Abstract
Background: Radioiodine-refractory differentiated thyroid cancer (RR-DTC) has a low 10-year patient-survival rate and is challenging to treat. Lenvatinib is a multikinase inhibitor approved for the treatment of RR-DTC. This study aims to assess Eastern Cooperative Oncology Group performance status (ECOG PS) and neutrophil-to-lymphocyte ratio (NLR) as prognostic markers for patients with RR-DTC treated with lenvatinib.
Methods: In this retrospective analysis of the Study of (E7080) LEnvatinib in Differentiated Cancer of the Thyroid (SELECT), patients randomly assigned to receive lenvatinib were classified according to baseline ECOG PS (0 or 1) or baseline NLR (≤3 or >3). The effects of baseline ECOG PS and NLR on progression-free survival (PFS), overall survival (OS), and objective response rate (ORR) were evaluated. In addition, the effects of baseline ECOG PS on the change in diameter of target lesions and correlations between baseline NLR and the sums of the diameters of target lesions were calculated.
Results: Among patients who received lenvatinib, patients with a baseline ECOG PS of 0 had statistically improved PFS (hazard ratio [HR] 0.52; 95% confidence interval [CI 0.35–0.77]; p = 0.001), OS (HR 0.42 [CI 0.26–0.69]; p = 0.0004), and ORR (odds ratio [OR] 3.51 [CI 2.02–6.10]; p < 0.0001) compared with patients with a baseline ECOG PS of 1. Patients who received lenvatinib with a baseline NLR ≤3 also had improved PFS (HR 0.43 [CI 0.29–0.65]; p < 0.0001) and OS (HR 0.48 [CI 0.29–0.78]; p = 0.0029) versus patients with a baseline NLR >3. Moreover, patients with a baseline NLR ≤3 had a trend toward increased ORR (OR 1.57 [CI 0.94–2.64]; p = 0.08) compared with patients with a baseline NLR >3. Treatment-emergent adverse events were generally similar among patients who received lenvatinib, irrespective of patients' ECOG PS at baseline.
Conclusion: Lower ECOG PS and NLR may provide prognostic value for improved efficacy in patients with RR-DTC. ClinicalTrials.gov no. NCT01321554.
Keywords: differentiated thyroid cancer, ECOG, lenvatinib, NLR, tumor size
Introduction
The incidence of cancer with thyroid as the site of origin has increased from 3.6 cases per 100,000 in 1973 to 8.7 cases per 100,000 in 2002, representing a 2.4-fold increase in the number of cases diagnosed/year (1). This increase was almost entirely attributable to an increase in papillary thyroid cancers (2), and because the incidence of tumors of ∼2–4 cm in diameter remained stable, this increase was thought to be due to more frequent diagnosis of subclinical tumors as a result of medical imaging (3). However, rates are also increasing for larger thyroid tumors and those with regional and distant spread (4); as such, thyroid cancer mortality has increased by 1.1% per year from 1994 to 2013 (5). Although most patients' differentiated thyroid cancer (DTC) can be cured with surgery, often followed by radioiodine therapy and thyroid-stimulating-hormone suppression, patients with unresectable locally recurrent and/or metastatic radioiodine-refractory (RR)-DTC have a 10-year survival rate of only 19% (6,7).
Lenvatinib is a tyrosine kinase inhibitor of vascular endothelial growth factor receptors 1–3, fibroblast growth factor receptors 1–4, platelet-derived growth factor receptor α, RET, and KIT (8–11). Lenvatinib was approved for the treatment of patients with locally recurrent or metastatic, progressive RR-DTC, based on the results of the phase 3 Study of (E7080) LEnvatinib in Differentiated Cancer of the Thyroid (SELECT) (12,13). In SELECT, median progression-free survival (PFS) was 18.3 months with lenvatinib compared with 3.6 months with placebo. Further analyses of SELECT have indicated that the efficacy and safety of lenvatinib is maintained across several categories of radioiodine refractoriness (i.e., when patients were stratified by RR inclusion criteria: including no radioiodine uptake, disease progression within 12 months of radioiodine therapy, and extensive cumulative radioiodine exposure) (14).
Despite this advancement in treatment, few prognostic factors have been described for RR-DTC. Eastern Cooperative Oncology Group performance status (ECOG PS)—a comprehensive measure of a patient's level of functioning (15)—is associated with survival and response to therapy in patients with multiple types of malignancies, including non-small cell lung cancer and esophageal cancer (16,17). Similarly, elevated neutrophil-to-lymphocyte ratio (NLR) is associated with more aggressive disease and decreased survival in patients with numerous types of solid tumors, including DTC (18,19). When NLR was retrospectively analyzed in patients specifically with RR-DTC treated with lenvatinib in a real-world setting, median overall survival (OS) was significantly longer in patients with lower baseline NLR (20). The mechanism by which elevated NLR is associated with more aggressive disease is not definitively known but may, in part, be explained by tumor-promoting consequences of inflammation (21). Moreover, results of a retrospective review of clinical records indicated that high baseline tumor burden was associated with a worse prognosis in patients with RR-DTC (22). Evidence has shown that treatment-emergent hypertension in patients receiving lenvatinib is associated with longer OS (23), but this correlation is not useful in making decisions regarding when to initiate lenvatinib treatment.
Treatment with tyrosine kinase inhibitors in RR-DTC is not curative and is associated with treatment-emergent adverse events (TEAEs); therefore, identification of prognostic markers could help physicians make the challenging decision of whether to initiate systemic therapy. This exploratory post hoc analysis assessed baseline ECOG PS and NLR as potential prognostic indicators in patients with RR-DTC treated with lenvatinib.
Methods
Patients
SELECT was a phase 3 randomized, double-blind, multicenter study that compared lenvatinib versus placebo in patients with RR-DTC. Full details of the study have been published (13). A brief summary of eligibility criteria can be found in the Supplementary Material S1. This analysis focuses on patients initially randomized to the lenvatinib arm of the study.
All patients provided written informed consent, and the study protocol was approved by all relevant institutional review bodies. The study was conducted in accordance with the provisions of the Declaration of Helsinki and local laws.
Study design
In SELECT, patients were randomly assigned (2:1) to receive oral lenvatinib at 24 mg/day or placebo in 28-day cycles (13). Tumor responses were assessed by an independent centralized imaging laboratory using Response Evaluation Criteria In Solid Tumors version 1.1 (RECIST v1.1) every 8 weeks during the randomization phase and every 12 weeks during the extension phase; they were confirmed by an independent imaging review (13). Patients initially randomly assigned to receive placebo were offered open-label lenvatinib on centrally confirmed disease progression. Primary and secondary objectives of SELECT have been previously reported (13). Adverse events (AEs) were categorized according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0.
Post hoc subgroup analyses and statistical methods
This exploratory post hoc subgroup analysis included patients from SELECT who were randomly assigned to receive lenvatinib, using the data cutoff date from the primary analysis (November 15, 2013). Patients were then divided into subgroups according to baseline ECOG PS (0 or 1) and baseline NLR of ≤3 or >3 (the median baseline NLR value for patients receiving lenvatinib was 3.1, thus a cutoff NLR value of 3 was utilized for this analysis). NLR was calculated based on peripheral blood cell counts. As corticosteroid treatment can impact NLR values, a separate analysis was conducted excluding 20 patients who were receiving concomitant corticosteroids at baseline to confirm the accuracy of the NLR data.
Efficacy analyses were conducted with patients who received lenvatinib and were grouped by either ECOG PS or NLR. The effects of baseline ECOG PS and baseline NLR on PFS, OS, and objective response rate (ORR) were evaluated separately. In addition, the effects of baseline ECOG PS on the change in the sums of the diameters of target lesions and correlations between baseline NLR and the sums of the diameters of target lesions were calculated. The number of metastatic sites involved was assessed in patients with a baseline ECOG PS 0 versus 1, as well as NLR ≤3 versus NLR >3. ECOG PS was also measured in patients with a baseline NLR ≤3 and NLR >3. Kaplan–Meier analyses for OS and PFS were also conducted on data from patients who had received placebo, grouped by either ECOG PS or NLR. These results are reported in the Supplementary Material S1.
Statistical analyses were performed using SAS (SAS Institute Inc.) version 9.4. PFS and OS were summarized by using Kaplan–Meier estimates; 95% confidence intervals (CIs) of the median were constructed with a generalized Brookmeyer and Crowley method; hazard ratios (HRs) between subgroups were estimated from an unstratified Cox proportional hazard model; and p-values were based on an unstratified log-rank test. The CIs for ORR were calculated by using asymptotic normal approximation; the odds ratios (OR) between subgroups and p-values were calculated by using the Chi-square test. Percentage changes from baseline to postbaseline nadir in the sums of diameters of target lesions and percentage changes over time according to ECOG PS at baseline (0 or 1) were plotted and assessed.
Multivariate analyses, which included ECOG PS, NLR, sums of the diameters of target lesions at baseline, and age, were conducted to determine whether any factors had a significant association with OS or PFS. Patients randomly assigned to lenvatinib who had a baseline ECOG PS of 0 or 1 and a nonmissing baseline NLR were included in these analyses. Associations with PFS and OS were based on maximum likelihood estimates for the covariates in the unstratified Cox proportional hazard model. Extent of correlation (negligible, low, etc.) is described as per Hinkle et al (24).
Given the post hoc nature of the analyses in this article, all reported p-values (including those for multivariate analyses) should be considered nominal.
Results
Patients
The baseline demographic and disease characteristics of patients from SELECT who were randomly assigned to receive lenvatinib were analyzed according to patients' ECOG PS and are shown in Table 1. Of the 261 patients in the lenvatinib arm, 144 (55.2%) had a baseline ECOG PS of 0 and 104 (39.8%) had a baseline ECOG PS of 1. Demographics and disease characteristics were generally similar among the two ECOG PS groups (Table 1). In addition, more patients with an ECOG PS of 1 had ≥4 metastatic sites (19.2%) by RECIST v1.1 compared with patients with an ECOG PS of 0 (9.0%).
Table 1.
Baseline Demographic and Disease Characteristics of Patients Randomly Assigned to Receive Lenvatinib, According to Eastern Cooperative Oncology Group Performance Status
| Parameter | Baseline ECOG PS 0 (n = 144) | Baseline ECOG PS 1 (n = 104) |
|---|---|---|
| Age, median, years (range) | 63.5 (30–80) | 63 (27–89) |
| Age group, n (%) | ||
| ≤65 years | 86 (59.7) | 64 (61.5) |
| >65 years | 58 (40.3) | 40 (38.5) |
| Weight, n (%) | ||
| <60 kg | 33 (22.9) | 22 (21.2) |
| ≥60 kg | 111 (77.1) | 82 (78.8) |
| Sum of target lesion diameters at baseline, median (mm) | 50.1 | 66.1 |
| No. of metastatic sites, n (%) | ||
| 0 | 3 (2.1) | 1 (1.0) |
| 1 | 45 (31.3) | 17 (16.3) |
| 2 | 46 (31.9) | 38 (36.5) |
| 3 | 37 (25.7) | 28 (26.9) |
| ≥4 | 13 (9.0) | 20 (19.2) |
| NLR level, n (%) | ||
| ≤3 | 76 (52.8) | 44 (42.3) |
| >3 | 68 (47.2) | 60 (57.7) |
| Absolute neutrophil count, 109/L, mean (SD) | 4.0 (1.7) | 4.5 (2.1) |
| Absolute lymphocyte count, 109/L, mean (SD) | 1.3 (0.5) | 1.3 (0.6) |
| Hypertension, n (%) | ||
| Yes | 81 (56.3) | 57 (54.8) |
| No | 63 (43.8) | 47 (45.2) |
| Diabetes, n (%) | ||
| Yes | 22 (15.3) | 14 (13.5) |
| No | 122 (84.7) | 90 (86.5) |
| Proteinuria, n (%) | ||
| Positive | 5 (3.5) | 4 (3.8) |
| Negative | 139 (96.5) | 100 (96.2) |
| Renal impairment,an (%) | ||
| Yes | 17 (11.8) | 10 (9.6) |
| No | 127 (88.2) | 94 (90.4) |
| Hepatic impairment,bn (%) | ||
| Yes | 19 (13.2) | 8 (7.7) |
| Mild | 17 (11.8) | 8 (7.7) |
| Moderate | 2 (1.4) | 0 |
| No | 125 (86.8) | 96 (92.3) |
These data include only patients with baseline ECOG PS values of 0 or 1.
Defined as baseline serum creatine clearance <60 mL/min.
Defined as a condition with Common Terminology Criteria for Adverse Events grade ≥1 for any of the following parameters: aspartate aminotransferase, alanine aminotransferase, and bilirubin at baseline; impairment is defined as mild if any parameter is grade 1; impairment is defined as moderate if any parameter is grade 2.
ECOG PS, Eastern Cooperative Oncology Group performance status; NLR, neutrophil-to-lymphocyte ratio; SD, standard deviation.
Patients in the lenvatinib arm were also analyzed according to NLR. A baseline NLR ≤3 was recorded for 121 (46.4%) patients, and 140 (53.6%) patients had a baseline NLR >3; the median baseline NLR for patients randomly assigned to receive lenvatinib was 3.1 (range: 0.7–36.3). Absolute neutrophil and lymphocyte counts by NLR are reported in Supplementary Table S1. Among patients with an NLR ≤3, 62.8% had an ECOG PS of 0 and 37.2% had an ECOG PS ≥1; and among patients with an NLR >3, 48.6% had an ECOG PS of 0 and 51.4% had an ECOG PS ≥1. There was no significant association between baseline NLR and baseline ECOG PS (p = 0.2414). The baseline numbers of metastatic sites analyzed according to NLR for patients randomly assigned to receive lenvatinib are shown in Supplementary Figure S1. There was a significant association between higher baseline NLR and presence of ≥3 metastatic sites (p = 0.0011). In general, no significant correlations were observed between age and NLR or age and ECOG PS.
Efficacy
Subgroup analysis by baseline ECOG PS (0 or 1) in patients in the lenvatinib arm
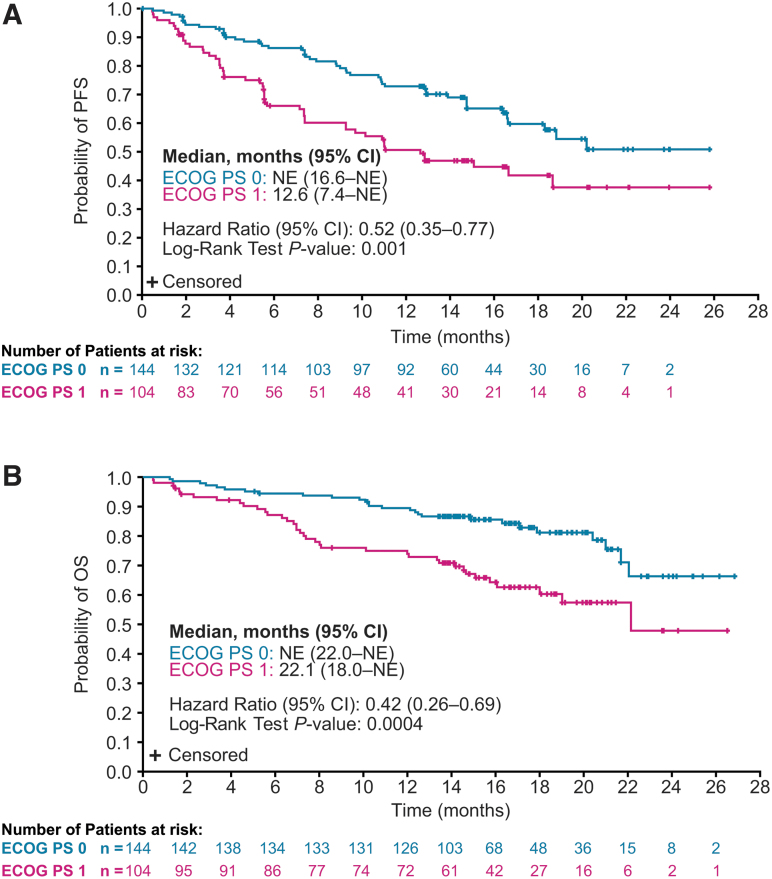
Among patients randomly assigned to lenvatinib, PFS was prolonged for those with a baseline ECOG PS of 0 compared with patients with a baseline ECOG PS of 1 (HR 0.52 [CI 0.35–0.77]; p = 0.001) (Fig. 1A). Similar results for the ECOG PS 0 and 1 groups were also observed for OS (HR 0.42 [CI 0.26–0.69]; p = 0.0004) (Fig. 1B). Tumor responses by baseline ECOG PS are summarized in Table 2.
FIG. 1.
Kaplan–Meier plots of PFS (A) and OS (B) in patients randomly assigned to receive lenvatinib, and with a baseline ECOG PS of 0 or 1. OS and PFS medians were calculated by Kaplan–Meier estimates, and the corresponding CIs were calculated with a generalized Brookmeyer and Crowley method. The HRs were estimated from an unstratified Cox proportional hazard model, and p-values were based on an unstratified log-rank test. CI, 95% confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio; NE, not estimable; OS, overall survival; PFS, progression-free survival.
Table 2.
Tumor Responses by Baseline Eastern Cooperative Oncology Group Performance Status in Patients Randomly Assigned to Receive Lenvatinib, as Assessed by Independent Imaging Review Using Response Evaluation Criteria In Solid Tumors Version 1.1
| Parameter | ECOG PS 0 (n = 144) | ECOG PS 1 (n = 104) |
|---|---|---|
| Best overall response, n (%) | ||
| Complete response | 3 (2.1) | 1 (1.0) |
| Partial response | 110 (76.4) | 52 (50.0) |
| Stable disease | 21 (14.6) | 32 (30.8) |
| Durable stable diseasea | 15 (10.4) | 20 (19.2) |
| Progressive disease | 7 (4.9) | 10 (9.6) |
| Unknown | 3 (2.1) | 9 (8.7) |
| Objective response rate, n (%) | 113 (78.5) | 53 (51.0) |
| [CI] | [71.8–85.2] | [41.4–60.6] |
| Difference, % [CI] | 27.5 [15.8–39.2] | |
| Odds ratiob [CI] | 3.51 [2.02–6.10] | |
| p | <0.0001 | |
Stable disease with a duration of ≥23 weeks.
The odds ratio and p-value were calculated by using the Chi-square test, and corresponding CIs were generated based on asymptotic normal approximation.
CI, 95% confidence interval.
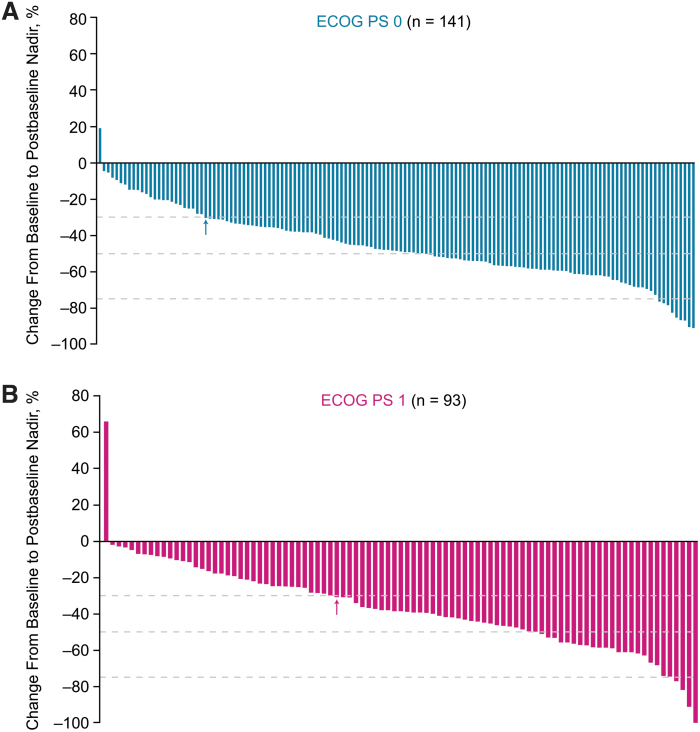
The ORR in patients with an ECOG PS of 0 was 78.5% [CI 71.8–85.2] versus 51.0% [CI 41.4–60.6] in patients with an ECOG PS of 1 (OR 3.51 [CI 2.02–6.10]; p < 0.0001). Percent changes in the sums of diameters of target lesions from baseline to postbaseline nadir according to baseline ECOG PS are shown in Figure 2. Mean maximum percent decrease in tumor size was numerically greater in patients with a baseline ECOG PS of 0 (−46.1%) compared with patients with a baseline ECOG PS of 1 (−37.2%) (Supplementary Fig. S2A). The shrinkage in the sums of diameters of target lesions increased over time, irrespective of patients' ECOG PS at baseline (Supplementary Fig. S2B).
FIG. 2.
Percentage change from baseline to postbaseline nadir in the sums of diameters of target lesions in patients randomly assigned to receive lenvatinib, and with a baseline ECOG PS of 0 (A) or 1 (B). n = the number of patients with both baseline and at least 1 postbaseline target lesion assessment. Arrows indicate the 30% threshold for partial response, according to RECIST version 1.1. RECIST, Response Evaluation Criteria In Solid Tumors.
Subgroup analysis by baseline NLR in patients in the lenvatinib arm
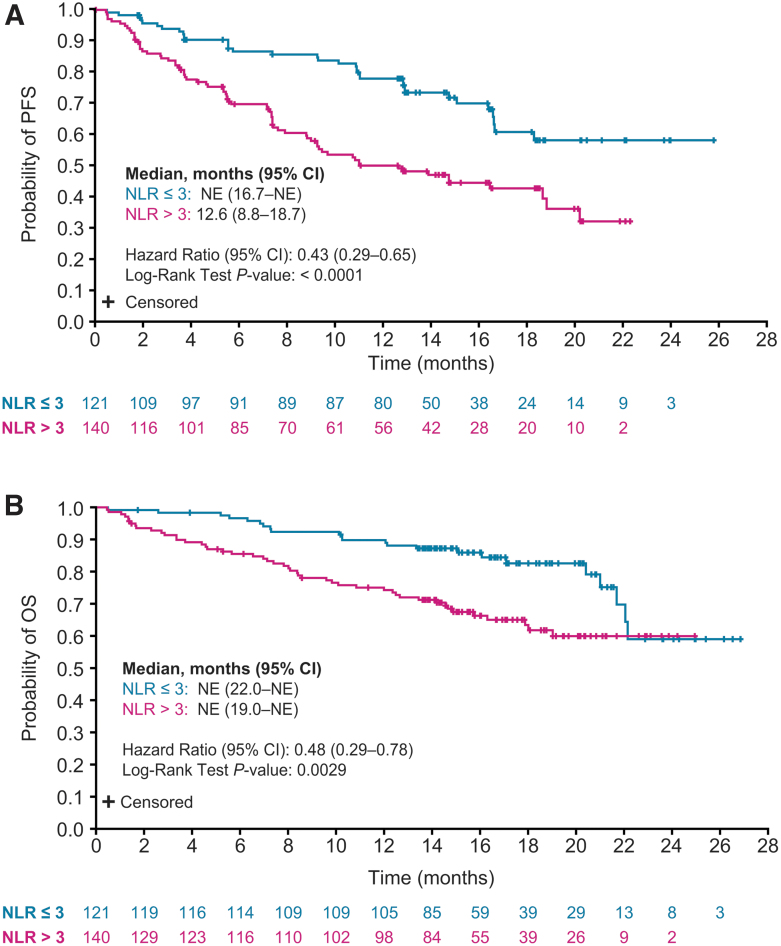
PFS was prolonged in patients with an NLR ≤3 versus patients with an NLR >3 (HR 0.43 [CI 0.29–0.65]; p < 0.0001) (Fig. 3A). Patients with an NLR ≤3 also had improved OS compared with patients with an NLR >3 (HR 0.48 [CI 0.29–0.78]; p = 0.0029) (Fig. 3B). When patients receiving concomitant corticosteroids at baseline (n = 20, 7.7%) were excluded from analysis, median PFS and OS values by baseline NLR were similar to those of the overall lenvatinib patient population (Supplementary Fig. S3).
FIG. 3.
Kaplan–Meier plots of PFS (A) and OS (B) in patients randomly assigned to receive lenvatinib by NLR (≤3 or >3). OS and PFS medians were calculated by Kaplan–Meier estimates, and the corresponding 95% CIs were calculated with a generalized Brookmeyer and Crowley method. The HRs were estimated from an unstratified Cox proportional hazard model, and p-values were based on an unstratified log-rank test. NLR, neutrophil-to-lymphocyte ratio.
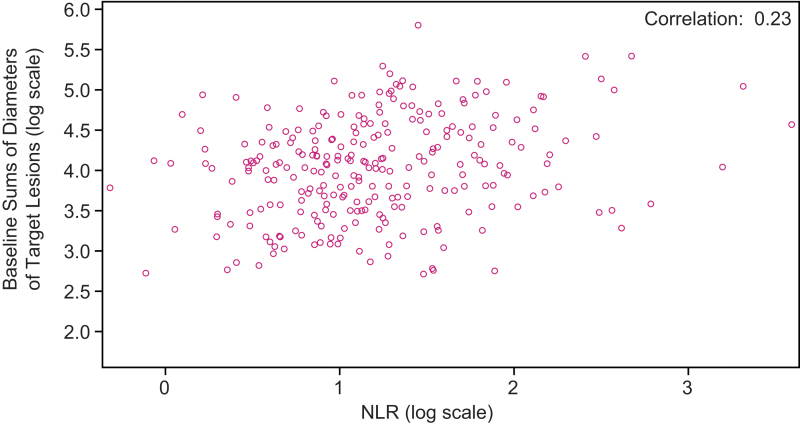
ORR was 70.2% [CI 62.1–78.4] for patients with an NLR ≤3 and 60.0% [CI 51.9–68.1] for patients with an NLR >3 (OR 1.57 [CI 0.94–2.64]; p = 0.08). Further, among patients without concomitant corticosteroid use at baseline, patients with an NLR ≤3 (n = 116) had an ORR of 71.6% [CI 63.3–79.8], and patients with an NLR >3 (n = 125) had an ORR of 63.2% [CI 54.8–71.7] (OR 1.46 [CI: 0.85–2.52]; p = 0.17) (Supplementary Table S2). Overall, the correlation (0.23) between sums of diameters of target lesions at baseline and NLR was negligible (Fig. 4).
FIG. 4.
Correlation between baseline sums of diameters of target lesions and NLR in patients randomly assigned to receive lenvatinib (log scales).
Results of the multivariate analyses, which included ECOG PS, sums of diameters of target lesions, NLR, and age at baseline
According to multivariate analyses, baseline ECOG PS (0 vs. 1) had a significant association with PFS (p = 0.0032) and OS (p = 0.0012) (Table 3). The sums of diameters of target lesions at baseline approached a significant association with PFS (p = 0.0587), but was not significantly associated with OS (p = 0.4145). Baseline NLR was significantly associated with PFS (p = 0.0009) and OS (p = 0.0128). Patient age at baseline was significantly associated with PFS (p = 0.0459), but not with OS (p = 0.2125).
Table 3.
Multivariate Analysis of Efficacy Endpoints with Baseline Characteristics in Patients Randomly Assigned to Receive Lenvatinib
| Baseline efficacy endpoints | p-value | Hazard ratio [CI] |
|---|---|---|
| Progression-free survival | ||
| ECOG PS (0 vs. 1) | 0.0032 | 0.55 [0.36–0.82] |
| Sums of target lesions | 0.0587 | 1.00 [1.00–1.01] |
| NLR | 0.0009 | 1.08 [1.03–1.12] |
| Age | 0.0459 | 1.02 [1.00–1.04] |
| Overall survival | ||
| ECOG PS (0 vs. 1) | 0.0012 | 0.43 [0.26–0.72] |
| Sums of target lesions | 0.4145 | 1.00 [1.00–1.01] |
| NLR | 0.0128 | 1.07 [1.02–1.14] |
| Age | 0.2125 | 1.02 [0.99–1.04] |
Subgroup analyses by baseline ECOG PS (0 or 1) and by baseline NLR in the placebo arm
PFS and OS data by baseline ECOG PS for patients who received placebo are provided in Supplementary Figure S4. Similar data by baseline NLR for patients who received placebo are provided in Supplementary Figure S5.
Safety
The median duration of treatment with lenvatinib was 14.3 months (range: 0.8–26.8) for patients with a baseline ECOG PS of 0 and 10.6 months (range: 0.2–26.5) for patients with a baseline ECOG PS of 1. The median dose intensity of lenvatinib was 16.2 mg/day/patient (range: 5.8–24.0) for patients with an ECOG PS of 0 at baseline and 17.6 mg/day/patient (range: 6.5–24.0) for patients with an ECOG PS of 1 at baseline.
An overview of TEAEs and the most common TEAEs by baseline ECOG PS are summarized in Supplementary Table S3. Any-grade TEAEs occurred in nearly all patients who received lenvatinib, irrespective of ECOG PS at baseline (ECOG PS 0, TEAEs in 100%; ECOG PS 1, TEAEs in 99%). TEAEs led to lenvatinib discontinuation in 11.1% of patients with an ECOG PS of 0 and in 19.2% of patients with an ECOG PS of 1; 73.6% of patients with an ECOG PS of 0 experienced a TEAE resulting in lenvatinib dose reduction, and 59.6% of patients with an ECOG PS of 1 had a TEAE resulting in dose reduction. TEAEs led to lenvatinib interruption in 81.9% of patients with an ECOG PS of 0 and in 82.7% of patients with an ECOG PS of 1. The most common TEAEs (data presented as ECOG PS 0/ECOG PS 1) were diarrhea (76.4%/53.8%), hypertension (72.2%/66.3%), decreased appetite (54.2%/51.9%), decreased weight (54.2%/47.1%), and nausea (46.5%/45.2%). Patients with an ECOG PS of 0 had fewer serious TEAEs (41.0%) than did patients with an ECOG PS of 1 (60.6%). Most patients experienced a treatment-related AE, irrespective of baseline ECOG PS (ECOG PS 0, 98.6%; ECOG PS 1, 95.2%). Percentages of patients who experienced treatment-related AEs of grade ≥3 were also similar between the ECOG PS groups (ECOG PS 0, 77.8%; ECOG PS 1, 72.1%).
Discussion
In SELECT, lenvatinib demonstrated a significant PFS benefit in patients with RR-DTC (13). This exploratory post hoc analysis of SELECT provides insights into markers of efficacy among patients with RR-DTC treated with lenvatinib. Specifically, patients with lower ECOG PS and lower NLR (largely driven by lower absolute neutrophil counts; Table 1 and Supplementary Table S1) at baseline had improved PFS, OS, and ORR.
The results of this post hoc analysis suggest that initiating treatment before a worsening in ECOG PS may improve clinical outcomes. Similarly, patients who are more fit and indicated for systemic therapy may benefit more from earlier treatment with lenvatinib. As expected, the sum of target lesion diameters at baseline was less in patients treated with lenvatinib with an ECOG PS of 0 compared with that measured in patients with an ECOG PS of 1 (Table 1). Nonetheless, patients with an ECOG PS of 0 experienced a higher ORR and greater percentage decrease in the sums of the diameters of target lesions, which was maintained over time (Supplementary Fig. S2). Patients with lower ECOG PS and NLR (regardless of treatment) also had improved survival outcomes (Figs. 1 and 3 and Supplementary Figs. S4 and S5). In a separate analysis of SELECT with updated data, durations of response with lenvatinib were shorter in patients with increased tumor burden (25). In combination with our analysis, these cumulative data suggest that, regardless of the disease-state marker, outcomes are improved when treatment with lenvatinib is initiated earlier in more fit patients with progressive RR-DTC. In addition, the higher ORR and greater tumor shrinkage in patients with an ECOG PS of 0 suggest that the improvement in clinical benefit seen in these patients is not solely because of a better performance status.
Evidence regarding the prognostic nature of NLR for thyroid cancers is somewhat equivocal. A study assessing NLR in patients with DTC versus benign thyroid nodules found a correlation between NLR and tumor size, but no significant difference in NLR between cancer and control groups (26). Similarly, a meta-analysis indicated that there was no significant difference in NLR between patients with DTC and patients with benign nodules (mean difference = 0.19 [CI −0.09 to 0.46]) (27). A large meta-analysis across solid tumors, however, did find an association between high NLR and a shorter OS (HR 1.81 [CI 1.67–1.97]; p < 0.001) (19). In an exploratory analysis of NLR in a phase 2 trial of lenvatinib, a trend indicating that baseline NLR could affect PFS was seen, although statistical significance was not reached (28). Our exploratory analysis of data from the phase 3 SELECT provides additional evidence of a relationship between lower baseline NLR and longer PFS and OS in patients with RR-DTC that was treated with lenvatinib.
This analysis was limited by the post hoc nature of the assessment. In addition, concurrent corticosteroid treatment can increase NLR (29), creating a potential confounding factor for these data. However, only 20 patients (7.7%) in the lenvatinib treatment arm were taking corticosteroids at baseline, and corticosteroid treatment did not appear to influence outcomes by NLR status (Supplementary Table S2 and Supplementary Fig. S3). This study also had a potential selection bias regarding tumor size, and an imbalance between number of metastatic sites at baseline between ECOG PS groups. In addition, tumor size was measured by the assessment of target lesions using RECIST v1.1, which is a surrogate for, but not absolute measure of, total tumor burden. There was also a difference in rates of lenvatinib dose reduction and discontinuation between ECOG PS 0 and 1, which could impact efficacy and create bias.
Lead-time bias may also have impacted the OS results observed in this analysis. For example, it is possible that patients with lower baseline ECOG PS and NLR were diagnosed earlier in the course of their disease; such early detection could make OS appear improved compared with patients diagnosed at a later disease stage, irrespective of treatment. Notably, however, PFS and ORR (measures of efficacy not impacted by lead time bias) were also improved in patients with lower ECOG PS. A further limitation is that this study was not powered to assess the predictive validity of ECOG PS or NLR; therefore, additional studies are needed to determine whether any of these indicators are predictive for lenvatinib versus placebo. Finally, ECOG PS may be assessed inconsistently by clinicians and may be unreliable as a prognostic marker, particularly in a real-world setting (30).
Despite these limitations, this analysis indicates that correlates of efficacy, including ECOG PS and NLR, may provide prognostic value and treatment guidance in patients with RR-DTC. For clinicians, deciding on the optimal time to initiate tyrosine kinase inhibitor therapy can be challenging because of the desire to avoid AEs (31), but this analysis suggests that, in patients with RR-DTC, it may be advantageous to start lenvatinib treatment before ECOG PS and NLR worsen, and tumor size increases.
Supplementary Material
Authors' Contributions
Study design: M.H.T., L.J.W., and R.X. Data acquisition: S.T., M.H.T., and J.C. Data analysis: R.X., M.H.T., and M.T. Data interpretation: All authors. Drafting the article and critical review of revisions: All authors. Final approval to submit: All authors.
Author Disclosure Statement
M.H.T. has performed consulting, is an advisory board member, and is on the speakers' bureaus for Bristol Myers Squibb and Eisai (honoraria). He is a consulting/advisory board member for Array Biopharma, Loxo Oncology, Blueprint Medicines, Bayer, Sanofi/Genzyme, Novartis, Arqule, and Merck (honoraria).
S.T. has received grants and personal fees from Eisai, Taiho, MSD, Chugai, Bayer, and AstraZeneca; and grants from Novartis.
J.C. has a scientific consultancy role (speaker and advisory roles) with Novartis, Pfizer, Ipsen, Exelixis, Bayer, Eisai, Advanced Accelerator Applications, Amgen, Sanofi, ITM, Sirlex, and Merck Serono. J.C. has received research grants from Novartis, Pfizer, AstraZeneca, Advanced Accelerator Applications, Eisai, and Bayer.
M.T. has received grants and personal fees from Eisai, MSD, Bristol Myers Squibb, Rakuten Medical, Pfizer, AstraZeneca, Bayer, and Ono Pharmaceutical. Personal fees from Merck, Serono, and LOXO.
S.L. has received research funding (inst) from Novartis and Sanofi Genzyme. She is an advisory board member for Eisai and Bayer.
N.K. reports grants and nonfinancial support for research funding from Eisai Co., Ltd, during the conduct of the study. Grants for research funding were received from AstraZeneca Co., Ltd, Bristol Myers Squibb, Pfizer Co., Ltd, and Chugai Pharmaceutical Co., Ltd. Grants and nonfinancial support for research funding were received from Ono Pharmaceutical Co., Ltd, outside the submitted work. Honoraria were received from Ono Pharmaceutical Co., Ltd, Bristol Myers Squibb, Merck Biopharma, AstraZeneca Co., Ltd, Merck Sharp & Dohme, Eisai, and Bayer.
C.E.D. is an employee of Eisai.
R.X. is an employee of Eisai.
B.R. has served in a consulting/advisory role for Eisai and LOXO; received honoraria from Eisai; and claims travel, accommodations, or expenses from Eisai and LOXO.
S.S. has served in a consulting/advisory role for Fortress Biotech and received grant support from Exelixis.
M.A.H. has served in a consulting role, is an advisory board member for Corcept and HRA Pharma (honoraria), and has received research support from Exelixis.
R.E. has served in a consulting/advisory role for Eisai, Exelixis, Sanofi Genzyme, and LOXO, and has served on speakers' bureaus for Eisai, Exelixis, Sanofi Genzyme, and LOXO.
L.J.W. has served in a consulting/advisory role with Bayer, Eisai, LOXO, and Merck.
Funding Information
This study was funded by Eisai Inc., Woodcliff Lake, NJ, USA, and also by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. Medical writing support was provided by Heather A. Mitchell, PhD, Oxford PharmaGenesis Inc., Newtown, PA, USA and was funded by Eisai Inc., Woodcliff Lake, NJ, USA, and also by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
Supplementary Material
References
- 1.Davies L, Welch HG. 2006Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA 295:2164–2167 [DOI] [PubMed] [Google Scholar]
- 2.Davies L, Welch HG. 2014Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg 140:317–322 [DOI] [PubMed] [Google Scholar]
- 3.Kent WD, Hall SF, Isotalo PA, et al. 2007Increased incidence of differentiated thyroid carcinoma and detection of subclinical disease. CMAJ 177:1357–1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Enewold L, Zhu K, Ron E, et al. 2009Rising thyroid cancer incidence in the United States by demographic and tumor characteristics, 1980–2005. Cancer Epidemiol Biomarkers Prev 18:784–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lim H, Devesa SS, Sosa JA, et al. 2017Trends in thyroid cancer incidence and mortality in the United States, 1974–2013. JAMA 317:1338–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carhill AA, Litofsky DR, Ross DS, et al. 2015Long-term outcomes following therapy in differentiated thyroid carcinoma: NTCTCS registry analysis 1987–2012. J Clin Endocrinol Metab 100:3270–3279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Durante C, Haddy N, Baudin E, et al. 2006Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab 91:2892–2899 [DOI] [PubMed] [Google Scholar]
- 8.Matsui J, Yamamoto Y, Funahashi Y, et al. 2008E7080, a novel inhibitor that targets multiple kinases, has potent antitumor activities against stem cell factor producing human small cell lung cancer H146, based on angiogenesis inhibition. Int J Cancer 122:664–671 [DOI] [PubMed] [Google Scholar]
- 9.Matsui J, Funahashi Y, Uenaka T, et al. 2008Multi-kinase inhibitor E7080 suppresses lymph node and lung metastases of human mammary breast tumor MDA-MB-231 via inhibition of vascular endothelial growth factor-receptor (VEGF-R) 2 and VEGF-R3 kinase. Clin Cancer Res 14:5459–5465 [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto Y, Matsui J, Matsushima T, et al. 2014Lenvatinib, an angiogenesis inhibitor targeting VEGFR/FGFR, shows broad antitumor activity in human tumor xenograft models associated with microvessel density and pericyte coverage. Vasc Cell 6:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tohyama O, Matsui J, Kodama K, et al. 2014Antitumor activity of lenvatinib (e7080): an angiogenesis inhibitor that targets multiple receptor tyrosine kinases in preclinical human thyroid cancer models. J Thyroid Res 2014:638747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lenvima (lenvatinib) [prescribing information] 2020Eisai Inc., Woodcliff Lake, NJ, USA. Available at http://www.lenvima.com/pdfs/prescribing-information.pdf (accessed March24, 2021)
- 13.Schlumberger M, Tahara M, Wirth LJ, et al. 2015Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med 372:621–630 [DOI] [PubMed] [Google Scholar]
- 14.Kiyota N, Robinson B, Shah M, et al. 2017Defining radioiodine-refractory differentiated thyroid cancer: efficacy and safety of lenvatinib by radioiodine-refractory criteria in the SELECT Trial. Thyroid 27:1135–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jang RW, Caraiscos VB, Swami N, et al. 2014Simple prognostic model for patients with advanced cancer based on performance status. J Oncol Pract 10:e335–341 [DOI] [PubMed] [Google Scholar]
- 16.Suh SY, Leblanc TW, Shelby RA, et al. 2011Longitudinal patient-reported performance status assessment in the cancer clinic is feasible and prognostic. J Oncol Pract 7:374–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song T, Wan Q, Yu W, et al. 2017Pretreatment nutritional risk scores and performance status are prognostic factors in esophageal cancer patients treated with definitive chemoradiotherapy. Oncotarget 8:98974–98984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee F, Yang PS, Chien MN, et al. 2018An increased neutrophil-to-lymphocyte ratio predicts incomplete response to therapy in differentiated thyroid cancer. Int J Med Sci 15:1757–1763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Templeton AJ, McNamara MG, Šeruga B, et al. 2014Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst 106:dju124. [DOI] [PubMed] [Google Scholar]
- 20.Fukuda N, Wang X, Ohmoto A, et al. 2020Sequential analysis of neutrophil-to-lymphocyte ratio for differentiated thyroid cancer patients treated with lenvatinib. In Vivo 34:709–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanahan D, Weinberg RA. 2011Hallmarks of cancer: the next generation. Cell 144:646–674 [DOI] [PubMed] [Google Scholar]
- 22.Suzuki C, Kiyota N, Imamura Y, et al. 2019Exploratory analysis of prognostic factors for lenvatinib in radioiodine-refractory differentiated thyroid cancer. Head Neck 41:3023–3032 [DOI] [PubMed] [Google Scholar]
- 23.Wirth LJ, Tahara M, Robinson B, et al. 2018Treatment-emergent hypertension and efficacy in the phase 3 study of (E7080) lenvatinib in differentiated cancer of the thyroid (SELECT). Cancer 124:2365–2372 [DOI] [PubMed] [Google Scholar]
- 24.Hinkle DE, Wiersma W, Jurs SG 2002 Applied Statistics for the Behavioral Sciences. Fifth edition. Houghton Mifflin, Boston, MA [Google Scholar]
- 25.Gianoukakis AG, Dutcus CE, Batty N, et al. 2018Prolonged duration of response in lenvatinib responders with thyroid cancer. Endocr Relat Cancer 25:699–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu CL, Lee JJ, Liu TP, et al. 2013Blood neutrophil-to-lymphocyte ratio correlates with tumor size in patients with differentiated thyroid cancer. J Surg Oncol 107:493–497 [DOI] [PubMed] [Google Scholar]
- 27.Liu JF, Ba L, Lv H, et al. 2016Association between neutrophil-to-lymphocyte ratio and differentiated thyroid cancer: a meta-analysis. Sci Rep 6:38551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takahashi S, Kiyota N, Yamazaki T, et al. 2019. A phase II study of the safety and efficacy of lenvatinib in patients with advanced thyroid cancer. Future Oncol 15:717–726 [DOI] [PubMed] [Google Scholar]
- 29.Boegemann M, Schlack K, Thomes S, et al. 2017 The role of the neutrophil to lymphocyte ratio for survival outcomes in patients with metastatic castration-resistant prostate cancer treated with abiraterone. Int J Mol Sci 18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Datta SS, Ghosal N, Daruvala R, et al. 2019How do clinicians rate patient's performance status using the ECOG performance scale? A mixed-methods exploration of variability in decision-making in oncology. Ecancermedicalscience 13:913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmidbauer B, Menhart K, Hellwig D, et al. 2017Differentiated thyroid cancer-treatment: state of the art. Int J Mol Sci 18:1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.