Highlights
-
•
Associations between neighborhood disadvantage and trajectories of the difference between brain-predicted age and chronological age (brainAGE), and moderating factors, were investigated during adolescence.
-
•
Neighborhood disadvantage was positively associated with brainAGE during early adolescence and a deceleration (decreasing brainAGE) thereafter
-
•
Temperamental effortful control moderated this association. In adolescents exposed to high neighborhood disadvantage, low effortful control was associated with delayed development during late adolescence
-
•
Temperamental effortful control and positive parenting were independently associated with brainAGE trajectory
Keywords: adolescence, socioeconomic status, temperamental effortful control, parenting, longitudinal, MRI, machine learning, brain age, brain structure, brain development
Abstract
Neighborhood disadvantage has consistently been linked to alterations in brain structure; however, positive environmental (e.g., positive parenting) and psychological factors (e.g., temperament) may buffer these effects. We aimed to investigate associations between neighborhood disadvantage and deviations from typical neurodevelopmental trajectories during adolescence, and examine the moderating role of positive parenting and temperamental effortful control (EC). Using a large dataset (n = 1313), a normative model of brain morphology was established, which was then used to predict the age of youth from a longitudinal dataset (n = 166, three time-points at age 12, 16, and 19). Using linear mixed models, we investigated whether trajectories of the difference between brain-predicted-age and chronological age (brainAGE) were associated with neighborhood disadvantage, and whether positive parenting (positive behavior during a problem-solving task) and EC moderated these associations. We found that neighborhood disadvantage was associated with positive brainAGE during early adolescence and a deceleration (decreasing brainAGE) thereafter. EC moderated this association such that in disadvantaged adolescents, low EC was associated with delayed development (negative brainAGE) during late adolescence. Findings provide evidence for complex associations between environmental and psychological factors, and brain maturation. They suggest that neighborhood disadvantage may have long-term effects on neurodevelopment during adolescence, but high EC could buffer these effects.
1. Introduction
Early experience of socioeconomic disadvantage is associated with lifelong negative cognitive and mental health outcomes (Forns et al., 2012; Koutra et al., 2012; Packard et al., 2011; Ruijsbroek et al., 2011). While socioeconomic disadvantage can be considered at the household level (e.g., family income), neighborhood disadvantage has been shown to have particularly negative effects on the individual, including lower academic achievement and greater physical and mental health problems (Leventhal & Brooks-Gunn, 2000). A disadvantaged neighborhood is one in which people generally have lower levels of income, employment, and education, and can be associated with several risk factors (e.g., inadequate access to healthcare, low resourced schools, and higher pollution and crime) for maladaptive outcomes (Evans, 2004; Santiago et al., 2011). While much work has been devoted to understanding the neurobiological effects of family-based measures of socioeconomic disadvantage, where studies have demonstrated household socioeconomic disadvantage to be associated with cortical thickness, surface area, and cortical and subcortical volume in adolescents (Hanson et al., 2011, 2015; Lawson et al., 2013; Machlin et al., 2020; Mackey et al., 2015; Noble et al., 2015), less is known about the effects of neighborhood disadvantage on the brain. Adolescence is a key developmental period (Dumontheil, 2016; Tamnes et al., 2017) during which brain structure may be vulnerable to effects of socioeconomic disadvantage (Farah, 2018; Lawson et al., 2013, 2017; Whittle et al., 2017). However, there has been little longitudinal work investigating the association between neighborhood disadvantage and brain structural development. Indeed, there is only one study to our knowledge; in the same sample as reported here, we previously found that neighborhood disadvantage was associated with increases in thickness/volume of temporal regions and parahippocampus and amygdala in youth (11 to 19 years; Whittle et al., 2017). As such, and given marked neurodevelopment across adolescence (Blakemore, 2012; Foulkes & Blakemore, 2018), further studies are needed to understand whether and how neighborhood disadvantage is associated with deviations from normative neurodevelopmental trajectories, and how these in turn may contribute to negative functional outcomes.
Early experiences play an important role in shaping brain development. For example, it is suggested that resource-rich low-stress environments are conducive to the optimal development of brain systems associated with executive and other types of cognitive function. In contrast, development may be altered in low-resource high-stress environments, such that the development of brain systems that are involved in adapting and reacting to stressful conditions, is potentially prioritized over the development of brain systems supporting executive functioning (Blair & Raver, 2016). Indeed, some developmental models (such as the stress acceleration hypothesis [SAH]; Callaghan & Tottenham, 2016) and life-history theory (Ellis & Del Giudice, 2019), propose that adverse experiences (including those that co-occur with socioeconomic disadvantage) potentially accelerate biological and neurodevelopmental processes to reach ‘adult-like’ functioning earlier, given that faster development may be advantageous under high stress conditions in an evolutionary context. Although theoretical models of stress-associated acceleration originally considered emotional circuits (Callaghan & Tottenham, 2016), the concept has often been applied more broadly (including in structural neuroimaging studies) suggesting generally accelerated neurobiological aging in response to adversity (e.g., Colich et al., 2020; Gur et al., 2019). Existing research investigating associations between neighborhood disadvantage and adolescent structural gray matter development has found mixed support for these models. For example, while cross-sectional literature has found neighborhood-disadvantage-associated accelerated development (i.e., higher proportions of individuals <18 years misclassified as adults; Gur et al., 2019), our previous longitudinal study (as mentioned above) reported findings that reflect disadvantage-associated maturational lag of temporal, parahippocampal, and subcortical regions (Whittle et al., 2017). Studies investigating other SES indicators have similarly found inconsistent effects (Barch et al., 2020; Hair et al., 2015; Hanson et al., 2013; Lawson et al., 2013; Mackey et al., 2015; Noble et al., 2015; Romeo et al., 2018). Further, longitudinal resting-state functional connectivity work on the same sample as investigated here has also shown that different neural circuits show adversity-associated accelerated versus delayed development (Rakesh et al., 2021a).
Past work, however, has not quantified normative development, and deviations from said development at an individual level, which would be beneficial for testing hypotheses about accelerated versus delayed development. Further, despite neighborhood disadvantage having unique effects on childhood behavioral (Leventhal & Brooks-Gunn, 2000) and brain development (Gard et al., 2021; Taylor et al., 2020; Rakesh et al., 2021b), no work to our knowledge has examined neighborhood-disadvantage-associated deviations from typical brain development trajectories at an individual level. Using machine learning models, an individual’s brain age can be predicted based on neuroimaging data, thus allowing quantification of the extent to which an individual deviates from typical neurodevelopment. This is computed as the difference between an individual’s brain-predicted-age and chronological age and is referred to as the brain age gap estimate (brainAGE) (Cole & Franke, 2017). brainAGE values close to zero indicate typical development, whereas positive and negative brainAGE values suggest older and younger brains, respectively. To our knowledge, no studies have directly tested hypotheses regarding the association between neighborhood disadvantage and deviations from typical brainAGE developmental trajectories using longitudinal designs. Longitudinal work permits investigation of whether deviations in brainAGE associated with socioeconomic disadvantage stay constant, or are more pronounced at particular developmental stages (i.e., converge/diverge with development).
Further, while neighborhood disadvantage has been shown to be associated with negative outcomes, positive environmental or psychological factors may attenuate such negative effects. For example, positive caregiving (e.g., higher parental warmth and sensitivity) has been shown to buffer the negative effects of disadvantage on socio-emotional outcomes and school readiness (Sheridan et al., 2010). Indeed, it has been previously demonstrated that positive parenting buffers the effects of neighborhood disadvantage on structural (Whittle et al., 2017; Ziegler et al., 2020) and functional (Brody et al., 2019; Rakesh et al., 2021b) neurodevelopment. Further, studies have shown that certain facets of temperament (e.g., effortful control) are also associated with neurodevelopment (Vijayakumar et al., 2014), and mitigate the negative consequences of socioeconomic disadvantage on academic functioning (Wang et al., 2017). Individuals with high effortful control have been shown to possess greater behavioral and emotion regulation, which could contribute to this mitigating effect (Eisenberg et al., 2006). Whether positive factors such as positive parenting and effortful control moderate the association between neighborhood disadvantage and brainAGE is unknown. Given the large number of youth that face neighborhood disadvantage (Annie E. Casey Foundation, 2014), understanding the role of potential moderators such as positive parenting and temperament, is important for targeting prevention efforts.
The aim of the present study was to investigate whether neighborhood disadvantage is associated with accelerated or delayed structural neurodevelopment during adolescence (represented by brainAGE trajectory), and whether positive parenting and/or temperamental effortful control have buffering effects. In order to address these aims, we used a normative model of brain age based on data from the openly available Philadelphia Neurodevelopmental Cohort (PNC) dataset (n = 1313), and applied it to an independent longitudinal dataset (three time-points, n = 166).
Given conflicting findings regarding the association between disadvantage (generally speaking) and accelerated versus delayed adolescent brain development, we did not make specific directional hypotheses. Based on our previous work with this sample, we might expect that neighborhood disadvantage would be associated with decreasing brain age (negative trajectory), and that positive parenting and effortful control would moderate this relationship such that brainAGE would normalize (i.e., be close to zero) in those with a) higher levels of positive parenting, and/or b) higher levels of effortful control. Alternatively, consistent with the SAH, we might expect that high neighborhood disadvantage would be associated with accelerated development (i.e., positive brainAGE trajectory). Exploratory analyses also investigated whether i) positive parenting and effortful control were independently associated with brainAGE trajectories, and ii) given past evidence for male-specific effects of neighborhood disadvantage and positive parenting on brain structure (i.e., dorsal frontal thickness; Whittle et al., 2017), as well as the suggestion that boys may be more vulnerable to effects of neighborhood disadvantage (Chetty & Hendren, 2018), sex would moderate associations.
2. Methods
2.1. Participants and Procedures
2.1.1. Philadelphia Neurodevelopmental Cohort (PNC)
Recruitment procedures and assessment protocols for the original PNC sample can be found in Calkins et al. (2015). The present study included 1313 participants (mean age 14.5 years, SD = 3.43, 659 F) for whom a structural magnetic resonance imaging (MRI) scan was acquired. This sample was obtained after excluding participants on the basis of image quality, medical conditions, and missing data (n = 285; for details see Cropley et al., 2020). Structural MRI scans from this final PNC sample were used to train the normative brain age model described in subsequent sections.
2.1.2. Orygen Adolescent Development Study (OADS)
Participants were derived from a larger Australian longitudinal cohort of 2453 adolescents, of which 245 agreed to participate in longitudinal research, (see Yap et al. (2011) for details). From this larger sample, 177 community-residing adolescents completed structural MRI scans 1-3 times (average ages = 13 [T1], 17 [T2], and 19 [T3] years). Following exclusions based on missing data and poor image quality, 166 participants were included in the final analysis. For more information see Whittle et al. (2017). Of these, 73 participants (44.0%) had 3 scans, 55 (33.1%) had 2 scans, and 38 (22.9%) had 1 scan. Participants in the current study did not differ from the original sample in key demographic variables at baseline (age, sex, and neighborhood disadvantage; p > 0.5). The research was approved by the human research ethics committee at The University of Melbourne, Australia, and written informed consent was obtained from each child and a parent and/or guardian. IQ was measured using the Wechsler Intelligence Scale for Children IV (WISC IV) (Wechler, 2003) [see SI for details]). See SI for MRI acquisition protocol of the PNC and OADS.
2.2. Measures
2.2.1. Neighborhood Disadvantage
We used the Socio-Economic Indexes for Areas (SEIFA) Index of Relative Socio-economic Disadvantage (IRSD; Pink, 2008) to operationalize neighborhood disadvantage. The SEIFA-IRSD is a summary measure that characterizes relative disadvantage within neighborhoods based on Australian census data (e.g., percentage of households in the neighborhood that have low income or people that have low skilled occupations). Percentiles were used for analyses, and high-percentile scores indicate relatively greater neighborhood disadvantage.
2.2.2. Positive Parenting
Each mother-child pair completed a Problem Solving Interaction (PSI) task at T1. Five issues for discussion endorsed as occurring frequently and generating the highest intensity of anger (by the mother and the child) were selected based on an Issues Checklist (Prinz et al., 1979). Maternal behavior was coded using the Living in Family Environments (LIFE) micro-coding scheme (Hops et al., 1995), and frequency of maternal expression of positive behavior (rate per minute) was calculated.
2.2.3. Effortful Control
Effortful control was assessed at T1 using participants’ responses to the Early Adolescent Temperament Questionnaire-Revised (EATQ-R) (Capaldi & Rothbart, 1992; Ellis & Rothbart, 2001). See Supplementary Information (SI) for details of the measures and distributions.
2.3. Statistical Analyses
2.3.1. MRI processing
Of note, in the OADS, different scanners were used at T1 versus T2 and T3. Steps were taken to ensure that there were no confounds associated with multi-site scanning (see Dennison et al., 2013; Vijayakumar et al., 2016; Whittle et al., 2017). Image processing for both datasets was conducted using automated pipelines in Freesurfer v6.0 (through the longitudinal stream for the OADS). Data was extracted based on the Desikan-Killiany parcellation scheme resulting in 111 measures: 34 cortical regions (each with volume, thickness, and area), seven subcortical volumes (nucleus accumbens, amygdala, caudate, hippocampus, pallidum, putamen, and thalamus), 1 lateral ventricle volume, and intra-cranial volume (ICV).
2.3.2. Prediction of brain age and calculation of brainAGE
Using data from the PNC sample, a support vector regression (SVR) model (using 10-fold cross-validation) was used to predict each individual’s age (in the PNC sample) based on the 111 measures listed above (i.e., the normative model; Cropley et al., 2020). All measures were standardized. Model performance within the PNC sample was quantified in a previous study (see Cropley et al., 2020 and Section 3.2 for model performance within the PNC sample). The present study used the previously trained normative model to predict the age of the OADS sample at each time point. brainAGE was calculated by subtracting chronological age from the predicted age. Based on recent recommendations regarding bias associated with ‘regression to the mean’ (Le et al., 2018), we regressed out the effect of age on brainAGE (across the whole sample with a cross-sectional model including all timepoints). Positive and negative brainAGE values reflect older and younger brains, respectively. Validity of the model was quantified by computing the mean absolute error (MAE) and the Pearson correlation coefficient between the brain-predicted-age and chronological age for the OADS sample. Further, in order to enhance our understanding of the novel brainAGE metric, we examined associations between change in brainAGE and change in brain morphology (thickness, surface area, and volume of 34 regions). See SI for details.
2.3.3. Relationships between brainAGE, and neighborhood disadvantage, positive parenting, and Effortful Control
Linear mixed models (LMM), which permit the use of all available data (Gibbons et al., 2010), were used to examine the relationship between neighborhood disadvantage and brainAGE trajectory (brainAGE predicted by an interaction between chronological age and neighborhood disadvantage). For model equations see SI. Next, we separately examined whether positive parenting and effortful control moderated the effect of neighborhood disadvantage on brainAGE trajectory. We controlled for multiple comparisons using the false discovery rate (PFDR < 0.05). We covaried for sex and IQ in our models.
Given that the study is centered around the salience of the neighborhood context and that recent work has shown unique associations between neighborhood disadvantage (as compared to family indices of SES) and alterations in brain structure (Gard et al., 2021; Hyde et al., 2020; Taylor et al., 2020; Tomlinson et al., 2020; Whittle et al., 2017), we covaried for parent education (assessed using the Australian National University Four scale) as well as the experience of childhood maltreatment (total score on the total score on the Childhood Trauma Questionnaire [CTQ]; Bernstein et al. 1994; see SI for details) in order to test whether effects held when accounting for household SES and other experiences of adversity.
2.3.4. Exploratory analyses
Exploratory analyses were conducted to examine the independent relationships between i) positive parenting and ii) temperamental effortful control, and brainAGE trajectory using LMM. We also explored whether sex moderated all aforementioned associations. For significant longitudinal effects, follow-up analyses were run to examine cross-sectional relationships at each time point using ordinary least squares (OLS) regression. In addition to covariates mentioned above, we also controlled for neighborhood disadvantage in analyses where it was not the variable of interest. P values < 0.05 were considered significant for exploratory analyses.
For main and exploratory analyses, to evaluate the robustness of findings, we evaluated the normality of model residuals, and in cases where these residuals were not normally distributed, we re-ran our models after winsorizing our variables of interest (5th-95th percentile; Liao et al., 2016). If residuals were found to not be normally distributed in the models with winsorized data, we used robust regression/LMM to verify if findings remained significant (using unwinsorized data). Results from these analyses are presented in SI and are described in text below in cases where findings become non-significant with winsorized data/robust models.
2.3.5. Regional localization of association between brainAGE and neighborhood disadvantage/positive parenting/effortful control
To determine which cerebral loci contributed the most to significant associations, we re-trained the SVR model nine times on the PNC dataset, with specific brain lobes/regions (i.e., frontal/parietal/temporal/occipital/insula/cingulate/subcortical/ICV/ventricles) excluded in each incidence of the training, and then used to the model to compute the brain-predicted-age of the OADS sample. Thereafter, we re-ran LMMs to determine the strength of the relationship between predictors and brainAGE trajectory (computed after a lobe was omitted from the data), and recorded the t-statistic value of the association (i.e., between neighborhood disadvantage/effortful control/positive parenting and brainAGE). Larger decreases in t-statistic values correspond to the lobe being important for the association. If a relatively strong decrease in t-statistic values was found for a lobe, the process was repeated excluding regions within that specific lobe. Of note, the magnitude of decreases in t-stat values chosen was subjective and only to localize the relationships observed in Section 2.5.
2.3.6. Mediation models and functional outcomes
In exploratory analyses we also investigated the mediating role of brainAGE trajectory (obtained as random age slopes from LMM) in the relationship between neighborhood disadvantage and age-19 outcomes [i.e., depression and anxiety symptoms (based on Center for Epidemiologic Studies Depression Scale (Radloff, 1977) and Beck Anxiety Inventory (Beck et al., 1988)) as well as problematic substance use (Rakesh et al., 2020) and academic performance]. See SI for details of methods and results.
3. Results
3.1. Demographic Information
The PNC sample consisted of 1313 youth (659 F; aged 8-21, M = 14.5 ± 5.4 years). Demographic information for the OADS sample can be found in Table 1.
Table 1.
Demographic information.
| OADS Sample Characteristics | |
|---|---|
| N total (N females) | 166 (86 F) |
| T1 age, y | 12.79 (0.425) |
| T2 age, y | 16.70 (0.518) |
| T3 age, y | 19.08 (0.460) |
| Delay time from T1 to T2, y | 3.83 (0.204) |
| Delay time from T2 to T3, y | 2.38 (0.219) |
| Estimated FSIQ | 107.86 (15.60) |
| IRSD | 37.07 (26.69) |
| Maternal positivity during PSI | 1.71 (0.62) |
| DNCYr12, N/total N (%) | 21/127 (16.5) |
Unless otherwise stated, values represent mean and standard deviation (in parentheses).
Abbreviations: DNCYr12, did not complete year 12/senior year school; FSIQ, full-scale IQ; IRSD, Index of Relative Socioeconomic Disadvantage (neighborhood disadvantage (scale 1-100, with higher scores indicating relatively more disadvantage)); See Supplementary Figure S1 for the distribution of IRSD; PSI, problem-solving interaction; T1, time 1; T2, time 2; T3, time 3. The sample included in the present study did not differ from the original sample in key demographic characteristics (age [at baseline], sex, and neighborhood disadvantage, p > .5)
3.2. Brain age prediction based on brain morphology
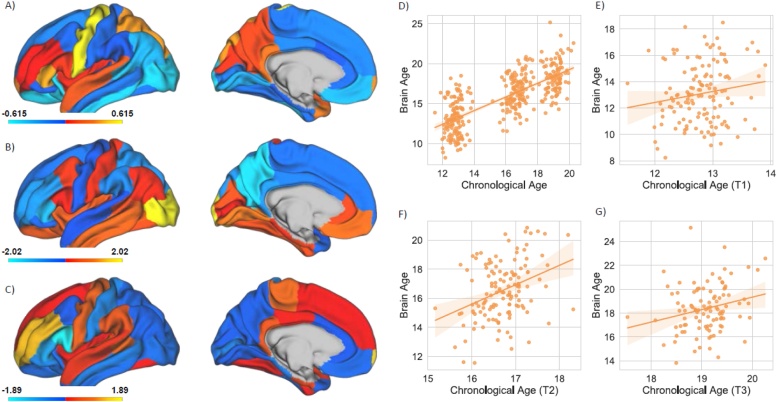
In a previous study we successfully predicted chronological age from brain structural features with high accuracy in the PNC (Cropley et al., 2020). The beta weights for each feature in the age prediction model are depicted in Fig. 1A,B,C. When training the SVR on the whole PNC sample, structural brain measures predicted chronological age with a mean absolute error (MAE) of 1.43 years and a correlation between chronological and predicted age of r = 0.79, p < 0.0001. Applying the trained model on the OADS cohort yielded adequate results with a MAE of 1.64 years (r = 0.74, p < 0.0001; Fig. 1D) across the three time points.
Fig. 1.
The beta weights for each individual cortical region for thickness (A), volume (B), and surface area (C) in the prediction of age have been visualized based on the Desikan-Killiany parcellation scheme. Warmer colors correspond to positive beta weights whereas cooler colors correspond to negative beta weights. Only one hemisphere is shown due to the averaging of left and right hemispheres for each region (see Supplementary Figure S2 for beta weights of subcortical structures, ICV, and ventricles). The correlation between corrected predicted age and chronological age have been depicted for all time points (D), and Time 1, 2, and 3 (E,F,G). Age estimates at individual time-points: T1 = 1.78 years MAE (r = 0.16, p = 0.06; Fig. 1E); T2 = 1.50 years MAE (r = 0.35, p < 0.0001; Fig. 1F); T3 = 1.62 years MAE (r = 0.24, p = 0.01; Fig. 1G).
3.3. Relationships between predictor variables and brainAGE
3.3.1. Neighborhood disadvantage
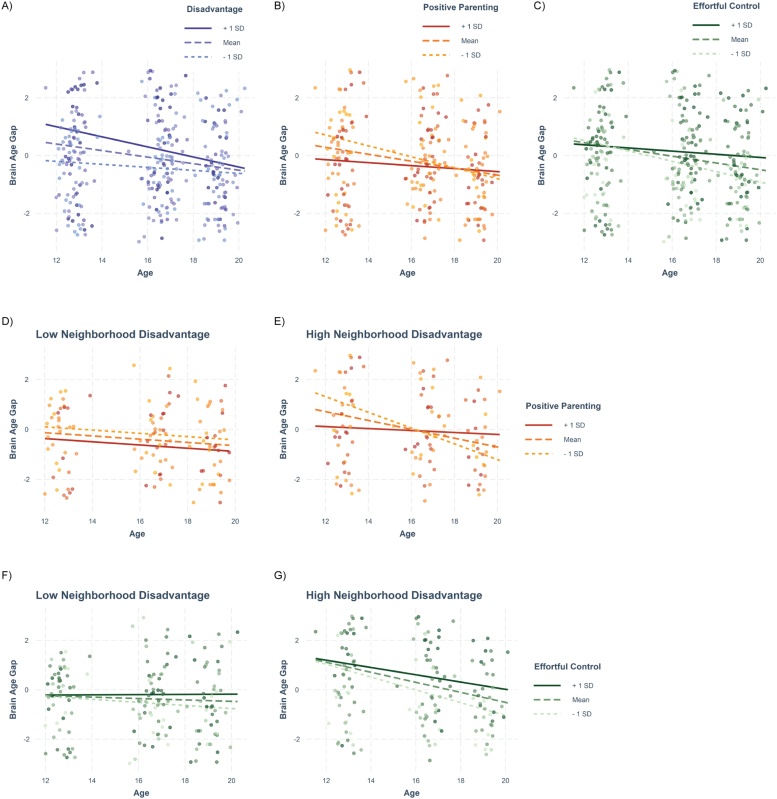
There was a significant effect of neighborhood disadvantage on brainAGE trajectory (t = -2.36, p = 0.019; Fig. 2A), whereby higher disadvantage was associated with a decrease in brainAGE across adolescence. Results were also significant when covarying for parent education and childhood maltreatment (t = -2.31, p = 0.021). Cross-sectionally, disadvantage was associated with positive brainAGE at T1, but not T2 or T3 (Fig. 3A).
Fig. 2.
Relationships between neighborhood disadvantage, positive parenting, effortful control and brainAGE. Developmental trajectories are represented for the brainAGE for adolescents with relatively high and low (A) neighborhood disadvantage, (B) positive maternal behavior, and (C) effortful control. Effect of positive maternal behavior on brainAGE trajectories in adolescents with relatively high (D) and low neighborhood disadvantage (E) based on a mean ± 1SD for visualization. Effect of effortful control on brainAGE trajectories in adolescents with relatively low (F) and high neighborhood disadvantage (G) based on a median split for visualization. brainAGE = brain age gap estimate. Blue/purple, red/orange, and greens reflect models with neighborhood disadvantage, positive parenting, and effortful control as moderators, respectively.
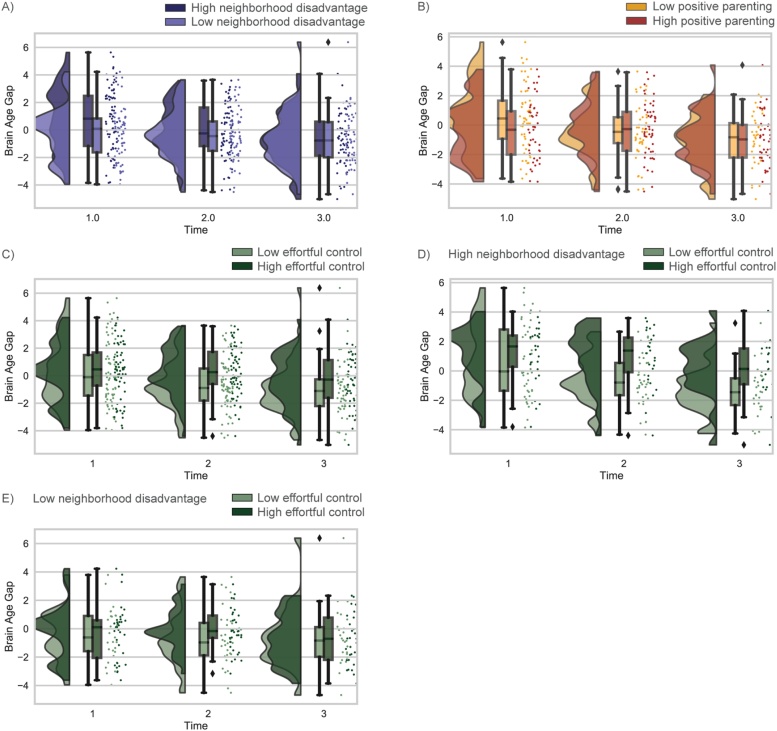
Fig. 3.
Cross-sectional differences are represented in raincloud plots at Time 1, 2, and 3 for the brainAGE for adolescents with relatively high and low neighborhood disadvantage (A) positive maternal behavior (B), and effortful control (C) based on a median split of the data for visualization. Effect of effortful control on brainAGE trajectories in adolescents with relatively high (D) and low neighborhood disadvantage (E) at Time 1, 2 and 3. Blue/purple, red/orange, and greens reflect models with neighborhood disadvantage, positive parenting, and effortful control as moderators, respectively.
Cross-sectional relationships: Cross-sectionally, neighborhood disadvantage was associated with positive brainAGE at T1 (F(126,3) = 5.60, R2 = 0.12, p = 0.001), but not T2 (F(128,3) = 3.50, R2 = 0.08, p = 0.098) or T3 (F(99,3) = 0.675, R2 = 0.02, p = 0.838).
Cross-sectionally, the moderating role of effortful control on relationship between neighborhood disadvantage and brainAGE was found to be significant at T3 (F(95,5) = 1.66, R2 = 0.08, p = 0.048), but not T2 (F(121,5) = 4.63, R2 =0.16, p=0.131), or T1 (F(118,5) = 3.48, R2 = 0.13, p = 0.267).
Cross-sectionally, the relationship between positive parenting and brainAGE was found to be significant at T1 (F(90,4) = 5.04, R2 = 0.15, p = 0.046), but not T2 (F(90,4) = 2.9, R2 = 0.08, p = 0.88) or T3 (F(74,4) = 1.69, R2 = 0.08, p = 0.411).
Cross-sectionally, the association between effortful control and brainAGE was significant at T2 (F(123,4) = 4.45, R2 = 0.097, p = 0.002), but not T1 (F(120,4) = 3.96, R2 = 0.086, p = 0.71) or T3 (F(96,4) = 1.07, R2 = 0.004, p = 0.37).
Abbreviations: brainAGE=brain age gap estimate. * = p < 0.05.
3.3.2. Moderating role of positive parenting and effortful control
Positive parenting did not moderate the relationship between neighborhood disadvantage and brainAGE trajectory (t = 1.92, p = 0.056; Fig. 2D,E). Effortful control was found to significantly moderate the relationship between neighborhood disadvantage and brainAGE trajectory (t = 2.19, p = 0.029; Fig. 2F,G), whereby disadvantaged individuals with high effortful control did not demonstrate the same negative trajectory of brainAGE as those with low effortful control. Unstandardized residuals were found to not be normally distributed. Results remained significant with winsorized data (p = 0.04). Residuals for models with winsorized outliers were also not normally distributed, and findings were no longer significant with robust LMM (p = 0.082). Cross-sectionally, this relationship was found to be significant at T3, but not T1 or T2 such that in disadvantaged adolescents, low effortful control was related to negative brainAGE values at T3.
3.3.3. Exploratory analyses
We found a significant effect of positive parenting on brainAGE trajectory (t = 2.40, p = 0.017; Fig. 2B), whereby low maternal positivity was associated with a decrease in brainAGE across adolescence. Cross-sectionally, this effect was found to be significant at T1, but not T2 or T3 (Fig. 3B). We also found a significant effect of effortful control on brainAGE trajectory (t = 2.45, p = 0.015; Fig. 2C). Cross-sectionally, this effect was significant at T2, but not T1 or T3.
Sex was not found to moderate the relationship between neighborhood disadvantage and brainAGE trajectory (t = 1.16, p = 0.244). Sex also did not moderate the relationship between neighborhood disadvantage, positive parenting/effortful control and brainAGE trajectory (t = -0.83, p = 0.407; t = .396, p = 0.692).
3.4. Brain regions contributing to the association between environmental variables and brainAGE
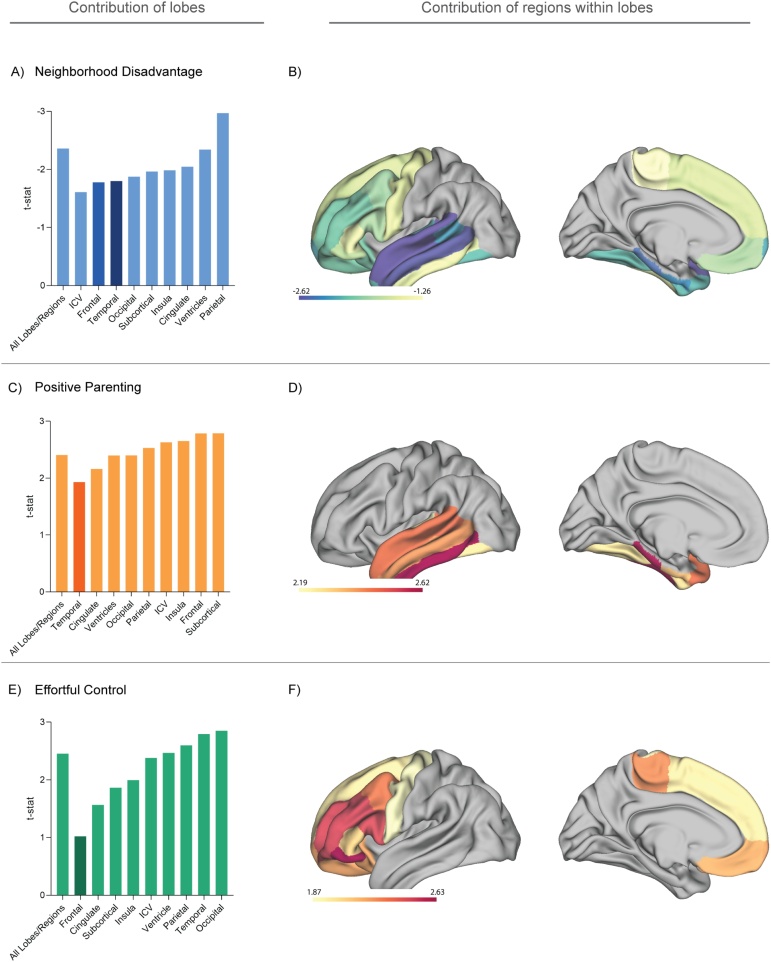
The greatest reduction in t-statistic values (and hence greatest contribution) for neighborhood disadvantage was observed with ICV, frontal lobe (particularly Paracentral, Parstriangularis, Precentral, Superiorfrontal regions), and temporal lobe (particularly Inferiortemporal) exclusion (Fig. 4A,B). The greatest reduction in t-statistic values for positive parenting was found when excluding the temporal lobe (Fig. 4C,D). Finally, for the association between effortful control and brainAGE trajectory, the greatest reduction in t-statistic values was observed when excluding the frontal lobe and the cingulate region (Fig. 4E,F). See Table S7-9.
Fig. 4.
Brain regions contributing to the association between environmental variables and brainAGE. Cortical renderings of the t statistic values of models for results in 3.3. (A) t stat values for the relationship between neighborhood disadvantage and change in brainAGE with the exclusion of each lobe/region and (B) regions within the frontal and temporal lobes; (C) t stat values for the relationship between positive parenting and change in brainAGE with the exclusion of each lobe/region and (D) regions within the temporal lobe, (E) t stat values for the relationship between temperamental effortful control and change in brainAGE with the exclusion of each lobe and (F) regions within the frontal lobe. Darker colors in the bar plots represent the lobes being depicted in panels B, D, and F. Blue/purple, red/orange, and green in A, C, and E reflect t-stat values from models with neighborhood disadvantage, positive parenting, and effortful control as moderators, respectively. Warm and cool colors in B, D, and F reflect positive and negative t-stat values respectively. See the Supplementary Tables S7-9.
Abbreviations: brainAGE=brain age gap estimate, ICV = Intracranial volume
4. Discussion
In a longitudinal sample, we found that neighborhood disadvantage was associated with brainAGE trajectories from early to late adolescence, whereby high disadvantage was associated with positive brainAGE (reflecting an older brain-predicted-age than chronological age) during early adolescence and a deceleration (i.e., decrease) through to late adolescence. Further, we found that temperamental effortful control moderated the relationship between disadvantage and change in brainAGE, such that low effortful control was associated with a decelerating brainAGE trajectory in disadvantaged individuals and negative brainAGE (younger brain-predicted-age than chronological age) during late adolescence.
Our longitudinal findings suggest that neighborhood disadvantage is associated with a decelerating brainAGE trajectory. This finding could indicate delayed brain development, and is consistent with other work finding neighborhood and other types of disadvantage to be associated with delayed brain maturation (Barch et al., 2020; Hair et al., 2015; Hanson et al., 2013; Whittle et al., 2017), as interpreted by authors. In the only previous longitudinal study to our knowledge (in the same sample of adolescents) we reported that higher neighborhood disadvantage was associated with relative cortical thickening in the parahippocampal and temporal cortices (Whittle et al., 2017), which we interpreted as delayed development.
Consideration of our cross-sectional findings adds more nuance to this interpretation, however. Disadvantage was associated with positive brainAGE during early adolescence, which appeared to ‘normalize’ (i.e., was close to zero) by late adolescence. As such, our cross-sectional findings during early adolescence could be interpreted as consistent with the SAH (i.e., positive brainAGE may be interpreted as advanced development; Cole & Franke, 2017; Cropley et al., 2020). Previous cross-sectional work in youth has identified a pattern of neighborhood, and other types of socioeconomic disadvantage to be associated with lower cortical thickness (suggested to be a proxy for advanced brain age/development; Lawson et al., 2013; Mackey et al., 2015; Piccolo et al., 2016). A recent meta-analysis examining the association between different types of adversity and biological aging in childhood/adolescence reported an association between low SES and accelerated cortical-thinning in frontoparietal, default mode, and visual regions (Colich et al., 2020), again consistent with advanced brain age. As such, our findings highlight that neighborhood disadvantage may not be associated either with advanced or delayed brain development; rather, it may be associated with different patterns across the adolescent period.
While our findings of a significant cross-sectional association between neighborhood disadvantage and advanced brainAGE during early adolescence is consistent with the SAH, stress acceleration does not explain the changes seen in brainAGE trajectories across adolescence. Indeed, recent longitudinal work using brainAGE has shown decelerated/negative trajectories of brainAGE associated with mood disorders (de Nooij et al., 2020); however, with no knowledge of changes that occur after late adolescence, and no relationships with functioning (see SI), it is challenging to comment on the meaning of adversity-related associations with reducing brainAGE trajectories, and interpret them as either adaptive or maladaptive. However, we speculate that this might reflect either: 1) that disadvantaged individuals tend to “normalize” by late adolescence, or 2) disadvantaged individuals may proceed to have decelerated development post late adolescence (indeed, this trend was present during late adolescence for disadvantaged individuals with low effortful control), which could reflect stress-associated early acceleration followed by delays in development. It is possible that development does not follow a simple linear trajectory of stress-associated acceleration of aging processes from adolescence to adulthood (de Nooij et al., 2020). In addition, it is important to acknowledge that positive and negative brainAGE values could also reflect atypical developmental patterns, not simply advanced or delayed development (which only implies a temporal shift of normative patterns).
Of note, increases in the brainAGE metric were associated with greater cortical thinning/volumetric loss/surface area reduction (see SI). This lends credence to the notion that cortical thinning (as well change in the other metrics) is associated with advanced aging, and highlights the clinical utility of the brainAGE tool – i.e., its ability to map individual deviations from typical development both cross-sectionally and longitudinally.
Our finding of the association between neighborhood disadvantage and brainAGE trajectory being driven by temporal and frontal lobes is consistent with our previous longitudinal work (Whittle et al., 2017), in addition to other longitudinal work on family-SES and brain development (Hair et al., 2015). Neighborhood disadvantage has consistently been linked to alterations in cognitive and language development, likely due to a combination of different factors, such as school quality (usually associated with neighborhood income; Leventhal & Brooks-Gunn, 2000), the presence of role models and socializing influences for parents (which in turn would impact parent-child interactions), and lower access to other resources in the community (e.g., parks, libraries) (Kohen et al., 2008). Given the role of temporal and frontal regions in language and cognition (Duncan & Owen, 2000; Price, 2010), our findings are consistent with this notion. Although speculative, brainAGE could represent a neural mechanism by which neighborhood disadvantage impacts cognitive and language development.
Importantly, accounting for household SES and other experiences of adversity did not substantially change our findings of neighborhood-disadvantage-associated change in brainAGE. In line with past work (Gard et al., 2021; Rakesh et al., 2021a; Taylor et al., 2020; Rakesh et al., 2021b), this finding shows that the effects of neighborhood context are above and beyond family-level and other adversities such as parent education and childhood maltreatment, and highlights the salience of the neighborhood environment in shaping brain development. Future work should disentangle the specific mechanisms that may be driving these effects (i.e., stress/exposure to violence and/or lower access to resources [e.g., playgrounds, libraries, low-resourced schools]; Hyde et al., 2020).
Contrary to hypotheses, and in contrast to our previous finding from the sample (Whittle et al., 2017), positive parenting did not moderate the association between neighborhood disadvantage and brain development. Contrasting findings could be attributed to the utilization of different metrics (brainAGE based on thickness/area/volume across the whole brain in the present study vs the use thickness and region-specific findings in Whittle et al., 2017). However, we found an independent effect of positive parenting on brainAGE trajectory, whereby less maternal positivity was found to be associated with positive brainAGE during early adolescence and deceleration thereafter. Of note, this relationship was found to be driven by temporal regions. Given the known role of these regions in language and cognitive functions such as social cognition (Price, 2010; Zahn et al., 2007), and other work suggesting that parenting is considered important for both language development (Perkins et al., 2013) and social cognition (Steele et al., 2002), it is possible that temporally shifted patterns of neurodevelopment in cortical temporal regions may have implications for language and cognitive abilities, and/or other aspects of adolescent functioning.
We found that temperamental effortful control moderated the relationship between neighborhood disadvantage and brainAGE trajectory, such that individuals with low effortful control exhibited a deceleration in brainAGE from early- to late adolescence and the brain-predicted-age of individuals was younger(i.e., lower) than their actual age in late adolescence. Conversely, individuals with high effortful control exhibited a more stable brainAGE trajectory. The combination of high neighborhood disadvantage and low effortful control may thus be associated with more protracted or delayed development after early adolescence, becoming evident by late adolescence. Conversely, high effortful control may partially buffer the effects of socioeconomic disadvantage on brainAGE. Previous work suggests that effortful control has the capacity to alleviate some of the negative consequences of socioeconomic disadvantage on academic functioning (Wang et al., 2017); however, in the absence of relationships with outcomes in the present study (see SI), it is difficult to interpret whether this relationship with brainAGE trajectory is indeed a buffering mechanism. In addition, given that certain model assumptions were not met (i.e., normality of residuals) and findings were not significant with robust LMM, we make our interpretations very cautiously. Further, effortful control was also independently associated with brainAGE trajectory, whereby low effortful control was associated with deceleration in brainAGE. This relationship was found to be driven by frontal and cingulate regions, which is consistent with prior work highlighting the importance of these regions for functions central to effortful control including inhibitory and attentional control (Milham et al., 2001; Vijayakumar et al., 2014) as well as emotion regulation (Etkin et al., 2011; Ochsner et al., 2012).
While our study has strengths, such as the longitudinal design and observational measures of parenting, some limitations must be considered. First, childhood is also considered a sensitive neurodevelopmental period (Giedd et al., 1999); however, we were only able to assess effects from early to late adolescence. Future work should examine effects of socioeconomic disadvantage, and the potential buffering effects of positive parenting and temperamental factors (such as effortful control), on neurodevelopment during childhood. Second, the PNC recruited participants from pediatric clinics in the United States (Calkins et al., 2015), whereas the OADS participants were from the community in Australia. However, the model performed well on the OADS sample, which speaks to the broader utility of the brainAGE metric. However, given our findings of disadvantage-associated alterations in brainAGE trajectories, it is possible that brainAGE models would not perform well across training and test cohorts with drastically different environmental exposures. Training datasets should therefore be chosen that have a large socio-demographically diverse sample. Third, given past associations between puberty and brain development (Blakemore et al., 2010), and disadvantage and pubertal development (Sun et al., 2017), changes in brainAGE could also be associated with changes in pubertal status. Future work should examine associations between brainAGE and pubertal development. Fourth, although in testing the SAH, our assumption is that neighborhood disadvantage is a proxy for stress, we did not directly investigate stress exposure. Future work should include measures of perceived stress to complement analyses. Fifth, we did not examine the association between change in disadvantage/parenting and change in brain age. Future work should account for changes in these time-varying constructs when looking at relationships with brain development using extended longitudinal designs. Further, given that timing of disadvantage also plays a role in brain development (Gard et al., 2021), future work should examine the role of timing of exposure in the relationship between neighborhood disadvantage and brain development. Sixth, given that some variables were not temporally separated, we could not conduct mediation analyses (Maxwell et al., 2011; Maxwell & Cole, 2007). However, based on findings from some past research (Luby et al., 2013), it is possible that parenting behavior acts as a mediator, rather than a moderator, between neighborhood disadvantage and brain development – an open question for future studies. Finally, the data collected for this study utilized two scanners; however, inter-scanner reliability was previously assessed and validated in a robust manner (Dennison et al., 2013; Vijayakumar et al., 2016; Whittle et al., 2017).
In sum, the present study provides evidence for the impact of socioeconomic disadvantage, positive parenting, and temperament, on brain maturational trajectories, whereby higher disadvantage and lower positive parenting were found to be associated with increased brainAGE during early adolescence, and a decrease thereafter, and low effortful control was associated with negative brainAGE in late adolescence in those with high neighborhood disadvantage. Our findings highlight the importance of studying the interplay between neighborhood disadvantage and other environmental and psychological factors, in the prediction of neurodevelopment, in order to advance our understanding of the consequences of socioeconomic disadvantage on long-term functioning and mental health.
Data Sharing Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgements
DR was supported by a Melbourne Research Scholarship (MRS), VC was supported by a National Health and Medical Research Council (NHMRC) Investigator Grant (1177370), AZ was supported by an NHMRC Senior Research Fellowship (ID: 1136649), SW was supported by an NHMRC Career Development Fellowship (ID: 1125504).
The Philadelphia Neurodevelopment Cohort sample is a publicly available data set. Support for the collection of the data sets was provided by grant RC2MH089983 awarded to Raquel Gur and RC2MH089924 awarded to Hakon Hakonarson. All subjects were recruited through the Center for Applied Genomics at The Children’s Hospital in Philadelphia. Database of Genotypes and Phenotypes study afccession: phs000607.v2.p2
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.dcn.2021.101002.
Contributor Information
Divyangana Rakesh, Email: divyangana.rakesh@gmail.com.
Sarah Whittle, Email: swhittle@unimelb.edu.au.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Annie E. Casey Foundation . The Annie E. Casey Foundation; 2014. The 2014 KIDS COUNT Data Book.https://www.aecf.org/resources/2019-kids-count-data-book/ [Google Scholar]
- Barch D.M., Shirtcliff E.A., Elsayed N.M., Whalen D., Gilbert K., Vogel A.C., Tillman R., Luby J.L. Testosterone and hippocampal trajectories mediate relationship of poverty to emotion dysregulation and depression. Proceedings of the National Academy of Sciences of the United States of America. 2020;117(36):22015–22023. doi: 10.1073/pnas.2004363117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A.T., Epstein N., Brown G., Steer R.A. An inventory for measuring clinical anxiety: Psychometric properties. Journal of Consulting and Clinical Psychology. 1988;56(6):893–897. doi: 10.1037/0022-006X.56.6.893. [DOI] [PubMed] [Google Scholar]
- Bernstein D.P., Fink L., Handelsman L., Foote J., Lovejoy M., Wenzel K., Sapareto E., Ruggiero J. Initial reliability and validity of a new retrospective measure of child abuse and neglect. American Journal of Psychiatry. 1994;151(8):1132–1136. doi: 10.1176/ajp.151.8.1132. [DOI] [PubMed] [Google Scholar]
- Blair C., Raver C.C. Poverty, Stress, and Brain Development: New Directions for Prevention and Intervention. Academic Pediatrics. 2016;16(3):S30–S36. doi: 10.1016/j.acap.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore S.J. Imaging brain development: The adolescent brain. NeuroImage. 2012;61(2):397–406. doi: 10.1016/j.neuroimage.2011.11.080. [DOI] [PubMed] [Google Scholar]
- Blakemore S.J., Burnett S., Dahl R.E. The role of puberty in the developing adolescent brain. Human Brain Mapping. 2010;31(6):926–933. doi: 10.1002/hbm.21052. Wiley-Blackwell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody G.H., Yu T., Nusslock R., Barton A.W., Miller G.E., Chen E., Holmes C., McCormick M., Sweet L.H. The Protective Effects of Supportive Parenting on the Relationship Between Adolescent Poverty and Resting-State Functional Brain Connectivity During Adulthood. Psychological Science. 2019;30(7):1040–1049. doi: 10.1177/0956797619847989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins M.E., Merikangas K.R., Moore T.M., Burstein M., Behr M.A., Satterthwaite T.D., Ruparel K., Wolf D.H., Roalf D.R., Mentch F.D., Qiu H., Chiavacci R., Connolly J.J., Sleiman P.M.A., Gur R.C., Hakonarson H., Gur R.E. The Philadelphia Neurodevelopmental Cohort: Constructing a deep phenotyping collaborative. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2015;56(12):1356–1369. doi: 10.1111/jcpp.12416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan B.L., Tottenham N. The Stress Acceleration Hypothesis: Effects of early-life adversity on emotion circuits and behavior. Current Opinion in Behavioral Sciences. 2016;7:76–81. doi: 10.1016/j.cobeha.2015.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capaldi D.M., Rothbart M.K. Development and Validation of an Early Adolescent Temperament Measure. The Journal of Early Adolescence. 1992;12(2):153–173. doi: 10.1177/0272431692012002002. [DOI] [Google Scholar]
- Chetty R., Hendren N. The impacts of neighborhoods on intergenerational mobility II: County-level estimates. Quarterly Journal of Economics. 2018;133(3):1163–1228. doi: 10.1093/QJE/QJY006. [DOI] [Google Scholar]
- Cole J.H., Franke K. Predicting Age Using Neuroimaging: Innovative Brain Ageing Biomarkers. Trends in Neurosciences. 2017;40(12):681–690. doi: 10.1016/j.tins.2017.10.001. [DOI] [PubMed] [Google Scholar]
- Colich N.L., Rosen M.L., Williams E.S., McLaughlin K.A. Biological aging in childhood and adolescence following experiences of threat and deprivation: A systematic review and meta-analysis. Psychological Bulletin. 2020 doi: 10.1037/bul0000270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cropley V.L., Tian Y., Fernando K., Mansour S., Pantelis C., Cocchi L., Zalesky A. Brain-predicted age associates with psychopathology dimensions in youth. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. 2020;0(0) doi: 10.1016/j.bpsc.2020.07.014. [DOI] [PubMed] [Google Scholar]
- de Nooij L., Harris M.A., Hawkins E.L., Clarke T.-K., Shen X., Chan S.W.Y., Ziermans T.B., McIntosh A.M., Whalley H.C. Longitudinal trajectories of brain age in young individuals at familial risk of mood disorder from the Scottish Bipolar Family Study. Wellcome Open Research. 2020;4:206. doi: 10.12688/wellcomeopenres.15617.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennison M., Whittle S., Yücel M., Vijayakumar N., Kline A., Simmons J., Allen N.B. Mapping subcortical brain maturation during adolescence: Evidence of hemisphere- and sex-specific longitudinal changes. Developmental Science. 2013;16(5):772–791. doi: 10.1111/desc.12057. [DOI] [PubMed] [Google Scholar]
- Dumontheil I. Adolescent brain development. Current Opinion in Behavioral Sciences. 2016;10:39–44. doi: 10.1016/j.cobeha.2016.04.012. [DOI] [Google Scholar]
- Duncan J., Owen A.M. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends in Neurosciences. 2000;23(10):475–483. doi: 10.1016/S0166-2236(00)01633-7. Elsevier Current Trends. [DOI] [PubMed] [Google Scholar]
- Eisenberg N., Fabes R.A., Shepard S.A., Murphy B.C., Guthrie I.K., Jones S., Friedman J., Poulin R., Maszk P. Contemporaneous and Longitudinal Prediction of Children’s Social Functioning from Regulation and Emotionality. Child Development. 2006;68(4):642–664. doi: 10.1111/j.1467-8624.1997.tb04227.x. [DOI] [PubMed] [Google Scholar]
- Ellis B.J., Del Giudice M. Developmental Adaptation to Stress: An Evolutionary Perspective. Annual Review of Psychology. 2019;70:111–139. doi: 10.1146/annurev-psych-122216-011732. Annual Reviews Inc. [DOI] [PubMed] [Google Scholar]
- Ellis L., Rothbart M. Revision of the early adolescent temperament questionnaire. Society for Research in Child Development. 2001;81:1–5. http://www.bowdoin.edu/∼sputnam/rothbart-temperament-questionnaires/pdf/lesa-ellis-srcd-poster-reprint.pdf [Google Scholar]
- Etkin A., Egner T., Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences. 2011;15(2):85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans G.W. The Environment of Childhood Poverty. American Psychologist. 2004;59(2):77–92. doi: 10.1037/0003-066X.59.2.77. [DOI] [PubMed] [Google Scholar]
- Farah M.J. Socioeconomic status and the brain: Prospects for neuroscience-informed policy. Nature Reviews Neuroscience. 2018;19(7):428–438. doi: 10.1038/s41583-018-0023-2. Nature Publishing Group. [DOI] [PubMed] [Google Scholar]
- Forns J., Torrent M., Garcia-Esteban R., Cáceres A., Pilar Gomila M., Martinez D., Morales E., Julvez J., Grimalt J.O., Sunyer J. Longitudinal association between early life socio-environmental factors and attention function at the age 11 years. Environmental Research. 2012;117:54–59. doi: 10.1016/j.envres.2012.04.007. [DOI] [PubMed] [Google Scholar]
- Foulkes L., Blakemore S.-J.J. Studying individual differences in human adolescent brain development. Nature Neuroscience. 2018;21(3):315–323. doi: 10.1038/s41593-018-0078-4. [DOI] [PubMed] [Google Scholar]
- Gard A.M., Maxwell A.M., Shaw D.S., Mitchell C., Brooks‐Gunn J., McLanahan S.S., Forbes E.E., Monk C.S., Hyde L.W. Beyond family‐level adversities: Exploring the developmental timing of neighborhood disadvantage effects on the brain. Developmental Science. 2021;24(1):e12985. doi: 10.1111/desc.12985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd J.N., Blumenthal J., Jeffries N.O., Castellanos F.X., Liu H., Zijdenbos A., Paus T., Evans A.C., Rapoport J.L. Brain development during childhood and adolescence: A longitudinal MRI study [2] Nature Neuroscience. 1999;2(10):861–863. doi: 10.1038/13158. Plenum. [DOI] [PubMed] [Google Scholar]
- Gur R.E., Moore T.M., Rosen A.F.G., Barzilay R., Roalf D.R., Calkins M.E., Ruparel K., Scott J.C., Almasy L., Satterthwaite T.D., Shinohara R.T., Gur R.C. Burden of Environmental Adversity Associated with Psychopathology, Maturation, and Brain Behavior Parameters in Youths. JAMA Psychiatry. 2019;76(9):966–975. doi: 10.1001/jamapsychiatry.2019.0943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hair N.L., Hanson J.L., Wolfe B.L., Pollak S.D. Association of Child Poverty, Brain Development, and Academic Achievement. JAMA Pediatrics. 2015;169(9):822–829. doi: 10.1001/jamapediatrics.2015.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson J.L., Chandra A., Wolfe B.L., Pollak S.D. Association between income and the hippocampus. PloS One. 2011;6(5):e18712. doi: 10.1371/journal.pone.0018712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson J.L., Hair N., Shen D.G., Shi F., Gilmore J.H., Wolfe B.L., Pollak S.D. Family poverty affects the rate of human infant brain growth. PloS One. 2013;8(12):e80954. doi: 10.1371/journal.pone.0080954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson J.L., Nacewicz B.M., Sutterer M.J., Cayo A.A., Schaefer S.M., Rudolph K.D., Shirtcliff E.A., Pollak S.D., Davidson R.J. Behavioral problems after early life stress: Contributions of the hippocampus and amygdala. Biological Psychiatry. 2015;77(4):314–323. doi: 10.1016/j.biopsych.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde L.W., Gard A.M., Tomlinson R.C., Burt S.A., Mitchell C., Monk C.S. An ecological approach to understanding the developing brain: Examples linking poverty, parenting, neighborhoods, and the brain. American Psychologist. 2020;75(9):1245–1259. doi: 10.1037/amp0000741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohen D.E., Leventhal T., Dahinten V.S., McIntosh C.N. Neighborhood Disadvantage: Pathways of Effects for Young Children. Child Development. 2008;79(1):156–169. doi: 10.1111/j.1467-8624.2007.01117.x. [DOI] [PubMed] [Google Scholar]
- Koutra K., Chatzi L., Roumeliotaki T., Vassilaki M., Giannakopoulou E., Batsos C., Koutis A., Kogevinas M. Socio-demographic determinants of infant neurodevelopment at 18 months of age: Mother-Child Cohort (Rhea Study) in Crete, Greece. Infant Behavior and Development. 2012;35(1):48–59. doi: 10.1016/j.infbeh.2011.09.005. [DOI] [PubMed] [Google Scholar]
- Lawson G.M., Camins J.S., Wisse L., Wu J., Duda J.T., Cook P.A., Gee J.C., Farah M.J. Childhood socioeconomic status and childhood maltreatment: Distinct associations with brain structure. PLOS ONE. 2017;12(4):e0175690. doi: 10.1371/journal.pone.0175690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson G.M., Duda J.T., Avants B.B., Wu J., Farah M.J. Associations between children’s socioeconomic status and prefrontal cortical thickness. Developmental Science. 2013;16(5):641–652. doi: 10.1111/desc.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le T.T., Kuplicki R.T., McKinney B.A., Yeh H.-W., Thompson W.K., Paulus M.P. A Nonlinear Simulation Framework Supports Adjusting for Age When Analyzing BrainAGE. Frontiers in Aging Neuroscience. 2018;10:317. doi: 10.3389/fnagi.2018.00317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal T., Brooks-Gunn J. The Neighborhoods They Live in: The Effects of Neighborhood Residence on Child and Adolescent Outcomes. Psychological Bulletin. 2000;126(2):309–337. doi: 10.1037/0033-2909.126.2.309. [DOI] [PubMed] [Google Scholar]
- Liao H., Li Y., Brooks G. Outlier impact and accommodation methods: Multiple comparisons of Type I error rates. Journal of Modern Applied Statistical Methods. 2016;15(1):23. [Google Scholar]
- Luby J., Belden A., Botteron K., Marrus N., Harms M.P., Babb C., Nishino T., Barch D. The effects of poverty on childhood brain development: the mediating effect of caregiving and stressful life events. JAMA Pediatrics. 2013;167(12):1135–1142. doi: 10.1001/jamapediatrics.2013.3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machlin L., McLaughlin K.A., Sheridan M.A. Brain structure mediates the association between socioeconomic status and attention‐deficit/hyperactivity disorder. Developmental Science. 2020;23(1):e12844. doi: 10.1111/desc.12844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey A.P., Finn A.S., Leonard J.A., Jacoby-Senghor D.S., West M.R., Gabrieli C.F.O., Gabrieli J.D.E. Neuroanatomical Correlates of the Income-Achievement Gap. Psychological Science. 2015;26(6):925–933. doi: 10.1177/0956797615572233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell S.E., Cole D.A. Bias in cross-sectional analyses of longitudinal mediation. Psychological Methods. 2007;12(1):23–44. doi: 10.1037/1082-989X.12.1.23. [DOI] [PubMed] [Google Scholar]
- Maxwell S.E., Cole D.A., Mitchell M.A. Bias in Cross-Sectional Analyses of Longitudinal Mediation: Partial and Complete Mediation Under an Autoregressive Model. Multivariate Behavioral Research. 2011;46(5):816–841. doi: 10.1080/00273171.2011.606716. [DOI] [PubMed] [Google Scholar]
- Milham M.P., Banich M.T., Webb A., Barad V., Cohen N.J., Wszalek T., Kramer A.F. The relative involvement of anterior cingulate and prefrontal cortex in attentional control depends on nature of conflict. Cognitive Brain Research. 2001;12(3):467–473. doi: 10.1016/S0926-6410(01)00076-3. [DOI] [PubMed] [Google Scholar]
- Noble K.G., Houston S.M., Brito N.H., Bartsch H., Kan E., Kuperman J.M., Akshoomoff N., Amaral D.G., Bloss C.S., Libiger O., Schork N.J., Murray S.S., Casey B.J., Chang L., Ernst T.M., Frazier J.A., Gruen J.R., Kennedy D.N., Van Zijl P. Family income, parental education and brain structure in children and adolescents. Nature Neuroscience. 2015;18(5):773–778. doi: 10.1038/nn.3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner K.N., Silvers J.A., Buhle J.T. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Annals of the New York Academy of Sciences. 2012;1251(1):E1–E24. doi: 10.1111/j.1749-6632.2012.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard C.J., Bezlyak V., McLean J.S., Batty G.D., Ford I., Burns H., Cavanagh J., Deans K.A., Henderson M., McGinty A., Millar K., Sattar N., Shiels P.G., Velupillai Y.N., Tannahill C. Early life socioeconomic adversity is associated in adult life with chronic inflammation, carotid atherosclerosis, poorer lung function and decreased cognitive performance: A cross-sectional, population-based study. BMC Public Health. 2011;11(1):1–16. doi: 10.1186/1471-2458-11-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins S.C., Finegood E.D., Swain J.E. Poverty and language development: Roles of parenting and stress. In Innovations in Clinical Neuroscience. 2013;10(4):10–19. Matrix Medical Communications. /pmc/articles/PMC3659033/?report=abstract. [PMC free article] [PubMed] [Google Scholar]
- Piccolo L.R., Merz E.C., He X., Sowell E.R., Noble K.G. Age-related differences in cortical thickness vary by socioeconomic status. PLoS ONE. 2016;11(9):e0162511. doi: 10.1371/journal.pone.0162511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pink B. Australian Bureau of Statistics (ABS); Canberra: 2008. Information paper: an introduction to socio-economic indexes for areas (SEIFA), 2006. [Google Scholar]
- Price C.J. The anatomy of language: A review of 100 fMRI studies published in 2009. Annals of the New York Academy of Sciences. 2010;1191(1):62–88. doi: 10.1111/j.1749-6632.2010.05444.x. Blackwell Publishing Inc. [DOI] [PubMed] [Google Scholar]
- Prinz R.J., Foster S., Kent R.N., O’Leary K.D. Multivariate assessment of conflict in distressed and nondistressed mother-adolescent dyads. Journal of Applied Behavior Analysis. 1979;12(4):691–700. doi: 10.1901/jaba.1979.12-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff L.S. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Applied Psychological Measurement. 1977;1(3):385–401. doi: 10.1177/014662167700100306. [DOI] [Google Scholar]
- Rakesh D., Lv J., Zalesky A., Allen N.B., Lubman D.I., Yücel M., Whittle S. Altered resting functional connectivity patterns associated with problematic substance use and substance use disorders during adolescence. Journal of Affective Disorders. 2020;279:599–608. doi: 10.1016/j.jad.2020.10.051. [DOI] [PubMed] [Google Scholar]
- Rakesh D., Kelly C., Vijayakumar N., Zalesky A., Allen N.B., Whittle S. Unraveling the Consequences of Childhood Maltreatment: Deviations From Typical Functional Neurodevelopment Mediate the Relationship Between Maltreatment History and Depressive Symptoms. Biol. Psychiatry: Cogn. Neurosci. Neuroimaging. 2021;6(3):329–342. doi: 10.1016/j.bpsc.2020.09.016. [DOI] [PubMed] [Google Scholar]
- Rakesh D., Zalesky A., Whittle S. Similar but distinct ? Effects of different socioeconomic indicators on resting state functional connectivity: findings from the Adolescent Brain Cognitive Development (ABCD) Study®. Dev. Cogn. Neurosci. 2021:101005. doi: 10.1016/J.DCN.2021.101005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo R.R., Christodoulou J.A., Halverson K.K., Murtagh J., Cyr A.B., Schimmel C., Chang P., Hook P.E., Gabrieli J.D.E.E. Socioeconomic Status and Reading Disability: Neuroanatomy and Plasticity in Response to Intervention. Cerebral Cortex (New York, N.Y. : 1991) 2018;28(7):2297–2312. doi: 10.1093/cercor/bhx131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruijsbroek A., Wijga A.H., Kerkhof M., Koppelman G.H., Smit H.A., Droomers M. The development of socio-economic health differences in childhood: Results of the Dutch longitudinal PIAMA birth cohort. BMC Public Health. 2011:11. doi: 10.1186/1471-2458-11-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago C.D.C., Wadsworth M.E., Stump J. Socioeconomic status, neighborhood disadvantage, and poverty-related stress: Prospective effects on psychological syndromes among diverse low-income families. Journal of Economic Psychology. 2011;32(2):218–230. doi: 10.1016/j.joep.2009.10.008. [DOI] [Google Scholar]
- Sheridan S.M., Knoche L.L., Edwards C.P., Bovaird J.A., Kupzyk K.A. Parent engagement and school readiness: Effects of the getting ready intervention on preschool children’s social-emotional competencies. Early Education and Development. 2010;21(1):125–156. doi: 10.1080/10409280902783517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele M., Steele H., Johansson M. Maternal predictors of children’s social cognition: An attachment perspective. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2002;43(7):861–872. doi: 10.1111/1469-7610.00096. [DOI] [PubMed] [Google Scholar]
- Sun Y., Mensah F.K., Azzopardi P., Patton G.C., Wake M. Childhood social disadvantage and pubertal timing: A national birth cohort from Australia. Pediatrics. 2017;139(6) doi: 10.1542/peds.2016-4099. [DOI] [PubMed] [Google Scholar]
- Tamnes C.K., Herting M.M., Goddings A.L., Meuwese R., Blakemore S.J., Dahl R.E., Güroğlu B., Raznahan A., Sowell E.R., Crone E.A., Mills K.L. Development of the cerebral cortex across adolescence: A multisample study of inter-related longitudinal changes in cortical volume, surface area, and thickness. Journal of Neuroscience. 2017;37(12):3402–3412. doi: 10.1523/JNEUROSCI.3302-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R.L., Cooper S.R., Jackson J.J., Barch D.M. Assessment of Neighborhood Poverty, Cognitive Function, and Prefrontal and Hippocampal Volumes in Children. JAMA Network Open. 2020;3(11):e2023774. doi: 10.1001/jamanetworkopen.2020.23774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson R.C., Burt S.A., Waller R., Jonides J., Miller A.L., Gearhardt A.N., Peltier S.J., Klump K.L., Lumeng J.C., Hyde L.W. Neighborhood poverty predicts altered neural and behavioral response inhibition. NeuroImage. 2020;209(December 2019):116536. doi: 10.1016/j.neuroimage.2020.116536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayakumar N., Allen N.B., Youssef G., Dennison M., Yücel M., Simmons J.G., Whittle S. Brain development during adolescence: A mixed-longitudinal investigation of cortical thickness, surface area, and volume. Human Brain Mapping. 2016;37(6):2027–2038. doi: 10.1002/hbm.23154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayakumar N., Whittle S., Dennison M., Yücel M., Simmons J., Allen N.B. Development of temperamental effortful control mediates the relationship between maturation of the prefrontal cortex and psychopathology during adolescence: A 4-year longitudinal study. Developmental Cognitive Neuroscience. 2014;9:30–43. doi: 10.1016/j.dcn.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Soden B., Deater-Deckard K., Lukowski S.L., Schenker V.J., Willcutt E.G., Thompson L.A., Petrill S.A. Development in reading and math in children from different SES backgrounds: the moderating role of child temperament. Developmental Science. 2017;20(3) doi: 10.1111/desc.12380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechler D. 4th ed. Tex, Harcourt Assessment; San Antonio: 2003. Wechsler Intelligence Scale for Children. [Google Scholar]
- Whittle S., Vijayakumar N., Simmons J.G., Dennison M., Schwartz O., Pantelis C. Role of positive parenting in the association between neighborhood social disadvantage and brain development across adolescence. JAMA Psychiatry. 2017;74(8):824–832. doi: 10.1001/jamapsychiatry.2017.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap M.B.H., Allen N.B., O’Shea M., Di Parsia P., Simmons J.G., Sheeber L. Early adolescents’ temperament, emotion regulation during mother-child interactions, and depressive symptomatology. Development and Psychopathology. 2011;23(1):267–282. doi: 10.1017/S0954579410000787. [DOI] [PubMed] [Google Scholar]
- Zahn R., Moll J., Krueger F., Huey E.D., Garrido G., Grafman J. Social concepts are represented in the superior anterior temporal cortex. Proceedings of the National Academy of Sciences. 2007;104(15):6430–6435. doi: 10.1073/pnas.0607061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler G., Moutoussis M., Hauser T.U., Fearon P., Bullmore E.T., Goodyer I.M., Fonagy P., Jones P.B., Lindenberger U., Dolan R.J. Childhood socio-economic disadvantage predicts reduced myelin growth across adolescence and young adulthood. Human Brain Mapping. 2020;41(12):3392–3402. doi: 10.1002/hbm.25024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.