Abstract
The purpose of this study was to evaluate oxygen-enhanced pulmonary imaging at 0.55T with 3D stack-of-spirals ultrashort-TE (UTE) acquisition. Oxygen-enhanced pulmonary MRI offers the measurement of regional lung ventilation and perfusion using inhaled oxygen as a contrast agent. Low-field MRI systems equipped with contemporary hardware can provide high-quality structural lung imaging by virtue of the prolonged T2*. Fortuitously, the T1 relaxivity of oxygen increases at lower field strengths, which is expected to improve the sensitivity of oxygen-enhanced lung MRI. We implemented a breath-held T1-weighted 3D stack-of-spirals UTE acquisition with a 7ms spiral-out readout. Measurement repeatability was assessed using five repetitions of oxygen-enhanced lung imaging in healthy volunteers (n=7). The signal intensity at both normoxia and hyperoxia was strongly dependent on lung tissue density modulated by breath-hold volume during the 5 repetitions. A voxel-wise correction for lung tissue density improved the repeatability of percent signal enhancement maps (coefficient of variation = 34 ± 16%). Percent signal enhancement maps were compared in 15 healthy volunteers and 10 patients with lymphangioleiomyomatosis (LAM), a rare cystic disease known to reduce pulmonary function. We measured a mean percent signal enhancement of 9.0 ± 3.5% at 0.55T in healthy volunteers, and reduced signal enhancement in patients with LAM (5.4 ± 4.8%, p = 0.02). The heterogeneity, estimated by the percentage of lung volume exhibiting low enhancement, was significantly increased in patients with LAM compared with healthy volunteers (11.1 ± 6.0% vs 30.5 ± 13.1%, p = 0.01), illustrating the capability to measure regional functional deficits.
Keywords: low-field MRI, lung, oxygen-enhanced MRI, lung function, LAM
Graphical Abstract

1. INTRODUCTION
MR imaging is an attractive method for combined assessment of lung structure and regional function 1, 2. Regional ventilation imaging by means of hyperpolarized 3He and 129Xe gases has been widely used to assess ventilation defects in patients 3–6. However, hyperpolarized gas imaging requires expensive equipment and institutional experience. Oxygen-enhanced MRI is an accessible alternative to evaluate regional lung function, which uses inhaled 100% oxygen as a contrast agent 7. Due to the T1 relaxivity properties of paramagnetic oxygen, inhaled 100% oxygen shortens tissue T1; thereby increasing signal intensity on T1-weighted images, compared with inhalation of room air. The regional signal enhancement represents a combination of ventilation and perfusion 8, and is calculated via subtraction of images acquired during hyperoxia (100% oxygen) and normoxia (room air, 21% oxygen). Oxygen-enhanced functional MRI has shown promise for assessing regional ventilation and perfusion deficits in several lung diseases 9–12.
Recently, we described a 0.55T MRI configuration with contemporary hardware and software which offers opportunities for lung imaging 13, 14. This low-field MRI configuration provides improved B0 field homogeneity, and associated prolonged T2*. Lung parenchymal T2* is approximately 10ms at 0.55T, compared with T2* ≤ 2ms at 1.5T 1, resulting in reduced image artifact from susceptibility gradients in the lungs. Coincidentally, T1 relaxivity of paramagnetic oxygen is also higher at low field strengths 15. We previously showed that the increased T1 relaxivity of oxygen at 0.55T (r1 = 4.7e-4 mmHg−1s−1 at 0.55T vs. r1 = 3.3e-4 mmHg−1s−1 at 1.5T) results in more T1 shortening in the lung parenchyma between normoxia and hyperoxia, compared with 1.5T 14. We sought to exploit this property of lower field MRI for oxygen-enhanced functional lung imaging.
Inversion-recovery turbo spin echo acquisitions have most commonly been used for oxygen enhanced lung imaging 7, 16, 17. More recent studies have implemented radial ultra-short-TE (UTE) imaging for time-efficient whole-lung coverage oxygen-enhanced imaging at 1.5T 11, 18. At 0.55T, spiral acquisitions are attractive because of their SNR-efficiency, and because the B0 uniformity and prolonged T2* at 0.55T reduces off-resonance blurring compared to higher field strengths 19. Therefore, we propose to use breath-held stack-of-spirals 3D imaging for oxygen-enhanced MRI at 0.55T. To our knowledge, spiral UTE has not been employed for oxygen-enhanced MRI at 1.5T or 3T. In addition to oxygen enhancement related to regional ventilation and perfusion, parenchymal signal intensity is also modulated by changes in lung tissue density during respiration, and therefore is strongly dependent on breath-hold position 20. The repeatability of oxygen-enhanced functional MRI with variable breath-hold position is not well characterized.
The purposes of this study were: 1. To implement a breath-held three-dimensional stack-of-spirals UTE sequence for oxygen-enhanced imaging at 0.55T, 2. to assess the magnitude, heterogeneity, and repeatability of signal enhancement using this method, and 3. to explore the utility of this technique at 0.55T by comparing patients with lymphangioleoimyomatosis (LAM) to healthy volunteers.
2. MATERIALS AND METHODS
2.1. Patient population
Imaging of research subjects was approved prospectively by our local Institutional Review Board (clinicaltrials.gov NCT03331380). All subjects consented in writing. Fifteen healthy volunteers (aged 33±14 years, 7 women) and ten patients with LAM (age 46±8 years, 10 women) underwent oxygen-enhanced functional MRI at 0.55T. LAM is a rare cystic lung disease that primarily affects women. LAM is known to cause reduced global pulmonary function and regional ventilation/perfusion deficits 21–23. Patients with LAM underwent pulmonary function tests (spirometry and gas diffusing capacity) within 2 days of the MRI exam, performed according to American Thoracic Society guidelines. Standard measurements of forced expiratory volume in 1 second (FEV1) and diffusing capacity of lung for carbon monoxide (DLCO) were reported from pulmonary function tests.
2.2. Imaging system and methods
We modified a commercial 1.5 T system to operate at 0.55T (prototype MAGNETOM Aera, Siemens Healthcare, Erlangen, Germany). This system configuration uses a superconducting closed-bore magnet design, and the receiver architecture and gradient specifications (maximum amplitude = 45mT/m, maximum slew rate = 200T/m/s) were maintained from the commercial system. A six-channel body receiver array coil and an eighteen-channel spine receiver array coil were modified to operate at 0.55T for chest imaging.
We used a prototype 3D stack-of-spirals T1-weighted UTE sequence for oxygen-enhanced functional imaging (spoiled gradient echo, TE/TR = 0.15/8.54ms, spiral-out readout = 7ms, 16 spiral interleaves per kz, flip angle = 17°, FOV = 450mm x 450mm x 320mm, matrix = 128×128, 32 coronal slices, spatial resolution = 3.5mm x 3.5 mm x 10mm, bandwidth = 975Hz/Px, signal averages = 2, scan time = 9 s) 24. Signal acquisition used a 7ms constant density spiral-out readout, and reconstruction was performed using the measured gradient system impulse response function for trajectory correction 25. The nine second breath-held acquisition provided volumetric coverage of the lungs.
2.3. Percent oxygen-enhancement maps
Oxygen was inhaled at 15L/min through a non-rebreather face mask, and hyperoxia images were acquired after three minutes of equilibration. Normoxia images were registered to hyperoxia images using a b-spline 3D non-rigid registration algorithm 26. The lung parenchyma, excluding major vasculature, was segmented from registered images by a semi-automated active contour segmentation, corrected manually. Registration and segmentation were performed in MATLAB R2020a (Mathworks, Natick, MA).
Previous studies have demonstrated that parenchymal signal intensity varies with lung volume, due to changes in lung tissue density, which is approximated using a “sponge model” for MRI and CT 20, 27. When the lung is inflated during inspiration, tissue density is lower, resulting in lower signal intensity compared with expiration. Therefore, differences in lung tissue density can impact the measured signal enhancement between normoxia and hyperoxia. Percent signal enhancement (PSE) maps were calculated twice, with and without correction for tissue density:
- “Uncorrected PSE map”: PSE maps were calculated by the subtraction of normoxia images () from hyperoxia images (), normalized to the normoxia images, following image registration (Eq. 1).
Eq. 1 - “Corrected PSE map”: Zha et al proposed voxel-wise correction for differing lung density using a sponge model that assumes conservation of mass between inspiration and expiration 11. The deformation fields calculated during 3D image registration were used to estimate local changes in tissue density. The Jacobian determinant of the deformation fields () approximates the expansion () or contraction () of an individual voxel between the two states. Local signal intensity is scaled by to correct for changes in tissue density (Eq. 2).
Eq. 2
2.4. Measurements of oxygen-enhancement and heterogeneity in healthy volunteers and patients
Mean PSE in the lungs of healthy volunteers and patients was used to represent the global response to hyperoxia. Mean PSE was measured across the central 5 slices (50mm). An anterior-posterior gradient in PSE has been previously reported, and we selected the central 50mm to be representative of the lung 11. The percentage of low-enhancement voxels was used as a surrogate for regional functional deficits. A histogram of lung PSE with limits of −5% to 30% was divided into 10 equally spaced bins, and image voxels in the lowest intensity bin were labeled as “low-enhancement”. We compared the mean PSE and the low enhancement percent in patients with LAM and healthy volunteers using unpaired Student’s t-tests.
2.5. Repeatability of oxygen-enhancement mapping
To assess the within-session repeatability of the oxygen-enhancement measurements, five repetitions of 3D UTE images were acquired during normoxia and five repetitions of 3D UTE images were acquired during hyperoxia (Figure 1). Each 3D UTE image was acquired in a separate breath-hold at end-inspiration. Repeatability was assessed in seven of the healthy volunteers.
Figure 1:
Schematic diagram of repeatability study. Five repetitions of normoxia and hyperoxia imaging are used to generate five PSE maps by pairing acquisitions chronologically. Oxygen (100%) is administered at 15L/min using a non-rebreather face mask. Repeatability of PSE mapping was assessed in 7 healthy volunteers.
We evaluated the relationship between lung volume (ie. lung tissue density) and lung parenchymal signal intensity at normoxia and hyperoxia using each repetition. The five pairs of normoxia and hyperoxia images, paired chronologically, were used to assess PSE mapping repeatability. Both uncorrected PSE maps (Eq 1) and corrected PSE maps (Eq 2) were computed. We expect higher repeatability from the corrected PSE maps due to the local correction for lung tissue density. Repeatability of mean PSE and low-enhancement percent was measured using the coefficient of variation (CV = standard deviation/mean*100%), Bland-Altman analysis, and intraclass correlation coefficient (ICC). Bland-Altman analysis included only the first two measurements, whereas CV and ICC used all measurement repetitions.
3. RESULTS
3.1. Stack-of-spirals UTE imaging
Figure 2 provides example images from 0.55T generated using 3D stack-of-spirals UTE imaging during normoxia (Figure 2A) and hyperoxia (Figure 2B) in a healthy volunteer. This 9s breath-held acquisition generated high-quality 3D UTE images that provided visualization of the lung parenchyma and large vasculature without observable image blurring caused by the spiral readout. A histogram analysis (Figure 2C) shows the signal enhancement in the lungs between normoxia and hyperoxia. The resulting PSE maps (Figure 2D) show regionally uniform signal enhancement in six coronal slices in the lung of a healthy volunteer.
Figure 2:
3D stack-of-spirals UTE images from 0.55T shown in 6 slices in a healthy volunteer at A) normoxia and B) hyperoxia. Images have been scaled for lung visualization. C) Lung signal intensity histograms demonstrate signal enhancement on T1-weighted imaging with hyperoxia, which results in signal enhancement visible in PSE maps (D).
3.2. Repeatability of PSE mapping
Signal intensity variation with lung tissue density was evident from repeated measurement of UTE imaging within an imaging session. Figure 3 shows the mean signal intensity versus breath-hold lung volume during normoxia and hyperoxia in seven healthy volunteers. Signal intensity during hyperoxia was consistently higher than during normoxia for equivalent lung volume, as expected. The within-subject variability in inspiratory breath-held lung volume was 12 ± 8% (range 5% to 24%) across healthy volunteers, which resulted in signal intensity variation of 10 ± 6% (range 5% to 25%). This variability in signal intensity illustrates the importance of compensating for lung tissue density when calculating PSE maps.
Figure 3:
Signal intensity versus breath-held lung inflation volume plotted from five repeated measurements in seven healthy volunteers. Signal intensity varies with lung tissue density; however, hyperoxia signal intensity is consistently higher than normoxia for equivalent lung volume within an individual for a given breath-hold volume.
Figure 4 provides single-slice PSE maps in one healthy volunteer across five repetitions of oxygen-enhanced imaging calculated with and without correction (Eqs. 1 and 2). Poor repeatability is evident using the uncorrected PSE map calculation (Figure 4A, CV = 140%, mean PSE range = −9% to 21%). PSE maps generated from images with inconsistent breath holds, where lung volume between normoxia and hyperoxia differed substantially (ΔVolume>10%, outlined in red), are clear outliers (mean PSE = −9% and 32%). The application of to compensate changes in lung tissue density (Eq. 2) and excluding measurements with ΔVolume >10% improved the repeatability of PSE mapping in this example healthy volunteer (Figure 4B, CV = 28%, mean PSE range = 6% to 11%).
Figure 4:
Five repetitions of percent oxygen enhancement mapping (1 slice) illustrated in one healthy volunteer A) using uncorrected PSE mapping (Eq. 1) and B) using corrected PSE mapping (Eq. 2). The poor repeatability of the uncorrected PSE maps (A) is most evident from Repetitions # 2 and #5. These two data sets have Δvolume >10% and are outlined in red. Local compensation for lung tissue density (Eq. 2) generates more consistent PSE maps (B). Coefficients of variation in this volunteer were 140% for uncorrected PSE maps, and 28% following pixel-wise correction. MPSE = mean percent signal enhancement, LEP = low enhancement percent
Table 1 summarizes the CV, Bland-Altman bias and limits of agreement, and ICC repeatability assessments of mean PSE and low enhancement percent. Across seven healthy volunteers, mean PSE CV was reduced from 68 ± 42% to 34 ± 16%, on average, by applying the det(JT) correction and excluding cases with ΔVolume>10%. The Bland-Altman 95% limits of agreement were reduced from 27.1% to 5.98%, and ICC was improved from 0.46 to 0.82 for measured mean PSE using the det(JT) correction. Bland-Altman plots are provided in Supplementary Figure 1. In addition, the variation of the measured low enhancement percent was reduced from CV = 85 ± 45% to CV = 41 ± 17%. These results indicate that voxel-wise correction improves the measurement repeatability by compensating signal intensity changes from differing lung tissue density.
Table 1:
Repeatability assessment of uncorrected and corrected mean percent signal enhancement (PSE) measurements and low enhancement percent measurements using intra-study repeated measured from 7 healthy volunteers. The coefficients of variation and intraclass correlation coefficient (ICC) are calculated based on all measurements in each volunteer, whereas the Bland-Altman bias is calculated from only the first 2 measurements.
| Coefficient of variation | Bland-Altman bias (bias ± 95% limits of agreement) | ICC | ||
|---|---|---|---|---|
| Uncorrected PSE mapping (Eqn 1) | Mean PSE | 68 ± 42 % | −2.21 ± 27.1 % | 0.46 |
| Low enhancement percent | 85 ± 45 % | 5.83 ± 70.1 % | 0.17 | |
| Corrected PSE mapping (Eqn 2) | Mean PSE | 34 ± 16 % | −0.81 ± 5.98 % | 0.82 |
| Low enhancement percent | 41 ± 18 % | 2.36 ± 16.8 % | 0.66 |
3.3. Oxygen enhancement in healthy volunteers and patients with LAM
Figure 5 provides corrected PSE maps in a single slice from five example healthy volunteers and five example patients with LAM. Overall, the signal enhancement is similar between healthy volunteers and the PSE maps are spatially homogenous. We measured mean PSE of 9.0 ± 3.5% using 3D stack-of-spirals UTE imaging in healthy volunteers (n = 15). This is higher than previous reports using 3D radial UTE at 1.5T (6.6 ± 1.8%) 18, which is expected due to the increased T1 relaxivity of oxygen at 0.55T compared with 1.5T. Low enhancement regions were scarce in the healthy volunteers, as expected (low enhancement percent = 11.1 ± 6.0%).
Figure 5:
Corrected PSE maps in a single slice for (A) five different healthy volunteers and (B) five different patients with LAM. Healthy volunteers demonstrate homogenous regional signal enhancement across subjects, whereas reduced mean PSE and regional functional deficits are evident in patients with LAM. MPSE = mean percent signal enhancement, LEP = low enhancement percent
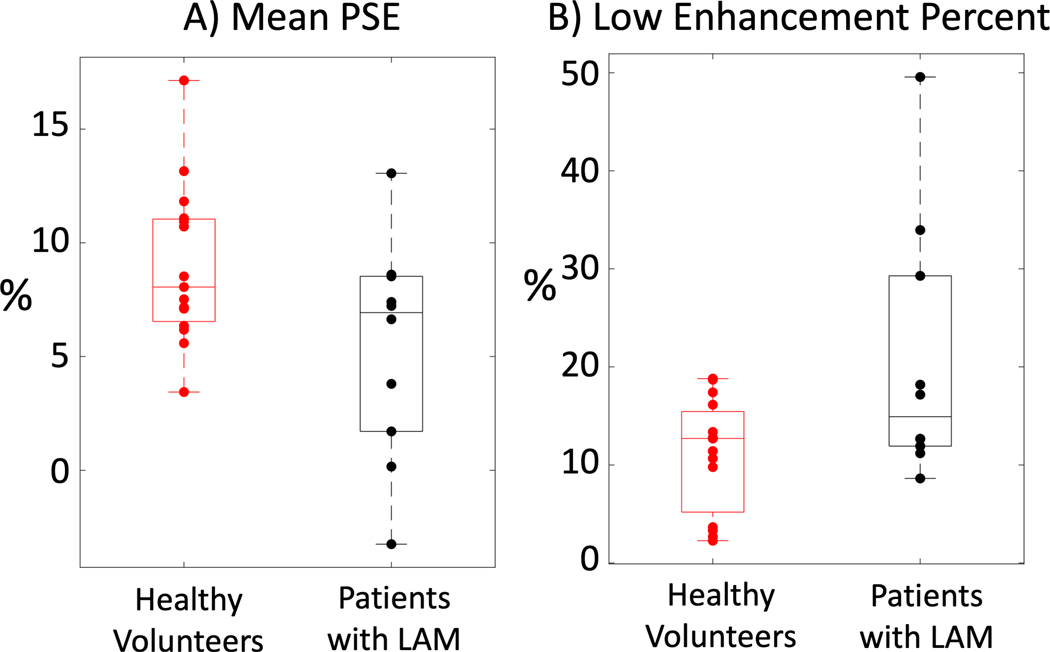
Compared with healthy volunteers, patients with LAM (Figure 5B) show reduced PSE and increased heterogeneity with clear regions of functional deficits. Clinical pulmonary function tests indicated that both FEV1 and DLCO were impaired in the 10 patients with LAM (FEV1 = 72.6± 14.6%, DLCO = 60.2 ± 14.9%). Mean PSE was significantly lower in patients with LAM (5.4 ± 4.8%, n = 10, p = 0.02) compared with healthy volunteers, and there was a significantly higher percentage of low enhancement voxels in patients (20.5 ± 13.1%, p = 0.01) compared with healthy volunteers. Box plots comparing mean PSE and low enhancement percent in healthy volunteers and patients with LAM are provided in Figure 6 and the results are summarized in Table 2.
Figure 6:
Box-and-whisker plots comparing A) mean PSE and B) low enhancement percent between 15 healthy volunteers and 10 patients with LAM. Both parameters were significantly different (p<0.05) between the healthy volunteer and patient groups.
Table 2:
Summary of oxygen-enhancement in healthy volunteers and patients with LAM. Mean percent signal enhancement (PSE) and low-enhancement percent were calculated in both populations. P-values from non-paired students’ t-tests are provided to compare the populations.
| Healthy Volunteers (n = 15) | Patients with LAM (n=10) | t-test p-value | |
|---|---|---|---|
| Mean PSE | 9.0 ± 3.5% | 5.4 ± 4.8% | 0.02 |
| Low enhancement percent | 11.1 ± 6.0% | 20.5 ± 13.1% | 0.01 |
4. DISCUSSION
In this study, we implemented oxygen-enhanced functional lung imaging at 0.55T to exploit the higher T1 relaxivity of oxygen at 0.55T compared with 1.5T. We used 3D stack-of-spirals UTE imaging to generate percent signal enhancement maps and compared healthy volunteers to patients with LAM. Using this technique at 0.55T, we generated high-quality spiral UTE images in a short 9s breath-hold. Reduced percent signal enhancement and increased regional heterogeneity was observed in the patients compared with healthy volunteers. In addition, the correction for lung tissue density variation with breath-hold volume was demonstrated to improve measurement repeatability.
We developed a 0.55T MRI system that combines contemporary clinical hardware and imaging methods with lower field strength. For lung imaging, the improved B0 field homogeneity of this contemporary 0.55T system design offers significant advantages. The prolonged T2* provides high-quality images of the lung parenchyma. We selected a spiral UTE acquisition to exploit the relative increase in T2* for improved SNR-efficiency. Off-resonance blurring was not observed in our study with 7ms spiral readout.
We measured more signal enhancement at 0.55T (9.0 ± 3.5%) compared to prior radial UTE acquisitions at 1.5T (6.6 ± 1.8%) 18. This is attributed to the increase in T1 relaxivity of oxygen at 0.55T. Comparisons of PSE between studies is challenging because this measurement is dependent on both imaging parameters and image processing methods. We did not pursue direct comparison between field strengths for our T1-weighted stack-of-spirals sequence because spiral imaging can generate substantial blurring at 1.5T, compared to 0.55T, due to the short T2* in the lung, and because native tissue T1 changes nonuniformly with field strength. One previous study has compared free-breathing high-resolution stack-of-spirals UTE at 1.5T and 3T with matched parameters and demonstrated inferior performance at 3T 28. Other oxygen-enhancement methods have used inversion recovery acquisitions, which can offer increased T1 weighting and higher PSE (eg. 31.8 ± 2.7% in Ohno et al 17), but these are usually 2D Cartesian turbo spin echo techniques that will have different sensitivity to blood flow and T2*.
The repeatability of oxygen-enhanced functional measurements is not well established. Reduced PSE has been used as an indication of disease using oxygen-enhanced imaging, and therefore measurement precision is important. We specifically demonstrated that the intra-study repeatability of measured PSE is dependent on breath-hold lung volume during normoxia and hyperoxia. In our study, correction of pixel-wise signal intensity using the deformation fields from image registration, originally proposed for free-breathing acquisitions by Zha et al 11, improved the repeatability of both the mean PSE and the low enhancement percent. Zha et al also assessed oxygen-enhancement repeatability between two separate imaging sessions using their free-breathing isotropic radial UTE approach and found good agreement in mean PSE. The authors reported narrower Bland-Altman limits of agreement (LOA) for mean PSE compared to our study (LOA = 1.73% in 11 vs LOA = 5.98% in our study), and slightly higher ICCs (0.90 vs 0.82). We attribute our lower repeatability to the anisotropic resolution and breath-hold position variability, or the lower SNR of breath-held acquisitions at 0.55T compared to free-breathing at 1.5T. Future work will focus on higher-resolution free-breathing protocols to reduce variability. Zha et al also reported only moderate spatial agreement of ventilation defects using the Dice coefficient using both oxygen-enhanced imaging and hyperpolarized gas imaging, indicating that regional functional assessment may be dynamic in nature 11. Regional repeatability was not assessed in our study.
We studied patients with LAM, who may have impaired pulmonary function as the disease progresses, and demonstrated regional functional deficits using oxygen-enhanced imaging. Hyperpolarized gas imaging studies often calculate the ventilation defect percent, but here we used low enhancement percent as a surrogate for regional functional deficits instead. Future work will optimize the calculation of the ventilation defect percent metric for our oxygen-enhancement technique, as previously shown for radial UTE oxygen-enhanced MRI at 1.5T 29. One previous study in patients with LAM used hyperpolarized gas MRI and identified heterogeneity in cyst ventilation 23. Ventilation measurements using hyperpolarized noble gases offer increased sensitivity and specificity compared with oxygen-enhanced imaging. However, these methods are costly and require dedicated hardware and specialized expertise. Oxygen inhalation offers advantages over hyperpolarized gas since it is readily available in most clinical MRI environments. Other non-contrast ventilation/perfusion methods such as Fourier-decomposition 30 and phase-resolved functional lung (PREFUL) 31 could also be explored at 0.55T in the future.
Most contrast agents, including exogenous agents such as gadolinium, and physiological agents such as oxygen or carbon dioxide, result in changes to underlying physiology. Molecular oxygen is a vasoconstrictor in the peripheral vasculature 32 and in the brain 33, but is a vasodilator in the lung 34. In the current study, it is anticipated that inhalation of 100% oxygen would increase pulmonary blood volume and perfusion via vasodilation 35. This increase in blood flow could lead to local T1 changes, in addition to the T1 shortening caused by the T1 relaxation properties of dissolved paramagnetic oxygen. The observed oxygen-enhancement is consistent with either increases in oxygen concentration, flow, or both. A compensation for flow-induced changes in T1 may be required when assessing oxygen-enhanced lung MRI.
Some limitations of this study include the use of receiver array coil hardware that has been modified from 1.5T, rather than optimized for 0.55T. There are opportunities to improve image quality using optimal receiver coil designs. The use of breath-held acquisitions instead of free-breathing is a limitation that will be addressed by future work. Also, our study did not evaluate spatial agreement of functional defects during repeated measures. Additional patient studies at 0.55T are required to assess oxygen-enhanced MRI imaging of lung function in other diseases.
CONCLUSION
We have implemented oxygen-enhanced functional lung imaging using a 0.55T MRI system equipped with contemporary hardware and software using a 3D stack-of-spirals UTE sequence. The dependence of parenchymal signal intensity on lung inflation volume was demonstrated, and repeatability of oxygen-enhancement mapping was improved using a regional signal intensity correction in post-processing. This method was used to reveal regional functional deficits in patients with LAM compared with healthy volunteers.
Supplementary Material
Bland-Altman plots comparing uncorrected PSE mapping (Eqn 1) and corrected PSE mapping (Eqn 2) for A) mean PSE and B) low enhancement percent. Bland-Altman analysis is performed for the first two within-session repeated measurements in 7 healthy volunteers. The limits of agreement are substantially narrower using corrected PSE mapping for both mean PSE and low enhancement percent.
Acknowledgements:
The authors would like to acknowledge the assistance of Siemens Healthcare in the modification of the MRI system for operation at 0.55T under an existing cooperative research agreement (CRADA) between NHLBI and Siemens Healthcare. We thank Peg Lowery, Jennifer Henry and Tania Machado for their assistance in subject recruitment, and Amanda Jones and Patricia Julien-Williams for evaluation of LAM patients, in addition to Delaney McGuirt, Christine Mancini, and Kendall O’Brien for their technical expertise acquiring MR images.
Grant Support: This work was supported by the Division of Intramural Research, National Heart, Lung, and Blood Institute, National Institutes of Health (Z01-HL006213, Z01-HL006257).
Disclosures: The authors are investigators on a US Government Cooperative Research and Development Agreement (CRADA) with Siemens Healthcare. Siemens participated in the modification of the MRI system from 1.5T to 0.55T.
Abbreviations:
- CV
Coefficient of variation
- DLCO
Diffusing capacity of lung for carbon monoxide
- FEV1
Forced expiratory volume in 1 second
- LAM
Lymphangioleiomyomatosis
- PSE
Percent Signal Enhancement
- UTE
Ultrashort TE
Footnotes
Availability of supporting data:
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1.Wild JM, Marshall H, Bock M, et al. MRI of the lung (1/3): methods. Insights Imaging. August2012;3(4):345–53. doi: 10.1007/s13244-012-0176-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wielputz M, Kauczor HU. MRI of the lung: state of the art. Diagn Interv Radiol. Jul-Aug 2012;18(4):344–53. doi: 10.4261/1305-3825.DIR.5365-11.0 [DOI] [PubMed] [Google Scholar]
- 3.Roos JE, McAdams HP, Kaushik SS, Driehuys B. Hyperpolarized Gas MR Imaging: Technique and Applications. Magn Reson Imaging Clin N Am. May2015;23(2):217–29. doi: 10.1016/j.mric.2015.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kern AL, Vogel-Claussen J. Hyperpolarized gas MRI in pulmonology. Br J Radiol. April2018;91(1084):20170647. doi: 10.1259/bjr.20170647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fain S, Schiebler ML, McCormack DG, Parraga G. Imaging of lung function using hyperpolarized helium-3 magnetic resonance imaging: Review of current and emerging translational methods and applications. J Magn Reson Imaging. December2010;32(6):1398–408. doi: 10.1002/jmri.22375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mugler JP, 3rd, Altes TA. Hyperpolarized 129Xe MRI of the human lung. Journal of magnetic resonance imaging : JMRI. February2013;37(2):313–31. doi: 10.1002/jmri.23844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edelman RR, Hatabu H, Tadamura E, Li W, Prasad PV. Noninvasive assessment of regional ventilation in the human lung using oxygen-enhanced magnetic resonance imaging. Nat Med. Nov 1996;2(11):1236–9. [DOI] [PubMed] [Google Scholar]
- 8.Kruger SJ, Nagle SK, Couch MJ, Ohno Y, Albert M, Fain SB. Functional imaging of the lungs with gas agents. J Magn Reson Imaging. February2016;43(2):295–315. doi: 10.1002/jmri.25002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jakob PM, Wang T, Schultz G, Hebestreit H, Hebestreit A, Hahn D. Assessment of human pulmonary function using oxygen-enhanced T(1) imaging in patients with cystic fibrosis. Magn Reson Med. May2004;51(5):1009–16. doi: 10.1002/mrm.20051 [DOI] [PubMed] [Google Scholar]
- 10.Nakagawa T, Sakuma H, Murashima S, Ishida N, Matsumura K, Takeda K. Pulmonary ventilation-perfusion MR imaging in clinical patients. J Magn Reson Imaging. October2001;14(4):419–24. [DOI] [PubMed] [Google Scholar]
- 11.Zha W, Kruger SJ, Johnson KM, et al. Pulmonary ventilation imaging in asthma and cystic fibrosis using oxygen-enhanced 3D radial ultrashort echo time MRI. J Magn Reson Imaging. May2018;47(5):1287–1297. doi: 10.1002/jmri.25877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang WJ, Niven RM, Young SS, Liu YZ, Parker GJ, Naish JH. Dynamic oxygen-enhanced magnetic resonance imaging of the lung in asthma -- initial experience. Eur J Radiol. Feb 2015;84(2):318–26. doi: 10.1016/j.ejrad.2014.10.021 [DOI] [PubMed] [Google Scholar]
- 13.Campbell-Washburn AE. 2019 ATS BEAR Cage Winning Proposal: Lung Imaging Using High-performance Low-field MRI. Am J Respir Crit Care Med. April162020;doi: 10.1164/rccm.201912-2505ED [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Campbell-Washburn AE, Ramasawmy R, Restivo MC, et al. Opportunities in Interventional and Diagnostic Imaging by Using High-performance Low-Field-Strength MRI. Radiology. October12019:190452. doi: 10.1148/radiol.2019190452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mirhej ME. Proton spin relaxation by paramagnetic molecular oxygen. Canadian Journal of Chemistry. 1965;43((5)):1130–1138. [Google Scholar]
- 16.Ohno Y, Hatabu H, Higashino T, et al. Centrically reordered inversion recovery half-Fourier single-shot turbo spin-echo sequence: improvement of the image quality of oxygen-enhanced MRI. Eur J Radiol. November2004;52(2):200–5. doi: 10.1016/j.ejrad.2004.02.007 [DOI] [PubMed] [Google Scholar]
- 17.Ohno Y, Hatabu H, Takenaka D, Adachi S, Van Cauteren M, Sugimura K. Oxygen-enhanced MR ventilation imaging of the lung: preliminary clinical experience in 25 subjects. AJR Am J Roentgenol. July2001;177(1):185–94. doi: 10.2214/ajr.177.1.1770185 [DOI] [PubMed] [Google Scholar]
- 18.Kruger SJ, Fain SB, Johnson KM, Cadman RV, Nagle SK. Oxygen-enhanced 3D radial ultrashort echo time magnetic resonance imaging in the healthy human lung. NMR Biomed. Dec 2014;27(12):1535–41. doi: 10.1002/nbm.3158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Restivo MC, Ramasawmy R, Bandettini WP, Herzka DA, Campbell-Washburn AE. Efficient spiral in-out and EPI balanced steady-state free precession cine imaging using a high-performance 0.55T MRI. Magn Reson Med. April142020;doi: 10.1002/mrm.28278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pusterla O, Bauman G, Bieri O. Three-dimensional oxygen-enhanced MRI of the human lung at 1.5T with ultra-fast balanced steady-state free precession. Magn Reson Med. January2018;79(1):246–255. doi: 10.1002/mrm.26665 [DOI] [PubMed] [Google Scholar]
- 21.Avila NA, Chen CC, Chu SC, et al. Pulmonary lymphangioleiomyomatosis: correlation of ventilation-perfusion scintigraphy, chest radiography, and CT with pulmonary function tests. Radiology. February2000;214(2):441–6. doi: 10.1148/radiology.214.2.r00fe41441 [DOI] [PubMed] [Google Scholar]
- 22.Taveira-DaSilva AM, Julien-Williams P, Jones AM, Stylianou M, Moss J. Rates of change in FEV1 and DLCO as potential indicators for mTOR inhibitor therapy in premenopausal lymphangioleiomyomatosis patients. Eur Respir J. April2018;51(4)doi: 10.1183/13993003.02258-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walkup LL, Roach DJ, Hall CS, et al. Cyst Ventilation Heterogeneity and Alveolar Airspace Dilation as Early Disease Markers in Lymphangioleiomyomatosis. Ann Am Thorac Soc. Aug 2019;16(8):1008–1016. doi: 10.1513/AnnalsATS.201812-880OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mugler III JP, Fielden SW, Meyer CH, et al. Breath-hold UTE Lung Imaging using a Stack-of-Spirals Acquisition. 2015: [Google Scholar]
- 25.Campbell-Washburn AE, Xue H, Lederman RJ, Faranesh AZ, Hansen MS. Real-time distortion correction of spiral and echo planar images using the gradient system impulse response function. Magn Reson Med. June2016;75(6):2278–85. doi: 10.1002/mrm.25788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rueckert D, Sonoda LI, Hayes C, Hill DL, Leach MO, Hawkes DJ. Nonrigid registration using free-form deformations: application to breast MR images. IEEE Trans Med Imaging. August1999;18(8):712–21. doi: 10.1109/42.796284 [DOI] [PubMed] [Google Scholar]
- 27.Staring M, Bakker ME, Stolk J, Shamonin DP, Reiber JH, Stoel BC. Towards local progression estimation of pulmonary emphysema using CT. Med Phys. February2014;41(2):021905. doi: 10.1118/1.4851535 [DOI] [PubMed] [Google Scholar]
- 28.Chassagnon G, Martin C, Ben Hassen W, et al. High-resolution lung MRI with Ultrashort-TE: 1.5 or 3 Tesla? Magn Reson Imaging. September2019;61:97–103. doi: 10.1016/j.mri.2019.04.015 [DOI] [PubMed] [Google Scholar]
- 29.Zha W, Nagle SK, Cadman RV, Schiebler ML, Fain SB. Three-dimensional Isotropic Functional Imaging of Cystic Fibrosis Using Oxygen-enhanced MRI: Comparison with Hyperpolarized (3)He MRI. Radiology. January2019;290(1):229–237. doi: 10.1148/radiol.2018181148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bauman G, Puderbach M, Deimling M, et al. Non-contrast-enhanced perfusion and ventilation assessment of the human lung by means of fourier decomposition in proton MRI. Magn Reson Med. September2009;62(3):656–64. doi: 10.1002/mrm.22031 [DOI] [PubMed] [Google Scholar]
- 31.Voskrebenzev A, Gutberlet M, Klimes F, et al. Feasibility of quantitative regional ventilation and perfusion mapping with phase-resolved functional lung (PREFUL) MRI in healthy volunteers and COPD, CTEPH, and CF patients. Magn Reson Med. April2018;79(4):2306–2314. doi: 10.1002/mrm.26893 [DOI] [PubMed] [Google Scholar]
- 32.Mathieu D, Favory R, Collet F, Linke J-C, Wattel F. Physiologic Effects of Hyperbaric Oxygen on Hemodynamics and Microcirculation. In: Mathieu D, ed. Handbook on Hyperbaric Medicine. Springer, Dordrecht; 2006. [Google Scholar]
- 33.Bulte DP, Chiarelli PA, Wise RG, Jezzard P. Cerebral perfusion response to hyperoxia. J Cereb Blood Flow Metab. January2007;27(1):69–75. doi: 10.1038/sj.jcbfm.9600319 [DOI] [PubMed] [Google Scholar]
- 34.Euler USv, Llijestrand G Observations on the Pulmonary Arterial Blood Pressure in the Cat. Acta Physiologica Scandinavica. 1946;12:301–320. [Google Scholar]
- 35.Ley S, Puderbach M, Risse F, et al. Impact of oxygen inhalation on the pulmonary circulation: assessment by magnetic resonance (MR)-perfusion and MR-flow measurements. Invest Radiol. May2007;42(5):283–90. doi: 10.1097/01.rli.0000258655.58753.5d [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Bland-Altman plots comparing uncorrected PSE mapping (Eqn 1) and corrected PSE mapping (Eqn 2) for A) mean PSE and B) low enhancement percent. Bland-Altman analysis is performed for the first two within-session repeated measurements in 7 healthy volunteers. The limits of agreement are substantially narrower using corrected PSE mapping for both mean PSE and low enhancement percent.