Abstract
This paper presents the results of the research on the use of fish oil (FO) in combination with soybean oil (SO) in laying hens diet on physical and chemical properties of fresh eggs and those stored in a refrigerator for 28 d at + 4°C. Fatty acids (FA) profile, as well as thiobarbituric acid reactive substances (TBARS) values in yolks are also presented. The following feeding treatments have been used: C (control, without FO), E1 (0.3% FO + 4.7% SO), E2 (0.6% FO + 4.4% SO), E3 (0.9% FO + 4.1% SO), E4 (1.2% FO + 3.8% SO) and E5 (1.5% FO + 3.5% SO). Laying hens diets were balanced at the level of 176.10 g/kg crude protein and 11.50 MJ/kg ME. The results of the study showed that feeding treatments affected the relative shares of the eggs basic parts (P < 0.05). The egg storage duration significantly reduced Haugh units (HU), egg and albumen egg weight, and increased the yolk color intensity (P < 0.001). Fish oil share increment in the diets resulted in the EPA (eicosapentaenoic FA) content increase from 10.27 to 20.10 mg/100 g egg; DHA (docosahexaenoic FA) from 105.44 to 236.87 mg/100 g egg and ∑ n-3 PUFA (polyunsatureated FA) from 204.59 to 327.35 mg/100 g egg. The ∑ n-6 PUFA/∑ n-3 PUFA ratio decreased from 8.69 (C group) to 4.54 (E5 group). TBARS values were affected by feeding treatments as well as treatment-storage interactions (P < 0.01).
Key words: egg quality, eicosapentaenoic acid, docosahexaenoic acid n-6/n-3 polyunsaturated fatty acid ratio, TBARS
INTRODUCTION
Essential fatty acids (FA) eicosapentaenoic (EPA) and docosahexaenoic (DHA) are important for the organism growth and development having a special role in the prevention of coronary heart disease, hypertension, inflammation, autoimmune diseases, and cancer (Gogus and Smith, 2010; Fraeye et al., 2012; Pottel et al., 2014; Stupin et al., 2018). They cannot be synthesized in the human body but must be ingested through food. The diet of our ancestors contained lean meat and fish. It was richer in fruits and vegetables, and poorer in calories compared to today's diet. The daily meal contained less saturated FAs and in approximately equal ratios of n-6 and n-3 polyunsaturated FAs (Simopoulos, 1999). The ratio of n-6 polyunsaturated (n-6 PUFA) and n-3 polyunsaturated fatty acids (n-3 PUFA) was considerably more favorable (4:1) than today, since in human nutrition in Western countries this ratio reaches an unfavorable 25:1 (Simopoulos, 2010). Linoleic acid (LA) is a precursor of n-6 PUFA, and α-linolenic acid (ALA) is a precursor of n-3 PUFA. In poultry metabolism, LA is transformed into arachidonic fatty acid (AA) whereas ALA is metabolized into EPA and DHA. DHA synthesis is limited if the LA: ALA ratio is high since there is competition for the enzyme desaturase in both processes. At best, ALA is converted into EPA and DHA up to 4% according to Shahidi and Ambigaipalan (2018), and even less according to some authors. Today, researchers are focusing on enriching eggs with EPA and DHA because of their benefits to human health (Lee et al., 2019). Fraeye et al. (2012) recommended the use of sources rich in EPA and DHA in the laying hens diets. The vegetable and fish oil combination in the laying hens feeding has been effective (Kralik et al., 2008a, b; Škrtić et al., 2008; Lemahieu et al., 2015; Brelaz et al., 2019) in enriching eggs with n-3 PUFA. Fish oil is added to feed in limited quantities due to the possible occurrence of undesirable odors in eggs (Lawlor et al., 2010). Research of some authors indicated that the different fish oils added to laying hens feed resulted in a significant n-3 PUFA increase in egg yolks (Cherian et al., 2007; Kralik et al., 2008b; Mariod et al., 2015; Brelaz et al., 2019). Eggs with high n-3 PUFA content are called “Enriched” eggs (Cherian et al., 2007; Dong et al., 2018). Increasing of the ALA content along with EPA and DHA, including reduction of vegetable oils containing high levels of LA, is necessary to achieve a healthier meal for humans (Simopoulos, 2000).
Our study aimed to investigate the effect of the fish oil (0; 0.3; 0.6; 0.9; 1.2, and 1.5%), added into laying hens diets, on the physicochemical properties of eggs, the n-3 PUFA content in yolk lipids as well as oxidative changes in fresh eggs and those stored for 28 d in a refrigerator at + 4°C.
MATERIALS AND METHODS
Laying Hens and Feed
TETRA SL laying hens (540) were included in the study. They were divided into 6 groups: control and 5 groups with different fish oil shares (C 0.0, E1 0.3, E2 0.6, E3 0.9, E4 1.2, and E5 1.5%). The difference up to 5% was compensated by soybean oil. Each group consisted of 90 laying hens (10 repetitions × 9 hens). The laying hens’ groups were housed in the same facility in the enriched cages. The laying hens were 47 wk old at the beginning of the experiment. The housing conditions corresponded to the technological and microclimatic recommendations for the mentioned laying hens’ hybrid. Procedures on animals, necessary to obtain data presented in this paper, were conducted in accordance with Animal Protection Act (Narodne novine, 2017 and Narodne novine, 2019), and other valid legal acts determining welfare of farm animals, and are eligible for publication. Table 1 shows the composition of the basic diet modified for the purposes of the research according to the above fish and soybean oil combinations. The diets were of the same isoprotein (176.10 g/kg) and isoenergetic (11.50 MJ/kg ME) composition. Feeding and watering were ad libitum. The study, according to feeding treatments, lasted for 35 d.
Table 1.
Composition of basic diet for laying hens (control).
| Ingredients | % | Chemical analysis of diet | g/kg⁎⁎ |
|---|---|---|---|
| Corn Toasted soybean Soybean cake Sunflower cake Dehydrated alfalfa Limestone Monocalcium phosphate Yeast Salt Soybean oil Sal - CURB Nanofeed - zeolite Premix* Methionine |
48.50 3.00 22.33 5.00 1.67 10.33 1.33 0.50 0.33 5.00 0.33 0.33 1.20 0.15 |
Moisture Crude ash Crude protein Crude fat Crude fibre |
93.00 193.63 176.10 77.00 34.00 |
| Energy value of diet | |||
| Total | 100.00 | ME, MJ/kg | 11.50 |
Diets differed in the oil contents: C group 5% SO; E1 group 0.3% FO + 4.7% SO: E2 group 0.6% FO + 4.4% SO; E3 group 0.9% FO + 4.1% SO; E4 group 1.2% FO + 3.8% SO; E5 group 1.5% FO + 3.5% SO.
Premix (1 kg) contained: vitamin A 834000 IU, vitamin D3 208500 IU, vitamin E3 2085 mg, vitamin K3 167 mg, vitamin B1 150 mg, vitamin B2 374 mg, vitamin B6 200 mg, vitamin B12 918 μg, vitamin C 1860 mg, niacin 2085 mg, pantothenic acid 584 mg, folic acid 75 mg, biotin 7 mg, choline chloride 33600 mg, iron 2520 mg, iodine 76 mg, copper 425 mg, manganese 5640 mg, zinc 5175 mg, canthaxanthin 300 mg, selenium 0.30 mg (E1 group) and 0.45 mg (E2 group).
Reference methods applied for chemical analysis of diet: moisture HRN ISO 6496; ash HRN EN ISO 5984; crude protein HRN ISO 5983-2; crude fat HRN ISO 6492; crude fibre HRN EN ISO 6865, modified (Croatia standards, 2001; 2004; 2010).
Chemical analyses of the diet were conducted in 2 parallels, and the average was showed in the table. The egg samples, from the control and experimental groups, were collected for analysis after the laying hens were fed the modified diets for 5 wk.
Physical and Chemical Properties of Eggs
Aiming to calculate the shape index, the length and the width of the eggs were measured using a sliding scale, and these measurements were used to calculate the shape index by the following formula: SI = (egg width [mm] /egg length [mm]) × 100. The weights of eggs and their basic parts were measured by electronic scale BBK 422-6 DXS (Mettler Toledo, Albstadt, Germany). The shell strength was measured using automatic device Eggshell Force Gauge Model-II (Robotmation Co, Ltd., Tokyo, Japan), and the values were given in kg/cm2. The shell thickness was measured in the middle of the egg using an electronic micrometer and the average value was used. The pH values of albumen and yolk were measured using a digital pH meter MT Seven Easy (Mettler Toledo, Schwerzenbach, Switzerland) in fresh eggs and those stored for 28 d at +4°C. The albumen height, Haugh units (HU) and yolk color were measured by the EggMulti-Tester EMT-5200 device (Robotmation Co, Ltd.).
L class of eggs, 63 to 73 g according to Regulation on the quality of eggs (Narodne novine, 2006) was used for researching physical and chemical properties. Physical and chemical properties of eggs were tested on 360 eggs (60 eggs per group, 30 fresh and 30 eggs stored 28 d at + 4°C).
FA Analysis
Preparation of samples for the determination of FAs was performed by the method of Csapo et al. (1986). Gas liquid chromatography was done on a Bruker 430-GC apparatus (Billerica, MA), equipped with a FAMEWAX (RESTEK, Bellefonte, PA) type capillary column (30 m × 0.32 mm internal diameter, 0.25-µm film) and flame ionization detector. Characteristic operating conditions were as follows: injector temperature: 220°C, detector temperature: 230°C, helium flow: 25 mL/min. The oven temperature was graded: from 50 to 225°C: 6.0°C/min, 21 min at 225°C. A FA standard mixture (Supelco 37 Component FAME Mix) was used to identify individual FA in the chromatogram. Individual acids in diets and yolk lipids are expressed as a percentage of total FAs. The formula according to Stibilj et al. (1999) was used to calculate the FAs in mg/100 g of eggs. The FA content was determined on a total of 60 fresh eggs (10 samples per group).
Lipid Oxidation
Oxidation of lipids in yolks of fresh and stored eggs (28 d at 4°C) was determined using the TBARS (thiobarbituric acid reactive substances) value (μg malondialdehyde [MDA]/g egg yolk). The samples were prepared as follows: 10% trichloroacetic acid was added to the weighed egg yolk, the mixture was homogenized and centrifuged for 15 min at 5,500 × g, 4°C. After centrifugation, a solution of thiobarbituric acid (pH 2.5) was added to the supernatant. Then, the tubes were closed and immersed in water bath at 95°C for 30 min. After cooling, distilled water was added and the mixture was centrifuged for 15 minu at 5,500 × g, 4°C. The content of the colored product formed by the reaction of lipid peroxidation products with thiobarbituric acid was measured spectrophotometrically at 534 nm. The values obtained were compared with the standard curve prepared using standard malondialdehyde tetrabutylammonium salt (Sigma-Aldrich, Switzerland) and expressed as μg MDA/g of egg yolk. In total, 72 eggs were used for oxidation determination (6 per group in each term of measurement – fresh and stored eggs).
Statistical Analysis
The research results were processed by Statistica software (Tibco Software Inc, 2018). Statistical parameters presented were arithmetic mean (), SEM or SD. Testing of significant difference within a group and between the groups was conducted using the GLM procedure of multivariate analysis of variance (6 × 2). The calculated F value was compared to the critical theoretic F value at a significance level of 5%. Significance of differences between mean values was determined by Fisher's LSD test. Linear regression was used to show the association between the increase in fish oil content in laying hens and omega-3 deposition in egg yolks.
RESULTS AND DISCUSSION
Table 2 shows the individual FAs and their sums in total FAs according to feeding treatments: C (control group), and from E1 to E5 are experimental groups with the addition of 0.3, 0.6, 0.9, 1.2, and 1.5% fish oil in the laying hens diets. The laying hens of the C group were fed diet containing only ALA (4.04%), while laying hens of other groups were fed diets containing ALA, EPA, and DHA in certain shares depending on the addition of fish oil to the diets. The control diet (C group) contained 4.04% ∑ n-3 PUFA, and by increasing the fish oil share in the diets, the content of ∑ n-3 PUFA increased from 4.40 to 9.01%. At the same time, the n-6/n3 PUFA ratio decreased from 8.88 (0.3% fish oil) to 4.76 (1.5% fish oil).
Table 2.
Fatty acids in feed (% in total fatty acids).
| Feeding treatment |
||||||
|---|---|---|---|---|---|---|
| Fatty acid | C | E1 | E2 | E3 | E4 | E5 |
| Myristic (C14:0) | 0.17 | 1.07 | 0.59 | 0.90 | 1.38 | 1.71 |
| Pentadecanoic (C15:0) | 0.03 | 0.17 | 0.09 | 0.15 | 0.22 | 0.25 |
| Palmitic (C16:0) | 14.69 | 20.82 | 15.76 | 15.16 | 15.42 | 13.65 |
| Heptadecanoic (C17:0) | 0.13 | 0.25 | 0.14 | 0.21 | 0.25 | 0.24 |
| Stearic (C18:0) | 5.52 | 6.65 | 4.92 | 5.02 | 4.74 | 3.60 |
| Arachidic (C20:0) | 0.47 | 0.40 | 0.41 | 0.43 | 0.40 | 0.31 |
| Behenic (C22:0) | 0.91 | 0.24 | 0.18 | 0.40 | 0.19 | 0.26 |
| ∑ SFA | 21.92 | 29.61 | 22.08 | 22.27 | 22.60 | 20.03 |
| Palmitoleic (C16:1) | 0.24 | 0.89 | 0.65 | 0.92 | 1.34 | 1.72 |
| Oleic (C18:1 cis+trans) | 28.27 | 24.92 | 26.61 | 25.76 | 24.65 | 24.67 |
| Eicosenoic (C20:1) | 0.18 | 0.63 | 0.46 | 0.65 | 0.68 | 0.79 |
| Erucic (C22:1) | 1.17 | 0.45 | 0.54 | 1.12 | 0.41 | 0.91 |
| Σ MUFA | 29.86 | 26.89 | 28.26 | 28.45 | 27.08 | 28.09 |
| Linoleic (C18:2 n-6) | 44.17 | 39.09 | 44.40 | 43.64 | 42.90 | 42.87 |
| ∑ n-6 PUFA | 44.17 | 39.09 | 44.40 | 43.64 | 42.90 | 42.87 |
| α-linolenic (C18:3 n-3) | 4.04 | 3.38 | 4.39 | 3.88 | 4.06 | 4.15 |
| Eicosapentaenoic (C20:5 n-3) | 0.00 | 0.64 | 0.56 | 0.88 | 1.30 | 1.76 |
| Docozahexaenoic (C22:6 n-3) | 0.00 | 0.38 | 0.30 | 0.88 | 2.05 | 3.10 |
| ∑ n-3 PUFA | 4.04 | 4.40 | 5.25 | 5.64 | 7.41 | 9.01 |
| ∑ n-6/∑ n-3 PUFA | 10.93 | 8.88 | 8.46 | 7.74 | 5.79 | 4.76 |
Diets differed in the oil contents: C group 5% SO; E1 group 0.3% FO + 4.7% SO: E2 group 0.6% FO + 4.4% SO; E3 group 0.9% FO + 4.1% SO; E4 group 1.2% FO + 3.8% SO; E5 group 1.5% FO + 3.5% SO.
Tables 3 and 4 show indicators of the impact of feeding treatments (C, E1, E2, C3, E4, and E5) and storage time (fresh egg on first day and storage at 4°C in the refrigerator for 28 d), on the quality of table eggs. Feeding treatments and storage time did not have a significant influence (P ˃ 0.05) on the value of the shape index (%) and the shell thickness (mm). The egg shape index was uniform in all groups whereas in fresh eggs it averaged about 75.57% while in the stored ones 75.94%. The shell thickness ranged from 0.415 (E2) do 0.424 (E1) for fresh eggs and from 0.413 (E3) to 0.430 (C) mm for stored eggs. Feeding treatments and storage time affected (P < 0.05) the shares of eggs basic parts (shell, yolk, and egg white), weight and color of yolk whereas the storage time affected (P < 0.05) egg weight, shell strength, albumen weight, albumen height, HU, and yolk pH (P ˂ 0.05). Feeding treatment affected shell weight and albumen pH (P ˂ 0.05) while the interaction of treatment action and storage time was significant for yolk color and albumen pH (P ˂ 0.001). The egg storage duration reduced the average albumen height by 0.65 mm that is, from 5.61 mm to 4.96 mm, respectively. Furthermore, the egg storage duration also affected the decrease in HU values (P < 0.01), while the yolk color intensity increased (P < 0.01). However, the yolk color was also affected by the feeding treatment (P < 0.001). The decomposition products in yolks and albumen affected the pH values of yolks and albumen which increased during the storage.
Table 3.
Effect of feeding treatments and storage time on egg quality indicators ().
| Feeding treatment | Storage time | Egg shape index% | Egg weight g | Eggshell strength kg/cm2 | Eggshell thickness mm | Eggshell share % | Yolk share % | Albumen share % |
|---|---|---|---|---|---|---|---|---|
| C | Fresh | 75.95 | 65.48abc | 3.04ab | 0.421 | 12.67b | 25.73def | 61.59ab |
| Stored | 76.17 | 64.23bc | 2.84b | 0.430 | 12.88b | 28.35a | 58.75f | |
| E1 | Fresh | 74.55 | 67.19a | 2.86b | 0.424 | 12.78b | 26.85bcd | 60.36bcde |
| Stored | 74.38 | 65.35abc | 2.74b | 0.415 | 12.95b | 28.06ab | 58.96ef | |
| E2 | Fresh | 75.83 | 67.17a | 2.92ab | 0.415 | 12.36b | 25.38ef | 52.24a |
| Stored | 76.34 | 64.01c | 2.64b | 0.416 | 12.90b | 27.93abc | 59.15def | |
| E3 | Fresh | 75.76 | 65.63abc | 3.00ab | 0.421 | 12.35b | 25.48ef | 62.12a |
| Stored | 76.36 | 66.44ab | 3.06ab | 0.413 | 12.67b | 26.43de | 60.89abc | |
| E4 | Fresh | 75.77 | 65.17abc | 3.35a | 0.420 | 13.01b | 26.52de | 60.45bcd |
| Stored | 76.39 | 64.51bc | 2.63b | 0.421 | 12.99b | 26.88bcd | 60.13bcdef | |
| E5 | Fresh | 75.57 | 65.82abc | 2.84b | 0.417 | 12.90b | 26.06f | 62.03a |
| Stored | 75.98 | 65.80abc | 2.72b | 0.417 | 13.74a | 26.73cde | 59.52cdef | |
| SEM | 0.719 | 0.847 | 0.153 | 0.005 | 0.246 | 0.482 | 0.530 | |
| Source of variation | ||||||||
| Feeding treatment | 0.164 | 0.414 | 0.378 | 0.671 | 0.021 | 0.008 | 0.015 | |
| Storage time | 0.381 | 0.037 | 0.010 | 0.726 | 0.017 | ˂0.001 | ˂0.001 | |
| Interaction | 0.994 | 0.237 | 0.193 | 0.677 | 0.570 | 0.127 | 0.072 | |
Diets differed in the oil contents: C group 5% SO; E1 group 0.3% FO + 4.7% SO: E2 group 0.6% FO + 4.4% SO; E3 group 0.9% FO + 4.1% SO; E4 group 1.2% FO + 3.8% SO; E5 group 1.5% FO + 3.5% SO.
= mean value
Storage time: fresh eggs (1 d after laying) and stored eggs (28 d in a refrigerator at 4°C).
The exponents above the numbers in the columns indicate statistical significance between the groups at the significance level P ˂ 0.05.
Table 4.
Effect of feeding treatment and storage time on the internal quality of eggs ().
| Feeding treatment | Storagetime | Shell weightg | Yolk weightg | Albumen weightg | Albumen heightmm | HU | Yolk color | Albumen pH | Yolk pH |
|---|---|---|---|---|---|---|---|---|---|
| C | Fresh | 8.28bc | 16.83df | 40.36abc | 6.01a | 74.29a | 11.85cd | 8.51d | 5.81c |
| Stored | 8.27bc | 18.17ab | 37.77d | 5.14bcd | 69.41bcd | 11.05f | 8.81bc | 6.24a | |
| E1 | Fresh | 8.59ab | 18.04ab | 40.55abc | 5.75ab | 71.65abc | 12.05bc | 8.76ab | 5.79c |
| Stored | 8.47bc | 18.34a | 38.53cd | 4.69de | 67.14bc | 12.55a | 8.84bc | 5.99abc | |
| E2 | Fresh | 8.29bc | 17.05edf | 41.82a | 5.30bcd | 68.81bcd | 11.90cd | 8.74bc | 5.86bc |
| Stored | 8.25bc | 17.88abc | 37.87d | 5.20bcde | 67.11bc | 12.45a | 8.77bc | 6.09abc | |
| E3 | Fresh | 8.10c | 16.72df | 60.81ab | 5.69ab | 72.68ab | 11.70cde | 8.73c | 5.82bc |
| Stored | 8.40bc | 17.58abcd | 40.45abc | 5.20bcde | 66.57bc | 12.35ab | 8.79bc | 6.05abc | |
| E4 | Fresh | 8.48bc | 17.27bcde | 39.41bcd | 5.40abc | 69.68abcd | 11.35ef | 8.73c | 5.87bc |
| Stored | 8.38bc | 17.27bcde | 38.85bcd | 4.88cde | 65.31c | 12.30ab | 8.92a | 5.88abc | |
| E5 | Fresh | 8.48bc | 16.44f | 40.83ab | 5.49abc | 70.42abcd | 11.60de | 8.73c | 5.83bc |
| Stored | 8.95a | 17.46abcd | 39.38bcd | 4.65e | 65.34c | 12.65a | 8.81bc | 6.18ab | |
| SEM | 0.164 | 0.327 | 0.733 | 0.230 | 1.925 | 0.136 | 0.034 | 0.131 | |
| Sources of variation | |||||||||
| Feeding treatment | 0.029 | 0.006 | 0.249 | 0.235 | 0.472 | ˂0.001 | ˂0.001 | 0.817 | |
| Storage time | 0.380 | ˂0.001 | ˂0.001 | ˂0.001 | ˂0.001 | ˂0.001 | 0.503 | 0.022 | |
| Interaction | 0.356 | 0.368 | 0.141 | 0.342 | 0.826 | ˂0.001 | ˂0.001 | 0.172 | |
Diets differed in the oil contents: C group 5% SO; E1 group 0.3% FO + 4.7% SO: E2 group 0.6% FO + 4.4% SO; E3 group 0.9% FO + 4.1% SO; E4 group 1.2% FO + 3.8% SO; E5 group 1.5% FO + 3.5% SO.
= mean value.
Storage time: fresh eggs (1 d after laying) and stored eggs (28 d in a refrigerator at 4°C)
The exponents above the numbers in the columns indicate statistical significance between groups at the significance level P ˂ 0.05.
According to the contemporary literature (Tabidi, 2011; Kralik et al., 2014, 2020a), egg quality indicators, among many, are: weight, form index, yolk index, albumen thick layer height, HU, and shells thickness. Campo et al. (2000) and Halaj et al. (2000) pointed out that the pH values of yolk and albumen are important for egg freshness. The internal quality is characterized by albumen compact structure that should be clear without any stains. Egg storage conditions and duration reduce the nutritive value of eggs (Silversides and Budgell, 2004). Adamski et al. (2017) stated that the egg storage duration affected the albumen weight loss, change of albumen structure as well as increase of yolk and albumen pH value, being the case in this study where egg storage time affected (P < 0.05) egg weight, strength and share of shell, as well as yolk percentage increase and albumen percentage decrease (P < 0.001). The study shows that our results are consistent with the results of Silversides and Budgell (2004), as well as Adamski et al. (2017) in terms of the change in the relative shares of the eggs basic parts. During the egg storage, due to the water and gases passage from the albumen via the shell pores, the albumen weight decreased whereas the changes depended on the temperature and storage duration. The weight loss of the eggs stored in the refrigerator at + 4°C in this study was 1.90% in group C, and in the experimental groups E1 2.73, E2 2.17, E3 0.28, E4 1.01, and E5 1.5%. Perić et al. (2017) pointed out that egg quality depended on storage duration and laying hens age whereas both factors had a negative effect. Adamski et al. (2017) found out that during egg storage there was a decrease in albumen weight and increase in yolk volume. The same authors found out HU decrease from 72.27 to 71.60 at 4°C. By our results, regarding HU, it can be concluded that the eggs of E3 and E5 groups meet the original quality even after 28 d of refrigerator storage at 4°C. The decline of HU values depending on the storage duration and conditions was also confirmed by Scott and Silversides (2000), Samli et al. (2005) as well as Brown et al. (2018). A HU values decrease during storage of eggs in the refrigerator was also determined by Kralik et al. (2020a) who stated that HU decreased from 79.79 to 74.05 (P ˂ 0.001). Also, they noticed that, due to fish oil and microalgae share increase in the laying hens feed, there was a HU decrease in the eggs. However, differences were not significant (C = 78.92; E1 = 76.63 and E2 = 75.21; P = 0.07).
Ceylan et al. (2011) stated that the addition of fish and vegetable oils to the laying hen diets neither affected eggs weight, shares of basic parts in the egg nor the HU values of fresh eggs. Biochemical processes in yolk and albumen affected pH increase during the storage. At higher pH values, ovomucin was broken down faster in the albumen being partially converted into the yolk. In our study, albumen changes were affected by feeding treatments and interaction (P < 0.001) while in yolk by the storage duration (P < 0.05). Perić et al. (2017) pointed out that as eggs aged the yolk became lighter. The authors explained it as a consequence of the water transition from albumen into the yolk which is being diluted.
The results of our research are not consistent with their statements since the color of the yolk became darker during the storage. Ceylan et al. (2011) stated that the type and level of oil in hens feed affected the yolk color (P ˂ 0.01). Thus, they stated that the darkest yolk was in the eggs of laying hens fed a diet supplemented with fish oil in the amount of 1.5%. Our results are in line with their research results. As for the paper on the influence of laying hens on the omega 3 eggs production, Kralik et al. (2020a) stated that feeding treatment, egg storage duration as well as their interaction affected the yolk color intensity (P ˂ 0.001). The authors stated that the storage time of eggs enriched in omega-3 FA affected the yolk color intensity which became darker (fresh = 12.45 compared to storage = 13.26) being in harmony with our study.
The results of the FA content in mg/100 g of eggs are shown in Table 5. Significant differences in saturated FAs (SFA) were found out in pentadecaenoic (C15:0, P = 0.047), palmitic (C16:0, P = 0.014), stearic acid (C18:0, P = 0.006), as well as in ∑ SFA (P = 0.011). Also, significant differences (P < 0.001) of the total monounsaturated FAs (MUFA) were found out in palmitoleic (C16:1), heptadecenoic (C17:1), oleic (C18:1 cis + trans, P = 0.007), eicosanoic acid (C20:1, P = 0.016), as well as in ∑ MUFA (P = 0.003). Significant differences were also determined in polyunsaturated FAs (PUFA) in the content of linoleic (C18:2, P = 0.044), arachidonic acid (C20: 4 n-6, P < 0.001), as well as in ∑ n-6 PUFA (P = 0.021). The ∑ n-6/∑ n-3 PUFA ratio in the yolks of the group C eggs was 8.69 whereas in the groups with fish oil added into the laying hens feed it decreased from 7.33 to 4.54 (P < 0.001). In the control group of eggs, apart from ALA (99.15 mg/100 g of egg) and EPA in traces, DHA was (105.44 mg/100 g of egg) also found out although the diet fed laying hens of this group contained only ALA. It means that long-chain FAs are formed in the laying hens’ metabolism by the elongation and saturation processes. Differences in the content of ALA (P = 0.009), EPA, DHA and the content of ∑ n-3 PUFA between the egg groups were statistically highly significant (P < 0.001; Table 5).
Table 5.
Fatty acid profile in eggs (mg fatty acids/100 g eggs; )
| Fatty acid | C | E1 | E2 | E3 | E4 | E5 | SEM | P value |
|---|---|---|---|---|---|---|---|---|
| Myristic (C14:0) | 20.20 | 21.83 | 24.37 | 26.04 | 27.80 | 25.39 | 1.81 | 0.065 |
| Pentadecanoic (C15:0) | 4.18c | 4.13c | 4.57bc | 4.98abc | 5.62ab | 6.09a | 0.48 | 0.047 |
| Palmitic (C16:0) | 1585.93a | 1036.63bc | 1102.29bc | 1048.55bc | 892.94c | 1275.12ab | 128.2 | 0.014 |
| Heptadecanoic (C17:0) | 15.48 | 10.18 | 11.27 | 10.72 | 10.07 | 14.16 | 1.64 | 1.129 |
| Stearic (C18:0) | 564.06a | 309.23b | 339.91b | 320.45b | 245.86b | 381.67b | 53.1 | 0.006 |
| Heneicosanoic (C21:0) | 16.84 | 15.11 | 13.55 | 15.90 | 14.37 | 12.26 | 1.04 | 0.058 |
| Σ SFA | 2206.68a | 1397.11b | 1495.96b | 1426.65b | 1196.66b | 1714.71ab | 180.6 | 0.011 |
| Palmitoleic (C16:1) | 141.54b | 214.23a | 207.72a | 222.64a | 229.50a | 234.95a | 13.25 | ˂0.001 |
| Heptadecenoic (C17:1) | 11.50c | 15.33b | 16.55ab | 16.17ab | 18.78a | 17.68ab | 1.05 | ˂0.001 |
| Oleic (C18:1 cis+trans) | 2616.80b | 3194.05a | 3163.20a | 3184.77a | 3432.13a | 3175.97a | 133.5 | 0.007 |
| Eicosenoic (C20:1) | 12.38c | 15.21ab | 14.34bc | 16.21ab | 16.81a | 15.53ab | 0.84 | 0.016 |
| Σ MUFA | 2782.22b | 3438.81a | 3401.81a | 3439.80a | 3697.22a | 3444.13a | 140.8 | 0.003 |
| Linoleic (C18:2 n-6) | 1637.96a | 1724.57a | 1665.41a | 1671.58a | 1635.40a | 1389.15b | 71.7 | 0.044 |
| Eicosadienoic (C20:2 n-6) | 10.02 | 12.85 | 11.50 | 12.03 | 11.95 | 10.97 | 0.87 | 0.316 |
| Arachidonic (C20:4 n-6) | 130.52a | 140.22a | 125.20ab | 127.45a | 111.35b | 85.69 c | 5.45 | ˂0.001 |
| Σ n-6 PUFA | 1778.50a | 1877.64a | 1802.11a | 1811.07a | 1758.70a | 1485.81b | 75.4 | 0.021 |
| α-linolenic (C18:3 n-3) | 99.15a | 96.97a | 87.51a | 93.83a | 87.20a | 70.37 b | 5.225 | 0.009 |
| Eicosapentaenoic (C20:5 n-3) | * | 10.27d | 11.17d | 12.07c | 16.64b | 20.10a | 0.772 | ˂0.001 |
| Docosahexaenoic (C22:6 n-3) | 105.44e | 151.19d | 173.44cd | 188.59bc | 215.57ab | 236.87a | 12.76 | ˂0.001 |
| Σ n-3 PUFA | 204.59c | 258.44b | 272.12b | 294.49ab | 319.42a | 327.35a | 14.76 | ˂0.001 |
| n-6/n-3 PUFA | 8.69a | 7.33b | 6.63bc | 6.16cd | 5.58d | 4.54e | 0.282 | ˂0.001 |
Diets differed in the oil contents: C group 5% SO; E1 group 0.3% FO + 4.7% SO: E2 group 0.6% FO + 4.4% SO; E3 group 0.9% FO + 4.1% SO; E4 group 1.2% FO + 3.8% SO; E5 group 1.5% FO + 3.5% SO.
Abbreviations: MUFA, monounsaturated fatty acids; PUFA, polyunsaturated fatty acids; SFA, saturated fatty acids.
Detected in traces; = mean value.
Letters above the mean values in the rows indicate statistical significance at the level of P < 0.05.
Fish oil addition into the laying hens feed affected the eggs enrichment with ∑ n-3 PUFA (Lawlor et al., 2010). Mariod et al. (2015) stated that fish oil is a rich source of n-3 PUFA, and poorer in n-6 PUFA whereas the LA content is low. Ceylan et al. (2011) in their study, added 1.5 and 3.0% fish oil into laying hens feed which significantly (P < 0.01) increased the DHA content in yolk lipids. They found out 0.61 and 0.72% ALA as well as 2.43 and 3.16% DHA in the total FAs, respectively. The authors did not identify EPA in the yolk lipids while the content of linoleic and arachidonic acids as well as ALA and DHA was close to that one reported by Baucells et al. (2000) and Mazalli et al. (2004). According to a study by Basmacioglu et al. (2003), by using fish oil in laying hens diet, in yolk lipids can be deposited 0.71 ALA, 0.18 EPA, and 3.29% DHA in total FAs. These values are similar to the reported ones by Ceylan et al. (2011). In our study, an increasing trend of ALA, EPA, and DHA in yolks along with fish oil content increase in laying hens feed was also recorded. It is in line with results of the abovementioned authors. Trziszka et al. (2011) pointed out that fish and flaxseed oil combination significantly (P < 0.01) increased DHA content in yolk lipids. Yalcın and Unal (2010) obtained shares of n-3 PUFA in yolk lipids by adding 1.5% fish oil into the laying hens diet after 30 d of feeding: 0.49 ALA, 0.82 EPA, and 4.92% DHA (calculated mg/100 g eggs: 41.08 ALA, 68.74 EPA, and 412.44 DHA). Cachaldora et al. (2006) in their study found that increasing the proportion of marine fish oil in diet affects the increase in DHA content and decreased AA content in egg yolks, which is consistent with our results. A weaker synthesis of AA in the yolk was established by Ceylan et al. (2011) while adding fish oil in the laying hens diet. Mazalli et al. (2004) explained that there was a competition in the n-3 PUFA and n-6 PUFA metabolism in the enzyme Δ6 desaturase which directed FA biosynthesis. They have observed that the inclusion of flaxseed oil, which has a high level of LNA (n-3 PUFA), decreased the synthesis of AA (n-6 PUFA) from LA (n-6 PUFA), because LNA competes with LA by the same Δ6 desaturase enzyme. In our study, fish oil, which is rich in n-3 PUFA, was added to diet, and we assume that the decrease in AA content was caused by the same mechanism as suggested by Mazalli et al. (2004). Gonzalez-Esquerra and Leeson (2000) enriched eggs with 150 to 200 mg DHA/50 g egg and 45 to 60 mg EPA/50 g egg by adding fish oil into the laying hens feed at 60 g/kg. de Carvalho et al. (2009) achieved 187 mg DHA/yolk that is, total of 218 mg/yolk ∑ n-3 PUFA when using 2.4% fish oil in the diets.
Lawlor et al. (2010) used encapsulated fish oil in the laying hens diet (2, 4, and 6%). The eggs produced contained 65 to 73 mg ALA, 12-40 mg EPA and 96-262 mg DHA per egg. Hayat et al. (2009) pointed out that ∑ n-3 PUFA content increase in yolk lipids was accompanied by ∑ n-6 PUFA content decrease, especially AA. The authors believe that biosynthesis processes depend on the desaturase enzyme used in the conversion of ALA into EPA, as well as the conversion of LA into AA. Fredriksson et al. (2006) as well as Wu et al. (2019) stated that laying hens could convert ALA or EPA into DHA by their metabolism and deposit it in yolks, which is confirmed by our research. Lemahieu et al. (2015) pointed out that fish oil was a good source of EPA and DHA, and added to laying hens mainly enriched eggs with DHA and much less with EPA. It is in line with the results of our study. Yalcin (2017) found out that fish oil could be added up to 1.5% to laying hens diets to preserve the organoleptic properties of eggs. Brelaz et al. (2019) investigated the fish oil use (from fish processing waste) in the laying hens feed aiming to affect performance, egg quality and sensory properties of eggs. The oil contained 12.19% n-3 PUFA and 8.57% n-6 PUFA. Supplementation of oil to the feed was about, 0.5, 1.0, 1.5, 2.0, 2.5, and 3.0%. No effect on egg quality was found out. However, higher oil levels adversely affected consumption, egg weight, and odor. EPA and DHA are more rapidly incorporated into plasma and lipids membrane resulting in a faster functional effect compared to ALA. The n-6/n-3 PUFA ratio reduction is necessary to decrease a negative effect of AA excess and the eicosanoids formation. This case may occur if there is too much LA and AA in the feed and an adequate n-3 PUFA supply is not possible (Simopoulos, 2000). The phenomenon can be overcome if feeds containing vegetable oil rich in LA are reduced to prevent conversion encourage into AA. At the same time, the consumption of feeds rich in n-3 PUFA should be increased. Aiming to reduce a possible negative fish oil effect on the organoleptic eggs properties, the combination of oils was designed in our study so that the fish oil percentage did not exceed 1.5%.
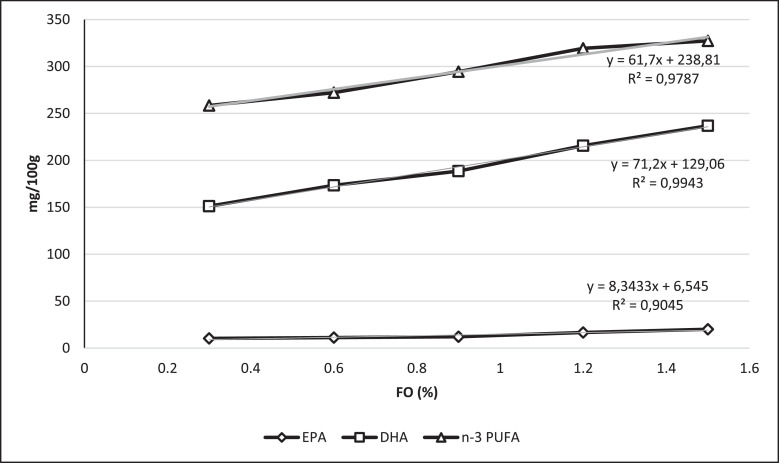
Figure 1 shows the correlation between the fish oil share increase in the laying hens diets on the EPA, DHA and n-3 PUFA deposition in egg yolk using linear regression. The values of the determination coefficients of EPA, DHA, and ∑ n-3 PUFA contents (R2 = 0.9045, 0.9943, and 0.9787, respectively) show that a high part of the content variability of these FAs in eggs (mg/100 g) can be explained by regression parameters. There was a significant correlation of EPA, DHA, and ∑ n-3 PUFA contents ((r = ); 0.951, 0.997, and 0.989; respectively) in yolk lipids and fish oil share increase in laying hens diets.
Figure 1.
Regression of EPA, DHA, and n-3 PUFA (mg/100 g egg) on the FO percentage in diets. Abbreviations: EPA, eicosapentaenoic acid; DHA, docosahexaenoic; n-3 PUFA, n-3 polyunsaturated fatty acids.
Table 6 shows the results of the lipid oxidation in egg yolks. The content of MDA is used as an indicator of lipid oxidation in egg yolk, which increases during egg storage, and is presented as TBARS. Accordingly, Cherian et al. (2007) pointed out that TBARS values were suitable for oxidation presentation in egg yolks. In our study, fresh yolk of the control group of eggs contained 0.892 μg MDA/g, and after 28 d of refrigerator storage, the MDA content increased to 1,016 µg MDA/g yolk. The n-3 PUFA content increased by 60.25% in the C to E5 group of eggs leading to TBARS values increase from 1,016 to 1,241 µg MDA/g in the stored eggs. Statistically significant (P < 0.01) impact of the feeding treatment as well as the interaction of treatment and storage time (fresh and stored eggs) on the value of lipid oxidation in yolks was determined by the variance analysis. Egg yolks of all groups contained a significant share of polyunsaturated FAs susceptible to oxidation, which also affected the TBARS values movement in both fresh eggs and eggs stored in the refrigerator for 28 d. Kralik et al. (2020b) found out that the eggs storage time had a significant effect on TBARS values between the fresh eggs and those stored for 28 d in the refrigerator (control group, 0.89: 1.08 μg MDA/g yolk, respectively; P = 0.026). Akter et al. (2014) found out similar TBARS values in the eggs also stored for 28 d in the refrigerator.
Table 6.
Lipid oxidation in the fresh and stored egg yolks ( ± sd; µg MDA/g)
| Feeding treatment | Storage time | µg MDA/g |
|---|---|---|
| C | Fresh | 0.892 ± 0.03f |
| Stored | 1.016 ± 0.10cdef | |
| E1 | Fresh | 0.939 ± 0.08def |
| Stored | 1.146 ± 0.02abcd | |
| E2 | Fresh | 0.976 ± 0.20bcdef |
| Stored | 1.111 ± 0.12abc | |
| E3 | Fresh | 0.990 ± 0.08fe |
| Stored | 1.141 ± 0.11abcde | |
| E4 | Fresh | 1.025 ± 0.21a |
| Stored | 1.200 ± 0.13ab | |
| E5 | Fresh | 1.185 ± 0.12cdef |
| Stored | 1.241 ± 0.11a | |
| Effect | P value | |
| Feeding treatment | 0.002 | |
| Storage time | 0.252 | |
| Interaction | 0.005 | |
Diets differed in the oil contents: C group 5% SO; E1 group 0.3% FO + 4.7% SO: E2 group 0.6% FO + 4.4% SO; E3 group 0.9% FO + 4.1% SO; E4 group 1.2% FO + 3.8% SO; E5 group 1.5% FO + 3.5% SO.
Storage time: fresh eggs (1 d after laying) and stored eggs (28 d in a refrigerator at 4°C).
= mean value.
Letters above the mean values in the row indicate statistical significance at the level P < 0.05.
As for the study dealing with the oxidative stability of lipids in omega-3 eggs, Ren et al. (2013) also pointed out that the TBARS value increase in egg yolks was significant during the egg storage (P ˂ 0.025). Their results are consistent with ours. Kralik et al. (2020a) reported a significant effect of feeding treatment on TBARS values in the stored eggs (P = 0.020). The authors stated that the lipid oxidation in the yolks of the experimental groups was more intensive, compared to the control group, since those eggs had a higher content of unsaturated FAs which were more susceptible to oxidative processes. The results of our research are in line with theirs.
Our study showed that the eggs storage for 28 d in a refrigerator at 4°C led to the original egg quality decrease whereas the yolks TBARS values increased. Also, the results indicated that fish oil share increase from 0.3 to 1.5% combined with soybean oil (from 3.5 to 4.7%) in laying hens diets resulted in the EPA content increase from 1.08 to 1.96, DHA from 1.43 to 2.24 and ∑ n-3 PUFA from 1.26 to 1.60 times. At the same time, there was a significant correlation between fish oil share increase in the laying hens diet and the content of EPA, DHA and ∑ n-3 PUFA in yolk lipids.
ACKNOWLEDGMENTS
This study is supported by the European Structural and Investment Funds grant for the Croatian National Scientific Center of Excellence for Personalized Health Care (grant #KK.01.1.1.01.0010) and by Ministry of Science and Education of the Republic of Croatia
DISCLOSURES
There are no conflicts of interests for any of the authors in the manuscript.
REFERENCES
- Adamski M., Kuźniacka J., Czarnecki R., Kucharska-Gaca J., Kowalska E. Variation in egg quality traits depending on storage conditions. Polish J. Nat. Sci. 2017;32:39–47. [Google Scholar]
- Akter Y., Kasim A., Omar H., Sazili A.Q. Effect of storage time and temperature on the quality characteristics of chicken eggs. J. Food Agric. Environ. 2014;12:87–92. [Google Scholar]
- Basmacioǧlu H., Çabuk M., Ünal K., Özkan K., Akkan S., Yalçin H. Effects of dietary fish oil and flax seed on cholesterol and fatty acid composition of egg yolk and blood parameters of laying hens. South Afr. J. Anim. Sci. 2003;33:266–273. [Google Scholar]
- Baucells M.D., Crespo N., Barroeta A.C., López-Ferrer S., Grashorn M.A. Incorporation of different polyunsaturated fatty acids into eggs. Poult. Sci. 2000;79:51–59. doi: 10.1093/ps/79.1.51. [DOI] [PubMed] [Google Scholar]
- Brelaz K.C.B.T.R., Cruz F.G.G., Brasil R.J.M., Silva A.F., Rufino J.P.F., Costa V.R., Viana Filho G.B. Fish waste oil in laying hens* Diets. Rev. Bras. Cienc. Avic. 2019;21:1–10. [Google Scholar]
- Brown C.A., Hamidu J.A., Adomako K., Okai M.A. Effects of omega-3 fatty acids enrichment with flaxseed oil and egg storage duration on egg quality. Livest. Res. Rural Dev. 2018;30:45. [Google Scholar]
- Cachaldora P., García-Rebollar P., Alvarez C., De Blas J.C., Méndez J. Effect of type and level of fish oil supplementation on yolk fat composition and n-3 fatty acids retention efficiency in laying hens. Br. Poult. Sci. 2006;47:43–49. doi: 10.1080/00071660500475541. [DOI] [PubMed] [Google Scholar]
- Campo J.L., Gil M.G., Muñoz I., Alonso M. Effects of breed, hen age, and egg storage on the indirect prediction of the albumen quality. Arch. Geflugelkd. 2000;64:109–114. [Google Scholar]
- de Carvalho P.R., Pita M.C.G., Neto E.P., de Mendonça C.X. Efficiency of PUFAs incorporation from marine sources in yolk egg's laying hens. Int. J. Poult. Sci. 2009;8:603–614. [Google Scholar]
- Ceylan N., Ciftçi I., Mızrak C., Kahraman Z., Efil H. Influence of different dietary oil sources on performance and fatty acid profile of egg yolk in laying hens. J. Anim. Feed Sci. 2011;20:71–83. [Google Scholar]
- Cherian G., Traber M.G., Goeger M.P., Leonard S.W. Conjugated linoleic acid and fish oil in laying hen diets: Effects on egg fatty acids, thiobarbituric acid reactive substances, and tocopherols during storage. Poult. Sci. 2007;86:953–958. doi: 10.1093/ps/86.5.953. [DOI] [PubMed] [Google Scholar]
- Csapo J., Sugar L., Horn A., Csapo-Kiss Z. Chemical composition of milk from red deer, roe and fallow deer kept in captivity. Acta Agron. Hungarica. 1986;36:359–372. [Google Scholar]
- Dong X.F., Liu S., Tong J.M. Comparative effect of dietary soybean oil, fish oil, and coconut oil on performance, egg quality and some blood parameters in laying hens. Poult. Sci. 2018;97:2460–2472. doi: 10.3382/ps/pey094. [DOI] [PubMed] [Google Scholar]
- Fraeye I., Bruneel C., Lemahieu C., Buyse J., Muylaert K., Foubert I. Dietary enrichment of eggs with omega-3 fatty acids: a review. Food Res. Int. 2012;48:961–969. [Google Scholar]
- Fredriksson, S., K. Elwinger, and J. Pickova. 2006. Food chemistry fatty acid and carotenoid composition of egg yolk as an effect of microalgae addition to feed formula for laying hens. 99:530–537.
- Gogus U., Smith C. N-3 omega fatty acids: a review of current knowledge. Int. J. Food Sci. Technol. 2010;45:417–436. [Google Scholar]
- Gonzalez-Esquerra R., Leeson S. Effect of feeding hens regular or deodorized menhaden oil on production parameters, yolk fatty acid profile, and sensory quality of eggs. Poult. Sci. 2000;79:1597–1602. doi: 10.1093/ps/79.11.1597. [DOI] [PubMed] [Google Scholar]
- Halaj M., Halaj P., Golian J., Valasek F., Moravcik F., Mélen M. The influence of storage time and temperature on weight loss in eggs and yolk pigmentation. Acta Fytotech. Zootech. 2000;3:52–54. [Google Scholar]
- Hayat Z., Cherian G., Pasha T.N., Khattak F.M., Jabbar M.A. Effect of feeding flax and two types of antioxidants on egg production, egg quality, and lipid composition of eggs. J. Appl. Poult. Res. 2009;18:541–551. [Google Scholar]
- Kralik G., Gajčević Z., Škrtić Z. The effect of different oil supplementations on laying performance and fatty acid composition of egg yolk. Ital. J. Anim. Sci. 2008;7:173–183. [Google Scholar]
- Kralik G., Grčević M., Hanžek D., Margeta P., Galović O., Kralik Z. Feeding to produce N-3 fatty acid-enriched table eggs. J. Poult. Sci. 2020;57:138–147. doi: 10.2141/jpsa.0190076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kralik Z., Kralik G., Grčević M., Galović D. Effect of storage period on the quality of table eggs. Acta Agrar. Kaposváriensis. 2014;18:200–206. [Google Scholar]
- Kralik G., Kralik Z., Grčević M., Hanžek D., Margeta P., Galović O. Enrichment of table eggs with lutein. Poljoprivreda. 2020;26:56–63. [Google Scholar]
- Kralik G., Škrtić Z., Suchý P., Straková E., Gajčević Z. Feeding fish oil and linseed oil to laying hens to increase the n-3 PUFA of egg yolk. Acta Vet. Brno. 2008;77:561–568. [Google Scholar]
- Lawlor J.B., Gaudette N., Dickson T., House J.D. Fatty acid profile and sensory characteristics of table eggs from laying hens fed diets containing microencapsulated fish oil. Anim. Feed Sci. Technol. 2010;156:97–103. [Google Scholar]
- Lee S.A., Whenham N., Bedford M.R. Review on docosahexaenoic acid in poultry and swine nutrition: Consequence of enriched animal products on performance and health characteristics. Anim. Nutr. 2019;5:11–21. doi: 10.1016/j.aninu.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemahieu C., Bruneel C., Ryckebosch E., Muylaert K., Buyse J., Foubert I. Impact of different omega-3 polyunsaturated fatty acid (n-3 PUFA) sources (flaxseed, Isochrysis galbana, fish oil and DHA Gold) on n-3 LC-PUFA enrichment (efficiency) in the egg yolk. J. Funct. Foods. 2015;19:821–827. [Google Scholar]
- Mariod A.A., Mukhtar M.A.E., Salih M.E., Herwan T. Effect of addition of fish oil on the performance parameters of laying hens and the fatty acid composition of their egg yolk. Am. J. Food Sci. Heal. 2015;1:38–42. [Google Scholar]
- Mazalli M.R., Faria D.E., Salvador D., Ito D.T. A comparison of the feeding value of different sources of fat for laying hens: 2. Lipid, cholesterol, and vitamin E profiles of egg yolk. J. Appl. Poult. Res. 2004;13:280–290. [Google Scholar]
- Narodne novine (Official Gazette of the Republic of Croatia) 115/2006. Rulebook on egg quality. 2006. https://narodne-novine.nn.hr/clanci/sluzbeni/2006_10_115_2561.html. Accessed July 2021.
- Narodne novine (Official Gazette of the Republic of Croatia) 102/2017. Animal Protection Act, 2017. https://narodne-novine.nn.hr/clanci/sluzbeni/2017_10_102_2342.html. Accessed July 2021.
- Narodne novine (Official Gazette of the Republic of Croatia) 32/2019. Animal Protection Act, 2019. https://narodne-novine.nn.hr/clanci/sluzbeni/2019_03_32_656.html. Accessed July 2021.
- Perić L., Đukić Stojčić M., Bjedov S. The effect of storage and age of hens on the quality of table eggs. Adv. Res. Life Sci. 2017;1:64–67. [Google Scholar]
- Pottel L., Lycke M., Boterberg T., Foubert I., Pottel H., Duprez F., Goethals L., Debruyne P. Omega-3 fatty acids: physiology, biological sources and potential applications in supportive cancer care. Phytochem. Rev. 2014;13:223–244. [Google Scholar]
- Ren Y., Perez T.I., Zuidhof M.J., Renema R.A., Wu J. Oxidative stability of omega-3 polyunsaturated fatty acids enriched eggs. J. Agric. Food Chem. 2013;61:11595–11602. doi: 10.1021/jf403039m. [DOI] [PubMed] [Google Scholar]
- Samli H.E., Agma A., Senkoylu N. Effects of storage time and temperature on egg quality in old laying hens. J. Appl. Poult. Res. 2005;14:548–553. [Google Scholar]
- Scott T.A., Silversides F.G. The effect of storage and strain of hen on egg quality. Poult. Sci. 2000;79:1725–1729. doi: 10.1093/ps/79.12.1725. [DOI] [PubMed] [Google Scholar]
- Shahidi F., Ambigaipalan P. Omega-3 polyunsaturated fatty acids and their health benefits. Annu. Rev. Food Sci. Technol. 2018;9:345–381. doi: 10.1146/annurev-food-111317-095850. [DOI] [PubMed] [Google Scholar]
- Silversides F.G., Budgell K. The relationships among measures of egg albumen height, pH, and whipping volume. Poult. Sci. 2004;83:1619–1623. doi: 10.1093/ps/83.10.1619. [DOI] [PubMed] [Google Scholar]
- Simopoulos A.P. Essential fatty acids in health and chronic disease. Am. J. Clin. Nutr. 1999;70:560–569. doi: 10.1093/ajcn/70.3.560s. [DOI] [PubMed] [Google Scholar]
- Simopoulos A.P. Human requirement for N-3 polyunsaturated fatty acids. Poult. Sci. 2000;79:961–970. doi: 10.1093/ps/79.7.961. [DOI] [PubMed] [Google Scholar]
- Simopoulos A.P. The omega-6/omega-3 fatty acid ratio: health implications. OCL. 2010;17:267–275. [Google Scholar]
- Škrtić Z., Kralik G., Gajčević Z., Hanžek D., Bogut I. Effect of different source of oils on fatty acid profile and organoleptic traits of eggs. Acta Agric. Slov. 2008;92(Suppl. 2):129–134. [Google Scholar]
- Stibilj V., Koman Rajšp M., Holcman A. Fatty acid composition of eggs enriched with omega-3 fatty acids on the market. Kmet. Zooteh. 1999;74:27–36. [Google Scholar]
- Stupin A., Rasic L., Matic A., Stupin M., Kralik Z., Kralik G., Grcevic M., Drenjancevic I. Omega-3 polyunsaturated fatty acids-enriched hen eggs consumption enhances microvascular reactivity in young healthy individuals. Appl. Physiol. Nutr. Metab. 2018;43:988–995. doi: 10.1139/apnm-2017-0735. [DOI] [PubMed] [Google Scholar]
- Tabidi M.H. Impact of storage period and quality on composition of table egg. Adv. Environ. Biol. 2011;5:856–861. [Google Scholar]
- Tibco Software Inc. 2018. Statistica. Accessed March 2021. https://www.tibco.com/products/tibco-statistica.
- Trziszka T., Dobrzanski Z., Kazmierska M., Tronina L., Skiba M. Effect of dietary humic-fatty preparations on egg quality in Lohmann Brown hens. Arch. Geflugelkd. 2011;75:84–90. [Google Scholar]
- Wu Y.B., Li L., Wen Z.G., Yan H.J., Yang P.L., Tang J., Xie M., Hou S.S. Dual functions of eicosapentaenoic acid-rich microalgae: enrichment of yolk with n-3 polyunsaturated fatty acids and partial replacement for soybean meal in diet of laying hens. Poult. Sci. 2019;98:350–357. doi: 10.3382/ps/pey372. [DOI] [PubMed] [Google Scholar]
- Yalcin H. Pages 373–381 in Egg Innovations and Strategies for Improvements. Elsevier Inc.; London, UK: 2017. Supplemental fish oil and its impact on n-3 fatty acids in eggs. [Google Scholar]
- Yalcın H., Unal M.K. The enrichment of hen eggs with ω-3 fatty acids. J. Med. Food. 2010;13:610–614. doi: 10.1089/jmf.2008.0024. [DOI] [PubMed] [Google Scholar]