Abstract
Background
Atrial fibrillation (AF) associated ischemic stroke has worse functional outcomes, less effective recanalization, and increased rates of hemorrhagic complications after intravenous thrombolysis (IVT). Limited data exist about the effect of AF on procedural and clinical outcomes after mechanical thrombectomy (MT).
Objective
To determine whether recanalization efficacy, procedural speed, and clinical outcomes differ in AF associated stroke treated with MT.
Methods
We performed a retrospective cohort study of the Stroke Thrombectomy and Aneurysm Registry (STAR) from January 2015 to December 2018 and identified 4169 patients who underwent MT for an anterior circulation stroke, 1517 (36.4 %) of whom had comorbid AF. Prospectively defined baseline characteristics, procedural outcomes, and clinical outcomes were reported and compared.
Results
AF predicted faster procedural times, fewer passes, and higher rates of first pass success on multivariate analysis (p<0.01). AF had no effect on intracranial hemorrhage (aOR 0.69, 95% CI 0.43 to 1.12) or 90-day functional outcomes (aOR 1.17, 95% CI 0.91 to 1.50) after MT, although patients with AF were less likely to receive IVT (46% vs 54%, p<0.0001).
Conclusions
In patients treated with MT, comorbid AF is associated with faster procedural time, fewer passes, and increased rates of first pass success without increased risk of intracranial hemorrhage or worse functional outcomes. These results are in contrast to the increased hemorrhage rates and worse functional outcomes observed in AF associated stroke treated with supportive care and or IVT. These data suggest that MT negates the AF penalty in ischemic stroke.
INTRODUCTION
Atrial fibrillation (AF) remains prevalent, undertreated, and a common cause of acute ischemic stroke (AIS).1,2 Large registry studies have demonstrated that comorbid AF is an independent predictor of poor functional outcome and increased mortality after an ischemic stroke.3–6 This is partly explained by covariate older age and medical comorbidities; however, AF associated strokes predicted larger territories of hypoperfusion and larger infarct volumes.3,7,8
The benefit of intravenous thrombolysis (IVT) with alteplase is also modified by comorbid AF. Comorbid AF independently increases the risk of intracranial hemorrhage after IVT, although this may be secondary to the larger infarct burden.7,9,10 Consistent with this observation, recanalization rates after IVT have been reported to be lower in AF associated stroke.11,12
Together, these data suggest that the AF associated cardioemboli may have distinct histologic characteristics that underlie these observed clinical differences. The rise of endovascular therapy for AIS has facilitated analysis of acute clots, driving advances in clot science. A number of studies have reported that fibrin-rich thrombi correlate with atheroembolic strokes, whereas erythrocyte-rich thrombi are more often associated with cardioembolic stroke, although this has recently been challenged.13–15
Whether these histologic differences predict responsiveness to mechanical reperfusion remains unclear. A secondary analysis of the MR CLEAN trial reported a non-significant trend towards decreased benefit of mechanical thrombectomy (MT) in patients with AF, while a subsequent meta-analysis demonstrated no interaction between AF and functional outcomes.16,17 Similarly, single-center reports have recently reported worse outcomes for AF associated stroke treated with MT for acute large vessel occlusions, secondary to both increased clot size and more resistant clots.18 Conversely, a national registry study assessing post-thrombectomy outcomes found no difference in either in-hospital or discharge outcomes between patients with or without AF, whereas a separate single-center study suggested higher rates of recanalization with AF associated stroke.19,20 Whether AF associated clots are more readily retrievable, or harder to remove, remains unclear.
We therefore aim to assess whether recanalization efficacy, procedural time, and hemorrhagic complications differ in AF associated large vessel occlusions undergoing MT using a large multicenter, international dataset.
METHODS
Study population
Patient data were reviewed from the Stroke Thrombectomy and Aneurysm Registry (STAR), which includes all patients (18 years of age or older) undergoing MT for AIS at 15 comprehensive stroke centers between January 2015 and December 2018. Only patients treated for anterior circulation emergent large vessel occlusions (internal carotid artery, M1, A1 or M2) with modern endovascular devices that were described in the 2015 major thrombectomy trials or after were included. Patients were allocated to the AF group if they had an established diagnosis of AF prior to presentation with AIS, or if AF was diagnosed during the stroke work-up prior to discharge. To guard against confounding comorbid AF and carotid atheroembolism, patients were excluded from analysis if they had both AF and underwent carotid angioplasty or stenting during the thrombectomy. The registry did not assess the completeness of the stroke workup; patients were therefore not excluded due to the presence or absence of any specific diagnostic tests. Additionally, data on antithrombotic use and comorbid heart failure or valvular disease are not currently reported in the registry. This study is covered by approval from institutional review boards at each participating institution, and informed consent was waived given the retrospective design of the study.
Mechanical thrombectomy
Patient selection for MT was based on operator judgment and discussion with patient families. It was not influenced by this study. Participating centers used different selection criteria for patient eligibility. Investigators had no uniform onset-to-groin cut-off point for offering intervention. The frontline thrombectomy approach used was based on operator preference and included aspiration thrombectomy (or a direct aspiration first pass technique), stent retriever, primary combined approach or, in a few cases, intracranial angioplasty and stenting. Success of recanalization was reported using the modified Thrombolysis in Cerebral Infarction (TICI) score obtained by the operator at the end of the procedure.21 Postprocedural hemorrhage was assessed using postoperative CT or MRI performed at 24 hours after the procedure.
Data collection
Demographic data, admission deficits, severity scores, onset-to-groin time, and IVT use were reviewed from patient charts. Procedure notes and imaging reports were reviewed for technical variables, reperfusion scores (TICI), and hemorrhage scores. Postprocedural hemorrhage was scored by neuroradiologists based on European Cooperative Acute Stroke Study II (ECASS II) criteria.22 Successful recanalization was defined as a TICI score of 2b or more.
Clinical outcomes
The modified Rankin Scale (mRS) score was the primary outcome measure. mRS scores were obtained during routinely scheduled follow-up visits with stroke neurologists or advanced practice providers at 90-days post-stroke (±14 days). If patients were discharged to a nursing home or hospice, telephone encounters were used. Telephone encounters with family were used to confirm mortality of deceased patients. A good outcome was defined as a mRS score 0–2. Postprocedural National Institutes of Health Stroke Scale (NIHSS) scores (within 24 hours), NIHSS at discharge, and/or follow-up were also available for a subset of patients.
Complications
Procedural notes were reviewed for intraoperative complications, including the type of complication and need for intervention. Additionally, postprocedural hemorrhage was evaluated by a neuroradiologist on postoperative CT or MRI imaging (24 hours) based on ECASS II criteria.22 Symptomatic intracranial hemorrhage (sICH) was defined as postprocedural hemorrhage associated with an increase of at least 4 on the NIHSS.
Statistical analysis
Statistical analyses were performed in SPSS v.25 (IBM) and GraphPad Prism 9 (GraphPad, California, USA). Univariate testing was performed using Student’s t-test, Mann-Whitney test, or χ2 test for parametric, non-parametric, and categorical variables, respectively. Multivariate analysis was then performed using independent models for different outcome measures. Variables included in regression included predetermined variables (age, sex, admission NIHSS score, comorbidities) and variables with p<0.1 on univariate testing. To avoid bias in excluding patients with incomplete data, we used multiple imputations to handle missing baseline variables (race, onset-to-groin, sex, and other comorbidities), and Rubin’s rule was then used to approximate coefficients. A total of 10 imputations was performed for each model. Logistic regression models were used for categorical variables (eg, good outcome), and linear regression models were used for continuous variables (eg, procedure time). A p value <0.05 was considered statistically significant.
RESULTS
Demographic data
A total of 5621 patients underwent MT for AIS at 15 stroke centers during the study period, of whom 4169 had an anterior circulation stroke and were included. Among the included subset, 1517 (36.4%) patients had comorbid AF that was diagnosed either before, or at the time of, presentation.
Table 1 reports patient baseline and presentation characteristics. Patients with AF were more likely to be older, female, white, and have vascular risk factors, including hypertension and hyperlipidemia (p<0.05). At presentation, patients with AF had higher NIHSS scores on admission, lower Alberta Stroke Program Early CT Score (ASPECTS), and a lower rate of IVT with tissue plasminogen activator (tPA; p<0.05). There was no difference in onset-to-groin time or pre-stroke mRS scores between the two groups (p>0.05, table 1). The distribution of occluded vessels per group is shown in online supplemental figure 1 and was comparable between the two groups. The majority of patients presented with M1 occlusions (AF vs no AF, 57% vs 56%, p>0.05).
Table 1.
Patient demographic, admission, technical, radiographic, and clinical outcome variables
| Variable | AF |
No AF |
Test | Standardized mean difference | P value | ||
|---|---|---|---|---|---|---|---|
| N | Mean (SD) Median (IQR) N (%) |
N | Mean (SD) Median (IQR) N (%) |
||||
| Demographics | |||||||
| Age | 1517 | 76 (11) | 2652 | 65 (15) | t-test | 0.75 | 0.001 |
| Female | 1517 | 877 (58%) | 2651 | 1299 (49%) | Chi-sq | 0.02 | |
| White race | 1133 | 754 (67%) | 2107 | 1241 (59%) | Chi-sq | 0.001 | |
| Comorbidities | |||||||
| Diabetes | 1511 | 453 (30%) | 2650 | 721 (27%) | Chi-sq | 0.06 | |
| Hypertension | 1517 | 1252 (83%) | 2652 | 1860 (70%) | Chi-sq | 0.001 | |
| Hyperlipidemia | 1514 | 668 (44%) | 2652 | 979 (37%) | Chi-sq | 0.001 | |
| Prior stroke | 1070 | 196 (18%) | 1982 | 287 (14%) | Chi-sq | 0.006 | |
| Pre-stroke mRS score 0–2 | 1085 | 986 (91%) | 2022 | 1871 (93%) | Chi-sq | 0.121 | |
| Admission variables | |||||||
| Admission NIHSS score | 1502 | 16 (6) | 2630 | 15 (7) | t-test | 0.15 | 0.001 |
| ASPECTS | 1112 | 8 (2) | 1889 | 9 (3) | MW | 0.37 | 0.015 |
| IV tPA use | 1401 | 651 (46%) | 2498 | 1340 (54%) | Chi-sq | 0.001 | |
| Onset-to-groin time (hours) | 1139 | 5.9 (7.2) | 2147 | 6.1 (8.4) | t-test | 0.02 | 0.476 |
| Procedural variables | |||||||
| Procedure time (min) | 1331 | 51 (43) | 2382 | 56 (44) | t-test | 0.10 | 0.002 |
| Total attempts | 1368 | 2.1 (1.6) | 2439 | 2.3 (1.7) | MW | 0.12 | 0.001 |
| First pass success | 902 | 377 (42%) | 1574 | 552 (35%) | Chi-sq | 0.001 | |
| Final TICI score | 1437 | 2477 | Chi-sq | 0.301 | |||
| 0–2a | 233 (16%) | 369 (15%) | |||||
| 2b–3 | 1204 (84%) | 2108 (85%) | |||||
| ICA angioplasty | 1437 | 0 (0.0%) | 1481 | 82 (6%) | Chi-sq | 0.001 | |
| Complications | 909 | 78 (9%) | 1841 | 157 (9%) | Chi-sq | 0.999 | |
| Hemorrhage (any type) | 1322 | 300 (23%) | 2382 | 571 (24%) | Chi-sq | 0.396 | |
| Hemorrhage (PH2/sICH) | 1165 | 89 (8%) | 2197 | 160 (7%) | Chi-sq | 0.729 | |
| Outcome | |||||||
| mRS score: discharge | 796 | 4 (3) | 1319 | 3 (3) | MW | 0.12 | 0.001 |
| mRS score: 90 days | 1368 | 4 (2) | 2439 | 3 (3) | MW | 0.30 | 0.001 |
| mRS score 0–2 | 1368 | 426 (31%) | 2434 | 1029 (42%) | Chi-sq | 0.001 | |
| mRS score 3–6 | 1368 | 942 (69%) | 2434 | 1405 (58%) | Chi-sq | 0.001 | |
| Mortality: 90 days | 1368 | 354 (26%) | 2434 | 408 (17%) | Chi-sq | 0.001 | |
Statistical tests were done using t-tests, chi squared tests (Chi-sq), or Mann-Whitney (MW) tests. A standardized mean difference >0.10 is considered meaningful.
AF, atrial fibrillation; ASPECTS, Alberta Stroke Program Early CT Score; ICA, internal carotid artery; IV tPA, intravenous tissue plasminogen activator; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; PH2, parenchymal hematoma type II (ECASS II criteria); sICH, symptomatic intracranial hemorrhage; TICI, thrombolysis in cerebral infarction.
Procedural metrics
Univariate analysis for procedural variables is also shown in table 1, notable for faster procedural time in patients with AF (51 min vs 56 min, p=0.002). Comorbid AF was associated with fewer total number of thrombectomy attempts (mean 2.2 vs 2.4, p=0.016) and a higher rate of first pass success (42% vs 35%, p=0.001). Angiographic outcomes were similar between the two groups, with similar rates of TICI 2b and TICI 3 reperfusion.
To better determine whether comorbid AF is an independent predictor of faster recanalization and a lower number of attempts, we performed multivariate analyses while controlling for potential confounders (figure 1). Using multivariate linear regression, comorbid AF was an independent predictor of shorter procedure time (adjusted coefficient (-) 5.4, p<0.001) and fewer attempts to achieve recanalization (adjusted coefficient (-) 0.2, p<0.001). On multivariate logistic regression for predictors of first pass success, AF was associated with higher odds of first pass success (adjusted OR=1.29, p=0.008, figure 1). Additional predictors of first pass success and procedure time are reported in figure 1.
Figure 1.
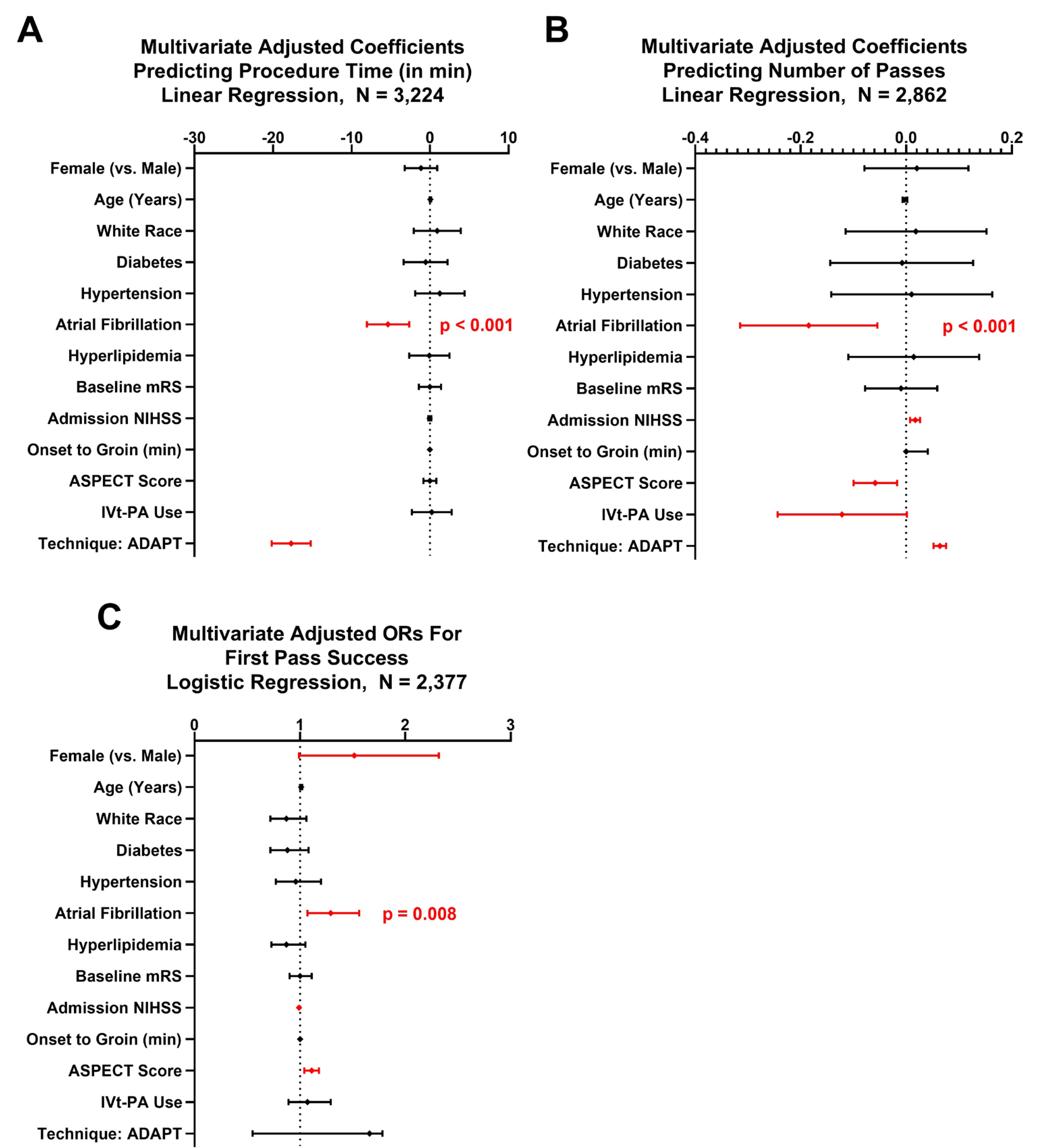
Multivariate regression analyses for predictors of procedure time (A), number of attempts (B), and success of first pass (C). Shown are adjusted ORs or estimates with error bars representing 95% confidence intervals (CIs). Significant estimates (p<0.05) are highlighted in red.
Postprocedural hemorrhage
Given the association of AF with sICH after IVT, we also tested whether AF was an independent risk factor for sICH after MT. There was no significant difference in rates of sICH or parenchymatous hematoma type 2 (PH2) between patients with or without AF with univariate analysis (table 1). On multivariate analysis for predictors of sICH and or PH2 (sICH/PH2), neither IVT nor AF were independent predictors of postprocedural sICH/PH2 in the full cohort or in successfully recanalized patients only (TICI 2b or higher,table 2). Only advanced age and lower ASPECT scores were independently associated with higher rates of sICH/PH2 in the full cohort (table 2).
Table 2.
Multivariate logistic regression for predictors of postprocedural sICH/PH2 hemorrhage
| Variable | Coefficient 95% CI | P value | |
|---|---|---|---|
|
| |||
| Full cohort | |||
| Model: logistic regression, n=1823 | |||
| Age | 1.02 | 1 to 1.03 | 0.026 |
| White race | 1.06 | 0.7 to 1.6 | 0.798 |
| Diabetes mellitus | 1.44 | 0.94 to 2.19 | 0.090 |
| Hypertension | 1.13 | 0.66 to 1.93 | 0.655 |
| Hyperlipidemia | 1.02 | 0.68 to 1.54 | 0.915 |
| Atrial fibrillation | 0.76 | 0.49 to 1.18 | 0.219 |
| Pre-stroke mRS score 0–2 | 0.94 | 0.47 to 1.89 | 0.867 |
| Admission NIHSS score | 1.01 | 0.98 to 1.04 | 0.576 |
| IV tPA use | 1.32 | 0.87 to 2 | 0.189 |
| Onset-to-groin | 1.00 | 0.99 to 1.01 | 0.290 |
| ASPECTS | 0.89 | 0.79 to 0.99 | 0.038 |
| Successful recanalization | 0.89 | 0.51 to 1.55 | 0.674 |
| Attempts | 1.02 | 0.91 to 1.14 | 0.762 |
| IA tPA | 1.28 | 0.77 to 2.14 | 0.339 |
| TICI 2b–3 Cohort | |||
| Model: logistic regression, n=1614 | |||
| Age | 1.02 | 1.01 to 1.04 | 0.007 |
| White race | 1.03 | 0.66 to 1.6 | 0.914 |
| Diabetes mellitus | 1.52 | 0.96 to 2.4 | 0.071 |
| Hypertension | 0.9 | 0.51 to 1.59 | 0.724 |
| Hyperlipidemia | 1.2 | 0.77 to 1.86 | 0.425 |
| Atrial fibrillation | 0.69 | 0.43 to 1.12 | 0.133 |
| Pre-stroke mRS score 0–2 | 1 | 0.47 to 2.16 | 0.997 |
| Admission NIHSS score | 1.01 | 0.98 to 1.04 | 0.623 |
| IV tPA use | 1.28 | 0.82 to 2.01 | 0.283 |
| Onset-to-groin | 1 | 1 to 1 | 0.385 |
| ASPECTS | 0.89 | 0.78 to 1 | 0.057 |
| Attempts | 0.97 | 0.85 to 1.1 | 0.618 |
| IA tPA | 1.4 | 0.81 to 2.4 | 0.228 |
ASPECTS, Alberta Stroke Program Early CT Score; IA tPA, intra-arterial tissue plasminogen activator; IV tPA, intravenous tissue plasminogen activator; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; PH2, parenchymal hematoma type II (ECASS-II criteria); sICH, symptomatic intracranial hemorrhage; TICI, thrombolysis in cerebral infarction.
Functional outcome
On univariate analysis, patients with comorbid AF had worse functional outcomes at discharge along with increased mortality and decreased rates of a good functional outcome at 90 days (table 1). However, patients with AF were significantly older and had worse presenting deficits (table 1). We assessed whether this effect was attributed to confounding variables using multivariate analysis. When controlling for age, admission NIHSS score, and ASPECT scores on admission, AF was not an independent predictor of good outcome at 90 days (aOR 1.17, 0.91–1.50, p=0.224, table 3). This suggests that the worse outcomes observed in AF associated strokes can probably be attributed to poor presenting deficits in addition to advanced age in this group.
Table 3.
Multivariate logistic regression for predictors of good outcome (MRS 0–2) at 90 Days in full cohort
| Variable | Coefficient | 95% CI | P value |
|---|---|---|---|
| Full cohort controlling for AF | |||
| Model: logistic regression, n=3024 | |||
| Age | 0.97 | 0.96 to 0.97 | 0.001 |
| Diabetes mellitus | 0.71 | 0.55 to 0.91 | 0.008 |
| Hypertension | 0.7 | 0.53 to 0.91 | 0.009 |
| Hyperlipidemia | 1.3 | 1.03 to 1.64 | 0.029 |
| Atrial fibrillation | 1.17 | 0.91 to 1.5 | 0.224 |
| Pre-stroke mRS score 0–2 | 7.84 | 3.96 to 15.52 | 0.001 |
| Admission NIHSS score | 0.9 | 0.89 to 0.92 | 0.001 |
| IV tPA use | 1.28 | 1.02 to 1.6 | 0.034 |
| Onset-to-groin | 1 | 1 to 1 | 0.734 |
| ASPECTS | 1.23 | 1.14 to 1.32 | 0.001 |
| Number of attempts | 0.79 | 0.73 to 0.85 | 0.001 |
| Successful reperfusion (TICI 2b+) | 2.51 | 1.73 to 3.64 | 0.001 |
AF, atrial fibrillation; ASPECTS, Alberta Stroke Program Early CT Score; IV tPA, intravenous tissue plasminogen activator; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; TICI, thrombolysis in cerebral infarction score.
DISCUSSION
AF has previously been shown to be associated with worse functional outcomes, larger infarcts, decreased rates of recanalization, and increased rates of hemorrhagic complications after IVT.3–6 This has been hypothesized to be due to larger embolized clots, larger territories at risk, and the lack of pre-ischemic conditioning.3,7,8,23 Notably, these data come from the IVT era and predate the widespread availability of MT. These associations have not yet been explored in the setting of MT, with or without bridging therapy.
Here, we report for the first time that MT in patients with comorbid AF is associated with faster recanalization time, fewer passes, and higher rates of first pass success. Despite decreased procedural times, patients with AIS with comorbid AF have worse functional outcomes, consistent with observations from the pre-endovascular era.3–6 Our data suggest that these worse outcomes are attributable to increased age, decreased ASPECTS, and more severe deficits at onset, but not AF in adjusted models.
AF has been consistently shown to independently predict intracranial hemorrhage in the pre-endovascular era, with or without IVT.3,5,8–10,20 In contrast to these reports, AF is not associated with increased intracranial hemorrhage in patients undergoing MT, either in the full cohort or those who achieved good angiographic reperfusion (table 2). This is even more striking when considering the likely enrichment of anticoagulant use in the AF cohort who underwent MT. In contrast, patients with AF receiving anticoagulants are largely excluded from IVT and are nevertheless more likely to have an intracranial hemorrhage after IVT, further supporting a differential effect of MT and IVT on post-reperfusion hemorrhage rates. Our observations extend the recently reported experiences in randomized controlled trials, demonstrating that AF does not interact with MT outcomes in a large, international registry.
These results also raise a novel question in the ongoing debate about bridging therapy for large vessel occlusions.24,25 Given that IVT complications are increased in patients with AF associated stroke, our observation of equivalent clinical and hemorrhagic outcomes with MT raises the question of how AF modifies the effect of bridging therapy in these patients.7,9,10 The enrichment of IVT complications in patients with AF in the pre-endovascular era suggests that patients with AF may be a particularly high-risk subgroup who may benefit from a direct-to-thrombectomy approach at thrombectomy-capable centers. Further investigation will be needed to assess the efficacy and safety of bridging therapy in these patients.
Strengths of our study include leveraging a large multicenter database with over 5000 thrombectomies, characterizing the real-world experience and outcomes across a spectrum of large academic institutions. Nevertheless, our study has a number of limitations. First, the stroke mechanism in patients without AF was not available for each patient. Instead, we used comorbid AF as a surrogate of the stroke mechanism, probably underestimating the rate of non-cardioembolic stroke in patients with comorbid AF. Estimates vary of the rate of non-cardioembolic strokes that occur in patients with AF, largely due to lacunar or carotid disease.26,27 Nevertheless, because we selected for large vessel occlusions, lacunar contributions are unlikely. Additionally, patients with treated carotid disease were excluded from the AF cohort. Second, angiographic outcomes (final mTICI), hemorrhagic complications, and functional outcomes were scored locally and not centrally adjudicated. Third, as a retrospective registry, we cannot exclude selection bias, particularly with decisions for continued recanalization attempts to improve the angiographic outcome. Fourth, antithrombotic data are not currently reported in the STAR registry, therefore limiting commentary on the concomitant use of antiplatelets and/or anticoagulants and the risk of hemorrhagic complications. Finally, we did not assess posterior circulation occlusions given the center-to-center variability in inclusion criteria and the paucity of randomized data to guide decision making. Whether these results extend to posterior circulation strokes remains unclear.
CONCLUSIONS
In patients treated with MT, comorbid AF is associated with faster procedural time, fewer passes, and increased rates of first pass success without increased risk of intracranial hemorrhage. These results are in contrast to the increased hemorrhage rates reported in AF associated stroke treated with supportive care and or thrombolysis. Together, these results suggest that AF associated stroke has a differential response to IVT and MT, and that MT negates the AF penalty in ischemic stroke.
Supplementary Material
Acknowledgments
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests RMS: consulting and teaching agreements with Penumbra, Abbott, Medtronic, InNeuroCo, and Cerenovus. MNP: travel grants/honoraria–Phenox, Stryker, Siemens. ASA: consultant–Balt, Johnson and Johnson, Leica, Medtronic, Microvention, Penumbra, Scientia, Siemens, and Stryker; research support–Cerenovus, Microvention, Penumbra, and Siemens; and shareholder–Bendit, Cerebrotech, Endostream, Magneto, Marblehead, Neurogami, Serenity, Synchron, Triad Medical, Vascular Simulations. PJ: consultant-Medtronics, Microvention. AMS: consultant–Penumbra, Microvention, and Pulsar Vascular; travel grants/honoraria–Penumbra, Pulsar Vascular, Microvention, Stryker. AR: consulting agreement with Stryker, Cerenovus, and Microvention.
Footnotes
Additional material is published online only. To view, please visit the journal online (http://dx.doi.org/10.1136/neurintsurg-2020-016720).
Patient consent for publication Not required.
Provenance and peer review Not commissioned; internally peer reviewed.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
REFERENCES
- 1.Healey JS, Oldgren J, Ezekowitz M, et al. Occurrence of death and stroke in patients in 47 countries 1 year after presenting with atrial fibrillation: a cohort study. Lancet 2016;388:1161–9. [DOI] [PubMed] [Google Scholar]
- 2.Ogilvie IM, Newton N, Welner SA, et al. Underuse of oral anticoagulants in atrial fibrillation: a systematic review. Am J Med 2010;123:638–45. [DOI] [PubMed] [Google Scholar]
- 3.Saposnik G, Gladstone D, Raptis R, et al. Atrial fibrillation in ischemic stroke: predicting response to thrombolysis and clinical outcomes. Stroke 2013;44:99–104. [DOI] [PubMed] [Google Scholar]
- 4.Seet RCS, Zhang Y, Wijdicks EF, et al. Relationship between chronic atrial fibrillation and worse outcomes in stroke patients after intravenous thrombolysis. Arch Neurol 2011;68:1454–8. [DOI] [PubMed] [Google Scholar]
- 5.Steger C, Pratter A, Martinek-Bregel M, et al. Stroke patients with atrial fibrillation have a worse prognosis than patients without: data from the Austrian Stroke Registry. Eur Heart J 2004;25:1734–40. [DOI] [PubMed] [Google Scholar]
- 6.Kimura K, Minematsu K, Yamaguchi T, et al. Atrial fibrillation as a predictive factor for severe stroke and early death in 15,831 patients with acute ischaemic stroke. J Neurol Neurosurg Psychiatry 2005;76:679–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tu HTH, Campbell BCV, Christensen S, et al. Pathophysiological determinants of worse stroke outcome in atrial fibrillation. Cerebrovasc Dis 2010;30:389–95. [DOI] [PubMed] [Google Scholar]
- 8.Zhang J-B, Ding Z-Y, Yang Y, et al. Thrombolysis with alteplase for acute ischemic stroke patients with atrial fibrillation. Neurol Res 2010;32:353–8. [DOI] [PubMed] [Google Scholar]
- 9.Paciaroni M, Agnelli G, Corea F, et al. Early hemorrhagic transformation of brain infarction: rate, predictive factors, and influence on clinical outcome: results of a prospective multicenter study. Stroke 2008;39:2249–56. [DOI] [PubMed] [Google Scholar]
- 10.Wahlgren N, Ahmed N, Eriksson N, et al. Multivariable analysis of outcome predictors and adjustment of main outcome results to baseline data profile in randomized controlled trials: Safe Implementation of Thrombolysis in Stroke-MOnitoring STudy (SITS-MOST). Stroke 2008;39:3316–22. [DOI] [PubMed] [Google Scholar]
- 11.Kimura K, Iguchi Y, Yamashita S, et al. Atrial fibrillation as an independent predictor for no early recanalization after IV-t-PA in acute ischemic stroke. J Neurol Sci 2008;267:57–61. [DOI] [PubMed] [Google Scholar]
- 12.Tandberg Askevold E, Naess H, Thomassen L. Predictors for recanalization after intravenous thrombolysis in acute ischemic stroke. J Stroke Cerebrovasc Dis 2007;16:21–4. [DOI] [PubMed] [Google Scholar]
- 13.Kim SK, Yoon W, Kim TS, et al. Histologic analysis of retrieved clots in acute ischemic stroke: correlation with stroke etiology and gradient-echo MRI. AJNR Am J Neuroradiol 2015;36:1756–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marder VJ, Chute DJ, Starkman S, et al. Analysis of thrombi retrieved from cerebral arteries of patients with acute ischemic stroke. Stroke 2006;37:2086–93. [DOI] [PubMed] [Google Scholar]
- 15.Sporns PB, Hanning U, Schwindt W, et al. Ischemic stroke: what does the histological composition tell us about the origin of the thrombus? Stroke 2017;48:2206–10. [DOI] [PubMed] [Google Scholar]
- 16.Smaal JA, de Ridder IR, Heshmatollah A, et al. Effect of atrial fibrillation on endovascular thrombectomy for acute ischemic stroke. A meta-analysis of individual patient data from six randomised trials: results from the HERMES collaboration. Eur Stroke J 2020;5:245–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heshmatollah A, Fransen PSS, Berkhemer OA, et al. Endovascular thrombectomy in patients with acute ischaemic stroke and atrial fibrillation: a MR CLEAN subgroup analysis. EuroIntervention 2017;13:996–1002. [DOI] [PubMed] [Google Scholar]
- 18.Giray S, Ozdemir O, Baş DF, Bas DF, et al. Does stroke etiology play a role in predicting outcome of acute stroke patients who underwent endovascular treatment with stent retrievers? J Neurol Sci 2017;372:104–9. [DOI] [PubMed] [Google Scholar]
- 19.Munir MB, Alqahtani F, Beltagy A, et al. Comparative outcomes of mechanical thrombectomy for acute ischemic stroke in patients with and without atrial fibrillation. J Vasc Interv Radiol 2017;28:1604–5. [DOI] [PubMed] [Google Scholar]
- 20.Xiaohua Pan GL, Li Y, Wang B, et al. Is atrial fibrillation a prognostic predictor for patients with acute ischemic stroke treated with thrombectomy? Int J Clin Exp Med 2016;9:6819–24. [Google Scholar]
- 21.Higashida RT, Furlan AJ, Roberts H, et al. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke 2003;34:e109–37. [DOI] [PubMed] [Google Scholar]
- 22.Larrue V, von Kummer R, del Zoppo G, et al. Hemorrhagic transformation in acute ischemic stroke. Potential contributing factors in the European Cooperative Acute Stroke Study. Stroke 1997;28:957–60. [DOI] [PubMed] [Google Scholar]
- 23.Guglielmi V, LeCouffe NE, Zinkstok SM, et al. Collateral circulation and outcome in atherosclerotic versus cardioembolic cerebral large vessel occlusion. Stroke 2019;50:3360–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hassan AE, Ringheanu VM, Preston L, et al. IV tPA is associated with increase in rates of intracerebral hemorrhage and length of stay in patients with acute stroke treated with endovascular treatment within 4.5 hours: should we bypass IV tPA in large vessel occlusion? J Neurointerv Surg 2020. doi: 10.1136/neurintsurg-2020-016045. [Epub ahead of print: 03 Jul 2020]. [DOI] [PubMed] [Google Scholar]
- 25.Goyal N, Tsivgoulis G, Pandhi A, et al. Impact of pretreatment with intravenous thrombolysis on reperfusion status in acute strokes treated with mechanical thrombectomy. J Neurointerv Surg 2019;11:1073–9. [DOI] [PubMed] [Google Scholar]
- 26.Chang Y-J, Ryu S-J, Lin S-K. Carotid artery stenosis in ischemic stroke patients with nonvalvular atrial fibrillation. Cerebrovasc Dis 2002;13:16–20. [DOI] [PubMed] [Google Scholar]
- 27.Katsi V, Georgiopoulos G, Skafida A, et al. Noncardioembolic stroke in patients with atrial fibrillation. Angiology 2019;70:299–304. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.


