Highlights
-
•
MEG connectivity to emotional faces in ASD and typical controls 6–39 years of age was investigated.
-
•
Distinct age-related changes in connectivity were observed in the groups to happy and angry faces.
-
•
Age-related between-group differences in functional connectivity were found in gamma band.
-
•
Emotion-specific age-related between-group differences were seen in beta.
-
•
Findings highlight specific neurodevelopmental trajectories to emotional faces in ASD vs. TD.
Keywords: ASD, Emotional face processing, Functional connectivity, Age-related changes, Development, Magnetoencephalography
Abstract
Impairments in social functioning are hallmarks of autism spectrum disorder (ASD) and atypical functional connectivity may underlie these difficulties. Emotion processing networks typically undergo protracted maturational changes, however, those with ASD show either hyper- or hypo-connectivity with little consensus on the functional connectivity underpinning emotion processing. Magnetoencephalography was used to investigate age-related changes in whole-brain functional connectivity of eight regions of interest during happy and angry face processing in 190 children, adolescents and adults (6–39 years) with and without ASD. Findings revealed age-related changes from child- through to mid-adulthood in functional connectivity in controls and in ASD in theta, as well as age-related between-group differences across emotions, with connectivity decreasing in ASD, but increasing for controls, in gamma. Greater connectivity to angry faces was observed across groups in gamma. Emotion-specific age-related between-group differences in beta were also found, that showed opposite trends with age for happy and angry in ASD. Our results establish altered, frequency-specific developmental trajectories of functional connectivity in ASD, across distributed networks and a broad age range, which may finally help explain the heterogeneity in the literature.
1. Introduction
A central feature of autism spectrum disorder (ASD) is social dysfunction, with a particular deficit in processing emotional faces (American Psychiatric Association, 2013). The ability to perceive and infer emotions from faces is critical for successful social interactions; emotional faces convey an abundance of social cues that help one understand the expresser’s feelings and intentions (Rump et al., 2009). Emotion recognition difficulties in young children through to adults are seen in those with ASD (Harms et al., 2010), as demonstrated in two meta-analyses showing less impairment for happy faces (Uljarevic and Hamilton, 2013), but continued deficits with age (Lozier et al., 2014). These difficulties are exacerbated when emotions are complex, subtle or implicitly presented (Frith, 2004).
Recent investigation of brain function in ASD has shifted in theoretical approach from the study of dysfunction of discrete ‘social brain’ areas to the interconnectedness of brain-wide networks, whereby spatially distinct brain regions interact to support socio-emotional function (Fries, 2005; Müller and Fishman, 2018). Evidence across modalities suggests that there are fundamental differences in functional connectivity in the ASD brain, which may underpin emotion processing deficits (Kana et al., 2016; Khan et al., 2013; Kleinhans et al., 2008; Leung et al., 2014; Mennella et al., 2017a; Safar et al., 2018, 2020; Sato et al., 2012). Findings from these studies have shown both increased and decreased patterns of connectivity among brain areas involved in emotion processing in children, adolescents and adults with ASD relative to typical controls (Kana et al., 2016; Khan et al., 2013; Kleinhans et al., 2008; Leung et al., 2014; Mennella et al., 2017a; Safar et al., 2018, 2020; Sato et al., 2012). Magnetoencephalography (MEG) studies using an implicit emotional faces task have shown reduced interregional functional connectivity in the beta and gamma frequency bands to angry faces in adults with ASD compared to typical controls (Mennella et al., 2017a; Safar et al., 2020). Similarly, using MEG and the same task, adolescents with ASD showed reduced connectivity in the beta range during implicit angry face processing (Leung et al., 2014). However, Safar et al. (2018) found that children with ASD demonstrated increased alpha connectivity during happy face perception. This pattern of results suggests an altered neurodevelopmental trajectory of emotional face processing in ASD.
In typical development, it is well established that face and emotional face processing networks undergo protracted development; studies report increases in functional connectivity with development among core and extended face processing areas, with continued specialization of these networks with age (Cohen Kadosh et al., 2011; He et al., 2015; Joseph et al., 2012; Song et al., 2015; Zhou et al., 2016). However, little work has considered age-related differences in task-based functional connectivity for emotional face processing in ASD (Lynn et al., 2018; Mamashli et al., 2018). In a study of 7–21year-olds (48 controls, 37 ASD), relations between age and functional connectivity during an emotional face paradigm were examined using MEG (Mamashli et al., 2018). They focused only on local alpha-gamma phase-amplitude coupling of the right fusiform gyrus, and alpha long-range connectivity of the right fusiform to the left precuneus, left anterior cingulate cortex and left inferior frontal gyrus. Their results showed a significant positive correlation with age in phase-amplitude coupling in typical controls, while a negative correlation was found in ASD. Alpha connectivity was also positively correlated in typical controls, yet negatively correlated in those with the disorder. Although interesting, this study was very limited in scope, analyzing long-range connectivity only of the right fusiform and in the alpha frequency band. Investigating maturational changes in extended functional networks underlying emotion processing in ASD and typical controls across a wide age range, and identifying differences between ASD and typical development, is necessary to better understand the neurodevelopmental trajectory of social dysfunction in ASD.
Using MEG, we investigated age-related changes in task-based whole-brain functional connectivity of eight regions of interest (ROIs) during the implicit perception of happy and angry faces in 190 children, adolescents and adults (6–39years) with and without ASD. The bilateral fusiform gyri, amygdalae, insulae and anterior cingulate cortices (ACC) were selected as ROIs as they are core and extended face processing regions (Haxby et al., 2002) and in previous research from our group were sensitive to differences in functional connectivity in ASD (Safar et al., 2018). MEG is well-suited to study neurophysiological functional connectivity, as it affords both high temporal and good spatial resolution (Hari and Salmelin, 2012). We hypothesized age-related changes in functional connectivity to emotional faces across childhood through to middle adulthood in ASD and in typical development. We also expected flattened developmental trajectories of functional connectivity to emotional faces in ASD participants to angry faces, based on research showing a lack of improvement in angry face recognition accuracy with age in ASD relative to their typically developing counterparts (Rump et al., 2009); thus, emotion-specific differences in functional connectivity with age between groups. Based on the MEG findings described above and the unique role of neural oscillations in distinct frequency bands for emotion processing, we expected that age-related group differences in functional connectivity would implicate different frequency ranges, offering insight into the diverse processes that differ in ASD.
2. Methods
2.1. Participants
Data from 190 children, adolescents and adults were included in the analysis: 83 with ASD (Mage = 19.5 years, SD = 9.17, Mdnage = 19.18 years, range = 7.02–39.52 years, 59 males) and 107 age- and sex-matched typically developing (TD) controls (Mage = 19.92 years, SD = 8.83, Mdnage = 20.98 years, range = 6.62–38.79 years, 74 males). Data from 20 additional participants were excluded from the analysis due to: inadequate number of clean MEG trials in each condition (n = 15), poor accuracy in task performance (n = 3), poor head localization (n = 1), and IQ > 3 SD from the mean (n = 1). All participants with ASD were high functioning, with Full Scale IQ scores within the normal range. Information regarding IQ and clinical ASD diagnoses can be found in the Supplemental Information. The Hospital for Sick Children Research Ethics Board approved the study protocol. All adult participants and parents provided informed written consent; all child participants gave informed verbal assent.
2.2. The emotional faces task
Participants completed an implicit emotional faces task during MEG data recording. Happy and angry faces (25 faces per condition, 12 males) were extracted from the NimStim Set of Facial Expressions (Tottenham et al., 2009). Each trial consisted of a happy or angry face paired with a scrambled version of that same face (the target stimulus) on either side of a central fixation cross for 80 ms followed by a 1300–1500 ms inter-stimulus-interval. Participants attended to the fixation cross and rapidly indicated the left or right position of the target stimulus relative to the fixation cross using a button-box, ignoring the emotional faces, probing implicit emotion processing. Implicit emotional face processing is suggested to be more challenging for those with ASD versus explicit judgment, due to its automatic and rapid processing requirements (Frith and Frith, 2008; Frith, 2004). Participants saw a total of 50 trials per emotion, presented twice in each hemifield, totalling 200 randomized trials. For further details on the task stimuli, see Safar et al. (2020).
2.3. MEG and MRI data acquisition
A 151-channel CTF system (CTF MEG International Services LP, Coquitlam, BC, Canada) was used to acquire MEG data. Data were continuously sampled at 600 Hz with an online 150 Hz antialiasing filter. A third-order spatial gradient was applied to attenuate external noise. To track continuous head movement, fiducial coils were placed at left and right pre-auricular and nasion locations. Following MEG data acquisition, fiducial coils were substituted for radio-opaque markers for MRI co-registration.
MRI data were acquired on either a Siemens 3.0 T MAGNETOM Trio (with a 12 channel head coil) or PrismaFIT (with a 20 channel head and neck coil) scanner. A T1-weighted structural image was acquired for every participant (Trio: TR/TE = 2300/2.96 ms, FA = 9 °, FOV = 240 × 256 mm, # slices = 192, resolution = 1.0 mm isotropic; PrismaFIT: TR/TE = 1870/3.14 ms, FA = 9 °, FOV = 240 × 256 mm, # slices = 192, resolution = 0.8 mm isotropic).
2.4. MEG preprocessing and source estimation
MEG preprocessing and source estimation were performed using the FieldTrip toolbox (Oostenveld et al., 2011) in MATLAB. Correct trials were epoched from -500–1500 ms relative to stimulus onset by emotional face, and independent component analysis was applied to suppress ocular and cardiac artefacts; components were rejected based on visual inspection. Additionally, trials were rejected if the MEG signal exceeded 2000 fT or if head movement exceeded 5 mm from the initial median head position. There was no difference in head movement between ASD (M=0.97, SD=0.62, Mdn = 0.77) and control (M=0.84, SD=0.63, Mdn=0.57) groups, (U = 3711, z=−1.94, p = 0.052; for further details see Supplemental Information). The number of happy and angry face trials included in the analyses following artefact rejection did not differ between groups (Happy: U = 3740 z=−1.864, p = 0.062; Angry: U = 3825.5, z=−1.638, p = 0.101).
The continuous MEG data were filtered using a 4th order Butterworth band-pass filter at 1–150 Hz, and a discrete Fourier transform notch filter at 60 and 120 Hz was used to suppress line noise. A single-shell head model for each participant was generated based on anatomical data from their structural MRI. The centroids of the 90 cortical and subcortical source locations of the Automated Anatomical Labelling (AAL) atlas (Tzourio-Mazoyer et al., 2002) were computed in standard template space (ICBM 152; Fonov et al., 2009) and nonlinearly warped to analogous head space locations for each individual. A linearly constrained minimum variance beamformer (Van Veen et al., 1997) with 5% regularization was used to estimate the broadband time series of source activity for each of the 90 AAL sources. The amplitudes of the reconstructed time series at each source were normalized by the estimated amplitude of projected noise to obtain the neural activity index (Van Veen et al., 1997).
2.5. Functional connectivity
Following source estimation, the time series data at each source location were filtered into theta (4–7 Hz), alpha (8–14 Hz), beta (15–29 Hz) and gamma (30–55 Hz) frequency bands. The Hilbert transform was computed to extract the time series of instantaneous phase values for each source location and frequency. The phase data were epoched into 1000 ms segments, -400–600 ms relative to stimulus onset. Functional connectivity was measured using the phase difference derivative (PDD; Tewarie et al., 2019) between each pair of regions. PDD measures the stability of phase relations by assuming that phase-locking occurs when the phase difference between the two timeseries remains approximately constant over time (Tewarie et al., 2019).
PDD values between the eight selected AAL ROIs (bilateral fusiform gyri, amygdalae, insulae and ACC) and each of the other 90 AAL brain regions were extracted yielding a 90-by-8 adjacency matrix for each sample in the timeseries, for each emotion and frequency band, for each participant. PDD values were normalized relative to the baseline period (-200–0 ms). The time window of interest chosen for statistical analysis was 200–400 ms following stimulus onset and the normalized PDD values were averaged across this time window. This time window was selected based on assessment of task-based changes in mean whole brain PDD strength across the time series, collapsed across participants, group and emotion, and is consistent with previous neurophysiological reports that have found between-group differences in interregional phase synchronization during this latency window to emotional faces (Mennella et al., 2017a; Wright et al., 2012).
2.6. Statistical analyses
To test for between-group differences in reaction time and accuracy, we conducted a 2-by-2 repeated measures ANCOVA (with emotion (happy, angry) as a within subjects factor, and group (control, ASD) as a between subjects factor) controlling for age. For functional connectivity, the network based statistics (NBS), a non-parametric approach that is optimal for the analysis of large networks while accounting for the family-wise error rate (FWER), was used (Zalesky et al., 2012, 2010). We tested the full ANCOVA model, presented term by term in the Results, to gain a comprehensive understanding of the networks underpinning group differences in emotion processing and interactions with age and emotion, for each of the frequency bands.
First, relations between age and functional connectivity, within control and ASD groups, for each emotion were tested. Second, we tested the main effect of group on functional connectivity, with age as a covariate, and the group-by-age interaction to emotional faces. Third, we tested the main effect of emotion, with age as a covariate, and the emotion-by-age interaction. Last, we tested the group-by-emotion interaction, and the group-by-age-by-emotion interaction. The primary component-forming thresholds were chosen based on the sparsity of the networks, such that the networks comprised 5% of total possible network connections. Post-hoc Pearson correlations were run between age and the mean network connectivity strength for significant group-by-age and group-by-age-by-emotion interactions, and relations between within-group functional connectivity and age to determine directionality of effects and to provide a measure of effect size (Pearson r); a post-hoc paired t-test was run on the mean network connectivity strength to determine the effect size (Cohen’s d) for the main effect of emotion.
3. Results
3.1. Behavioural
For reaction time, there were no main effects of emotion (F(1187) = 1.79, p = 0.182, ηp2 = 0.009) or group (F(1187) = 0.013, p = 0.911, ηp2 = 0.000067), and no emotion-by-group (F(1187) = 0.967, p = 0.327, ηp2 = 0.005) or emotion-by-age (F(1187) = 1.685, p = 0.196, ηp2 = 0.009) interactions. Age was significantly related to reaction time, (F(1187) = 103.883, p < 0.001, ηp2 = 0.357) with faster reaction times with increasing age.
Similarly for accuracy, there were no main effects of emotion (F(1187) = 1.975, p = 0.162, ηp2 = 0.01) or group (F(1187) = 0.058, p = 0.809, ηp2 = 0.0003), and no emotion-by-group (F(1187) = 0.151, p = 0.698, ηp2 = 0.001) or emotion-by-age (F(1187) = 2.101, p = 0.149, ηp2 = 0.011) interactions. The covariate age was significantly related to accuracy (F(1187) = 45.13, p < 0.001, ηp2 = 0.194) as accuracy increased with age.
3.2. Within-group functional connectivity changes with age
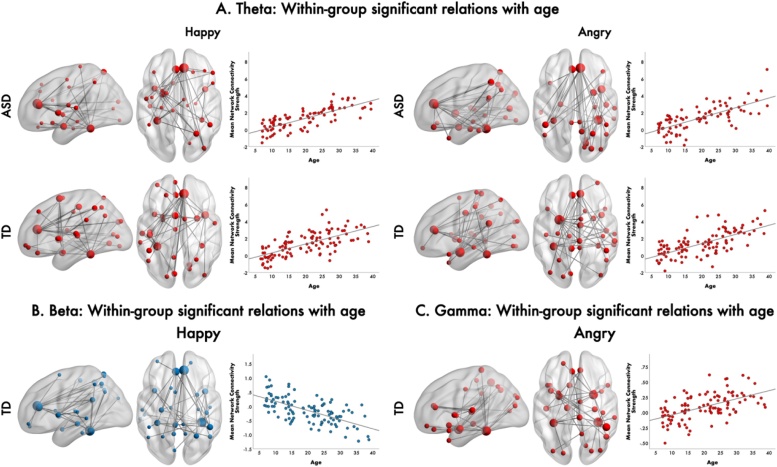
Functional connectivity in the ASD group was positively correlated with age in the theta frequency band to happy (F = 6.45, 35 edges, 30 nodes, pcorr = 0.004 (FWER-corrected and Bonferroni-corrected for multiple comparisons across emotion; r = 0.768, pcorr< 0.001), and angry (F = 7.85, 35 edges and 28 nodes, pcorr<0.002; r = 0.708, pcorr<0.001) faces (Fig. 1a). In the TD group, we also found a positive correlation between functional connectivity and age in the theta band to happy (F = 8.1, 35 edges, 32 nodes, pcorr<0.002); (r = 0.661, pcorr<0.001) and angry (F = 8.425, 35 edges, 33 nodes, pcorr<0.002; r = 0.658, pcorr<0.001) faces.
Fig. 1.
Functional connectivity correlations with age in ASD and TD, 200-400 ms following face onset. In the theta band, increased functional connectivity with age was found to happy and angry faces in ASD and TD (a). In the beta band, in controls only, we found decreased functional connectivity with age to happy faces (b), while in the gamma band, in TDs, increased functional connectivity with age to angry faces was found (c). All networks are represented in the glass brains, where node size is scaled by degree. The mean network connectivity strength across age for each of these networks is plotted in the scatter plots on the right.
Other within group effects were seen only in the TDs. In the beta band, decreased functional connectivity with age to happy faces (F = 7.05, 35 edges, 30 nodes, pcorr = 0.002; r= −0.636, pcorr<0.001) (Fig. 1b), with hubs in the right ACC and fusiform gyrus; in the gamma band, increased connectivity with age to angry faces (F = 5.30, 35 edges, 32 nodes, pcorr = 0.022; r = 0.553, pcorr<0.001) (Fig. 1c), with largely inferior connections, anchored in the right hemisphere. Further information can be found in Supplemental Information.
3.3. Emotion-specific functional connectivity
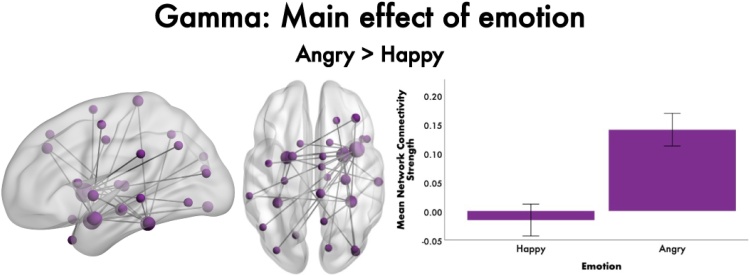
The only main effect of emotion, while controlling for age, was in the gamma frequency band (F = 5.25, 35 edges, 30 nodes, pcorr = 0.01; t(189)= −8.265,p<0.001, d = 1.14, Fig. 2), showing greater functional connectivity to angry than happy faces. The network encompassed five ROIs (bilateral fusiform gyri, amygdalae and right insula), with most connections anchored in the right hemisphere, particularly in the right insula and amygdala. The right insula was connected to occipital, parietal, temporal, limbic, and subcortical regions. The right amygdala connections were mainly between occipital, temporal and limbic brain areas. No significant emotion-by-age interactions were found (all pcorr>0.05, FWER-corrected).
Fig. 2.
Main effect of emotion on functional connectivity, 200-400 ms following face onset. In the gamma band, a significant main effect of emotion was found, indicating increased functional connectivity to angry compared to happy faces. The network is represented in the glass brain, where node size is scaled by degree. The mean network connectivity strength for happy and angry faces for the network is plotted in the bar graph on the right.
3.4. Between-group differences in functional connectivity
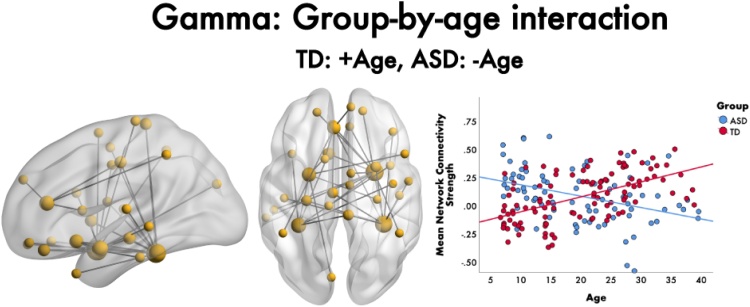
No main effects of group were found on functional connectivity across emotion while controlling for age, in any of the frequency bands, nor group-by-emotion interactions (all pcorr>0.05, FWER-corrected). However, a significant group-by-age interaction was found in the gamma frequency band across emotions (F = 5.1, 36 edges, 33 nodes, pcorr = 0.016; Fig. 3), indicating a different age-related trajectory of functional connectivity to emotional faces depending on group. In ASD, age was negatively correlated with mean network connectivity strength (r= −0.395, p = 0.0002), but positively correlated in TDs (r= 0.557, p < 0.0001). The network contained connections involving six ROIs (bilateral fusiform gyri, amygdalae, right insula and left ACC), with many inferior connections extending to orbital frontal brain areas. The majority of network connections were anchored in the right amygdala and fusiform gyrus. The right amygdala was connected to limbic, parietal and temporal brain areas, as well as the left superior frontal gyrus. The right fusiform connections were primarily to orbitofrontal and limbic regions.
Fig. 3.
Group-by-age interaction on functional connectivity to emotional faces, 200-400 ms following face onset. In the gamma band, a significant group-by-age interaction was found to emotional faces. The network showed increasing functional connectivity with age in the TDs, but decreasing with age in the ASD group. This network is represented in the glass brain, where node size is scaled by degree. The mean network connectivity strength across age for the network for each group is plotted in the scatter plot on the right.
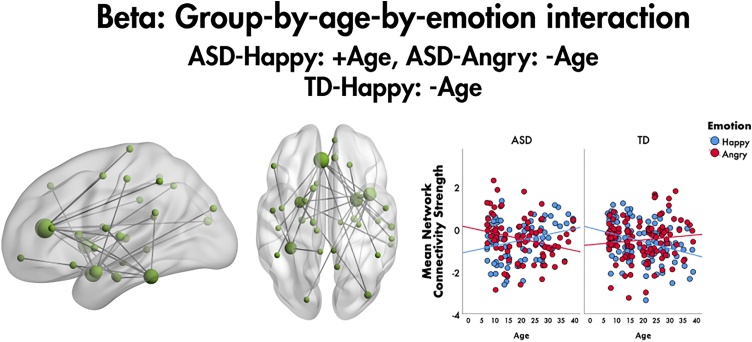
A significant group-by-emotion-by-age interaction was found in the beta frequency band (F = 4.9, 35 edges, 34 nodes, pcorr = 0.025). In ASD, age was positively correlated with mean network connectivity strength to happy faces, r = 0.276, p = 0.012, while to angry faces, age was negatively correlated with mean network connectivity strength, r= −0.24, p = 0.029. In the TD group, to happy faces, age was negatively correlated with mean network connectivity strength (r= −0.312, p = 0.001), while no significant correlation was found between age and connectivity strength to angry faces (r= 0.109, p = 0.265). The network included connections involving six ROIs (bilateral ACC, amygdalae, left fusiform gyrus and right insula), with the highest number of connections in the left ACC (Fig. 4). The left ACC was functionally connected to parietal brain areas, as well as the left thalamus, right middle occipital lobe and right superior temporal gyrus. The right insula and amygdala were also highly connected within the network. The right insula to primarily limbic brain areas; the right amygdala was connected to the right thalamus, occipital and superior temporal regions and right frontal areas.
Fig. 4.
Group-by-age-by-emotion interaction on functional connectivity to emotional faces, 200-400 ms following face onset. In the beta band, this significant group-by-age-by-emotion interaction was found to emotional faces, showing distinct age-related patterns of connectivity depending on emotion and group. This network is represented in the glass brain, where node size is scaled by degree. The mean network connectivity strength across age for the network is plotted for the two emotions by group in the scatter plots on the right.
4. Discussion
We leveraged MEG to investigate age-related changes in functional connectivity from early childhood to mid-adulthood during the implicit processing of happy and angry faces in ASD and typical development, in the largest study to date. We found age-specific changes in happy and angry face processing networks in ASD and TD, as well as age-related group differences in functional connectivity. We also observed enhanced functional connectivity to angry faces, irrespective of group, while emotion-specific age-related differences in connectivity were seen between groups. We did not see significant differences between groups in either theta or alpha bands. This suggests that the long-range connectivity patterns underlying face processing (in theta) are largely intact in ASD, and that some of the attentional and/or mnemonic processes associated with faces were also largely intact with this task. Between-group differences were found only in the higher frequency bands, particularly gamma, which plays an important role in excitatory inhibitory imbalance in the brain, which is thought to be an underlying deficit in ASD (Rojas and Wilson, 2014). We discuss these various findings in detail below.
4.1. Age-related differences in functional connectivity between ASD and TD groups
ASD and TD groups demonstrated differing age-related patterns of functional connectivity following the presentation of emotional faces in the gamma frequency range. We found that functional connectivity increased with age in TD but decreased in ASD. This pattern of results is consistent with a developmental model, which proposes that heterogeneity in the direction of functional connectivity reported in the literature is influenced by developmental factors, initiated by the onset of adolescence (Uddin et al., 2013). Adolescence is a stage of major structural and functional changes in neural development, along with increased social pressure and is a particularly vulnerable period for those with ASD (Picci et al., 2015; Uddin et al., 2013). By tracking changes in functional connectivity from early childhood to mid-adulthood, we are the first to determine that those with ASD show an altered maturational course of functional connectivity for emotional face processing compared to a TD population, demonstrating support for the disordinal developmental model (an option suggested by Uddin, 2015).
A similar pattern of age-related altered long-range connectivity in ASD has been reported using MEG to emotional faces in the alpha range (Mamashli et al., 2018). The authors reported a positive correlation between age and coherence among the four regions investigated in controls, while coherence was negatively correlated with age between the right fusiform and inferior frontal gyrus in ASD. However, this study was limited in sample size and age range, and coherence was only examined in alpha and among the four areas. Thus, in contrast, we demonstrate an atypical maturational trajectory of emotional neural circuitry, with whole-brain analyses, in an extended network in the gamma band and over a broader age range, which encompasses many brain areas known to be critically involved in diverse mechanisms for emotion processing (Adolphs, 2002) and undergoes very protracted developmental change.
Gamma band synchrony is fundamental to sensory and perceptual processing (Simon and Wallace, 2016) including implicit and explicit emotion perception (Luo et al., 2007; Safar et al., 2020; Uhlhaas et al., 2011), and is a key mechanism in the perceptual binding/integration of emotion details from faces (Liu et al., 2012; Sun et al., 2012). Alterations in gamma activity during the perception of faces and emotional faces have been reported in ASD (Sun et al., 2012; Wilson et al., 2007; Wright et al., 2012). For instance, Wright et al. (2012) found reduced early visual gamma responses in occipital brain areas to emotional faces in those with ASD, providing evidence for disrupted integration of facial features/holistic face processing, or a bias for distinct features – consistent with the central coherence theory in ASD (Happé, 2005; Jolliffe and Baron-Cohen, 1997). An imbalance in excitatory and inhibitory synaptic transmission mediating the generation of synchronous gamma oscillations is thought to contribute to disrupted local gamma function and long-range connectivity in autism (Canitano and Pallagrosi, 2017; Rojas and Wilson, 2014).
The gamma network (Fig. 3) included connections among core and extended face and emotional face processing regions, with the right fusiform and right amygdala being the most highly connected. We observed that the right fusiform was primarily connected to orbital frontal areas. Dynamic causal modelling studies also report task-modulated feedforward and feedback connectivity between right fusiform and the prefrontal cortex during the perception of emotional and socially rewarding information from faces and top-down enhancement of visual perception, respectively (Fairhall and Ishai, 2007; Ishai, 2008; Mechelli et al., 2004; Summerfield et al., 2006; Zhao et al., 2018). Furthermore, we found that the right amygdala was highly connected to widespread limbic, parietal, temporal and prefrontal brain areas within the network. The amygdalae play an integral role in evaluating and directing attention to salient and biologically relevant affective information from faces (Pessoa and Adolphs, 2010; Phelps and LeDoux, 2005). Several studies have suggested a role for increased gamma band activity in the amygdala in automatic processing and evaluation of emotional faces in early perception, as well as the binding of perceptual representations with emotional meaning (Liu et al., 2015; Oya et al., 2002; Sato et al., 2011). Buttressing our results of decreasing connectivity in this gamma-based network with age in autism, task-based studies of emotional face processing have shown altered functional connectivity of the amygdalae in autism (Monk et al., 2010; Sato et al., 2019; Swartz et al., 2013). Of particular relevance, the fusiform gyri and amygdalae were shown to be recruited when facial expressions are perceived in an implicit manner (Critchley et al., 2000), and the engagement and functional connectivity of these regions were found to be reduced in adults with ASD during the implicit processing of emotional information, yet preserved for explicit processing (Kana et al., 2016; Kovarski et al., 2019; Wong et al., 2008). In the present study, reduced connectivity of the right fusiform gyrus and amygdala with age in ASD may reflect the disrupted or delayed maturation of well-established emotional face processing areas and their circuitry, while age-related increased functional connectivity in controls demonstrates the protracted development of connectivity of these regions into adulthood. It is well established that phase synchrony mediated interregional functional connectivity enables dynamic communication among brain areas coordinating information transfer and supporting perceptual and cognitive processes across the brain (Fries, 2005, 2015). Therefore, our findings of decreasing gamma band connectivity with age suggest atypical recruitment and integration of the key brain regions mediating the processing of emotional faces in ASD, particularly in the evaluation and direction of attention to faces.
4.2. Increased functional connectivity to angry faces
A main effect of emotion, while controlling for age, revealed greater functional connectivity to angry than happy faces, also in the gamma range (Fig. 2). This is concordant with electroencephalography (EEG) studies that showed early enhanced gamma band activity and interregional phase synchrony to negatively valenced or aversive stimuli over positively valenced or neutral stimuli (Keil et al., 2001; Martini et al., 2012), and to auditory stimuli presented in a negative affective context (Garcia-Garcia et al., 2010). Increased gamma oscillations to emotional faces of high (i.e., anger and fear) and low arousal (i.e., happy and sad) have also been reported (Balconi and Lucchiari, 2008), as well as heightened high gamma activity to arousing emotional stimuli versus neutral stimuli (Keil et al., 2001).
The right insula and amygdala were the major hubs within the network. The right insula is critical for the interoceptive perception and experience of affective information, such as anger and disgust, related to bodily states (Craig, 2002; Menon and Uddin, 2010). In addition, the bilateral insulae are engaged in processing angry faces (Ziaei et al., 2017), and the right insula is recruited in scenarios of angry behaviour of others (Mazzola et al., 2016). The amygdalae are known to be critically involved in the processing of emotionally salient facial expressions, such as those expressing threat (Morris et al., 1998, 1996; Öhman, 2002; Suslow et al., 2006). Recent work in mice showed that the central amygdalae are a relay station, receiving top-down connections from the insular cortex, which directly excites amygdala output (Ponserre et al., 2020). Furthermore, the insulae and the amygdalae are two components of the salience network (Seeley et al., 2007), along with the ACC, which identifies, integrates and directs attention to salient biological/emotional internal states and external stimuli (Menon, 2011).
Interestingly, there were no group-by-emotion effects. Previous studies have shown that despite reported amygdala atypicalities (Monk et al., 2010; Sato et al., 2019; Swartz et al., 2013), those with ASD demonstrate, similar to controls, a threat-detection advantage (i.e., quicker and more accurate detection of angry vs. happy faces), however in ASD the effect is not as robust (Ashwin et al., 2006; Krysko and Rutherford, 2009). Therefore, we suggest that greater involvement of our gamma network to angry faces anchored in the right amygdala and insula supports enhanced angry detection across both ASD and controls, over a wide age range. Importantly, it is this same network that supports enhanced angry processing in both TD and ASD groups. This may be specific to gamma and this network, as a few studies do report atypical MEG broadband neural activity in ASD to angry vs. happy faces (Leung et al., 2018, 2019).
The greater involvement of these core regions in our network, across age, to angry more than happy faces suggests enhanced recruitment and communication among these key regions and further underscores the critical role they have for angry face processing and the greater salience of angry faces. Our results also highlight the important role of gamma in processing negative affect. It is worth noting that the group-by-age interaction to emotional faces in the gamma band was found in a different network than the emotion main effect, anchored in the right amygdala and fusiform, with the right fusiform highly connected to orbitofrontal areas. Thus, when examining the effect of age on emotional face processing in ASD and controls, we observed this distinct network of reduced connectivity underlying emotion processes (i.e., evaluation and direction of attention to faces) in ASD compared to controls.
4.3. Emotion-specific age-related group differences in functional connectivity
We observed emotion-specific differences in functional connectivity with age in ASD compared to TD in the beta frequency band (Fig. 4). Beta oscillations have been associated with the processing of visual and emotional stimuli (Güntekin and Başar, 2014, 2010; Luckhardt et al., 2017), while in autism, reduced beta connectivity in emotional face processing tasks was reported in adolescents and young adults (Leung et al., 2014; Mennella et al., 2017a). Across a wide age range, we demonstrated that functional connectivity to happy faces in beta increased with age in ASD, while connectivity to angry faces decreased with age. In contrast, in controls, functional connectivity decreased with age to happy expressions, while no age-related association was seen for angry expressions. In typical development, the earliest expression to be discriminated and recognized is happy (Leppänen and Nelson, 2006), while the recognition of negatively valenced expressions, such as angry, undergoes prolonged development into adolescence (Thomas et al., 2007). Several studies have reported impaired recognition of negatively valenced faces in ASD compared to typical controls; but difficulties recognizing happy faces are less established (Greimel et al., 2014; Harms et al., 2010; Humphreys et al., 2007). Unlike typical development, proficiency in recognizing negative expressions in those with ASD does not show the same advances with age (Rump et al., 2009), suggesting that adults with ASD do not reach comparable performance to typical adults.
Functional connectivity studies report disrupted angry face connectivity in adults with ASD, while the neural circuitry underpinning happy face processing is largely preserved (Leung et al., 2014; Mennella et al., 2017a; Safar et al., 2020). These findings are consistent with our results of angry-specific connectivity decreasing with age in ASD. In addition, it is possible that atypically heightened engagement of neural circuitry underpinning happy faces from child to adulthood in ASD suggests increased compensatory recruitment with age underlies the relatively intact happy face processing in ASD (see Uljarevic and Hamilton, 2013), while the TDs require less engagement with age to process happy faces, the easiest emotion to recognize. Beta oscillations play a central role in the top-down attentional control of affective information (Miskovic and Schmidt, 2010; Schnitzler and Gross, 2005; Kajal et al., 2020), and beta phase synchronization has been shown to be modulated by task difficulty, such that increased beta phase synchrony is associated with greater task difficulty and performance errors (Rueda-Delgado et al., 2017). Furthermore, the most highly connected region in this network was the left ACC. Similar to our beta network, previous research has shown that the left anterior cingulate is engaged during top-down face processing as part of a network, involving the right fusiform, bilateral amygdalae, right hippocampus, left inferior parietal lobule, occipito-temporal areas and left STS (Li et al., 2009). In addition, in TD children compared to adolescents and adults, increased functional connectivity of the anterior cingulate during enhanced top-down attention was thought to reflect a compensatory response due to reduced functional integrity of this brain area (Hwang et al., 2014). Thus, although those with ASD can process happy faces, our findings of age-related atypically increased beta phase synchronization, primarily involving the left ACC, is consistent with the notion that happy face processing does not become easier with age in those with ASD, requiring increased engagement of the beta band network to maintain good performance. However, because we did not collect behavioural emotion recognition data in the current study, specific relations between functional connectivity and emotion recognition ability cannot be assessed, limiting our conclusions.
A limitation of the present study is that it is unclear whether our results will hold when facial expressions are presented explicitly. The implicit task in this study entails automatic and rapid processing of facial expressions, where deficits in perception cannot be compensated by learned strategies and increased experience with age. This can be achieved for the explicit processing of emotions in ASD (Frith and Frith, 2008; Frith, 2004) and thus explicit emotion recognition can be relatively preserved (Kana et al., 2016; Kovarski et al., 2019). It will be important for future work to investigate the neurodevelopmental trajectory of explicit emotional face processing in those with the disorder. Another area of future work would benefit from the inclusion of a number of different time windows, to assess either early perceptual processes (50−150 ms) or later consolidation or memory effects (300−600 ms) with emotional faces, or frequency-specific time windows of maximal connectivity. A final limitation is our inclusion of only high-functioning participants, due to the difficulty in obtaining reliable neuroimaging measures during task performance in lower functioning individuals. Thus, our results are not generalizable to either those with or without autism who are lower functioning.
In conclusion, we observed age-related changes in MEG connectivity underpinning implicit emotion processing in ASD and in typical controls, as well as age-related between-group differences across emotions. These findings demonstrate that emotional neural networks undergo protracted maturational change in those with and without ASD, and highlight an altered developmental trajectory of emotional processing networks in those with ASD in the higher frequency bands. We also found emotion-specific differences in functional connectivity with age between groups, suggesting that even happy faces remain difficult to process in those with ASD. These distinct patterns of development support the model that discrepancies in the reported functional connectivity in the literature can be resolved by accounting for age, a critical contribution to a greater understanding of the neurodevelopmental trajectory of social dysfunction in this population. The results are also important in demonstrating frequency specific changes, further helping us understand the neural underpinning of emotional face processing in both typical and atypical development.
Data availability
Data are available from the corresponding author upon reasonable request.
Declaration of Competing Interest
The authors have no conflicts of interest to declare.
Acknowledgments
This research was supported by Brain Canada-Kids Brain Health Network, Health Canada and the Canadian Institutes of Health Research (CIHR) [MOP-119541; MOP-142379].
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.dcn.2021.101003.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Hari R., Salmelin R. Magnetoencephalography: From SQUIDs to neuroscience.n Neuroimage 20th Anniversary Special Edition. Neuroimage. 2012;61(2):386–396. doi: 10.1016/j.neuroimage.2011.11.074. [DOI] [PubMed] [Google Scholar]
- Adolphs R. Neural systems for recognizing emotion. Current Opinion in. Neurobiology. 2002;12(2):169–177. doi: 10.1016/S0959-4388(02)00301-X. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association Diagnostic and statistical manual of mental disorders. Arlington. 2013 doi: 10.1176/appi.books.9780890425596.744053. [DOI] [Google Scholar]
- Ashwin C., Wheelwright S., Baron-Cohen S. Finding a face in the crowd: testing the anger superiority effect in Asperger Syndrome. Brain Cogn. 2006;61(1):78–95. doi: 10.1016/j.bandc.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Balconi M., Lucchiari C. Consciousness and arousal effects on emotional face processing as revealed by brain oscillations. A gamma band analysis. Int. J. Psychophysiol. 2008;67(1):41–46. doi: 10.1016/j.ijpsycho.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Canitano R., Pallagrosi M. Autism spectrum disorders and schizophrenia spectrum disorders: Excitation/inhibition imbalance and developmental trajectories. Front. Psychiatry. 2017;8:1–7. doi: 10.3389/fpsyt.2017.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen Kadosh K., Cohen Kadosh R., Dick F., Johnson M.H. Developmental changes in effective connectivity in the emerging core face network. Cereb. Cortex. 2011;21(6):1389–1394. doi: 10.1093/cercor/bhq215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig A.D. How do you feel? Interoception: The sense of the physiological condition of the body. Nat. Rev. Neurosci. 2002;3(8):655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Critchley H.D., Daly E.M., Bullmore E.T., Williams S.C., Van Amelsvoort T., Robertson D.M., Rowe a, Phillips M., McAlonan G., Howlin P., Murphy D.G. The functional neuroanatomy of social behaviour: changes in cerebral blood flow when people with autistic disorder process facial expressions. Brain. 2000;123(11):2203–2212. doi: 10.1093/brain/123.11.2203. [DOI] [PubMed] [Google Scholar]
- Fairhall S.L., Ishai A. Effective connectivity within the distributed cortical network for face perception. Cereb. Cortex. 2007;17(10):2400–2406. doi: 10.1093/cercor/bhl148. [DOI] [PubMed] [Google Scholar]
- Fonov V.S., Evans A.C., McKinstry R.C., Almli C.R., Collins D.L. Unbiased nonlinear average age-appropriate brain templates from birth to adulthood. Neuroimage. 2009;47:S102. doi: 10.1016/s1053-8119(09)70884-5. [DOI] [Google Scholar]
- Fries P. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn. Sci. (Regul. Ed.) 2005;9:474–480. doi: 10.1016/j.tics.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Fries P. Rhythms for cognition: communication through coherence. Neuron. 2015;88:220–225. doi: 10.1016/j.neuron.2015.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith U. Emanuel Miller lecture: confusions and controversies about Asperger syndrome. J. Child Psychol. Psychiatry Allied Discip. 2004;45(4):672–686. doi: 10.1111/j.1469-7610.2004.00262.x. [DOI] [PubMed] [Google Scholar]
- Frith C.D., Frith U. Implicit and explicit processes in social cognition. Neuron. 2008;60(3):503–510. doi: 10.1016/j.neuron.2008.10.032. [DOI] [PubMed] [Google Scholar]
- Garcia-Garcia M., Yordanova J., Kolev V., Domínguez-Borràs J., Escera C. Tuning the brain for novelty detection under emotional threat: the role of increasing gamma phase-synchronization. Neuroimage. 2010;49(1):1038–1044. doi: 10.1016/j.neuroimage.2009.07.059. [DOI] [PubMed] [Google Scholar]
- Greimel E., Schulte-Rüther M., Kamp-Becker I., Remschmidt H., Herpertz-Dahlmann B., Konrad K. Impairment in face processing in autism spectrum disorder: a developmental perspective. J. Neural Transm. 2014;121(9):1171–1181. doi: 10.1007/s00702-014-1206-2. [DOI] [PubMed] [Google Scholar]
- Güntekin B., Başar E. Event-related beta oscillations are affected by emotional eliciting stimuli. Neurosci. Lett. 2010;483(3):173–178. doi: 10.1016/j.neulet.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Güntekin B., Başar E. A review of brain oscillations in perception of faces and emotional pictures. Neuropsychologia. 2014;58:33–51. doi: 10.1016/j.neuropsychologia.2014.03.014. [DOI] [PubMed] [Google Scholar]
- Happé F. Handbook of Autism and Pervasive Developmental Disorders. 2005. The weak Central coherence account of autism; pp. 640–649. ch24. [DOI] [Google Scholar]
- Harms M.B., Martin A., Wallace G.L. Facial emotion recognition in autism spectrum disorders: a review of behavioral and neuroimaging studies. Neuropsychol. Rev. 2010;20(3):290–322. doi: 10.1007/s11065-010-9138-6. [DOI] [PubMed] [Google Scholar]
- Haxby J.V., Hoffman E.A., Gobbini M.I. Human neural systems for face recognition and social communication. Biol. Psychiatry. 2002;51(1):59–67. doi: 10.1016/s0006-3223(01)01330-0. 51, 59–67. [DOI] [PubMed] [Google Scholar]
- He W., Garrido M.I., Sowman P.F., Brock J., Johnson B.W. Development of effective connectivity in the core network for face perception. Hum. Brain Mapp. 2015;36(6):2161–2173. doi: 10.1002/hbm.22762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys K., Minshew N., Leonard G.L., Behrmann M. A fine-grained analysis of facial expression processing in high-functioning adults with autism. Neuropsychologia. 2007;45(4):685–695. doi: 10.1016/j.neuropsychologia.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Hwang S., White S.F., Nolan Z.T., Sinclair S., Blair R.J.R. Neurodevelopmental changes in the responsiveness of systems involved in top down attention and emotional responding. Neuropsychologia. 2014;62:277–285. doi: 10.1016/j.neuropsychologia.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishai A. Let’s face it: it’s a cortical network. Neuroimage. 2008;40(2):415–419. doi: 10.1016/j.neuroimage.2007.10.040. [DOI] [PubMed] [Google Scholar]
- Jolliffe T., Baron-Cohen S. Are people with autism and Asperger syndrome faster than normal on the embedded Figures test? J. Child Psychol. Psychiatry Allied Discip. 1997;38(5):527–534. doi: 10.1111/j.1469-7610.1997.tb01539.x. [DOI] [PubMed] [Google Scholar]
- Joseph J.E., Swearingen J.E., Clark J.D., Benca C.E., Collins H.R., Corbly C.R., Gathers A.D., Bhatt R.S. The changing landscape of functional brain networks for face processing in typical development. Neuroimage. 2012;63(3):1223–1236. doi: 10.1016/j.neuroimage.2012.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajal D.S., Fioravanti C., Elshahabi A., Ruiz S., Sitaram R., Braun C. Involvement of top-down networks in the perception of facial emotions: a magnetoencephalographic investigation. Neuroimage. 2020;222 doi: 10.1016/j.neuroimage.2020.117075. [DOI] [PubMed] [Google Scholar]
- Kana R.K., Patriquin M.A., Black B.S., Channell M.M., Wicker B. Altered medial frontal and superior temporal response to implicit processing of emotions in autism. Autism Res. 2016;9:55–66. doi: 10.1002/aur.1496. [DOI] [PubMed] [Google Scholar]
- Keil A., Müller M.M., Gruber T., Wienbruch C., Stolarova M., Elbert T. Effects of emotional arousal in the cerebral hemispheres: a study of oscillatory brain activity and event-related potentials. Clin. Neurophysiol. 2001;112(11):2067–2068. doi: 10.1016/S1388-2457(01)00654-X. [DOI] [PubMed] [Google Scholar]
- Khan S., Gramfort A., Shetty N.R., Kitzbichler M.G., Ganesan S., Moran J.M., Lee S.M., Gabrieli J.D.E., Tager-Flusberg H.B., Joseph R.M., Herbert M.R., Hamalainen M.S., Kenet T. Local and long-range functional connectivity is reduced in concert in autism spectrum disorders. Proc. Natl. Acad. Sci. 2013;110:3107–3112. doi: 10.1073/pnas.1214533110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinhans N.M., Richards T., Sterling L., Stegbauer K.C., Mahurin R., Johnson L.C., Greenson J., Dawson G., Aylward E. Abnormal functional connectivity in autism spectrum disorders during face processing. Brain. 2008;131:1000–1012. doi: 10.1093/brain/awm334. [DOI] [PubMed] [Google Scholar]
- Kovarski K., Mennella R., Wong S.M., Dunkley B.T., Taylor M.J., Batty M. Enhanced early visual responses during implicit emotional faces processing in autism Spectrum disorder. J. Autism Dev. Disord. 2019;49(3):871–886. doi: 10.1007/s10803-018-3787-3. [DOI] [PubMed] [Google Scholar]
- Krysko K.M., Rutherford M.D. A threat-detection advantage in those with autism spectrum disorders. Brain Cogn. 2009;69(3):472–480. doi: 10.1016/j.bandc.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Leppänen J.M., Nelson C.A. The development and neural bases of facial emotion recognition. Adv. Child Dev. Behav. 2006;34:207–246. doi: 10.1016/S0065-2407(06)80008-X. [DOI] [PubMed] [Google Scholar]
- Leung R.C., Annette X.Y., Wong S.M., Taylor M.J., Doesburg S.M. Reduced beta connectivity during emotional face processing in adolescents with autism. Mol. Autism. 2014;5:51. doi: 10.1186/2040-2392-5-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung R.C., Pang E.W., Anagnostou E., Taylor M.J. Young adults with autism spectrum disorder show early atypical neural activity during emotional face processing. Front. Hum. Neurosci. 2018;12:57. doi: 10.3389/fnhum.2018.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung R.C., Pang E.W., Brian J.A., Taylor M.J. Happy and angry faces elicit atypical neural activation in children with autism spectrum disorder. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 2019;4(12):1021–1030. doi: 10.1016/j.bpsc.2019.03.013. [DOI] [PubMed] [Google Scholar]
- Li J., Liu J., Liang J., Zhang H., Zhao J., Huber D.E. A distributed neural system for top-down face processing. Neurosci. Lett. 2009;451(1):6–10. doi: 10.1016/j.neulet.2008.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T.Y., Hsieh J.C., Chen Y.S., Tu P.C., Su T.P., Chen L.F. Different patterns of abnormal gamma oscillatory activity in unipolar and bipolar disorder patients during an implicit emotion task. Neuropsychologia. 2012;50(7):1514–1520. doi: 10.1016/j.neuropsychologia.2012.03.004. [DOI] [PubMed] [Google Scholar]
- Liu T.Y., Chen Y.S., Hsieh J.C., Chen L.F. Asymmetric engagement of amygdala and its gamma connectivity in early emotional face processing. PLoS One. 2015;10(1) doi: 10.1371/journal.pone.0115677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozier L.M., Vanmeter J.W., Marsh A.A. Impairments in facial affect recognition associated with autism spectrum disorders: a meta-analysis. Dev. Psychopathol. 2014;26(4pt1):933–945. doi: 10.1017/S0954579414000479. [DOI] [PubMed] [Google Scholar]
- Luckhardt C., Kröger A., Cholemkery H., Bender S., Freitag C.M. Neural correlates of explicit versus implicit facial emotion processing in ASD. J. Autism Dev. Disord. 2017;47(7):1944–1955. doi: 10.1007/s10803-017-3141-1. [DOI] [PubMed] [Google Scholar]
- Luo Q., Holroyd T., Jones M., Hendler T., Blair J. Neural dynamics for facial threat processing as revealed by gamma band synchronization using MEG. Neuroimage. 2007;34(2):839–847. doi: 10.1016/j.neuroimage.2006.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn A.C., Padmanabhan A., Simmonds D., Foran W., Hallquist M.N., Luna B., O’Hearn K. Functional connectivity differences in autism during face and car recognition: underconnectivity and atypical age-related changes. Dev. Sci. 2018;21(1) doi: 10.1111/desc.12508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamashli F., Khan S., Bharadwaj H., Losh A., Pawlyszyn S.M., Hämäläinen M.S., Kenet T. Maturational trajectories of local and long-range functional connectivity in autism during face processing. Hum. Brain Mapp. 2018;39:4094–4104. doi: 10.1002/hbm.24234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini N., Menicucci D., Sebastiani L., Bedini R., Pingitore A., Vanello N., Milanesi M., Landini L., Gemignani A. The dynamics of EEG gamma responses to unpleasant visual stimuli: from local activity to functional connectivity. Neuroimage. 2012;60(2):922–932. doi: 10.1016/j.neuroimage.2012.01.060. [DOI] [PubMed] [Google Scholar]
- Mazzola V., Arciero G., Fazio L., Lanciano T., Gelao B., Popolizio T., Vuilleumier P., Bondolfi G., Bertolino A. What impact does an angry context have upon us? The effect of anger on functional connectivity of the right insula and superior temporal gyri. Front. Behav. Neurosci. 2016;10:109. doi: 10.3389/fnbeh.2016.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechelli A., Price C.J., Friston K.J., Ishai A. Where bottom-up meets top-down: neuronal interactions during perception and imagery. Cereb. Cortex. 2004;14:1256–1265. doi: 10.1093/cercor/bhh087. [DOI] [PubMed] [Google Scholar]
- Mennella R., Leung R.C., Taylor M.J., Dunkley B.T. Disconnection from others in autism is more than just a feeling: whole-brain neural synchrony in adults during implicit processing of emotional faces. Mol. Autism. 2017;8(1):1–12. doi: 10.1186/s13229-017-0123-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn. Sci. (Regul. Ed.) 2011;15(10):483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Menon V., Uddin L.Q. Saliency, switching, attention and control: a network model of insula function. Brain Struct. Funct. 2010;214(5–6):655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miskovic V., Schmidt L.A. Cross-regional cortical synchronization during affective image viewing. Brain Res. 2010;1362:102–111. doi: 10.1016/j.brainres.2010.09.102. [DOI] [PubMed] [Google Scholar]
- Monk C.S., Weng S.J., Wiggins J.L., Kurapati N., Louro H.M.C., Carrasco M., Maslowsky J., Risi S., Lord C. Neural circuitry of emotional face processing in autism spectrum disorders. J. Psychiatry Neurosci. 2010;35(2):105. doi: 10.1503/jpn.090085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J.S., Frith C.D., Perrett D.I., Rowland D., Young A.W., Calder A.J., Dolan R.J. A differential neural response in the human amygdala to fearful and happy facial expressions. Nature. 1996;383(6603):812–815. doi: 10.1038/383812a0. [DOI] [PubMed] [Google Scholar]
- Morris J.S., Ohrnan A., Dolan R.J. Conscious and unconscious emotional learning in the human amygdala. Nature. 1998;393(6684):467–470. doi: 10.1038/30976. [DOI] [PubMed] [Google Scholar]
- Müller R.A., Fishman I. Brain connectivity and neuroimaging of social networks in autism. Trends Cogn. Sci. (Regul. Ed.) 2018;22(12):1103–1116. doi: 10.1016/j.tics.2018.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öhman A. Automaticity and the amygdala: nonconscious responses to emotional faces. Curr. Dir. Psychol. Sci. 2002;11(2):62–66. doi: 10.1111/1467-8721.00169. [DOI] [Google Scholar]
- Oostenveld R., Fries P., Maris E., Schoffelen J.M. FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput. Intell. Neurosci. 2011;2011:1. doi: 10.1155/2011/156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oya H., Kawasaki H., Howard M.A., Adolphs R. Electrophysiological responses in the human amygdala discriminate emotion categories of complex visual stimuli. J. Neurosci. 2002;22(21):9502–9512. doi: 10.1523/jneurosci.22-21-09502.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L., Adolphs R. Emotion processing and the amygdala: from a “low road” to “many roads” of evaluating biological significance. Nat. Rev. Neurosci. 2010;12(7) doi: 10.1038/nrn2920. 425–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps E.A., LeDoux J.E. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48(2):175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Picci Giorgia, Gotts Stephen J., Scherf Suzanne K. A two-hit model of autism: Adolescence as the second hit. Clin. Psychol. Sci. 2015;3(3):349–371. doi: 10.1177/2167702614540646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponserre M., Peters C., Fermani F., Conzelmann K.K., Klein R. The insula cortex contacts distinct output streams of the central Amygdala. J. Neurosci. 2020;40(46):8870–8882. doi: 10.1523/JNEUROSCI.0567-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas D.C., Wilson L.B. γ-band abnormalities as markers of autism spectrum disorders. Biomark. Med. 2014;8:353–368. doi: 10.2217/bmm.14.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueda-Delgado L.M., Solesio-Jofre E., Mantini D., Dupont P., Daffertshofer A., Swinnen S.P. Coordinative task difficulty and behavioural errors are associated with increased long-range beta band synchronization. Neuroimage. 2017;146:883–893. doi: 10.1016/j.neuroimage.2016.10.030. [DOI] [PubMed] [Google Scholar]
- Rump K.M., Giovannelli J.L., Minshew N.J., Strauss M.S. The development of emotion recognition in individuals with autism. Child Dev. 2009;80(5):1434–1447. doi: 10.1111/j.1467-8624.2009.01343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safar K., Wong S.M., Leung R.C., Dunkley B.T., Taylor M.J. Increased functional connectivity during emotional face processing in children with autism Spectrum disorder. Front. Hum. Neurosci. 2018;12 doi: 10.3389/fnhum.2018.00408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safar K., Yuk V., Wong S.M., Leung R.C., Anagnostou E., Taylor M.J. Emotional face processing in autism spectrum disorder: effects in gamma connectivity. Biol. Psychol. 2020;149 doi: 10.1016/j.biopsycho.2019.107774. [DOI] [PubMed] [Google Scholar]
- Sato W., Kochiyama T., Uono S., Matsuda K., Usui K., Inoue Y., Toichi M. Rapid amygdala gamma oscillations in response to fearful facial expressions. Neuropsychologia. 2011;49$:612–617. doi: 10.1016/j.neuropsychologia.2010.12.025. [DOI] [PubMed] [Google Scholar]
- Sato W., Toichi M., Uono S., Kochiyama T. Impaired social brain network for processing dynamic facial expressions in autism spectrum disorders. BMC Neurosci. 2012;13(1):99. doi: 10.1186/1471-2202-13-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato W., Kochiyama T., Uono S., Yoshimura S., Kubota Y., Sawada R., Sakihama M., Toichi M. Atypical Amygdala–Neocortex Interaction During Dynamic Facial Expression Processing in Autism Spectrum Disorder. Front. Hum. Neurosci. 2019;13:351. doi: 10.3389/fnhum.2019.00351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzler A., Gross J. Normal and pathological oscillatory communication in the brain. Nat. Rev. Neurosci. 2005;6(4):285–296. doi: 10.1038/nrn1650. [DOI] [PubMed] [Google Scholar]
- Seeley W.W., Menon V., Schatzberg A.F., Keller J., Glover G.H., Kenna H., Reiss A.L., Greicius M.D. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon D.M., Wallace M.T. Dysfunction of sensory oscillations in autism Spectrum disorder. Neurosci. Biobehav. Rev. 2016;68:848–861. doi: 10.1016/j.neubiorev.2016.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y., Zhu Q., Li J., Wang X., Liu J. Typical and atypical development of functional connectivity in the face network. J. Neurosci. 2015;35(43):14624–14635. doi: 10.1523/JNEUROSCI.0969-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerfield C., Egner T., Greene M., Koechlin E., Mangels J., Hirsch J. Predictive codes for forthcoming perception in the frontal cortex. Science. 2006;314(5803):1311–1314. doi: 10.1126/science.1132028. [DOI] [PubMed] [Google Scholar]
- Sun L., Grützner C., Bölte S., Wibral M., Tozman T., Schlitt S., Poustka F., Singer W., Freitag C.M., Uhlhaas P.J. Impaired gamma-band activity during perceptual organization in adults with autism spectrum disorders: evidence for dysfunctional network activity in frontal-posterior cortices. J. Neurosci. 2012;32(28):9563–9573. doi: 10.1523/JNEUROSCI.1073-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suslow T., Ohrmann P., Bauer J., Rauch A.V., Schwindt W., Arolt V., Heindel W., Kugel H. Amygdala activation during masked presentation of emotional faces predicts conscious detection of threat-related faces. Brain Cogn. 2006;61(3):243–248. doi: 10.1016/j.bandc.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Swartz J.R., Wiggins J.L., Carrasco M., Lord C., Monk C.S. Amygdala habituation and prefrontal functional connectivity in youth with autism spectrum disorders. J. Am. Acad. Child Adolesc. Psychiatry. 2013;52(1):84–93. doi: 10.1016/j.jaac.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewarie P., Hunt B.A.E., O’Neill G.C., Byrne A., Aquino K., Bauer M., Mullinger K.J., Coombes S., Brookes M.J. Relationships between neuronal oscillatory amplitude and dynamic functional connectivity. Cereb. Cortex. 2019;29(6):2668–2681. doi: 10.1093/cercor/bhy136. [DOI] [PubMed] [Google Scholar]
- Thomas L.A., De Bellis M.D., Graham R., LaBar K.S. Development of emotional facial recognition in late childhood and adolescence: REPORT. Dev. Sci. 2007;10(5):547–558. doi: 10.1111/j.1467-7687.2007.00614.x. [DOI] [PubMed] [Google Scholar]
- Tottenham N., Tanaka J.W., Leon A.C., McCarry T., Nurse M., Hare T.A., Marcus D.J., Westerlund A., Casey B.J., Nelson C. The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Res. 2009;168:242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N., Mazoyer B., Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Uddin L.Q. Idiosyncratic connectivity in autism: developmental and anatomical considerations. Trends Neurosci. 2015;38:261–263. doi: 10.1016/j.tins.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin L.Q., Supekar K., Menon V. Reconceptualizing functional brain connectivity in autism from a developmental perspective. Front. Hum. Neurosci. 2013;7:458. doi: 10.3389/fnhum.2013.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas P.J., Pipa G., Neuenschwander S., Wibral M., Singer W. A new look at gamma? High- (&60 Hz) γ-band activity in cortical networks: function, mechanisms and impairment. Prog. Biophys. Mol. Biol. 2011;105(1–2):14–28. doi: 10.1016/j.pbiomolbio.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Uljarevic M., Hamilton A. Recognition of emotions in autism: a formal meta-analysis. J. Autism Dev. Disord. 2013;43(7):1517–1526. doi: 10.1007/s10803-012-1695-5. [DOI] [PubMed] [Google Scholar]
- Van Veen B., van Drongelen W., Yuchtman M., Suzuki A. Localization of brain electrical activity via linearly constrained minimum variance spatial filtering. IEEE Trans. Biomed. Eng. 1997;44:867–880. doi: 10.1109/10.623056. [DOI] [PubMed] [Google Scholar]
- Wilson T.W., Rojas D.C., Reite M.L., Teale P.D., Rogers S.J. Children and adolescents with autism exhibit reduced MEG steady-state gamma responses. Biol. Psychiatry. 2007;62(3):192–197. doi: 10.1016/j.biopsych.2006.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong T.K.W., Fung P.C.W., Chua S.E., McAlonan G.M. Abnormal spatiotemporal processing of emotional facial expressions in childhood autism: dipole source analysis of event-related potentials. Eur. J. Neurosci. 2008;28(2):407–416. doi: 10.1111/j.1460-9568.2008.06328.x. [DOI] [PubMed] [Google Scholar]
- Wright B., Alderson-Day B., Prendergast G., Bennett S., Jordan J., Whitton C., Gouws A., Jones N., Attur R., Tomlinson H., Green G. Gamma activation in young people with autism spectrum disorders and typically-developing controls when viewing emotions on faces. PLoS One. 2012;7 doi: 10.1371/journal.pone.0041326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalesky A., Fornito A., Bullmore E.T. Network-based statistic: identifying differences in brain networks. Neuroimage. 2010;53:1197–1207. doi: 10.1016/j.neuroimage.2010.06.041. [DOI] [PubMed] [Google Scholar]
- Zalesky A., Cocchi L., Fornito A., Murray M.M., Bullmore E.D. Connectivity differences in brain networks. Neuroimage. 2012;60:1055–1062. doi: 10.1016/j.neuroimage.2012.01.068. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Zhen Z., Liu X., Song Y., Liu J. The neural network for face recognition: insights from an fMRI study on developmental prosopagnosia. Neuroimage. 2018;169:151–161. doi: 10.1016/j.neuroimage.2017.12.023. [DOI] [PubMed] [Google Scholar]
- Zhou G., Liu J., Ding X.P., Fu G., Lee K. Development of effective connectivity during own- and other-race face processing: a granger causality analysis. Front. Hum. Neurosci. 2016;10:474. doi: 10.3389/fnhum.2016.00474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziaei M., Ebner N.C., Burianová H. Functional brain networks involved in gaze and emotional processing. Eur. J. Neurosci. 2017;45(2):312–320. doi: 10.1111/ejn.13464. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available from the corresponding author upon reasonable request.