Abstract
Large B-cell lymphomas, with an estimated 150,000 new cases annually worldwide, represent almost 30% of all cases of non-Hodgkin’s lymphoma. Patients typically present with progressive lymphadenopathy, extranodal disease, or both and require therapy. Despite the advanced stage at presentation in the majority of patients, more than 60% can be cured with R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) immunochemotherapy (Fig. 1A). Patients with treatment failure after R-CHOP often have a poor outcome — in particular, those with disease that is refractory to frontline or subsequent therapies — although some patients can have a durable remission and be cured after secondary therapies. Over the past two decades, improved insights into large B-cell lymphomas, in terms of epidemiology, prognostic factors, and biologic heterogeneity, have led to a refinement of disease classification and the development of new therapeutic approaches.
PATHOLOGICAL FEATURES AND MOLECULAR CLASSIFICATION
Diagnosis of large B-cell lymphomas relies on a detailed examination of tumor tissue, best achieved with an excisional biopsy specimen evaluated by an expert hematopathologist.5 In addition to morphologic characteristics, an accurate lymphoma classification requires specialized tests, including immunohistochemistry, flow cytometry, fluorescence in situ hybridization (FISH), and molecular testing. Biopsy specimens obtained by fine-needle aspiration are inadequate for pathological assessment. Although specimens from core biopsy are frequently used, they are often insufficient for a complete evaluation, and core biopsy should be performed only if excisional biopsy is not feasible.
The updated World Health Organization (WHO) classification has refined the categorization of large B-cell lymphomas, which are a heterogeneous collection of clinicopathological entities (Table 1),6 of which diffuse large B-cell lymphoma, not otherwise specified (DLBCL, NOS), is the most common. A detailed review of each disorder is beyond the scope of this article, and thoughtful management often requires consultative review.
Table 1.
Pathological and Clinical Characteristics of Large B-Cell Lymphomas According to the World Health Organization (WHO) Classification.*
| WHO Denomination | Diagnostic Features | Clinical Features and Outcome |
|---|---|---|
| Diffuse large B-cell lymphoma, NOS | ||
| Diffuse large B-cell lymphoma, NOS (accounts for >80% of cases of large B-cell lymphomas) Molecular subtypes: GCB subtype, about 60%; ABC subtype, about 25–30%; unclassifiable, about 10–15%; new molecular entities recently characterized |
Diffuse proliferation of medium or large lymphoid B cells typically expressing CD19, CD20, CD22, CD79a, PAX5, and surface or cytoplasmic immunoglobulin; molecular techniques (e.g., GEP) or IHC-based algorithms recommended to classify subtypes | Median age: 65–70 yr, nodal presentation most common, 30–40% of cases are primary extranodal; ABC subtype overrepresented among patients with primary extranodal lymphoma and elderly patients; prognosis varies |
| Other large B-cell lymphomas | ||
| T-cell/histiocyte-rich large B-cell lymphoma (rare) | Few large B cells embedded in a background of T cells and histiocytes; distinguish from nodular lymphocyte-predominant Hodgkin lymphoma | Commonly found in middle-aged men, advanced stage with extranodal involvement (liver, spleen, bone marrow); poor prognosis |
| Primary diffuse large B-cell lymphoma of the CNS (rare) | Typically ABC subtype; frequent loss of H LA class I/II; frequent mutation of MYD88 | Exclusively in CNS or intraocular region, rare systemic involvement; poor prognosis; specialized treatment with CNS-penetrating agents, with or without radiation therapy, required; targeted therapies under investigation |
| Primary cutaneous diffuse large B-cell lymphoma, leg type (rare) | Typically ABC subtype; frequent mutation of MYD88; distinguish from other cutaneous B-cell lymphoma | Typically in elderly patients and women; presents with skin nodules in lower legs; 10–15% of cases arise in other sites; poor prognosis |
| EBV-positive diffuse large B-cell lymphoma, NOS (rare) | Variable histologic features, including Hodgkin-like lesions, monomorphic to polymorphic patterns; EBV detectable in tumor and frequently in serum | Typically in patients older than 50 yr; more frequent in Asia and Latin America than elsewhere; extranodal involvement common; prognosis varies |
| EBV-positive mucocutaneous ulcer (rare) | Polymorphic infiltrate with frequent Hodgkin-like cells; EBV detectable in tumor | Presents as localized, ulcerated lesions in oral mucosa, intestine, or skin; dissemination is rare; commonly occurs as iatrogenic or age-related disease in immunocompromised patients; favorable prognosis; consider reduction of immunosuppressive therapy |
| Diffuse large B-cell lymphoma associated with chronic inflammation (rare) | Morphologically similar to DLBCL, NOS but strongly associated with EBV; also called pyothorax-associated lymphoma, when associated with chronic pyothorax | Occurs in context of chronic inflammation, involving pleural cavity or other sites such as bone and joints; male predominance; poor prognosis |
| Lymphomatoid granulomatosis (rare) | EBV-driven angiocentric and angiodestructive lymphoproliferation with reactive T cells; grade based on proportion of EBV-positive B cells and cytologic features | Commonly involves extranodal sites (lung >90%); often in context of immunodeficiency; prognosis varies; no standard therapy |
| Large B-cell lymphoma with IRF4 rearrangement (rare) | Strong expression of IRF4/MUM1, usually with IRF4 rearrangement; diffuse-to-follicular morphologic features; distinguish from pediatric-type follicular lymphoma | Commonly in children and young adults; typically involves Waldeyer’s ring or cervical lymph nodes; favorable prognosis |
| Primary mediastinal (thymic) large B-cell lymphoma (around 6% of large B-cell cases) | Putative thymic B-cell origin; medium-to-large B cells, frequently with sclerosis; distinctive phenotype (CD30, CD23, PDL1, PDL2) and unique GEP signature; frequent 9p21 amplification, genomic alterations of CIITA | Typically in young adults, female predominance; mediastinal prominence with local invasion; can involve other nodal or extranodal sites (kidney and liver); prognosis varies; DA-EPOCH-R an option |
| Intravascular large B-cell lymphoma (rare) | Lymphoma cells exclusively within lumina of small or intermediate vessels; bone marrow and skin biopsy may be useful to establish diagnosis | Wide intravascular dissemination (lung, bone marrow, skin, CNS, kidney), often associated with fever of unknown origin or neurologic or cutaneous symptoms; poor prognosis |
| ALK-positive large B-cell lymphoma (rare) | ALK-positive large B cells, immunoblastic features and plasma-cell phenotype, typically CD20-negative | Typically in young men with generalized lymphadenopathy; prognosis varies |
| Plasmablastic lymphoma (rare) | Immunoblastic or plasmablastic B cells, plasma-cell phenotype (CD138-positive, CD20-negative), often EBV-positive; distinguish from multiple myeloma | Often associated with HIV infection or immunosuppression; frequently extranodal; poor prognosis; consider more intensive regimens |
| HHV8-positive diffuse large B-cell lymphoma (rare) | HHV8-positive IgM lambda plasmablasts; often associated with HHV8-positive multicentric Castleman disease | Often associated with HIV infection; lymphadenopathy and splenomegaly are common; poor prognosis; no standard therapy |
| Primary effusion lymphoma (rare) | Immunoblastic or plasmablastic B cells, HHV8-positive and usually EBV-positive; plasma-cell phenotype lacking usual B-cell markers; CD20-negative | Often associated with HIV infection or immunosuppression; presents as pleural, pericardial, or peritoneal serous effusions, often without detectable tumor mass; poor prognosis; DA-EPOCH an option |
| High-grade B-cell lymphoma | ||
| High-grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements or both (double-hit or triple-hit lymphoma) (4–8% of large B-cell cases) | Variable morphology, including DLBCL, B-cell lymphoma unclassifiable (with features intermediate between DLBCL and Burkitt lymphoma), and blastoid features; MYC and BCL2 and/or BCL6 rearrangements, detected by FISH | Frequently aggressive clinical presentation; higher risk of CNS involvement; poor prognosis; consider more intensive immunochemotherapy regimens, such as DA-EPOCH-R |
| High-grade B-cell lymphoma, NOS (rare) | Heterogeneous category; often has morphologic features intermediate between DLBCL and Burkitt lymphoma; lacks MYC and BCL2 and/or BCL6 rearrangements | Frequently aggressive clinical presentation; increased risk of CNS involvement; poor prognosis; consider more intensive immunochemotherapy regimens |
| B-cell lymphoma, unclassifiable | ||
| B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma and classic Hodgkin lymphoma (grey-zone lymphoma) (rare) | Overlapping morphologic or immunophenotypic features, or both, between DLBCL and classic Hodgkin lymphoma | Male predominance, younger age (20–40 yr); mediastinal presentation most common (80% of cases) but can occur in other sites; prognosis varies; no standard therapy, consider therapy suitable for DLBCL or Hodgkin lymphoma |
Data are based on the updated WHO classification, and the terminology adheres to that of the WHO.6 For most rare entities, confirmation of the diagnosis by a hematopathologist with expertise in lymphoma is highly recommended. Also, since clinical management of these rare entities may evolve rapidly, with new therapies under investigation in clinical trials, consultation with a hemato-oncologist specializing in lymphoid cancer is recommended. The standard regimen for many of these entities continues to be R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone), although rituximab would be omitted in patients with lymphomas that are CD20-negative. Follicular lymphoma grades 3A and 3B also contain a variable but substantial proportion of large B cells; although treatment for follicular lymphoma grade 3B is commonly the same as treatment for DLBCL, it is not included in this table. Post-transplantation lymphoproliferative disorders may also present as large B-cell lymphoma but are beyond the scope of this review and generally require individualized management.7 Finally, transformed indolent lymphoma may present as various forms of large B-cell lymphoma; the presence of the indolent lymphoma component (follicular, marginal-zone, lymphoplasmacytic, chronic lymphocytic leukemia), when known, is usually noted in the pathological report, together with the diagnosis of the large B-cell lymphoma entity. Treatment of transformed lymphoma is generally directed at the large B-cell lymphoma but must take into account the underlying indolent disease, as well as likely prior therapies. ABC denotes activated B-cell–like, CNS central nervous system, DA-EPOCH-R dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin with rituximab, DLBCL diffuse large B-cell lymphoma, EBV Epstein–Barr virus, FISH fluorescence in situ hybridization, GCB germinal center B-cell–like, GEP gene expression profiling, HHV8 human herpesvirus 8, HIV human immunodeficiency virus, IHC immunohistochemistry, and NOS not otherwise specified.
This review focuses primarily on DLBCL, NOS (henceforth referred to simply as DLBCL), which is also highly heterogeneous. Gene expression profiling has delineated two distinct molecular subtypes of DLBCL, the germinal center B-cell–like (GCB) subtype and the activated B-cell–like (ABC) subtype; 10 to 15% of cases are unclassifiable.1 These subtypes are believed to arise from different stages of lymphoid differentiation (cell of origin), relying on separate oncogenic mechanisms, with the ABC subtype having an inferior outcome (3-year progression-free survival, approximately 40 to 50%, vs. 75% with the GCB subtype).8,9 The ABC subtype of DLBCL is characterized by chronic B-cell receptor signaling and activation of nuclear factor κB, whereas the GCB subtype expresses genes commonly detected in germinal center B cells, including BCL6 and EZH2 (Fig. 1C). This phenotypic distinction is relevant because targeted agents may be preferentially active in one subtype. Although gene expression profiling is rarely performed in clinical practice, platforms suitable for routine care may soon be available.9 Alternatively, immunohistochemistry-based algorithms, such as the Hans algorithm (Table 2), can be used to dichotomize cases as GCB and non-GCB (the latter comprising the ABC subtype and the majority of unclassified cases), although these algorithms provide only an approximation of gene expression profiling, with a risk of misclassification.10
Figure 1 (facing page). Outcomes of Diffuse Large B-Cell Lymphoma (DLBCL), Risk Factors, and Biologic Features.
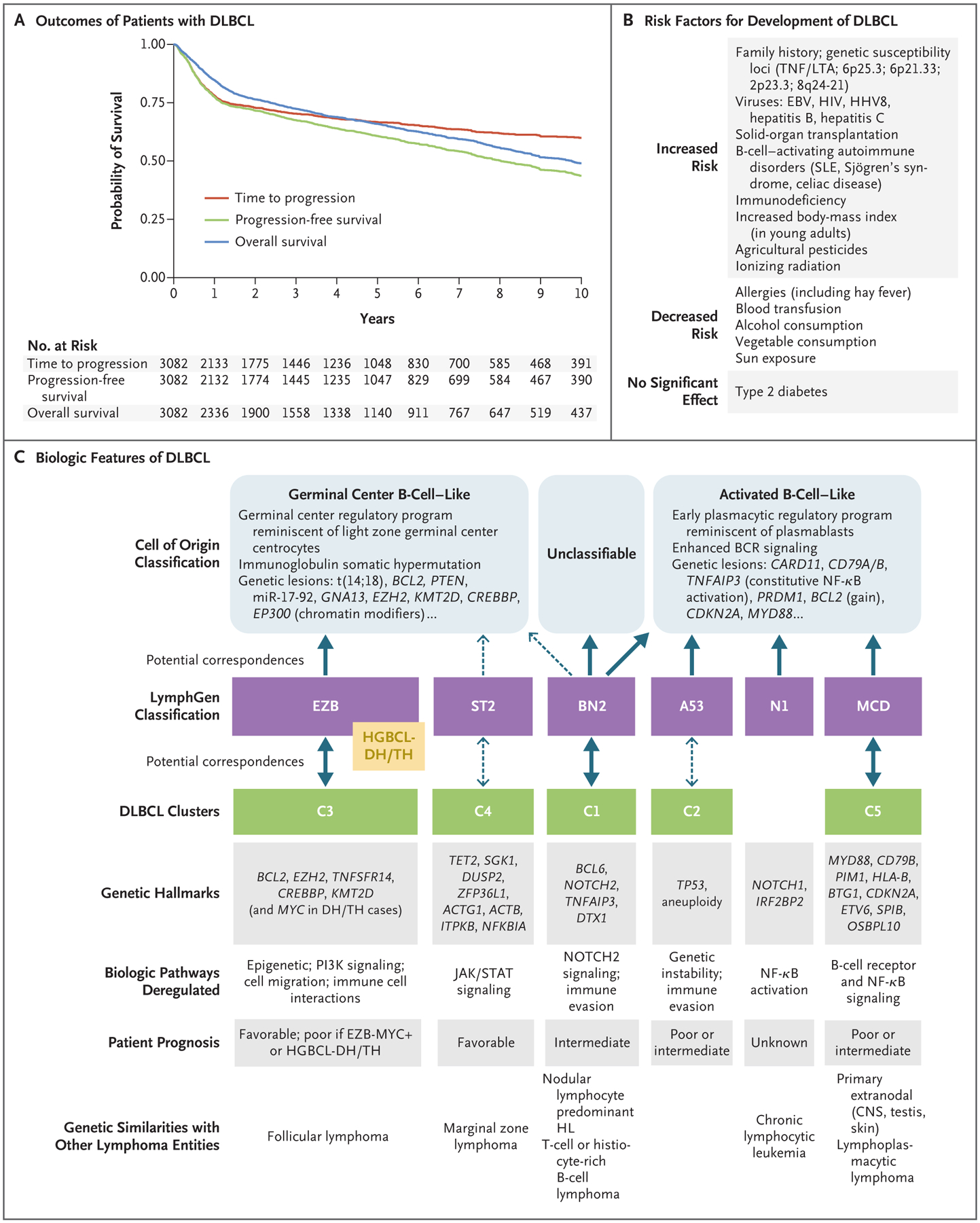
Panel A shows Kaplan–Meier survival estimates for all patients with newly diagnosed DLBCL treated with R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) in British Columbia, Canada (2001–2019). Time to progression (TTP) is measured from the date of diagnosis to the date of disease progression or death from lymphoma, with deaths from unrelated causes censored. This curve highlights that the risk of DLBCL progression is highest within the first 2 years, followed by a lower risk of progression for up to 10 years. Progression-free survival (PFS) is measured from the date of diagnosis to the date of progression or death from any cause. Given that the median age of patients with DLBCL is in the mid-60s, the difference between the TTP and PFS curves reflects the competing risk of death from unrelated causes. The marginal difference between the PFS and overall survival (OS) curves reflects the limited number of patients cured with secondary therapies, although new therapies may improve overall survival. Panel B shows reported risk factors for the development of DLBCL. Panel C shows the heterogeneous biologic features that reflect insights gained over the past 20 years. Gene expression profiling originally delineated two molecular subtypes, germinal center B-cell–like and activated B-cell–like, which are believed to arise from different stages of B-cell lymphoid differentiation (cell of origin), with gene expression resembling their normal B-cell counterparts.1 Distinct functional profiles and genetic aberrations have been identified within the two subtypes, but heterogeneity within these subtypes has also been recognized. On the basis of the results of in-depth genomic analyses, new taxonomies for DLBCL have been proposed, designated as the LymphGen classification2 and DLBCL clusters.3 These taxonomies further refine DLBCL genomic classification and may better delineate distinct biologic entities. The postulated associations between cell-of-origin molecular subtypes and these new genomic entities are denoted by solid arrows, indicating robust associations; dashed arrows indicate weaker associations or uncertain associations. Genetic hallmarks based on recurring genomic aberrations and resultant deregulated genetic pathways have been identified within entities, which are associated with varied prognoses. DLBCL with a MYC rearrangement and a concurrent rearrangement in BCL2, BCL6, or both (double-hit [DH] or triple-hit [TH] lymphoma) is currently classified as a high-grade B-cell lymphoma (HGBCL-DH/TH). HGBCL-DH/TH cases, together with cases with an EZB subtype with a MYC DH gene signature (EZB-MYC+),2,4 largely cluster with the EZB subtype and harbor biologic features associated with a poor clinical outcome. BCR denotes B-cell receptor, CNS central nervous system, EBV Epstein–Barr virus, HHV8 human herpesvirus 8, HIV human immunodeficiency virus, HL Hodgkin’s lymphoma, miR-17–92 microRNA cluster 17–92, NF-κB nuclear factor κB, PI3K phosphatidylinositol 3-kinase, SLE systemic lupus erythematosus, and TNF/LTA, tumor necrosis factor/lymphotoxin alpha.
Table 2.
Biologic Factors Associated with Outcomes in Patients with DLBCL.*
| Biomarker | Methodology | Prognostic Significance | Other Implications |
|---|---|---|---|
| Cell-of-origin molecular classification | Various technologies (gene array, digital expression profiling, multiplex RT-PCR-based methods) | ABC subtype is associated with poor prognosis | ABC subtype may be associated with an increased risk of CNS relapse |
| Cell-of-origin IHC-based algorithms | Various IHC-based algorithms to assign molecular subtype; most commonly the Hans algorithm† | Non-GCB subtype is associated with poor prognosis, although this is not confirmed in some studies | Dichotomizes patients into GCB and non-GCB subgroups and represents an approximation of molecular subtype as assessed by GEP |
| Double- or triple-hit rearrangement involving MYC and either BCL2 or BCL6 or both | FISH is used primarily in clinical practice; the use of break-apart probes is recommended; GEP-based assays may identify additional cases with double-hit signature undetected by FISH with similar biologic features and outcome‡ | Double- or triple-hit cases are associated with poor prognosis; poor prognosis may be limited to cases in which the MYC translocation partner is an immunoglobulin gene locus | Now classified by the WHO as high-grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements; majority of cases are GCB subtype; may benefit from more intensive therapies |
| MYC and BCL2 protein expression | IHC measurement to estimate the percentage of cells expressing MYC or BCL2 protein or both; 40% cutoff threshold for MYC and 50% for BCL2 | Double expression of MYC and BCL2 or expression of BCL2 alone is associated with worse prognosis | May have prognostic significance mainly in GCB-type DLBCL; MYC-BCL2 double expression may be associated with an increased risk of CNS relapse |
| Proliferation index | IHC measurement of proliferation marker Ki67; no established cutoff threshold | Higher proliferation may be associated with poorer prognosis, although it has not consistently been shown to be an independent prognostic marker | High proliferation rate (>80%) may increase suspicion that patient has high-grade B-cell lymphoma (with or without double- or triple-hit rearrangements) |
| TP53 | PCR, NGS, or gene array for detection of mutation or deletion of TP53 | TP53 mutations in the DNA-binding domain are associated with poor prognosis | May cluster with a genetic subset of DLBCL |
| CDKN2A | Gene array, FISH, or PCR for detection of deletion of the CDKN2A locus or loss of the 9p21 region | Deletion of the CDKN2A locus or loss of the 9p21 is associated with poor prognosis | May cluster with some genetic subsets of DLBCL |
| MHC class II | IHC measurement of partial or complete loss of MHC class II expression | Loss of expression of MHC class II may be associated with a poor prognosis (more frequent in non-GCB subtype) | Primarily observed in primary mediastinal B-cell lymphoma and in tumors with EZH2 mutations |
| Lymphocyte count and lymphocyte:monocyte ratio | Measured in peripheral blood; low lymphocyte count (<1 × 109/liter) or low lymphocyte:monocyte ratio (various cutoff thresholds) | Low lymphocyte count or low lymphocyte:monocyte ratio is associated with poor prognosis | May have implications for immunebased therapies |
| Host genetics | Single nucleotide variation in 5q23.2 or 6q21 (PCR or single nucleotide polymorphism array) | Single nucleotide variation in 5q23.2 or 6q21 is associated with poor prognosis | Further investigation is needed |
The list of select biologic factors correlated with outcomes in patients with DLBCL is based on reproducible observations, including validation in independent patient cohorts. NGS denotes next-generation sequencing, and RT-PCR reverse-transcriptase polymerase chain reaction.
The Hans algorithm is as follows: GCB: CD10+ or CD10−BCL6+MUM1−; non-GCB: CD10−BCL6−MUM1+ or CD10−BCL6+MUM1+ or CD10− BCL6−MUM1−.
The information on methods for detecting additional cases with the use of a double-hit gene-expression signature is from Ennishi et al.4
Detailed analyses of molecular aberrations (including gene mutations and copy-number gains or losses) have led to proposals of new taxonomies for DLBCL, yielding unique, genetically defined subtypes beyond the cell of origin2,3 (Fig. 1C). These newly proposed classification schemes may better delineate distinct biologic entities, providing greater potential for individualized therapeutic interventions. However, further validation and development of reproducible molecular assays will be required before clinical application is feasible.
In addition to the molecular heterogeneity of DLBCL described above, recurrent genetic rearrangements of clinical significance can be detected by FISH. A MYC rearrangement is seen in 12% of cases, whereas a MYC rearrangement concurrent with a rearrangement in BCL2, BCL6, or both occurs in 4 to 8% of cases with morphologic features of DLBCL, the majority of which are the GCB subtype, in which BCL2 rearrangements occur exclusively.11,12 These cases are now classified as “high-grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements,” commonly referred to as double- or triple-hit lymphoma, and are associated with a poor outcome after R-CHOP.6,11 Data suggest that the adverse outcome associated with double- or triple-hit high-grade B-cell lymphoma is primarily evident when MYC is translocated with an immunoglobulin gene partner (rarely assessed in clinical practice) and that concurrent rearrangements involving BCL2 or BCL6 have similar prognostic significance.11 Retrospective series suggesting that R-CHOP may be insufficient in such cases prompted the use of more intensive therapies, such as dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin with rituximab (DA-EPOCH-R), which may be associated with improved outcomes and are currently recommended in appropriate cases.13
In contrast to the relative rarity of double- or triple-hit high-grade B-cell lymphoma detected by FISH, overexpression of MYC protein as measured by immunohistochemical analysis occurs in approximately 45% of cases and overexpression of BCL2 protein occurs in approximately 65% of cases (in the absence of dual rearrangement of MYC and BCL2).12 The overexpression of both MYC and BCL2, occurring in approximately 30% of cases of DLBCL, termed double-expressor lymphoma, is associated with a worse prognosis than single or no overexpression of either MYC or BCL2.14 Double-expressor lymphoma is not a discrete biologic entity, since it can occur in both the GCB and ABC subtypes as a result of varied underlying molecular mechanisms, but it is more common in the ABC subtype, which may in part mediate the prognostic implications.
EPIDEMIOLOGIC FEATURES
The median age at diagnosis of DLBCL is in the mid-60s; 30% of patients are older than 75 years of age. Although the majority of patients present without a history of lymphoma, DLBCL can arise as a transformation from an underlying known or occult low-grade B-cell lymphoma. Epidemio-logic studies support a complex and multifactorial cause of DLBCL, with risk factors including genetic features, clinical characteristics, and immune dysregulation, as well as viral, environmental, or occupational exposures15 (Fig. 1B). Although DLBCL is not considered a heritable disease, genomewide association studies have identified multiple genetic susceptibility loci, implicating pathways involved with immune function.16 Screening procedures are not available.
STAGING AND RESPONSE ASSESSMENT
Staging and response assessment should be performed in accordance with Ann Arbor staging and the Lugano classification criteria5,17,18 (see Tables S1 and S2 in the Supplementary Appendix, available with the full text of this article at NEJM.org). In recent years, because of its higher sensitivity, 18F-fluorodeoxyglucose positron-emission tomography with computed tomography (PET-CT) has replaced CT.17 The total metabolic tumor volume at diagnosis may also be prognostic.19 Staging bone marrow biopsy is positive in 15 to 20% of cases and, when concordant large B cells are present, is associated with a poor prognosis.20 Bone marrow biopsy is no longer mandatory in patients who have undergone PET-CT staging, although low-volume disease or discordant indolent lymphoma (which does not alter the outcome) may occasionally be missed.5,21 End-of-treatment response evaluation is best performed by means of PET-CT, with interpretation according to the Deauville five-point scale (Table S3), with uptake in the mediastinum and liver used as reference points. A score of 1 or 2 and probably 3 is considered to indicate a complete metabolic response.17
The response during therapy can be assessed with the use of CT to detect nonresponding or progressive disease. Studies evaluating the merit of interim PET-CT have yielded conflicting results, although PET-CT after two to four cycles of treatment appears to be prognostic, particularly when the response is assessed with the use of quantitative methods.22 However, treatment modification based solely on interim PET-CT findings has not been shown to alter the outcome and thus is not recommended outside of clinical trials.17 Recently, circulating tumor DNA has shown promise as an interim response-assessment tool and is being actively investigated.23
Although data are limited, routine post-treatment surveillance imaging has not been shown to affect the outcome and is generally discouraged.5 Patients should be clinically monitored every 3 months for 2 years, then every 6 to 12 months.5 Patients who remain event-free for 2 years from the time of diagnosis have an expected overall survival that is almost similar to survival in the general, age-matched population.24 However, physicians should monitor patients for long-term risks, including late infectious complications, autoimmune disorders, secondary cancers, and cardiovascular events.
PROGNOSTIC FACTORS
The International Prognostic Index (IPI) remains the primary clinical tool for predicting outcomes and for stratifying patients in clinical trials.25 The IPI has been validated and refined in the modern era, with the National Comprehensive Cancer Network IPI (NCCN-IPI) allowing greater discrimination among high-risk patients26–28 (Table 3). However, these clinical indexes cannot be used to identify patients at very high risk or to discern biologic heterogeneity. Numerous biologic factors have been correlated with outcomes (Table 2). However, they have yet to be integrated into a validated prognostic index.
Table 3.
Clinical Indexes for Predicting Outcomes in Patients with DLBCL.*
| Prognostic Index, Clinical Factors, and Risk Categories | Proportion of Patients | Estimated 5-yr PFS | Estimated 5-yr OS |
|---|---|---|---|
| percent | |||
| IPI | |||
| Age, >60 yr; LDH, >ULN; Ann Arbor stage III or IV; ECOG performance status, >1; no. of extranodal sites of disease, >1 | |||
| Risk categories | |||
| Low (0 or 1 factor) | 34 | 81 | 88 |
| Low-intermediate (2 factors) | 23 | 67 | 76 |
| High-intermediate (3 factors) | 23 | 58 | 67 |
| High (4 or 5 factors) | 20 | 46 | 54 |
| R-IPI | |||
| Age, >60 yr; LDH, >ULN; Ann Arbor stage III or IV; ECOG performance status, >1; no. of extranodal sites of disease, >1 | |||
| Risk categories | |||
| Very good (0 factors) | 9 | 87 | 93 |
| Good (1 or 2 factors) | 48 | 74 | 81 |
| Poor (3–5 factors) | 43 | 53 | 61 |
| NCCN-IPI | |||
| Age, >40 to ≤60 yr (1 point), >60 to ≤75 yr (2 points), >75 yr (3 points); LDH ratio, >1 to ≤3 (1 point), >3 (2 points); Ann Arbor stage III or IV (1 point); ECOG performancestatus score, ≥2 (1 point); extranodal disease: lymphoma involvement in bone marrow, CNS, liver or GI tract, or lung (1 point) | |||
| Risk categories | |||
| Low (0 or 1 point) | 13 | 86 | 92 |
| Low-intermediate (2 or 3 points) | 41 | 75 | 84 |
| High-intermediate (4 or 5 points) | 36 | 54 | 63 |
| High (6–8 points) | 10 | 43 | 49 |
The three commonly used clinical prognostic indexes established over the past 30 years are based on the most discriminating clinical variables.25,27,28 The 5-year progression-free survival (PFS) and overall survival (OS) estimates are derived from a large international collaboration involving 2124 patients with DLBCL who were treated between 1998 and 2009 with frontline rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) or a variant in seven multicenter, randomized clinical trials.26 An age-adjusted International Prognostic Index (IPI), for patients who are 60 years of age or younger, has been designed that has only three factors: stage, lactate dehydrogenase (LDH) level, and performance status.25 A stage-modified IPI, designed for patients with limited-stage disease, has four factors: age (>60 years), stage (I or II), LDH level, and performance status.29 Other clinical factors associated with a poor outcome have been identified, but many have not retained prognostic significance in multivariable models including the presence of B symptoms, largest tumor diameter (≥7.5 cm or, more commonly, ≥10 cm used as a threshold), elevated serum β2-microglobulin level, low hemoglobin and serum albumin levels, and bone marrow involvement (although concordant bone marrow involvement with large B cells present has been shown to be an independent factor in some studies20). Recently, baseline total metabolic tumor volume, assessed with 18F-fluorodeoxyglucose positron-emission tomography and computed tomography, has been identified as a potentially independent prognostic measure.19 ECOG denotes Eastern Cooperative Oncology Group, GI gastrointestinal, NCCN-IPI National Comprehensive Cancer Network IPI, R-IPI Revised IPI, and ULN upper limit of the normal range.
PRIMARY MANAGEMENT
ADVANCED-STAGE DISEASE
Treatment of DLBCL relies on systemic therapy. Most patients (approximately 70%) present with advanced-stage disease, and historically, eight cycles of CHOP was established as the preferred chemotherapeutic regimen. The addition of the anti-CD20 monoclonal antibody rituximab subsequently led to a significant improvement in overall survival.30 A dose-intensive regimen of rituximab combined with doxorubicin, cyclophosphamide, vindesine, bleomycin, and prednisone (R-ACVBP) has been the only regimen providing a survival advantage over R-CHOP in patients with an age-adjusted IPI score of 1 (on a scale of 0 to 3, with higher scores indicating greater risk).31 However, clinically significant toxic effects curtailed its use. Attempts to improve outcomes by intensifying chemotherapy, with or without stem-cell transplantation, or by decreasing the interval between R-CHOP cycles to 14 days have not yielded a survival benefit (Table S4). In a randomized trial involving unselected patients with DLBCL, DA-EPOCH-R was associated with greater toxic effects and did not improve progression-free or overall survival in the overall cohort, as compared with R-CHOP.32 It is noteworthy that high-risk patients were underrepresented in this trial, and on post hoc analysis, patients with an IPI score of 3 to 5 (on a scale of 0 to 5, with higher scores indicating greater risk) had improved progression-free survival with DA-EPOCH-R, although there was no significant difference in overall survival between the two regimens. Although treatment with DA-EPOCH-R has shown encouraging outcomes in patients with double- or triple-hit high-grade B-cell lymphoma and those with primary mediastinal B-cell lymphoma, its use for patients with high-risk DLBCL remains investigational. A study of the anti-CD20 monoclonal antibody obinutuzumab did not show that it provided an additional benefit, as compared with rituximab.33 This study showed no added value of eight cycles of CHOP as compared with six cycles, thereby confirming six cycles of R-CHOP every 3 weeks as the standard of care.34,35
The value of consolidative radiation therapy after immunochemotherapy has not been proved. Patients with a complete metabolic response on post-treatment PET-CT have a favorable outcome without the use of radiation therapy.33,36 Whereas biopsy and further systemic therapy may be warranted in patients with a positive finding on PET-CT, consolidative radiation therapy may be considered in some patients without evidence of disease progression who have residual positive sites on PET-CT that are amenable to radiation therapy.36
Evaluating new therapies for patients with disease that is resistant to chemotherapy is a priority. However, in view of the biologic heterogeneity of DLBCL, targeted agents may benefit only select subgroups of patients, requiring biomarker assessment. Several large, randomized trials have evaluated the addition of new agents to R-CHOP (Table S4). Whereas the addition of the proteasome inhibitor bortezomib showed no benefit,37,38 the addition of the Bruton tyrosine kinase inhibitor ibrutinib yielded mixed findings. A phase 3 trial comparing ibrutinib and R-CHOP with R-CHOP alone in patients with non-GCB DLBCL (selected on the basis of immunohistochemical testing) showed no significant difference in outcomes between groups in the intention-to-treat population, but a secondary analysis suggested a survival benefit with the addition of ibrutinib for patients younger than 60 years of age; toxic effects in older patients impeded treatment with R-CHOP.39 The use of ibrutinib with R-CHOP requires further validation.
A randomized phase 2 trial evaluating the addition of lenalidomide to R-CHOP (R2-CHOP) in unselected patients suggested an improvement in progression-free and overall survival,40 but the definitive phase 3 trial involving patients with the ABC subtype of DLBCL (selected by means of gene expression profiling) showed no added value of lenalidomide.41 Several phase 3 trials failed to show a survival benefit of maintenance therapy after R-CHOP, with agents such as rituximab,42 enzastaurin,43 everolimus,44 or lenalidomide,45 adding to prior negative studies of maintenance chemotherapy.
Outside of clinical trials, R-CHOP has prevailed as the standard of care for DLBCL, regardless of the immunohistochemical profile or molecular subtype. However, the negative findings in recent trials should be interpreted in the context of numerous limitations. Delays incurred by biomarker testing probably led to selection bias, with underrepresentation of higher-risk patients that were in need of immediate treatment,46 limiting the statistical power to detect a benefit. Most important, biologic heterogeneity due to the molecular complexity of DLBCL, despite enrichment for cell of origin, may have limited the ability to detect a benefit within more discrete subgroups of patients. Future trials will need to have adaptive designs in order to maximize the likelihood of success.
LIMITED-STAGE DISEASE
Approximately 30% of patients present with limited-stage disease, commonly defined as stage I or II disease that is nonbulky (largest mass, <7.5 to 10 cm) and anatomically localized, without systemic symptoms. These patients tend to have low-risk clinical features and a favorable outcome, although a pattern of delayed relapse has been recognized.47 Before the introduction of rituximab, the standard treatment consisted of three cycles of CHOP and involved-field radiation therapy, since it improved overall survival, as compared with eight cycles of CHOP.29 However, this survival advantage was lost with longer follow-up as a result of late relapses and second cancers probably related to the radiation therapy, suggesting that chemotherapy alone may be appropriate.47 With a 5-year overall survival rate in the range of 85 to 95% for patients with limited-stage disease, recent efforts have focused on limiting the number of chemotherapy cycles or omitting radiation therapy (Table S4).
A randomized trial has confirmed that treatment with four cycles of R-CHOP alone is sufficient for patients 60 years of age or younger with nonbulky stage I or II disease (largest mass, <7.5 cm) who have no age-adjusted IPI risk factors (Eastern Cooperative Oncology Group [ECOG] performance status score of 0 or 1, on a scale of 0 to 5, with higher numbers indicating greater disability; and a normal lactate dehydrogenase level).48 PET-CT tailored therapy has been explored in patients with broader inclusion criteria. In a phase 3 trial, patients who had a complete metabolic response as indicated by PET-CT assessment after four cycles of R-CHOP did not benefit from the addition of radiation therapy, although patients with at least one IPI risk factor received six cycles of R-CHOP.49 Results from a phase 2 trial and a population-based analysis have shown that treatment with four cycles of R-CHOP alone appears to be sufficient in patients who have a complete metabolic response as indicated by PET-CT after three cycles of R-CHOP.50,51 Optimal management has not been fully defined for patients with a positive interim PET-CT assessment or for those with a high stage-modified IPI score or disease that has high-risk biologic features (few of whom have been included in recent trials).
PATIENTS FOR WHOM STANDARD THERAPY IS NOT FEASIBLE
Approximately 20 to 25% of patients are not candidates for treatment with standard frontline therapy such as R-CHOP because of poor fitness related to age, coexisting medical conditions, or cardiac dysfunction. Patients with a good baseline performance status whose functional status has been compromised by lymphoma may be considered for standard therapy. Comprehensive geriatric assessment or simple functional testing may be useful to identify patients for whom a modified approach is warranted. For such patients, dose-reduced versions of R-CHOP, such as R-mini-CHOP, may be used with curative intent.52 A short prephase of glucocorticoids, with or without vincristine, may improve the side-effect profile associated with treatment.53 In patients with a contraindication to anthracycline, substitution with gemcitabine or etoposide may provide satisfactory results, whereas trials of alternative anthracyclines or cardioprotective agents have not provided convincing evidence of safety or efficacy.54,55
CENTRAL NERVOUS SYSTEM PROPHYLAXIS
Recurrence of disease in the central nervous system (CNS), occurring in 3 to 5% of patients, is a devastating event, with median overall survival of less than 6 months.56–58 CNS recurrence is often manifested early after the completion of therapy, suggesting the presence of occult CNS involvement at diagnosis. The CNS-IPI risk model, which includes the five IPI risk factors and the presence of renal or adrenal involvement, stratifies patients into risk categories, with 12% of patients having a high risk of CNS recurrence (10 to 12% risk).58 Other factors may augment this risk, including ABC subtype, double expression of MYC and BCL2, and testicular involvement at presentation.56–58 The role of CNS prophylaxis that incorporates systemic CNS-penetrating agents remains unproved and controversial.59,60 Prophylactic intrathecal chemotherapy is no longer recommended for patients with DLBCL.61
MANAGEMENT OF RELAPSED OR REFRACTORY DISEASE
Approximately 10 to 15% of patients treated with R-CHOP have primary refractory disease (i.e., an incomplete response or a relapse within 6 months after treatment), and an additional 20 to 25% will have a relapse after an initial response, typically within the first 2 years.24 Outcomes remain poor for patients in whom frontline treatment fails, particularly patients with refractory disease, for whom the median overall survival is approximately 6 months.62 Patients with late relapses (>2 years after treatment) have somewhat better outcomes, although relapse with indolent lymphoma can occur, underscoring the need for repeat biopsy.63
TRANSPLANTATION-ELIGIBLE PATIENTS
Treatment with high-dose chemotherapy and autologous stem-cell transplantation (ASCT) offers the best chance of cure in patients with chemotherapy-sensitive relapsed or refractory disease, but because of advanced age and coexisting medical conditions, only half of such patients are considered to be candidates for transplantation. The commonly used platinum-based salvage regimens (rituximab with dexamethasone, high-dose cytarabine, and cisplatin [R-DHAP], rituximab with ifosfamide, carboplatin, and etoposide [R-ICE], and rituximab with gemcitabine, dexamethasone, and cisplatin [R-GDP]) have shown similar efficacy in randomized trials, and the choice of regimen may depend on institutional preference or the side-effect profile.64,65 Approximately 50% of patients have a response to initial salvage therapy and then undergo ASCT, with an overall cure rate in the range of 25 to 35%.64,65 Allogeneic transplantation may also be curative; however, the advantage of graft-versus-tumor effect is offset by higher treatment-related mortality. In light of the availability of new agents, the role of allogeneic transplantation in patients in whom ASCT has failed is unclear.
TRANSPLANTATION-INELIGIBLE PATIENTS
Patients who are not candidates for ASCT because of poor fitness due to age or coexisting medical conditions, those who do not have a response to salvage therapy, and those who have a relapse after ASCT are classified as transplantation ineligible. Ultimately, the majority of patients with relapsed or refractory DLBCL fall into this category, and sequential single-agent chemotherapy or a multiagent regimen with an acceptable side-effect profile, such as rituximab, gemcitabine, and oxaliplatin (R-GemOx), has frequently been used with palliative intent.66 However, the availability of novel agents, including chimeric antigen receptor (CAR) T-cell therapy, has provided alternatives with the potential for durable disease control and an apparent survival advantage, as compared with conventional therapy.
CAR T-CELL THERAPY
CAR T-cell therapy, a gene-modified cellular treatment, represents a major paradigm shift in the management of relapsed or refractory DLBCL. The first approved products involve autologous T cells targeting CD19. In pivotal trials, axicabtagene ciloleucel, tisagenlecleucel, and lisocabtagene maraleucel have been associated with overall and complete response rates in the range of 52 to 82% and 40 to 54%, respectively, among patients with relapsed or refractory aggressive B-cell lymphoma67–69 (Table 4). Updated follow-up of the pivotal study of axicabtagene ciloleucel showed that 37% of patients had ongoing complete responses at a median follow-up of 27 months.88 However, the reports of outcomes are likely to be optimistic because of patient selection. These CAR T-cell products have received regulatory approval for patients with relapsed or refractory aggressive B-cell lymphoma who have received at least two lines of systemic therapy, and randomized studies are evaluating the possibility of replacing ASCT with CAR T-cell therapy.
Table 4.
Select Agents in Development for the Treatment of DLBCL.*
| Class and Agent | Target | Clinical Trial Phase | Overall Response Rate | Complete Response Rate | Study |
|---|---|---|---|---|---|
| percent | |||||
| CAR T-cell therapy† | |||||
| Axicabtagene ciloleucel | CD19 | 1 | 82 | 54 | Neelapu et al.68 |
| Tisagenlecleucel | CD19 | 2 | 52 | 40 | Schuster et al.69 |
| Lisocabtagene maraleucel | CD19 | 1 | 73 | 53 | Abramson et al.67 |
| Monoclonal antibodies | |||||
| Tafasitamab | CD19 | 2a | 26 | 6 | Jurczak et al.70 |
| Tafasitamab plus lenalidomide | CD19 | 2 | 60 | 43 | Salles et al.71 |
| Antibody-drug conjugates | |||||
| Loncastuximab tesirine | CD19 | 1 | 42 | 23 | Hamadani et al.72 |
| Brentuximab vedotin | CD30 | 2 | 44 | 17 | Jacobsen et al.73 |
| Polatuzumab vedotin | CD79b | 1 | 52‡ | 13‡ | Palanca-Wessels et al.74 |
| Polatuzumab vedotin plus BR vs. BR | CD79b | 2, randomized | 45 vs. 17.5 | 40 vs. 17.5 | Sehn et al.75 |
| Bispecific antibodies | |||||
| Blinatumomab | CD19-CD3 | 2 | 43 | 19 | Viardot et al76 |
| Mosunetuzumab | CD20-CD3 | 1/1b | 35§ | 19§ | Schuster et al.77 |
| Glofitamab | CD20-CD3 | 1/1b | 41 | 29 | Hutchings et al.78 |
| Odronextamab | CD20-CD3 | 1 | 42¶ | 35¶ | Bannerji et al.79 |
| Epcoritamab | CD20-CD3 | 1/2 | 76‖ | 32‖ | Hutchings et al.80 |
| NF-κB and BCR modifiers | |||||
| Ibrutinib | BTK | 1/2 | 37 ABC, 5 GCB | 16 ABC, 0 GCB | Wilson et al.81 |
| Lenalidomide vs. investigator’s choice | Multiple, NF-κB | 2, randomized | 28 vs. 12 | 10 vs. 2 | Czuczman et al.82 |
| Agents with other targets | |||||
| Venetoclax | BCL2 | 1 | 18 | 12 | Davids et al.83 |
| Selinexor | XPO1 | 2b | 28 | 12 | Kalakonda et al.84 |
| Checkpoint inhibitors | |||||
| Nivolumab | PD-1 | 2 | ≤10 | ≤3 | Ansell et al.85 |
| Magrolimab | CD47 | 1b | 40 | 33 | Advani et al.86 |
| Epigenetic modifiers | |||||
| Tazemetostat | EZH2 | 2 | 17 EZH2 mt, 17 EZH2 wt | 3 EZH2 mt, 9 EZH2 wt | Ribrag et al.87 |
Results from early clinical trials involving patients with relapsed or refractory DLBCL are shown. BCL2 denotes B-cell lymphoma 2, BCR B-cell receptor, BR bendamustine plus rituximab, BTK Bruton’s tyrosine kinase, EZH2 enhancer of zeste homologue 2, mt mutant, NF-κB nuclear factor κB, PD-1 programmed cell death protein 1, wt wild type, and XPO1 exportin 1.
The three CD19-specific chimeric antigen receptor (CAR) T-cell products differ in the nature of the CAR construct and in the manufacturing processes (axicabtagene ciloleucel comprises bulk T cells retrovirally transduced with a receptor containing the CD28 costimulatory domain; tisagenlecleucel comprises bulk T cells lentivirally transduced with a receptor containing the 4−1BB costimulatory molecule; and lisocabtagene maraleucel comprises a 1:1 mix of CD4+ and CD8+ T cells separately transduced with a lentiviral vector coding for a receptor with the 4−1BB costimulatory domain). The bispecific CD3–CD20 antibodies present several differences in the antigen recognition domains of the antibodies and the number of binding sites to CD20, as well as in the route of administration (intravenous vs. subcutaneous). For these bispecific antibodies, early data from dose-escalation studies are presented.
Results pertain to patients receiving a dose of 1.8 mg per square meter of body-surface area or higher.
Results pertain to the aggressive non-Hodgkin’s lymphoma cohort.
Results pertain to patients receiving a dose of 80 mg or higher.
Results pertain to patients receiving a dose of 12 mg or higher.
Treatment with CAR T-cell therapy is associated with distinct toxic effects and may not be appropriate for all patients. The reported rate of grade 3 to 4 cytokine release syndrome and neurologic toxic effects has ranged from 2 to 22% and 10 to 28%, respectively.67–69 Currently, use of CAR T-cell therapy remains impeded by potential toxic effects, inadequate bridging therapy for patients with rapidly evolving disease, the requirement for specialized care, and economic considerations, with cost-effectiveness analyses placing it at a level that may not be feasible in some clinical settings.89 Ongoing development, including evaluation of constructs directed at alternative or multiple targets, as well as allogeneic “off-the-shelf” products, is likely to expand options in the future.
NOVEL THERAPIES
Despite the advance of CAR T-cell therapy, novel therapies are needed for relapsed or refractory DLBCL. Numerous agents are undergoing evaluation, and selected drugs of interest are listed in Table 4.
Antibody–drug conjugates allow selective delivery of cytotoxic agents to tumor cells with the use of targeted antibodies. Polatuzumab vedotin is an antibody–drug conjugate targeting CD79b, a component of the B-cell receptor complex.74 The combination of polatuzumab vedotin and bendamustine–rituximab has received regulatory approval on the basis of a randomized phase 2 trial involving transplantation-ineligible patients that showed a significant improvement in the rates of complete metabolic response, progression-free survival, and overall survival, as compared with bendamustine–rituximab alone.75 A phase 3 trial evaluating polatuzumab vedotin as a replacement for vincristine in R-CHOP in previously untreated patients has been completed, and the results are pending. Additional antibody–drug conjugates are undergoing clinical evaluation.72,73
Selinexor, a selective inhibitor of the nuclear export protein XPO1, leading to nuclear accumulation of tumor suppressor proteins, has also received regulatory approval for patients with relapsed or refractory DLBCL who have received at least two lines of therapy, on the basis of a phase 2 study showing modest single-agent activity.84 Tafasitamab is a humanized anti-CD19 monoclonal antibody providing a modest benefit as a single agent,70 but results from a phase 2 study of tafasitamab in combination with lenalidomide showed efficacy, leading to regulatory approval for patients with DLBCL who are transplantation-ineligible.71 Since this agent has the same target as CD19-directed CAR T-cell therapy, appropriate sequencing of these options needs to be assessed.
Various other immunotherapeutic approaches are under investigation. Despite efficacy in primary mediastinal B-cell lymphoma, programmed cell death protein 1 (PD-1) inhibitors have failed to show a benefit in patients with DLBCL.85 Magrolimab, a macrophage immune checkpoint inhibitor blocking the “don’t eat me” molecule CD47, appears to synergize with rituximab, enhancing macrophage cellular phagocytosis, and has shown encouraging activity in an early clinical trial.86
By targeting antigens on both tumor cells and T cells, bispecific antibodies induce T-cell activation, leading to cell-mediated cytotoxicity. Bispecific antibodies have shown potential in relapsed or refractory DLBCL, with durable remissions observed. Blinatumomab, a bispecific T-cell engager directed against CD3 and CD19, is active in DLBCL, but the development of this agent is hindered by a continuous infusion schedule and associated neurotoxicity.76 Several full-length bispecific antibodies targeting CD3 and CD20, which are in development, have a longer half-life, allowing for administration every 3 to 4 weeks, including the possibility of subcutaneous delivery. An ongoing phase 1–1b study of mosunetuzumab has shown promising response rates among patients with relapsed or refractory DLBCL, including patients in whom CAR T-cell therapy had failed, with durable responses observed.77 Additional agents targeting CD3 and CD20 that are in development and have shown preliminary efficacy are glofitamab, odronextamab, and epcoritamab.78–80
Other agents targeting apoptosis (the BCL2 inhibitor venetoclax), the B-cell receptor pathway (the Bruton tyrosine kinase inhibitor ibrutinib, as well as lenalidomide), and epigenetic regulators (the EZH2 inhibitor tazemetostat) have shown limited single-agent activity and are being explored in various combinations.81–83,87 As additional agents become available, the sequencing of rational synergistic combinations, guided by patient characteristics and underlying biologic features that are based on validated molecular assays and predictive biomarkers, would be the desired goal.
A decision tree for management of large B-cell lymphomas is provided in Figure 2.
Figure 2 (facing page). Algorithm for the Management of Large B-Cell Lymphomas.
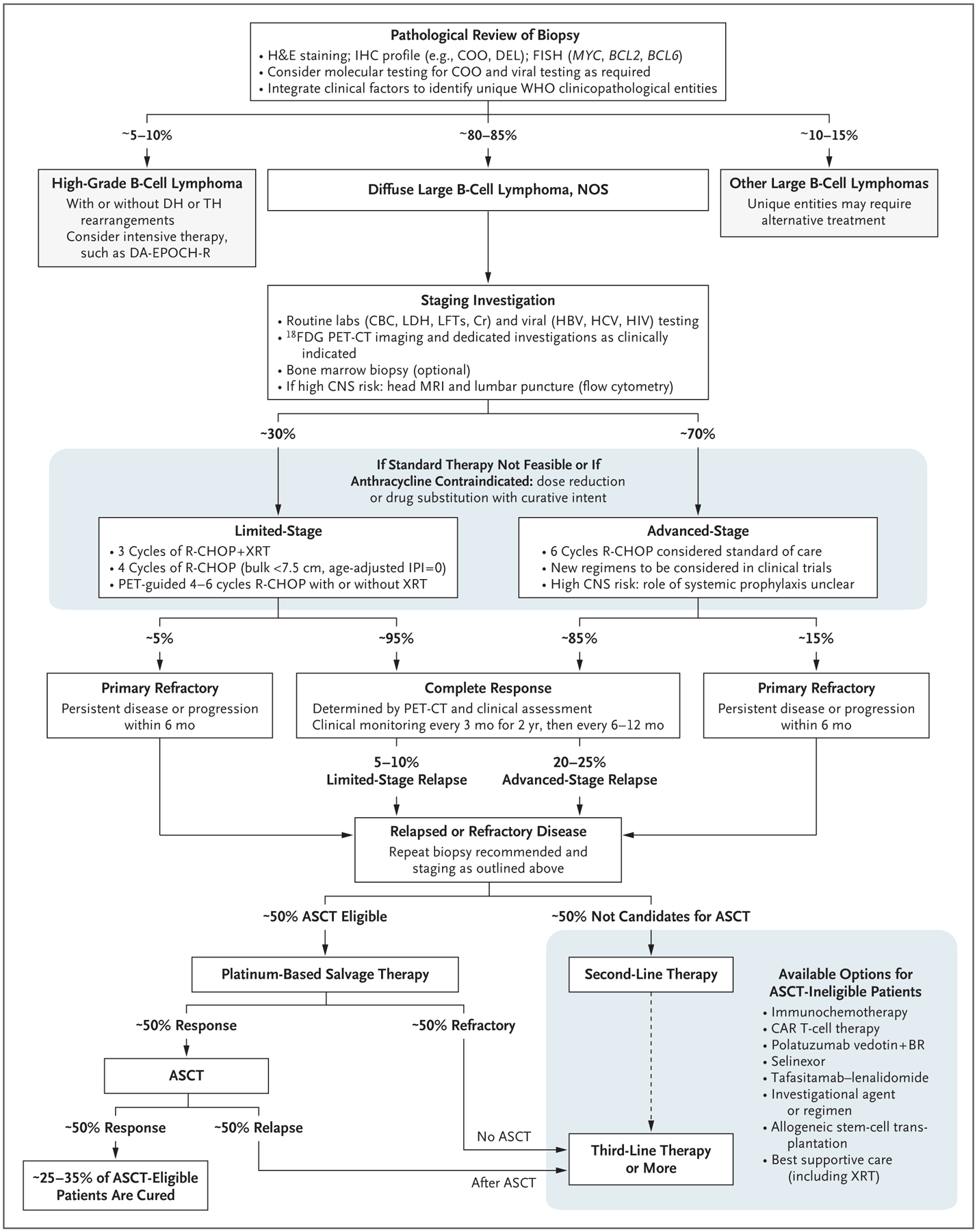
Diagnostic confirmation is based on careful pathological review of biopsy material (preferably from an excisional biopsy). Clinical and pathological features should be used to categorize patients according to the World Health Organization (WHO) classification for lymphoid cancers in order to identify patients with large B-cell lymphomas who may require alternative therapies. Routine staging investigations should be performed to distinguish patients with limited-stage disease (typically defined as Ann Arbor stage I or II, with nonbulky mass <7.5 to 10 cm, without systemic symptoms and with disease that can be encompassed by a radiation field) from those with advanced-stage disease. Evaluation of patients with a high risk of CNS involvement should include magnetic resonance imaging (MRI) of the brain and cytologic evaluation of cerebrospinal fluid, with flow cytometry to rule out occult CNS involvement. Patients with limited-stage disease may be treated with an abbreviated course of immunochemotherapy, with or without radiation therapy. Standard therapy for patients with advanced-stage disease is six cycles of R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) immunochemotherapy, regardless of the immunohistochemical (IHC) profile (e.g., double-expressor lymphoma [DEL]) or molecular subtype. Outcomes in patients with high-risk DLBCL remain unsatisfactory with R-CHOP, and clinical trials should strongly be considered. Although CNS-penetrating systemic therapy, such as high-dose methotrexate with R-CHOP, can be considered for patients at high risk for CNS recurrence, the value of this approach remains unproven. Response should be assessed with 18F-fluorodeoxyglucose positron-emission tomography and computed tomography (18F-FDG PET-CT), according to the Lugano classification criteria, with interpretation according to the Deauville five-point scale.5,17,18 Patients with evidence of relapsed or refractory disease should undergo repeat biopsy and staging to optimize further therapy. Patients who are eligible for autologous stem-cell transplantation (ASCT eligible) should receive platinum-based salvage therapy, with those who have a response proceeding to ASCT. Patients who do not have a response to salvage therapy or who have a relapse after ASCT, as well as those who are not candidates for ASCT because of age and coexisting medical conditions, are considered to be ASCT ineligible. There are numerous treatment alternatives for these patients, and selection of therapy should be individualized on the basis of disease and clinical characteristics of the patient. Based on regulatory approvals, some options may be indicated only for third-line therapy and beyond (e.g., CAR T-cell therapy at present) and therefore thoughtful sequencing of available therapies is instrumental. Clinical trials incorporating new agents should strongly be considered at all phases of therapy. CBC denotes complete blood count, COO cell of origin, Cr creatinine, DA-EPOCH-R dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin with rituximab, FISH fluorescence in situ hybridization, H&E hematoxylin and eosin, HBV hepatitis B virus, HCV hepatitis C virus, LDH lactate dehydrogenase, LFTs liver-function tests, NOS not otherwise specified, and XRT radiation therapy.
Supplementary Material
Acknowledgments
Dr. Sehn reports receiving advisory board fees from AbbVie, AstraZeneca, Gilead, Genentech, Janssen, Merck, Takeda, Apobiologix, Acerta, Celgene, Kite, Karyopharm, Morphosys, Lund-beck, TG Therapeutics, Verastem, Sandoz, Incyte, Novartis, Genmab, and Debiopharm, grant support and advisory board fees from Teva, and advisory board fees and lecture fees from Roche and Seattle Genetics; and Dr. Salles, receiving honoraria from Amgen, BMS, Acerta, AbbVie, Janssen, Merck, Gilead/Kite, Morphosys, Servier, Celgene, Roche/Genentech, and Takeda, honoraria and consulting fees from Novartis and Epizyme, advisory board fees from Pfizer, Autolus, Allogene, Beigene, Debiopharm, Genmab, and Velosbio, and consulting fees from Miltenyi and IPSEN. No other potential conflict of interest relevant to this article was reported.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
We thank Drs. Joseph M. Connors, David W. Scott, and Ahmet Dogan for their helpful comments on an earlier version of the manuscript.
References
- 1.Rosenwald A, Wright G, Chan WC, et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med 2002; 346: 1937–47. [DOI] [PubMed] [Google Scholar]
- 2.Wright GW, Huang DW, Phelan JD, et al. A probabilistic classification tool for genetic subtypes of diffuse large B cell lymphoma with therapeutic implications. Cancer Cell 2020; 37(4): 551–568.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chapuy B, Stewart C, Dunford AJ, et al. Molecular subtypes of diffuse large B cell lymphoma are associated with distinct pathogenic mechanisms and outcomes. Nat Med 2018; 24: 679–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ennishi D, Jiang A, Boyle M, et al. Double-hit gene expression signature defines a distinct subgroup of germinal center B-cell-like diffuse large B-cell lymphoma. J Clin Oncol 2019; 37: 190–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol 2014; 32: 3059–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Thiele J. WHO classification of tumors of the haematopoietic and lymphoid tissues. Lyon, France: International Agency for Research on Cancer, 2017. [Google Scholar]
- 7.Dierickx D, Habermann TM. Post-transplantation lymphoproliferative disorders in adults. N Engl J Med 2018; 378: 549–62. [DOI] [PubMed] [Google Scholar]
- 8.Lenz G, Wright G, Dave SS, et al. Stromal gene signatures in large-B-cell lymphomas. N Engl J Med 2008; 359: 2313–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scott DW, Mottok A, Ennishi D, et al. Prognostic significance of diffuse large B-cell lymphoma cell of origin determined by digital gene expression in formalin-fixed paraffin-embedded tissue biopsies. J Clin Oncol 2015; 33:2848–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meyer PN, Fu K, Greiner TC, et al. Immunohistochemical methods for predicting cell of origin and survival in patients with diffuse large B-cell lymphoma treated with rituximab. J Clin Oncol 2011; 29: 200–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosenwald A, Bens S, Advani R, et al. Prognostic significance of MYC rearrangement and translocation partner in diffuse large B-cell lymphoma: a study by the Lunenburg Lymphoma Biomarker Consortium. J Clin Oncol 2019; 37: 3359–68. [DOI] [PubMed] [Google Scholar]
- 12.Scott DW, King RL, Staiger AM, et al. High-grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements with diffuse large B-cell lymphoma morphology. Blood 2018; 131: 2060–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petrich AM, Gandhi M, Jovanovic B, et al. Impact of induction regimen and stem cell transplantation on outcomes in double-hit lymphoma: a multicenter retrospective analysis. Blood 2014; 124: 2354–61. [DOI] [PubMed] [Google Scholar]
- 14.Johnson NA, Slack GW, Savage KJ, et al. Concurrent expression of MYC and BCL2 in diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol 2012; 30: 3452–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cerhan JR, Kricker A, Paltiel O, et al. Medical history, lifestyle, family history, and occupational risk factors for diffuse large B-cell lymphoma: the InterLymph Non-Hodgkin Lymphoma Subtypes Project. J Natl Cancer Inst Monogr 2014; 2014: 15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cerhan JR, Berndt SI, Vijai J, et al. Genome-wide association study identifies multiple susceptibility loci for diffuse large B cell lymphoma. Nat Genet 2014; 46: 1233–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barrington SF, Mikhaeel NG, Kostakoglu L, et al. Role of imaging in the staging and response assessment of lymphoma: consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J Clin Oncol 2014; 32: 3048–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheson BD, Ansell S, Schwartz L, et al. Refinement of the Lugano Classification lymphoma response criteria in the era of immunomodulatory therapy. Blood 2016; 128: 2489–96. [DOI] [PubMed] [Google Scholar]
- 19.Vercellino L, Cottereau A-S, Casasnovas O, et al. High total metabolic tumor volume at baseline predicts survival independent of response to therapy. Blood 2020; 135: 1396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sehn LH, Scott DW, Chhanabhai M, et al. Impact of concordant and discordant bone marrow involvement on outcome in diffuse large B-cell lymphoma treated with R-CHOP. J Clin Oncol 2011; 29: 1452–7. [DOI] [PubMed] [Google Scholar]
- 21.Rutherford SC, Herold M, Hiddemann W, et al. Impact of bone marrow biopsy on response assessment in immunochemotherapy-treated lymphoma patients in GALLIUM and GOYA. Blood Adv 2020; 4: 1589–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Casasnovas R-O, Meignan M, Berriolo-Riedinger A, et al. SUVmax reduction improves early prognosis value of interim positron emission tomography scans in diffuse large B-cell lymphoma. Blood 2011; 118: 37–43. [DOI] [PubMed] [Google Scholar]
- 23.Kurtz DM, Scherer F, Jin MC, et al. Circulating tumor DNA measurements as early outcome predictors in diffuse large B-cell lymphoma. J Clin Oncol 2018; 36: 2845–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maurer MJ, Ghesquières H, Jais J-P, et al. Event-free survival at 24 months is a robust end point for disease-related outcome in diffuse large B-cell lymphoma treated with immunochemotherapy. J Clin Oncol 2014; 32: 1066–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.The International Non-Hodgkin’s Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin’s lymphoma. N Engl J Med 1993; 329: 987–94. [DOI] [PubMed] [Google Scholar]
- 26.Ruppert AS, Dixon JG, Salles G, et al. International prognostic indices in diffuse large B-cell lymphoma: a comparison of IPI, R-IPI, and NCCN-IPI. Blood 2020; 135: 2041–8. [DOI] [PubMed] [Google Scholar]
- 27.Sehn LH, Berry B, Chhanabhai M, et al. The revised International Prognostic Index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood 2007; 109: 1857–61. [DOI] [PubMed] [Google Scholar]
- 28.Zhou Z, Sehn LH, Rademaker AW, et al. An enhanced International Prognostic Index (NCCN-IPI) for patients with diffuse large B-cell lymphoma treated in the rituximab era. Blood 2014; 123: 837–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller TP, Dahlberg S, Cassady JR, et al. Chemotherapy alone compared with chemotherapy plus radiotherapy for localized intermediate- and high-grade non-Hodgkin’s lymphoma. N Engl J Med 1998; 339: 21–6. [DOI] [PubMed] [Google Scholar]
- 30.Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med 2002; 346: 235–42. [DOI] [PubMed] [Google Scholar]
- 31.Récher C, Coiffier B, Haioun C, et al. Intensified chemotherapy with ACVBP plus rituximab versus standard CHOP plus rituximab for the treatment of diffuse large B-cell lymphoma (LNH03–2B): an open-label randomised phase 3 trial. Lancet 2011; 378: 1858–67. [DOI] [PubMed] [Google Scholar]
- 32.Bartlett NL, Wilson WH, Jung S-H, et al. Dose-adjusted EPOCH-R compared with R-CHOP as frontline therapy for diffuse large B-cell lymphoma: clinical outcomes of the phase III Intergroup Trial Alliance/CALGB 50303. J Clin Oncol 2019; 37: 1790–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vitolo U, Trněný M, Belada D, et al. Obinutuzumab or rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone in previously untreated diffuse large B-cell lymphoma. J Clin Oncol 2017; 35: 3529–37. [DOI] [PubMed] [Google Scholar]
- 34.Pfreundschuh M, Schubert J, Ziepert M, et al. Six versus eight cycles of bi-weekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20+ B-cell lymphomas: a randomised controlled trial (RICOVER-60). Lancet Oncol 2008; 9: 105–16. [DOI] [PubMed] [Google Scholar]
- 35.Sehn LH, Congiu A, Culligan DJ, et al. No added benefit of eight versus six cycles of CHOP when combined with rituximab in previously untreated diffuse large B-cell lymphoma patients: results from the international phase III GOYA study. Blood 2018; 132: Suppl 1: 783. abstract. [Google Scholar]
- 36.Freeman CL, Savage KJ, Villa D, et al. Long-Term Results of PET-Guided Radiation in Advanced-Stage Diffuse Large B-Cell Lymphoma Patients Treated with R-CHOP. Blood 2020September1 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 37.Davies A, Cummin TE, Barrans S, et al. Gene-expression profiling of bortezomib added to standard chemoimmunotherapy for diffuse large B-cell lymphoma (REMoDL-B): an open-label, randomised, phase 3 trial. Lancet Oncol 2019; 20: 649–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leonard JP, Kolibaba KS, Reeves JA, et al. Randomized phase II study of R-CHOP with or without bortezomib in previously untreated patients with non-germinal center B-cell-like diffuse large B-cell lymphoma. J Clin Oncol 2017; 35: 3538–46. [DOI] [PubMed] [Google Scholar]
- 39.Younes A, Sehn LH, Johnson P, et al. Randomized phase III trial of ibrutinib and rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone in non-germinal center B-cell diffuse large B-cell lymphoma. J Clin Oncol 2019; 37: 1285–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nowakowski GS, Hong F, Scott DW, et al. Addition of lenalidomide to R-CHOP improves outcomes in newly diagnosed diffuse large B-cell lymphoma in a randomized phase II US Intergroup study ECOG-ACRIN E1412. J Clin Oncol 2021February8(Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nowakowski GS, Chiappella A, Gascoyne R, et al. ROBUST: a phase III study of lenalidomide plus R-CHOP versus placebo plus R-CHOP in previously untreated patients with ABC-type diffuse large B-cell lymphoma. J Clin Oncol (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Habermann TM, Weller EA, Morrison VA, et al. Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma. J Clin Oncol 2006; 24: 3121–7. [DOI] [PubMed] [Google Scholar]
- 43.Crump M, Leppä S, Fayad L, et al. Randomized, double-blind, phase III trial of enzastaurin versus placebo in patients achieving remission after first-line therapy for high-risk diffuse large B-cell lymphoma. J Clin Oncol 2016; 34: 2484–92. [DOI] [PubMed] [Google Scholar]
- 44.Witzig TE, Tobinai K, Rigacci L, et al. Adjuvant everolimus in high-risk diffuse large B-cell lymphoma: final results from the PILLAR-2 randomized phase III trial. Ann Oncol 2018; 29: 707–14. [DOI] [PubMed] [Google Scholar]
- 45.Thieblemont C, Tilly H, Gomes da Silva M, et al. Lenalidomide maintenance compared with placebo in responding elderly patients with diffuse large B-cell lymphoma treated with first-line rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol 2017; 35: 2473–81. [DOI] [PubMed] [Google Scholar]
- 46.Maurer MJ, Ghesquières H, Link BK, et al. Diagnosis-to-treatment interval is an important clinical factor in newly diagnosed diffuse large B-cell lymphoma and has implication for bias in clinical trials. J Clin Oncol 2018; 36: 1603–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stephens DM, Li H, LeBlanc ML, et al. Continued risk of relapse independent of treatment modality in limited-stage diffuse large B-cell lymphoma: final and long-term analysis of Southwest Oncology Group Study S8736. J Clin Oncol 2016; 34: 2997–3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Poeschel V, Held G, Ziepert M, et al. Four versus six cycles of CHOP chemotherapy in combination with six applications of rituximab in patients with aggressive B-cell lymphoma with favourable prognosis (FLYER): a randomised, phase 3, non-inferiority trial. Lancet 2019; 394: 2271–81. [DOI] [PubMed] [Google Scholar]
- 49.Lamy T, Damaj G, Soubeyran P, et al. R-CHOP 14 with or without radiotherapy in nonbulky limited-stage diffuse large B-cell lymphoma. Blood 2018; 131:174–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Persky DO, Li H, Stephens DM, et al. Positron emission tomography-directed therapy for patients with limited-stage diffuse large B-cell lymphoma: results of Intergroup National Clinical Trials Network Study S1001. J Clin Oncol 2020; 38: 3003–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sehn LH, Scott DW, Villa D, et al. Long-term follow-up of a PET-guided approach to treatment of limited-stage diffuse large B-cell lymphoma (DLBCL) in British Columbia (BC). Blood 2019; 134: Suppl 1: 401. abstract. [Google Scholar]
- 52.Peyrade F, Jardin F, Thieblemont C, et al. Attenuated immunochemotherapy regimen (R-miniCHOP) in elderly patients older than 80 years with diffuse large B-cell lymphoma: a multicentre, single-arm, phase 2 trial. Lancet Oncol 2011; 12: 460–8. [DOI] [PubMed] [Google Scholar]
- 53.Peyrade F, Bologna S, Delwail V, et al. Combination of ofatumumab and reduced-dose CHOP for diffuse large B-cell lymphomas in patients aged 80 years or older: an open-label, multicentre, single-arm, phase 2 trial from the LYSA group. Lancet Haematol 2017; 4(1): e46–e55. [DOI] [PubMed] [Google Scholar]
- 54.Fields PA, Townsend W, Webb A, et al. De novo treatment of diffuse large B-cell lymphoma with rituximab, cyclophosphamide, vincristine, gemcitabine, and prednisolone in patients with cardiac comorbidity: a United Kingdom National Cancer Research Institute trial. J Clin Oncol 2014; 32: 282–7. [DOI] [PubMed] [Google Scholar]
- 55.Moccia AA, Schaff K, Freeman C, et al. Long-term outcomes of R-CEOP show curative potential in patients with DLBCL and a contraindication to anthracyclines. Blood Adv (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Klanova M, Sehn LH, Bence-Bruckler I, et al. Integration of cell of origin into the clinical CNS International Prognostic Index improves CNS relapse prediction in DLBCL. Blood 2019; 133: 919–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Savage KJ, Slack GW, Mottok A, et al. Impact of dual expression of MYC and BCL2 by immunohistochemistry on the risk of CNS relapse in DLBCL. Blood 2016; 127: 2182–8. [DOI] [PubMed] [Google Scholar]
- 58.Schmitz N, Zeynalova S, Nickelsen M, et al. CNS International Prognostic Index: a risk model for CNS relapse in patients with diffuse large B-cell lymphoma treated with R-CHOP. J Clin Oncol 2016; 34: 3150–6. [DOI] [PubMed] [Google Scholar]
- 59.Cheah CY, Herbert KE, O’Rourke K, et al. A multicentre retrospective comparison of central nervous system prophylaxis strategies among patients with high-risk diffuse large B-cell lymphoma. Br J Cancer 2014; 111: 1072–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Orellana-Noia V, Reed DR, Sen JM, et al. CNS prophylaxis during front-line therapy in aggressive non-Hodgkin lymphomas: real-world outcomes and practice patterns from 19 US academic institutions. Blood 2020; 136: Suppl 1: 478. abstract. [Google Scholar]
- 61.Eyre TA, Djebbari F, Kirkwood AA, Collins GP. Efficacy of central nervous system prophylaxis with stand-alone intrathecal chemotherapy in diffuse large B-cell lymphoma patients treated with anthracycline-based chemotherapy in the rituximab era: a systematic review. Haematologica 2020; 105: 1914–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Crump M, Neelapu SS, Farooq U, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood 2017; 130: 1800–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang Y, Farooq U, Link BK, et al. Late relapses in patients with diffuse large B-cell lymphoma treated with immunochemotherapy. J Clin Oncol 2019; 37: 1819–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Crump M, Kuruvilla J, Couban S, et al. Randomized comparison of gemcitabine, dexamethasone, and cisplatin versus dexamethasone, cytarabine, and cisplatin chemotherapy before autologous stem-cell transplantation for relapsed and refractory aggressive lymphomas: NCIC-CTG LY.12. J Clin Oncol 2014; 32: 3490–6. [DOI] [PubMed] [Google Scholar]
- 65.Gisselbrecht C, Glass B, Mounier N, et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol 2010; 28: 4184–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mounier N, El Gnaoui T, Tilly H, et al. Rituximab plus gemcitabine and oxaliplatin in patients with refractory/relapsed diffuse large B-cell lymphoma who are not candidates for high-dose therapy: a phase II Lymphoma Study Association trial. Haematologica 2013; 98: 1726–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Abramson JS, Palomba ML, Gordon LI, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet 2020; 396: 839–52. [DOI] [PubMed] [Google Scholar]
- 68.Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N Engl J Med 2017; 377: 2531–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med 2019; 380: 45–56. [DOI] [PubMed] [Google Scholar]
- 70.Jurczak W, Zinzani PL, Gaidano G, et al. Phase IIa study of the CD19 antibody MOR208 in patients with relapsed or refractory B-cell non-Hodgkin’s lymphoma. Ann Oncol 2018; 29: 1266–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Salles G, Duell J, González Barca E, et al. Tafasitamab plus lenalidomide in relapsed or refractory diffuse large B-cell lymphoma (L-MIND): a multicentre, prospective, single-arm, phase 2 study. Lancet Oncol 2020; 21: 978–88. [DOI] [PubMed] [Google Scholar]
- 72.Hamadani M, Radford J, Carlo-Stella C, et al. Final results of a phase 1 study of loncastuximab tesirine in relapsed/refractory B-cell non-Hodgkin lymphoma. Blood 2020November19 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jacobsen ED, Sharman JP, Oki Y, et al. Brentuximab vedotin demonstrates objective responses in a phase 2 study of relapsed/refractory DLBCL with variable CD30 expression. Blood 2015; 125: 1394–402. [DOI] [PubMed] [Google Scholar]
- 74.Palanca-Wessels MCA, Czuczman M, Salles G, et al. Safety and activity of the anti-CD79B antibody-drug conjugate polatuzumab vedotin in relapsed or refractory B-cell non-Hodgkin lymphoma and chronic lymphocytic leukaemia: a phase 1 study. Lancet Oncol 2015; 16: 704–15. [DOI] [PubMed] [Google Scholar]
- 75.Sehn LH, Herrera AF, Flowers CR, et al. Polatuzumab vedotin in relapsed or refractory diffuse large B-cell lymphoma. J Clin Oncol 2020; 38: 155–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Viardot A, Goebeler M-E, Hess G, et al. Phase 2 study of the bispecific T-cell engager (BiTE) antibody blinatumomab in relapsed/refractory diffuse large B-cell lymphoma. Blood 2016; 127: 1410–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schuster S, Bartlett N, Assouline S, et al. Mosunetuzumab induces complete remissions in poor prognosis non-Hodgkin lymphoma patients, including those who are resistant to or relapsing after chimeric antigen receptor T-cell (CAR-T) therapies, and is active in treatment through multiple lines. Blood 2019; 134: Suppl 1: 6. abstract.31273004 [Google Scholar]
- 78.Hutchings M, Morschhauser F, Iacoboni G, et al. Glofitamab, a novel, bivalent CD20 targeting T-cell engaging bispecific antibody, induces durable complete remissions in relapsed/refractory B-cell lymphoma: a phase I trial. J Clin Oncol (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bannerji R, Allan JA, Arnason JE, et al. Odronextamab (REGN1979), a human CD20 × CD3 bispecific antibody, induces durable, complete responses in patients with highly refractory B-cell non-Hodgkin lymphoma, including patients refractory to CAR T therapy. Presented at the 62nd American Society of Hematology virtual Annual Meeting and Exposition, December5–8, 2020. abstract. [Google Scholar]
- 80.Hutchings M, Mous R, Clausen MR, et al. Subcutaneous epcoritamab induces complete responses with an encouraging safety profile across relapsed/refractory B-cell non-Hodgkin lymphoma subtypes, including patients with prior CAR-T therapy: updated dose escalation data. Blood 2020; 136: Suppl 1: 45–6. abstract. [Google Scholar]
- 81.Wilson WH, Young RM, Schmitz R, et al. Targeting B cell receptor signaling with ibrutinib in diffuse large B cell lymphoma. Nat Med 2015; 21: 922–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Czuczman MS, Trněný M, Davies A, et al. A phase 2/3 multicenter, randomized, open-label study to compare the efficacy and safety of lenalidomide versus investigator’s choice in patients with relapsed or refractory diffuse large B-cell lymphoma. Clin Cancer Res 2017; 23: 4127–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Davids MS, Roberts AW, Seymour JF, et al. Phase I first-in-human study of venetoclax in patients with relapsed or refractory non-Hodgkin lymphoma. J Clin Oncol 2017; 35: 826–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kalakonda N, Maerevoet M, Cavallo F, et al. Selinexor in patients with relapsed or refractory diffuse large B-cell lymphoma (SADAL): a single-arm, multinational, multicentre, open-label, phase 2 trial. Lancet Haematol 2020; 7(7): e511–e522. [DOI] [PubMed] [Google Scholar]
- 85.Ansell SM, Minnema MC, Johnson P, et al. Nivolumab for relapsed/refractory diffuse large B-cell lymphoma in patients ineligible for or having failed autologous transplantation: a single-arm, phase II study. J Clin Oncol 2019; 37: 481–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Advani R, Flinn I, Popplewell L, et al. CD47 blockade by Hu5F9-G4 and rituximab in non-Hodgkin’s lymphoma. N Engl J Med 2018; 379: 1711–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ribrag V, Morschhauser F, McKay P, et al. Interim results from an ongoing phase 2 multicenter study of tazemetostat, an EZH2 inhibitor, in patients with relapsed or refractory (R/R) diffuse large B-cell lymphoma (DLBCL). Blood 2018; 132: Suppl 1: 4196. abstract. [Google Scholar]
- 88.Locke FL, Ghobadi A, Jacobson CA, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1–2 trial. Lancet Oncol 2019; 20: 31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lin JK, Muffly LS, Spinner MA, Barnes JI, Owens DK, Goldhaber-Fiebert JD. Cost effectiveness of chimeric antigen receptor T-cell therapy in multiply relapsed or refractory adult large B-cell lymphoma. J Clin Oncol 2019; 37: 2105–19. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


