Key Points
Question
Do focal 22q11.22 deletions identify patients with IKZF1 alterations who will have worse outcomes in childhood B-cell acute lymphoblastic leukemia (B-ALL)?
Findings
In this cohort study of 1310 patients with B-ALL in 6 independent cohorts, focal 22q11.22 deletions were common (39.5%) in B-ALL and, when co-occurring with IKZF1 alterations, were associated with poor outcomes compared with patients with IKZF1 alterations alone (5-year event-free survival rates, 43.3% vs 68.5%; 5-year overall survival rates, 66.9% vs 83.9%).
Meaning
The study results suggest that 22q11.22 deletions are associated with very poor clinical outcomes in patients with B-ALL with IKZF1 alterations.
Abstract
Importance
Alterations in the IKZF1 gene drive B-cell acute lymphoblastic leukemia (B-ALL) but are not routinely used to stratify patients by risk because of inconsistent associations with outcomes. We describe a novel deletion in 22q11.22 that was consistently associated with very poor outcomes in patients with B-ALL with IKZF1 alterations.
Objective
To determine whether focal deletions within the λ variable chain region in chromosome 22q11.22 were associated with patients with B-ALL with IKZF1 alterations with the highest risk of relapse and/or death.
Design, Setting, and Participants
This cohort study included 1310 primarily high-risk pediatric patients with B-ALL who were taken from 6 independent clinical cohorts, consisting of 3 multicenter cohorts (AALL0232 [2004-2011], P9906 [2000-2003], and patients with Down syndrome who were pooled from national and international studies) and 3 single-institution cohorts (University of Utah [Salt Lake City], Children’s Hospital of Philadelphia [Philadelphia, Pennsylvania], and St. Jude Children’s Hospital [Memphis, Tennessee]). Data analysis began in 2011 using patients from the older studies first, and data analysis concluded in 2021.
Exposures
Focal 22q11.22 deletions.
Main Outcomes and Measures
Event-free and overall survival was investigated. The hypothesis that 22q11.22 deletions stratified the prognostic effect of IKZF1 alterations was formulated while investigating nearby deletions in VPREB1 in 2 initial cohorts (n = 270). Four additional cohorts were then obtained to further study this association (n = 1040).
Results
This study of 1310 patients with B-ALL (717 male [56.1%] and 562 female patients [43.9%]) found that focal 22q11.22 deletions are frequent (518 of 1310 [39.5%]) in B-ALL and inconsistent with physiologic V(D)J recombination. A total of 299 of 1310 patients with B-ALL had IKZF1 alterations. Among patients with IKZF1 alterations, more than half shared concomitant focal 22q11.22 deletions (159 of 299 [53.0%]). Patients with combined IKZF1 alterations and 22q11.22 deletions had worse outcomes compared with patients with IKZF1 alterations and wild-type 22q11.22 alleles in every cohort examined (combined cohorts: 5-year event-free survival rates, 43.3% vs 68.5%; hazard ratio [HR], 2.18; 95% CI, 1.54-3.07; P < .001; 5-year overall survival rates, 66.9% vs 83.9%; HR, 2.05; 95% CI, 1.32-3.21; P = .001). While 22q11.22 deletions were not prognostic in patients with wild-type IKZF1 , concomitant 22q11.22 deletions in patients with IKZF1 alterations stratified outcomes across additional risk groups, including patients who met the IKZF1plus criteria, and maintained independent significance in multivariate analysis for event-free survival (HR, 2.05; 95% CI, 1.27-3.29; P = .003) and overall survival (HR, 1.83; 95% CI, 1.01-3.34; P = .05).
Conclusions and Relevance
This cohort study suggests that 22q11.22 deletions identify patients with B-ALL and IKZF1 alterations who have very poor outcomes and may offer a new genetic biomarker to further refine B-ALL risk stratification and treatment strategies.
This cohort study examines whether focal deletions within the λ variable chain region in chromosome 22q11.22 were associated with patients with B-ALL with IKZF1 alterations with the highest risk of relapse and/or death.
Introduction
Acute lymphoblastic leukemia (ALL) is one of the leading causes of death in children.1,2,3 Somatic alterations of the gene IKZF1 occur in approximately 15% of pediatric patients with B-cell ALL (B-ALL)4 and have been associated with very poor outcomes, including an initial report of a 5-year event-free survival (EFS) rate of 25% in a high-risk cohort.5 When this cohort of patients was clustered according to gene expression profile,6 IKZF1 alterations clustered within patients with the worst outcomes (22 of 24; 4-year relapse-free survival rate, 21%). However, IKZF1 alterations were also present in 6 of 21 patients in a cluster with excellent outcomes (relapse-free survival rate, 95%), suggesting that the prognostic effect of IKZF1 alterations may be context dependent. Many others have observed the deleterious effects of IKZF1 alterations in patients with B-ALL,7,8,9,10,11,12,13,14,15 although the strength of these findings varies across different cohorts.16 Current known prognostic modifiers of IKZF1 alterations include concomitant occurrence in the high risk B-ALL subgroups Ph-positive and Ph-like ALL,17 occurrence with codeletions in BTG1,18 ERG,19,20 and/or occurrence in the setting of IKZF1plus (defined as IKZF1 alterations occurring in the absence of ERG deletions, but additionally having any of the following: CDKN2A deletion, homozygous CDKN2B deletion, PAX5 deletion, or a PAR1 deletion).14
Our group previously described focal somatic deletions of the gene VPREB1. While located within the λ variable light-chain region, these deletions occurred independently of normal V(D)J recombination in B-ALL.21 In this article, we identify a second recurrent somatic focal deletion in the λ light chain that is nearly 80 kilobases (kb) upstream from VPREB1 on chromosome 22q11.22. In this study, we investigated this deletion as part of VPREB1 and discovered that 22q11.22 focal deletions were independently associated with very poor outcomes, but only in combination with IKZF1 alterations located on chromosome 7. The presence of a combined 22q11.22 focal deletion and IKZF1 alteration (hereafter referred to as a double deletion) identified pediatric patients with B-ALL with very poor outcomes, whereas patients with IKZF1 alterations with wild-type (WT) 22q11.22 had relatively better outcomes.
Risk adapted therapy that includes prognostic genetic alterations and early treatment response, defined by end of induction minimal residual disease (MRD), has substantially contributed to improved survival and reduced toxic effects in patients with B-ALL.22 Recognizing the substantially worse outcomes of patients with B-ALL with this concomitant deletion may help to stratify patients with IKZF1 alterations into higher and lower risk categories and suggests that 22q11.22 deletions are another marker for informing risk-adapted therapy in B-ALL.
Methods
Patients and Samples
Patient data were collected from multiple independent cohorts of pediatric patients with a new diagnosis of B-ALL for whom leukemia and germline copy number profiling had been previously performed using microarray-based comparative genomic hybridization. Cohorts included participants in Utah,21 Children’s Oncology Group (COG) P9906,5,23 COG AALL0232,24,25 St Jude Hospital (Memphis, Tennessee),5,26 Children’s Hospital of Philadelphia (CHOP; Philadelphia, Pennsylvania), and a group of patients with Down syndrome who were pooled from the previously described cohorts with additional cases from UKALL, AIEOP, Texas Children’s Hospital, and COG.27 Single-nucleotide variation analysis was performed on all data sets to ensure no duplicate patients were analyzed. All samples were obtained with written informed consent under institutional review board–approved protocols. Only patients with known IKZF1, 22q11.22, and event-free survival status were selected for analysis. While treated with similar chemotherapy backbones, differences in treatment and underlying clinical characteristics existed between the cohorts, with P9906 and AALL0232 specifically focusing on high-risk patients with B-ALL. Patients from the AALL0232 cohort with available genomic data were enriched for relapse (27% overall) because COG prioritized patients who relapsed within 3 years of diagnosis for genomic analyses (https://ocg.cancer.gov/programs/target).
Data Analysis
Copy number data from leukemia and germline CEL files were visualized with Nexus Copy Number 8.0 (BioDiscovery, Inc). Hemizygous and homozygous 22q11.22 deletions were observed, and the 2 deletion types were pooled for all analyses. Quantitative polymerase chain reaction was used to assess the validity of microarray calls of 22q11.22 deletions (eFigure 1 in the Supplement). Genomic coordinates assessed for 22q11.22 deletions are described in eTable 1 in the Supplement. Alterations in IKZF1 were defined as a loss of coding exons or an inactivating point variation. Somatic variation data were limited to the P9906 cohort and accounted for only 7 of 299 patients who were classified as having an IKZF1 alteration.17 The term double deletion was used to describe a combined 22q11.22 deletion and IKZF1 alteration irrespective of the type of IKZF1 alteration (somatic deletion or somatic variation) or zygosity of the deletion (hemizygous or homozygous). End of induction MRD was measured by flow cytometry.
Statistical Analysis
Events were defined as leukemia relapse, induction failure, secondary malignancy, or death, with censoring occurring at the date of last follow-up. Significance in univariate analyses that compared patient subgroups was calculated with the log-rank test. The Kaplan-Meier method was used to calculate survival rates, and survival curves for each dichotomous covariate were assessed to identify any crossing that would violate the assumption of proportional hazards. The effect of 22q11.22 deletions in patients with concomitant IKZF1 deletions across all combined cohorts was adjusted for cohort membership. Subgroup and interaction term analyses investigated potential modifiers of double deletions on outcome, and sensitivity analyses assessed the effect of each cohort. Multivariate analyses were performed with Cox proportional hazards stepwise regression using backward elimination and a retention threshold of P < .05. Separate models were determined based on all patients and the subset of patients who were BCR-ABL1 negative, the status of which is a determinant for some treatment protocols. Cohort membership had forced inclusion in all models as a design variable, and additional covariates included currently used B-ALL prognostic factors. We were unable to include all B-ALL cytogenetic subtypes in the multivariate analysis. However, given the prognostic importance of and association with IKZF1 alterations, BCR-ABL1 translocations were included as a variable. Two-sided P values were used in all analyses, and statistical significance was set at P < .05. No adjustment for multiple comparisons was performed. The analyses were performed with SAS, version 9.4 (SAS Institute). See eMethods in the Supplement for further details regarding patients, data, and the statistical analysis.
Results
A copy number analysis of de novo patients with B-ALL (see eTables 2 and 3 in the Supplement for clinical characteristics) revealed frequent focal deletions within the λ light chain at the 22q11.22 locus (518 of 1310 [39.5%]), approximately 80 kb upstream of VPREB1. The 22q11.22 deletions were variable in size, spanning up to 142 kb, with the most common recurring region just under 12 kb in length (eFigure 2 in the Supplement). For the purposes of this analysis, focal 22q11.22 deletions were defined as including this common recurring region alone and not extending to the VJ junction because the latter deletions would have likely arisen from physiologic V(D)J recombination.21
Association of 22q11.22 and IKZF1 Double Deletions With Poor Outcomes
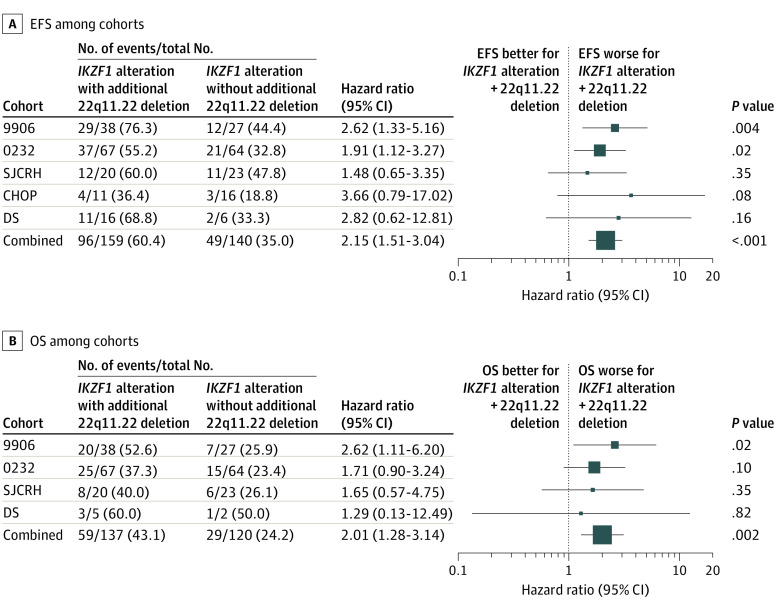
Focal 22q11.22 deletions were initially investigated in the Utah (n = 56) and P9906 (n = 214) cohorts and were associated with a significantly worse outcome. However, as IKZF1 alterations were also associated with very poor outcomes in a separate report of the P9906 cohort,5 we investigated the association between IKZF1 alterations and focal 22q11.22 deletions. We found that patients with WT IKZF1 alleles and a focal 22q11.22 deletion had similar outcomes to the remainder of the cohort; however, patients with a combined 22q11.22 and IKZF1 double deletion had significantly worse outcomes than patients with IKZF1 alterations with WT 22q11.22 (P9906 cohort: 5-year EFS, 26.4% vs 59%; hazard ratio [HR], 2.62; 95% CI, 1.33-5.16; P = .004; overall-survival [OS], 64.2% vs 92.4%; HR, 2.62; 95% CI, 1.11-6.2; P = .02). Several additional cohorts were evaluated (AALL0232, n = 555; St. Jude Children’s Research Hospital, n = 235; CHOP, n = 160; and a Down syndrome cohort, n = 90) and demonstrated the same pattern of 22q11.22 deletions that were associated with worse outcomes in patients with IKZF1 alterations (Figure 1; eFigures 3 and eFigure 4 in the Supplement).
Figure 1. Association of Concomitant 22q11.22 Deletions With Survival Outcomes Among Patients With IKZF1 Alterations.
A, Shown for each cohort are the unadjusted hazard ratios for the effect of 22q11.22 deletions on event-free survival (EFS) in persons having IKZF1 alterations. The combined row shows the overall hazard ratio for all persons from a proportional hazards model adjusted for the study cohorts. B, Shown for each cohort are the unadjusted hazard ratios for the association of 22q11.22 deletions with overall survival (OS) in persons having IKZF1 alterations. The combined row shows the overall hazard ratio for all persons from a proportional hazards model adjusted for the study cohorts. The Utah cohort not shown because of small numbers (7 patients with a 22q11.22 + IKZF1 double deletion, of whom 3 died and none had other events; 4 patients with an IKZF1 alteration and WT 22q11.22, of whom all patients survived and none had events). Overall survival data were not available for the Children’s Hospital of Philadelphia (CHOP) cohort. DS indicates Down syndrome; SJCRH, St. Jude Children’s Research Hospital.
All cohorts were then combined to facilitate an analysis of different clinical subgroups irrespective of their original study. More than half of the patients with IKZF1 alterations had a 22q11.22 deletion (159 of 299 [53.2%]), with double deletions representing 159 of 1310 participants (12.1%). When all patients in the study were considered, patients with IKZF1 alterations and WT 22q11.22 had decreased survival compared with patients with WT IKZF1 (5-year EFS, 68.5% vs 81.2%; HR, 1.84; 95% CI, 1.34-2.51; P < .001; OS, 83.9% vs 87.3%; HR, 1.73; 95% CI, 1.15-2.60; P = .01). Patients with 22q11.22 and IKZF1 double deletions had even worse outcomes when compared with patients with IKZF1 alterations and WT 22q11.22 for 5-year EFS (43.3% vs 68.5%; HR, 2.18; 95% CI, 1.54-3.07; P < .001) and OS (66.9% vs 83.9%; HR, 2.05; 95% CI, 1.32-3.21; P = .001) (Figure 2). Sensitivity analyses found that no single cohort markedly affected the results (eTable 4 in the Supplement). Patients with 22q11.22 deletions and WT IKZF1 had no worse prognosis than patients with WT 22q11.22.
Figure 2. Association of Concomitant 22q11.22 Deletions With Survival Outcome Among Patients With IKZF1 Alterations.
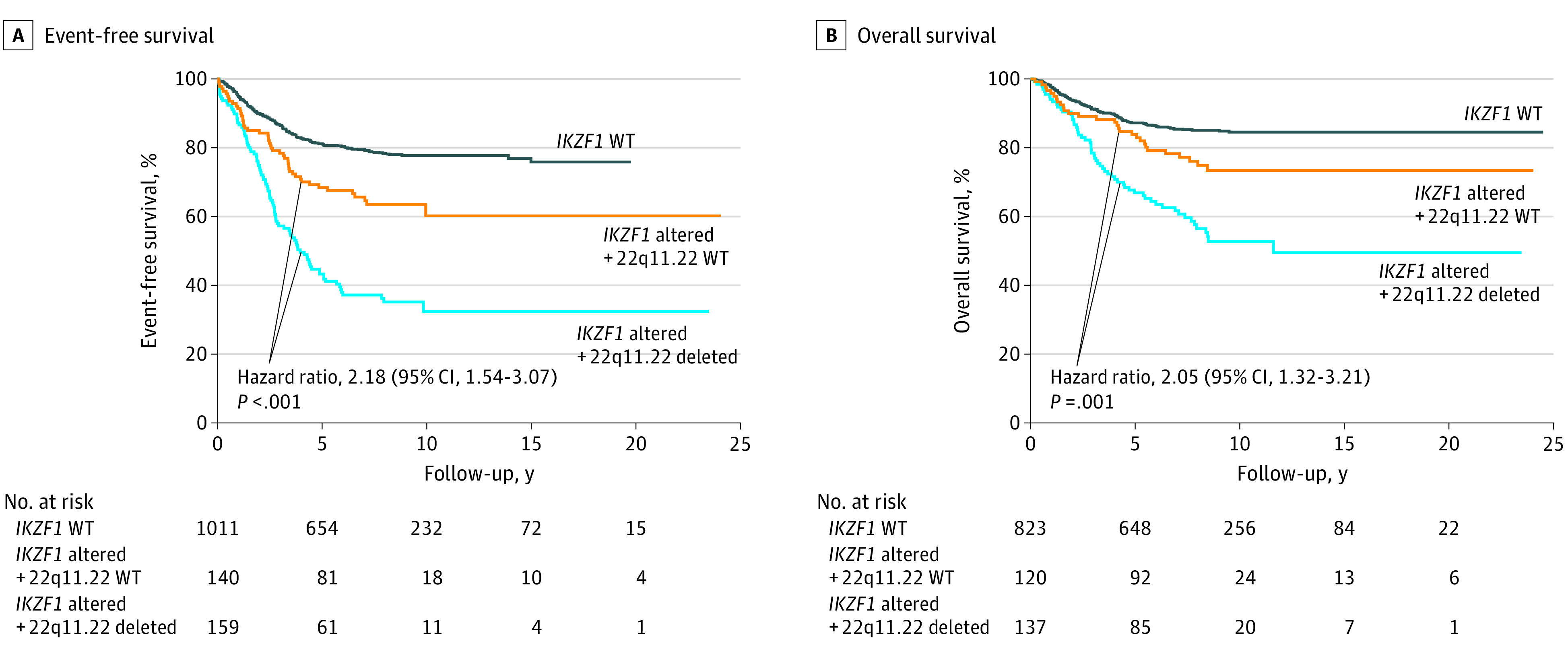
When all patients in the study were considered (n = 1310), the presence of a 22q11.22 deletion among patients with IKZF1 alterations was associated with significantly worse outcomes. A, Five-year event-free survival: 22q11.22 + IKZF1 double deletion, 43.3%; 22q11.22 wild type (WT) + IKZF1 alteration, 68.5%; and IKZF1 WT, 81.2%. For reference, the 5-year event-free survival rate for all patients was 75.2%. B, Five-year overall survival: 22q11.22 + IKZF1 double deletion, 66.9%; 22q11.22 WT + IKZF1 alteration, 83.9%; and IKZF1 WT, 87.3%. For reference, the 5-year overall survival rate for all patients was 84.3%. Shown are unadjusted hazard ratios; P values are from a log rank test. Overall survival data were not available for all patients.
Subgroup Analyses
The 22q11.22 deletions were common among patients with ETV6-RUNX1 translocations (67 or 100 [67.0%]), BCR-ABL1 translocations (27 of 51 [52.9%]), and a Ph-like gene expression signature (72 of 98 [73.5%]) but were underrepresented or absent in patients with TCF3-PBX1 translocations (4 of 85 [4.7%]) or KMT2A rearrangements (0) (eTable 3 in the Supplement). Within cytogenetic subtypes, we observed similar proportions of 22q11.22 deletions and IKZF1 alterations, except in ETV6-RUNX1 cases, which exhibited frequent 22q11.22 deletions but rarely had IKZF1 alterations (eFigure 5 in the Supplement).
The 22q11.22 and IKZF1 double deletions were common in Ph-like ALL (53 of 98 [54.1%]) and exhibited a trend toward worse outcomes compared with Ph-like patients with IKZF1 alterations and WT 22q11.22 (5-year EFS, 40.3% vs 57.3%; P = .08). The same trend remained among known non–Ph-like patients with IKZF1 alterations (5-year EFS, 58.6% vs 76.7%; P = .15) (eFigure 6 in the Supplement). For Ph-positive patients with an IKZF1 alteration, poor outcomes were observed regardless of 22q11.22 status (eFigure 7 in the Supplement). Among the 90 patients with Down syndrome who were examined, most with an IKZF1 alteration shared a 22q11.22 focal deletion (16 of 22 [72.7%]) and fared poorly (5-year EFS, 34.7% vs 62.5%; P = .16) (eFigure 3 in the Supplement).
Intragenic ERG deletions occurred infrequently (36 of 1164 [3.1%]), with insufficient numbers for meaningful analysis of the effect of 22q11.22 and IKZF1 double deletions. The effect of 22q11.22 deletions was also examined among patients who met the criteria for the recently reported poor outcome IKZF1plus subgroup.14 As our microarray data could not accurately assess the PAR1 region nor discriminate between CDKN2A vs CDKN2B deletions, we defined IKZF1plus by codeletion of IKZF1 with either PAX5 or homozygous CDKN2A/B deletion in the absence of ERG deletions. While this excludes patients with IKZF1plus alterations based solely on a PAR1 deletion, these were a small number of patients with IKZF1plus alterations in the original report (9 of 63 [14.3%]).14 Among patients with IKZF1 alterations, IKZF1plus deletions were significantly correlated with 22q11.22 deletions (Pearson correlation, 0.21; P < .001); however, those with IKZF1plus and a 22q11.22 deletion had significantly worse outcomes compared with patients with IKZF1plus and WT 22q11.22 (5-year EFS, 32.5% vs 68.8%; HR, 3.02; 95% CI, 1.65-5.55; P < .001; 5-year OS, 56.4% vs 80.4%; HR, 2.61; 95% CI, 1.26-5.42; P = .01). Analysis of patients with IKZF1plus alterations, including hemizygous CDKN2A/B deletions, found similar results (eFigure 8 in the Supplement).
Patients with 22q11.22 + IKZF1 double deletions were also enriched in patients who had positive MRD (≥0.01%) at the end of induction 70 of 244 [28.7%] vs 43 of 567 [7.6%]; (P < .001) and in National Cancer Institute (NCI) high-risk vs standard-risk patients28 (138 of 957 [14.4%] vs 19 of 332 [5.7%]; P < .001). Regardless of MRD or NCI risk group, 22q11.22 deletions maintained prognostic relevance among patients with IKZF1 alterations (eFigures 9-11 in the Supplement).
Multivariate Analyses
To determine the independent prognostic ability of 22q11.22 status to identify patients with IKZF1 alterations who were at higher risk, we performed a multivariate analysis within patients with IKZF1 alterations that assessed the following covariates: end of induction MRD positivity, BCR-ABL1 translocation, 22q11.22 deletion, central nervous system disease group, age, initial white blood cell count, cohort, and sex. Several showed highly significant univariate association with survival (eTable 5 in the Supplement). As most patients with IKZF1 alterations who were eligible for multivariate analysis were NCI high risk, (199 of 203 [98.0%]), NCI standard-risk patients were excluded. Interaction term analyses with 22q11.22 status identified a significant interaction with BCR-ABL1 translocations (eFigure 12 in the Supplement) for EFS and OS. As a result, BCR-ABL1–positive and negative patients combined and BCR-ABL1–negative patients alone were analyzed in separate multivariate models (eTable 5 in the Supplement). In eligible NCI high-risk patients with IKZF1 alterations who were negative for a BCR-ABL1 translocation (181 [91.0%]), deletion of 22q11.22 was a significantly independent risk factor for EFS (HR, 2.05; 95% CI, 1.27-3.29; P = .003) and OS (HR, 1.83; 95% CI, 1.01-3.34; P = .05) (eTable 6 in the Supplement). When patients with IKZF1 alterations with a BCR-ABL1 translocation were also included, the significant interaction observed between patients having 22q11.22 deletions and BCR-ABL1 translocation status for EFS and OS in unadjusted analyses was found to persist in multivariate assessments (eTable 6 in the Supplement). In a nested model comparison that included IKZF1plus as an additional variable among BCR-ABL1–negative patients, 22q11.22 status had a greater association than IKZF1plus status with EFS (χ2 = 7.06; P = .008 vs χ2 = 4.84; P = .03), whereas IKZF1plus status had a greater association than 22q11.22 status with OS (χ2 = 5.34; P = .02; vs χ2 = 2.77; P = .10) (eTable 7 in the Supplement).
Potential Functional Associations
Often, 22q11.22 and VPREB1 deletions occur together as a single deletion. In 173 of 509 patients with 22q11.22 deletions (34.0%), the deletion extended to include VPREB1. Comparatively, nearly all deletions of VPREB1 extended to the 22q11.22 common recurring region (173 of 199 [86.9%]) (eFigure 2 in the Supplement). However, no significant difference in outcome was observed in 22q11.22 and IKZF1 double deletion cases with vs without VPREB1 deletion (5-year EFS, 44.0% vs 42.7%; HR, 1.10; 95% CI, 0.73-1.65; P = .66) (eFigure 13 in the Supplement). We hypothesized that the 22q11.22 region could contain regulatory elements for VPREB1 and thus affect VPREB1 even when not included in the deletion. However, we found no association between 22q11.22 deletions and VPREB1 expression in the P9906 cohort with paired gene expression data available. Aside from VPREB1, no known genes or micro RNAs were located within the observed 22q11.22 deletions. The pseudogene SOCS2P2 is located near (but not within) the 22q11.22 common recurring region, and patients with 22q11.22 deletions had upregulated SOCS2 expression (eFigure 14 in the Supplement). While pseudogenes can affect parent gene expression,29 the functional effects and potential causality of this association are unknown. In the P9906 cohort, we found no significant differential expression of nearby genes that could be implicated in cancer progression (MAPK1, SMARCB1, PIK3IP1, BCL2L13, BCR, PRAME, CHEK2, and TOP3B). Finally, we assessed the proportion of the genome that was affected by copy number alterations for patients in the AALL0232 cohort. We observed that 22q11.22 deletions did not increase genomic instability, but rather that patients with a 22q11.22 and IKZF1 double deletion had the highest percentage of diploid regions (eFigure 15 in the Supplement).
Discussion
In our analysis of a cohort of 1310 pediatric patients with B-ALL, we identified that 518 (39.5%) had recurrent focal deletions in chromosome 22q11.22 within the λ variable chain region that did not follow physiologic light chain rearrangement.21 More than half of patients with IKZF1 alterations shared a 22q11.22 deletion (159 of 299 [53.2%]), with the combined double deletion being associated with very poor prognosis in 6 independent clinical cohorts despite underlying differences between cohorts. The 22q11.22 and IKZF1 double deletions were more frequent in NCI high-risk and MRD-positive patients, but nonetheless retained an independently adverse prognostic significance in multivariate analysis.
In acute myeloid leukemia, the prognostic effect of a genetic driver can be significantly modified by the presence or absence of other genetic alterations.30 Similarly, outcomes in patients with IKZF1 alterations are increasingly being recognized to be context specific.4,14,16,20,31 Recently, IKZF1plus was introduced as a means to identify patients with IKZF1 alterations who are at highest risk.14 However, assessing IKZF1plus requires the assessment of multiple genes (eg, CDKN2A, CDKN2B, PAX5, PAR1, and ERG) and only has prognostic significance in MRD-positive patients. In contrast, deletion of the single locus 22q11.22 was associated with worse outcomes in patients with IKZF1 alterations independently of MRD and even successfully stratified patients with IKZF1plus into those with relatively good vs very poor outcomes.
Among the most important modifiers of outcomes for IKZF1 alterations are their occurrence as cooperating lesions in Ph-positive or Ph-like ALL,17 both of which historically have poor outcomes. Targeted therapy has markedly improved outcomes in Ph-positive ALL, and studies are ongoing to evaluate the same for Ph-like ALL.17,32 Patients with Ph-like ALL commonly contained a 22q11.22 and IKZF1 double deletion. Nonetheless, in patients with known Ph-like and non–Ph-like ALL, a trend toward worse outcomes was observed when patients with IKZF1 alterations were stratified by 22q11.22 deletion status. Ph-positive (BCR-ABL1) patients with IKZF1 alterations appeared to have poor outcomes irrespective of 22q11.22 status, although these Ph-positive patients were largely treated before the era of tyrosine kinase inhibitor therapy. Currently, it is unknown how targeted therapies for Ph-positive and Ph-like ALL may affect the prognostic effect of 22q11.22 + IKZF1 double deletions, and further work is needed.
A functional basis for the association between 22q11.22 deletions and adverse outcomes in ALL remains unknown and beyond the scope of this article. Some have suggested that genomic instability and increased copy number alterations contribute to poor outcomes in patients with IKZF1 alterations,33 and the 22q11.22 region is known for genomic instability because of low copy repeats, which facilitate nonallelic homologous recombination.34 However, the 22q11.22 deletion is located more than 1 MB downstream of these regions that are often involved in DiGeorge syndrome, and 22q11.22 and IKZF1 double deleted B-ALL samples showed no increased genomic instability, which was consistent with prior reports.4,35 Approximately 90% or more of IKZF1 alterations in pediatric B-ALL are deletions (primarily intragenic deletions that create dominant negative isoforms), with somatic point variations being a rare cause of IKZF1 alterations.5,16,36 Prognosis in IKZF1 alterations remains independent of the type of dominant-negative IKAROS isoform.37 The prognostic effect of somatic IKZF1 point variations is less certain35; however, somatic point variations accounted for very few patients with IKZF1 alterations in this study. While we found no association between 22q11.22 deletions and VPREB1 expression in the P9906 cohort, given the proximity and inclusion of VPREB1 in many 22q11.22 deletions, this remains an area for further functional investigation.
The ability of 22q11.22 + IKZF1 double deletions to robustly predict very poor prognosis could be used to identify pediatric patients with B-ALL who might benefit from novel therapies, such as immunotherapy, retinoids,38 or focal adhesion kinase inhibition.39 Moreover, risk stratification using these double deletions could lessen overtreatment of lower-risk patients with IKZF1 alterations with aggressive therapy,40 which is particularly important in patients with Down syndrome given their increased risk for toxic effects.
Limitations
The limitations of our study included the retrospective nature during a time that preceded the routine identification of Ph-like patients with ALL and the use of targeted therapies for Ph-positive and Ph-like ALL. Also, gene expression data were limited to the P9906 cohort alone, which precluded a more expansive analysis regarding 22q11.22 deletions and VPREB1 expression.
Conclusions
Focal deletions in chromosome 22q11.22 within the λ variable chain region identify a specific subset of patients with B-ALL with IKZF1 alterations who have very poor outcomes. Further studies are needed to better understand the functional consequences of 22q11.22 and IKZF1 double deletions and how to best assess and incorporate this predictive marker into clinical studies to improve patient outcomes. These data add to the increasing evidence that genetic interactions in individual cancer cells may determine their therapeutic sensitivity.
eMethods. Further Details on Samples and Data Analysis
eTable 1. Probe Density and Coordinates along Chromosome 22q11.22 and IKZF1 Coding Region
eTable 2. Patient Characteristics by Cohort
eTable 3. Patient Characteristics by 22q11.22 and IKZF1 Status
eTable 4. Sensitivity Analysis
eTable 5. Univariate Analyses of Event-free Survival and Overall Survival in NCI High Risk Patients with an IKZF1 Alteration
eTable 6. Multivariate Analyses of Event-free Survival and Overall Survival in NCI High Risk Patients with an IKZF1 Alteration
eTable 7. Additional Assessment of IKZF1plus in Multivariate Models of Patients Lacking a BCR-ABL1 Translocation
eFigure 1. Mean Copy Number Status Validation Using qPCR
eFigure 2. 22q11.22 Deletions in B-ALL.
eFigure 3. Patients with a 22q11.22 + IKZF1 Double Deletion Have Very Poor Event-free Survival (Individual Cohorts)
eFigure 4. Patients with a 22q11.22 + IKZF1 Double Deletion Have Very Poor Overall Survival (Individual Cohorts)
eFigure 5. Association of ALL Subtypes and 22q11.22 Deletions or IKZF1 Alterations in the AALL0232 Cohort
eFigure 6. Patients with a 22q11.22 + IKZF1 Double Deletion Have Worse Outcomes in Both Ph-like ALL and Non-Ph-like ALL
eFigure 7. Ph+ (BCR-ABL1+) Patients with IKZF1 Alterations Have Poor Outcomes Regardless of 22q11.22 Status
eFigure 8. 22q11.22 Deletions Stratify Outcomes among IKZF1plus Patients
eFigure 9. 22q11.22 + IKZF1 Double Deletion Patients Maintain Trend of Worse Outcomes when Compared to IKZF1 Altered and WT 22q11.22 Patients in Both MRD Positive and MRD Negative Settings
eFigure10. 22q11.22 + IKZF1 Double Deletion Patients Have Worse Outcomes when Compared to Patients without a Double Deletion in Both MRD Positive and MRD Negative Settings
eFigure 11. 22q11.22 + IKZF1 Double Deletion Patients Have Worse Outcomes in Both NCI High Risk and Standard Risk Patients
eFigure 12. Interaction-term Analysis
eFigure 13. Presence or Absence of VPREB1 Deletions Do Not Affect Outcome
eFigure 14. SOCS2 Gene Expression is Upregulated in Patients with 22q11.22 deletions
eFigure 15. 22q11.22 + IKZF1 Double Deletion Patients Do Not Have Increased Genomic Instability
References
- 1.Siegel DA, King J, Tai E, Buchanan N, Ajani UA, Li J. Cancer incidence rates and trends among children and adolescents in the United States, 2001-2009. Pediatrics. 2014;134(4):e945-e955. doi: 10.1542/peds.2013-3926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hunger SP, Mullighan CG. Acute lymphoblastic leukemia in children. N Engl J Med. 2015;373(16):1541-1552. doi: 10.1056/NEJMra1400972 [DOI] [PubMed] [Google Scholar]
- 3.Phillips SM, Padgett LS, Leisenring WM, et al. Survivors of childhood cancer in the United States: prevalence and burden of morbidity. Cancer Epidemiol Biomarkers Prev. 2015;24(4):653-663. doi: 10.1158/1055-9965.EPI-14-1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olsson L, Johansson B. Ikaros and leukaemia. Br J Haematol. 2015;169(4):479-491. doi: 10.1111/bjh.13342 [DOI] [PubMed] [Google Scholar]
- 5.Mullighan CG, Su X, Zhang J, et al. ; Children’s Oncology Group . Deletion of IKZF1 and prognosis in acute lymphoblastic leukemia. N Engl J Med. 2009;360(5):470-480. doi: 10.1056/NEJMoa0808253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harvey RC, Mullighan CG, Wang X, et al. Identification of novel cluster groups in pediatric high-risk B-precursor acute lymphoblastic leukemia with gene expression profiling: correlation with genome-wide DNA copy number alterations, clinical characteristics, and outcome. Blood. 2010;116(23):4874-4884. doi: 10.1182/blood-2009-08-239681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dörge P, Meissner B, Zimmermann M, et al. IKZF1 deletion is an independent predictor of outcome in pediatric acute lymphoblastic leukemia treated according to the ALL-BFM 2000 protocol. Haematologica. 2013;98(3):428-432. doi: 10.3324/haematol.2011.056135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuiper RP, Waanders E, van der Velden VH, et al. IKZF1 deletions predict relapse in uniformly treated pediatric precursor B-ALL. Leukemia. 2010;24(7):1258-1264. doi: 10.1038/leu.2010.87 [DOI] [PubMed] [Google Scholar]
- 9.van der Veer A, Waanders E, Pieters R, et al. Independent prognostic value of BCR-ABL1-like signature and IKZF1 deletion, but not high CRLF2 expression, in children with B-cell precursor ALL. Blood. 2013;122(15):2622-2629. doi: 10.1182/blood-2012-10-462358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ofverholm I, Tran AN, Heyman M, et al. Impact of IKZF1 deletions and PAX5 amplifications in pediatric B-cell precursor ALL treated according to NOPHO protocols. Leukemia. 2013;27(9):1936-1939. doi: 10.1038/leu.2013.92 [DOI] [PubMed] [Google Scholar]
- 11.Olsson L, Castor A, Behrendtz M, et al. Deletions of IKZF1 and SPRED1 are associated with poor prognosis in a population-based series of pediatric B-cell precursor acute lymphoblastic leukemia diagnosed between 1992 and 2011. Leukemia. 2014;28(2):302-310. doi: 10.1038/leu.2013.206 [DOI] [PubMed] [Google Scholar]
- 12.Yamashita Y, Shimada A, Yamada T, et al. IKZF1 and CRLF2 gene alterations correlate with poor prognosis in Japanese BCR-ABL1-negative high-risk B-cell precursor acute lymphoblastic leukemia. Pediatr Blood Cancer. 2013;60(10):1587-1592. doi: 10.1002/pbc.24571 [DOI] [PubMed] [Google Scholar]
- 13.Krentz S, Hof J, Mendioroz A, et al. Prognostic value of genetic alterations in children with first bone marrow relapse of childhood B-cell precursor acute lymphoblastic leukemia. Leukemia. 2013;27(2):295-304. doi: 10.1038/leu.2012.155 [DOI] [PubMed] [Google Scholar]
- 14.Stanulla M, Dagdan E, Zaliova M, et al. ; TRANSCALL Consortium; International BFM Study Group . IKZF1plus defines a new minimal residual disease-dependent very-poor prognostic profile in pediatric B-cell precursor acute lymphoblastic leukemia. J Clin Oncol. 2018;36(12):1240-1249. doi: 10.1200/JCO.2017.74.3617 [DOI] [PubMed] [Google Scholar]
- 15.Buitenkamp TD, Pieters R, Gallimore NE, et al. Outcome in children with Down’s syndrome and acute lymphoblastic leukemia: role of IKZF1 deletions and CRLF2 aberrations. Leukemia. 2012;26(10):2204-2211. doi: 10.1038/leu.2012.84 [DOI] [PubMed] [Google Scholar]
- 16.Stanulla M, Cavé H, Moorman AV. IKZF1 deletions in pediatric acute lymphoblastic leukemia: still a poor prognostic marker? Blood. 2020;135(4):252-260. doi: 10.1182/blood.2019000813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roberts KG, Li Y, Payne-Turner D, et al. Targetable kinase-activating lesions in Ph-like acute lymphoblastic leukemia. N Engl J Med. 2014;371(11):1005-1015. doi: 10.1056/NEJMoa1403088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scheijen B, Boer JM, Marke R, et al. Tumor suppressors BTG1 and IKZF1 cooperate during mouse leukemia development and increase relapse risk in B-cell precursor acute lymphoblastic leukemia patients. Haematologica. 2017;102(3):541-551. doi: 10.3324/haematol.2016.153023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clappier E, Auclerc MF, Rapion J, et al. An intragenic ERG deletion is a marker of an oncogenic subtype of B-cell precursor acute lymphoblastic leukemia with a favorable outcome despite frequent IKZF1 deletions. Leukemia. 2014;28(1):70-77. doi: 10.1038/leu.2013.277 [DOI] [PubMed] [Google Scholar]
- 20.Zaliova M, Zimmermannova O, Dörge P, et al. ERG deletion is associated with CD2 and attenuates the negative impact of IKZF1 deletion in childhood acute lymphoblastic leukemia. Leukemia. 2014;28(1):182-185. doi: 10.1038/leu.2013.282 [DOI] [PubMed] [Google Scholar]
- 21.Mangum DS, Downie J, Mason CC, et al. VPREB1 deletions occur independent of lambda light chain rearrangement in childhood acute lymphoblastic leukemia. Leukemia. 2014;28(1):216-220. doi: 10.1038/leu.2013.223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schultz KR, Pullen DJ, Sather HN, et al. Risk- and response-based classification of childhood B-precursor acute lymphoblastic leukemia: a combined analysis of prognostic markers from the Pediatric Oncology Group (POG) and Children’s Cancer Group (CCG). Blood. 2007;109(3):926-935. doi: 10.1182/blood-2006-01-024729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bowman WP, Larsen EL, Devidas M, et al. Augmented therapy improves outcome for pediatric high risk acute lymphocytic leukemia: results of Children’s Oncology Group trial P9906. Pediatr Blood Cancer. 2011;57(4):569-577. doi: 10.1002/pbc.22944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hunger SP, Loh ML, Whitlock JA, et al. ; COG Acute Lymphoblastic Leukemia Committee . Children’s Oncology Group’s 2013 blueprint for research: acute lymphoblastic leukemia. Pediatr Blood Cancer. 2013;60(6):957-963. doi: 10.1002/pbc.24420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larsen EC, Devidas M, Chen S, et al. Dexamethasone and high-dose methotrexate improve outcome for children and young adults with high-risk B-acute lymphoblastic leukemia: a report from Children’s Oncology Group Study AALL0232. J Clin Oncol. 2016;34(20):2380-2388. doi: 10.1200/JCO.2015.62.4544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mullighan CG, Goorha S, Radtke I, et al. Genome-wide analysis of genetic alterations in acute lymphoblastic leukaemia. Nature. 2007;446(7137):758-764. doi: 10.1038/nature05690 [DOI] [PubMed] [Google Scholar]
- 27.Loudin MG, Wang J, Leung HC, et al. Genomic profiling in Down syndrome acute lymphoblastic leukemia identifies histone gene deletions associated with altered methylation profiles. Leukemia. 2011;25(10):1555-1563. doi: 10.1038/leu.2011.128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith M, Arthur D, Camitta B, et al. Uniform approach to risk classification and treatment assignment for children with acute lymphoblastic leukemia. J Clin Oncol. 1996;14(1):18-24. doi: 10.1200/JCO.1996.14.1.18 [DOI] [PubMed] [Google Scholar]
- 29.Milligan MJ, Lipovich L. Pseudogene-derived lncRNAs: emerging regulators of gene expression. Front Genet. 2015;5:476. doi: 10.3389/fgene.2014.00476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Papaemmanuil E, Gerstung M, Bullinger L, et al. Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med. 2016;374(23):2209-2221. doi: 10.1056/NEJMoa1516192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Irving JA, Enshaei A, Parker CA, et al. Integration of genetic and clinical risk factors improves prognostication in relapsed childhood B-cell precursor acute lymphoblastic leukemia. Blood. 2016;128(7):911-922. doi: 10.1182/blood-2016-03-704973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cario G, Leoni V, Conter V, Baruchel A, Schrappe M, Biondi A. BCR-ABL1-like acute lymphoblastic leukemia in childhood and targeted therapy. Haematologica. 2020;105(9):2200-2204. doi: 10.3324/haematol.2018.207019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palmi C, Valsecchi MG, Longinotti G, et al. What is the relevance of Ikaros gene deletions as a prognostic marker in pediatric Philadelphia-negative B-cell precursor acute lymphoblastic leukemia? Haematologica. 2013;98(8):1226-1231. doi: 10.3324/haematol.2012.075432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Emanuel BS. Molecular mechanisms and diagnosis of chromosome 22q11.2 rearrangements. Dev Disabil Res Rev. 2008;14(1):11-18. doi: 10.1002/ddrr.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olsson L, Albitar F, Castor A, et al. Cooperative genetic changes in pediatric B-cell precursor acute lymphoblastic leukemia with deletions or mutations of IKZF1. Genes Chromosomes Cancer. 2015;54(5):315-325. doi: 10.1002/gcc.22245 [DOI] [PubMed] [Google Scholar]
- 36.Lana T, de Lorenzo P, Bresolin S, et al. Refinement of IKZF1 status in pediatric Philadelphia-positive acute lymphoblastic leukemia. Leukemia. 2015;29(10):2107-2110. doi: 10.1038/leu.2015.78 [DOI] [PubMed] [Google Scholar]
- 37.Boer JM, van der Veer A, Rizopoulos D, et al. Prognostic value of rare IKZF1 deletion in childhood B-cell precursor acute lymphoblastic leukemia: an international collaborative study. Leukemia. 2016;30(1):32-38. doi: 10.1038/leu.2015.199 [DOI] [PubMed] [Google Scholar]
- 38.Churchman ML, Low J, Qu C, et al. Efficacy of retinoids in IKZF1-mutated BCR-ABL1 acute lymphoblastic leukemia. Cancer Cell. 2015;28(3):343-356. doi: 10.1016/j.ccell.2015.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Churchman ML, Evans K, Richmond J, et al. Synergism of FAK and tyrosine kinase inhibition in Ph+ B-ALL. JCI Insight. 2016;1(4):86082. doi: 10.1172/jci.insight.86082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hunger SP. More is not always better: the perils of treatment intensification in pediatric acute lymphoblastic leukemia. J Clin Oncol. 2019;37(19):1601-1603. doi: 10.1200/JCO.19.00889 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Further Details on Samples and Data Analysis
eTable 1. Probe Density and Coordinates along Chromosome 22q11.22 and IKZF1 Coding Region
eTable 2. Patient Characteristics by Cohort
eTable 3. Patient Characteristics by 22q11.22 and IKZF1 Status
eTable 4. Sensitivity Analysis
eTable 5. Univariate Analyses of Event-free Survival and Overall Survival in NCI High Risk Patients with an IKZF1 Alteration
eTable 6. Multivariate Analyses of Event-free Survival and Overall Survival in NCI High Risk Patients with an IKZF1 Alteration
eTable 7. Additional Assessment of IKZF1plus in Multivariate Models of Patients Lacking a BCR-ABL1 Translocation
eFigure 1. Mean Copy Number Status Validation Using qPCR
eFigure 2. 22q11.22 Deletions in B-ALL.
eFigure 3. Patients with a 22q11.22 + IKZF1 Double Deletion Have Very Poor Event-free Survival (Individual Cohorts)
eFigure 4. Patients with a 22q11.22 + IKZF1 Double Deletion Have Very Poor Overall Survival (Individual Cohorts)
eFigure 5. Association of ALL Subtypes and 22q11.22 Deletions or IKZF1 Alterations in the AALL0232 Cohort
eFigure 6. Patients with a 22q11.22 + IKZF1 Double Deletion Have Worse Outcomes in Both Ph-like ALL and Non-Ph-like ALL
eFigure 7. Ph+ (BCR-ABL1+) Patients with IKZF1 Alterations Have Poor Outcomes Regardless of 22q11.22 Status
eFigure 8. 22q11.22 Deletions Stratify Outcomes among IKZF1plus Patients
eFigure 9. 22q11.22 + IKZF1 Double Deletion Patients Maintain Trend of Worse Outcomes when Compared to IKZF1 Altered and WT 22q11.22 Patients in Both MRD Positive and MRD Negative Settings
eFigure10. 22q11.22 + IKZF1 Double Deletion Patients Have Worse Outcomes when Compared to Patients without a Double Deletion in Both MRD Positive and MRD Negative Settings
eFigure 11. 22q11.22 + IKZF1 Double Deletion Patients Have Worse Outcomes in Both NCI High Risk and Standard Risk Patients
eFigure 12. Interaction-term Analysis
eFigure 13. Presence or Absence of VPREB1 Deletions Do Not Affect Outcome
eFigure 14. SOCS2 Gene Expression is Upregulated in Patients with 22q11.22 deletions
eFigure 15. 22q11.22 + IKZF1 Double Deletion Patients Do Not Have Increased Genomic Instability