Abstract
Objective: To characterize patients with diabetes treated with a tubeless insulin pump (Omnipod® Insulin Management System; Insulet Corp., Acton, MA), and to evaluate the frequency of acute complications with long-term use of the system.
Methods: This retrospective analysis of the German/Austrian Diabetes Patienten Verlaufsdokumentation (DPV) registry included data from 3657 patients with diabetes (n = 3582 type 1, n = 25 type 2, n = 50 latent autoimmune diabetes in adults/other) treated with a tubeless insulin pump. Hemoglobin A1c (HbA1c) levels and frequency of diabetic ketoacidosis (DKA) and severe hypoglycemia (SH) were compared between 1 year pre- and 1 year (n = 2911) or up to 3 years (n = 1311) post-tubeless insulin pump initiation and compared with a contemporary cohort on multiple daily injections (MDI) with 3-year data (n = 1874).
Results: Patients using tubeless insulin pump therapy had a median age of 13.7 years [interquartile range 10.8, 17.3], diabetes duration 3.7 years [1.7, 8.0], and HbA1c 7.5% [6.9, 8.2]. In patients with 3 years of follow-up data (n = 1311), the percentage with ≥1 episode of DKA, SH (Level 3, requiring assistance), and SH (coma) event with prior treatment was 6.3%, 5.5%, and 1.7%, respectively. After 3 years of tubeless insulin pump therapy, the frequency of DKA, SH (Level 3), and SH (coma) decreased to 2.2%, 4.1%, and 0.5%, respectively. Both DKA and SH remained significantly lower compared with MDI after adjustment in multiple regression analysis. High treatment retention rates (>90%) were observed.
Conclusion: Real-world registry data document that tubeless insulin pump therapy is associated with good glycemic control and a low frequency of DKA and SH in an age group prone to acute complications.
Keywords: Hypoglycemia, Diabetic ketoacidosis, CSII, DPV, Omnipod, patch pump
Introduction
Severe hypoglycemia (SH) and diabetic ketoacidosis (DKA) are acute complications of diabetes that can require treatment through emergency department visits and hospitalization, and can quickly escalate to life-threatening situations.1–8 These acute complications can also have a negative impact on patients' overall well-being and quality of life and contribute to diabetes-related psychological distress,9–14 in addition to the added burden of costs to patients, their families, and the health care system.15–20
Despite continued innovation in diabetes treatments and technologies, DKA and SH rates remain relatively high worldwide, with large national registry data showing estimates of 2% to 16% of patients affected per year, varying by age group, treatment modality, country, and other factors.2–8,21–29 Encouragingly, recent studies suggest that insulin pump use is associated with a lower proportion of subjects with acute complications than multiple daily injections (MDI) in youth with type 1 diabetes.3–6
A previous retrospective analysis of German/Austrian Diabetes Patienten Verlaufsdokumentation (DPV) registry data indicated that treatment with a tubeless insulin pump (Omnipod® Insulin Management System; Insulet Corp., Acton, MA) may be associated with improvement in glycemic outcomes in youth with type 1 diabetes compared with treatment with MDI.30 The objective of this study was to characterize patients of all ages with diabetes treated with a tubeless insulin pump in the German/Austrian DPV registry and to evaluate the frequency of acute complications after tubeless insulin pump initiation, which has not previously been reported.
Research Design and Methods
The German/Austrian/Swiss/Luxembourgian DPV registry has been described previously.31 In short, the DPV initiative collects data on patients with diabetes mellitus every 6 months using DPV software and the anonymized data are sent to the University of Ulm for aggregation into the database. The DPV initiative was established in 1995, approved by the University of Ulm Ethics Committee, and data collection was approved by local review boards.
We evaluated the clinical characteristics of all patients with diabetes treated with a tubeless insulin pump, as well as glycemic control and the frequency of acute complications post-tubeless insulin pump initiation compared with prior treatment. Patients with any diabetes diagnosis (type 1, type 2, and latent autoimmune diabetes in adults [LADA]/other) who initiated treatment with a tubeless insulin pump from January 1, 2013, to March 2019 were included in the summary of demographics (entire cohort).
Glycemic control and frequencies of acute complications over time were analyzed for patients who had at least 1 year of data before tubeless insulin pump initiation and at least 1 year of follow-up data available (referred to as total cohort), and for a subgroup of those patients with 3 years of follow-up data available. In addition, we compared this with MDI patients from the same centers that also had 3 years data available during this time period. For the MDI group, the “Year Prior” corresponds to the third year before last year evaluated.
Outcome measures
The demographics, including age, hemoglobin A1c (HbA1c), insulin dose, and body mass index-standard deviation score (BMI-SDS) using the international pediatric reference data from the World Health Organization (WHO) (www.who.int/childgrowth/standards/bmi_for_age/en/), of patients using the tubeless insulin pump were summarized, both overall and stratified by age group. Glycemic control and proportion of subjects with DKA and SH were analyzed for the year before switch to tubeless insulin pump (using prior treatment modality) and at 1, 2, and 3 years post-tubeless insulin pump initiation.
DKA was defined as pH <7.3 or bicarbonate concentration <15 mmol/L.32 To avoid skewing of the analysis, single patients with multiple DKA events per year were counted as 1. SH was evaluated both as SH (Level 3), defined as blood glucose (BG) <70 mg/dL or <3.9 mmol/L and requiring assistance from another person to actively administer carbohydrates, glucagon, or intravenous glucose, and SH (coma), defined as loss of consciousness or occurrence of seizures.33 Retention rate on tubeless insulin pump therapy was also determined.
Statistical methods
Results are presented as median (interquartile range, IQR) or mean (standard deviation) for continuous variables, and as proportions for binary variables. Kruskal–Wallis test was used for group comparisons of continuous variables. Nonparametric statistics were used because most outcome measurements were not normally distributed. Chi-squared test was used for comparison of dichotomous variables.
Multiple regression models were applied for the outcome variables HbA1c, total daily dose of insulin, and BMI SDS, and logistic regression models were applied for SH and DKA to control for differences in age, gender, and diabetes duration between treatment groups. Mathematical details of the regression models, as well as the implementation in the SAS software, are described elsewhere.34 Two-sided hypotheses were used throughout the analysis. A P-value <0.05 was considered statistically significant. The statistical analysis software package SAS, version 9.4 (SAS Institute, Carey, NC), was used for all analyses.
Results
The German/Austrian DPV registry includes 3657 patients with diabetes using the tubeless insulin management system. Within this cohort, 2911 patients had at least 1 year of data using prior treatment and 1 year of follow-up data post-tubeless insulin pump initiation. A subgroup of 1311 patients had 3 years of follow-up data post-tubeless insulin pump initiation.
Clinical characteristics
Clinical characteristics of the entire cohort of patients using the tubeless insulin pump as of March 2019 are summarized in Table 1. The distribution of patients by diagnosis is primarily type 1 diabetes (n = 3582, 98%), with a small number of patients with type 2 diabetes (n = 25) and LADA/other diagnoses (n = 50). Overall, patients were median (IQR): age 13.7 years (10.8, 17.3), diabetes duration 3.7 years (1.7, 8.0), and HbA1c 7.5% (6.9, 8.2). The majority of patients were <20 years old (n = 3023, 83%). Continuous glucose monitor (CGM) use was higher in the pediatric age group than in adults. The prior treatment modality for patients with type 1 diabetes was 58.5% MDI, 38.1% other pump, 3.2% tubeless insulin pump as initial therapy, and 0.3% unknown. The median duration of tubeless insulin pump use was 1.1 (0.1–2.7) years overall and comparable for the subset of patients with type 1 diabetes: 1.3 (0.1–2.3) years.
Table 1.
Clinical Characteristics of Tubeless Insulin Management System Users by Age Group (Entire Cohort)
| Age category, year |
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 0 to <5 | 5 to <10 | 10 to <15 | 15 to <20 | 20 to <30 | 30 to <40 | >40 | Overall | |
| n (%) | 77 (2.2) | 532 (15.4) | 1505 (39.8) | 909 (25.2) | 182 (4.6) | 161 (4.5) | 291 (8.4) | 3657 |
| Age, year | 3.9 (3.2, 4.3) | 7.8 (6.8, 8.9) | 12.3 (11.0, 13.4) | 16.4 (15.5, 17.5) | 24.7 (22.1, 27.2) | 34.2 (31.9, 37.1) | 50.9 (46.3, 57.9) | 13.7 (10.8, 17.3) |
| HbA1c, %a | 7.2 (6.9, 8.2) | 7.2 (6.7, 7.7) | 7.5 (6.7, 7.7) | 7.7 (7.0, 8.6) | 7.6 (6.8, 8.5) | 7.5 (6.8, 8.4) | 7.6 (7.1, 8.3) | 7.5 (6.9, 8.2) |
| Insulin dose, U/(kg·day)a | 0.65 (0.47, 0.78) | 0.66 (0.54, 0.77) | 0.76 (0.61, 0.95) | 0.80 (0.64, 1.0) | 0.60 (0.42, 0.77) | 0.47 (0.36, 0.60) | 0.47 (0.37, 0.50) | 0.71 (0.55, 0.90) |
| BMI-SDSa | 0.96 (0.07, 1.53) | 0.45 (−0.04, 0.92) | 0.36 (−0.25, 1.07) | 0.57 (−0.09, 1.20) | 0.80 (0.24, 1.68) | 0.79 (0.18, 1.69) | 1.22 (0.48, 2.01) | 0.55 (−0.09, 1.22) |
| DKA, %b | 1.3 | 4.5 | 3.6 | 3.6 | 3.8 | 3.7 | 2.7 | 3.9 |
| SH (coma), %b | 2.6 | 0.7 | 1.6 | 1.8 | 0 | 1.8 | 2.0 | 1.5 |
| SH (Level 3), %b | 9.0 | 5.4 | 5.8 | 4.7 | 1.9 | 1.0 | 5.5 | 5.4 |
| SMBG/daya | 7 (5, 9) | 6.5 (5, 8) | 5 (4, 7) | 5 (3.5, 6) | 5 (3.5, 6) | 4 (2, 6) | 4 (4, 6) | 5 (4, 7) |
| CGM (%)a | 33.8 | 38.6 | 37.6 | 31.4 | 18.6 | 17.4 | 13.7 | 32.7 |
| Previous therapy (tethered pump/MDI%)a | 58/42 | 35/65 | 23/76 | 27/73 | 66/33 | 81/17 | 77/18 | 38/59 |
| Retention rate (%) | 100 | 96.4 | 94.8 | 83.3 | 94.0 | 95.7 | 96.6 | 92.4 |
Results are presented as median (IQR).
In a small number of patients from each age group, data were missing from each measure (up to 9% of patients for HbA1c, 11% for BMI-SDS, 16% for insulin dose, and 18% for SMBG). For better comparison, SDS-BMI was also calculated in the adult population using the 18-year old reference values.
BMI-SDS, body mass index-standard deviation score; CGM, continuous glucose monitor; DKA, diabetic ketoacidosis; HbA1c, hemoglobin A1c; IQR, interquartile range; MDI, multiple daily injections; SH, severe hypoglycemia.
Glycemic control and frequency of acute complications for 3 years
The change in glycemic control and frequency of acute complications with long-term tubeless insulin pump use compared with prior treatment are summarized for patients with at least 1 year of data before switching to a tubeless insulin pump and at least 1 year of follow-up data (total cohort; n = 2911). In this population, there were n = 2873 diagnosed with type 1 diabetes, n = 10 with type 2 diabetes, and n = 28 with LADA/other. Fifty-eight percent had switched from MDI, 38% had switched from a tethered pump, and 3% started from onset with tubeless therapy, with children switching more frequently from MDI and adults more often from tethered pumps (Table 1). In the total cohort compared with the year before switch over the next 3 years, a continuous reduction of DKA was seen already in the first year (5.6% [(n = 2912] vs. 3.2% vs. 2.9% [n = 1921] vs. 2.2% [n = 1336])), and from year 2 a reduction in SH (level 3) (5.0% vs. 5.4% vs. 4.5% vs. 4.1%) and hypoglycemia with coma (1.3% vs. 1.4% vs. 0.9% vs. 0.6%) respectively, was seen, whereas Hba1c showed only a mild age-related increase from 7.5% to 7.7%. As the number of individuals with available data declined over the 3-year period, the analysis was repeated for the subgroup of patients (n = 1311) with data available for all 3 years of follow-up and a corresponding MDI cohort from the same centers (Table 2).
Table 2.
Glycemic Control and Frequency of Acute Complications for 3 Years of Tubeless Insulin Pump Therapy in Patients with 3-Year Follow-Up Compared with Prior Treatment and with Multiple Daily Injection Patients with 3-Year Data from the Same Centers During the Same Time Period
| |
MDIa |
Tubeless-pumpb |
||||||
|---|---|---|---|---|---|---|---|---|
| Year prior |
|
Year prior |
Post-tubeless insulin pump |
|||||
| Parameter | (n = 1874) | 1 year (n = 1874) | 2 years (n = 1874) | 3 years (n = 1874) | (n = 1311) | 1 year (n = 1311) | 2 years (n = 1311) | 3 years (n = 1311) |
| Age, year | 12.3 | 13.4 | 14.4 | 15.4 | 11.5 | 12.4 | 13.5 | 14.5 |
| Diabetes duration, year | 3.4 | 4.4 | 5.4 | 6.4 | 3.3 | 4.2 | 5.3 | 6.2 |
| HbA1c, %c | 7.4 | 7.5 | 7.6 | 7.8 | 7.5 | 7.4 | 7.7 | 7.7 |
| Insulin dose, U/(kg·day)c | 0.83 | 0.87 | 0.92 | 0.95 | 0.77 | 0.74 | 0.77 | 0.79 |
| BMI-SDSc | 0.41 | 0.46 | 0.49 | 0.54 | 0.41 | 0.49 | 0.53 | 0.59 |
| DKA, %d | 3.1 | 3.0 | 3.4 | 3.3 | 6.3 | 3.7 | 3.1 | 2.2 |
| SH (coma), %d | 2.5 | 2.4 | 1.9 | 1.9 | 1.7 | 1.4 | 1.1 | 0.5 |
| SH (level 3), %d | 8.4 | 7.3 | 6.4 | 6.3 | 5.6 | 6.3 | 5.0 | 4.1 |
| SMBG/day | 6 | 6 | 5 | 5 | 6 | 6 | 5 | 5 |
| CGM (%) | 1.6 | 1.5 | 6.6 | 29.2 | 6.7 | 16.3 | 31.0 | 44.8 |
Data are shown as median or proportion.
Patients with type 1 diabetes on MDI in same centers with >10 tubeless pumps and 3 years follow-up, same treatment years. For the MDI group, the “Year Prior” corresponds to the third year before last year evaluated.
Patients who had at least 1 year of data before tubeless insulin pump initiation and 3 years of follow-up data available.
In a small number of patients, data were missing from each measure (total cohort: up to 7% for HbA1c, 8% for BMI-SDS, and 9% for insulin dose; subgroup with 3 years. follow-up: up to 4% for HbA1c, 5% for BMI-SDS, and 5% for insulin dose).
Patients with ≥1 event per year, %. SH, severe hypoglycemia.
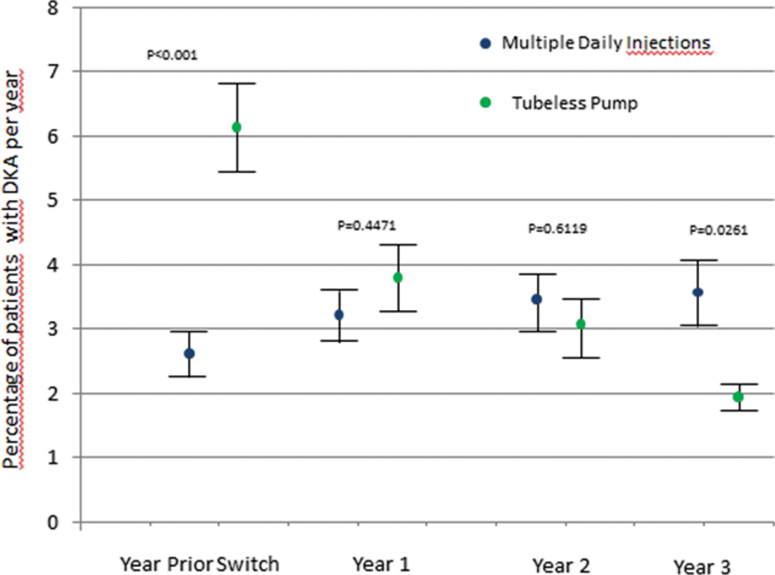
Initially, CGM use was higher in the tubeless pump group, and after increasing over time in both cohorts, CGM was used approximately by one-third of the patients in either group (Table 2). The annual rate of DKA was 6.3% with prior treatment, and decreased to 3.7%, 3.1%, and 2.2% in years 1, 2, and 3, respectively, of tubeless insulin pump therapy (Fig. 1). The frequency of SH (coma) was 1.7% with prior treatment, and decreased after 1, 2, and 3 years of tubeless insulin pump therapy to 1.4%, 1.1%, and 0.5%, respectively. The rate of SH (Level 3) was 5.5% with prior treatment and increased slightly to 6.3% in the first year of tubeless insulin pump therapy. In year 2, the proportion of subjects with SH (Level 3) was lower than with prior treatment and continued to decrease in year 3 (5.0% and 4.1%, respectively).
FIG. 1.
Data are shown for the cohort of 1311 patients with 3 years of follow-up data post-tubeless insulin pump initiation. Comparison of the estimated mean ± SEM of DKA (top panel) and SH (Level 3) (bottom panel) adjusted for age, diabetes duration, gender, baseline-HbA1c with either MDI (n = 1874) or tubeless pump (n = 1311) treated at the same centers during the same 3-year time period. For the MDI group, the “Year Prior” corresponds to the third year before last year evaluated. DKA, diabetic ketoacidosis; HbA1c, hemoglobin A1c; MDI, multiple daily injection.
The HbA1c was 7.5% with prior therapy, decreased slightly to 7.4% in the first year of tubeless insulin pump therapy, and increased to 7.7% in years 2 and 3. The decrease in the frequency of DKA, SH (coma), and SH (Level 3) ranged from 29% to 76% with tubeless insulin pump use compared with prior treatment in the total cohort and the subgroup with 3 years of follow-up data. The difference between tubeless pump and MDI remained significant after adjustment for age, diabetes duration, gender, and baseline HbA1c (Fig. 1) even after accounting for the differences in CGM use between pump and MDI (data not shown).
Retention rate
Discontinuation of tubeless insulin pump treatment in patients with type 1 diabetes was 7.4% and occurred after 0.9 ± 1.2 year. The retention rate was well above 90% for all age groups, except for young adults aged 15 to 20 years (retention rate was >80% for this age group) (Table 1). The discontinuation rate in the total cohort of 2911 patients was similar (7.5%).
Discussion
This retrospective analysis of the German/Austrian DPV registry is the first evaluation of acute complications with long-term tubeless insulin pump therapy, as well as the first characterization of tubeless insulin pump users of all ages outside of the United States. This analysis demonstrated that tubeless insulin pump therapy is associated with a low rate of SH and DKA in a primarily pediatric and adolescent population that is prone to these acute complications. The frequency of DKA and SH decreased after 3 years of tubeless insulin pump use compared with prior treatment modality and compares favorably with previous reports of youth using traditional insulin pumps from this and other large registries.3–8,27
Much of the decline of DKA was driven by the significantly higher rate at baseline compared with MDI. Potentially DKA episodes have contributed to the decision to switch the therapeutic regimen and/or led to additional measures. Nevertheless, over time the frequency of DKA and SH declines progressively after the switch and was found to be significantly lower than in a contemporary MDI group from the same centers after adjustment for common variables of influence such as age, diabetes duration, gender, HbA1c, or CGM use. Of course, this statistical difference may still be an artifact due to the limitations of real-world data and would need confirmation in a proper randomized controlled trial. Although the frequency of acute complications decreased in the 3 years after initiation of tubeless insulin pump therapy, glycemic control as measured by HbA1c remained consistent with or better than overall population data from this and other large registries.3,21,35,36 It is interesting that the HbA1c for people of ages from 15 to 30 years is higher than for other age groups, pointing to the challenges of the transition period from pediatric to adult care.
Glycemic control improved in the first year of tubeless insulin pump therapy, but increased moderately in years 2 and 3. Improvements in glycemic control with use of the tubeless pump in the first year were also reported in a previous analysis of the DPV registry30 and elsewhere.37 The trend toward an increased HbA1c beyond the first year may be explained by the expected age-related worsening in glycemic control associated with adolescents going through puberty, which is consistent with observations made previously both with tubeless30 and tethered pumps.38
In this retrospective analysis, high treatment retention rates (>90%) were observed in patients with type 1 diabetes of most age groups after 1 year of tubeless insulin pump use; young adults aged 15 to 20 years had a retention rate >80%. The high treatment retention is comparable with other real-world studies in pediatric and adult tubeless pump users.39,40
Adolescents have been reported to have the highest proportion of subjects with DKA, between 5.6% and 8.4% in the 13- to 17-year age group,4 and thus the comparatively low DKA frequency of 2.2% observed after 3 years of tubeless insulin pump therapy in this study is of clinical interest. Many younger tubeless insulin pump users had switched from MDI treatment, whereas in the age group >30 years, the majority of patients switched from a pump with catheter to a tubeless pump. This may account, in part, for the low proportion of subjects with DKA that is consistent with other reports, indicating that MDI use is associated with significantly higher rates of DKA in youth compared with traditional insulin pump use.3,6,21,25 However, the risk of DKA is still of concern as interruptions in insulin delivery and infusion site issues can quickly cause hyperglycemia, which can progress to DKA if untreated.25,33,41,42
Although several aspects of insulin pump therapy may contribute to improved glycemic outcomes, one may argue that the required pump site change at 72 h with this tubeless insulin pump system may potentially alleviate some of the infusion site issues that contribute to hyperglycemia and the increased potential for DKA.40,43 Of note, the frequency of DKA of 2.2% in this study compares favorably with the frequency of DKA reported for insulin pump users overall from the U.S. T1D Exchange (T1DX) registry (5.2% [4] and the German/Austrian DPV registry (3.4% [(3] to 5.2%).4
The proportion of subjects with SH (coma) was low initially but continued to decrease each year for 3 years' tubeless insulin pump use compared with prior treatment. The general limitation to real-world data is lacking information on the ascertainment rate that may underestimate the prevalence of SH. This finding of low SH (coma) is also consistent with previous research in which a significantly lower frequency of SH with insulin pump use has been observed compared with MDI.3,6,21 The frequency of 0.5% SH (coma) is lower than that reported for youth overall in the U.S. T1DX registry (4.9%)5 and for youth using insulin pumps in the German/Austrian DPV registry (1.8%).3 The decrease in SH (coma) observed with tubeless insulin pump use is important, as it may be associated with a commensurate decrease in hospital admissions and cost per year, as well as a decreased risk of mortality.44–46
Although the proportion of subjects with SH (Level 3) increased slightly during the first year of tubeless insulin pump therapy, in year 2, it was lower than the year before tubeless insulin pump initiation and continued to decrease further over time. In comparison, a recent study by Karges and colleagues of youth in the German/Austrian DPV registry found SH (Level 3) frequency to be 7.3% in MDI users and 5.5% in pump users, with a significantly higher rate of events per 100 patient-years in MDI users (P < 0.001).3 It is possible that SH is not frequently reported due to the inconsistency with which patients may interpret the definition of requiring assistance in the context of their experiences that could add variability to the results, potentially leading to a higher margin of error associated with this outcome.7,8 Nevertheless, both the improvements in DKA and hypoglycemia compare favorably with the contemporary MDI group in these centers.
Key strengths of this study include the large sample size, long-term follow-up, and the robust nature of the German/Austrian DPV registry. Limitations of the study are inherent in a retrospective design. In contrast to controlled trials, such real-world data on declining frequencies of acute complications associated with tubeless pumps preclude conclusions on causality. A direct comparison of these groups is compromised as no data is available on the clinical decision making for choosing MDI or tubeless pumps. Potential influencing factors such as educational status of the patient and/or their parents, training on nutrition, psychological support, or other additional care related to diabetes are not captured by the DPV registry.
At the time of the analysis, the only available tubeless pump in the DPV registry was the Omnipod. Possibly, similar associations could be seen with tethered pumps or other brands of tubeless pumps that are not currently available in Germany or Austria. In addition, other treatment changes made during the time period studied may have affected the results, including the adoption or discontinuation of a CGM34,35,47 or adjustments to pump therapy parameters. The principles of DPV preclude analyses that directly compare single commercial entities with each other. Thus, no comparative analysis was done between different brands of pumps or tubeless and tethered pumps. The results of the study may be influenced by the inclusion of some adults >20 years of age in the 3-year outcome data; however, the median age is indicative of a primarily pediatric/adolescent population. Compared with other large cohort studies, the study population was in fairly good glycemic control that may limit the generalizability of our findings.
Conclusions
This large retrospective analysis of the German/Austrian DPV registry demonstrated that tubeless insulin pump therapy is associated with a low frequency of SH and DKA in a primarily pediatric and adolescent population that is prone to these acute complications. Despite the typical age-dependent increase in HbA1c through adolescence, glycemic control with tubeless insulin pump use compares favorably with other large registry data.21,34 In addition, high treatment retention rates were observed in patients with type 1 diabetes of all ages initiating tubeless insulin pump use.
Acknowledgments
Special thanks to A. Hungele and R. Ranz for DPV documentation software support, and K. Fink and E. Bollow for DPV data management support (all clinical data manager, University of Ulm). Moreover, many thanks to all participating DPV centers for contributing anonymized data to this study (see hereunderSupporting Information). We also thank Irene Hadjiyianni, PhD, Lauren M. Huyett, PhD, and Jennifer E. Layne, PhD, of Insulet Corp. for their support.
Centers that have contributed to the German/Austrian Diabetes Patienten Verlaufsdokumentation (DPV) registry analysis:
Aachen—Innere RWT H, Aachen—Uni-Kinderklinik RWTH, Aalen Kinderklinik, Ahlen St. Franziskus Kinderklinik, Aidlingen Praxisgemeinschaft, Altötting Zentrum Inn-Salzach, Altötting-Burghausen Innere Medizin, Amberg Kinderklinik St. Marien,vAmstetten Klinikum Mostviertel Kinderklinik, Arnsberg-Hüsten Karolinenhosp. Kinderabteilung, Asbach Kamillus-Klinik Innere, Aue Helios Kinderklink, Augsburg IV. Med. Klinik, Augsburg Josefinum Kinderklinik, Augsburg Kinderklinik Zentralklinikum, Aurich Kinderklinik, Bad Aibling Internist. Praxis, Bad Driburg/Bad Hermannsborn Innere, Bad Hersfeld Innere, Bad Hersfeld Kinderklinik, Bad Kreuznach-St.Marienwörth-Innere, Bad Kreuznach-Viktoriastift, Bad Krozingen Klinik Lazariterhof Park-Klinikum, Bad Kösen Kinder-Rehaklinik, Bad Lauterberg Diabeteszentrum Innere, Bad Mergentheim—Diabetesfachklinik, Bad Mergentheim—Gemeinschaftspraxis DM-dorf Althausen, Bad Oeynhausen Herz-und Diabeteszentrum NRW, Bad Orb Spessart Klinik, Bad Orb Spessart Klinik Reha, Bad Reichenhall Kreisklinik Innere Med,Bad Salzungen Kinderklinik, Bad Säckingen Hochrheinklinik Innere, Bad Waldsee Kinderarztpraxis, Bautzen Oberlausitz KK, Bayreuth Innere Medizin, Berchtesgaden CJD, Berchtesgaden MVZ Innere Med, Berlin DRK-Kliniken Innere, Berlin DRK-Kliniken Pädiatrie, Berlin Endokrinologikum, Berlin Evang. Krankenhaus Königin Elisabeth, Berlin Klinik St. Hedwig Innere, Berlin Lichtenberg—Kinderklinik, Berlin Oskar Zieten Krankenhaus Innere, Berlin Parkklinik Weissensee, Berlin Schlosspark-Klinik Innere, Berlin St. Josephskrankenhaus Innere, Berlin Virchow-Kinderklinik, Berlin Vivantes Hellersdorf Innere, Bern Universitätsklinik InselSpital Innere Medizin, Bielefeld Kinderklinik Gilead, Bocholt Kinderklinik, Bochum Universitäts St. Josef, Bochum Universitätskinderklinik St. Josef, Bonn Uni-Kinderklinik, Bottrop Kinderklinik,Bottrop Knappschaftskrankenhaus Innere, Braunfels-Wetzlar Innere, Braunschweig Kinderarztpraxis,Bremen—Kinderklinik Nord, Bremen—Mitte Innere, Bremen Zentralkrankenhaus Kinderklinik, Bremerhaven Kinderklinik, Bruchweiler Edelsteinklinik Kinder-Reha Böblingen Kinderklinik, Castrop-Rauxel Rochus-Hospital, Celle Klinik für Kinder—und Jugendmedizin, Chemnitz Kinderklinik, Chemnitz-Hartmannsdorf Innere Medizin—DIAKOMED-1, Coburg Innere Medizin, Coburg Kinderklinik, Coesfeld Kinderklinik, Coesfeld/Dülmen Innere Med., Darmstadt Innere Medizin, Darmstadt Kinderklinik Prinz. Margaret, Datteln Vestische Kinderklinik, Deggendorf Gemeinschaftspraxis, Deggendorf Kinderklinik, Deggendorf Medizinische Klinik II, Deggendorf Pädiatrie-Praxis, Delmenhorst Kinderklinik, Dessau Kinderklinik, Detmold Kinderklinik, Dinslaken Kinderklinik, Dornbirn Innere Medizin, Dornbirn Kinderklinik, Dortmund Kinderklinik, Dortmund Knappschaftskrankenhaus Innere, Dortmund Medizinische Kliniken Nord, Dortmund-Hombruch Marienhospital, Dortmund-St. Josefshospital Innere, Dortmund-West Innere, Dresden Neustadt Kinderklinik, Dresden Uni-Kinderklinik, Duisburg Evang. und Johanniter Krhs Innere, Duisburg Malteser Rhein-Ruhr St. Anna Innere, Duisburg Malteser St. Johannes Duisburg Sana Kinderklinik, Duisburg-Huckingen, Duisburg-Huckingen Malteser Rhein-Ruhr ST. Johannes, Duisburg-St.Johannes Helios, Düren-Birkesdorf Kinderklinik, Düsseldorf Uni-Kinderklinik, Eberswalde Klinikum Barnim Werner Forßmann—Innere, Eisleben Lutherstadt Helios-Klinik, Erfurt Kinderklinik, Erlangen Uni Innere Medizin, Erlangen Uni-Kinderklinik, Essen Diabetes-Schwerpunktpraxis, Essen Elisabeth Kinderklinik, Essen Kinderarztpraxis, Essen Uni-Kinderklinik, Esslingen Klinik für Kinder und Jugendliche, Eutin Kinderklinik, Eutin St.-Elisabeth Innere, Feldkirch Kinderklinik, Filderstadt Kinderklinik, Flensburg Diakonissen Kinderklinik, Forchheim Diabeteszentrum SPP, Frankenthal Kinderarztpraxis, Frankfurt Diabeteszentrum Rhein-Main-Erwachsenendiabetologie (Bürgerhospital), Frankfurt Diabeteszentrum Rhein-Main-pädiat. Diabetologie (Clementine-Hospital), Frankfurt Uni-Kinderklinik, Frankfurt Uni-Klinik Innere, Frankfurt-Sachsenhausen Innere, Freiburg Kinder-MVZ, Freiburg St. Josef Kinderklinik, Freiburg Uni Innere, Freiburg Uni-Kinderklinik, Freudenstadt Kinderklinik Friedberg Innere Klinik, Friedrichshafen Kinderklinik, Fulda Innere Medizin, Fulda Kinderklinik, Fürth Kinderklinik, Gaissach Fachklinik der Deutschen Rentenversicherung Bayern Süd, Garmisch-Partenkirchen Kinderklinik, Geislingen Klinik Helfenstein Innere, Gelnhausen Innere, Gelnhausen Kinderklinik, Gelsenkirchen Kinderklinik Marienhospital, Gera Kinderklinik, Gießen Ev. Krankenhaus Mittelhessen, Gießen Uni-Kinderklinik, Graz Uni-Kinderklinik, Greifswald Uni-Kinderklinik, Göppingen Innere Medizin, Göppingen Kinderklinik am Eichert, Görlitz Städtische Kinderklinik, Göttingen Uni Gastroenterologie, Göttingen Uni-Kinderklinik, Güstrow Innere, Hachenburg Kinderpraxis, Hagen Kinderklinik, Halberstadt Innere Med. AMEOS Klinik, Halberstadt Kinderklinik AMEOS, Halle Uni-Kinderklinik, Halle-Dölau Städtische Kinderklinik, Hamburg Altonaer Kinderklinik, Hamburg Endokrinologikum, Hamburg Kinderklinik Wilhelmstift, Hamburg-Nord Kinder-MVZ, Hameln Kinderklinik Hamm Kinderklinik, Hanau Kinderklinik, Hanau St. Vincenz—Innere, Hannover Henriettenstift—Innere, Hannover Kinderklinik MHH, Hannover Kinderklinik auf der Bult, Haren Kinderarztpraxis, Heide Kinderklinik, Heidelberg St. Josefskrankenhaus, Heidelberg Uni-Kinderklinik, Heidelberg Uniklinik Innere, Heidenheim Arztpraxis Allgemeinmed, Heidenheim Kinderklinik, Heilbronn Innere Klinik, Heilbronn Kinderklinik, Herdecke Kinderklinik, Herford Innere Med I, Herford Kinderarztpraxis, Herford Klinikum Kinder & Jugendliche, Heringsdorf Inselklinik, Hermeskeil Kinderpraxis, Herne Evan. Krankenhaus Innere, Herten St. Elisabeth Innere Medizin, Herzberg Kreiskrankenhaus Innere, Hildesheim GmbH—Innere, Hildesheim Kinderarztpraxis, Hildesheim Kinderklinik, Hinrichsegen-Bruckmühl Diabetikerjugendhaus, Hof Kinderklinik, Homburg Uni-Kinderklinik Saarland, Idar Oberstein Innere, Ingolstadt Klinikum Innere, Innsbruck Uni-Kinderklinik Innsbruck Universitätsklinik Innere, Iserlohn Innere Medizin, Itzehoe Kinderklinik, Jena Uni-Kinderklinik, Kaiserslautern Kinderarztpraxis, Kaiserslautern-Westpfalzklinikum Kinderklinik, Kamen Klinikum Westfalen Hellmig Krankenhaus, Karlsburg Klinik für Diabetes & Stoffwechsel, Karlsruhe Städtische Kinderklinik, Kassel Klinikum Kinder—und Jugendmedizin, Kassel Rot-Kreuz-Krankenhaus Innere, Kassel Städtische Kinderklinik, Kaufbeuren Innere Medizin, Kempen Heilig Geist—Innere, Kempen Heilig Geist-KHS—Innere, Kempten Oberallgäu Kinderklinik, Kiel Städtische Kinderklinik, Kiel Universitäts-Kinderklinik, Kirchen DRK Krankenhaus Kinderklinik, Kirchheim-Nürtingen Innere, Klagenfurt Innere Med I, Kleve Innere Medizin, Koblenz Kemperhof 1. Med. Klinik, Koblenz Kinderklinik Kemperhof, Konstanz Innere Klinik, Konstanz Kinderklinik, Krefeld Alexianer Innere, Krefeld Innere Klinik, Krefeld Kinderklinik, Krefeld-Uerdingen St. Josef Innere, Kreischa-Zscheckwitz Klinik Bavaria, Köln Kinderklinik Amsterdamerstrasse, Köln Uni-Kinderklinik Landau/Annweiler Innere, Landesklinikum Korneuburg Stockerau, Landshut Kinderklink, Lappersdorf Kinderarztpraxis, Leer Kreiskrankenhaus—Kinderabt., Leipzig Uni-Kinderklinik, Leoben LKH Kinderklinik, Leverkusen Kinderklinik, Lienz BKH Kinderklinik, Lienz BKH Pädiatrie, Lienz Diabetesschwerpunktpraxis für Kinder und Jugendliche, Lilienthal Diabeteszentrum, Limburg Innere Medizin, Lindenfels Luisenkrankenhaus Innere, Lindenfels Luisenkrankenhaus Innere 2, Lingen Kinderklinik St. Bonifatius, Linz AKH—2. Med, Linz Krankenhaus Barmherzige Schwestern Kardiologie Abt. Int. II, Linz Krankenhaus der Barmherzigen Schwestern Kinderklinik, Linz Landes-Kinderklinik, Lippstadt Evangelische Kinderklinik, Ludwigsburg Innere Medizin, Ludwigsburg Kinderklinik, Ludwigshafen Kinderklinik St.Anna-Stift, Ludwigshafen diabetol. SPP, Luxembourg—Centre Hospitalier, Lübeck Uni-Kinderklinik, Lübeck Uni-Klinik Innere Medizin, Lüdenscheid Hilfswerk Kinder & Jugendliche, Lüdenscheid Märkische Kliniken—Kinder & Jugendmedizin, Lünen Klinik am Park, Magdeburg Städtisches Klinikum Innere, Magdeburg Uni-Kinderklinik, Mainz Uni-Kinderklinik Malchower See Rehaklinik, Mannheim Uni-Kinderklinik, Mannheim Uniklinik Innere Medizin, Marburg—UKGM Endokrinologie & Diabetes, Marburg Uni-Kinderklinik, Marktredwitz Innere Medizin, Marpingen-SPP, Mechernich Kinderklinik, Meissen Kinderklinik Elblandklinikum, Memmingen Internistische Praxis, Memmingen Kinderklinik, Merzig Kinderklinik, Minden Kinderklinik, Moers—St. Josefskrankenhaus Innere, Moers Kinderklinik, Murnau am Staffelsee—diabetol. SPP, Mutterstadt Kinderarztpraxis, Mödling Kinderklinik, Mölln Reha-Klinik Hellbachtal, Mönchengladbach Kinderklinik Rheydt Elisabethkrankenhaus, Mühlacker Enzkreiskliniken Innere, Mühldorf am Inn Kinderarztpraxis, München 3. Orden Kinderklinik, München Diabetes-Zentrum Süd, München Kinderarztpraxis diabet. SPP, München Schwerpunktpraxis, München von Haunersche Kinderklinik, München-Gauting Kinderarztzentrum, München-Harlaching Kinderklinik, München-Schwabing Kinderklinik, Münster Clemens-Hospital Innere, Münster Herz Jesu Innere, Münster St. Franziskus Kinderklinik Münster Uni-Kinderklinik, Münster pädiat. Schwerpunktpraxis, Nagold Kreiskrankenhaus Innere, Nauen Havellandklinik, Neuburg Kinderklinik, Neumarkt Innere, Neunkirchen Innere Medizin,Neunkirchen Marienhausklinik Kohlhof Kinderklinik, Neuruppin Kinderklinik, Neuss Lukaskrankenhaus Kinderklinik, Neuwied Kinderklinik Elisabeth, Neuwied Marienhaus Klinikum St. Elisabeth Innere, Nidda Bad Salzhausen Klinik Rabenstein/Innere-1 Reha, Nidda Bad Salzhausen Klinik Rabenstein/Innere-2 Reha, Nürnberg Cnopfsche Kinderklinik, Nürnberg Med. Klinik 4, Nürnberg Zentrum f Neugeb./Kinder & Jugendl., Oberhausen Innere, Oberhausen Kinderklinik, Oberhausen Kinderpraxis, Oberhausen St.Clemens Hospitale Sterkrade, Oberndorf Gastroenterologische Praxis Schwerpunkt Diabetologie, Offenbach/Main Innere Medizin, Offenbach/Main Kinderklinik, Offenburg Kinderklinik, Oldenburg Kinderklinik, Oldenburg Schwerpunktpraxis, Oschersleben MEDIGREIF Bördekrankenhaus, Osnabrück Christliches Kinderhospital, Osterkappeln Innere, Ottobeuren Kreiskrankenhaus, Oy-Mittelberg Hochgebirgsklinik Kinder-Reha, Paderborn St. Vincenz Kinderklinik Papenburg Marienkrankenhaus Kinderklinik, Passau Kinderarztpraxis, Passau Kinderklinik, Pforzheim Kinderklinik, Pfullendorf Innere Medizin, Pirmasens Städtisches Krankenhaus Innere, Plauen Vogtlandklinikum, Prenzlau Krankenhaus Innere, Rastatt Gemeinschaftspraxis, Rastatt Kreiskrankenhaus Innere, Ravensburg Kinderklink St. Nikolaus, Recklinghausen Dialysezentrum Innere, Regensburg Kinderklinik St. Hedwig, Remscheid Kinderklinik, Rendsburg Kinderklinik, Reutlingen Kinderarztpraxis, Reutlingen Kinderklinik, Reutlingen Klinikum Steinenberg Innere, Rheine Mathiasspital Kinderklinik, Ried Innkreis Barmherzige Schwestern, Rodalben St. Elisabeth, Rosenheim Innere Medizin, Rosenheim Kinderklinik, Rosenheim Schwerpunktpraxis, Rostock Uni-Kinderklinik, Rostock Universität Innere Medizin, Rotenburg/Wümme Agaplesion Diakonieklinikum Kinderabteilung, Rüsselsheim Kinderklinik, Saaldorf-Surheim Diabetespraxis, Saalfeld Thüringenklinik Kinderklinik, Saarbrücken Kinderklinik Winterberg, Saarbrücken Kinderklinik Winterberg 2, Saarlouis Kinderklinik Salzburg Universitäts-Kinderklinik, Scheibbs Landesklinikum, Scheidegg Prinzregent Luitpold, Scheidegg Reha-Kinderklinik Maximilian, Schw. Gmünd Stauferklinik Kinderklinik, Schweinfurt Kinderklinik, Schwerin Innere Medizin, Schwerin Kinderklinik, Schwäbisch Hall Diakonie Innere Medizin, Schwäbisch Hall Diakonie Kinderklinik, Siegen Kinderklinik, Singen—Hegauklinik Kinderklinik, Singen Kinderarztpraxis, Sinsheim Innere, Spaichingen Innere, St. Augustin Kinderklinik, St. Pölten Universitäts-Kinderklinik, St. Pölten Universitätsklinik Innere, Stade Kinderklinik, Stolberg Kinderklinik, Stuttgart Bethesda Agaplesion, Stuttgart Olgahospital Kinderklinik, Suhl Kinderklinik, Sylt Rehaklinik, Tettnang Innere Medizin, Timmendorfer Strand, Traunstein Kinderklinik, Traunstein diabetol. Schwerpunktpraxis, Trier Kinderklinik der Borromäerinnen, Trostberg Innere, Tübingen Uni-Kinderklinik, Ulm Agaplesion Bethesda-Krankenhaus, Ulm Endokrinologikum Ulm Schwerpunktpraxis Bahnhofsplatz, Ulm Uni Innere Medizin, Ulm Uni-Kinderklinik, Vechta Kinderklinik, Viersen Kinderkrankenhaus St. Nikolaus, Villach Kinderklinik, Villingen-Schwenningen SPP, Villingen-Schwenningen Schwarzwald Baar Klinikum Kinderklinik, Villingen-Schwenningen Schwarzwald-Baar-Klinikum Innere, Waldshut Kinderpraxis, Waldshut-Tiengen Kinderpraxis Biberbau, Wangen Oberschwabenklinik Innere Medizin, Waren-Müritz Kinderklinik, Weiden Kinderklinik, Weingarten Kinderarztpraxis, Weisswasser Kreiskrankenhaus, Wels Innere, Wels Klinikum Pädiatrie, Wernberg-Köblitz SPP, Wetzlar Schwerpunkt-Praxis, Wien 3. Med. Hietzing Innere, Wien Preyersches Kinderspital, Wien Rudolfstiftung, Wien SMZ Ost Donauspital, Wien Uni Innere Med III, Wien Uni-Kinderklinik, Wien Wilhelminenspital 5. Med. Abteilung, Wiesbaden Horst-Schmidt-Kinderkliniken, Wiesbaden Kinderklinik DKD, Wilhelmshaven Reinhard-Nieter-Kinderklinik, Wilhelmshaven St. Willehad Innere, Winnenden Rems-Murr Kinderklinik, Wismar Kinderklinik Wittenberg Innere Medizin, Wittenberg Kinderklinik, Wolgast Innere Medizin, Worms—Weierhof, Worms Kinderklinik, Wuppertal Kinderklinik, Zweibrücken Ev. KH. Innere, Zweibrücken Kinderarztpraxis.
Contributor Information
Collaborators: for the DPV Initiative, A. Hungele, R. Ranz, K. Fink, E. Bollow, Irene Hadjiyianni, Lauren M. Huyett, Jennifer E. Layne, and Aachen—Innere RWT H,Aachen—Uni-Kinderklinik RWTH,Aalen Kinderklinik, Ahlen St. Franziskus Kinderklinik, Aidlingen Praxisgemeinschaft, Altötting Zentrum Inn-Salzach, Altötting-Burghausen Innere Medizin, Amberg Kinderklinik St. Marien,vAmstetten Klinikum Mostviertel Kinderklinik, Arnsberg-Hüsten Karolinenhosp. Kinderabteilung, Asbach Kamillus-Klinik Innere, Aue Helios Kinderklink, Augsburg IV. Med. Klinik, Augsburg Josefinum Kinderklinik, Augsburg Kinderklinik Zentralklinikum, Aurich Kinderklinik, Bad Aibling Internist. Praxis, Bad Driburg/Bad Hermannsborn Innere, Bad Hersfeld Innere, Bad Hersfeld Kinderklinik, Bad Kreuznach-St.Marienwörth-Innere, Bad Kreuznach-Viktoriastift, Bad Krozingen Klinik Lazariterhof Park-Klinikum, Bad Kösen Kinder-Rehaklinik, Bad Lauterberg Diabeteszentrum Innere, Bad Mergentheim—Diabetesfachklinik, Bad Mergentheim—Gemeinschaftspraxis DM-dorf Althausen, Bad Oeynhausen Herz-und Diabeteszentrum NRW, Bad Orb Spessart Klinik, Bad Orb Spessart Klinik Reha, Bad Reichenhall Kreisklinik Innere Med,Bad Salzungen Kinderklinik, Bad Säckingen Hochrheinklinik Innere, Bad Waldsee Kinderarztpraxis, Bautzen Oberlausitz KK, Bayreuth Innere Medizin, Berchtesgaden CJD, Berchtesgaden MVZ Innere Med, Berlin DRK-Kliniken Innere, Berlin DRK-Kliniken Pädiatrie, Berlin Endokrinologikum, Berlin Evang. Krankenhaus Königin Elisabeth, Berlin Klinik St. Hedwig Innere, Berlin Lichtenberg—Kinderklinik, Berlin Oskar Zieten Krankenhaus Innere, Berlin Parkklinik Weissensee, Berlin Schlosspark-Klinik Innere, Berlin St. Josephskrankenhaus Innere, Berlin Virchow-Kinderklinik, Berlin Vivantes Hellersdorf Innere, Bern Universitätsklinik InselSpital Innere Medizin, Bielefeld Kinderklinik Gilead, Bocholt Kinderklinik, Bochum Universitäts St. Josef, Bochum Universitätskinderklinik St. Josef, Bonn Uni-Kinderklinik, Bottrop Kinderklinik,Bottrop Knappschaftskrankenhaus Innere, Braunfels-Wetzlar Innere, Braunschweig Kinderarztpraxis,Bremen—Kinderklinik Nord, Bremen—Mitte Innere, Bremen Zentralkrankenhaus Kinderklinik, Bremerhaven Kinderklinik, Bruchweiler Edelsteinklinik Kinder-Reha Böblingen Kinderklinik, Castrop-Rauxel Rochus-Hospital, Celle Klinik für Kinder—und Jugendmedizin, Chemnitz Kinderklinik, Chemnitz-Hartmannsdorf Innere Medizin—DIAKOMED-1, Coburg Innere Medizin, Coburg Kinderklinik, Coesfeld Kinderklinik, Coesfeld/Dülmen Innere Med., Darmstadt Innere Medizin, Darmstadt Kinderklinik Prinz. Margaret, Datteln Vestische Kinderklinik, Deggendorf Gemeinschaftspraxis, Deggendorf Kinderklinik, Deggendorf Medizinische Klinik II, Deggendorf Pädiatrie-Praxis, Delmenhorst Kinderklinik, Dessau Kinderklinik, Detmold Kinderklinik, Dinslaken Kinderklinik, Dornbirn Innere Medizin, Dornbirn Kinderklinik, Dortmund Kinderklinik, Dortmund Knappschaftskrankenhaus Innere, Dortmund Medizinische Kliniken Nord, Dortmund-Hombruch Marienhospital, Dortmund-St. Josefshospital Innere, Dortmund-West Innere, Dresden Neustadt Kinderklinik, Dresden Uni-Kinderklinik, Duisburg Evang. und Johanniter Krhs Innere, Duisburg Malteser Rhein-Ruhr St. Anna Innere, Duisburg Malteser St. Johannes Duisburg Sana Kinderklinik, Duisburg-Huckingen, Duisburg-Huckingen Malteser Rhein-Ruhr ST. Johannes, Duisburg-St.Johannes Helios, Düren-Birkesdorf Kinderklinik, Düsseldorf Uni-Kinderklinik, Eberswalde Klinikum Barnim Werner Forßmann—Innere, Eisleben Lutherstadt Helios-Klinik, Erfurt Kinderklinik, Erlangen Uni Innere Medizin, Erlangen Uni-Kinderklinik, Essen Diabetes-Schwerpunktpraxis, Essen Elisabeth Kinderklinik, Essen Kinderarztpraxis, Essen Uni-Kinderklinik, Esslingen Klinik für Kinder und Jugendliche, Eutin Kinderklinik, Eutin St.-Elisabeth Innere, Feldkirch Kinderklinik, Filderstadt Kinderklinik, Flensburg Diakonissen Kinderklinik, Forchheim Diabeteszentrum SPP, Frankenthal Kinderarztpraxis, Frankfurt Diabeteszentrum Rhein-Main-Erwachsenendiabetologie (Bürgerhospital), Frankfurt Diabeteszentrum Rhein-Main-pädiat. Diabetologie (Clementine-Hospital), Frankfurt Uni-Kinderklinik, Frankfurt Uni-Klinik Innere, Frankfurt-Sachsenhausen Innere, Freiburg Kinder-MVZ, Freiburg St. Josef Kinderklinik, Freiburg Uni Innere, Freiburg Uni-Kinderklinik, Freudenstadt Kinderklinik Friedberg Innere Klinik, Friedrichshafen Kinderklinik, Fulda Innere Medizin, Fulda Kinderklinik, Fürth Kinderklinik, Gaissach Fachklinik der Deutschen Rentenversicherung Bayern Süd, Garmisch-Partenkirchen Kinderklinik, Geislingen Klinik Helfenstein Innere, Gelnhausen Innere, Gelnhausen Kinderklinik, Gelsenkirchen Kinderklinik Marienhospital, Gera Kinderklinik, Gießen Ev. Krankenhaus Mittelhessen, Gießen Uni-Kinderklinik, Graz Uni-Kinderklinik, Greifswald Uni-Kinderklinik, Göppingen Innere Medizin, Göppingen Kinderklinik am Eichert, Görlitz Städtische Kinderklinik, Göttingen Uni Gastroenterologie, Göttingen Uni-Kinderklinik, Güstrow Innere, Hachenburg Kinderpraxis, Hagen Kinderklinik, Halberstadt Innere Med. AMEOS Klinik, Halberstadt Kinderklinik AMEOS, Halle Uni-Kinderklinik, Halle-Dölau Städtische Kinderklinik, Hamburg Altonaer Kinderklinik, Hamburg Endokrinologikum, Hamburg Kinderklinik Wilhelmstift, Hamburg-Nord Kinder-MVZ, Hameln Kinderklinik Hamm Kinderklinik, Hanau Kinderklinik, Hanau St. Vincenz—Innere, Hannover Henriettenstift—Innere, Hannover Kinderklinik MHH, Hannover Kinderklinik auf der Bult, Haren Kinderarztpraxis, Heide Kinderklinik, Heidelberg St. Josefskrankenhaus, Heidelberg Uni-Kinderklinik, Heidelberg Uniklinik Innere, Heidenheim Arztpraxis Allgemeinmed, Heidenheim Kinderklinik, Heilbronn Innere Klinik, Heilbronn Kinderklinik, Herdecke Kinderklinik, Herford Innere Med I, Herford Kinderarztpraxis, Herford Klinikum Kinder & Jugendliche, Heringsdorf Inselklinik, Hermeskeil Kinderpraxis, Herne Evan. Krankenhaus Innere, Herten St. Elisabeth Innere Medizin, Herzberg Kreiskrankenhaus Innere, Hildesheim GmbH—Innere, Hildesheim Kinderarztpraxis, Hildesheim Kinderklinik, Hinrichsegen-Bruckmühl Diabetikerjugendhaus, Hof Kinderklinik, Homburg Uni-Kinderklinik Saarland, Idar Oberstein Innere, Ingolstadt Klinikum Innere, Innsbruck Uni-Kinderklinik Innsbruck Universitätsklinik Innere, Iserlohn Innere Medizin, Itzehoe Kinderklinik, Jena Uni-Kinderklinik, Kaiserslautern Kinderarztpraxis, Kaiserslautern-Westpfalzklinikum Kinderklinik, Kamen Klinikum Westfalen Hellmig Krankenhaus, Karlsburg Klinik für Diabetes & Stoffwechsel, Karlsruhe Städtische Kinderklinik, Kassel Klinikum Kinder—und Jugendmedizin, Kassel Rot-Kreuz-Krankenhaus Innere, Kassel Städtische Kinderklinik, Kaufbeuren Innere Medizin, Kempen Heilig Geist—Innere, Kempen Heilig Geist-KHS—Innere, Kempten Oberallgäu Kinderklinik, Kiel Städtische Kinderklinik, Kiel Universitäts-Kinderklinik, Kirchen DRK Krankenhaus Kinderklinik, Kirchheim-Nürtingen Innere, Klagenfurt Innere Med I, Kleve Innere Medizin, Koblenz Kemperhof 1. Med. Klinik, Koblenz Kinderklinik Kemperhof, Konstanz Innere Klinik, Konstanz Kinderklinik, Krefeld Alexianer Innere, Krefeld Innere Klinik, Krefeld Kinderklinik, Krefeld-Uerdingen St. Josef Innere, Kreischa-Zscheckwitz Klinik Bavaria, Köln Kinderklinik Amsterdamerstrasse, Köln Uni-Kinderklinik Landau/Annweiler Innere, Landesklinikum Korneuburg Stockerau, Landshut Kinderklink, Lappersdorf Kinderarztpraxis, Leer Kreiskrankenhaus—Kinderabt., Leipzig Uni-Kinderklinik, Leoben LKH Kinderklinik, Leverkusen Kinderklinik, Lienz BKH Kinderklinik, Lienz BKH Pädiatrie, Lienz Diabetesschwerpunktpraxis für Kinder und Jugendliche, Lilienthal Diabeteszentrum, Limburg Innere Medizin, Lindenfels Luisenkrankenhaus Innere, Lindenfels Luisenkrankenhaus Innere 2, Lingen Kinderklinik St. Bonifatius, Linz AKH—2. Med, Linz Krankenhaus Barmherzige Schwestern Kardiologie Abt. Int. II, Linz Krankenhaus der Barmherzigen Schwestern Kinderklinik, Linz Landes-Kinderklinik, Lippstadt Evangelische Kinderklinik, Ludwigsburg Innere Medizin, Ludwigsburg Kinderklinik, Ludwigshafen Kinderklinik St.Anna-Stift, Ludwigshafen diabetol. SPP, Luxembourg—Centre Hospitalier, Lübeck Uni-Kinderklinik, Lübeck Uni-Klinik Innere Medizin, Lüdenscheid Hilfswerk Kinder & Jugendliche, Lüdenscheid Märkische Kliniken—Kinder & Jugendmedizin, Lünen Klinik am Park, Magdeburg Städtisches Klinikum Innere, Magdeburg Uni-Kinderklinik, Mainz Uni-Kinderklinik Malchower See Rehaklinik, Mannheim Uni-Kinderklinik, Mannheim Uniklinik Innere Medizin, Marburg—UKGM Endokrinologie & Diabetes, Marburg Uni-Kinderklinik, Marktredwitz Innere Medizin, Marpingen-SPP, Mechernich Kinderklinik, Meissen Kinderklinik Elblandklinikum, Memmingen Internistische Praxis, Memmingen Kinderklinik, Merzig Kinderklinik, Minden Kinderklinik, Moers—St. Josefskrankenhaus Innere, Moers Kinderklinik, Murnau am Staffelsee—diabetol. SPP, Mutterstadt Kinderarztpraxis, Mödling Kinderklinik, Mölln Reha-Klinik Hellbachtal, Mönchengladbach Kinderklinik Rheydt Elisabethkrankenhaus, Mühlacker Enzkreiskliniken Innere, Mühldorf am Inn Kinderarztpraxis, München 3. Orden Kinderklinik, München Diabetes-Zentrum Süd, München Kinderarztpraxis diabet. SPP, München Schwerpunktpraxis, München von Haunersche Kinderklinik, München-Gauting Kinderarztzentrum, München-Harlaching Kinderklinik, München-Schwabing Kinderklinik, Münster Clemens-Hospital Innere, Münster Herz Jesu Innere, Münster St. Franziskus Kinderklinik Münster Uni-Kinderklinik, Münster pädiat. Schwerpunktpraxis, Nagold Kreiskrankenhaus Innere, Nauen Havellandklinik, Neuburg Kinderklinik, Neumarkt Innere, Neunkirchen Innere Medizin,Neunkirchen Marienhausklinik Kohlhof Kinderklinik, Neuruppin Kinderklinik, Neuss Lukaskrankenhaus Kinderklinik, Neuwied Kinderklinik Elisabeth, Neuwied Marienhaus Klinikum St. Elisabeth Innere, Nidda Bad Salzhausen Klinik Rabenstein/Innere-1 Reha, Nidda Bad Salzhausen Klinik Rabenstein/Innere-2 Reha, Nürnberg Cnopfsche Kinderklinik, Nürnberg Med. Klinik 4, Nürnberg Zentrum f Neugeb./Kinder & Jugendl., Oberhausen Innere, Oberhausen Kinderklinik, Oberhausen Kinderpraxis, Oberhausen St.Clemens Hospitale Sterkrade, Oberndorf Gastroenterologische Praxis Schwerpunkt Diabetologie, Offenbach/Main Innere Medizin, Offenbach/Main Kinderklinik, Offenburg Kinderklinik, Oldenburg Kinderklinik, Oldenburg Schwerpunktpraxis, Oschersleben MEDIGREIF Bördekrankenhaus, Osnabrück Christliches Kinderhospital, Osterkappeln Innere, Ottobeuren Kreiskrankenhaus, Oy-Mittelberg Hochgebirgsklinik Kinder-Reha, Paderborn St. Vincenz Kinderklinik Papenburg Marienkrankenhaus Kinderklinik, Passau Kinderarztpraxis, Passau Kinderklinik, Pforzheim Kinderklinik, Pfullendorf Innere Medizin, Pirmasens Städtisches Krankenhaus Innere, Plauen Vogtlandklinikum, Prenzlau Krankenhaus Innere, Rastatt Gemeinschaftspraxis, Rastatt Kreiskrankenhaus Innere, Ravensburg Kinderklink St. Nikolaus, Recklinghausen Dialysezentrum Innere, Regensburg Kinderklinik St. Hedwig, Remscheid Kinderklinik, Rendsburg Kinderklinik, Reutlingen Kinderarztpraxis, Reutlingen Kinderklinik, Reutlingen Klinikum Steinenberg Innere, Rheine Mathiasspital Kinderklinik, Ried Innkreis Barmherzige Schwestern, Rodalben St. Elisabeth, Rosenheim Innere Medizin, Rosenheim Kinderklinik, Rosenheim Schwerpunktpraxis, Rostock Uni-Kinderklinik, Rostock Universität Innere Medizin, Rotenburg/Wümme Agaplesion Diakonieklinikum Kinderabteilung, Rüsselsheim Kinderklinik, Saaldorf-Surheim Diabetespraxis, Saalfeld Thüringenklinik Kinderklinik, Saarbrücken Kinderklinik Winterberg, Saarbrücken Kinderklinik Winterberg 2, Saarlouis Kinderklinik Salzburg Universitäts-Kinderklinik, Scheibbs Landesklinikum, Scheidegg Prinzregent Luitpold, Scheidegg Reha-Kinderklinik Maximilian, Schw. Gmünd Stauferklinik Kinderklinik, Schweinfurt Kinderklinik, Schwerin Innere Medizin, Schwerin Kinderklinik, Schwäbisch Hall Diakonie Innere Medizin, Schwäbisch Hall Diakonie Kinderklinik, Siegen Kinderklinik, Singen—Hegauklinik Kinderklinik, Singen Kinderarztpraxis, Sinsheim Innere, Spaichingen Innere, St. Augustin Kinderklinik, St. Pölten Universitäts-Kinderklinik, St. Pölten Universitätsklinik Innere, Stade Kinderklinik, Stolberg Kinderklinik, Stuttgart Bethesda Agaplesion, Stuttgart Olgahospital Kinderklinik, Suhl Kinderklinik, Sylt Rehaklinik, Tettnang Innere Medizin, Timmendorfer Strand, Traunstein Kinderklinik, Traunstein diabetol. Schwerpunktpraxis, Trier Kinderklinik der Borromäerinnen, Trostberg Innere, Tübingen Uni-Kinderklinik, Ulm Agaplesion Bethesda-Krankenhaus, Ulm Endokrinologikum Ulm Schwerpunktpraxis Bahnhofsplatz, Ulm Uni Innere Medizin, Ulm Uni-Kinderklinik, Vechta Kinderklinik, Viersen Kinderkrankenhaus St. Nikolaus, Villach Kinderklinik, Villingen-Schwenningen SPP, Villingen-Schwenningen Schwarzwald Baar Klinikum Kinderklinik, Villingen-Schwenningen Schwarzwald-Baar-Klinikum Innere, Waldshut Kinderpraxis, Waldshut-Tiengen Kinderpraxis Biberbau, Wangen Oberschwabenklinik Innere Medizin, Waren-Müritz Kinderklinik, Weiden Kinderklinik, Weingarten Kinderarztpraxis, Weisswasser Kreiskrankenhaus, Wels Innere, Wels Klinikum Pädiatrie, Wernberg-Köblitz SPP, Wetzlar Schwerpunkt-Praxis, Wien 3. Med. Hietzing Innere, Wien Preyersches Kinderspital, Wien Rudolfstiftung, Wien SMZ Ost Donauspital, Wien Uni Innere Med III, Wien Uni-Kinderklinik, Wien Wilhelminenspital 5. Med. Abteilung, Wiesbaden Horst-Schmidt-Kinderkliniken, Wiesbaden Kinderklinik DKD, Wilhelmshaven Reinhard-Nieter-Kinderklinik, Wilhelmshaven St. Willehad Innere, Winnenden Rems-Murr Kinderklinik, Wismar Kinderklinik Wittenberg Innere Medizin, Wittenberg Kinderklinik, Wolgast Innere Medizin, Worms—Weierhof, Worms Kinderklinik, Wuppertal Kinderklinik, Zweibrücken Ev. KH. Innere, Zweibrücken Kinderarztpraxis
Authors' Contributions
All named authors met the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article. T.D. drafted the article. Reinhard W. Holl designed the analysis and interpreted the results. A.S. checked the statistical program and critically reviewed and commented on the article. All authors had full access to all the study data and contributed to the interpretation, critically reviewed the article, and approved the final version for submission.
Author Disclosure Statement
Dr. T.D. has received research support or has consulted for Abbott, AstraZeneca, Bayer, Boehringer, DexCom, Insulet Corp., Eli Lilly, Medtronic, NovoNordisk, Roche. Dr. B.R.-M. has received research support or has consulted for, Bayer, Boehringer, Eli Lilly, Medtronic, NovoNordisk, Roche, and Sanofi. Dr. A.F. has consulted and/or has received lecture support from Eli Lilly, Boehringer Ingelheim, MSD, Astra Zeneca, and Sanofi. Dr. T.B. received research support and honoraria for lecture and travel support from Astra Zeneca, DexCom, Medtronic, NovoNordisk, Roche, Sanofi, and Ypsomed. The other authors have no disclosures.
Supporting Information - Acknowledgments
Funding Information
This study was funded by Insulet Corporation. Additional support has been received by the Federal Ministry of Education and Research within the German Center for Diabetes Research (DZD; Grant No. 82DZD14A02), the German Diabetes Association (DDG), and the German Robert Koch Institute. The funding sources had no involvement in the content presented in this article.
References
- 1.Gibb FW, Teoh WL, Graham J, Lockman KA: Risk of death following admission to a UK hospital with diabetic ketoacidosis. Diabetologia 2016;59:2082–2087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benoit SR, Zhang Y, Geiss LS, et al. : Trends in diabetic ketoacidosis hospitalizations and in-hospital mortality—United States, 2000–2014. MMWR Morb Mortal Wkly Rep 2018;67:362–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karges B, Schwandt A, Heidtmann B, et al. : Association of insulin pump therapy vs insulin injection therapy with severe hypoglycemia, ketoacidosis, and glycemic control among children, adolescents, and young adults with type 1 diabetes. JAMA 2017;318:1358–1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maahs DM, Hermann JM, Holman N, et al. : Rates of diabetic ketoacidosis: international comparison with 49,859 pediatric patients with type 1 diabetes from England, Wales, the U.S., Austria, and Germany. Diabetes Care 2015;38:1876–1882 [DOI] [PubMed] [Google Scholar]
- 5.Haynes A, Hermann JM, Miller KM, et al. : Severe hypoglycemia rates are not associated with HbA1c: a cross-sectional analysis of 3 contemporary pediatric diabetes registry databases. Pediatr Diabetes 2017;18:643–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lindner LME, Gontscharuk V, Bachle C, et al. : Severe hypoglycemia and diabetic ketoacidosis in young persons with preschool onset of type 1 diabetes mellitus: an analysis of three nationwide population-based surveys. Pediatr Diabetes 2018;19:713–720 [DOI] [PubMed] [Google Scholar]
- 7.Weinstock RS, Xing D, Maahs DM, et al. : Severe hypoglycemia and diabetic ketoacidosis in adults with type 1 diabetes: results from the T1D Exchange clinic registry. J Clin Endocrinol Metab 2013;98:3411–3419 [DOI] [PubMed] [Google Scholar]
- 8.Cengiz E, Xing D, Wong JC, et al. : Severe hypoglycemia and diabetic ketoacidosis among youth with type 1 diabetes in the T1D Exchange clinic registry. Pediatr Diabetes 2013;14:447–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wild D, von Maltzahn R, Brohan E, et al. : A critical review of the literature on fear of hypoglycemia in diabetes: implications for diabetes management and patient education. Patient Educ Couns 2007;68:10–15 [DOI] [PubMed] [Google Scholar]
- 10.Van Name MA, Hilliard ME, Boyle CT, et al. : Nighttime is the worst time: parental fear of hypoglycemia in young children with type 1 diabetes. Pediatr Diabetes 2018;19:114–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Varni JW, Delamater AM, Hood KK, et al. : Diabetes symptoms predictors of health-related quality of life in adolescents and young adults with type 1 or type 2 diabetes. Qual Life Res 2018;27:2295–2303 [DOI] [PubMed] [Google Scholar]
- 12.Anderbro TC, Amsberg S, Moberg E, et al. : A longitudinal study of fear of hypoglycaemia in adults with type 1 diabetes. Endocrinol Diabetes Metab 2018;1:e00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rechenberg K, Whittemore R, Grey M: Anxiety in youth with type 1 diabetes. J Pediatr Nurs 2017;32:64–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martyn-Nemeth P, Schwarz Farabi S, Mihailescu D, et al. : Fear of hypoglycemia in adults with type 1 diabetes: impact of therapeutic advances and strategies for prevention—a review. J Diabetes Complications 2016;30:167–177 [DOI] [PubMed] [Google Scholar]
- 15.Tieder JS, McLeod L, Keren R, et al. : Variation in resource use and readmission for diabetic ketoacidosis in children's hospitals. Pediatrics 2013;132:229–236 [DOI] [PubMed] [Google Scholar]
- 16.Icks A, Strassburger K, Baechle C, et al. : Frequency and cost of diabetic ketoacidosis in Germany—study in 12,001 paediatric patients. Exp Clin Endocrinol Diabetes 2013;121:58–59 [DOI] [PubMed] [Google Scholar]
- 17.Shrestha SS, Zhang P, Barker L, Imperatore G: Medical expenditures associated with diabetes acute complications in privately insured U.S. youth. Diabetes Care 2010;33:2617–2622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morgan CL, Peters JR, Dixon S, Currie CJ: Estimated costs of acute hospital care for people with diabetes in the United Kingdom: a routine record linkage study in a large region. Diabet Med 2010;27:1066–1073 [DOI] [PubMed] [Google Scholar]
- 19.Geller AI, Shehab N, Lovegrove MC, et al. : National estimates of insulin-related hypoglycemia and errors leading to emergency department visits and hospitalizations. JAMA Intern Med 2014;174:678–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.American Diabetes Association: Economic costs of diabetes in the U.S. in 2017. Diabetes Care 2018;41:917–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller KM, Foster NC, Beck RW, et al. : Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D Exchange clinic registry. Diabetes Care 2015;38:971–978 [DOI] [PubMed] [Google Scholar]
- 22.Karges B, Rosenbauer J, Holterhus PM, et al. : Hospital admission for diabetic ketoacidosis or severe hypoglycemia in 31,330 young patients with type 1 diabetes. Eur J Endocrinol 2015;173:341–350 [DOI] [PubMed] [Google Scholar]
- 23.Beck RW, Tamborlane WV, Bergenstal RM, et al. : The T1D Exchange clinic registry. J Clin Endocrinol Metab 2012;97:4383–4389 [DOI] [PubMed] [Google Scholar]
- 24.Farsani SF, Brodovicz K, Soleymanlou N, et al. : Incidence and prevalence of diabetic ketoacidosis (DKA) among adults with type 1 diabetes mellitus (T1D): a systematic literature review. BMJ Open 2017;7:e016587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoshina S, Andersen GS, Jørgensen ME, et al. : Treatment modality–dependent risk of diabetic ketoacidosis in patients with type 1 diabetes: danish adult diabetes database study. Diabetes Technol Ther 2018;20:229–234 [DOI] [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention: National Diabetes Statistics Report. Atlanta, GA: 2017 [Google Scholar]
- 27.Fritsch M, Rosenbauer J, Schober E, et al. : Predictors of diabetic ketoacidosis in children and adolescents with type 1 diabetes. Experience from a large multicentre database. Pediatr Diabetes 2011;12(4 Pt 1):307–312 [DOI] [PubMed] [Google Scholar]
- 28.Zhong VW, Juhaeri J, Mayer-Davis EJ: Trends in hospital admission for diabetic ketoacidosis in adults with type 1 and type 2 diabetes in England, 1998–2013: a retrospective cohort study. Diabetes Care 2018;41:1870. [DOI] [PubMed] [Google Scholar]
- 29.Rosenbauer J, Dost A, Karges B, et al. : Improved metabolic control in children and adolescents with type 1 diabetes: a trend analysis using prospective multicenter data from Germany and Austria. Diabetes Care 2012;35:80–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Danne T, Schwandt A, Biester T, et al. : Long-term study of tubeless insulin pump therapy compared to multiple daily injections in youth with type 1 diabetes: data from the German/Austrian DPV registry. Pediatr Diabetes 2018;19:979–984 [DOI] [PubMed] [Google Scholar]
- 31.Biester T, Grimsmann JM, Heidtmann B, et al. : Intermittently scanned glucose values for continuous monitoring: cross-sectional analysis of glyceamic control and hypoglycaemia in 1809 children and adolescents with type 1 diabetes. Diabetes Technol Ther 2021;23:160–167 [DOI] [PubMed] [Google Scholar]
- 32.Wolfsdorf JI, Glaser N, Agus M, et al. : ISPAD Clinical Practice Consensus Guidelines 2018: diabetic ketoacidosis and the hyperglycemic hyperosmolar state. Pediatr Diabetes 2018;19Suppl 27:155–177 [DOI] [PubMed] [Google Scholar]
- 33.Agiostratidou G, Anhalt H, Ball D, et al. : Standardizing clinically meaningful outcome measures beyond HbA1c for type 1 diabetes: a consensus report of the American Association of Clinical Endocrinologists, the American Association of Diabetes Educators, the American Diabetes Association, the Endocrine Society, JDRF International, The Leona M. and Harry B. Helmsley Charitable Trust, the Pediatric Endocrine Society, and the T1D Exchange. Diabetes Care 2017;40:1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Littell RC, Milliken GA, Stroup WW, et al. : SAS for Mixed Models, 2nd ed. Cary, NC: SAS Press, 2006, ISBN:978-1-59047-500-3 [Google Scholar]
- 35.DeSalvo DJ, Miller KM, Hermann JM, et al. : Continuous glucose monitoring and glycemic control among youth with type 1 diabetes: international comparison from the T1D Exchange and DPV Initiative. Pediatr Diabetes 2018;19:1271–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sherr JL, Hermann JM, Campbell F, et al. : Use of insulin pump therapy in children and adolescents with type 1 diabetes and its impact on metabolic control: comparison of results from three large, transatlantic paediatric registries. Diabetologia 2016;59:87–91 [DOI] [PubMed] [Google Scholar]
- 37.Mehta SN, Tinsley LJ, Kruger D, et al. : Improved glycemic control following transition to tubeless insulin pump therapy in adults with type 1 diabetes. Clin Diabetes 2020;39:72–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnson SR, Cooper MN, Jones TW, Davis EA: Long-term outcome of insulin pump therapy in children with type 1 diabetes assessed in a large population-based case–control study. Diabetologia 2013;56:2392–2400 [DOI] [PubMed] [Google Scholar]
- 39.Rachmiel M, Levy-Shraga Y, Gruber N, et al. : Comparing insulin pump devices in real life: the AWeSoMe Study Group Prospective Experience. Diabetes Technol Ther 2019;21:138–145 [DOI] [PubMed] [Google Scholar]
- 40.Leelarathna L, Roberts S, Hindle A, et al. : Comparison of different insulin pump makes under routine care conditions in adults with Type 1 diabetes. Diabet Med 2017;34:1372–1379 [DOI] [PubMed] [Google Scholar]
- 41.Ziegler A, Williams T, Yarid N, et al. : Fatalities due to failure of continuous subcutaneous insulin infusion devices: a report of six cases. J Forensic Sci 2019;64:275–280 [DOI] [PubMed] [Google Scholar]
- 42.Zisser H: Quantifying the impact of a short-interval interruption of insulin-pump infusion sets on glycemic excursions. Diabetes Care 2008;31:238–239 [DOI] [PubMed] [Google Scholar]
- 43.Thethi TK, Rao A, Kawji H, et al. : Consequences of delayed pump infusion line change in patients with type 1 diabetes mellitus treated with continuous subcutaneous insulin infusion. J Diabetes Complications 2010;24:73–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bronstone A, Graham C: The potential cost implications of averting severe hypoglycemic events requiring hospitalization in high-risk adults with type 1 diabetes using real-time continuous glucose monitoring. J Diabetes Sci Technol 2016;10:905–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lung TW, Petrie D, Herman WH, et al. : Severe hypoglycemia and mortality after cardiovascular events for type 1 diabetic patients in Sweden. Diabetes Care 2014;37:2974–2981 [DOI] [PubMed] [Google Scholar]
- 46.McCoy RG, Van Houten HK, Ziegenfuss JY, et al. : Increased mortality of patients with diabetes reporting severe hypoglycemia. Diabetes Care 2012;35:1897–1901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tauschmann M, Hermann JM, Freiberg C, et al. : A multicenter analysis of 3,553 subjects from the DPV registry. Diabetes Care 2020;43:e40–e42 [DOI] [PubMed] [Google Scholar]