A.S., Hii C.L., Law C.L., Suzannah S., Djaeni M. Effects of water blanching on polyphenol reaction kinetics and quality of cocoa beans. AIP Conf. Proc. 2015;1699 [Google Scholar]
- 38.Urbańska B., Kowalska J. Comparison of the total polyphenol content and antioxidant activity of chocolate obtained from roasted and unroasted cocoa beans from different regions of the world. Antioxidants. 2019;8 doi: 10.3390/antiox8080283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Djikeng F.T., Teyomnou W.T., Tenyang N., Tiencheu B., Morfor A.T., Touko B.A.H., Houketchang S.N., Boungo G.T., Karuna M.S.L., Ngoufack F.Z., Womeni H.M. Effect of traditional and oven roasting on the physicochemical properties of fermented cocoa beans. Heliyon. 2018;4 doi: 10.1016/j.heliyon.2018.e00533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Santhanam Menon A., Hii C.L., Law C.L., Shariff S., Djaeni M. Effects of drying on the production of polyphenol-rich cocoa beans. Dry. Technol. 2017;35:1799–1806. [Google Scholar]
- 41.Albertini B., Schoubben A., Guarnaccia D., Pinelli F., Della Vecchia M., Ricci M., Di Renzo G.C., Blasi P. Effect of fermentation and drying on cocoa polyphenols. J. Agric. Food Chem. 2015;63:9948–9953. doi: 10.1021/acs.jafc.5b01062. [DOI] [PubMed] [Google Scholar]
- 42.Carrillo L.C., Londoño-Londoño J., Gil A. Comparison of polyphenol, methylxanthines and antioxidant activity in Theobroma cacao beans from different cocoa-growing areas in Colombia. Food Res. Int. 2014;60:273–280. [Google Scholar]
- 43.Sukha D.A., Bharath S.M., Ali N.A., Umaharan P. An assessment of the quality attributes of the Imperial College Selections (ICS) cacao (Theobroma cacao L.) clones. Acta Hortic. 2014;1047:237–244. [Google Scholar]
- 44.Zapata Bustamante S., Tamayo Tenorio A., Alberto Rojano B. Efecto de la fermentación sobre la actividad antioxidante de diferentes clones de cacao Colombiano. Rev. Cuba. Plantas Med. 2013;18:391–404. [Google Scholar]
- 45.de Oliveira T.B., Genovese M.I. Chemical composition of cupuassu (Theobroma grandiflorum) and cocoa (Theobroma cacao) liquors and their effects on streptozotocin-induced diabetic rats. Food Res. Int. 2013;51:929–935. [Google Scholar]
- 46.Żyżelewicz D., Budryn G., Oracz J., Antolak H., Kręgiel D., Kaczmarska M. The effect on bioactive components and characteristics of chocolate by functionalization with raw cocoa beans. Food Res. Int. 2018;113:234–244. doi: 10.1016/j.foodres.2018.07.017. [DOI] [PubMed] [Google Scholar]
- 47.Gültekin-Özgüven M., Berktaş I., Özçelik B. Influence of processing conditions on procyanidin profiles and antioxidant capacity of chocolates: optimization of dark chocolate manufacturing by response surface methodology. LWT - Food Sci. Technol. 2016;66:252–259. [Google Scholar]
- 48.Belščak-Cvitanović A., Durgo K., Gačina T., Horžić D., Franekić J., Komes D. Comparative study of cytotoxic and cytoprotective activities of cocoa products affected by their cocoa solids content and bioactive composition. Eur. Food Res. Technol. 2012;234:173–186. [Google Scholar]
- 49.Todorovic V., Milenkovic M., Vidovic B., Todorovic Z., Sobajic S. Correlation between antimicrobial, antioxidant activity, and polyphenols of alkalized/nonalkalized cocoa powders. J. Food Sci. 2017;82:1020–1027. doi: 10.1111/1750-3841.13672. [DOI] [PubMed] [Google Scholar]
- 50.Todorovic V., Redovnikovic I.R., Todorovic Z., Jankovic G., Dodevska M., Sobajic S. Polyphenols, methylxanthines, and antioxidant capacity of chocolates produced in Serbia. J. Food Compos. Anal. 2015;41:137–143. [Google Scholar]
- 51.Sarriá B., Martínez-López S., Sierra-Cinos J.L., Garcia-Diz L., Goya L., Mateos R., Bravo L. Effects of bioactive constituents in functional cocoa products on cardiovascular health in humans. Food Chem. 2015;174:214–218. doi: 10.1016/j.foodchem.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 52.Khan N., Monagas M., Andres-Lacueva C., Casas R., Urpí-Sardà M., Lamuela-Raventós R.M., Estruch R. Regular consumption of cocoa powder with milk increases HDL cholesterol and reduces oxidized LDL levels in subjects at high-risk of cardiovascular disease. Nutr. Metab. Cardiovasc. Dis. 2012;22:1046–1053. doi: 10.1016/j.numecd.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 53.Sarriá B., Mateos R., Sierra-Cinos J.L., Goya L., García-Diz L., Bravo L. Hypotensive, hypoglycaemic and antioxidant effects of consuming a cocoa product in moderately hypercholesterolemic humans. Food Funct. 2012;3:867–874. doi: 10.1039/c2fo10267f. [DOI] [PubMed] [Google Scholar]
- 54.Chen Y.F., Roan H.Y., Lii C.K., Huang Y.C., Wang T.S. Relationship between antioxidant and antiglycation ability of saponins, polyphenols, and polysaccharides in Chinese herbal medicines used to treat diabetes. J. Med. Plants Res. 2011;5:2322–2331. [Google Scholar]
- 55.Roda A., Lambri M. Changes in antioxidants and sensory properties of Italian chocolates and related ingredients under controlled conditions during an eighteen-month storage period. Nutrients. 2019;11:2719. doi: 10.3390/nu11112719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gammone M.A., Efthymakis K., Pluchinotta F.R., Bergante S., Tettamanti G., Riccioni G., Orazio N.D. Impact of chocolate on the cardiovascular health. Front. Biosci. - Landmark. 2018;23:852–864. doi: 10.2741/4620. [DOI] [PubMed] [Google Scholar]
- 57.Torri L., Frati A., Ninfali P., Mantegna S., Cravotto G., Morini G. Comparison of reduced sugar high quality chocolates sweetened with stevioside and crude stevia ‘green’ extract. J. Sci. Food Agric. 2017;97:2346–2352. doi: 10.1002/jsfa.8045. [DOI] [PubMed] [Google Scholar]
- 58.Godočiková L., Ivanišová E., Árvay J., Petrová J., Kačániová M. The comparison of biological activity of chocolates made by different technological procedures. Potravinarstvo. 2016;10:316–322. [Google Scholar]
- 59.Belščak-Cvitanović A., Komes D., Dujmović M., Karlović S., Biškić M., Brnčić M., Ježek D. Physical, bioactive and sensory quality parameters of reduced sugar chocolates formulated with natural sweeteners as sucrose alternatives. Food Chem. 2015;167:61–70. doi: 10.1016/j.foodchem.2014.06.064. [DOI] [PubMed] [Google Scholar]
- 60.Fernández V., Yee A., Sulbarán B., Peña J. Actividad antioxidante y contenido de polifenoles en chocolates comerciales venezolanos Antioxidant activity and polyphenol content in Venezuelan commercial chocolates. Rev. Fac. Agron. 2014;31:129–144. [Google Scholar]
- 61.Loffredo L., Perri L., Catasca E., Pignatelli P., Brancorsini M., Nocella C., De Falco E., Bartimoccia S., Frati G., Carnevale R., Violi F. Dark chocolate acutely improves walking autonomy in patients with peripheral artery disease. J. Am. Heart Assoc. 2014;3:1–11. doi: 10.1161/JAHA.114.001072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee E.S., Kum J.Y., Hwang Y.O., Tu O.J., Bin Jo H., Kim J.H., Chae Y.Z. Comparative study on antioxidant capacities and polyphenolic contents of commercially available cocoa-containing products. J. Korean Soc. Food Sci. Nutr. 2012;41:1356–1362. [Google Scholar]
- 63.Rodríguez-Ramiro M.M.I., Ramos S., López-Oliva E., Agis-Torres A., Gómez-Juaristi M., Mateos R., Bravo L., Goya L. Cocoa-rich diet prevents azoxymethane-induced colonic preneoplastic lesions in rats by restraining oxidative stress and cell proliferation and inducing apoptosis. Mol. Nutr. Food Res. 2011;55:1895–1899. doi: 10.1002/mnfr.201100363. [DOI] [PubMed] [Google Scholar]
- 64.Prayoga R.D., Murwani R., Anwar S. Polyphenol extracts from low quality cocoa beans: antioxidant, antibacterial and food colouring properties. Int. Food Res. J. 2013;20:3275–3281. [Google Scholar]
- 65.Oracz J., Nebesny E., Żyżelewicz D. Changes in the flavan-3-ols, anthocyanins, and flavanols composition of cocoa beans of different Theobroma cacao L. groups affected by roasting conditions. Eur. Food Res. Technol. 2015;241:663–681. [Google Scholar]
- 66.Nazaruddin R., Seng L.K., Hassan O., Said M. Effect of pulp preconditioning on the content of polyphenols in cocoa beans (Theobroma Cacao) during fermentation. Ind. Crop. Prod. 2006;24:87–94. [Google Scholar]
- 67.Quao J. University of Ghana; 2010. Effect of Pulp Preconditioning on the Biochemical Qualities and Flavour Precursor Formation during Fermentation of Ghanaian cocoa Beans. [Google Scholar]
- 68.Counet C., Ouwerx C., Rosoux D., Collin S. Relationship between procyanidin and flavor contents of cocoa liquors from different origins. J. Agric. Food Chem. 2004;52:6243–6249. doi: 10.1021/jf040105b. [DOI] [PubMed] [Google Scholar]
- 69.Schwan R.F. CRC Press; Boca Ratón, FL: 2015. Cocoa and Coffee Fermentations. [Google Scholar]
- 70.Niemenak N., Rohsius C., Elwers S., Omokolo Ndoumou D., Lieberei R. Comparative study of different cocoa (Theobroma cacao L.) clones in terms of their phenolics and anthocyanins contents. J. Food Compos. Anal. 2006;19:612–619. [Google Scholar]
- 71.Wollgast J., Anklam E. Review on polyphenols in Theobroma cacao: changes in composition during the manufacture of chocolate and methodology for identification and quantification. Food Res. Int. 2000;33:423–447. [Google Scholar]
- 72.ICONTEC Internacional . Cacao en grano; 2003. Norma Técnica Colombiana NTC 1252. [Google Scholar]
- 73.Guehi J.G., Irie S.G., Zahouli B., Fae A., Ban-Koffi L., Nemlin Effect of drying methods on the chemical quality traits of Cocoa Raw Material. Adv. J. Food Sci. Technol. 2010;2:184–190. [Google Scholar]
- 74.Gil M. Universidad Nacional de Colombia - Medellín; 2018. Aproximación quimiométrica del balance entre los compuestos neoformados y los responsables de la calidad desarrollados durante las etapas de poscosecha de cacaos especiales (Theobroma cacao L.) cultivados en Antioquia. [Google Scholar]
- 75.Mazor Jolić S., Radojčic Redovnikovic I., Marković K., Ivanec Šipušić D., Delonga K. Changes of phenolic compounds and antioxidant capacity in cocoa beans processing. Int. J. Food Sci. Technol. 2011;46:1793–1800. [Google Scholar]
- 76.Lee de L.H., Kang N.J., Lee K.M., Lee B.K., Kim J.H., Lee K.W. Cocoa polyphenols attenuate hydrogen peroxide-induced inhibition of gap-junction intercellular communication by blocking phosphorylation of connexin 43 via the MEK/ERK signaling pathway. J. Nutr. Biochem. 2010;21:680–686. doi: 10.1016/j.jnutbio.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 77.de Oliveira T.B., Rogero M.M., Genovese M.I. Poliphenolic-rich extracts from cocoa (Theobroma cacao L.) and cupuassu (Theobroma grandiflorum Willd. Ex Spreng. K. Shum) liquors: a comparison of metabolic effects in high-fat fed rats. PharmaNutrition. 2015;3:20–28. [Google Scholar]
- 78.Roda A., Lambri M. Changes in antioxidants and sensory properties of Italian chocolates and related ingredients under controlled conditions during an eighteen-month storage period. Nutrients. 2019;11 doi: 10.3390/nu11112719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Qin S., Hou D.X. Multiple regulations of Keap1/Nrf2 system by dietary phytochemicals. Mol. Nutr. Food Res. 2016;60:1731–1755. doi: 10.1002/mnfr.201501017. [DOI] [PubMed] [Google Scholar]
- 80.Flores M.E.J. Cocoa flavanols: natural agents with attenuating effects on metabolic syndrome risk factors. Nutrients. 2019;11 doi: 10.3390/nu11040751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Scapagnini G., Davinelli S., Di Renzo L., De Lorenzo A., Olarte H.H., Micali G., Cicero A.F., Gonzalez S. Cocoa bioactive compounds: significance and potential for the maintenance of skin health. Nutrients. 2014;6:3202–3213. doi: 10.3390/nu6083202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Baird L., Yamamoto M. The molecular mechanisms regulating the KEAP1-NRF2 pathway. Mol. Cell Biol. 2020;40 doi: 10.1128/MCB.00099-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Da Costa R.M., Rodrigues D., Pereira C.A., Silva J.F., Alves J.V., Lobato N.S., Tostes R.C. Nrf2 as a potential mediator of cardiovascular risk in metabolic diseases. Front. Pharmacol. 2019;10:1–12. doi: 10.3389/fphar.2019.00382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Qader M., Xu J., Yang Y., Liu Y., Cao S. Natural Nrf2 activators from juices, wines, coffee, and cocoa. Beverages. 2020;6:1–24. [Google Scholar]
- 85.He F., Ru X., Wen T. NRF2, a transcription factor for stress response and beyond. Int. J. Mol. Sci. 2020;21:1–23. doi: 10.3390/ijms21134777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mehrabani S A.R. Arab A, Mohammadi H, the effect of cocoa consumption on markers of oxidative stress: a systematic review and meta-analysis of interventional studies. Compl. Ther. Med. 2020;48:102240. doi: 10.1016/j.ctim.2019.102240. [DOI] [PubMed] [Google Scholar]
- 87.Petrone A.B., Gaziano J.M., Djoussé L. Effects of dark chocolate and cocoa products on endothelial function: a meta-analysis. Curr. Nutr. Rep. 2013;2:267–273. [Google Scholar]
- 88.Gong F., Yao S., Wan J., Gan X. Chocolate consumption and risk of heart failure: a Meta-Analysis of prospective studies. Nutrients. 2017;9:402. doi: 10.3390/nu9040402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Baharum Z., Akim A.M., Taufiq-Yap Y.H., Hamid R.A., Kasran R. In vitro antioxidant and antiproliferative activities of methanolic plant part extracts of Theobroma cacao. Molecules. 2014;19:18317–18331. doi: 10.3390/molecules191118317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Martín M.Á., Serrano A.B.G., Ramos S., Pulido M.I., Bravo L., Goya L. Cocoa flavonoids up-regulate antioxidant enzyme activity via the ERK1/2 pathway to protect against oxidative stress-induced apoptosis in HepG2 cells. J. Nutr. Biochem. 2010;21:196–205. doi: 10.1016/j.jnutbio.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 91.Cordero-Herrera I., Martín M.A., Goya L., Ramos S. Cocoa flavonoids protect hepatic cells against high-glucose-induced oxidative stress: relevance of MAPKs. Mol. Nutr. Food Res. 2015;59:597–609. doi: 10.1002/mnfr.201400492. [DOI] [PubMed] [Google Scholar]
- 92.Lee D.E., Kang N.J., Lee K.M., Lee B.K., Kim J.H., Lee K.W., Lee H.J. Cocoa polyphenols attenuate hydrogen peroxide-induced inhibition of gap-junction intercellular communication by blocking phosphorylation of connexin 43 via the MEK/ERK signaling pathway. J. Nutr. Biochem. 2010;21:680–686. doi: 10.1016/j.jnutbio.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 93.Chidambaram S.B., Bhat A., Ray B., Sugumar M., Muthukumar P., Manivasagam T., Justin A., Essa M.M., Guillemin G.J., Kishore M., Chidambaram S.B., Bhat A., Ray B., Sugumar M., Muthukumar P., Manivasagam T., Justin A., Sakharkar K. Cocoa beans improve mitochondrial biogenesis via PPAR γ/PGC1 α dependent signalling pathway in Cocoa beans improve mitochondrial biogenesis via PPAR γ/PGC1 α dependent signalling pathway in MPP + intoxicated human neuroblastoma cells ( SH-SY5Y ) †. Nutr. Neurosci. 2018:1–10. doi: 10.1080/1028415X.2018.1521088. [DOI] [PubMed] [Google Scholar]
- 94.Oleaga C., García M., Solé A., Ciudad C.J., Izquierdo-Pulido M., Noé V. CYP1A1 is overexpressed upon incubation of breast cancer cells with a polyphenolic cocoa extract. Eur. J. Nutr. 2012;51:465–476. doi: 10.1007/s00394-011-0231-2. [DOI] [PubMed] [Google Scholar]
- 95.Martins T.F., Palomino O.M., Álvarez-cilleros D., Martín M.A., Ramos S., Goya L. 2020. Cocoa Flavanols Protect Human Endothelial Cells from Oxidative Stress. [DOI] [PubMed] [Google Scholar]
- 96.Guo Y., Pan W., Liu S., Shen Z., Xu Y., Hu L. ERK/MAPK signalling pathway and tumorigenesis (Review) Exp. Ther. Med. 2020;19:1997–2007. doi: 10.3892/etm.2020.8454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kim J.E., Son J.E., Jung S.K., Kang N.J., Lee C.Y., Lee K.W., Lee H.J. Cocoa polyphenols suppress TNF-α-induced vascular endothelial growth factor expression by inhibiting phosphoinositide 3-kinase (PI3K) and mitogen-activated protein kinase kinase-1 (MEK1) activities in mouse epidermal cells. Br. J. Nutr. 2010;104:957–964. doi: 10.1017/S0007114510001704. [DOI] [PubMed] [Google Scholar]
- 98.Romier-Crouzet B., Van De Walle J., During A., Joly A., Rousseau C., Henry O., Larondelle Y., Schneider Y.J. Inhibition of inflammatory mediators by polyphenolic plant extracts in human intestinal Caco-2 cells. Food Chem. Toxicol. 2009;47:1221–1230. doi: 10.1016/j.fct.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 99.Dugo L., Belluomo M.G., Fanali C., Russo M., Cacciola F., MacCarrone M., Sardanelli A.M. Effect of cocoa polyphenolic extract on macrophage polarization from proinflammatory M1 to anti-inflammatory M2 state. Oxid. Med. Cell. Longev. 2017;2017:6293740. doi: 10.1155/2017/6293740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Taparia S.S., Khanna A. Procyanidin-rich extract of natural cocoa powder causes ROS-mediated caspase-3 dependent apoptosis and reduction of pro-MMP-2 in epithelial ovarian carcinoma cell lines. Biomed. Pharmacother. 2016;83:130–140. doi: 10.1016/j.biopha.2016.06.019. [DOI] [PubMed] [Google Scholar]
- 101.Rodríguez-Ramiro I., Ramos S., Bravo L., Goya L., Martín M.Á. Procyanidin B2 induces Nrf2 translocation and glutathione S-transferase P1 expression via ERKs and p38-MAPK pathways and protect human colonic cells against oxidative stress. Eur. J. Nutr. 2012;51:881–892. doi: 10.1007/s00394-011-0269-1. [DOI] [PubMed] [Google Scholar]
- 102.Rodríguez-Ramiro I., Ramos S., Bravo L., Goya L., Martín M.Á. Procyanidin B2 and a cocoa polyphenolic extract inhibit acrylamide-induced apoptosis in human Caco-2 cells by preventing oxidative stress and activation of JNK pathway. J. Nutr. Biochem. 2011;22:1186–1194. doi: 10.1016/j.jnutbio.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 103.Da Silva M., Jaggers G.K., Verstraeten S.V., Erlejman A.G., Fraga C.G., Oteiza P.I. Large procyanidins prevent bile-acid-induced oxidant production and membrane-initiated ERK1/2, p38, and Akt activation in Caco-2 cells. Free Radic. Biol. Med. 2012;52:151–159. doi: 10.1016/j.freeradbiomed.2011.10.436. [DOI] [PubMed] [Google Scholar]
- 104.Rebollo-Hernanz M., Zhang Q., Aguilera Y., Martín-Cabrejas M.A., de Mejia E.G. Relationship of the phytochemicals from coffee and cocoa by-products with their potential to modulate biomarkers of metabolic syndrome in vitro. Antioxidants. 2019;8:8080279. doi: 10.3390/antiox8080279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Żyżelewicz D., Zakłos-Szyda M., Juśkiewicz J., Bojczuk M., Oracz J., Budryn G., Miśkiewicz K., Krysiak W., Zduńczyk Z., Jurgoński A. Cocoa bean (Theobroma cacao L.) phenolic extracts as PTP1B inhibitors, hepatic HepG2 and pancreatic β-TC3 cell cytoprotective agents and their influence on oxidative stress in rats. Food Res. Int. 2016;89:946–957. [Google Scholar]
- 106.Rowley T.J., Bitner B.F., Ray J.D., Lathen D.R., Smithson A.T., Dallon B.W., Plowman C.J., Bikman B.T., Hansen J.M., Dorenkott M.R., Goodrich K.M., Ye L., O’Keefe S.F., Neilson A.P., Tessem J.S. Monomeric cocoa catechins enhance β-cell function by increasing mitochondrial respiration. J. Nutr. Biochem. 2017;49:30–41. doi: 10.1016/j.jnutbio.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 107.Martín M.Á., Cordero-Herrera I., Bravo L., Ramos S., Goya L. Cocoa flavanols show beneficial effects in cultured pancreatic beta cells and liver cells to prevent the onset of type 2 diabetes. Food Res. Int. 2014;63:400–408. [Google Scholar]
- 108.Martín M.Á., Ramos S., Cordero-Herrero I., Bravo L., Goya L. Cocoa phenolic extract protects pancreatic beta cells against oxidative stress. Nutrients. 2013;5:2955–2968. doi: 10.3390/nu5082955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Spadafranca A., Martinez Conesa C., Sirini S., Testolin G. Effect of dark chocolate on plasma epicatechin levels, DNA resistance to oxidative stress and total antioxidant activity in healthy subjects. Br. J. Nutr. 2010;103:1008–1014. doi: 10.1017/S0007114509992698. [DOI] [PubMed] [Google Scholar]
- 110.Leyva-Soto A., Chavez-Santoscoy R.A., Lara-Jacobo L.R., Chavez-Santoscoy A.V., Gonzalez-Cobian L.N. Daily consumption of chocolate rich in flavonoids decreases cellular genotoxicity and improves biochemical parameters of lipid and glucose metabolism. Molecules. 2018;23:1–12. doi: 10.3390/molecules23092220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Vázquez-Agell M., Urpi-Sarda M., Sacanella E., Camino-López S., Chiva-Blanch G., Llorente-Cortés V., Tobias E., Roura E., Andres-Lacueva C., Lamuela-Raventós R.M., Badimon L., Estruch R. Cocoa consumption reduces NF-κB activation in peripheral blood mononuclear cells in humans. Nutr. Metab. Cardiovasc. Dis. 2013;23:257–263. doi: 10.1016/j.numecd.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 112.Carnevale R., Loffredo L., Pignatelli P., Nocella C., Bartimoccia S., di Santo S., Martino F., Catasca E., Perri L., Violi F. Dark chocolate inhibits platelet isoprostanes via NOX2 down-regulation in smokers. J. Thromb. Haemost. 2012;10:125–132. doi: 10.1111/j.1538-7836.2011.04558.x. [DOI] [PubMed] [Google Scholar]
- 113.Davinelli S., Corbi G., Zarrelli A., Arisi M., Calzavara-Pinton P., Grassi D., De Vivo I., Scapagnini G. Short-term supplementation with flavanol-rich cocoa improves lipid profile, antioxidant status and positively influences the AA/EPA ratio in healthy subjects. J. Nutr. Biochem. 2018;61:33–39. doi: 10.1016/j.jnutbio.2018.07.011. [DOI] [PubMed] [Google Scholar]
- 114.Sarriá B., Martínez-López S., Sierra-Cinos J.L., García-Diz L., Mateos R., Bravo L. Regular consumption of a cocoa product improves the cardiometabolic profile in healthy and moderately hypercholesterolaemic adults. Br. J. Nutr. 2014;111:122–134. doi: 10.1017/S000711451300202X. [DOI] [PubMed] [Google Scholar]
- 115.Parsaeyan N., Mozaffari-Khosravi H., Absalan A., Mozayan M.R. Beneficial effects of cocoa on lipid peroxidation and inflammatory markers in type 2 diabetic patients and investigation of probable interactions of cocoa active ingredients with prostaglandin synthase-2 (PTGS-2/COX-2) using virtual analysis. J. Diabetes Metab. Disord. 2014;13:1–9. doi: 10.1186/2251-6581-13-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ibero-Baraibar I., Suárez M., Arola-Arnal A., Zulet M.A., Martinez J.A. Cocoa extract intake for 4 weeks reduces postprandial systolic blood pressure response of obese subjects, even after following an energy-restricted diet. Food Nutr. Res. 2016;60 doi: 10.3402/fnr.v60.30449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hong M.Y., Nulton E., Shelechi M., Hernández L.M., Nemoseck T. Effects of dark chocolate on azoxymethane-induced colonic aberrant crypt foci. Nutr. Cancer. 2013;65:677–685. doi: 10.1080/01635581.2013.789542. [DOI] [PubMed] [Google Scholar]
- 118.Zhang H.J., Deng A.J., Zhang Z.H., Yu Z.H., Liu Y., Peng S.Y., Wu L.Q., Qin H.L., Wang W.J. The protective effect of epicatechin on experimental ulcerative colitis in mice is mediated by increasing antioxidation and by the inhibition of NF-κB pathway. Pharmacol. Rep. 2016;68:514–520. doi: 10.1016/j.pharep.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 119.Giacometti J., Muhvić D., Pavletić A., Dudarić L. Cocoa polyphenols exhibit antioxidant, anti-inflammatory, anticancerogenic, and anti-necrotic activity in carbon tetrachloride-intoxicated mice. J. Funct. Foods. 2016;23:177–187. [Google Scholar]
- 120.Hong MY N.T., Nulton E., Shelechi M., Hernández L.M. Effects of dark chocolate on azoxymethane-induced colonic aberrant crypt foci. Nutr Cancer. 2013;65:677–685. doi: 10.1080/01635581.2013.789542. [DOI] [PubMed] [Google Scholar]
- 121.Cordero-Herrera R.S.I., Martín M.A., Goya L. Cocoa flavonoids protect hepatic cells against high-glucose-induced oxidative stress: relevance of MAPKs. Mol. Nutr. Food Res. 2015;59:597–609. doi: 10.1002/mnfr.201400492. [DOI] [PubMed] [Google Scholar]
- 122.Castro M.C., Villagarcía H., Nazar A., Arbeláez L.G., Massa M.L., Del Zotto H., Ríos J.L., Schinella G.R., Francini F. Cacao extract enriched in polyphenols prevents endocrine-metabolic disturbances in a rat model of prediabetes triggered by a sucrose rich diet. J. Ethnopharmacol. 2020;247:112263. doi: 10.1016/j.jep.2019.112263. [DOI] [PubMed] [Google Scholar]
- 123.Fernández-Vallinas S., Miguel M., Aleixandre A. Long-term antihypertensive effect of a soluble cocoa fiber product in spontaneously hypertensive rats. Food Nutr. Res. 2016;60 doi: 10.3402/fnr.v60.29418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Quiñones M., Muguerza B., Miguel M., Aleixandre A. Evidence that nitric oxide mediates the blood pressure lowering effect of a polyphenol-rich cocoa powder in spontaneously hypertensive rats. Pharmacol. Res. 2011;64:478–481. doi: 10.1016/j.phrs.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 125.Janevski M., Antonas K.N., Sullivan-Gunn M.J., McGlynn M.A., Lewandowski P.A. The effect of cocoa supplementation on hepatic steatosis, reactive oxygen species and LFABP in a rat model of NASH. Comp. Hepatol. 2011;10:1–13. doi: 10.1186/1476-5926-10-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cordero-Herrera I., Martín M.Á., Goya L., Ramos S. Cocoa intake ameliorates hepatic oxidative stress in young Zucker diabetic fatty rats. Food Res. Int. 2015;69:194–201. doi: 10.1002/mnfr.201400746. [DOI] [PubMed] [Google Scholar]
- 127.Żyżelewicz D., Oracz J., Bojczuk M., Budryn G., Jurgoński A., Juśkiewicz J., Zduńczyk Z. Effects of raw and roasted cocoa bean extracts supplementation on intestinal enzyme activity, biochemical parameters, and antioxidant status in rats fed a high-fat diet. Nutrients. 2020;12:1–17. doi: 10.3390/nu12040889. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.