Abstract
The present series of studies examine the impact of systemically administered therapeutics on peripheral nerve injury (males; unilateral sciatic chronic constriction injury [CCI])-induced suppression of voluntary wheel running, across weeks after dosing cessation. Following CCI, active phase running distance and speed are suppressed throughout the 7-week observation period. A brief course of morphine, however, increased active phase running distance and speed throughout this same period, an effect apparent only in sham rats. For CCI rats, systemic co-administration of morphine with antagonists of either P2X7 (A438079) or TLR4 ((+)-naloxone) (receptors critical to the activation of NLRP3 inflammasomes and consequent inflammatory cascades) returned running behavior of CCI rats to that of shams through 5+ weeks after dosing ceased. This is a striking difference in effect compared to our prior CCI allodynia results using systemic morphine plus intrathecal delivery of these same antagonists, wherein a sustained albeit partial suppression of neuropathic pain was observed. This may point to actions of the systemic drugs at multiple sites along the neuraxis, modulating injury-induced, inflammasome-mediated effects at the injured sciatic nerve and/or dorsal root ganglia, spinal cord, and potentially higher levels. Given that our data to date point to morphine amplifying neuroinflammatory processes put into motion by nerve injury, it is intriguing to speculate that co-administration of TLR4 and/or P2X7 antagonists can intervene in these inflammatory processes in a beneficial way. That is, that systemic administration of such compounds may suppress inflammatory damage at multiple sites, rapidly and persistently returning neuropathic animals to sham levels of response.
Keywords: (+)-naloxone, A438079, circadian, neuropathic pain, neuropathy, rats
1 |. INTRODUCTION
Outcome measures used to evaluate nociception (pain) in rodents typically rely on evoked withdrawal responses to applied mechanical or thermal stimuli. However, such withdrawal responses are generated by experimenter-delivered stimuli, rather than being spontaneous, self-motivated behaviors by the animal. Sole reliance on evoked withdrawal responses has come under increasing criticism in this research field, leading to the search for more naturalistic behavioral indices of pain (Deuis, Dvorakova, & Vetter, 2017; Larson, Wilcox, & Fairbanks, 2019; Mogil & Crager, 2004; Tappe-Theodor, King, & Morgan, 2019; Yezierski & Hansson, 2018).
An additional concern regarding standard pain tests is that the animals’ responses are typically assessed during the inactive (light) phase of the rodent’s circadian cycle, rather than during the active (dark) phase. As circadian variations in pain behaviors have been noted, including for neuropathic pain (Burish, Chen, & Yoo, 2019; Koyanagi et al., 2016; Perissin, Facchin, & Porro, 2000), testing in the active phase of the animals may be predicted to have ecological and physiological relevance, but bears systematic comparison to measures made in the inactive phase.
Taken together, such issues lead to a consideration of inactive versus active phase voluntary wheel running as outcome measures in rats experiencing neuropathic pain. Voluntary wheel running may improve the translation of preclinical findings as expression of this behavior is entirely of the rodent’s own volition, with maximal expression of this behavior during the rodent’s natural active phase. Thus, voluntary wheel running may present a more integrated model for evaluating pain (Cobos et al., 2012; Grace, Strand, Maier, & Watkins, 2014; Kandasamy, Calsbeek, & Morgan, 2016; Mogil, 2009; Tappe-Theodor et al., 2019).
The only prior study of wheel running after sciatic chronic constriction injury (CCI) found a suppression of voluntary wheel running during the inactive (light) phase, but not during the active (dark) phase, when rats were given access to running wheels for just 1 hr per day (Whitehead et al., 2017). Constraining the daily duration of rodents’ access to running wheels has the effect of increasing both the motivation to run and the novelty of the wheel (Novak, Burghardt, & Levine, 2012). Providing continuous (24 hr/day, 7 days/week) access to running wheels may provide the most naturalistic expression of spontaneous behavior.
Notably, no prior study to our knowledge has examined the effect of peripheral nerve injury on subsequent voluntary wheel running when rats are housed with continuous free access to a running wheel, nor have any prior studies examined the impact of therapeutic drugs on this spontaneous behavior. This is the design of the experiments presented here, wherein rats had continuous free access to a running wheel throughout the multi-week experiment. This decreases the novelty of the wheel, as well as more accurately measures subtle differences in the rats’ daily choice to run, such as changes over the circadian cycle, across days, in response to sham versus CCI surgery, and in response to pharmacological interventions predicted to suppress neuropathic pain.
2 |. MATERIALS AND METHODS
2.1 |. Animals
Pathogen-free adult male Fischer 344 (F344) rats, 10 weeks old on arrival (Envigo, Indianapolis, IN) were used in all experiments. Males were the subjects of this study given that the Department of Defense funding required this one sex to be used. Upon arrival, rats were pair-housed for up to 1 week during acclimation to the colony, and then single-housed for 1 week before the study began. Single housing has not previously been found to significantly impair post-surgical recovery (Jirkof, Cesarovic, Rettich, Fleischmann, & Arras, 2012). Colony rooms are temperature-controlled (23 ± 3°C) and light-controlled (12 hr light–dark cycle; lights on at 07:00 hr) rooms with standard rodent chow and water available ad libitum. All procedures were approved by the Institutional Animal Care and Use Committee of the University of Colorado Boulder. Random assignment and blinded procedures for group assignments were used throughout these studies. The following experimental design was tested across six experiments: 2 (sham control vs. CCI) × 2(saline control vs. morphine) × 3(saline control vs. (+)-naloxone, vs. A438079). With a sample size of six for each group, an ANOVA will have 80% power to detect an effect size of 0.2534 in a variable among the eight groups, assuming a type I error rate of 0.05 (nQuery Advisor 7.0). Each experimental group included 5–6 rats; a total of 65 rats were used in this study.
2.2 |. Drugs
Morphine was gifted from the National Institute on Drug Abuse Drug Supply Program (NDSP) Division of Therapeutics and Medical Consequences, Research Triangle Institute, NC. The Toll-like receptor 4 (TLR4) antagonist (+)-Naloxone was synthesized by Dr. Kenner Rice (National Institute on Drug Abuse/National Institute on Alcohol Abuse and Alcoholism, Bethesda) for use in these studies. The P2X7 antagonist A438079 was obtained commercially (Tocris Bioscience). Endotoxin-free physiological saline (Hospira Inc.) was the vehicle for each.
2.3 |. Drug administration
Morphine ((−)-morphine) was administered subcutaneously (s.c.) at 5 mg/kg (calculated as free base), twice daily for 5 days. This was administered at approximately 09:00 hr and 17:00 hr. Equivolume saline vehicle (1 ml/kg) was administered s.c. to controls. (+)-Naloxone, A438079, or equivolume saline vehicle was administered s.c. twice daily for 5 days immediately before morphine or saline injections. (+)-Naloxone and A438079 were administered s.c. at 20 mg/kg (Lewis et al., 2012; Wang et al., 2019) and at 1 mg/kg (McGaraughty et al., 2007), respectively. Dosing began upon full expression of CCI-induced neuropathic pain (Day 10 post-surgery) and concluded on Day 14 post-surgery.
2.4 |. CCI surgery
Neuropathic pain was induced using the unilateral CCI model of sciatic nerve injury (Bennett & Xie, 1988). Surgery was aseptically performed under isoflurane anesthesia at mid-thigh level of the left hind leg as previously described (Milligan et al., 2004). The sciatic nerve was gently isolated, and four sterile chromic gut sutures (cuticular 4–0 WebGut; Patterson Veterinary, Devens, MA) were loosely tied around the nerve. For sham surgery, the sciatic nerve was isolated, but no chromic gut sutures were tied around the nerve. Rats were monitored postoperatively until fully ambulatory and then returned to their home cage.
2.5 |. Voluntary wheel running
Rats were single-housed in SCURRY rat running wheel chambers (Lafayette Instruments, Lafayette, IN). Unrestricted access to running wheels was continuously provided throughout the experiment, beginning 7 days before CCI surgery, and continuing for 42 days after surgery. Running data were collected by computer (SCURRY activity systems software, Lafayette, IN) 24 hr per day for the entire study. Parameters analyzed were total daily distance traveled and maximum running speed. On days when systemic injections were administered (Days 10–14 post-surgery), the hour required to administer drugs to all of the rats and the hour immediately following were discarded from data analysis as not reflective of undisturbed behavior (Tappe-Theodor et al., 2019).
2.6 |. Rotarod
The rotarod (IITC Life Science, Woodland Hills, CA) test was used to evaluate motor coordination. The rotarod consisted of a 9.53-cm-diameter drum that rotated slowly at 10 RPM fixed speed for 180 s. At 180 s the rotarod stopped, and the trial ended. Rats were acclimated and trained on rotarod three times each on three separate days prior to collecting baseline data, for a total of nine acclimations trials. During training, rats were placed immediately back on the rotarod after falling up to five times, for a total of 180 s running time each trial. Rats were returned to their home cage to rest for 15 min between trials. For data collection, rats were placed on the rotarod and time in seconds until fall was recorded. Rats were placed in the home cage after falling. Data for each time point tested were calculated as highest score in seconds out of three trails, with 15 min to rest between trials.
2.7 |. Statistics
Differences between treatment groups in the time course of running distance and maximum speed were determined using two-way repeated measures ANOVA followed by Sidak post hoc and by unpaired t test of area under curve (AUC) analysis of the mean values for time points after treatment.
3 |. RESULTS
3.1 |. Experiment 1: CCI persistently reduces maximum running speed and distance, compared to shams, only during the active (dark) phase of the circadian cycle
Experiment 1 examined whether CCI of the sciatic nerve would differentially suppress voluntary running behavior during the inactive versus active portions of the circadian cycle, compared to sham surgery. Rats were allowed unlimited access to running wheels for 1 week prior to, and 6 weeks after, surgery. As Experiments 3–6 examine the impact of 5 days of twice daily treatment with systemic drugs beginning 10 days post-surgery, subjects in Experiment 1 received equivolume saline on this same schedule. This served to verify, prior to proceeding with drug studies, whether this repeated disruption of rats with in-cage running wheels would markedly alter ongoing behavior in a manner that would confound interpretation of effects observed.
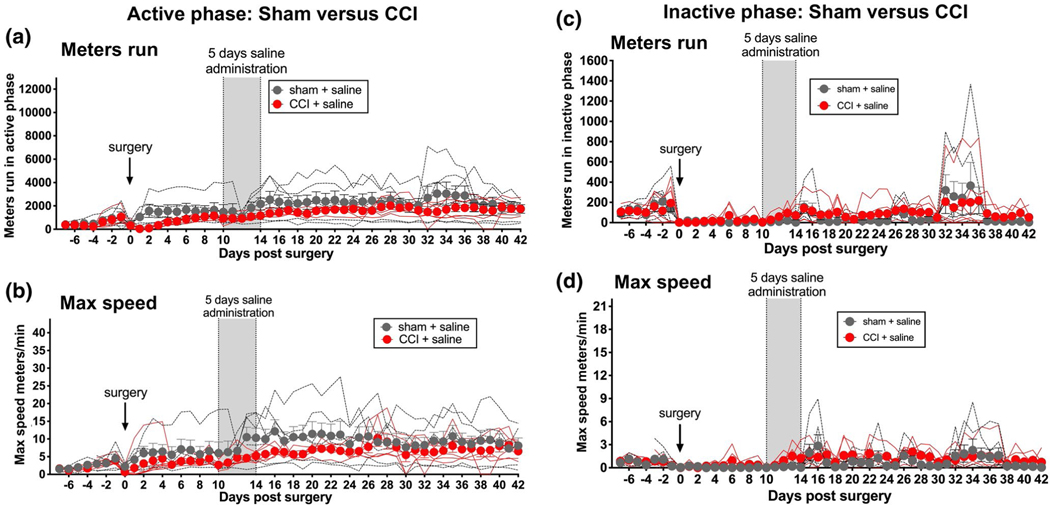
Running distance and speed were continuously recorded for the entire 49 days of running wheel access (Figure 1). Prior to surgery, no differences in running distance or maximum speed were observed between groups (Days −7 to −1), either during the active (Figure 1a,c) or inactive (Figure 1e,g) phases of the circadian activity cycle.
FIGURE 1.
Enduring reduction of active (dark) phase voluntary wheel running by sciatic nerve injury. Unilateral sciatic nerve chronic constriction injury (CCI), which induces prolonged and stable neuropathic pain, induced prolonged and stable suppression of voluntary wheel running distance (a) and maximal running speed (b), relative to sham controls in the active phase. Five days of twice daily systemic saline (vertical gray bar) was administered beginning on Day 10 after surgery so to provide a regimen comparable to that followed in subsequent experiments. No such changes were observed in these same rats in the light phase (c, d). n = 5–6 per group
CCI significantly reduced both active phase running distance (Figure 1a; Table 1 unpaired t test of AUC for Days 1–42 after surgery: p < 0.01) and active phase maximum speed (Figure 1b; Table 1 unpaired t test of AUC for Days 1–42 after surgery: p < 0.01), relative to sham controls. As expected, 5 days of twice daily saline did not alter running behaviors, beyond the effect of CCI alone. This conclusion was based on analysis of behavior for Days 15–42 (i.e., after the conclusion of saline dosing), calculated as a change from pre-injection behavior (mean of recordings on Days 8 and 9 after surgery, just prior to initiation of systemic injections). Behaviors after 5 days of saline dosing did not change relative to behavior before dosing.
TABLE 1.
Area under the curve analysis
| Active phase |
Inactive phase |
||||
|---|---|---|---|---|---|
| Group | Meters run | Max speed | Meters run | Max speed | Figure |
|
| |||||
| Sham + saline | 83,947 (6,552) | 3,586 (30) | 2,285 (687) | 26 (7) | 1 |
| CCI + saline | 56,745 (2,497)** | 244 (14)** | 3,352 (579) | 42 (5) | 1 |
| CCI + morphine + saline | 54,747 (2,769) | 249 (16) | 2,753 (618) | 25 (5) | 2, S2 |
| CCI + morphine + (+)-NLX | 85,577 (7,015)### | 406 (29)# | 3,290 (822) | 44 (11) | 2, S2 |
| CCI + morphine + A438079 | 74,045 (4,609)### | 348 (25)# | 3,341 (555) | 51 (10) | 2, S2 |
| CCI + saline + saline | 53,655 (2,470) | 190 (12) | 4,203 (617) | 47 (5) | 2, S4 |
| CCI + saline + (+)-NLX | 58,583 (3,297) | 228 (21) | 2,766 (554) | 26 (5) | 2, S4 |
| CCI + saline + A438079 | 49,544 (4,067) | 181 (18) | 3,508 (565) | 33 (5) | 2, S4 |
| Sham + morphine + saline | 82,317 (6,256) | 392 (28) | 4,919 (832) | 53 (9) | 3, S5 |
| Sham + morphine + (+)-NLX | 89,742 (8,101) | 433 (33) | 4,952 (1,056) | 71.08 (16) | 3, S5 |
| Sham + morphine + A438079 | 101,727 (7,561) | 421 (28) | 4,117 (726) | 34 (7) | 3, S5 |
| Sham + saline | 62,152 (5,834) | 260 (25) | 2,124 (682) | 24 (6) | 4 |
| Sham + morphine | 82,317 (6,256)$ | 392 (28)$$ | 2,815 (645) | 39 (8) | 4 |
Note: Relative to Sham + saline:
p < 0.01;Relative to CCI + morphine + saline:
p < 0.05,
p < 0.001; Relative to Sham + saline:
p < 0.05,
p < 0.01.
During the inactive (light) phase, sham and CCI groups exhibited comparable running behavior across the entire 42 days after surgery (distance: Figure 1c, Table 1; maximum speed: Figure 1d, Table 1). Both groups exhibited a general depression in running in the days following surgery. No reliable between-group differences were observed across the 5 weeks following 5 days of saline treatment.
3.2 |. Experiment 2. Unilateral CCI does not compromise rotarod performance
The active phase decrease in wheel running by unilateral CCI in Experiment 1 raised the question as to whether this reflected a confound of an ongoing physical impairment that made CCI rats incapable of motor movements necessary for locomotion, relative to shams. To test this, forced motor performance, as measured by the rotarod test, was assessed across 5 weeks after CCI versus sham surgery. A separate group of rats was used for this experiment, to avoid disturbing voluntary wheel running behavior. To parallel the procedures of Experiments 1 and 3 (below), rats again received 5 days of twice daily injections of either saline (paralleling Experiment 1) or morphine (paralleling Experiment 3) beginning on Day 10 after either surgery. Because there have been indications in the literature that opioids, albeit at higher doses, can compromise motor function after injury (Hook et al., 2007), the present morphine dosing regimen was included to assess whether unexpected motoric compromise occurred, prior to undertaking the studies below.
Analyses of rotarod performance revealed that there were no differences in motor performance between groups either before surgery, Day 9 after surgery (i.e., prior to saline/morphine dosing), or across time through 5 weeks after cessation of saline/morphine dosing. Hence, observations of depressed voluntary wheel running behavior in the studies reported here do not appear to reflect a physical impairment that makes rats incapable of running (Figure S1).
3.3 |. Experiment 3. Long after cessation of 5 days of morphine co-administered with either TLR4 or P2X7 antagonists, active phase running distance and active phase maximum running speed are persistently enhanced in CCI rats, relative to 5 days of morphine alone
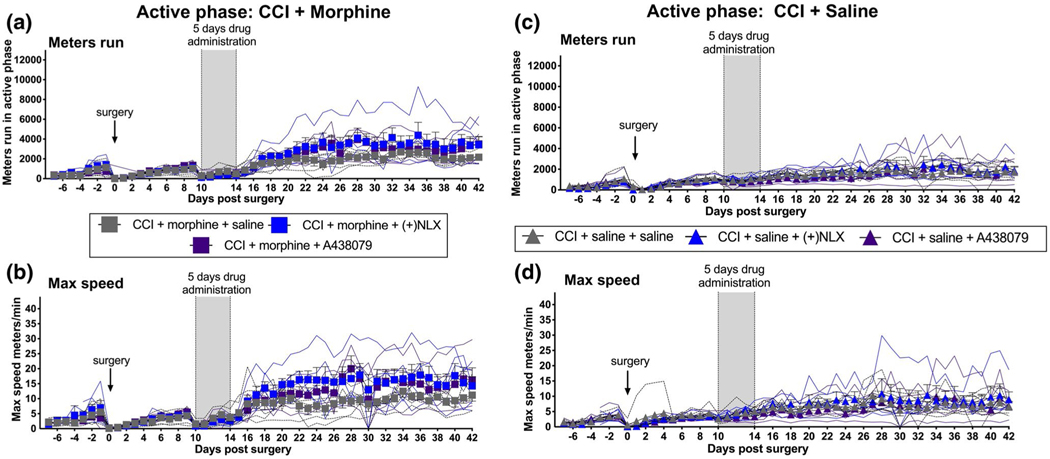
We have previously reported that 5 days of co-administration of the TLR4 antagonist (+)-naloxone ((+)-NLX) (Wang et al., 2016) or the P2X7 antagonist A438079 (King, 2007) with morphine, beginning on Day 10 after CCI, prevents morphine-induced potentiation of allodynia by suppressing the spinal glia-activating, neuroinflammatory effects of morphine (Grace et al., 2018; Grace, Strand, et al., 2016). Notably, the suppression of morphine-induced potentiation of allodynia by (+)-NLX and A438079 co-administration with morphine persists for at least 2 months after all drug dosing ceases (Grace, Strand, et al., 2016). To determine whether administration of these antagonists along with morphine would also prevent suppression of active phase voluntary wheel running by CCI (Figure 2a,b, Table 1), rats were given a 5-day course of morphine along with either saline (vehicle), (+)-NLX, or A438079. Systemic dosing was chosen for all drugs in this study, given concern that indwelling intrathecal catheters may alter voluntary wheel running. As above, running distance and maximum speed were recorded for 7 days prior to, and for 42 days after, surgery.
FIGURE 2.
Enduring potentiation of CCI active (dark) phase voluntary wheel running by 5 days of systemic morphine co-administered with systemic P2X7 or TLR4 inhibitors; no such change in the absence of morphine. In unilateral CCI rats, systemic co-administration of 5 days of morphine with either (+)-NLX (non-opioid TLR4 antagonist) or A438079 (P2X7 antagonist) (vertical gray bar) resulted in enhanced active phase running distance (a) and maximum running speed (b), relative to CCI rats treated with morphine plus saline (vehicle). This potentiation of voluntary wheel running continued through the 5-week observation after all drug dosing ceased. No such potentiation of running behavior was observed in response to identical A438079 or (+)-NLX dosing (vertical gray bar) in the absence of co-administered morphine (c, d). n = 5–6 per group
Prior to surgery, there were no reliable differences between groups for distance run or maximum speed, either during the active phase (Figure 2a,b) or inactive phase (Figure S2a,b). CCI surgery again depressed both active (p < 0.001) and inactive phase distance run (p < 0.01) and active phase (p < 0.001) maximum speed, relative to pre-surgery performance levels (comparing 1 day prior to vs. 1 day after CCI) (Figure 2a,b; Figure S2a,b).
Across the 4 weeks after cessation of drug dosing (Days 15–42 post-surgery), co-administration of either the TLR4 antagonist (+)-NLX or the P2X7 antagonist A438079 during the 5 days of morphine treatment markedly potentiated both active phase distance run (Figure 2a; Table 1, AUC: F(2,13) = 10.56, p < 0.01; post hoc morphine + saline vs. morphine + (+)NLX: p < 0.001; post hoc morphine + saline vs. morphine + A438079: p < 0.001) and active phase maximum speed (Figure 2b, Table 1; AUC: F(2,13) = 12.49, p < 0.001; post hoc morphine + saline v.s morphine + (+)NLX: p < 0.05; post hoc morphine + saline vs. morphine + A438079: p < 0.05). This enduring potentiation of running distance and maximum speed by these antagonists was despite neither antagonist having any effect on voluntary running during the 5 days drug dosing (Figure 2a,b; Figure S2a,b). No reliable enhancement of either running distance or maximum speed was observed during the inactive (light) phase (Figure S2a,b).
3.4 |. Experiment 4. In the absence of either morphine or neuropathic pain, TLR4 and P2X7 antagonists do not potentiate wheel running
Potentiation of active phase running distance and active phase maximum running speed observed in Experiment 3 (where TLR4 and P2X7 antagonists were co-administered with morphine in CCI rats) raises the question of whether these effects require co-administration of morphine and/or the presence of neuropathic pain. As a first test, sham-operated rats received saline co-administered with either saline (vehicle), (+)-NLX, or A438079 in a design parallel to that for Experiment 3, above, and active phase running analyzed. As differences in post-surgery activity levels were noted across the three sham groups prior to drug injections, data collected post-drugs for each group were analyzed as a difference from the mean of Days 8 and 9 (last days prior to initiation of drug dosing). This adjustment allowed for an unbiased examination of drug effects on running behavior. As evident in Figure S3a,b, neither the TLR4 nor the P2X7 antagonist had any effect on running distance or maximum speed. Thus, the results of Experiment 3 cannot be simply accounted for by effects of these drugs in the absence of morphine and/or nerve injury. The marked differences in behavior recorded for TLR4 and P2X7 inhibitors co-administered with morphine in CCI rats (Experiment 3) versus these inhibitors co-administered with saline in sham rats (Experiment 4) suggest an interaction between the presence of either neuropathic pain and/or morphine with these receptor antagonists that creates the potentiation of running behaviors observed in Experiment 3. The studies that follow examine this possibility.
3.5 |. Experiment 5. In the absence of morphine, TLR4 and P2X7 antagonists do not potentiate active phase voluntary wheel running in the presence of neuropathic pain
Potentiation of active phase running distance and active phase maximum speed observed in Experiment 3 (where TLR4 and P2X7 antagonists were co-administered with 5 days of twice daily morphine in CCI rats) raises the question of whether this is simply an enduring effect of TLR4 and P2X7 receptor blockade per se, versus an interaction with the co-administered morphine. To address this, CCI rats received 5 days of twice daily saline co-administered with either saline (vehicle), (+)-NLX, or A438079 in a design parallel to that for Experiment 3, above.
There were again no differences between groups in either active phase (Figure 2c,d, Table 1) or inactive phase (Figure S4a,b, Table 1) running distance or maximum speed, either prior to surgery (days −7 to −1) or prior to drug administration (Days 0–9). Running distance and maximum speed were again suppressed post-CCI in both active (comparing 1 day prior to vs. 1 day after CCI; distance: p < 0.001; max speed: p < 0.001) and inactive phases (comparing 1 day prior to vs. 1 day after CCI; distance: p < 0.001; max speed: p < 0.05). During the 5 days of drug dosing, neither drug affected running distance (Figure 2c; Figure S4a) or maximum running speed (Figure 2d; Figure S4b) in either activity phase, similar to Experiment 3.
Importantly, in contrast to the results of Experiment 3, neither (+)-NLX nor A438079 had any reliable effect on running distance or maximum running speed in CCI + Saline rats across the 5-week post-drug observation period, for either active (Figure 2c,d) or inactive (Figure S4a,b) phases. The marked differences in behavior recorded for TLR4 and P2X7 inhibition with (Experiment 3) versus without (Experiment 5) co-administration of morphine suggests that it is the interaction of morphine with these receptor antagonists in neuropathic rats that underlies potentiation of running behaviors, rather than a simple independent effect of these inhibitors alone.
3.6 |. Experiment 6. In the absence of neuropathic pain, TLR4 and P2X7 antagonists do not potentiate active phase voluntary wheel running in the presence of morphine
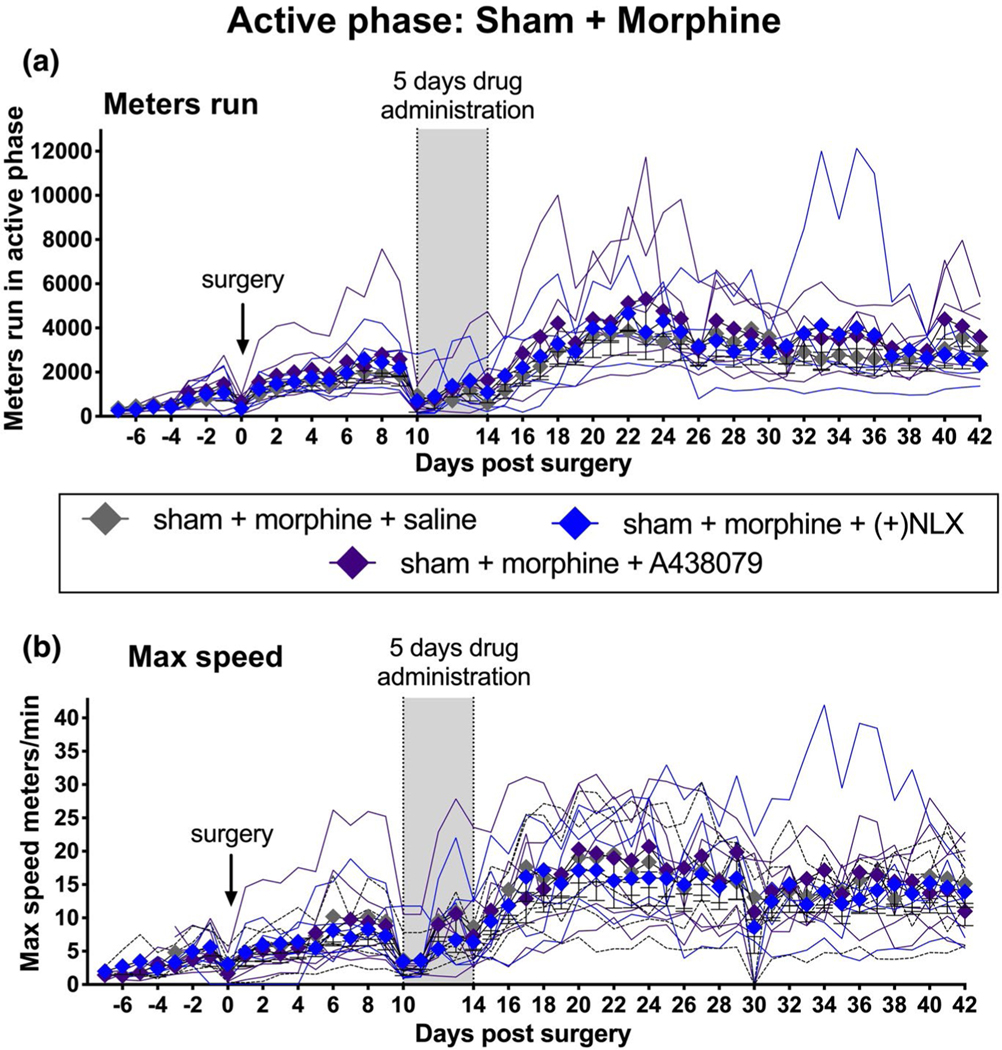
Potentiation of active phase running distance and active phase maximum running speed observed in Experiment 3 (where TLR4 and P2X7 antagonists were co-administered with morphine in CCI rats) raises the additional question of whether these same effects would occur in sham-operated rats; that is, in the absence of neuropathic pain. To address this, sham-operated rats received morphine co-administered with either saline (vehicle), (+)-NLX, or A438079 in a design parallel to that for Experiment 3, above.
There were again no differences between groups in either active phase (Figure 3a,b, Table 1) or inactive (Figure S5a,b, Table 1) phase running distance or maximum speed, either prior to surgery (days −7 to −1) or prior to drug administration (Days 0–9). Sham surgery itself did suppress inactive phase, but not the active phase, running distance (comparing 1 day prior to CCI vs. 1 day after CCI; p < 0.01). During the 5 days of drug dosing, neither drug affected running distance (Figure 3a; Figure S5a) or maximum running speed (Figure 3b; Figure S5b) in sham rats in either activity phase. This is similar to Experiments 3 and 4 in CCI rats.
FIGURE 3.
No potentiation of Sham active (dark) phase voluntary wheel running by 5 days of systemic morphine co-administered with systemic P2X7 or TLR4 inhibitors. To test whether potentiation of wheel running behavior arose simply as a result of co-administration of morphine with P2X7 or TLR4 antagonists, the paradigm was replicated in sham rats, instead of CCI. Systemic co-administration of morphine with either (+)-NLX nor A438079 (vertical gray bar) failed to alter either running distance (a) or maximum speed (b). n = 5–6 per group
Importantly, in contrast to the results from CCI rats in Experiment 3, neither (+)-NLX nor A438079, co-administered with morphine in sham rats, had any effect on running distance (Figure 3a, Table 1, Figure S5a) or maximum running speed (Figure 3b, Table 1, Figure S5b) across the 5-week post-drug observation period, for either active or inactive phases. The marked differences in behavior recorded for morphine co-administered with TLR4 and P2X7 inhibition with (Experiment 3) versus without (Experiment 5) the presence of peripheral nerve injury again suggest that it is the interaction of neuropathic pain with these receptor antagonists plus morphine that underlies potentiation of running behaviors.
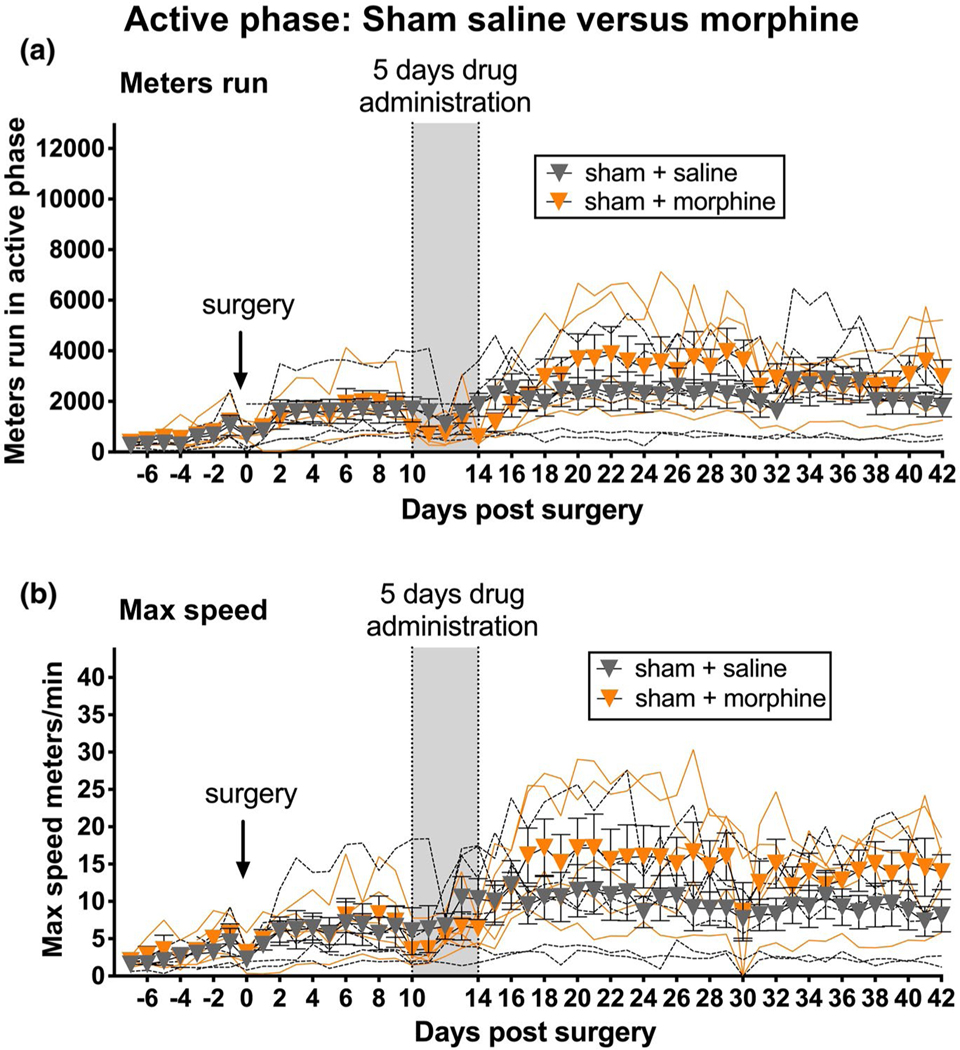
Additionally, a striking observation here was the apparent difference in running distance and speed of sham + morphine + saline rats here (Figure 3a,c) versus the sham + saline rats of Experiment 1 (Figure 1a,b). As these groups ran at the same time and under identical conditions, their data were compared here (Figure 4a,b). Analyzing AUC for Days 15–42 across these two groups (i.e., 5 weeks of observations after cessation of saline vs. morphine in shams) supported this impression, as both running distance (Table 1; unpaired t test; p < 0.05) and maximum running speed (Table 1; unpaired t test; p < 0.01) were significantly greater in sham rats administered 5 days of morphine, relative to shams receiving saline. This supports and extends prior literature demonstrating, after an initial suppression, enhancement of voluntary running by morphine (Domino, Vasko, & Wilson, 1976; Sisti & Lewis, 2001), albeit in studies where analyses were restricted to just the first few hours after morphine dosing rather than across 5 weeks after dosing cessation as in the present study.
FIGURE 4.
Enduring potentiation of sham active (dark) phase voluntary wheel running by 5 days of systemic morphine. In sham rats, systemic administration of 5 days of morphine (vertical gray bar) resulted in enhanced active phase running distance (a) and maximum running speed (b), relative to sham rats treated with saline (vehicle). This potentiation of voluntary wheel running continued through the 5-week observation after all dosing ceased. n = 5–6 per group
4 |. DISCUSSION
The present series of studies are the first to examine the impact of peripheral nerve injury and therapeutic drug treatment in male rodents with continuous free access to voluntary wheel running, analyzing both inactive (light) and active (dark) phases of the circadian activity cycle across an 8-week observation period. Males were the sole subjects as the Department of Defense funding source required that males would be used. Whether the results replicate in females awaits future studies. Regarding drug effects, the primary focus was on the enduring effects of treatments, long after drug dosing ceased, rather than on acute drug effects themselves. This focus is based on our prior studies of multi-week amplification of CCI-induced neuropathic pain by morphine (as well as other opioids (Green-Fulgham et al., 2019)), long after dosing ceases, and the amelioration of these negative consequences of morphine treatment by the antagonists under study here (Grace et al., 2018; Grace, Strand, et al., 2016). The data from the present series of experiments document that: (a) 5 days of morphine enduringly enhances active phase running distance and maximum speed in sham rats throughout the 5-week post-dosing observation period; (b) unilateral CCI suppresses, compared to sham controls, both distance run and maximum running speed, with the impact observed across the 6-week post-surgery period; (c) the impact of CCI on running distance and maximum speed was only observed in the active phase of the circadian cycle, as no between-group differences were found in the inactive phase attributable to nerve injury; (d) the impact of CCI on voluntary wheel running does not appear to indicate a physical motor impairment as CCI rats performed equally to shams on the rotarod test; (e) intriguingly, while co-administration of either an s.c. non-opioid TLR4 antagonist ((+)-Naloxone; (+)-NLX) or an s.c. P2X7 antagonist (A438079) with s.c. morphine had no effect on either active or inactive phase wheel running during the 5 days dosing period, both antagonists significantly potentiated both running distance and maximum running speed in the active phase (but not inactive phase) for several weeks after cessation of drug dosing; and (f) this persistent potentiation of active phase distance and speed by the TLR4 and P2X7 antagonists across the 5-week post-dosing period required both the presence of peripheral nerve injury (CCI) as well as the 5-day co-administration of morphine, as the antagonists had no effect on running distance or speed when delivered in the absence of co-administered morphine and/or in the absence of nerve injury. Remarkably, this brief (5 days) drug treatment, beginning 10 days after nerve injury, returned the running distance and speed of CCI rats to being comparable to those of sham animals across the 5-week observation period after all drug dosing ceased (compare Figure 2c,d with Figure 1c,d).
Clear differences between groups were observed during the active (dark) phase, but not during the inactive (light) phase. This may in part be attributable to rats displaying approximately 95% of their daily running in the active phase (Kandasamy et al., 2016; Pitzer, Kuner, & Tappe-Theodor, 2016), limiting sensitivity for observing group differences in the daytime, especially with free access to running wheels rather than very restricted access (Whitehead et al., 2017). Consistent with previous reports, the activity of the rats increased over time, which may be accounted for by the novel environment and/or training effects (Shyu, Andersson, & Thorén, 1984). Additionally, male rats (as studied here) typically run less than their female counterparts (Rosenfeld, 2017). While there are rat strains that are more robust runners than the Fischer 344 (F344) rats chosen for study here (Shyu et al., 1984), inter-individual variability in strains with higher capacity for running can confound data interpretation (Moraska & Fleshner, 2001; Novak et al., 2012; Sasse et al., 2008). Male F344s were chosen for use given that we have previously documented that morphine robustly enhances the magnitude and duration of CCI allodynia in male F344s, using the identical CCI method as employed in the present study (Grace et al., 2018; Grace, Strand, et al., 2016). Additionally, we have shown that the TLR4 and P2X7 inhibitors, under study in the present series of studies, each block morphine-induced enhancement of allodynia in males of this rat strain (Grace et al., 2018; Grace, Strand, et al., 2016). While amplification of CCI-induced neuropathic pain is also observed, actually to a greater extent, in female than male F344s (Grace et al., Unpub. Observ.), the pharmacological underpinnings of this effect in females have not as yet been elucidated. This combination of factors makes male F344 rats the ideal subjects for the current study.
Intriguingly, the observation that a brief (5 days) co-administration of morphine with either a non-opioid TLR4 ((+)-NLX) or P2X7 (A438079) antagonist potentiates voluntary running distance and speed through at least 5 weeks after all drug dosing ceases extends and contrasts our prior report where these drugs were studied for their effects on experimenter-evoked CCI allodynia (Grace et al., 2018; Grace, Strand, et al., 2016). In Grace, Strand, et al. (2016), the identical 5-day morphine regimen: (a) enhanced the magnitude and duration CCI-induced allodynia through at least 8 weeks in F344 rats after morphine dosing ceased, and (b) co-administration of either (+)-NLX or A438079 with morphine prevented morphine-induced enhancement of allodynia. That is, CCI-induced allodynia was still evident, but the allodynia expressed in rats receiving morphine plus either (+)-NLX or A438079 was comparable to CCI rats that received saline (Grace, Strand, et al., 2016) (compare Figure 1a to Figure 5a,b of Grace, Strand, et al. (2016)). In contrast, when voluntary wheel running serves as the outcome measure rather than experimenter-evoked allodynia, a quite different pattern of results emerges. First, there was no apparent amplification of CCI effects on running by the 5-day course of morphine, relative to saline-treated CCI rats through 5 weeks after drug cessation. And second, co-administration of morphine with either the TLR4 or P2X7 antagonist in CCI rats returned running behaviors to a level comparable to shams.
Regarding the first point, that CCI + morphine did not result in enhanced running relative to CCI + saline, it is notable that, in shams, 5 days of morphine enduringly potentiated active phase voluntary wheel running distance and maximum speed across the 5-week post-dosing observation period. One would have anticipated this same amplification of running distance and speed in CCI rats, as well. Instead, running distance and speed was comparable for CCI + saline and CCI + morphine groups. Given that CCI + morphine amplifies neuropathic pain, as measured by allodynia (Grace et al., 2018; Grace, Strand, et al., 2016), it is possible that the enduring enhancement of running by morphine, as observed in sham rats, offset the enhanced pain by CCI + morphine (Grace et al., 2018; Grace, Strand, et al., 2016), which would suppress running (Experiment 1), obscuring the ability to detect a change in running by CCI + morphine rats, compared to CCI + saline.
Regarding the second point, that co-administration of morphine with either the TLR4 or P2X7 antagonist in CCI rats returned running behaviors to a level comparable to shams, this is, indeed, a remarkable finding as it suggests not a simple, temporary reversal of nerve injury effects but rather an apparently permanent resolution (at least through the 5-week post-drug observation period) by blocking TLR4 or P2X7, but only during morphine dosing. Given that voluntary wheel running has been argued by some to be potentially more reflective of clinically relevant measure than experiment-evoked allodynia (Tappe-Theodor et al., 2019), these striking differences between allodynia versus voluntary running behaviors as outcome measures are intriguing. In addition, it has been argued that another feature that makes wheel running especially useful in screening drugs is that only treatments that produce antinociception in the absence of disruptive side effects will restore wheel running (Tappe-Theodor et al., 2019). Notably, both allodynia and wheel running as outcome measures, while distinct in their pattern of results, each provide clear support that TLR4 and P2X7 inhibition in the presence of morphine can each have marked positive and remarkably enduring effects on the behavior of neuropathic rats.
A caveat to consider when comparing wheel running versus experimenter-evoked endpoints is that the wheel running may influence pathophysiological processes systemically. For example, voluntary wheel running can increase expression of endogenous opioids, and can also promote anti-inflammatory immune cell phenotypes by reducing expression of cell surface receptors, inhibiting chemotaxis, and stimulating synthesis of anti-inflammatory mediators (Kawanishi, Yano, Yokogawa, & Suzuki, 2010; Koltyn, 2000; Nielsen & Pedersen, 2008; Pedersen, 2009; Pedersen & Hoffman-Goetz, 2000; Pedersen & Toft, 2000; Petersen & Pedersen, 2005, 2006; Singhal, Jaehne, Corrigan, & Baune, 2014; Walsh et al., 2011). Given the anti-nociceptive role of these processes, these events could alter the development of pain and potentially reduce effect sizes between surgery groups (Grace, Fabisiak, et al., 2016).
One potentially important, but as yet unexplored difference between the current series of experiments and our prior studies of morphine-induced potentiation of CCI allodynia and its amelioration by TLR4 and P2X7 antagonists (Grace, Strand, et al., 2016) lies in the route of drug administration. In both the present study and Grace et al. studies (Grace et al., 2018; Grace, Strand, et al., 2016), morphine was administered systemically. In contrast, while Grace et al. administered (+)-NLX and A438079 intrathecally in order to test for a spinal cord site of action (Grace et al., 2018; Grace, Strand, et al., 2016), the present study utilized systemic administration for these drugs because of a concern for the dorsally curved postures of rats in the running wheel, whether an indwelling catheter could alter the outcomes and/or create frank spinal damage.
Notably, by utilizing intrathecal drug delivery, Grace et al. implicated the spinal inflammasome pathway as creating opioid-induced amplification of neuropathic pain, utilizing a number of convergent pharmacological approaches (Grace et al., 2018; Grace, Strand, et al., 2016). This approach provided strong evidence that morphine induces, in the presence of ongoing neuropathic pain, the potent spinal expression of a wide array of Danger Associated Molecular Patterns (DAMPs; danger signals) known to activate glia to a neuroinflammatory, pain-enhancing state (Grace et al., 2018; Grace, Strand, et al., 2016). As documented by Grace et al. (2018) and Grace, Strand, et al. (2016) this, in turn, sets off the activation of the inflammasome/NLRP3 pathway in spinal cord via actions at P2X7 and TLR4, leading to the release of a host of pain-enhancing products (Grace et al., 2018; Grace, Strand, et al., 2016). Extending these concepts to the present study, it is intriguing to consider that systemic (instead of intrathecal) delivery of the P2X7 and TLR4 antagonist along with morphine may in fact now block inflammasome activation at multiple levels of the neuroaxis from the sciatic injury site, dorsal root ganglia, as well as spinal cord. Given that inflammasome activation has also been noted in injured sciatic nerve (Sun et al., 2019a, 2019b) and dorsal root ganglia (Yousuf et al., 2019), and given that systemic administration of P2X7 and systemic TLR4 antagonists along with morphine permanently returned wheel running behavior to sham levels throughout the 5-week observation period, these observations taken together suggest the potential for simultaneous actions at multiple sites for these inflammasome inhibitors along the pain pathway. Indeed, multiplicative interactions of drugs simultaneously acting at both peripheral and multiple central sites are well documented (Hayashida & Eisenach, 2008; Kolesnikov, Jain, Wilson, & Pasternak, 1996; Pasternak & Pan, 2013; Pavlovic & Bodnar, 1998; Rossi, Pasternak, & Bodnar, 1993; Yeung & Rudy, 1980). Furthermore, the multi-month lifespan of the leukocytes supports a possible role for these cells. If this is the basis for the present observation of systemic TLR4 and P2X7 inhibitors returning morphine-treated neuropathic rats to sham levels of running, it suggests that this same systemic drug administration approach would be worthy of study with allodynia as the outcome measure as well. That is, it predicts a more potent blockade of morphine- induced enhancement of neuropathic pain than observed with intrathecal antagonists alone.
Work from others implicates the mu-opioid receptor in the deleterious effects of opioids, including paradoxical hyperalgesia (Corder et al., 2017; Ferrini et al., 2013). However, we established that the enduring, pain amplifying effects of early post-trauma morphine was occurring not via classical opioid receptors, but rather TLR4 (Grace, Strand, et al., 2016). That is, knock down of the mu-opioid receptor failed to prevent morphine-induced pain amplification. Additionally, systemic administration of (+)-morphine (which, given severe stereo-selectivity of the opioid receptor, does not bind this receptor but does equally activate TLR4 (Hutchinson et al., 2010; Wang et al., 2012)), fully recapitulates the effects of (−)-morphine (Grace, Strand, et al., 2016). These results strongly implicate TLR4 rather than classical opioid receptors in the outcomes created. Furthermore, inactivating microglia prevented morphine-induced amplification of CCI-induced neuropathic pain, further emphasizing independence from the classical opioid receptor on neurons (Grace, Strand, et al., 2016). Whether such manipulation equally impact the voluntary wheel running as the outcome measure is as yet unknown but worthy of study.
In conclusion, the present series of studies have examined the impact that systemically administered therapeutic drugs have on suppression of wheel running behavior of neuropathic (CCI) rats, weeks after cessation of drug dosing. CCI suppressed active phase running distance and speed throughout the 7-week observation period. Morphine, however, enhanced active phase running distance and speed throughout the 7-week observation period, an effect apparent only in sham rats. Notably, co-administration of morphine with antagonists of either P2X7 or TLR4 (receptors critical to the activation of NLRP3 inflammasomes and consequent inflammatory cascades) returned the voluntary running behavior of CCI rats to that of shams through at least 5 weeks after dosing ceased. Future studies will need to define whether the striking effects of systemically administered TLR4 and P2X7 antagonists observed in the present study, enduringly returning nerve injury rat running distance and speed to that of shams, would also be observed with allodynia as the outcome measure. If so, this would likely point to multiple sites of action of the systemically administered drugs, modulating injury-induced effects, inflammasome-mediated effects at the injured sciatic nerve and/or dorsal root ganglia, in addition to spinal cord and potentially higher levels of the neuraxis. Given that our data to date point to morphine amplifying inflammatory processes put into motion by nerve injury, it is intriguing to speculate that co-administration of TLR4 and/or P2X7 antagonists can intervene in these inflammatory processes in a beneficial way, suppressing the inflammatory damage occurring at peripheral sites and thereby returning CCI animals to sham levels not only persistently but also more rapidly as well. As opioids remain the standard analgesic for chronic pain despite known long-term consequences, these results may indicate that co-administration of antagonists with morphine could be utilized to reduce the long-term deleterious effects of opioids, without sacrificing mu opioid receptor-mediated analgesia.
Supplementary Material
No impairment of motor function due to CCI or morphine. This experiment tested whether CCI, morphine, or their interaction would impair motor function as measured by the rotarod test. Data are from 1 week post morphine/saline administration. Time x treatment: F42,204 = 0.327, p > 0.999; time: F6,204 = 1.99, p = 0.068; treatment: F7,34 = 1.33, p = 0.366; n = 5–6 per group
No potentiation of CCI inactive (light) phase voluntary wheel running by 5 days of systemic morphine co-administered with systemic P2X7 or TLR4 inhibitors. This study tested whether potentiation of inactive phase wheel running behavior in CCI rats would result from systemic morphine co-administered with P2X7 or TLR4 antagonists. No effect of on either running distance (a) or maximum running speed (b) was observed. n = 5–6 per group
No potentiation of Sham active (dark) phase voluntary wheel running by 5 days of systemic saline co-administered with systemic P2X7 or TLR4 inhibitors. To test whether potentiation of wheel running behavior arose simply as a result of systemically administered P2X7 or TLR4 antagonists (in the absence of either CCI or morphine), the paradigm was replicated in sham rats. Systemic co-administration of saline with either (+)-NLX nor A438079 failed to alter either running distance (a) or maximum speed (b). Because, by chance, groups had different levels of running behavior prior to drug dosing, behavior in this Figure was calculated as the change from the running average of Days 8 and 9 (final days prior to initiation of drug dosing) in order to eliminate this bias. n = 5–6 per group
No potentiation of CCI inactive (light) phase voluntary wheel running by 5 days of systemic saline co-administered with systemic P2X7 or TLR4 inhibitors. This study tested whether potentiation of inactive phase wheel running behavior in CCI rats would result from systemic saline co-administered with P2X7 or TLR4 antagonists. No effect of on either running distance (a) or maximum running speed (b) was observed. n = 5–6 per group
No potentiation of sham inactive (light) phase voluntary wheel running by 5 days of systemic morphine co-administered with systemic P2X7 or TLR4 inhibitors. This study tested whether potentiation of inactive phase wheel running behavior in sham rats would result from morphine co-administered with P2X7 or TLR4 antagonists. No effect of on either running distance (a) or maximum running speed (b) was observed. n = 5–6 per group
Significance.
Evoked withdrawal responses to measure pain are increasingly criticized, stimulating the search for naturalistic behavioral indices. This study tests systemically administered therapeutics on nerve injury–induced suppression of voluntary wheel running, across weeks after dosing cessation. Nerve injury suppressed running distance and speed for 7+ weeks. A brief course of morphine increased running distance and speed for 5+ weeks, only in shams. Morphine plus antagonists of receptors that activate inflammatory cascades returned running of neuropathic rats to that of shams. This is strikingly different from results with intrathecal antagonists. This may point to systemic drugs acting simultaneously at multiple sites along the neuraxis.
ACKNOWLEDGMENTS
Morphine was gifted from the National Institute on Drug Abuse Drug Supply Program (NDSP) Division of Therapeutics and Medical Consequences, Research Triangle Institute, NC. The Toll-like receptor 4 (TLR4) antagonist (+)-Naloxone was synthesized by Dr. Kenner Rice (National Institute on Drug Abuse/National Institute on Alcohol Abuse and Alcoholism, Bethesda) for use in these studies. Supported by Department of Defense grant W81XWH-16-1-0161 (L.R.W.), NIH R01 DA044934 (L.R.W.), University of Texas Rising STARs Award (P.M.G.)., and by the National Institute on Alcohol Abuse and Alcoholism and the National Institute on Drug Abuse Intramural Research Programs.
Funding information
Supported by Department of Defense grant W81XWH-16-1-0161 (L.R.W.), NIH R01 DA044934 (L.R.W.), University of Texas Rising STARs Award (P.M.G.), and by the National Institute on Alcohol Abuse and Alcoholism and the National Institute on Drug Abuse Intramural Research Programs.
Footnotes
CONFLICT OF INTEREST
No conflicts of interest pertain to this work for any author.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Edited by Tuan Trang. Reviewed by Bradley Kerr, Theodore Price, and Michael Burton.
The peer review history for this article is available at https://publons.com/publon/10.1002/jnr.24645.
DECLARATION OF TRANSPARENCY
The authors, reviewers and editors affirm that in accordance to the policies set by the Journal of Neuroscience Research, this manuscript presents an accurate and transparent account of the study being reported and that all critical details describing the methods and results are present.
SUPPORTING INFORMATION
Additional Supporting Information may be found online in the Supporting Information section.
REFERENCES
- Bennett GJ, & Xie YK (1988). A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain, 33(1), 87–107. 10.1016/0304-3959(88)90209-6 [DOI] [PubMed] [Google Scholar]
- Burish MJ, Chen Z, & Yoo S-H (2019). Emerging relevance of circadian rhythms in headaches and neuropathic pain. Acta Physiologica, 225(1), e13161. 10.1111/apha.13161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobos EJ, Ghasemlou N, Araldi D, Segal D, Duong K, & Woolf CJ (2012). Inflammation-induced decrease in voluntary wheel running in mice: A nonreflexive test for evaluating inflammatory pain and analgesia. Pain, 153(4), 876–884. 10.1016/j.pain.2012.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corder G, Tawfik VL, Wang D, Sypek EI, Low SA, Dickinson JR, … Scherrer G. (2017). Loss of μ opioid receptor signaling in nociceptors, but not microglia, abrogates morphine tolerance without disrupting analgesia. Nature Medicine, 23(2), 164–173. 10.1038/nm.4262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuis JR, Dvorakova LS, & Vetter I. (2017). Methods used to evaluate pain behaviors in rodents. Frontiers in Molecular Neuroscience, 10, 284. 10.3389/fnmol.2017.00284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domino EF, Vasko MR, & Wilson AE (1976). Mixed depressant and stimulant actions of morphine and their relationship to brain acetylcholine. Life Sciences, 18(4), 361–376. 10.1016/0024-3205(76)90213-7 [DOI] [PubMed] [Google Scholar]
- Ferrini F, Trang T, Mattioli T-A, Laffray S, Del’Guidice T, Lorenzo L-E, … De Koninck Y. (2013). Morphine hyperalgesia gated through microglia-mediated disruption of neuronal Cl− homeostasis. Nature Neuroscience, 16(2), 183–192. 10.1038/nn.3295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace PM, Fabisiak TJ, Green-Fulgham SM, Anderson ND, Strand KA, Kwilasz AJ, … Watkins LR (2016). Prior voluntary wheel running attenuates neuropathic pain. Pain, 157(9), 2012–2023. 10.1097/j.pain.0000000000000607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace PM, Strand KA, Galer EL, Rice KC, Maier SF, & Watkins LR (2018). Protraction of neuropathic pain by morphine is mediated by spinal damage associated molecular patterns (DAMPs) in male rats. Brain, Behavior, and Immunity, 72, 45–50. 10.1016/j.bbi.2017.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace PM, Strand KA, Galer EL, Urban DJ, Wang X, Baratta MV, … Watkins LR (2016). Morphine paradoxically prolongs neuropathic pain in rats by amplifying spinal NLRP3 inflammasome activation. Proceedings of the National Academy of Sciences of the United States of America, 113(24), E3441–E3450. 10.1073/pnas.1602070113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace PM, Strand KA, Maier SF, & Watkins LR (2014). Suppression of voluntary wheel running in rats is dependent on the site of inflammation: Evidence for voluntary running as a measure of hind paw-evoked pain. Journal of Pain, 15(2), 121–128. 10.1016/j.jpain.2013.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green-Fulgham SM, Ball JB, Kwilasz AJ, Fabisiak T, Maier SF, Watkins LR, & Grace PM (2019). Oxycodone, fentanyl, and morphine amplify established neuropathic pain in male rats. Pain, 160(11), 2634–2640. 10.1097/j.pain.0000000000001652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashida K, & Eisenach JC (2008). Multiplicative interactions to enhance gabapentin to treat neuropathic pain. European Journal of Pharmacology, 598(1–3), 21–26. 10.1016/j.ejphar.2008.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hook MA, Liu GT, Washburn SN, Ferguson AR, Bopp AC, Huie JR, & Grau JW (2007). The impact of morphine after a spinal cord injury. Behavioural Brain Research, 179(2), 281–293. 10.1016/j.bbr.2007.02.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Lewis SS, Coats BD, Rezvani N, Zhang Y, Wieseler JL, … Watkins LR (2010). Possible involvement of toll-like receptor 4/myeloid differentiation factor-2 activity of opioid inactive isomers causes spinal proinflammation and related behavioral consequences. Neuroscience, 167(3), 880–893. 10.1016/j.neuroscience.2010.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirkof P, Cesarovic N, Rettich A, Fleischmann T, & Arras M. (2012). Individual housing of female mice: Influence on postsurgical behaviour and recovery. Laboratory Animals, 46(4), 325–334. 10.1258/la.2012.012027 [DOI] [PubMed] [Google Scholar]
- Kandasamy R, Calsbeek JJ, & Morgan MM (2016). Home cage wheel running is an objective and clinically relevant method to assess inflammatory pain in male and female rats. Journal of Neuroscience Methods, 263, 115–122. 10.1016/j.jneumeth.2016.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawanishi N, Yano H, Yokogawa Y, & Suzuki K. (2010). Exercise training inhibits inflammation in adipose tissue via both suppression of macrophage infiltration and acceleration of phenotypic switching from M1 to M2 macrophages in high-fat-diet-induced obese mice. Exercise Immunology Review, 16, 105–118. [PubMed] [Google Scholar]
- King BF (2007). Novel P2X7 receptor antagonists ease the pain. British Journal of Pharmacology, 151(5), 565–567. 10.1038/sj.bjp.0707266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolesnikov YA, Jain S, Wilson R, & Pasternak GW (1996). Peripheral morphine analgesia: Synergy with central sites and a target of morphine tolerance. Journal of Pharmacology and Experimental Therapeutics, 279(2), 502–506. [PubMed] [Google Scholar]
- Koltyn KF (2000). Analgesia following exercise: A review. Sports Medicine, 29(2), 85–98. 10.2165/00007256-200029020-00002 [DOI] [PubMed] [Google Scholar]
- Koyanagi S, Kusunose N, Taniguchi M, Akamine T, Kanado Y, Ozono Y, … Ohdo S. (2016). Glucocorticoid regulation of ATP release from spinal astrocytes underlies diurnal exacerbation of neuropathic mechanical allodynia. Nature Communications, 7, 13102. 10.1038/ncomms13102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson CM, Wilcox GL, & Fairbanks CA (2019). The study of pain in rats and mice. Comparative Medicine, 69(6), 555–570. 10.30802/AALAS-CM-19-000062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis SS, Loram LC, Hutchinson MR, Li C-M, Zhang Y, Maier SF, … Watkins LR (2012). (+)-Naloxone, an opioid-inactive toll-like receptor 4 signaling inhibitor, reverses multiple models of chronic neuropathic pain in rats. Journal of Pain, 13(5), 498–506. 10.1016/j.jpain.2012.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaraughty S, Chu KL, Namovic MT, Donnelly-Roberts DL, Harris RR, Zhang X-F, … Jarvis MF (2007). P2X7-related modulation of pathological nociception in rats. Neuroscience, 146(4), 1817–1828. 10.1016/j.neuroscience.2007.03.035 [DOI] [PubMed] [Google Scholar]
- Milligan ED, Zapata V, Chacur M, Schoeniger D, Biedenkapp J, O’Connor KA, … Watkins LR (2004). Evidence that exogenous and endogenous fractalkine can induce spinal nociceptive facilitation in rats. European Journal of Neuroscience, 20(9), 2294–2302. 10.1111/j.1460-9568.2004.03709.x [DOI] [PubMed] [Google Scholar]
- Mogil JS (2009). Animal models of pain: Progress and challenges. Nature Reviews. Neuroscience, 10(4), 283–294. 10.1038/nrn2606 [DOI] [PubMed] [Google Scholar]
- Mogil JS, & Crager SE (2004). What should we be measuring in behavioral studies of chronic pain in animals? Pain, 112(1–2), 12–15. 10.1016/j.pain.2004.09.028 [DOI] [PubMed] [Google Scholar]
- Moraska A, & Fleshner M. (2001). Voluntary physical activity prevents stress-induced behavioral depression and anti-KLH antibody suppression. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 281(2), R484–R489. 10.1152/ajpregu.2001.281.2.R484 [DOI] [PubMed] [Google Scholar]
- Nielsen S, & Pedersen BK (2008). Skeletal muscle as an immunogenic organ. Current Opinion in Pharmacology, 8(3), 346–351. 10.1016/j.coph.2008.02.005 [DOI] [PubMed] [Google Scholar]
- Novak CM, Burghardt PR, & Levine JA (2012). The use of a running wheel to measure activity in rodents: Relationship to energy balance, general activity, and reward. Neuroscience and Biobehavioral Reviews, 36(3), 1001–1014. 10.1016/j.neubiorev.2011.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak GW, & Pan Y-X (2013). Mu opioids and their receptors: Evolution of a concept. Pharmacological Reviews, 65(4), 1257–1317. 10.1124/pr.112.007138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlovic ZW, & Bodnar RJ (1998). Opioid supraspinal analgesic synergy between the amygdala and periaqueductal gray in rats. Brain Research, 779(1–2), 158–169. 10.1016/s0006-8993(97)01115-3 [DOI] [PubMed] [Google Scholar]
- Pedersen BK (2009). Edward F. Adolph distinguished lecture: Muscle as an endocrine organ: IL-6 and other myokines. Journal of Applied Physiology, 107(4), 1006–1014. 10.1152/japplphysiol.00734.2009 [DOI] [PubMed] [Google Scholar]
- Pedersen BK, & Hoffman-Goetz L. (2000). Exercise and the immune system: Regulation, integration, and adaptation. Physiological Reviews, 80(3), 1055–1081. 10.1152/physrev.2000.80.3.1055 [DOI] [PubMed] [Google Scholar]
- Pedersen BK, & Toft AD (2000). Effects of exercise on lymphocytes and cytokines. British Journal of Sports Medicine, 34(4), 246–251. 10.1136/bjsm.34.4.246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perissin L, Facchin P, & Porro CA (2000). Diurnal variations in tonic pain reactions in mice. Life Sciences, 67(12), 1477–1488. 10.1016/S0024-3205(00)00733-5 [DOI] [PubMed] [Google Scholar]
- Petersen AMW, & Pedersen BK (2005). The anti-inflammatory effect of exercise. Journal of Applied Physiology, 98(4), 1154–1162. 10.1152/japplphysiol.00164.2004 [DOI] [PubMed] [Google Scholar]
- Petersen AMW, & Pedersen BK (2006). The role of IL-6 in mediating the anti-inflammatory effects of exercise. Journal of Physiology and Pharmacology, 57(Suppl 10), 43–51. [PubMed] [Google Scholar]
- Pitzer C, Kuner R, & Tappe-Theodor A. (2016). EXPRESS: Voluntary and evoked behavioral correlates in neuropathic pain states under different housing conditions. Molecular Pain, 12. 10.1177/1744806916656635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld CS (2017). Sex-dependent differences in voluntary physical activity. Journal of Neuroscience Research, 95(1–2), 279–290. 10.1002/jnr.23896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi GC, Pasternak GW, & Bodnar RJ (1993). Synergistic brainstem interactions for morphine analgesia. Brain Research, 624(1–2), 171–180. 10.1016/0006-8993(93)90075-x [DOI] [PubMed] [Google Scholar]
- Sasse SK, Greenwood BN, Masini CV, Nyhuis TJ, Fleshner M, Day HEW, & Campeau S. (2008). Chronic voluntary wheel running facilitates corticosterone response habituation to repeated audiogenic stress exposure in male rats. Stress, 11(6), 425–437. 10.1080/10253890801887453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyu BC, Andersson SA, & Thorén P. (1984). Spontaneous running in wheels. A microprocessor assisted method for measuring physiological parameters during exercise in rodents. Acta Physiologica Scandinavica, 121(2), 103–109. 10.1111/j.1748-1716.1984.tb07435.x [DOI] [PubMed] [Google Scholar]
- Singhal G, Jaehne EJ, Corrigan F, & Baune BT (2014). Cellular and molecular mechanisms of immunomodulation in the brain through environmental enrichment. Frontiers in Cellular Neuroscience, 8, 97. 10.3389/fncel.2014.00097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisti HM, & Lewis MJ (2001). Naloxone suppression and morphine enhancement of voluntary wheel-running activity in rats. Pharmacology, Biochemistry, and Behavior, 70(2–3), 359–365. 10.1016/s0091-3057(01)00624–4 [DOI] [PubMed] [Google Scholar]
- Sun Q, Wang C, Yan B, Shi HX, Shi Y, Qu L, & Liang CX (2019a). [Expressions of Thioredoxin Interacting Protein/Nucleotide-binding Oligomerization Domain-like Receptor Protein 3 Inflammasome in the Sciatic Nerve of Streptozotocin-induced Diabetic Rats]. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. Acta Academiae Medicinae Sinicae, 41(6), 799–805. 10.3881/j.issn.1000-503X.11468 [DOI] [PubMed] [Google Scholar]
- Sun Q, Wang C, Yan B, Shi X, Shi Y, Qu L, & Liang X. (2019b). Jinmaitong ameliorates diabetic peripheral neuropathy through suppressing TXNIP/NLRP3 inflammasome activation in the streptozotocin-induced diabetic rat model. Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy, 12, 2145–2155. 10.2147/DMSO.S223842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tappe-Theodor A, King T, & Morgan MM (2019). Pros and cons of clinically relevant methods to assess pain in rodents. Neuroscience and Biobehavioral Reviews, 100, 335–343. 10.1016/j.neubiorev.2019.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh NP, Gleeson M, Shephard RJ, Gleeson M, Woods JA, Bishop NC, … Simon P. (2011). Position statement. Part one: Immune function and exercise. Exercise Immunology Review, 17, 6–63. [PubMed] [Google Scholar]
- Wang X, Loram LC, Ramos K, de Jesus AJ, Thomas J, Cheng K, … Yin H. (2012). Morphine activates neuroinflammation in a manner parallel to endotoxin. Proceedings of the National Academy of Sciences of the United States of America, 109(16), 6325–6330. 10.1073/pnas.1200130109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Northcutt AL, Cochran TA, Zhang X, Fabisiak TJ, Haas ME, … Watkins LR (2019). Methamphetamine activates toll-like receptor 4 to induce central immune signaling within the ventral tegmental area and contributes to extracellular dopamine increase in the nucleus accumbens shell. ACS Chemical Neuroscience, 10(8), 3622–3634. 10.1021/acschemneuro.9b00225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhang Y, Peng Y, Hutchinson MR, Rice KC, Yin H, & Watkins LR (2016). Pharmacological characterization of the opioid inactive isomers (+)-naltrexone and (+)-naloxone as antagonists of toll-like receptor 4. British Journal of Pharmacology, 173(5), 856–869. 10.1111/bph.13394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead RA, Lam NL, Sun MS, Sanchez J, Noor S, Vanderwall AG, … Milligan ED (2017). Chronic sciatic neuropathy in rat reduces voluntary wheel-running activity with concurrent chronic mechanical allodynia. Anesthesia and Analgesia, 124(1), 346–355. 10.1213/ANE.0000000000001662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung JC, & Rudy TA (1980). Multiplicative interaction between narcotic agonisms expressed at spinal and supraspinal sites of antinociceptive action as revealed by concurrent intrathecal and intracerebroventricular injections of morphine. Journal of Pharmacology and Experimental Therapeutics, 215(3), 633–642. [PubMed] [Google Scholar]
- Yezierski RP, & Hansson P. (2018). Inflammatory and neuropathic pain from bench to bedside: What went wrong? Journal of Pain, 19(6), 571–588. 10.1016/j.jpain.2017.12.261 [DOI] [PubMed] [Google Scholar]
- Yousuf MS, Noh M-C, Friedman TN, Zubkow K, Johnson JC, Tenorio G, … Kerr BJ (2019). Sensory neurons of the dorsal root ganglia become hyperexcitable in a T-cell-mediated MOG-EAE model of multiple sclerosis. Eneuro, 6(2). 10.1523/ENEURO.0024-192019 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
No impairment of motor function due to CCI or morphine. This experiment tested whether CCI, morphine, or their interaction would impair motor function as measured by the rotarod test. Data are from 1 week post morphine/saline administration. Time x treatment: F42,204 = 0.327, p > 0.999; time: F6,204 = 1.99, p = 0.068; treatment: F7,34 = 1.33, p = 0.366; n = 5–6 per group
No potentiation of CCI inactive (light) phase voluntary wheel running by 5 days of systemic morphine co-administered with systemic P2X7 or TLR4 inhibitors. This study tested whether potentiation of inactive phase wheel running behavior in CCI rats would result from systemic morphine co-administered with P2X7 or TLR4 antagonists. No effect of on either running distance (a) or maximum running speed (b) was observed. n = 5–6 per group
No potentiation of Sham active (dark) phase voluntary wheel running by 5 days of systemic saline co-administered with systemic P2X7 or TLR4 inhibitors. To test whether potentiation of wheel running behavior arose simply as a result of systemically administered P2X7 or TLR4 antagonists (in the absence of either CCI or morphine), the paradigm was replicated in sham rats. Systemic co-administration of saline with either (+)-NLX nor A438079 failed to alter either running distance (a) or maximum speed (b). Because, by chance, groups had different levels of running behavior prior to drug dosing, behavior in this Figure was calculated as the change from the running average of Days 8 and 9 (final days prior to initiation of drug dosing) in order to eliminate this bias. n = 5–6 per group
No potentiation of CCI inactive (light) phase voluntary wheel running by 5 days of systemic saline co-administered with systemic P2X7 or TLR4 inhibitors. This study tested whether potentiation of inactive phase wheel running behavior in CCI rats would result from systemic saline co-administered with P2X7 or TLR4 antagonists. No effect of on either running distance (a) or maximum running speed (b) was observed. n = 5–6 per group
No potentiation of sham inactive (light) phase voluntary wheel running by 5 days of systemic morphine co-administered with systemic P2X7 or TLR4 inhibitors. This study tested whether potentiation of inactive phase wheel running behavior in sham rats would result from morphine co-administered with P2X7 or TLR4 antagonists. No effect of on either running distance (a) or maximum running speed (b) was observed. n = 5–6 per group