Key Points
Question
Are general and central obesity and body composition associated with mortality after diagnosis among Black breast cancer survivors?
Findings
In this cohort study of 1891 Black breast cancer survivors, higher waist-to-hip ratio and body adiposity at approximately 10 months after diagnosis were associated with significantly worse overall and breast cancer–specific survival.
Meaning
This study’s findings suggest that simple measures of body fat distribution and body composition can be useful clinical tools to identify Black breast cancer survivors who have a higher risk of death.
Abstract
Importance
Obesity disproportionately affects Black women, who also have a higher risk of death after a breast cancer diagnosis compared with women of other racial/ethnic groups. However, few studies have evaluated the association of measures of adiposity with mortality among Black breast cancer survivors.
Objective
To assess the association of measures of adiposity with survival after a breast cancer diagnosis among Black women.
Design, Setting, and Participants
This prospective population-based cohort study comprised 1891 women with stage 0 to IV breast cancer who self-identified as African American or Black and were ages 20 to 75 years. The New Jersey State Cancer Registry was used to identify women living in 10 counties in New Jersey who were recruited from March 1, 2006, to February 29, 2020, and followed up until September 2, 2020.
Exposures
Measures of adiposity, including body mass index, body fat distribution (waist circumference and waist-to-hip ratio), and body composition (percent body fat and fat mass index), were collected during in-person interviews at approximately 10 months after breast cancer diagnosis.
Main Outcomes and Measures
All-cause and breast cancer–specific mortality.
Results
Among 1891 women, the mean (SD) age at breast cancer diagnosis was 54.5 (10.8) years. During a median follow-up of 5.9 years (range, 0.5-14.8 years), 286 deaths were identified; of those, 175 deaths (61.2%) were associated with breast cancer. A total of 1060 women (56.1%) had obesity, and 1291 women (68.3%) had central obesity. Higher adiposity, particularly higher waist-to-hip ratio, was associated with worse survival. Women in the highest quartile of waist-to-hip ratio had a 61% increased risk of dying from any cause (hazard ratio [HR], 1.61; 95% CI, 1.12-2.33) and a 68% increased risk of breast cancer death (HR, 1.68; 95% CI, 1.04-2.71) compared with women in the lowest quartile. The risks of all-cause and breast cancer–specific death were similarly high among women in the highest quartile for waist circumference (HR, 1.74 [95% CI, 1.26-2.41] and 1.64 [95% CI, 1.08-2.48], respectively), percent body fat (HR, 1.53 [95% CI, 1.09-2.15] and 1.81 [95% CI, 1.17-2.80]), and fat mass index (HR, 1.57 [95% CI, 1.11-2.22] and 1.74 [95% CI, 1.10-2.75]); however, the risk was less substantial for body mass index (HR, 1.26 [95% CI, 0.89-1.79] and 1.33 [95% CI, 0.84-2.10]). In analyses stratified by estrogen receptor status, menopausal status, and age, a higher waist-to-hip ratio was associated with a higher risk of all-cause death among women who had estrogen receptor–negative tumors (HR, 2.24; 95% CI, 1.14-4.41), women who were postmenopausal (HR, 2.15; 95% CI, 1.28-3.61), and women who were 60 years or older at diagnosis (HR per 0.10-U increase, 1.76; 95% CI, 1.37-2.26).
Conclusions and Relevance
In this population-based cohort study, central obesity and higher adiposity were associated with higher all-cause and breast cancer–specific mortality among Black breast cancer survivors. Simple measures of body fat distribution and body composition were found to be useful tools for identifying Black women with a higher risk of death after a breast cancer diagnosis.
This cohort study examines the association of obesity and body composition with all-cause and breast cancer–specific mortality among Black breast cancer survivors.
Introduction
Increasing obesity rates remain a substantial public health and clinical concern, with faster increases in prevalence among cancer survivors than the general population, particularly among non-Hispanic Black cancer survivors.1 Obesity is the second leading modifiable risk factor associated with incident cancer and cancer-associated death2 and is known to pose challenges for the clinical management of breast cancer.3 Because of the clinical importance of this issue, in its 2020 annual report, the American Society of Clinical Oncology identified the role of obesity in cancer risk and survival as a research priority.4 The association of obesity with increased postmenopausal breast cancer risk is well established,5 and the association between obesity and breast cancer prognosis is an active area of research. A meta-analysis of 82 cohort studies found a 35% increase in breast cancer–specific mortality and a 41% increase in overall mortality among women who had obesity before diagnosis.6
Few of these studies, however, have evaluated the association of obesity with breast cancer prognosis by hormone receptor status. Furthermore, most of the data on the association between obesity and breast cancer prognosis are based on studies using body mass index (BMI) as a marker of body adiposity because BMI calculation is based on weight and height, which are relatively easy measurements to collect in large studies. However, BMI is known to be an imperfect tool for characterizing body composition and body fat distribution.7,8 Increasing evidence suggests that body composition measured by computed tomography is an important factor associated with breast cancer prognosis.9,10 However, computed tomographic scans are not always available or feasible in clinical settings with low resources or in population-based prospective studies owing to cost and lack of portability. A more practical method of measuring body composition is bioelectrical impedance analysis, which has been validated against dual-energy x-ray absorptiometry and underwater weighing.11 However, to our knowledge, the association between measurement of body composition using bioelectrical impedance analysis and breast cancer mortality has not been evaluated among Black women.
Waist and hip circumferences are relatively easy measurements to obtain in any setting and can be used to identify central obesity, which is defined as a waist-to-hip ratio (WHR) of more than 0.85 or a waist circumference of more than 88 cm.12 Central obesity is a proxy for intra-abdominal visceral fat, which is more metabolically active and more substantially associated with insulin resistance and metabolic syndrome relative to subcutaneous fat.13 The role of central obesity in breast cancer mortality has received little attention; however, a meta-analysis of 4 studies found increased mortality among those with a high WHR.14 Furthermore, most of the data regarding general and central obesity are based on studies conducted among White women. Black women typically have higher lean mass and lower body fat for a given BMI than White women.15 Black women are also more likely to have general and central obesity and to experience worse breast cancer survival than any other racial or ethnic group.16 The objective of this study was to evaluate the association of BMI, body fat distribution (via WHR and waist circumference), and body composition (percent body fat and fat mass index [FMI]), which were measured at approximately 10 months after breast cancer diagnosis, with all-cause and breast cancer–specific mortality in a prospective cohort of Black breast cancer survivors.
Methods
Study Population
Participants were enrolled in the Women’s Circle of Health Study17 and the Women’s Circle of Health Follow-Up Study (WCHFS),18 an ongoing cohort study of Black breast cancer survivors that is built on the infrastructure of the Women’s Circle of Health Study. Our study used baseline data from participants enrolled in both studies using the same methods described in detail elsewhere.18 In brief, participants living in 10 New Jersey counties were identified through the New Jersey State Cancer Registry (NJSCR). Women with a diagnosis of ductal carcinoma in situ or invasive breast cancer (stages 0-IV) who self-identified as Black or African American, were aged 20 to 75 years at diagnosis, were able to speak English, and did not have a history of cancer (with the exception of nonmelanoma skin cancer) were eligible to participate. Men were excluded because male breast cancer is rare and likely associated with different etiologic and prognostic factors.19 All participants provided written informed consent during an in-person interview before data collection began, and the study protocol was approved by the institutional review boards of Rutgers University and Roswell Park Comprehensive Cancer Center. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cohort studies.
Data collection included a baseline in-person home interview at approximately 10 months after diagnosis followed by annual home visits or telephone interviews until 5 years after diagnosis. Computer-assisted interviewing was used to administer questionnaires that examined a range of factors, including sociodemographic and anthropometric characteristics, reproductive and lifestyle factors, medical history, weight and height at 1 year before diagnosis, and patient-reported outcomes.
Anthropometric measures, which were collected following a standardized protocol,18,20 included weight and height (to calculate BMI as a proxy for general adiposity; BMI is calculated as weight in kilograms divided by height in meters squared), body fat distribution (waist and hip circumference), and body composition (fat mass and percent body fat) using a portable bioelectrical impedance analysis scale (TBF-300A Total Body Composition Analyzer; Tanita). Waist and hip circumferences were recorded twice; if the difference between the 2 measures was greater than 2 cm, a third measurement was taken, and the 3 measurements were then averaged for the analyses. In the WCHFS, blood pressure measurements were also recorded after at least 5 minutes of rest using a clinically validated automated blood pressure monitor (IntelliSense model HEM-907XL; Omron) following American Heart Association protocol.21
At the interview, participants were asked to sign releases for medical records review, with approximately 98% of women providing consent. Information on breast cancer clinicopathologic characteristics, including American Joint Committee on Cancer stage, cancer grade, and hormone receptor status (ie, estrogen receptor and progesterone receptor status and human epidermal growth factor receptor 2 [ERBB2, formerly HER2] status), which were used to estimate tumor subtypes, was abstracted from pathology reports and/or NJSCR files. Tumors were classified into 3 distinct subtypes: luminal A (defined as estrogen receptor positive and/or progesterone receptor positive and ERBB2 negative), ERBB2-positive, and triple-negative breast cancer (defined as estrogen receptor negative, progesterone receptor negative, and ERBB2 negative). Because the statistical power to evaluate outcomes by tumor subtype was limited, we also classified women according to estrogen receptor status. Breast cancer treatment information was collected from medical records and, if unavailable, from NJSCR data or self-reports. For basic treatment information, self-reports were found to have good concordance with medical records in our study (κ = 0.91 for chemotherapy, κ = 0.74 for radiotherapy, and κ = 0.74 for lumpectomy and mastectomy), which was consistent with results reported by another study.22
Data on participants’ history of diabetes and hypertension were collected from medical records and, if unavailable, by self-reports during the in-person interviews. We also classified participants as having a history of hypertension if their measured systolic blood pressure was 140 mm Hg or higher or their diastolic blood pressure was 90 mm Hg or higher at the home visits.
From March 1, 2006, to February 29, 2020, a total of 1905 women (with first diagnosis in 2005) completed the baseline questionnaire. Five women were excluded because of missing baseline weight and height. An additional 9 women were excluded because they were underweight (BMI <18.5), and there were too few underweight women in the sample to allow a meaningful evaluation of the association between underweight status and mortality outcomes. After exclusions, 1891 women were included in the analysis.
Major outcomes of interest included breast cancer–specific and overall mortality. Outcome ascertainment was conducted through data linkage with NJSCR files to collect information on vital status, date of death, and cause of death for those who died during the follow-up period; these data were complemented by active annual follow-up of participants until September 2, 2020. The NJSCR files are updated annually using various sources, including Medicare, Medicaid, and the National Death Index, which allows for relatively complete follow-up data, even among participants who move to other states.
Statistical Analysis
Body mass index was categorized according to international weight classifications from the World Health Organization, which comprised normal weight (BMI, 18.5-24.9), overweight (BMI, 25.0-29.9), obese class 1 (BMI, 30.0-34.9), obese class 2 (BMI, 35.0 to 39.9), and obese class 3 (BMI, ≥40.0).13,23 We were unable to obtain anthropometric measurements for 72 women (3.8%); therefore, we used self-reported weight and height from the baseline interview in our BMI calculations. In previous analyses we found high concordance between BMI derived from self-reported and measured weight and height (intraclass correlation = 0.97).24 We repeated major analyses that excluded these participants, and results remained unchanged. Waist-to-hip ratio was computed as waist circumference divided by hip circumference. Fat mass index was calculated as fat mass in kilograms divided by height in meters squared. Waist circumference, WHR, FMI, and percent body fat were evaluated in quartiles, as continuous variables, and as dichotomous variables with cutoff points based on increased metabolic risk according to World Health Organization classifications12 (>88 cm for waist circumference and >0.85 for WHR) and classifications from other studies11 (>35% for abnormal percent body fat and ≥9.5 for FMI).
Cochran-Mantel-Haenszel tests were conducted to compare major factors of interest by BMI category. Survival time was calculated from the date of diagnosis to the date of death or the end of the follow-up period (September 2, 2020), whichever came first. Kaplan-Meier survival curves and log-rank tests were conducted to compare survival across categories of anthropometric variables of interest. Adjusted hazard ratios (HRs) and 95% CIs were estimated using multivariable Cox proportional hazards regression analysis, via a backward elimination approach with a significance threshold of P < .10 for covariate inclusion in the model and a priori knowledge of relevant factors. Main covariates of interest included age at diagnosis (continuous, as nonlinearity was not detected), location of birth (US vs non-US), educational level, marital status, health insurance status, household income, American Joint Committee on Cancer stage, tumor grade, tumor subtype, lifestyle factors relevant to breast cancer prognosis (physical activity, smoking, and alcohol intake 1 year before diagnosis), obesity-associated comorbidities (history of diabetes and hypertension), menopausal status, and cancer treatments received (surgery type and receipt of chemotherapy, radiotherapy, and/or endocrine therapy).
Hazard ratios and 95% CIs for breast cancer–specific mortality were based on competing risk analyses using the Fine and Gray subdistribution hazard model.25 We used the supremum test and Schoenfeld residuals to evaluate the proportional hazards assumption, and no violation was observed. Martingale and deviance residuals were also evaluated and indicated goodness of fit. To maximize statistical power, missing information on covariates was represented by an indicator category and incorporated into the models except for smoking status and surgery type (each was missing for 1 participant). Results remained unchanged when we repeated analyses excluding women assigned to those indicator categories. No multiple comparison adjustment was applied, as our study was not focused on multiple hypotheses.26,27
For ordinal variables, tests for trend were conducted by fitting the median value of each category as a continuous variable in the multivariable regression models.28 Stratified analyses by menopausal status, age group, and estrogen receptor status were also conducted, with P values for interaction computed. Two-sided P values were reported, and statistical significance was set at P = .05. All statistical analyses were performed using SAS software, version 9.4 (SAS Institute Inc).
Results
Among 1891 women, the mean (SD) age at breast cancer diagnosis was 54.5 (10.8) years (eTable 1 in the Supplement). During a median follow-up of 5.9 years (range, 0.5-14.8 years), 286 deaths were recorded, with 175 of those deaths (61.2%) associated with breast cancer. The distributions of relevant demographic and clinical characteristics for the overall cohort and by BMI are shown in eTable 1 and eTable 2 in the Supplement. Obesity was highly prevalent; 1060 women (56.1%) had obesity, and 559 of those (52.7%) had class 2 or 3 obesity. Central obesity was also common, with 1291 participants (68.3%) having a WHR greater than 0.85 (eTable 1 in the Supplement). Compared with women of normal weight, women with obesity were more likely to be older (74 women [24.6%] vs 363 women [34.2%] were >60 years, respectively), to have lower educational levels (103 women [34.2%] vs 440 women [41.4%] had a high school educational level or lower), and to be diagnosed with estrogen receptor–positive (200 women [66.4%] vs 761 women [71.7%]) or luminal A (131 women [43.5%] vs 544 women [51.2%]) tumors (eTable 1 and eTable 2 in the Supplement).
Kaplan-Meier curves and log-rank tests for selected anthropometric variables indicated significantly worse survival (both overall and breast cancer-specific survival) for women with a higher WHR (Figure). In multivariable analyses, the highest quartile of waist circumference was associated with worse overall survival compared with the lowest quartile (HR, 1.74; 95% CI, 1.26-2.41) (Table 1). A dose-response relationship was found between increasing WHR and all-cause mortality. Women in the highest quartile of WHR had a 61% increased risk of all-cause death (HR, 1.61; 95% CI, 1.12-2.33) compared with those in the lowest quartile, with similar associations observed for higher percent body fat (HR, 1.53; 95% CI, 1.09-2.15) and higher FMI (HR, 1.57; 95% CI, 1.11-2.22). In contrast, there was not a significant association between BMI categories and all-cause mortality, and there was no indication of a dose-response relationship. However, when modeled as a continuous variable, each 5.0-U increase in BMI was associated with an 11% increased risk of death from all causes (HR, 1.11; 95% CI, 1.02-1.20) (Table 1).
Figure. Kaplan-Meier Curves for All-Cause and Breast Cancer–Specific Survival by Body Mass Index (BMI) and Waist-to-Hip Ratio (WHR).
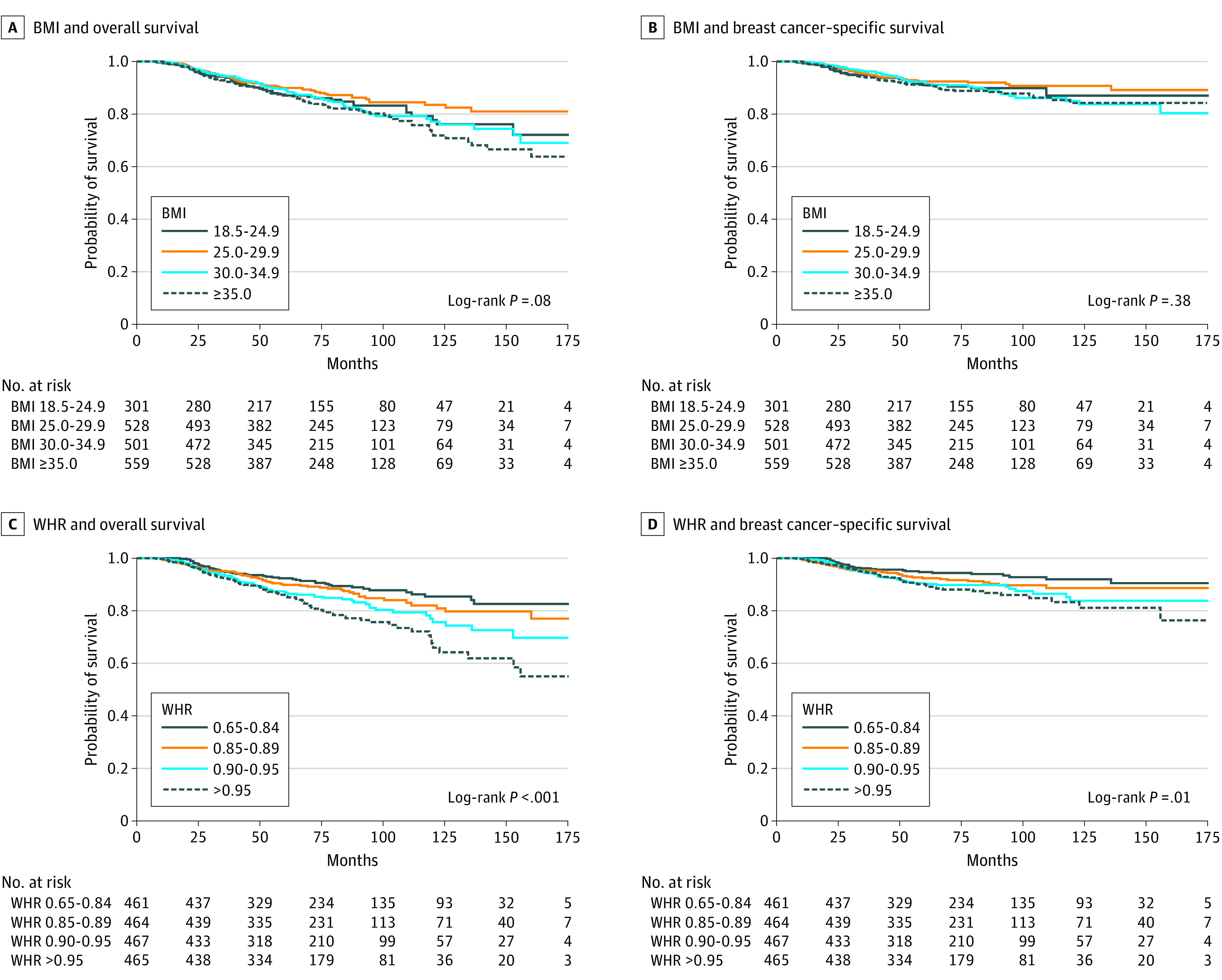
BMI is calculated as weight in kilograms divided by height in meters squared.
Table 1. Association of Body Size, Body Fat Distribution, and Body Composition With All-Cause and Breast Cancer–Specific Mortality Among Black Breast Cancer Survivors.
| Variable | Participants, No./total No. (%) | All-cause mortality | Breast cancer–specific mortality | ||||
|---|---|---|---|---|---|---|---|
| Total deaths | HR (95% CI)a | P value for trend | Deaths associated with breast cancer | HR (95% CI)b | P value for trend | ||
| Baseline BMI | |||||||
| Normal (18.5-24.9) | 301/1889 (15.9) | 47 | 1 [Reference] | .12 | 28 | 1 [Reference] | .12 |
| Overweight (25.0-29.9) | 528/1889 (28.0) | 64 | 0.94 (0.64-1.37) | 40 | 0.93 (0.57-1.53) | ||
| Obese class 1 (30.0-34.9) | 501/1889 (26.5) | 75 | 1.17 (0.80-1.67) | 48 | 1.27 (0.80-2.03) | ||
| Obese class 2-3 (≥35.0) | 559/1889 (29.6) | 100 | 1.26 (0.89-1.79) | 59 | 1.33 (0.84-2.10) | ||
| Per 5.0 U | 1889/1889 (100) | 286 | 1.11 (1.02-1.20) | NA | 175 | 1.11 (1.00-1.23) | NA |
| Waist circumference, cm | |||||||
| Quartile 1 (≤90.5) | 458/1857 (24.7) | 60 | 1 [Reference] | .19 | 39 | 1 [Reference] | .19 |
| Quartile 2 (90.6-100.1) | 465/1857 (25.0) | 47 | 0.91 (0.62-1.33) | 28 | 0.79 (0.48-1.32) | ||
| Quartile 3 (100.2-110.9) | 466/1857 (25.1) | 60 | 1.00 (0.69-1.44) | 40 | 1.03 (0.65-1.63) | ||
| Quartile 4 (>110.9) | 468/1857 (25.2) | 107 | 1.74 (1.26-2.41) | 61 | 1.64 (1.08-2.48) | ||
| Per 10.0 cm | 1857/1857 (100) | 274 | 1.14 (1.06-1.23) | NA | 168 | 1.11 (1.01-1.22) | NA |
| Waist-to-hip ratio | |||||||
| Quartile 1 (0.65-0.84) | 461/1857 (24.8) | 48 | 1 [Reference] | .03 | 28 | 1 [Reference] | .03 |
| Quartile 2 (0.85-0.89) | 464/1857 (25.0) | 59 | 1.07 (0.73-1.58) | 39 | 1.25 (0.75-2.07) | ||
| Quartile 3 (0.90-0.95) | 467/1857 (25.1) | 73 | 1.37 (0.94-2.00) | 47 | 1.47 (0.88-2.46) | ||
| Quartile 4 (>0.95) | 465/1857 (25.1) | 94 | 1.61 (1.12-2.33) | 54 | 1.68 (1.04-2.71) | ||
| Per 0.10 U | 1857/1857 (100) | 274 | 1.29 (1.11-1.50) | NA | 168 | 1.19 (0.99-1.44) | NA |
| Body fat, % | |||||||
| Quartile 1 (<37.1) | 440/1765 (24.9) | 61 | 1 [Reference] | .16 | 35 | 1 [Reference] | .16 |
| Quartile 2 (37.1-41.9) | 435/1765 (24.6) | 56 | 1.14 (0.79-1.65) | 38 | 1.29 (0.80-2.08) | ||
| Quartile 3 (42.0-46.5) | 447/1765 (25.3) | 58 | 1.08 (0.75-1.56) | 33 | 1.12 (0.68-1.85) | ||
| Quartile 4 (>46.5) | 443/1765 (25.1) | 82 | 1.53 (1.09-2.15) | 52 | 1.81 (1.17-2.80) | ||
| Per 5.0 U | 1765/1765 (100) | 257 | 1.10 (1.01-1.20) | NA | 158 | 1.13 (1.00-1.28) | NA |
| FMI | |||||||
| Quartile 1 (<9.9) | 435/1764 (24.7) | 55 | 1 [Reference] | .04 | 33 | 1 [Reference] | .03 |
| Quartile 2 (9.9-12.8) | 444/1764 (25.2) | 63 | 1.22 (0.85-1.75) | 41 | 1.27 (0.79-2.03) | ||
| Quartile 3 (12.9-16.5) | 442/1764 (25.0) | 61 | 1.22 (0.85-1.77) | 35 | 1.28 (0.79-2.09) | ||
| Quartile 4 (>16.5) | 443/1764 (25.1) | 79 | 1.57 (1.11-2.22) | 50 | 1.74 (1.10-2.75) | ||
| Per 5.0 U | 1764/1764 (100) | 258 | 1.18 (1.05-1.33) | NA | 159 | 1.20 (1.02-1.41) | NA |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); FMI, fat mass index (calculated as fat mass in kilograms divided by height in meters squared); HR, hazard ratio; NA, not applicable.
Hazard ratios and 95% CIs from Cox proportional hazards model were adjusted for age, income, smoking status, tumor stage, tumor subtype, and type of surgery.
Hazard ratios and 95% CIs from Fine and Gray subdistribution hazard model25 were adjusted for age, income, smoking status, tumor stage, tumor subtype, and type of surgery.
Similar risk estimates were observed for breast cancer–specific mortality using competing risk analyses (Table 1). The risk of breast cancer–associated death was substantially higher among women in the highest quartiles of waist circumference (HR, 1.64; 95% CI, 1.08-2.48), percent body fat (HR, 1.81; 95% CI, 1.17-2.80), and FMI (HR, 1.74; 95% CI, 1.10-2.75) compared with those in the lowest quartiles. In addition, women in the highest quartile of WHR had a 68% increased risk of breast cancer–specific death (HR, 1.68; 95% CI, 1.04-2.71). The risk of death among those in the highest category of BMI was less substantial and similar to the risk of death from all causes (HR, 1.33; 95% CI, 0.84-2.10), with an identical increased risk of 11% per 5.0-U increase in BMI (HR, 1.11; 95% CI, 1.00-1.23) when modeled as a continuous variable. Adjusting for estrogen receptor status in lieu of tumor subtype or further adjusting for diabetes did not change the results. Hypertension was not a significant covariate in the backward elimination analyses. We also repeated analyses excluding women with ductal carcinoma in situ and found no change in the observed associations. To further explore results for BMI, we repeated analyses controlling for WHR, but results remained unchanged. Because 159 women (8.4%) had normal BMI but high WHR (>0.85) (eTable 1 in the Supplement), we also repeated BMI analyses excluding these women, and results were again unchanged.
Associations between WHR and all-cause mortality tended to be more substantial among women with estrogen receptor–negative tumors (HR, 2.24; 95% CI, 1.14-4.41) (Table 2), women who were postmenopausal (HR, 2.15; 95% CI, 1.28-3.61) (Table 3), and women 60 years or older (HR per 0.10-U increase, 1.76; 95% CI, 1.37-2.26) (Table 4). Results for breast cancer–specific mortality by estrogen receptor status are shown in eTable 3 in the Supplement. Although an increased risk of breast cancer–specific death was observed for all anthropometric measures, particularly for estrogen receptor–negative tumors (eg, for highest quartile of percent body fat: HR, 2.04; 95% CI, 1.03-4.05; for highest category of BMI: HR, 1.51; 95% CI, 0.73-3.09), most 95% CIs included the null value (eTable 3 in the Supplement).
Table 2. Association of Body Size, Body Fat Distribution, and Body Composition With All-Cause Mortality by Estrogen Receptor Status Among Black Breast Cancer Survivors.
| Variable | Estrogen receptor–positive | Estrogen receptor–negative | P value for interaction | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Participants, No./total No. (%) | Total deaths | HR (95% CI)a | P value for trend | Participants, No./total No. (%) | Total deaths | HR (95% CI)a | P value for trend | ||
| Baseline BMI | |||||||||
| Normal (18.5-24.9) | 200/1332 (15.0) | 28 | 1 [Reference] | .12 | 93/506 (18.4) | 18 | 1 [Reference] | .11 | .91 |
| Overweight (25.0-29.9) | 372/1332 (27.9) | 38 | 0.93 (0.57-1.53) | 140/506 (27.7) | 25 | 0.92 (0.50-1.71) | |||
| Obese class 1 (30.0-34.9) | 347/1332 (26.1) | 45 | 1.02 (0.63-1.65) | 136/506 (26.9) | 28 | 1.23 (0.67-2.24) | |||
| Obese class 2-3 (≥35.0) | 413/1332 (31.0) | 65 | 1.20 (0.76-1.90) | 137/506 (27.1) | 35 | 1.34 (0.75-2.42) | |||
| Per 5.0 U | 1332/1332 (100) | 176 | 1.12 (1.02-1.24) | NA | 506/506 (100) | 106 | 1.11 (0.96-1.27) | NA | .87 |
| Waist circumference, cm | |||||||||
| Quartile 1 (<90.6) | 309/1314 (23.5) | 37 | 1 [Reference] | .18 | 139/495 (28.1) | 22 | 1 [Reference] | .20 | .36 |
| Quartile 2 (90.6-100.1) | 328/1314 (25.0) | 26 | 0.74 (0.44-1.24) | 116/495 (23.4) | 19 | 1.09 (0.59-2.04) | |||
| Quartile 3 (100.2-110.9) | 334/1314 (25.4) | 36 | 0.88 (0.55-1.40) | 123/495 (24.8) | 24 | 1.26 (0.70-2.28) | |||
| Quartile 4 (>110.9) | 343/1314 (26.1) | 68 | 1.57 (1.03-2.39) | 117/495 (23.6) | 38 | 1.90 (1.10-3.29) | |||
| Per 10.0 cm | 1314/1314 (100) | 167 | 1.16 (1.06-1.28) | NA | 495/495 (100) | 103 | 1.12 (0.99-1.27) | NA | .53 |
| Waist-to-hip ratio | |||||||||
| Quartile 1 (0.65-0.84) | 331/1314 (25.2) | 34 | 1 [Reference] | .03 | 120/495 (24.2) | 13 | 1 [Reference] | .02 | .36 |
| Quartile 2 (0.85-0.89) | 319/1314 (24.3) | 34 | 0.83 (0.51-1.35) | 130/495 (26.3) | 25 | 1.81 (0.92-3.57) | |||
| Quartile 3 (0.90-0.95) | 330/1314 (25.1) | 40 | 1.10 (0.69-1.77) | 126/495 (25.5) | 31 | 2.14 (1.09-4.19) | |||
| Quartile 4 (>0.95) | 334/1314 (25.4) | 59 | 1.38 (0.89-2.15) | 119/495 (24.0) | 34 | 2.24 (1.14-4.41) | |||
| Per 0.10 U | 1314/1314 (100) | 167 | 1.29 (1.06-1.57) | NA | 495/495 (100) | 103 | 1.31 (1.03-1.66) | NA | .90 |
| Body fat, % | |||||||||
| Quartile 1 (<37.1) | 293/1247 (23.5) | 37 | 1 [Reference] | .16 | 132/477 (27.7) | 23 | 1 [Reference] | .16 | .16 |
| Quartile 2 (37.1-41.9) | 311/1247 (24.9) | 35 | 1.19 (0.74-1.91) | 114/477 (23.9) | 21 | 1.05 (0.57-1.93) | |||
| Quartile 3 (42.0-46.5) | 322/1247 (25.8) | 36 | 1.04 (0.65-1.66) | 115/477 (24.1) | 22 | 1.34 (0.74-2.44) | |||
| Quartile 4 (>46.5) | 321/1247 (25.7) | 50 | 1.52 (0.98-2.35) | 116/477 (24.3) | 31 | 1.78 (1.03-3.09) | |||
| Per 5.0 U | 1247/1247 (100) | 158 | 1.09 (0.97-1.22) | NA | 477/477 (100) | 97 | 1.17 (1.02-1.34) | NA | .34 |
| FMI | |||||||||
| Quartile 1 (<9.9) | 294/1247 (23.6) | 35 | 1 [Reference] | .03 | 129/476 (27.1) | 19 | 1 [Reference] | .04 | .41 |
| Quartile 2 (9.9-12.8) | 314/1247 (25.2) | 36 | 1.18 (0.73-1.89) | 119/476 (25.0) | 27 | 1.38 (0.75-2.53) | |||
| Quartile 3 (12.9-16.5) | 316/1247 (25.3) | 38 | 1.06 (0.66-1.69) | 113/476 (23.7) | 23 | 1.79 (0.97-3.33) | |||
| Quartile 4 (>16.5) | 323/1247 (25.9) | 50 | 1.54 (0.98-2.41) | 115/476 (24.2) | 28 | 1.78 (0.99-3.23) | |||
| Per 5.0 U | 1247/1247 (100) | 159 | 1.19 (1.02-1.39) | NA | 476/476 (100) | 97 | 1.23 (1.01-1.49) | NA | .79 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); FMI, fat mass index (calculated as fat mass in kilograms divided by height in meters squared); HR, hazard ratio; NA, not applicable.
Hazard ratios and 95% CIs were adjusted for age, income, smoking status, tumor stage, and type of surgery.
Table 3. Association of Body Size, Body Fat Distribution, and Body Composition With All-Cause Mortality by Menopausal Status Among Black Breast Cancer Survivors.
| Variable | Premenopausal | Postmenopausal | P value for interaction | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Participants, No./total No. (%) | Total deaths | HR (95% CI)a | P value for trend | Participants, No./total No. (%) | Total deaths | HR (95% CI)a | P value for trend | ||
| Baseline BMI | |||||||||
| Normal (18.5-24.9) | 140/730 (19.2) | 19 | 1 [Reference] | .11 | 161/1159 (13.9) | 28 | 1 [Reference] | .11 | .37 |
| Overweight (25.0-29.9) | 211/730 (28.9) | 22 | 0.69 (0.37-1.29) | 317/1159 (27.3) | 42 | 1.14 (0.70-1.85) | |||
| Obese class 1 (30.0-34.9) | 187/730 (25.6) | 29 | 1.03 (0.56-1.89) | 314/1159 (27.1) | 46 | 1.22 (0.76-1.97) | |||
| Obese class 2-3 (≥35.0) | 192/730 (26.3) | 35 | 1.33 (0.75-2.37) | 367/1159 (31.7) | 65 | 1.26 (0.80-1.99) | |||
| Per 5.0 U | 730/730 (100) | 105 | 1.13 (1.00-1.29) | NA | 1159/1159 (100) | 181 | 1.09 (0.99-1.21) | NA | .54 |
| Waist circumference, cm | |||||||||
| Quartile 1 (<90.6) | 236/718 (32.9) | 33 | 1 [Reference] | .17 | 222/1139 (19.5) | 27 | 1 [Reference] | .19 | .49 |
| Quartile 2 (90.6-100.1) | 169/718 (23.5) | 13 | 0.54 (0.28-1.03) | 296/1139 (26.0) | 34 | 1.31 (0.78-2.19) | |||
| Quartile 3 (100.2-110.9) | 162/718 (22.6) | 23 | 0.88 (0.50-1.52) | 304/1139 (26.7) | 37 | 1.14 (0.69-1.88) | |||
| Quartile 4 (>110.9) | 151/718 (21.0) | 33 | 1.55 (0.93-2.59) | 317/1139 (27.8) | 74 | 2.12 (1.35-3.31) | |||
| Per 10.0 cm | 718/718 (100) | 102 | 1.10 (0.98-1.24) | NA | 1139/1139 (100) | 172 | 1.17 (1.07-1.29) | NA | .49 |
| Waist-to-hip ratio | |||||||||
| Quartile 1 (0.65-0.84) | 261/718 (36.3) | 29 | 1 [Reference] | .03 | 200/1139 (17.6) | 19 | 1 [Reference] | .02 | .05 |
| Quartile 2 (0.85-0.89) | 173/718 (24.1) | 30 | 1.14 (0.67-1.96) | 291/1139 (25.5) | 29 | 0.99 (0.55-1.78) | |||
| Quartile 3 (0.90-0.95) | 167/718 (23.3) | 24 | 1.03 (0.58-1.82) | 300/1139 (26.3) | 49 | 1.82 (1.06-3.12) | |||
| Quartile 4 (>0.95) | 117/718 (16.3) | 19 | 1.02 (0.56-1.89 | 348/1139 (30.6) | 75 | 2.15 (1.28-3.61) | |||
| Per 0.10 U | 718/718 (100) | 102 | 1.03 (0.791.34) | NA | 1139/1139 (100) | 172 | 1.47 (1.22-1.77) | NA | .06 |
| Body fat, % | |||||||||
| Quartile 1 (<37.1) | 214/697 (30.7) | 26 | 1 [Reference] | .16 | 226/1068 (21.2) | 35 | 1 [Reference] | .16 | .37 |
| Quartile 2 (37.1-41.9) | 184/697 (26.4) | 26 | 1.11 (0.63-1.95) | 251/1068 (23.5) | 30 | 1.13 (0.68-1.87) | |||
| Quartile 3 (42.0-46.5) | 148/697 (21.2) | 16 | 0.86 (0.45-1.65) | 299/1068 (28.0) | 42 | 1.18 (0.74-1.87) | |||
| Quartile 4 (>46.5) | 151/697 (21.7) | 32 | 1.66 (0.97-2.86) | 292/1068 (27.3) | 50 | 1.45 (0.92-2.27) | |||
| Per 5.0 U | 697/697 (100) | 100 | 1.09 (0.95-1.25) | NA | 1068/1068 (100) | 157 | 1.11 (0.99-1.24) | NA | .91 |
| FMI | |||||||||
| Quartile 1 (<9.9) | 204/697 (29.3) | 23 | 1 [Reference] | .03 | 231/1067 (21.6) | 32 | 1 [Reference] | .04 | .56 |
| Quartile 2 (9.9-12.8) | 182/697 (26.1) | 25 | 1.21 (0.68-2.16) | 262/1067 (24.6) | 38 | 1.20 (0.74-1.92) | |||
| Quartile 3 (12.9-16.5) | 145/697 (20.8) | 20 | 1.23 (0.66-2.29) | 297/1067 (27.8) | 41 | 1.18 (0.74-1.89) | |||
| Quartile 4 (>16.5) | 166/697 (23.8) | 32 | 1.70 (0.98-2.95) | 277/1067 (26.0) | 47 | 1.44 (0.91-2.29) | |||
| Per 5.0 U | 697/697 (100) | 100 | 1.20 (0.99-1.45) | NA | 1067/1067 (100) | 158 | 1.16 (0.99-1.36) | NA | .72 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); FMI, fat mass index (calculated as fat mass in kilograms divided by height in meters squared); HR, hazard ratio; NA, not applicable.
Hazard ratios and 95% CIs adjusted for age, income, smoking, tumor stage, tumor subtype, and type of surgery.
Table 4. Association of Body Size, Body Fat Distribution, and Body Composition With All-Cause Mortality by Age Among Black Breast Cancer Survivorsa.
| Variable | Age ≤45 y | Age 46-59 y | Age ≥60 y | P value for interaction |
|---|---|---|---|---|
| Baseline BMI | ||||
| Participants, No. | 415 | 878 | 596 | .91 |
| Total deaths | 68 | 121 | 97 | |
| HR (95% CI) per 5.0 U | 1.22 (1.01-1.49) | 1.06 (0.94-1.19) | 1.14 (0.99-1.31) | NA |
| Waist circumference, cm | ||||
| Participants, No. | 409 | 868 | 580 | .16 |
| Total deaths | 66 | 118 | 90 | |
| HR (95% CI) per 10.0 cm | 1.16 (0.97-1.40) | 1.07 (0.96-1.19) | 1.28 (1.12-1.46) | NA |
| Waist-to-hip ratio | ||||
| Participants, No. | 409 | 868 | 580 | .01 |
| Total deaths | 66 | 118 | 90 | |
| HR (95% CI) per 0.10 U | 0.90 (0.65-1.25) | 1.25 (0.97-1.60) | 1.76 (1.37-2.26) | NA |
| Body fat, % | ||||
| Participants, No. | 397 | 825 | 543 | .21 |
| Total deaths | 63 | 108 | 86 | |
| HR (95% CI) per 5.0 U | 1.13 (0.93-1.37) | 1.01 (0.89-1.14) | 1.20 (1.00-1.40) | NA |
| FMI | ||||
| Participants, No. | 397 | 826 | 541 | .56 |
| Total deaths | 63 | 109 | 86 | |
| HR (95% CI) per 5.0 U | 1.35 (1.0-1.80) | 1.06 (0.89-1.26) | 1.26 (1.02-1.57) | NA |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); FMI, fat mass index (calculated as fat mass in kilograms divided by height in meters squared); HR, hazard ratio; NA, not applicable.
Hazard ratios and 95% CIs were adjusted for age, income, smoking status, tumor stage, tumor subtype, and type of surgery.
Discussion
To our knowledge, this cohort study is the first to specifically focus on evaluating the association of measures of body fat distribution and body composition with mortality outcomes among Black breast cancer survivors, who have a disproportionate prevalence of excess body fat and an increased risk of more aggressive disease and breast cancer–associated death. We found that Black women with excess body fat were at higher risk of both death from all causes and breast cancer–specific death. The risk was more substantial for WHR and less substantial for BMI. To further explore the association between BMI and mortality, we conducted sensitivity analyses by repeating BMI analyses and excluding women who had central obesity but normal BMI because they have a higher risk of metabolic dysregulation and death29; therefore, including these women in the reference group may have distorted results. However, findings were essentially unchanged.
The few studies evaluating the association of obesity with mortality among Black breast cancer survivors reported no significant association between BMI before diagnosis and breast cancer–specific and/or all-cause mortality.30,31,32 Only 1 of these studies30 evaluated the association with mortality by estrogen receptor status, and findings suggested a decreased risk of all-cause and breast cancer–specific death among those who were estrogen receptor negative; however, 95% CIs included the null value. In the present study, there was increased all-cause mortality among those with a higher BMI, but a similar increased risk was observed by estrogen receptor status. Body mass index may not fully capture variations in fat and lean mass among women. For a given BMI, Black women typically have higher lean mass and lower fat mass than White women.15 Therefore, among Black women, waist circumference, WHR, FMI, and percent body fat may reflect adiposity better than BMI, which may explain why results were less substantial for BMI. Only the California Breast Cancer Survivorship Consortium32 has also evaluated WHR and, consistent with the present results, the consortium reported that Black women with a higher WHR had worse overall survival.
A number of possible physiological mechanisms underlying the association of excess body adiposity with cancer progression have been proposed, including high circulating insulin and/or insulinlike growth factor 1, altered adipokine levels (ie, increased leptin and decreased adiponectin), and systemic and tissue-level inflammation.33,34 These factors have been reported to downregulate antitumor immunity and promote tumor angiogenesis, growth, and metastasis.35 Consistent with the current findings, the Health, Eating, Activity, and Lifestyle (HEAL) study36 also found that a high WHR was associated with increases in all-cause and breast cancer–specific mortality; insulin resistance and inflammation appeared to mediate this association. However, the HEAL study included mostly White women; therefore, potential mechanisms among Black women warrant further investigation.
Strengths and Limitations
This study has several strengths. This prospective analysis of a cohort of Black breast cancer survivors from a large geographic area was specifically designed to assess the association of obesity with survivorship among Black women after a diagnosis of breast cancer. A previous analysis indicated that distributions of tumor characteristics among participants were similar to those of all cases in the target area, which provided support for the external validity of the present cohort.18 Another strength is that the time frame for the assessment of body adiposity was approximately 10 months after diagnosis for all participants; this period is optimal for informing weight management strategies among cancer survivors, as most have already received chemotherapy and experienced any associated weight changes during that time.37
This study also has limitations. We had limited power to conduct more granular analyses, such as evaluating associations by tumor subtype and menopausal status or examining changes in weight and body composition, as these analyses would have included a smaller subset of participants, further limiting statistical power. Furthermore, we did not broadly consider heart disease as a potential confounder. However, we did consider hypertension as a possible confounder, as it is a major risk factor for heart disease,38 and comorbid hypertension did not explain our results. Moreover, the complex and multilevel issues associated with disparities in both obesity and cancer should be noted; these issues include neighborhood- and health care system–level factors, which have implications for access to health care and nutritious food, as well as individual socioeconomic factors, health beliefs, cultural factors, and lifestyle behaviors. Although we were able to consider a wide range of lifestyle and clinical factors in our analyses, there may be some residual confounding from unmeasured factors.
Conclusions
Although measurements using dual-energy x-ray absorptiometry or computed tomography may be ideal when evaluating the association of body composition with breast cancer prognosis, the findings of the present study suggest that simple measures of central obesity (waist circumference and WHR) and body composition (percent body fat and FMI using a portable bioelectrical impedance analysis scale) are clinically useful tools for identifying Black breast cancer survivors at higher risk of death. These findings may be particularly relevant for primary care physicians, who are typically responsible for the long-term care of breast cancer survivors, and for clinical settings with limited resources.
eTable 1. Selected Participant Characteristics by Baseline BMI
eTable 2. Clinical Characteristics by Baseline BMI
eTable 3. Associations of Body Size, Body Fat Distribution, and Body Composition With Breast Cancer–Specific Mortality by Estrogen Receptor Status Among Black Breast Cancer Survivors
eTable 4. Precision Analyses: Estimated Half-width of 95% CIs for Main Results
References
- 1.Greenlee H, Shi Z, Sardo Molmenti CL, Rundle A, Tsai WY. Trends in obesity prevalence in adults with a history of cancer: results from the US National Health Interview Survey, 1997 to 2014. J Clin Oncol. 2016;34(26):3133-3140. doi: 10.1200/JCO.2016.66.4391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Islami F, Goding Sauer A, Miller KD, et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J Clin. 2018;68(1):31-54. doi: 10.3322/caac.21440 [DOI] [PubMed] [Google Scholar]
- 3.Lee K, Kruper L, Dieli-Conwright CM, Mortimer JE. The impact of obesity on breast cancer diagnosis and treatment. Curr Oncol Rep. 2019;21(5):41. doi: 10.1007/s11912-019-0787-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Markham MJ, Wachter K, Agarwal N, et al. Clinical cancer advances 2020: annual report on progress against cancer from the American Society of Clinical Oncology. J Clin Oncol. 2020;38(10):1081. doi: 10.1200/JCO.19.03141 [DOI] [PubMed] [Google Scholar]
- 5.Chan DSM, Abar L, Cariolou M, et al. World Cancer Research Fund International: Continuous Update Project—systematic literature review and meta-analysis of observational cohort studies on physical activity, sedentary behavior, adiposity, and weight change and breast cancer risk. Cancer Causes Control. 2019;30(11):1183-1200. doi: 10.1007/s10552-019-01223-w [DOI] [PubMed] [Google Scholar]
- 6.Chan DSM, Vieira AR, Aune D, et al. Body mass index and survival in women with breast cancer—systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol. 2014;25(10):1901-1914. doi: 10.1093/annonc/mdu042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bandera EV, Fay SH, Giovannucci E, et al. ; World Cancer Research Fund International Continuous Update Project Panel . The use and interpretation of anthropometric measures in cancer epidemiology: a perspective from the World Cancer Research Fund International Continuous Update Project. Int J Cancer. 2016;139(11):2391-2397. doi: 10.1002/ijc.30248 [DOI] [PubMed] [Google Scholar]
- 8.Slawinski CGV, Barriuso J, Guo H, Renehan AG. Obesity and cancer treatment outcomes: interpreting the complex evidence. Clin Oncol (R Coll Radiol). 2020;32(9):591-608. doi: 10.1016/j.clon.2020.05.004 [DOI] [PubMed] [Google Scholar]
- 9.Caan BJ, Cespedes Feliciano EM, Prado CM, et al. Association of muscle and adiposity measured by computed tomography with survival in patients with nonmetastatic breast cancer. JAMA Oncol. 2018;4(6):798-804. doi: 10.1001/jamaoncol.2018.0137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bradshaw PT, Cespedes Feliciano EM, Prado CM, et al. Adipose tissue distribution and survival among women with nonmetastatic breast cancer. Obesity (Silver Spring). 2019;27(6):997-1004. doi: 10.1002/oby.22458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peltz G, Aguirre MT, Sanderson M, Fadden MK. The role of fat mass index in determining obesity. Am J Hum Biol. 2010;22(5):639-647. doi: 10.1002/ajhb.21056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization . Waist circumference and waist-hip ratio: report of a WHO expert consultation. May 16, 2011. Accessed January 6, 2021. https://www.who.int/publications/i/item/9789241501491
- 13.Sung H, Siegel RL, Torre LA, et al. Global patterns in excess body weight and the associated cancer burden. CA Cancer J Clin. 2019;69(2):88-112. [DOI] [PubMed] [Google Scholar]
- 14.World Cancer Research Fund International . Diet, nutrition, physical activity and breast cancer survivors. Continuous Update Project, World Cancer Research Fund International; 2014. Accessed January 15, 2021. https://www.wcrf.org/wp-content/uploads/2021/03/Breast-Cancer-Survivors-2014-Report.pdf
- 15.Flegal KM, Shepherd JA, Looker AC, et al. Comparisons of percentage body fat, body mass index, waist circumference, and waist-stature ratio in adults. Am J Clin Nutr. 2009;89(2):500-508. doi: 10.3945/ajcn.2008.26847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bandera EV, Maskarinec G, Romieu I, John EM. Racial and ethnic disparities in the impact of obesity on breast cancer risk and survival: a global perspective. Adv Nutr. 2015;6(6):803-819. doi: 10.3945/an.115.009647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ambrosone CB, Ciupak GL, Bandera EV, et al. Conducting molecular epidemiological research in the age of HIPAA: a multi-institutional case-control study of breast cancer in African-American and European-American women. J Oncol. 2009;2009:871250. doi: 10.1155/2009/871250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bandera EV, Demissie K, Qin B, et al. The Women’s Circle of Health Follow-Up Study: a population-based longitudinal study of Black breast cancer survivors in New Jersey. J Cancer Surviv. 2020;14(3):331-346. doi: 10.1007/s11764-019-00849-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khan NAJ, Tirona M. An updated review of epidemiology, risk factors, and management of male breast cancer. Med Oncol. 2021;38(4):39. [DOI] [PubMed] [Google Scholar]
- 20.Bandera EV, Chandran U, Zirpoli G, et al. Body fatness and breast cancer risk in women of African ancestry. BMC Cancer. 2013;13:475. doi: 10.1186/1471-2407-13-475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pickering TG, Hall JE, Appel LJ, et al. ; Council on High Blood Pressure Research Professional and Public Education Subcommittee, American Heart Association . Recommendations for blood pressure measurement in humans: an AHA scientific statement from the Council on High Blood Pressure Research Professional and Public Education Subcommittee. J Clin Hypertens (Greenwich). 2005;7(2):102-109. doi: 10.1111/j.1524-6175.2005.04377.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cleveland RJ, Eng SM, Abrahamson PE, et al. Weight gain prior to diagnosis and survival from breast cancer. Cancer Epidemiol Biomarkers Prev. 2007;16(9):1803-1811. doi: 10.1158/1055-9965.EPI-06-0889 [DOI] [PubMed] [Google Scholar]
- 23.International Agency for Research on Cancer, World Health Organization . Weight Control and Physical Activity. IARC Press;2002. IARC Handbooks of Cancer Prevention; vol 6. [Google Scholar]
- 24.Qin B, Llanos AAM, Lin Y, et al. Validity of self-reported weight, height, and body mass index among African American breast cancer survivors. J Cancer Surviv. 2018;12(4):460-468. doi: 10.1007/s11764-018-0685-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fine JP, Gray RJ. A proportional hazards model for subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496-509. doi: 10.1080/01621459.1999.10474144 [DOI] [Google Scholar]
- 26.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1(1):43-46. doi: 10.1097/00001648-199001000-00010 [DOI] [PubMed] [Google Scholar]
- 27.Lash TL, VanderWeele TJ, Haneuse S, Rothman KJ. Fundamentals of epidemiologic data analysis. In: Modern Epidemiology. 4th ed. Lippincott Williams & Wilkins; 2021:392-393. [Google Scholar]
- 28.Brownstein NC, Cai J. Tests of trend between disease outcomes and ordinal covariates discretized from underlying continuous variables: simulation studies and applications to NHANES 2007-2008. BMC Med Res Methodol. 2019;19(1):2. doi: 10.1186/s12874-018-0630-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oliveros E, Somers VK, Sochor O, Goel K, Lopez-Jimenez F. The concept of normal weight obesity. Prog Cardiovasc Dis. 2014;56(4):426-433. doi: 10.1016/j.pcad.2013.10.003 [DOI] [PubMed] [Google Scholar]
- 30.Lu Y, Ma H, Malone KE, et al. Obesity and survival among Black women and White women 35 to 64 years of age at diagnosis with invasive breast cancer. J Clin Oncol. 2011;29(25):3358-3365. doi: 10.1200/JCO.2010.34.2048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Conroy SM, Maskarinec G, Wilkens LR, White KK, Henderson BE, Kolonel LN. Obesity and breast cancer survival in ethnically diverse postmenopausal women: the Multiethnic Cohort Study. Breast Cancer Res Treat. 2011;129(2):565-574. doi: 10.1007/s10549-011-1468-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kwan ML, John EM, Caan BJ, et al. Obesity and mortality after breast cancer by race/ethnicity: the California Breast Cancer Survivorship Consortium. Am J Epidemiol. 2014;179(1):95-111. doi: 10.1093/aje/kwt233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iyengar NM, Gucalp A, Dannenberg AJ, Hudis CA. Obesity and cancer mechanisms: tumor microenvironment and inflammation. J Clin Oncol. 2016;34(35):4270-4276. doi: 10.1200/JCO.2016.67.4283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiralerspong S, Goodwin PJ. Obesity and breast cancer prognosis: evidence, challenges, and opportunities. J Clin Oncol. 2016;34(35):4203-4216. doi: 10.1200/JCO.2016.68.4480 [DOI] [PubMed] [Google Scholar]
- 35.Picon-Ruiz M, Morata-Tarifa C, Valle-Goffin JJ, Friedman ER, Slingerland JM. Obesity and adverse breast cancer risk and outcome: mechanistic insights and strategies for intervention. CA Cancer J Clin. 2017;67(5):378-397. doi: 10.3322/caac.21405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.George SM, Bernstein L, Smith AW, et al. Central adiposity after breast cancer diagnosis is related to mortality in the Health, Eating, Activity, and Lifestyle study. Breast Cancer Res Treat. 2014;146(3):647-655. doi: 10.1007/s10549-014-3048-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anderson AS, Martin RM, Renehan AG, et al. ; UK NIHR Cancer and Nutrition Collaboration (Population Health Stream). Cancer survivorship, excess body fatness and weight-loss intervention—where are we in 2020? Br J Cancer. 2020;124(6):1057-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e596-e646. doi: 10.1161/CIR.0000000000000678Circulation [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Selected Participant Characteristics by Baseline BMI
eTable 2. Clinical Characteristics by Baseline BMI
eTable 3. Associations of Body Size, Body Fat Distribution, and Body Composition With Breast Cancer–Specific Mortality by Estrogen Receptor Status Among Black Breast Cancer Survivors
eTable 4. Precision Analyses: Estimated Half-width of 95% CIs for Main Results


