Abstract
This cohort study examines the association of the COVID-19 pandemic with total laryngectomy volumes among patients in Ontario, Canada.
Responding to the coronavirus disease 2019 (COVID-19) pandemic necessitated resource redistribution and disrupted health care access. Several groups offered recommendations to safeguard head and neck oncologic care in this setting.1,2,3 Patients with advanced head and neck cancers were prioritized. In cases of clinical equipoise, nonsurgical treatment modalities were preferred.4,5
It will take years to fully appreciate the downstream consequences of COVID-19 and the effects of these recommendations on head and neck cancer care. Most cancer registries with accurate staging information experience a significant time lag. Nonetheless, larynx cancer treatment may serve as an important litmus test. Larynx-preservation protocols are favored in localized disease (T1b, T2, T3), with total laryngectomy reserved for advanced cases (T4a). Increased total laryngectomy volume during the COVID-19 pandemic would provide early evidence of diagnostic delay and stage migration. Therefore, we sought to determine whether there were any differences in the total laryngectomy volumes between the pre- and peri-COVID periods.
Methods
This was a population-based retrospective cohort study in Ontario, Canada. The province of Ontario offers universal health care to its 14.6 million residents. All head and neck cancer care is provided at 7 high-volume designated cancer centers.6 A robust system is in place to support complete case capture. This provides a unique ability to study pandemic-related effects. These data sets were linked using unique encoded identifiers and analyzed at ICES, a nonprofit organization that has held patient-level health records for Ontario’s residents since 1986.
We defined a cohort of patients undergoing total laryngectomy using Canadian Institute for Health Information procedure codes (1GE89-total, 1GE91-radical). The 6-week volume of laryngectomy procedures between January 24, 2016, and February 13, 2021, were defined. The 6-week window was selected to minimize reidentification risk (ie, <6 observations at a given time point as per ICES policy). Segmented regression models were constructed to quantify (1) the surgical volume trend pre–COVID-19 (January 24, 2016-March 14, 2020), (2) the immediate decrease in surgical volume at the start of the pandemic (March 15, 2020; change in intercept), and (3) the surgical volume trend during the COVID-19 period (periperiod slope). This allowed for nearly 10 full months of data in the COVID-19 period. Distributions of patient characteristics in the pre–COVID-19 and COVID-19 period were compared. A standardized difference of 0.1 or greater indicates a meaningful difference between groups. All analyses were 2-sided, with P < .05 defined as significant.
The study was approved by the research ethics board at Sunnybrook Health Sciences Centre. The use of the data in this project is authorized under section 45 of Ontario’s Personal Health Information Protection Act (PHIPA) and does not require review by a research ethics board. Reporting was in line with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline. Statistical analysis was performed using SAS statistical software (Enterprise Guide 7.15; SAS Institute, Inc).
Results
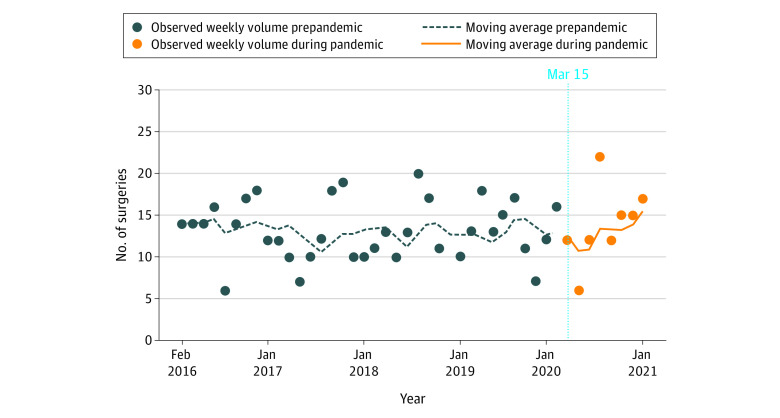
There were 576 eligible patients. A 21% drop in mean laryngectomy volume was observed at the start of the pandemic, though this quickly recovered by the next 6-week interval. The change in mean laryngectomy volume in the COVID-19 period was stable compared with the pre–COVID-19 period (relative rate, 1.00; 95% CI, 0.99-1.01; P = .86) (Figure). There was no clinically meaningful difference in patient characteristics between the pre–COVID-19 and COVID-19 groups (Table). The proportion of salvage laryngectomy cases in the pre–COVID-19 and peri–COVID-19 groups was similar (127 [27.3%] vs 28 [25.2%]), implying that borderline advanced cases were not more likely to be offered primary chemoradiation during the pandemic.
Figure. Total Laryngectomy Volume Before and During the COVID-19 Pandemic.
Data are presented in a 6-week interval and have been fitted with a 6-week moving average line. The dark-blue dashed line indicates the pre–COVID-19 period and the orange line the post–COVID-19 period. The vertical blue dotted line indicates the start of the COVID-19 pandemic.
Table. Distribution of Laryngectomy Procedures Performed Before and During the COVID-19 Pandemic.
| Variable | Laryngectomy patients by COVID-19 period, No (%) | Standardized differencea | P value | |
|---|---|---|---|---|
| Before COVID-19 (n = 465) | During COVID-19 (n = 111) | |||
| Age, y | ||||
| Mean (SD) | 65.67 (10.04) | 66.71 (8.72) | 0.11 | .32 |
| Median (IQR) | 66.0 (60.0-73.0) | 66.0 (61.0-73.0) | 0.08 | .48 |
| Sex on RPDB | ||||
| Female | 77 (16.6) | 18 (16.2) | 0.01 | .93 |
| Male | 388 (83.4) | 93 (83.8) | 0.01 | |
| Elixhauser Scoreb | ||||
| Mean (SD) | 2.37 (1.78) | 2.46 (1.73) | 0.05 | .68 |
| Median (IQR) | 2.0 (1.0-3.0) | 2.0 (1.0-3.0) | 0.06 | .66 |
| Income quintile | ||||
| 1 (lowest) | 137 (29.7) | 31 (28.2) | 0.03 | .99 |
| 2 | 96 (20.8) | 24 (21.8) | 0.03 | |
| 3 | 84 (18.2) | 21 (19.1) | 0.02 | |
| 4 | 66 (14.3) | 15 (13.6) | 0.02 | |
| 5 (highest) | 79 (17.1) | 19 (17.3) | 0 | |
| Immigrant | 37 (8.0) | 9 (8.1) | 0.01 | .81 |
| Deprivation quintilec | ||||
| 1 (least deprived) | 69 (15.2) | 16 (14.5) | 0.02 | .97 |
| 2 | 82 (18.0) | 23 (20.9) | 0.07 | |
| 3 | 81 (17.8) | 19 (17.3) | 0.01 | |
| 4 | 87 (19.1) | 23 (20.9) | 0.02 | |
| 5 (most deprived) | 136 (29.9) | 29 (26.4) | 0.04 | |
| Region | ||||
| Central | 88 (18.9) | 31 (27.9) | 0.21 | .10 |
| East | 122 (26.2) | 33 (29.7) | 0.08 | |
| North | 27 (5.8) | 7 (6.3) | 0.02 | |
| Toronto | 28 (6.0) | 7 (6.3) | 0.01 | |
| West | 200 (43.0) | 33 (29.7) | 0.28 | |
| Ruralityd | ||||
| 0-9 (least) | 290 (62.4) | 66 (59.5) | 0.06 | .81 |
| 10-30 | 90 (19.4) | 22 (19.8) | 0.01 | |
| 31-50 | 61 (13.1) | 15 (13.5) | 0.01 | |
| 51-70 | 13 (2.8) | 6 (5.4) | 0.13 | |
| ≥71 (most) | ≤5 | ≤5 | 0.07 | |
| Missing | ≤10 | ≤5 | 0.03 | |
| Urgente | 82 (17.6) | 20 (18.0) | 0.01 | .92 |
| Salvage laryngectomyf | 127 (27.3) | 28 (25.2) | 0.05 | .66 |
Abbreviations: IQR, interquartile range; RPDB, Registered Persons Database.
Standardized difference greater than 0.1 indicates a significant imbalance.
The Elixhauser comorbidity grouping is a well validated approach to assess comorbidities and uses a 5-year lookback window. A higher score indicates greater comorbidity.
Material deprivation is a composite measure of socioeconomic status, considering the proportion of a population without a high school diploma, single-parent families, receipt of government transfer payments, unemployment, low income, and living in dwellings that require major repair. Results are reported in quintiles.
Rural score was based on Rurality index score for Ontario version 2008. This measure takes into account community population and population density, travel time to nearest basic referral center and travel time to nearest advanced referral center.
Patients receiving urgent treatment arrived either by ambulance or through the emergency department.
We further classified laryngectomies as either primary or salvage. Whether or not a patient received curative radiation can be identified through the Ontario’s Cancer Activity Level Reporting System. Due to concerns about data completeness, however, we opted for a definition of more than 3 months from laryngeal cancer diagnosis to laryngectomy as indicative of salvage treatment.
Discussion
The current study did not show an increase in laryngectomy surgical volume during the COVID-19 pandemic. This study provides early, real-world evidence suggesting that Ontario’s head and neck oncology response may have mitigated adverse health outcomes stemming from broader diagnostic and treatment delays. Care has been equitable during the pandemic compared with the prepandemic period, with no group disproportionately affected. This work should be followed with more granular staging data and repeated in other jurisdictions because the present study results are limited in that they may not be directly transferrable.
References
- 1.Mehanna H, Hardman JC, Shenson JA, et al. Recommendations for head and neck surgical oncology practice in a setting of acute severe resource constraint during the COVID-19 pandemic: an international consensus. Lancet Oncol. 2020;21(7):e350-e359. doi: 10.1016/S1470-2045(20)30334-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Almeida JR, Noel CW, Forner D, et al. Development and validation of a surgical prioritization and ranking tool and navigation aid for head and neck cancer (SPARTAN-HN) in a scarce resource setting: response to the COVID-19 pandemic. Cancer. 2020;126(22):4895-4904. doi: 10.1002/cncr.33114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomson DJ, Palma D, Guckenberger M, et al. Practice recommendations for risk-adapted head and neck cancer radiation therapy during the COVID-19 pandemic: an ASTRO-ESTRO consensus statement. Int J Rad Onc Biol Phys. 2020;107(4):618-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.COVIDSurg Collaborative . Head and neck cancer surgery during the COVID-19 pandemic: an international, multicenter, observational cohort study. Cancer. 2021;127(14):2476-2488. [DOI] [PubMed] [Google Scholar]
- 5.Wu V, Noel CW, Forner D, et al. Considerations for head and neck oncology practices during the coronavirus disease 2019 (COVID-19) pandemic: Wuhan and Toronto experience. Head Neck. 2020;42(6):1202-1208. doi: 10.1002/hed.26205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eskander A, Goldstein DP, Irish JC. Health services research and regionalization of care—from policy to practice: the Ontario experience in head and neck cancer. Curr Oncol Rep. 2016;18(3):19. doi: 10.1007/s11912-016-0500-6 [DOI] [PubMed] [Google Scholar]