Abstract
Stem cell-based treatments have emerged as potentially effective approaches to delay the progression of amyotrophic lateral sclerosis (ALS). This study was designed as a single-center, prospective, and open-label study without a placebo control group to assess the safety and efficacy of concurrent intrathecal (IT) and intravenous (IV) administration of autologous bone marrow-derived mesenchymal stem cells (BM-MSCs) in patients with ALS. Autologous BM-MSCs were isolated and expanded under standard conditions. Fifteen patients were neurologically examined before BM-MSCs transplantation (1 × 10 6 cells/kg BW) to evaluate the rate of pre-treatment disease progression. To assess the safety and efficacy, patients were examined at 1, 3, and 6 months following the treatment with BM-MSCs. Adverse reactions were assessed, and the clinical outcome was determined by the evaluation of the ALS functional rating scale-revised (ALSFRS-R) and forced vital capacity (FVC). No serious adverse reaction was observed after combined IT and IV administration of BM-MSCs. The mean ALSFRS-R and FVC values remained stable during the first 3 months of the treatment. However, a significant reduction in ALSFRS-R and FVC levels was observed in these patients 6 months after BM-MSCs administration. Our study revealed that the concurrent IT and IV application of BM-MSCs in patients with ALS is a safe procedure. Furthermore, our data indicate a temporary delay in the progression of ALS after a single combined IT and IV administration of BM-MSCs. Further studies are required to explore if the repeated applications of BM-MSCs could prolong survival and delay the progression of ALS.
Keywords: Stem cell therapy, Clinical trial, Amyotrophic lateral sclerosis
1. Introduction
Amyotrophic lateral sclerosis (ALS) is a progressive neurological disease characterized by degeneration of the upper and lower motor neurons [1]. Despite extensive efforts to stop or reverse the progression, to date, no effective therapeutic approach is available for ALS [2,3]. Recently, stem cell-based therapies have emerged as a potentially novel approach in the treatment of ALS. Preclinical studies have suggested that transplanted stem cells can delay disease progression and prolong survival for people with ALS [4,5]. Evidence suggests that stem cells can generate new neural cells through the activation of cell proliferation and differentiation in the injury site, as well as to improve the survival of existing neural cells, presumably via the alleviation of neuro-inflammation [4,6]. Several types of stem cells have been applied in various ALS experimental models, such as bone marrow stem cells, mesenchymal stem cells (MSCs), embryonic stem cells, neural stem/progenitor cells, and induced pluripotent stem cells [[7], [8], [9], [10]].
MSCs have been attracting much attention for the treatment of ALS due to their ability to enhance the survival of neuronal cells and modulate inflammatory responses [11]. Furthermore, MSCs efficiently inhibit apoptosis of neurons and glial cells [4]. A considerable amount of investigations has been attempted to evaluate the effect of MSCs application in the treatment of ALS, trying to prove their inhibitory action on the progression of the disease [9,[12], [13], [14], [15]]. These studies suggest that several factors, such as the appropriate cell preparation, proper dose, and efficient delivery system are crucial to achieving optimal results after stem cell therapy for ALS. During the last few decades, several clinical trials have shown that various delivery systems, such as intrathecal (IT), intravenous (IV), intramuscular, and intraspinal, administration of MSCs can be safely utilized in the transplantation of MSCs in patients with ALS [[15], [16], [17], [18]]. The major goals of this study were to determine the safety as well as the efficacy of the concurrent IT and IV applications of autologous bone marrow-derived mesenchymal stem cells (BM-MSCs) in patients with ALS.
2. Materials and methods
2.1. Study design and ethical statement
This study was designed as a single-center, prospective, open-label study without a placebo control group to assess the safety and efficacy of IT and IV administrations of autologous BM-MSCs in ALS patients. This study was performed in accordance with the Declaration of Helsinki Ethical Principles and Good Clinical Practices and approved by the Ethics Committee of the Mashhad University of Medical Sciences, Mashhad, Iran (Reg. No: IR.MUMS.REC.1399.269). This clinical trial was registered with the Iranian Registry of Clinical Trials (ID: IRCT20160809029275N2). Informed consent was obtained from all patients. All patients were permanent residents or citizens of Iran.
2.2. Participants
In our trial, participants were between 18 and 75 years old with a history of ALS for at least 1 year. Fifteen patients (n = 15), who fulfilled El Escorial Criteria for definite ALS [19], were selected for this study. All patients had forced vital capacity (FVC) greater than 65% of the predicted average value for age, gender, and body size. Exclusion criteria were active systemic or local infection 1 week before cell therapy, ventilator dependency, cancer, any clinically serious medical conditions within the last six months (such as myocardial infarction, angina pectoris, congestive heart failure, etc.), and/or active systemic diseases.
2.3. Bone marrow extraction
Under local anesthesia, the bone marrow aspiration was performed on the posterior superior iliac crest while the patient was lying in a left or right lateral decubitus position. Approximately 12 ml of bone marrow blood was collected by a single aspiration from each patient. On the next day, the puncture site was examined, and the patient was then discharged if there were no complications.
2.4. Cell preparation and culture
The MSCs were isolated, expanded, and analyzed under good manufacturing practice conditions. The bone marrow mononuclear cells were isolated using Ficoll (Ficoll-Paque Premium; GE Healthcare Bio-Sciences, Uppsala, Sweden) density gradient centrifugation. The mononuclear cells (3 × 106 cells) were placed in a 175-cm2 flask (Thermo Scientific Nunc, Roskilde, Denmark) and cultured in enriched minimum essential medium-α (Lonza, Basel, Switzerland) containing 10% fetal bovine serum (Life Technologies, Grand Island, USA), and 1% penicillin-streptomycin (Biochrom, Berlin, Germany) in a humidified incubator at 37 °C under 5% CO2. The non-adherent cells were removed by replacing the medium. After removing the non-adherent cells, the culture medium was changed twice a week. When the BM-MSCs primary cultures reached 80% confluence, the cells were harvested using 0.125% trypsin-EDTA (Life Technologies, Darmstadt, Germany) and passaged.
To confirm sterility, the samples were analyzed for bacteria and mycoplasma by endotoxin and reverse transcription-polymerase chain reaction tests, respectively. The percentage of the viable cells before the transplantation was ranged between 95 and 98%. Finally, the autologous BM-MSCs product was released and transported under controlled temperature (2–8 °C) to the investigation site. All the above-mentioned procedures, such as BM-MSCs harvesting, processing, and product release, were performed in a Grade B cleanroom with GMP requirements.
2.5. Stem cell transplantation
All patients received concurrent IT and IV applications of autologous BM-MSCs in a clinical setting. BM-MSCs (1 × 106 cells/kg BW) in 2 ml normal saline solution were slowly injected IT for approximately 2 min using a standard lumbar puncture at the level of L3–L4. Furthermore, BM-MSCs (1 × 106 cells/kg) was infused intravenously in 50–60 ml of normal saline solution with a 22G intravascular catheter connected to a syringe dispenser at a rate of 120 ml/h.
2.6. Patient follow-up
The patients were neurologically examined 1 month before BM-MSCs transplantation to evaluate the rate of pre-treatment disease progression. The BM-MSCs have been applied both via IT and IV injections in 2 dosing cohorts (1 × 106 cells/kg IT and 1 × 106 cells/kg IV). The follow-up period was 6 months, with regular intervals between examinations at 1, 3, and 6 months. Safety was the primary objective of the current trial. To assess adverse effects after simultaneous IT and IV BM-MSCs delivery, all complaints of patients regarding their medical conditions, as well as any new neurological deficit, were registered. After the cell transplantation, patients were closely monitored for immediate adverse effects/reactions, both systemic (i.e., allergic reaction, fever, and sepsis) and local (pain, bleeding, local infection, urinary incontinence, paralysis, or sensory loss below the level of the injection site or other) for 3 days. During their 3-day stay in the hospital, neurological examinations and vital function monitoring (respiratory and heart rate, blood pressure, and body temperature) were performed every day. Serum biochemistry and whole blood analysis were evaluated to exclude liver or renal dysfunction, mineral imbalances, or systemic infection on days 1 and 3. If no complication occurred, patients were discharged after 3 days and followed up at regular intervals according to the study protocol.
2.7. Outcomes
The second aim of the study was to assess the effect of BM-MSC application on the progression of the disease using the ALS functional rating scale-revised (ALSFRS-R) and FVC assessments. The ALSFRS-R is a confirmed rating tool for monitoring the progression of disability in patients with ALS [20]. ALSFRS-R can assess respiratory symptoms and the degree of impairments in patients' abilities to perform independent daily activities [21]. As the ALSFRS-R could be influenced by the patient's mental status (i.e., depression) or by the subjective view of the investigators, the FVC examination was done to provide more objective data. The values of ALSFRS-R and FVC after the treatment were compared to the levels observed before the treatment.
2.8. Data analysis
Parameters were calculated as simple linear regressions to compare the pre- and post-intervention slopes (0–1, 0–3, and 0–6 months) of the ALS-FRS-R scores and the FVC values. The slopes were compared by paired t-test for correlated variables. All data were presented as mean ± standard error of the mean (SEM) and the significance level was regarded at P < 0.05.
3. Results
3.1. BM-MSCs isolation and cell characterization
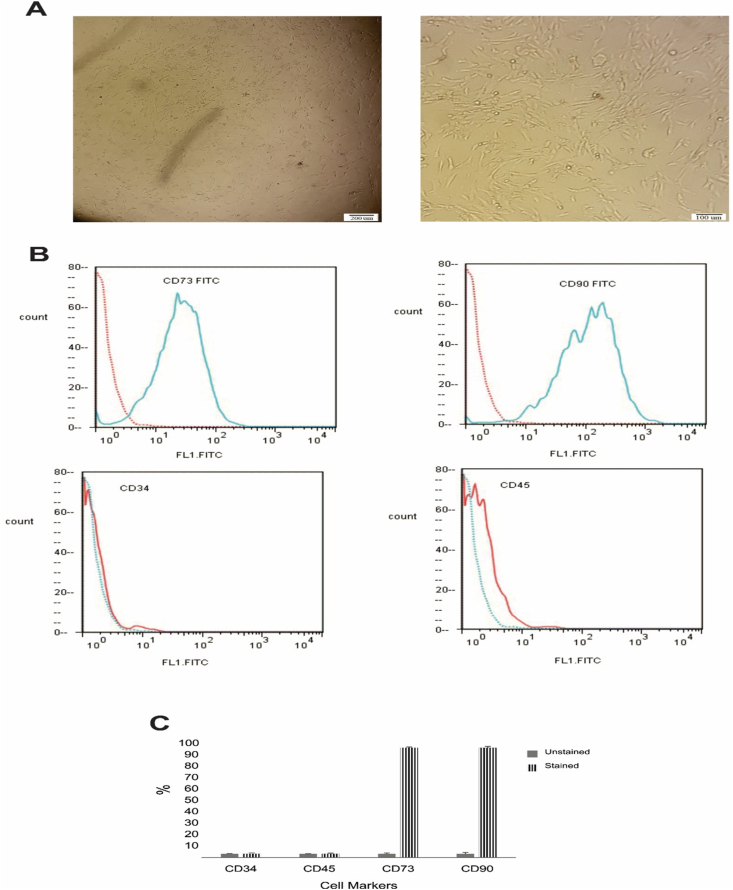
No side effects were observed during and after bone marrow collection in any of the patients. In cell culture, BM-MSCs were seen as fibroblastic-like and spindle-shaped cells (Fig. 1A). BM-MSCs highly expressed the specific markers of mesenchymal lineage CD73 and CD90. BM-MSCs did not express the hematopoietic stem cell markers CD34 and CD45 (Fig. 1B–C). The cells used for the transplantation were at the second or third passage. All sterility tests performed after each passage were negative. Using a doubling time assay, no significant cellular senescence was observed. We injected 1 × 106 BM-MSCs in saline solution through IT and IV injections. The viable BM-MSCs counts before the transplantation were ranged between 95 and 98%.
Fig. 1.
Mesenchymal stem cells characterization by flow cytometry. (A) Phase-contrast micrographs of MSCs derived from bone marrow on day 10 after seeding (left) and after passage 2 (right). (B) Representative data indicated that cells were positive for CD73 and CD90 as the MSCs markers, and negative for the CD34 and CD45as the hematopoietic markers. (C) The percentage of stained cells in comparison with unstained controls is shown for each marker.
3.2. Clinical characterization of patients
The demographic characteristics of the patients are presented in Table 1. The median age was 35 years (range 23–60 years), including 4 females and 11 males. Thirteen of 15 patients suffered from spinal-onset ALS and the other 2 had bulbar-spinal onset.
Table 1.
Clinical characterization of patients with amyotrophic lateral sclerosis enrolled in the clinical trial.
| Patient number | Age | Sex | ALS Symptoms | Disease Duration (Months) from first diagnostic |
|---|---|---|---|---|
| P1 | 55 | Female | spinal | 61 |
| P2 | 47 | Male | spinal | 67 |
| P3 | 60 | Male | bulbar/spinal | 12 |
| P4 | 36 | Male | spinal | 10 |
| P5 | 51 | Male | spinal | 55 |
| P6 | 37 | Female | spinal | 55 |
| P7 | 57 | Male | spinal | 31 |
| P8 | 35 | Male | spinal | 60 |
| P9 | 35 | Male | spinal | 72 |
| P10 | 52 | Male | spinal | 45 |
| P11 | 55 | Male | bulbar/spinal | 44 |
| P12 | 55 | Male | Spinal | 12 |
| P13 | 35 | Female | Spinal | 17 |
| P14 | 55 | Male | Spinal | 16 |
| P15 | 23 | Female | Spinal | 42 |
3.3. Safety assessment
To assess safety, all ALS patients were monitored for 3 days after the treatment with autologous BM-MSCs. As shown in Table 2, there were no serious adverse reactions in the 15 patients enrolled in this clinical trial during the follow-up period. On day 3, all patients experienced mild/moderate headaches similar to the headaches after a standard lumbar puncture. Patient No. 15 had an allergic reaction, respiratory distress, nausea, and vomiting that resolved after 24 h. Two participants (No. 3 and 13) had urinary incontinence that lasted 72 h after the cell administration.
Table 2.
Adverse effects after cell transplantation.
| Patient NO. | Parameters |
|||||||
|---|---|---|---|---|---|---|---|---|
| Headache | Fever | Allergic reaction | Urinary incontinence | Respiratory distress | Nausea | Vomiting | Neck stiffness | |
| P1 | + | NA | NA | NA | NA | NA | NA | NA |
| P2 | + | NA | NA | NA | NA | NA | NA | NA |
| P3 | + | NA | NA | + | NA | NA | NA | NA |
| P4 | + | NA | NA | NA | NA | NA | NA | NA |
| P5 | + | NA | NA | NA | NA | NA | NA | NA |
| P6 | + | NA | NA | NA | NA | NA | NA | NA |
| P7 | + | NA | NA | NA | NA | NA | NA | NA |
| P8 | + | NA | NA | NA | NA | NA | NA | NA |
| P9 | + | NA | NA | NA | NA | NA | NA | NA |
| P10 | + | NA | NA | NA | NA | NA | NA | NA |
| P11 | + | NA | NA | NA | NA | NA | NA | NA |
| P12 | + | + | NA | NA | NA | + | NA | NA |
| P13 | + | + | NA | + | NA | + | + | NA |
| P14 | + | + | NA | NA | NA | + | NA | NA |
| P15 | + | + | + | NA | + | + | + | NA |
Abbreviation: no-adverse effect (NA).
3.4. Efficacy assessments
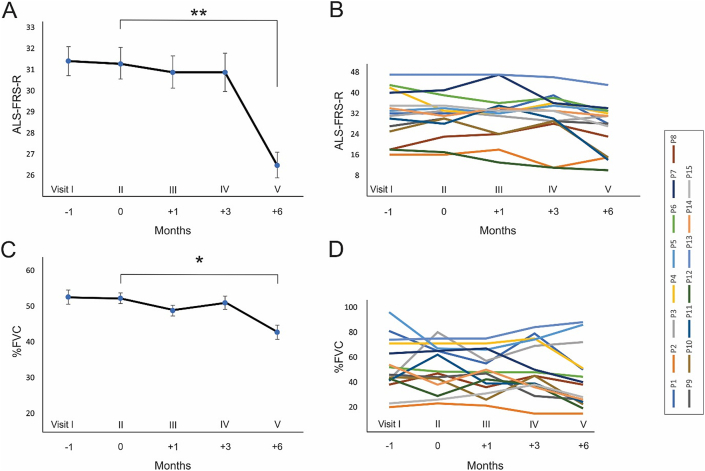
The alterations of the ALSFRS-R slope (monthly decline rate) were analyzed during the follow-up period. Fig. 2 shows the ALSFRS-R scores 1 month before as well as 1, 3, and 6 months after the treatment with BM-MSCs. The ALSFRS-R values remained stable for 3 months in the individual patients after BM-MSCs application (Fig. 2B). Regression analysis of ALSFRS-R scores in all patients indicated that there was no significant reduction in ALSFRS-R slopes after 1 (β = 0.81, R2 = 0.051; P = 0.812) and 3 (β = 0.86, R2 = 0.004; P = 0.985) months of BM-MSCs transplantation compared with the pre-implantation score (Fig. 2A–B). However, the six-month follow-up value of the ALSFRS-R declined significantly compared with the pre-treatment score (β = 0.80, R2 = 0.76; P < 0.01; Fig. 2A–B).
Fig. 2.
Clinical analysis of amyotrophic lateral sclerosis (ALS) patients during the follow-up period. (A) Regression analysis of ALS Functional Rating Scale–Revised (ALSFRS-R) score changes in 15 patients treated with autologous bone marrow-derived mesenchymal stem cells (BM-MSCs). The y-axis shows the ALSFRS-R scores, and the x-axis shows the clinical visits with comparing time courses in months. There was a significant reduction ALSFRS-R 6 months after the treatment. (B) Time courses of ALSFRS-R scores in the individual patients. (C) Regression analysis of forced vital capacity (FVC) changes in 15 patients (y-axis indicates the percentage of FVC and the x-axis indicates clinical visits with comparing time courses in months). There was a significant reduction in FVC 6 months after the application of BM-MSCs. D) FVC scores of the individual patient during the follow-up period.
Furthermore, FVC scores were evaluated and compared between the pre- and post-transplantation phases. There were no significant differences between FVC slopes at 1 (β = 0.64, R2 = 0.046; P = 0.665) and 3 (β = 0.78, R2 = 0.064; P = 0.270) months after BM-MSCs transplantation compared to the pre-implantation phase (Fig. 2C–D). The average values of the FVC were stable during 3 months of cell therapy in the individual patients (Fig. 2D). However, a six-month follow-up revealed a significant reduction of the FVC values compared with the pre-treatment values (β = 0.80, R2 = 0.62; P < 0.05; Fig. 2C–D).
4. Discussion
The present study revealed that the concurrent IT and IV injection of BM-MSCs in patients with ALS is a safe procedure with no major adverse events and provides indications of potential clinical benefits. During the follow-up period, the mild headache was the most frequent treatment-related adverse effect. Furthermore, ALSFRS-R and FVC values were stable after cell therapy during the 3-month follow-up. However, the ALSFRS-R scores and the FVC values significantly decreased 6 months after the cell therapy. Our data indicate a temporary reduction of ALS progression after a single application of BM-MSCs via concurrent IT and IV injections.
Both experimental and clinical investigations have shown temporary beneficial effects of stem cell-based treatments in neurodegenerative diseases. Administration of motor neurons derived from embryonic stem cells in the spinal cord of an ALS animal model exhibited temporary improvement of motor function before mutant rats succumbed to paralysis [22]. Transplantation of adult olfactory bulb neural precursor cells into the spinal cord of an ALS transgenic mouse model led to the temporary reduction of neuron degeneration and improvement of motor function [23]. Combined BM-MSCs with T-cell vaccination treatment of 7 patients with ALS resulted in temporary improvement of ALSFRS-R scores [24]. Both intramuscularly and intrathecally injection of BM-MSCs expressed neurotrophic factors in 14 patients with ALS temporarily reduced the progression of disease during 6 months of intervention [18]. It has been suggested that the microenvironment of the spinal cord of ALS is detrimental for transplanted stem cells, suggesting that transplanted cells can be targeted by the same hostile condition that leads to degeneration of endogenous cells [22,25]. Repeated injections of BM-MSCs have been suggested as a possible clinical effort to prolong the beneficial effect of stem cell therapy in patients with ALS, presumably via stem cell regulatory action on switching from pro- to anti-inflammatory conditions [26]. The beneficial action of BM-MSCs on damaged spinal cord neurons probably mediated through the secretion of neurotrophic factors, axonal regeneration, and myelin sheath repair [27]. However, it has been demonstrated that stem cell therapy through the secretion of anti-inflammatory cytokines and neurotrophic growth factors can modulate the immune system and influence the progression of ALS [28,29].
Several factors, such as the protocols of stem cell expansion, type and source of stem cells, number of stem cells, route of application, and the appropriate application method, exert a decisive impact on the success of any stem-cell-based therapeutic strategies for ALS [9,11]. The current trial has been demonstrated the safety and temporary efficacy of a high single dose of BM-MSCs through IV and IT injections. In keeping with our results, a previous study has shown that IV or IT transplantation of BM-derived stromal cells is a safe and feasible therapeutic approach [30]. Furthermore, several investigations have demonstrated that the application of MSCs can slow down the progression of ALS [[12], [13], [14], [15],31,32]. Besides, our results are consonant with the previous experimental studies on motor neuron diseases indicate the efficacy of BM-MSCs in targeting the pathological mechanisms and slowing the progression of the clinical symptoms [33,34]. So far, several clinical trials have been performed to explore the safety and efficacy of stem cells transplantation in patients with ALS. None of these studies have reported serious side effects, including tumorigenesis. Several clinical trials revealed that the application of MSCs in patients with ALS did not lead to any functional modification of the neural tissues, including chromosomal changes or cellular senescence [31,35]. Magnetic Resonance Imaging (MRI) evaluations did not show any structural alterations, including tumor formation, in the brain and spinal cord of ALS patients treated with IT application of MSCs during a 1–12 years follow-up period [12,14,31,35]. Although in the present study, we did not evaluate the MRI of patients during or after cell transplantation, no clinical evidence indicated severe side effects (Table 2).
The outcome of stem cell treatment in ALS patients can be modified by high expectations and psychosocial circumstances [15]. These factors could affect ALSFRS scores during the follow-up period [15]. In addition to ALSFRS scores, we assessed the FVC value as a valid variable in ALS patients. The FVC value obtained before any interventions represents a clinically worthwhile predictor of both survival and disease progression in patients with ALS [36]. In our study, the FVC values remained stable for 3 months and declined after 6 months of treatment, which supports evidence from previous observations [14,15].
It has been shown that the administration of MSCs can convert inflammatory microglia (M1) to anti-inflammatory (M2) cells, which are considered as one of their effective mechanisms in preventing ALS progression [37]. Previous clinical investigations have shown that the direct application of MSCs into the cervical and lumbar spinal cord of patients with ALS was not clinically beneficial [38]. Despite the safety and avoiding surgical interventions, IV application of MSCs, as a non-invasive and reproducible approach, requires large quantities of cells to reach the lesion site [39]. A part of these cells, however, are trapped in other organs, such as the lungs and lymph nodes, with a therapeutically questionable amount of MSCs reaching the lesion site [40]. IT injection of MSCs could be an effective strategy to deliver the cells to the target tissue and generate effective responses [12,14]. IT application of MSCs may increase their immunomodulatory and trophic effects directly on the CNS without producing systemic adverse effects [41]. In the present study, we investigated the effects of concurrent IV and IT injections of MSCs. Using simultaneous IT and IV application of MSCs may increase the chances of transplanted cells reaching the lesion site and improve the outcomes. Several experimental and clinical investigations have emphasized the superior efficacy of simultaneous IV and IT injections in ALS [42,43].
This study has some limitations. It is a phase I clinical trial with small sample size; therefore, randomized control trials with a larger number of patients and appropriate control groups are needed to confirm our findings. In addition, based on demographic data, the mean age of patients was generally low when contrasted to the other clinical studies. One of the probable clarifications is that more young patients participate in new clinical trials. Furthermore, a short pre-operation observation period (1 month) as well as a short follow-up period (6 months) cannot fully justify the long-term beneficial effects of our approach in preventing disease progression. Longer pre and post follow-up periods are required.
5. Conclusion
Our results demonstrated that the IT and IV transplantation of BM-MSCs in ALS patients is a safe procedure and could slow down the progression of the disease. To confirm the most effective methods of delivery and suitable doses in single or repeated applications of BM-MSCs treatment, further clinical investigations with more patients and longer follow-up periods as well as with the repetitive application of BM-MSCs are required.
Funding
This study was financially supported by Mashhad University of Medical Sciences and Parnia Stem Cell Institute.
Declaration of competing interest
N.K.F, A.G, and S.SN declare that they have no competing interests. J.T.A, A.R.B, and A.A have received research grants from Parnia Stem Cell Institute.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
Contributor Information
Sajad Sahab-Negah, Email: sahabnegahs@mums.ac.ir.
Ali Gorji, Email: gorjial@uni-muenster.de.
References
- 1.Armon C., Graves M.C., Moses D., Forté D.K., Sepulveda L., Darby S.M. Linear estimates of disease progression predict survival in patients with amyotrophic lateral sclerosis. Muscle Nerve. 2000;23(6):874–882. doi: 10.1002/(sici)1097-4598(200006)23:6<874::aid-mus5>3.0.co;2-u. Official Journal of the American Association of Electrodiagnostic Medicine. [DOI] [PubMed] [Google Scholar]
- 2.Miller R., Mitchell J., Lyon M., Moore D. Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND) Amyotroph Lateral Scler Other Motor Neuron Disord. 2003;4(3):191–206. official publication of the World Federation of Neurology, Research Group on Motor Neuron Diseases. [PubMed] [Google Scholar]
- 3.Al-Chalabi A., Andersen P.M., Chandran S., Chio A., Corcia P., Couratier P. July 2017 ENCALS statement on edaravone. Amyotroph Lateral Scler Frontotemporal Degener. 2017;18(7–8):471–474. doi: 10.1080/21678421.2017.1369125. [DOI] [PubMed] [Google Scholar]
- 4.Wahid S.F.A., Law Z.K., Ismail N.A., Lai N.M. Cell-based therapies for amyotrophic lateral sclerosis/motor neuron disease. Cochrane Database Syst Rev. 2019;(12) doi: 10.1002/14651858.CD011742.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zalfa C., Nodari L.R., Vacchi E., Gelati M., Profico D., Boido M. Transplantation of clinical-grade human neural stem cells reduces neuroinflammation, prolongs survival and delays disease progression in the SOD1 rats. Cell Death Dis. 2019;10(5):1–15. doi: 10.1038/s41419-019-1582-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu J., Wang F. Role of neuroinflammation in amyotrophic lateral sclerosis: cellular mechanisms and therapeutic implications. Front Immunol. 2017;8:1005. doi: 10.3389/fimmu.2017.01005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Csobonyeiova M., Polak S., Nicodemou A., Danisovic L. Induced pluripotent stem cells in modeling and cell-based therapy of amyotrophic lateral sclerosis. J Physiol Pharmacol. 2017 Oct;68(5):649–657. an official journal of the Polish Physiological Society. PubMed PMID: 29375039. Epub 2018/01/30. eng. [PubMed] [Google Scholar]
- 8.Hedges E.C., Mehler V.J., Nishimura A.L. The use of stem cells to model amyotrophic lateral sclerosis and frontotemporal dementia: from basic research to regenerative medicine. Stem Cell Int. 2016;2016:9279516. doi: 10.1155/2016/9279516. PubMed PMID: 26966440. Pubmed Central PMCID: PMC4761393. Epub 2016/03/12. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hajivalili M., Pourgholi F., Kafil H.S., Jadidi-Niaragh F., Yousefi M. Mesenchymal stem cells in the treatment of amyotrophic lateral sclerosis. Curr Stem Cell Res Ther. 2016;11(1):41–50. doi: 10.2174/1574888x10666150902095031. PubMed PMID: 26329483. Epub 2015/09/04. eng. [DOI] [PubMed] [Google Scholar]
- 10.Soler B., Fadic R., von Bernhardi R. Stem cells therapy in amyotrophic lateral sclerosis treatment. A critical view. Rev Neurol. 2011 Apr 1;52(7):426–434. PubMed PMID: 21425112. Epub 2011/03/23. Trasplante de celulas troncales como terapia para la esclerosis lateral amiotrofica. Una mirada critica. spa. [PubMed] [Google Scholar]
- 11.Lewis C.M., Suzuki M. Therapeutic applications of mesenchymal stem cells for amyotrophic lateral sclerosis. Stem Cell Res Ther. 2014;5(2):32. doi: 10.1186/scrt421. PubMed PMID: 25157751. Pubmed Central PMCID: Pmc4035799. Epub 2014/08/27. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mazzini L., Ferrero I., Luparello V., Rustichelli D., Gunetti M., Mareschi K. Mesenchymal stem cell transplantation in amyotrophic lateral sclerosis: a phase I clinical trial. Exp Neurol. 2010;223(1):229–237. doi: 10.1016/j.expneurol.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 13.Mazzini L., Mareschi K., Ferrero I., Vassallo E., Oliveri G., Boccaletti R. Autologous mesenchymal stem cells: clinical applications in amyotrophic lateral sclerosis. Neurol Res. 2006;28(5):523–526. doi: 10.1179/016164106X116791. [DOI] [PubMed] [Google Scholar]
- 14.Nabavi S.M., Arab L., Jarooghi N., Bolurieh T., Abbasi F., Mardpour S. Safety, feasibility of intravenous and intrathecal injection of autologous bone marrow derived mesenchymal stromal cells in patients with amyotrophic lateral sclerosis: an open label phase I clinical trial. Cell J (Yakhteh) 2019;20(4):592. doi: 10.22074/cellj.2019.5370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Syková E., Rychmach P., Drahorádová I., Konrádová Š., Růžičková K., Voříšek I. Transplantation of mesenchymal stromal cells in patients with amyotrophic lateral sclerosis: results of phase I/IIa clinical trial. Cell Transplant. 2017;26(4):647–658. doi: 10.3727/096368916X693716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geijo-Barrientos E., Pastore-Olmedo C., De Mingo P., Blanquer M., Gómez Espuch J., Iniesta F. Intramuscular injection of bone marrow stem cells in amyotrophic lateral sclerosis patients: a randomized clinical trial. Front Neurosci. 2020;14:195. doi: 10.3389/fnins.2020.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blanquer M., Moraleda J.M., Iniesta F., Gómez-Espuch J., Meca-Lallana J., Villaverde R. Neurotrophic bone marrow cellular nests prevent spinal motoneuron degeneration in amyotrophic lateral sclerosis patients: a pilot safety study. Stem cells. 2012;30(6):1277–1285. doi: 10.1002/stem.1080. [DOI] [PubMed] [Google Scholar]
- 18.Petrou P., Gothelf Y., Argov Z., Gotkine M., Levy Y.S., Kassis I. Safety and clinical effects of mesenchymal stem cells secreting neurotrophic factor transplantation in patients with amyotrophic lateral sclerosis: results of phase 1/2 and 2a clinical trials. JAMA Neurol. 2016;73(3):337–344. doi: 10.1001/jamaneurol.2015.4321. [DOI] [PubMed] [Google Scholar]
- 19.Brooks B.R., Miller R.G., Swash M., Munsat T.L. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000 Dec;1(5):293–299. doi: 10.1080/146608200300079536. official publication of the World Federation of Neurology, Research Group on Motor Neuron Diseases. PubMed PMID: 11464847. Epub 2001/07/24. eng. [DOI] [PubMed] [Google Scholar]
- 20.Hemmatian H., Bakker A.D., Klein-Nulend J., van Lenthe G.H. Aging, Osteocytes, and Mechanotransduction. Curr Osteoporos Rep. 2017 Oct;15(5):401–411. doi: 10.1007/s11914-017-0402-z. PubMed PMID: 28891009. Pubmed Central PMCID: Pmc5599455. Epub 2017/09/12. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cedarbaum J.M., Stambler N., Malta E., Fuller C., Hilt D., Thurmond B. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. J Neurol Sci. 1999;169(1–2):13–21. doi: 10.1016/s0022-510x(99)00210-5. [DOI] [PubMed] [Google Scholar]
- 22.López-González R., Kunckles P., Velasco I. Transient recovery in a rat model of familial amyotrophic lateral sclerosis after transplantation of motor neurons derived from mouse embryonic stem cells. Cell Transplant. 2009;18(10):1171–1181. doi: 10.3727/096368909X12483162197123. PubMed PMID: 19660174. Epub 2009/08/08. eng. [DOI] [PubMed] [Google Scholar]
- 23.Martin L.J., Liu Z., Chen K., Price A.C., Pan Y., Swaby J.A. Motor neuron degeneration in amyotrophic lateral sclerosis mutant superoxide dismutase-1 transgenic mice: mechanisms of mitochondriopathy and cell death. J Comp Neurol. 2007 Jan 1;500(1):20–46. doi: 10.1002/cne.21160. PubMed PMID: 17099894. Epub 2006/11/14. eng. [DOI] [PubMed] [Google Scholar]
- 24.Moviglia G.A., Moviglia-Brandolino M.T., Varela G.S., Albanese G., Piccone S., Echegaray G. Feasibility, safety, and preliminary proof of principles of autologous neural stem cell treatment combined with T-cell vaccination for ALS patients. Cell Transplant. 2012;21(Suppl 1):S57–S63. doi: 10.3727/096368912X633770. PubMed PMID: 22507681. Epub 2012/04/25. eng. [DOI] [PubMed] [Google Scholar]
- 25.Di Giorgio F.P., Boulting G.L., Bobrowicz S., Eggan K.C. Human embryonic stem cell-derived motor neurons are sensitive to the toxic effect of glial cells carrying an ALS-causing mutation. Cell Stem Cell. 2008 Dec 4;3(6):637–648. doi: 10.1016/j.stem.2008.09.017. PubMed PMID: 19041780. Epub 2008/12/02. eng. [DOI] [PubMed] [Google Scholar]
- 26.Oh K.W., Noh M.Y., Kwon M.S., Kim H.Y., Oh S.I., Park J. Repeated intrathecal mesenchymal stem cells for amyotrophic lateral sclerosis. Ann Neurol. 2018 Sep;84(3):361–373. doi: 10.1002/ana.25302. PubMed PMID: 30048006. Pubmed Central PMCID: Pmc6175096. Epub 2018/07/27. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nandoe Tewarie R.D., Hurtado A., Levi A.D., Grotenhuis J.A., Oudega M. Bone marrow stromal cells for repair of the spinal cord: towards clinical application. Cell Transplant. 2006;15(7):563–577. doi: 10.3727/000000006783981602. PubMed PMID: 17176609. Epub 2006/12/21. eng. [DOI] [PubMed] [Google Scholar]
- 28.Lech W., Figiel-Dabrowska A., Sarnowska A., Drela K., Obtulowicz P., Noszczyk B.H. Phenotypic, functional, and safety control at preimplantation phase of MSC-based therapy. Stem Cell Int. 2016;2016 doi: 10.1155/2016/2514917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marconi S., Bonaconsa M., Scambi I., Squintani G., Rui W., Turano E. Systemic treatment with adipose-derived mesenchymal stem cells ameliorates clinical and pathological features in the amyotrophic lateral sclerosis murine model. Neuroscience. 2013;248:333–343. doi: 10.1016/j.neuroscience.2013.05.034. [DOI] [PubMed] [Google Scholar]
- 30.Nabavi S.M., Arab L., Jarooghi N., Bolurieh T., Abbasi F., Mardpour S. Safety, feasibility of intravenous and intrathecal injection of autologous bone marrow derived mesenchymal stromal cells in patients with amyotrophic lateral sclerosis: an open label phase I clinical trial. Cell J. 2019 Jan;20(4):592–598. doi: 10.22074/cellj.2019.5370. PubMed PMID: 30124008. Pubmed Central PMCID: Pmc6099146. Epub 2018/08/21. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mazzini L., Mareschi K., Ferrero I., Vassallo E., Oliveri G., Nasuelli N. Stem cell treatment in amyotrophic lateral sclerosis. J Neurol Sci. 2008;265(1–2):78–83. doi: 10.1016/j.jns.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 32.Staff N.P., Madigan N.N., Morris J., Jentoft M., Sorenson E.J., Butler G. Safety of intrathecal autologous adipose-derived mesenchymal stromal cells in patients with ALS. Neurology. 2016;87(21):2230–2234. doi: 10.1212/WNL.0000000000003359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rando A., Pastor D. Intramuscular transplantation of bone marrow cells prolongs the lifespan of SOD1(G93A) mice and modulates expression of prognosis biomarkers of the disease. Stem Cell Res. Ther. 2018 Apr 6;9(1):90. doi: 10.1186/s13287-018-0843-z. PubMed PMID: 29625589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suzuki M., Svendsen C.N. Combining growth factor and stem cell therapy for amyotrophic lateral sclerosis. Trends Neurosci. 2008 Apr;31(4):192–198. doi: 10.1016/j.tins.2008.01.006. PubMed PMID: 18329734. Epub 2008/03/11. eng. [DOI] [PubMed] [Google Scholar]
- 35.Fagioli F., Mareschi K., Rustichelli D., Ferrero I., Mazzini L., Vercelli A., editors. 5th international satellite symposium AICC-GISM. 2014. Mesenchymal stem cells in amyotrophic lateral sclerosis. [Google Scholar]
- 36.Czaplinski A., Haverkamp L.J., Yen A.A., Simpson E.P., Lai E.C., Appel S.H. The value of database controls in pilot or futility studies in ALS. Neurology. 2006 Nov 28;67(10):1827–1832. doi: 10.1212/01.wnl.0000244415.48221.81. [DOI] [PubMed] [Google Scholar]
- 37.Frakes A.E., Ferraiuolo L., Haidet-Phillips A.M., Schmelzer L., Braun L., Miranda C.J. Microglia induce motor neuron death via the classical NF-κB pathway in amyotrophic lateral sclerosis. Neuron. 2014;81(5):1009–1023. doi: 10.1016/j.neuron.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Appel S.H., Armon C. AAN Enterprises; 2016. Stem cells in amyotrophic lateral sclerosis: ready for prime time? [DOI] [PubMed] [Google Scholar]
- 39.Mundra V., Gerling I.C., Mahato R.I. Mesenchymal stem cell-based therapy. Mol Pharm. 2013;10(1):77–89. doi: 10.1021/mp3005148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fischer U.M., Harting M.T., Jimenez F., Monzon-Posadas W.O., Xue H., Savitz S.I. Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect. Stem Cells Dev. 2009;18(5):683–692. doi: 10.1089/scd.2008.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saeed S., Soheila K., Sima K., Mandana M., Hosein P. A comprehensive review on the application of mesenchymal stem cell in the treatment of ALS patients. J Neurol Neurophysiol. 2018;9:471. [Google Scholar]
- 42.Forostyak S., Jendelova P., Kapcalova M., Arboleda D., Sykova E. Mesenchymal stromal cells prolong the lifespan in a rat model of amyotrophic lateral sclerosis. Cytotherapy. 2011;13(9):1036–1046. doi: 10.3109/14653249.2011.592521. [DOI] [PubMed] [Google Scholar]
- 43.Karussis D., Karageorgiou C., Vaknin-Dembinsky A., Gowda-Kurkalli B., Gomori J.M., Kassis I. Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch Neurol. 2010;67(10):1187–1194. doi: 10.1001/archneurol.2010.248. [DOI] [PMC free article] [PubMed] [Google Scholar]