Abstract
Introduction
It is essential to see if MRI can be used as an alternative to CT for the detection of retroperitoneal lymphadenopathy in patients with testicular neoplasms. By doing so, the amount of radiation received by these young patients might be reduced.
Material and methods
A systematic literature review was carried out in 5 databases between January 1984 until December 2020. The articles included were randomized and non-randomized clinical trials, cross-sectional studies, cohort, case and control, and retrospective studies that compare the accuracy of MRI against CT to detect retroperitoneal lymph nodes in patients with testicular neoplasms.
Results
The search string initially retrieved 222 non duplicated papers from which a total of 3 studies of diagnostic accuracy were included for analysis. These articles evaluated a total of 127 patients with testicular neoplasm; the sample size per study ranged from 25 to 52 patients, with a mean age between 29–34 years. MRI presented a sensitivity ranging from 98−80% and specificity of 100 % when read by an experienced radiologist. However, when it was read by a radiologist with 1 year of experience, the sensitivity dropped to 78 % and specificity to 91%.
Conclusion
This systematic literature review shows a knowledge gap since not much has been published regarding this topic; therefore, randomized clinical trials are mandatory. Research on when to use MRI over CT is necessary to reduce radiation exposure. The authors strongly suggest that readers start researching on this subject.
Abbreviations: CT, computed tomography; MRI, magnetic resonance imaging; ESMO, European Society for Medical Oncology; SWENOTECA, Swedish-Norwegian Testicular Cancer Project; PRISMA, the preferred reporting items for systematic review and meta-analysis; QUADAS-2, quality assessment of diagnostic accuracy studies-2; NPV, negative predictive value; PPV, positive predictive value; LNMRI, lymphotropic nanoparticle enhanced MRI; TRISST, trial of imaging and schedule in seminoma of the testis
Keywords: Testicular neoplasms, Tomography X-ray computed, Magnetic resonance imaging, Lymph node, Lymphatic metastasis
1. Introduction
Testicular cancer is a rare non-hematological malignancy representing 1 % of all neoplasms in men, affecting mainly patients between 15 and 49 years of age, with a high incidence in Northern Europe [1,2]. Incidence of testicular tumors has been rising worldwide in the past decade; nevertheless, the frequency of the disease may vary according to the geographical area, for example in Scandinavian countries, 6.7/100,000 men are affected by testicular neoplasms, in contrast to Japan in which 0.8/100,000 men suffer the disease [3].
All patients with testicular neoplasms must be followed closely for 5 years after primary treatment to monitor recurrences. The follow-up scheme may vary according to the tumor type, stage, and treatment established; however, it usually involves serum tumor markers, chest radiography (to seek for pulmonary metastasis), and abdominopelvic computed tomography (CT) (to look up for retroperitoneal lymphadenopathy) [[3], [4], [5], [6], [7], [8], [9]]. As seen, the follow-up scheme involves a high amount of radiation that may produce damage to the cellular genome, increasing the probability of other neoplasms in the future; therefore, low-dose CT and magnetic resonance imaging (MRI) are in constant research [5,10,11]. The European Society for Medical Oncology (ESMO) and the Swedish-Norwegian Testicular Cancer Project (SWENOTECA) is already recommending MRI for the detection of retroperitoneal lymphadenopathy in the follow-up of testicular germ cell tumors; nevertheless, the level of evidence is week (level III) [12,13].
The objective of this systematic literature review is to determine if MRI can be used as an alternative to CT for the detection of retroperitoneal lymphadenopathy in patients with testicular neoplasms to propose follow-up alternatives that involve less radiation.
2. Method
The Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) methodology was followed to develop this systematic literature review.
The protocol was registered in PROSPERO with the following registration code: CRD42020222883.
2.1. Eligibility criteria
The inclusion criteria were articles published in journals between January 1984 until December 2020, in English, Spanish or French, performed in humans, males with more than 19 years. The types of articles included were randomized and non-randomized clinical trials, cross-sectional studies, cohort, and case and control studies that compare the accuracy of CT and MRI to diagnose retroperitoneal lymph node spread in patients with testicular germ cell tumors. The exclusion criteria were articles that do not compare at any moment MRI against CT, papers with insufficient details regarding the outcome assessed (accuracy comparison between MRI and CT), and articles with a high risk of bias determined by the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) tool.
2.2. Sources of information
The search was conducted through the advanced search button in several databases, including the Medical Literature Analysis and Retrieval System Online (MEDLINE), SCOPUS, The Virtual Health Library (VHL), the Excerpta Medica database (EMBASE), and the Scientific Electronic Library Online (SCIELO). We chose these databases because it summarizes the most important articles around different regions of the world. In addition, a search in google scholar was driven to detect additional literature not published in standard databases; a snowball technique was applied by searching the references of the papers included in the systematic literature review to widen the information available.
2.3. Search
Broad keywords and specific mesh terms were used to guarantee that no articles were missed. The mesh terms used were: ((Testicular neoplasms) OR Seminoma) AND (((Tomography, X-Ray Computed) OR Tomography) OR Tomography, spiral computed) AND (((magnetic resonance imaging) OR Magnetic resonance spectroscopy) OR Diffusion magnetic resonance imaging) AND ((Lymph node) OR Lymphatic metastasis).
2.4. Study selection
All the papers identified through the search strategy were blindly loaded on Mendeley; the screening for duplicate records was performed using the Mendeley duplicate detection tool. Once duplicates were discarded, the screening was made based on title and abstract; the papers that passed the screening phase were then read thoroughly and subjected to a quality assessment. The whole process was performed independently by 2 authors, and if disagreements were presented, these were resolved by a third author.
2.5. Data extraction and synthesis
The 3 authors extracted the information from each paper saving it in a table, reporting the article's name, the journal, the number of participants, year of publication, authors, study type, intervention performed, results, conclusions, and bias. 1 author verified this information.
2.6. Quality assessment
Because all the articles included in the systematic review were diagnostic studies, the QUADAS-2 tool for diagnostic studies was implemented (https://www.bristol.ac.uk/population-health-sciences/projects/quadas/quadas-2/). Only studies with a low or acceptable probability of bias, with little concern regarding its applicability were included. The QUADAS-2 tool has 4 domains “patient selection,” “index test,” reference standard,” and “flow and timing.” Each domain is assessed regarding bias, and the first 3 domains are also evaluated respecting applicability. Each paper was reviewed twice by 2 authors, and if disagreements were presented, these were resolved by a third author.
3. Results
3.1. Search results
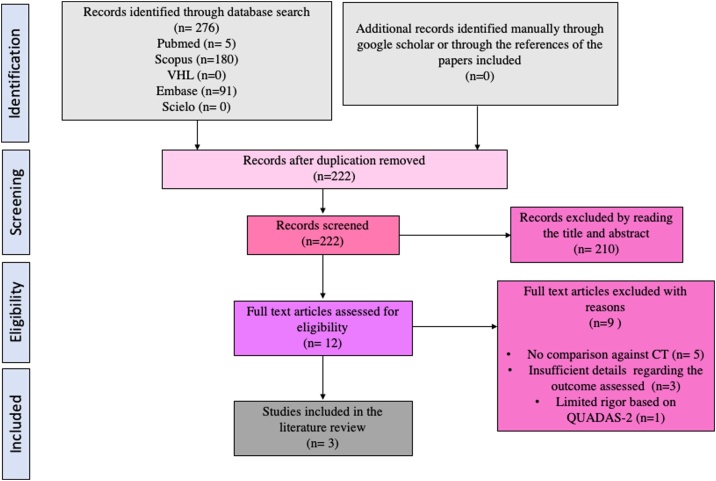
The search strategy resulted in 222 non-duplicate citations screened based on title and abstracts, of which 210 of them were not relevant to the research question. Leaving 12 articles that were read completely identifying that 5 of them did not compare MRI against CT, 3 of them provided insufficient details regarding the outcome assessed to be included in the study, and 1 of them had limited rigor determined by the QUADAS-2 tool, leaving a total of 3 papers that were included in the systematic literature review. The search results are better schematized in Fig. 1.
Fig. 1.
PRISMA Flow Diagram.
CT: Computed tomography.
3.2. Summary of studies
A total of 3 studies of diagnostic accuracy were included for analysis [[14], [15], [16]]. These articles evaluated a total of 127 patients; the sample size per study ranged from 25 to 52 patients, with a mean age between 29–34 years. All studies analyzed had a low or acceptable probability of bias, with little concern regarding its applicability in the QUADAS-2 tool for diagnostic studies. Just one study evaluated MRI with diffusion sequence [14]. In general, the studies' findings showed that MRI is equivalent to CT for the diagnosis of retroperitoneal lymphadenopathy in patients with testicular germ cell tumors. The criteria for considering a nodule as pathological were a size larger than 7−10 mm in short axis, non-homogeneous structure, irregular appearance, and restriction to diffusion (in cases it was performed). The following tables provide a detailed breakdown of each relevant finding (Table 1, Table 2, Table 3).
Table 1.
Description of included studies.
| Author, year | Study type | Diagnosis | Number of patients | Mean age | Index test | Reference standard test | Evaluator expertise | MRI machine | CT machine |
|---|---|---|---|---|---|---|---|---|---|
| Laukka et al. 2020. [14] | Prospective case-control | Testicular germ cell tumor stage I, II, III, IV | 50 | 33 | MRI with DWI | CT | One experienced radiologist | GE machine, Optima MR450w; 1.5 teslas MRI | Spiral CT machines provided by Siemens, Toshiba, and GE Medical systems, using 3-mm slice thickness |
| Sohaib et al. 2009. [15] | Prospective study | Testicular germ cell tumor stage I, II, III, IV | 52 | 34 | MRI and CT | CT (all radiologists' consensus) | Two experienced radiologists and one radiologist with 1 year of experience | Intera Release 9, Philips; 1.5 teslas MRI | GE LightSpeed 16 system; The images were reconstructed into 5 mm/2.5 mm section widths |
| Ellis et al. 1984. [16] | Cross-sectional study | Non seminomatous germ cell tumor | 25 | 29 | MRI and CT | Laparotomy biopsy | Four experienced radiologists | Investigational device manufactured by Technicare Corporation | Philips Tomoscan 310 third-generation scanner. Slice thickness was 12mm |
CT: Computed tomography; MRI: Magnetic resonance imaging; DWI: Diffusion-weighted imaging.
Table 2.
Outcomes of included studies.
| Author, year | MRI sensitivity | MRI Specificity | MRI PPV | MRI NPV | Lymph node size cut off value |
|---|---|---|---|---|---|
| Laukka et al. 2020. [14] | 98 % (88.5 %–99 %). | 100 % | 100 % | 80 % | 7 mm in short axis |
| Sohaib et al. 2009. [15] | Experienced radiologist: 96 % (86−100%) | Experienced radiologist: 100 % | Experienced radiologist: 100 % | Experienced radiologist: 91.6 % | 10 mm in short axis |
| Non-experienced radiologist: 65 % | |||||
| Non-experienced radiologist: 78 % (65−87%) | |||||
| Non-experienced radiologist: 95 % | |||||
| Non-experienced radiologist: 91 % | |||||
| Ellis et al. 1984. [16] | MRI: 80 % | Not mentioned | Not mentioned | Not mentioned | 10 mm in short axis |
| CT: 84 % |
CT: Computed tomography; MRI: Magnetic resonance imaging; DWI: Diffusion-weighted imaging.
Table 3.
QUADAS-2 results.
 |
3.3. Confirmation test/control
Ellis et al. [16] used retroperitoneal lymph node biopsy to confirm the CT and MRI findings; despite that, they evaluated the accuracy of MRI against CT. In contrast, Sohaib et al. [15] used all radiologists' consensus based on CT findings as a reference standard. Finally, Laukka et al. [14] compared MRI and CT findings using the Wilcoxon signed-rank test but did not implement any gold standard test to confirm the results. As seen, the confirmatory tests in the studies differ.
3.4. Sensitivity
Laukka et al. and Sohaib et al. both used a 1.5 teslas MRI machine, presenting similar MRI sensitivity compared to CT when read by an experienced radiologist, 98 % (95 % CI = 88.5–99 %) and 96 % (95 % CI = 86−100%) respectively. Nevertheless, Sohaib et al. also evaluated MRI compared to CT when read by a radiologist with 1 year of experience, reporting a sensitivity of 78 % (95 % CI = 65−87%).
Ellis et al. reported an MRI sensitivity of 80 % and a CT sensitivity of 84 % compared to laparotomy biopsy when read by four experienced radiologists; nevertheless, it is an old article published in 1984; therefore, it is expected that MRI sensitivity has improved substantially due to the complete introduction of diffusion sequence.
3.5. Specificity
Laukka et al. and Sohaib et al. reported 100 % MRI specificity when compared against CT for detecting retroperitoneal lymph nodes. Unfortunately, Ellis et al. did not provide enough data to calculate specificities in their papers.
3.6. Risk of bias within studies
Not all the studies used the same reference standard test to confirm the results, which may increase the risk of verification bias. Another concerning point is that 1 of the 3 papers included was an old study, which can affect the applicability of the results to daily clinical practice due to the rapid advance of technology in radiology; nevertheless, all 3 studies agreed with the same result.
4. Discussion
The main finding of this systematic literature review is the identification of a knowledge gap regarding this critical topic; therefore, the authors strongly suggest that readers start researching on this subject.
Hereby, we will provide a description and discussion of what is already published for readers to have the necessary information to run out future trials.
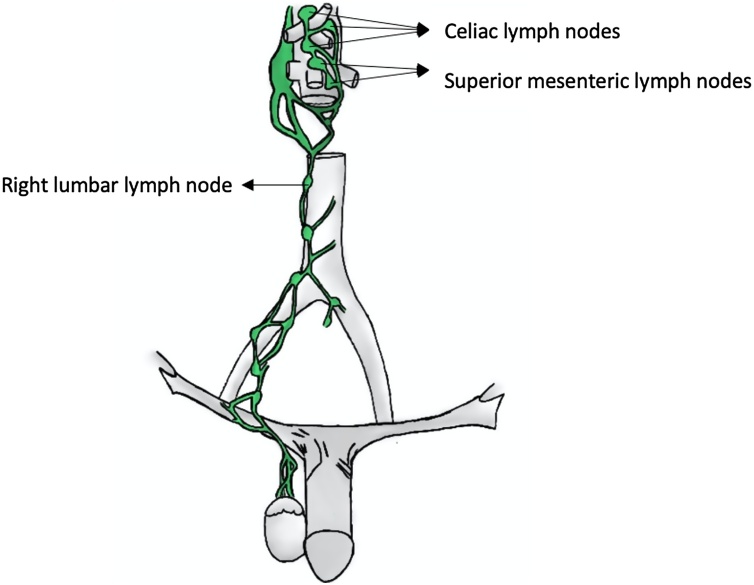
Testicular cancers metastasize through the lymphatic system, draining to the retroperitoneal lymph nodes in 88 % of cases (lumbar lymph nodes, celiac lymph nodes, superior mesenteric lymph nodes, and inferior mesenteric lymph nodes) (Fig. 2) [15,[17], [18], [19], [20]]. Therefore, some authors have suggested that the MRI technique should be directed and focused with greater detail on the retroperitoneal lymph nodes instead of focusing on inguinal and pelvic regions, reducing the time from 30−35 min to approximately 12−13 min [18]. These conclusions were supported by the ESMO and SWENOTECA, which recommended a contrast CT in the initial staging and retroperitoneum MRI in the follow-up after the initial treatment to reduce the radiation dose of these patients [12,13,21].
Fig. 2.
Testicular lymph node drainage.
Source: Elaborated by the authors.
After initial treatment, a close follow-up and surveillance of patients with testicular neoplasms should be done for at least the first 5 years to monitor possible recurrences (median time from diagnosis to relapse is 9 months). Currently, CT remains the first surveillance imaging modality to evaluate retroperitoneal lymph nodes (Fig. 3) [22,23]. The follow-up schedule varies according to the type of tumor, the stage, and the treatment established at the time of diagnosis; nevertheless, it implies high exposure to radiation for all these young patients, which may lead to an increased risk of other types of neoplasia in the future [3,9,24,25]. For this reason, we aimed to determine if MRI can replace CT for the detection of retroperitoneal lymphadenopathies in patients with testicular neoplasms.
Fig. 3.
CT showing retroperitoneal lymph node metastasis due to a testicular germ cell tumor.
Axial (a) and coronal (b) contrast CT showing retroperitoneal lumbar lymph node metastasis due to testicular germ cell tumor in a 30-year-old patient.
Currently, small studies have shown that CT and MRI may provide similar results when assessing the presence of retroperitoneal lymph node metastases; nevertheless, the evidence is limited due to the scanty amount of papers published [[14], [15], [16]].
MRI as a non-invasive staging method has two critical disadvantages: the high cost over CT and the limited number of institutions where it is available [24]. However, those limitations have been solved since nowadays there is a wide availability of institutions, MRI scanners, and the cost of this technique can be reduced by performing exclusively abdominal MRI instead of a thoraco-abdominal-pelvic MRI [25].
The best evidence available regarding this topic corresponds to a level of evidence 1b according to the Oxford Centre for Evidence-Based Medicine; in this prospective article, Sohaib et al. [15], evaluated the level of experience of the radiologist to detect retroperitoneal lymphadenopathy in patients with testicular germ cell tumors. They concluded that CT and MRI without diffusion sequence were comparable for detecting retroperitoneal lymph nodes as long as they were read by radiologists with more than 10 years of experience, showing a sensitivity of 96 % (95 % CI = 86−100%) and specificity of 100 %, when compared to CT. On the other hand, when a radiologist with one year of experience read them, the sensitivity drops to 78 % (95 % CI = 65−87%) and specificity to 91 % compared to CT. Another article with a level of evidence 1b was published by Laukka et al. [14] in 2020, in which 46 cases with retroperitoneal metastasis due to testicular cancer and 4 controls without abdominal metastasis were included. CT (3 mm thick slices) and MRI with diffusion sequence were performed. An experienced radiologist analyzed the results, comparing the ability to detect pathological lymph nodes on CT and MRI. The study concluded that there were no significant differences in detecting retroperitoneal metastases between CT and MRI with diffusion sequence, regardless of the lymph nodes' size. The main disadvantage of these articles is the fact that the gold standard used was CT instead of biopsy, which may lead to verification bias. Nevertheless, it is an understandable limitation since retroperitoneal biopsy in patients with testicular neoplasms is not frequently performed nowadays since imaging modalities and serum tumor markers can accurately detect relapses.
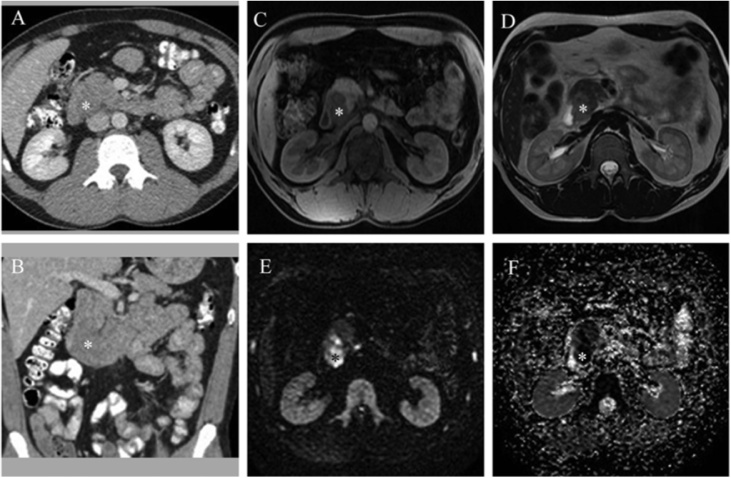
The two more recent studies included in the systematic literature review [14,15] evaluated the retroperitoneal spread of testicular germ cell tumor in all disease stages (I, II, III, IV). The criteria used for considering a nodule as pathological in MRI were size larger than 7−10 mm in short axis, non-homogeneous structure, irregular appearance, and restriction to diffusion (in cases it was performed) (Fig. 4). The lymph node size for considering the nodules pathological is under debate. Laukka et al. [14] established 7 mm as the threshold value for considering a node pathological. Nevertheless, Sohaib et al. [15] and Ellis et al. [16] recognized a pathological node if measured more than 10 mm. It is essential to highlight that prominent vessels, the regular appearance of the crus of the diaphragm, and intestinal loops may induce false-positive results on MRI without diffusion sequence (15). Therefore, it is crucial not only to look at the lymph node size but also at the intrinsic appearance of the node.
Fig. 4.
CT and MRI showing retroperitoneal lymph node metastasis due to a testicular germ cell tumor.
A 33-year-old patient with retroperitoneal metastasis in contact with the duodenum (asterisk). Contrast CT shows retroperitoneal lymphadenopathy, which does not stand out from the surrounding structures (A axial and B coronal). In non-contrast MRI T1 (C axial) and T2 (D axial) images, a retroperitoneal lymphadenopathy due to testicular cancer is visible and verified by diffusion images (E and F). Reprinted with permission from Laukka et al. and Taylor & Francis Ltd [14].
The appropriate threshold value to consider a retroperitoneal lymph node as pathological due to testicular cancer has been studied previously in CT but not in MRI, showing that the average size of metastatic nodes was <1 cm [26]. For this reason, it is recommended to decrease the size of lymph nodes considered pathological on CT from >1 cm to 0.7−0.8 cm, since if a size of >1 cm was used as the "cutoff value" to diagnose a metastatic node, 60 % of them would be missed since most are <1 cm [26]. In another study elaborated by Hilton et al., they concluded that retroperitoneal lymph nodes measuring ≥ 0.6 cm in CT had a sensitivity of 67 % and specificity of 83 % [27]. It is essential to highlight that 20–30 % of patients with clinical stage I non-seminomatous germ cell testicular cancer who have metastatic disease in the retroperitoneum could not be detected by imaging methods, especially if they measured <0.4 cm [26,28]. Studies that evaluate the most accurate cutoff value to determine a pathological retroperitoneal lymph node due to testicular cancer in MRI are required.
Mosavi et al. [25] conducted a prospective cohort study of 71 patients with histologically confirmed testicular germ cell tumors who underwent whole-body MRI staging, including diffusion sequence as a replacement of CT. The study concluded that whole-body MRI, including diffusion sequence, can replace CT in the follow-up of patients with testicular cancer since it is considered one of the best options providing added value to conventional MRI sequences, and without exposing patients at risk of nephropathy as occurs with contrast CT [21,29]. These conclusions are supported by Larsen et al. [23], who retrospectively studied 759 patients with stage 1 germ cell tumors using MRI with diffusion sequence; they confirmed relapse when the treatment was established in the medical records. The study suggests that pelvis and retroperitoneum MRI with diffusion sequence may be equivalent to abdominopelvic CT in patients with stage I testicular germ cell tumors. Nevertheless, none of the 2 studies mentioned compared the accuracy of CT and MRI; therefore, their suggestions are based on hypotheses, not on a direct comparison of both imaging methods. Studies evaluating the current accuracy between MRI and CT are lacking.
Hogeboom et al. [6] wanted to determine if specific radiological patterns in MRI were associated with the testicular lymph node metastasis histological subtype. Nevertheless, the study reported no association between the intensity of the node and the histological type.
Lymphotropic nanoparticle enhanced MRI (LNMRI) with Ferumoxtran-10 has been studied in small clinical trials to detect retroperitoneal lymphadenopathies in patients with testicular germ cell tumors, reporting safety, in addition to a sensitivity of 88.2 % and specificity of 92 % when lymph node biopsy was used as the gold standard, suggesting a better accuracy compared to simple MRI and CT. The pathological lymph node characteristics in LNMRI are nodules with a heterogeneous signal showing a central area of hyperintensity and peripheral decrease in signal intensity. Therefore, it could be an essential tool to differentiate benign vs. malignant nodules, eliminating the high radiation dose received by young patients in serial CT scans during the surveillance years. However, these results lack external validity due to the low sample size used in the study and the absence of randomization [30].
Finally, it is crucial to mention that the trial of imaging and schedule in seminoma of the testis (TRISST) is running, a large multicenter, randomized, prospective, non-inferiority trial with a factorial design including 660 patients. TRISST aims to compare the safety and effectiveness of MRI against CT for detecting retroperitoneal lymph nodes in patients with stage 1 testicular cancer [31].
5. Study limitations
There are several limitations regarding this study; the first is that the number of articles that compared MRI against CT for detecting retroperitoneal lymphadenopathy was scanty; moreover, one of the papers included was old. Only one study implemented weighted diffusion sequences. Also, the gold standard used in two studies was CT instead of biopsy, which may lead to verification bias. Nevertheless, it is an understandable limitation since retroperitoneal biopsy in patients with testicular neoplasms is not frequently performed.
The articles that assessed MRI but not CT were beyond the scope of this review; therefore, they were excluded from the result section because we aimed to compare the relative sensitivity and specificity of MRI against CT, which is the imaging modality currently used.
6. Conclusion
The evidence regarding MRI as an alternative for replacing CT to detect retroperitoneal lymphadenopathy in patients with testicular germ cell tumors is limited. Just one study compared CT against MRI with diffusion sequence. The reason that may contribute to the lack of diffusion sequence usage is that it was first introduced in 1980; therefore, it was not fully available at the year of the oldest study. This systematic literature review shows a knowledge gap since not much has been published regarding this topic. A similar conclusion was driven by Hansen et al. [19] in 2009 through a systematic literature review, in which they announced a lack of studies comparing MRI against CT to detect retroperitoneal lymphadenopathy due to testicular cancer—only reporting one article back in that time. Since then, just Laukka et al. [14] in 2020 have published an article comparing the accuracy of MRI against CT. Therefore, randomized clinical trials are mandatory. Research on when to use MRI over CT is necessary to reduce radiation exposure because there is still much uncertainty about the most appropriate method. The authors strongly suggest that readers start researching on this subject.
Ethical statement
This article does not involve human experimentation.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
There is no conflict of interest associated with this publication, and there has not been financial support for this work.
Acknowledgment
None.
References
- 1.Khan O., Protheroe A. Testis cancer. Postgrad. Med. J. 2007;83(Oct (984)):624–632. doi: 10.1136/pgmj.2007.057992. [Internet] Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park Jee Soo, Kim Jongchan, Elghiaty Ahmed, Ham W.S. Recent global trends in testicular cancer incidence and mortality. Medicine (United States) 2018;97(37):e12390. doi: 10.1097/MD.0000000000012390. [Internet]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kreydin Evgeniy I., Barrisford Glen W., Feldman Adam S., Preston Mark A. Testicular cancer: what the radiologist needs to know. Am. J. Roentgenol. 2013;200(Jun (6)):1215–1225. doi: 10.2214/AJR.12.10319. [Internet] Available from: [DOI] [PubMed] [Google Scholar]
- 4.Cassell A., Jalloh M., Ndoye M., Yunusa, Mbodji, Diallo Review of testicular tumor: diagnostic approach and management outcome in Africa. Res. Rep. Urol. 2020;12(1):35–42. doi: 10.2147/RRU.S242398. [Internet] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gilligan T., Lin D.W., Aggarwal R., Chism D., Cost N., Derweesh I.H. Testicular cancer, version 2.2020, NCCN clinical practice guidelines in oncology. JNCCN J. Natl. Compr. Cancer Netw. 2019;17(12):1529–1554. doi: 10.6004/jnccn.2019.0058. [Internet] Available from: [DOI] [PubMed] [Google Scholar]
- 6.Hogeboom, Hoekstra, Mooyaart, Sleijfer, Freling, Willemse P.H. The role of magnetic resonance imaging and computed tomography in the treatment evaluation of retroperitoneal lymph-node metastases of non-seminomatous testicular tumors. Eur. J. Radiol. 1991;13(1):31–36. doi: 10.1016/0720-048X(91)90052-W. [Internet] Available from: [DOI] [PubMed] [Google Scholar]
- 7.Brunereau L., Bruyère F., Linassier C., Baulieu The role of imaging in staging and monitoring testicular cancer. Diagn. Interv. Imaging. 2012;93(4):310–318. doi: 10.1016/j.diii.2012.01.014. [Internet] Available from: [DOI] [PubMed] [Google Scholar]
- 8.Baird Drew C., Garrett J., Meyers J.S.H. Testicular cancer: diagnosis and treatment. Am. Fam. Phys. 2018;97(4):261–268. https://pubmed.ncbi.nlm.nih.gov/29671528/ [Internet] Available from: [PubMed] [Google Scholar]
- 9.Tarin T.V., Sonn G., Shinghal R. Estimating the risk of cancer associated with imaging related radiation during surveillance for stage I testicular cancer Using computerized tomography. J. Urol. 2009;181(Feb (2)):627–633. doi: 10.1016/j.juro.2008.10.005. [Internet]. Available from: [DOI] [PubMed] [Google Scholar]
- 10.Chung P., O’Malley M.E., Jewett M.A.S., Bedard Philippe L., Panzarella T., Sturgeon J. Detection of relapse by low-dose computed tomography during surveillance in stage I testicular germ cell tumours. Eur. Urol. Oncol. 2019;2(Jul (4)):437–442. doi: 10.1016/j.euo.2018.08.031. [Internet] Available from: [DOI] [PubMed] [Google Scholar]
- 11.Lieng H., Warde P., Bedard P., Hamilton R.J., Hansen A.R., Jewett M.A.S. Recommendations for followup of stage I and II seminoma: the princess margaret cancer centre approach. J. Can. Urol. Assoc. 2018;12(Feb (2)):59–66. doi: 10.5489/cuaj.4531. [Internet] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Honecker F., Aparicio J., Berney D., Beyer J., Bokemeyer C., Cathomas R. ESMO consensus conference on testicular germ cell cancer: diagnosis, treatment and follow-up. Ann. Oncol. 2018;29(8):1658–1686. doi: 10.1093/annonc/mdy217. [Internet]. Available from: [DOI] [PubMed] [Google Scholar]
- 13.Tandstad Torgrim, Ståhl Olof, °kansson U.H., Wahlqvist R., Klepp O., Cavallin-Ståhl E. The SWENOTECA group: a good example of continuous binational and multidisciplinary collabor- ation for patients with testicular cancer in Sweden and Norway. Scand. J. Urol. 2016;50(1):9–13. doi: 10.3109/21681805.2015.1059360. [Internet]. Available from: [DOI] [PubMed] [Google Scholar]
- 14.Laukka M., Mannisto S., Beule A., Blomqvist C. Comparison between CT and MRI in detection of metastasis of the retroperitoneum in testicular germ cell tumors: a prospective trial. Acta Oncol. (Madr.) 2020;59(Jun (6)):660–665. doi: 10.1080/0284186X.2020.1725243. [Internet] Available from: [DOI] [PubMed] [Google Scholar]
- 15.Sohaib S.A., Koh D.M., Barbachano Y., Parkih, Husband, Dearnaley Prospective assessment of MRI for imaging retroperitoneal metastases from testicular germ cell tumours. Clin Radiol. 2009;64(Apr (4)):362–367. doi: 10.1016/j.crad.2008.10.011. [Internet] Available from: [DOI] [PubMed] [Google Scholar]
- 16.Ellis J.H., Bies J.R., Kopecky K.K., Klatte E.C., Rowland R.G., Al J.P.D. Comparison of NMR and CT imaging in the evaluation of metastatic retroperitoneal lymphadenopathy from testicular carcinoma. J. Comput. Assist. Tomogr. 1984;8(4):709–719. doi: 10.1097/00004728-198408000-00023. [Internet] Available from: [DOI] [PubMed] [Google Scholar]
- 17.Hale G.R., Teplitsky S., Truong H., Gold S.A., Bloom J.B., Agarwal Piyush K. Lymph node imaging in testicular cancer. Transl. Androl. Urol. 2018;7(Oct (5)):864–874. doi: 10.21037/tau.2018.07.18. [Internet] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rud E., Langberg C.W., Baco E., Lauritzen P., Sandbæk G. MRI in the follow-up of testicular cancer: less is more. Anticancer Res. 2019;39(6):2963–2968. doi: 10.21873/anticanres.13427. [Internet] Available from: [DOI] [PubMed] [Google Scholar]
- 19.Hansen J., Jurik A.G. Diagnostic value of multislice computed tomography and magnetic resonance imaging in the diagnosis of retroperitoneal spread of testicular cancer: a literature review. Acta Radiol. 2009;50(9):1064–1070. doi: 10.3109/02841850903220371. [Internet] Available from: [DOI] [PubMed] [Google Scholar]
- 20.Wilder R.B., Buyyounouski M.K., Efstathiou J.A., Beard Clair J. Radiotherapy treatment planning for testicular seminoma. Int. J. Radiat. Oncol. Biol. Phys. 2012;83(Jul (4)):445–452. doi: 10.1016/j.ijrobp.2012.01.044. [Internet] Available from: [DOI] [PubMed] [Google Scholar]
- 21.Padhani A.R., Liu G., Mu-Koh D., Chenevert T.L., Thoeny H.C., Takahara Taro. Diffusion-weighted magnetic resonance imaging as a cancer biomarker: consensus and recommendations. Neoplasia. 2009;11(2):102–125. doi: 10.1593/neo.81328. [Internet] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tasu J.-P., Faye N., Eschwege P., Rocher Laurence, Bléry M. Imaging of burned-out testis tumor. J. Ultrasound Med. 2003;22(5):515–521. doi: 10.7863/jum.2003.22.5.515. [Internet] Available from: [DOI] [PubMed] [Google Scholar]
- 23.Larsen S.K.A., Agerbæk M., Jurik A.G., Pedersen E.M. Ten years of experience with MRI follow-up of testicular cancer stage I: a retrospective study and an MRI protocol with DWI. Acta Oncol. (Madr.) 2020;59(11):1–8. doi: 10.1080/0284186X.2020.1794035. [Internet] Available from: [DOI] [PubMed] [Google Scholar]
- 24.Kok H.K., Leong S., Torreggiani W.C. Is magnetic resonance imaging comparable with computed tomography in the diagnosis of retroperitoneal metastasis in patients with testicular cancer? Can. Assoc. Radiol. J. 2014;65(3):196–198. doi: 10.1016/j.carj.2013.05.005. [Internet] Available from: [DOI] [PubMed] [Google Scholar]
- 25.Mosavi F., Laurell A., Ahlström H. Whole body MRI, including diffusion-weighted imaging in follow-up of patients with testicular cancer. Acta Oncol. (Madr.) 2015;54(Nov (10)):1763–1769. doi: 10.3109/0284186X.2015.1043027. [Internet]. Available from: [DOI] [PubMed] [Google Scholar]
- 26.Hudolin T., Kastelan Z., Knezevic N., Goluza E., Tomas D., Coric M. Correlation between retroperitoneal lymph node size and presence of metastases in nonseminomatous germ cell tumors. Int. J. Surg. Pathol. 2012;20(Feb (1)):15–18. doi: 10.1177/1066896911431452. [Internet] Available from: [DOI] [PubMed] [Google Scholar]
- 27.Hilton S., Herr H., Teitcher J.B., Begg C.B., Castéllino R.A. CT detection of retroperitoneal lymph node metastases in patients with clinical stage I testicular nonseminomatous germ cell cancer: assessment of size and distribution criteria. AJR Am. J. Roentgenol. 1997;169(1):521. doi: 10.2214/ajr.169.2.9242768. [Internet] Available from: [DOI] [PubMed] [Google Scholar]
- 28.Rassweiler J.J., Scheitlin W., Heidenreich A., Laguna M.P., Janetschek G. Laparoscopic retroperitoneal lymph node dissection: does it still have a role in the management of clinical stage I nonseminomatous testis cancer? A European perspective. Eur. Urol. 2008;54(5):1004–1019. doi: 10.1016/j.eururo.2008.08.022. [Internet] Available from: [DOI] [PubMed] [Google Scholar]
- 29.Thomas K.L., Jeong D., Montilla-Soler J., Feuerlein Sebastian. The role of diagnostic imaging in the primary testicular cancer: initial staging, response assessment and surveillance. Transl. Androl. Urol. 2020;9(Jan (1)):3–13. doi: 10.21037/tau.2019.07.01. [Internet] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harisinghani M.G., Saksena M., Ross R.W., Tabatabaei Shahin, Dahl D., McDougal S. A pilot study of lymphotrophic nanoparticle-enhanced magnetic resonance imaging technique in early stage testicular cancer: a new method for noninvasive lymph node evaluation. Urology. 2005;66(Nov (5)):1066–1071. doi: 10.1016/j.urology.2005.05.049. [Internet] Available from: [DOI] [PubMed] [Google Scholar]
- 31.Cafferty F.H., Gabe R., Huddart R.A., Rustin G., Williams M.P., Stenning S.P. UK management practices in stage 1 seminoma and the medical research council trial of imaging and schedule in seminoma testis managed with surveillance. Clin. Oncol. 2012;24(Feb (1)):25–29. doi: 10.1016/j.clon.2011.09.005. [Internet] Available from: [DOI] [PubMed] [Google Scholar]