Abstract
To analyze sex-specific relative and absolute risks associated with blood pressure (BP), we performed conventional and 24-hour ambulatory BP measurements in 9357 subjects (mean age, 52.8 years; 47% women) recruited from 11 populations. We computed standardized multivariable-adjusted hazard ratios for associations between outcome and systolic BP. During a course of 11.2 years (median), 1245 participants died, 472 of cardiovascular causes. The number of fatal combined with nonfatal events was 1080, 525, and 458 for cardiovascular and cardiac events and for stroke, respectively. In women and men alike, systolic BP predicted outcome, irrespective of the type of BP measurement. Women compared with men were at lower risk (hazard ratios for death and all cardiovascular events=0.66 and 0.62, respectively; P<0.001). However, the relation of all cardiovascular events with 24-hour BP (P=0.020) and the relations of total mortality (P=0.023) and all cardiovascular (P=0.0013), cerebrovascular (P=0.045), and cardiac (P=0.034) events with nighttime BP were steeper in women than in men. Consequently, per a 1-SD decrease, the proportion of potentially preventable events was higher in women than in men for all cardiovascular events (35.9% vs 24.2%) in relation to 24-hour systolic BP (1-SD, 13.4 mm Hg) and for all-cause mortality (23.1% vs 12.3%) and cardiovascular (35.1% vs 19.4%), cerebrovascular (38.3% vs 25.9%), and cardiac (31.0% vs 16.0%) events in relation to systolic nighttime BP (1-SD, 14.1 mm Hg). In conclusion, although absolute risks associated with systolic BP were lower in women than men, our results reveal a vast and largely unused potential for cardiovascular prevention by BP-lowering treatment in women.
Keywords: blood pressure, epidemiology, morbidity, risk factors, women
In the United States, cardiovascular disease kills ≈500 000 women each year, ≈1 every minute.1 Whereas 1 in 30 American women die of breast cancer, ≈1 in 3 dies from largely preventable cardiovascular disorders.1,2 Ninety percent of women have 1 or more risk factors for developing heart disease, but blood pressure (BP) remains the major reversible cardiovascular risk factor.
Conventional BP measurement by auscultation of the Korotkoff sounds is fraught with potential sources of error. Compared with conventional sphygmomanometry, ambulatory BP recordings have higher reproducibility and therefore provide a better estimate of a subject’s usual BP and cardiovascular prognosis.3-5 To our knowledge, no previous population study assessed the absolute and relative risks associated with BP on both conventional and ambulatory measurement in women compared with men and assessed the number of cardiovascular complications potentially preventable by lowering the ambulatory BP in women and men.
Methods
Study Population
As described in detail elsewhere,6 we constructed the International Database on Ambulatory blood pressure monitoring in relation to Cardiovascular Outcomes (IDACO). Studies were eligible for inclusion if they involved a random population sample, if baseline information on the ambulatory BP and cardiovascular risk factors was available, and if the subsequent follow-up included both fatal and nonfatal outcomes.
At the time of writing this report, the IDACO database included prospective studies from 11 centers (11 785 subjects). In line with previous reports, we excluded 252 participants (2.1%) because they were <18 years old at the moment of enrolment and 219 (1.9%) because their conventional BP had not been measured. We also excluded 493 (4.2%) and 1464 (12.4%) participants because their ambulatory recording included <30 readings during the whole day or <5 readings during nighttime, respectively. Thus, the number of subjects statistically analyzed totaled 9357. The participants were 2142 residents from Copenhagen, Denmark7; 1124 subjects from Noorderkempen, Belgium8; 1097 older men from Uppsala, Sweden9; 244 subjects from Novosibirsk, the Russian Federation10,11; 1312 inhabitants from Ohasama, Japan12; 349 villagers from the JingNing County, China13; 1372 subjects from Montevideo, Uruguay14; 165 subjects from Pilsen, the Czech Republic11; 934 subjects from Dublin, Ireland15; 310 subjects from Padova, Italy11; and 308 subjects from Kraków, Poland.11
BP Measurement
A detailed description of the methods used for conventional and ambulatory BP measurement is provided in the Expanded Methods section available online only at http://hyper.ahajournals.org. Hypertension was a conventional BP of at least 140 mm Hg systolic or 90 mm Hg diastolic or use of antihypertensive drugs.
Other Measurements
In all cohorts, we administered a questionnaire to obtain information on each subject’s medical history and smoking and drinking habits. Body mass index was body weight in kilograms divided by height in meters squared. We measured serum cholesterol and blood glucose by automated enzymatic methods.
Ascertainment of Events
We ascertained vital status and the incidence of fatal and nonfatal diseases from the appropriate sources in each country, as described in previous publications.6,9,12-14 Fatal and nonfatal strokes did not include transient ischemic attacks. Coronary events encompassed death from ischemic heart disease, sudden death, nonfatal myocardial infarction, and coronary revascularization. Cardiac events comprised coronary end points and fatal and nonfatal heart failure. The composite cardiovascular end point included all aforementioned end points plus cardiovascular mortality. In all outcome analyses, we only considered the first event within each category. The International Classification of Disease code numbers used to differentiate these events are available in Table I of the online-only Data Supplement available at http://hyper.ahajournals.org.
Statistical Methods
For database management and statistical analysis, we used SAS software, version 9.1.3 (SAS Institute, Cary, NC). For comparison of means and proportions, we applied the large-sample z test and the χ2 statistic, respectively. Statistical significance was a probability value of 0.05 or less on 2-sided tests.
Because in middle-aged and older subjects systolic BP is a stronger risk factor than is diastolic BP,16-18 we limited our analyses to systolic BP. We first plotted incidence rates by quintiles of the distributions of systolic BP while standardizing for cohort and age by the direct method. In dichotomous analyses, we considered 50 years of age as a cut-off limit because cardiovascular risk increases in postmenopausal women and because 50 years is close to the median age at menopause.19 We used Kaplan-Meier survival function estimates, plotted according to current recommendations,20 and the log-rank test to estimate and compare incidence rates by sex. We applied Cox regression to compute standardized hazard ratios (HRs), which express the risk for a 1-SD change in the independent variables. We checked the proportional-hazards assumption by the Kolmogorov-type supremum test and by testing the interaction terms between follow-up duration and the risk variable of interest. The HRs were adjusted for cohort, age, body mass index, smoking and drinking, serum cholesterol, history of cardiovascular disease, diabetes mellitus, and treatment with antihypertensive drugs. In analyses stratified by cohort, we pooled the participants recruited in the framework of the European Project on Genes in Hypertension (Kraków, Novosibirsk, Padova, and Pilsen).11
Results
Baseline Characteristics
The study population consisted of 6324 Europeans (67.6%), 1661 Asians (17.8%), and 1372 South Americans (14.7%). The 9357 participants included 4397 women (47.0%) and 3866 patients with hypertension on conventional BP measurement (41.3%). Mean (±SD) age was 52.8±15.7 years. The conventional BP averaged 130.4±20.4 mm Hg systolic and 79.5±11.6 mm Hg diastolic. For the 24-hour BP, these values were 123.7±14.1 and 73.7±8.4 mm Hg, respectively. At enrolment, 2676 participants (28.6%) were current smokers and 4618 (49.4%) reported intake of alcohol.
Table 1 shows the baseline characteristics of the participants by sex. With the exception of serum total cholesterol and antihypertensive treatment, women and men differed in their baseline characteristics. Cardiovascular risk factors were less frequent among women than in men. Among 1527 hypertensive women, 679 (44.5%) were untreated, 462 (30.3%) were treated but uncontrolled, and 386 (25.3%) were treated and controlled. Among 2339 hypertensive men, 1384 (59.2%) were untreated, 662 (28.3%) were treated but uncontrolled, and 239 (19.2%) were treated and controlled.
Table 1.
Baseline Characteristics of Participants by Sex
| Characteristics | Women (n=4397) |
Men (n=4960) |
|---|---|---|
| No. with characteristic (%) | ||
| Hypertension | 1527 (34.7) | 2339 (47.2) |
| Antihypertensive drug treatment | 848 (19.3) | 955 (19.3) |
| Diabetes mellitus | 243 (5.5) | 371 (7.5) |
| Current smokers | 945 (21.5) | 1731 (34.9) |
| Current drinkers | 1578 (35.9) | 3040 (61.3) |
| Previous cardiovascular disease | 232 (5.3) | 496 (10.0) |
| Age, y | 50.3±15.2 | 55.0±15.9 |
| Body mass index, kg/m2 | 24.8±4.5 | 25.8±3.9 |
| Blood pressure, mm Hg | ||
| Conventional systolic | 125.6±20.1 | 134.5±19.8 |
| 24-hour systolic | 119.9±13.4 | 127.0±13.8 |
| Daytime systolic | 126.0±14.3 | 133.7±14.8 |
| Nighttime systolic | 108.7±14.1 | 115.2±15.1 |
| Conventional diastolic | 77.1±11.4 | 81.7±11.3 |
| 24-hour diastolic | 71.6±8.1 | 75.6±8.3 |
| Daytime diastolic | 76.8±8.8 | 80.7±9.0 |
| Nighttime diastolic | 62.3±8.6 | 66.4±9.2 |
| Serum cholesterol, mmol/L | 5.63±1.18 | 5.64±1.16 |
All between-sex differences were significant (P<0.0001) with the exception of serum cholesterol (P=0.59) and antihypertensive treatment (P=0.059). Hypertension was a conventional BP of at least 140 mm Hg systolic or 90 mm Hg diastolic or use of antihypertensive drugs. Diabetes mellitus was use of antidiabetic drugs, a fasting blood glucose concentration ≥7.0 mmol/L, a random blood glucose concentration of ≥11.1 mmol/L, a self-reported diagnosis, or diabetes documented in practice or hospital records. Plus/minus values are mean±SD.
Table II (online only) lists the baseline characteristics of women and men by age class, with median year at menopause (50 years) as the cut-off. Comparing younger and older subjects revealed that all baseline characteristics in both sexes differed by age group. The only exception was the proportion of nondippers, defined as a night-to-day systolic pressure ratio of <0.90. Nondipping was significantly more frequent (P<0.01) among older women (31.3% vs 25.9%) and older men (29.7% vs 25.7%) than in younger subjects. In continuous analyses of the night-to-day ratio, however, the age differences disappeared in women (0.87 vs 0.86; P=0.25) as well as in men (0.86 vs 0.86; P=0.47).
Incidence of Events
In the overall study population, median follow-up was 11.2 years (5th to 95th percentile interval, 2.5 to 17.6 years). During 100 396 person-years of follow-up, 1245 participants died (12.4 per 1000 person-years), and 1080 experienced a fatal or nonfatal cardiovascular complication (10.8 per 1000 person-years). The cause of death was cardiovascular in 472 participants, noncardiovascular in 714, renal failure in 17, and unknown in 42. Considering cause-specific first cardiovascular events, the incidence of fatal and nonfatal stroke amounted to 88 and 370, respectively, and cardiac events consisted of 171 fatal and 438 nonfatal events.
Sex-Specific Incidence of Events in Unadjusted Analyses
Exploratory analyses, in which we plotted the incidence of events standardized for cohort and age, showed association between the incidence of total mortality and cardiovascular events and BP on conventional and ambulatory measurement in women as well as men (Figure 1). The cohort- and age-standardized rates were significantly higher in the top than in the bottom quintile (P≤0.0023) except for noncardiovascular mortality, which was not associated with BP in women (P=0.31) or men (P=0.77). The Kaplan-Meier survival function estimates showed a significantly lower incidence of total, cardiovascular, and noncardiovascular mortality (P≤0.001) and of all cardiovascular, cerebrovascular, cardiac, and coronary events (P≤0.0001) in women than in men (Figure 2).
Figure 1.
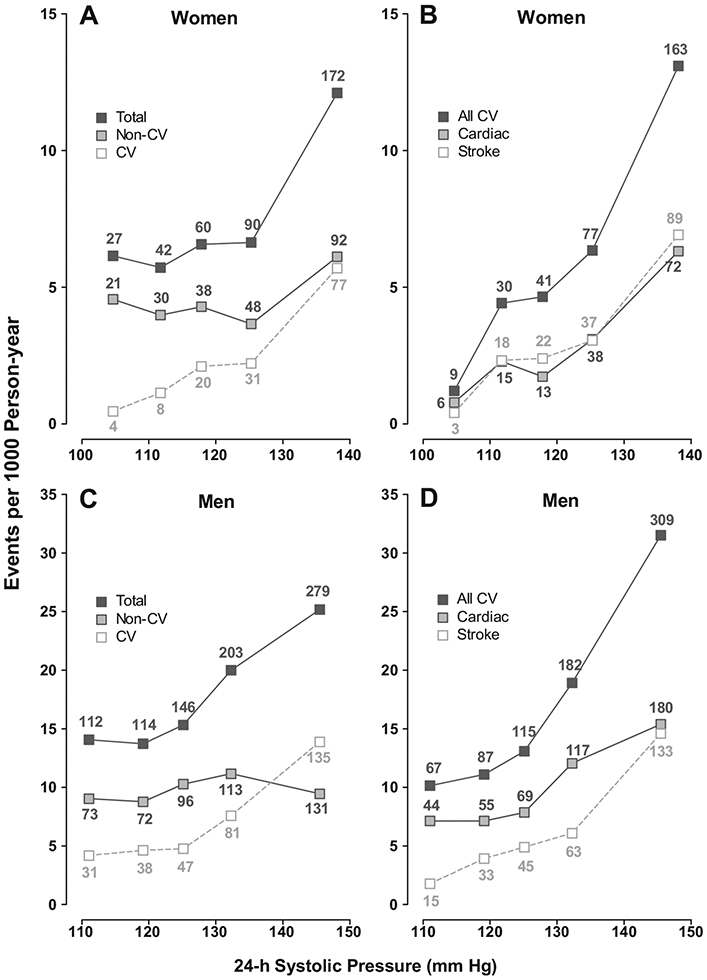
Incidence of total mortality (A, C) and all cardiovascular events (B, D) in relation to the 24-hour systolic BP in 4397 women (A, B) and 4960 men (C, D). Incidence rates were standardized for cohort and age by the direct method. Mortality rates are plotted separately for total, noncardiovascular (non-CV), and cardiovascular (CV) mortality. Cardiovascular events refer to the composite of all fatal plus nonfatal cardiovascular events. The number of end points contributing to the rates is presented.
Figure 2.
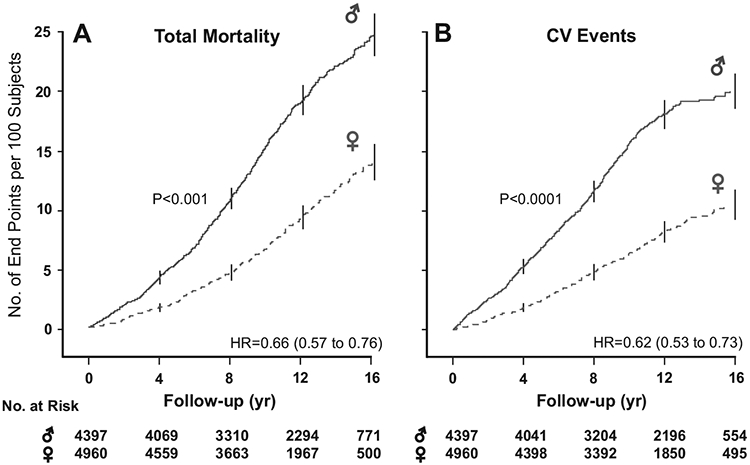
Kaplan-Meier survival function estimates for total mortality (A) and the composite of all fatal plus nonfatal cardiovascular events (B) in 4397 women and 4960 men. Follow-up time spans the 5th to 95th percentile interval. Numbers refer to women and men at risk at the beginning of each 4-year interval. Vertical lines represent the SE of the survival function estimates. HR refers to the hazard ratio, which expresses the risk of women compared with men, with adjustment applied for cohort, age, body mass index, smoking and drinking, serum total cholesterol, history of cardiovascular disease, presence of diabetes mellitus, and antihypertensive drug treatment at baseline.
Sex-Specific Incidence of Events in Multivariable-Adjusted Analyses
Relative Risk
Table 2 shows the multivariable-adjusted standardized HRs for mortality by sex. In women and men, systolic BP on conventional, 24-hour, and nighttime measurement was a significant predictor of total and cardiovascular mortality. Daytime systolic BP predicted total mortality in women and cardiovascular mortality in both sexes. The HRs relating total mortality to the 24-hour systolic BP or to the nighttime systolic BP were, respectively, slightly (P=0.097) or significantly (P=0.023) larger in women than in men (Table 2), whereas those associated with the conventional (P≤0.89) and daytime (P≤0.19) BPs were similar in both sexes. Except for nighttime BP in women, systolic BP did not predict noncardiovascular mortality and was significantly (P<0.001) higher in subjects dying of cardiovascular causes than in those dying of noncardiovascular diseases. For the 24-hour systolic BP, these levels were 132.9±14.4 versus 127.4±15.8 mm Hg (P=0.0008) in women and 135.7±16.3 versus 129.8±14.2 mm Hg (P<0.0001) in men.
Table 2.
Multivariable-Adjusted Standardized HRs for Mortality in Relation to Systolic BP by Sex
| Cause of Death | No. | Conventional | 24-Hour | Daytime | Nighttime |
|---|---|---|---|---|---|
| Total | |||||
| Women | 391 | 1.12 (1.00–1.25)* | 1.25 (1.12–1.38)‡ | 1.17 (1.05–1.30)† | 1.30 (1.17–1.44)‡ |
| Men | 854 | 1.14 (1.06–1.23)‡ | 1.12 (1.04–1.19)‡ | 1.06 (0.99–1.13) | 1.14 (1.07–1.20)‡ |
| P | 0.89 | 0.097 | 0.19 | 0.023 | |
| Noncardiovascular | |||||
| Women | 229 | 1.02 (0.88–1.19) | 1.13 (0.98–1.30) | 1.05 (0.91–1.21) | 1.22 (1.06–1.39)† |
| Men | 485 | 1.01 (0.91–1.12) | 0.98 (0.90–1.08) | 0.95 (0.86–1.04) | 1.04 (0.96–1.13) |
| P | 0.082 | 0.054 | 0.15 | 0.025 | |
| Cardiovascular | |||||
| Women | 140 | 1.36 (1.15–1.61)‡ | 1.52 (1.28–1.80)‡ | 1.45 (1.23–1.71)‡ | 1.50 (1.27–1.76)‡ |
| Men | 332 | 1.33 (1.19–1.49)‡ | 1.31 (1.18–1.44)‡ | 1.23 (1.11–1.37)‡ | 1.26 (1.16–1.37)‡ |
| P | 0.75 | 0.24 | 0.25 | 0.13 |
P indicates the significance of the sex difference in the HRs. No. refers to the number of deaths. The numbers at risk were 4397 women and 4960 men. The HRs (95% CIs) express the risk associated with a 1-SD increase in systolic BP. In women, the SDs of systolic BP were 20.1, 13.4, 14.3, and 14.1 mm Hg for the conventional, 24-hour, daytime, and nighttime BPs; in men, the corresponding SDs were 19.8, 13.8, 14.8, and 15.1 mm Hg, respectively. All models were adjusted for cohort, age, body mass index, smoking and drinking, serum total cholesterol, a history of cardiovascular disease, the presence of diabetes mellitus, and antihypertensive drug treatment at baseline. There were 42 deaths of unknown cause and 17 fatal renal deaths.
Significance of the HRs:
P<0.05
P<0.01
P<0.001.
Table 3 shows the multivariable-adjusted standardized HRs for all and cause-specific cardiovascular events by sex. In women and men, the 24-hour, daytime, and nighttime systolic BPs were significant predictors of all cardiovascular events, stroke, and cardiac and coronary complications. The conventional systolic BP predicted all cardiovascular events and stroke in women and men and cardiac and coronary events, but only in men. The HRs expressing the risk of the composite cardiovascular end point in relation to the 24-hour systolic BP (P=0.020) and the risk of all cardiovascular (P=0.0013), cerebrovascular (P=0.045), and cardiac (P=0.034) events in relation to the nighttime systolic BP were higher in women than in men (Table 3).
Table 3.
Multivariable-Adjusted Standardized HRs for Cardiovascular Events in Relation to Systolic BP by Sex
| Event | No. | Conventional | 24-Hour | Daytime | Nighttime |
|---|---|---|---|---|---|
| All cardiovascular | |||||
| Women | 320 | 1.26 (1.12–1.42)‡ | 1.56 (1.39–1.74)‡ | 1.45 (1.29–1.61)‡ | 1.54 (1.38–1.71)‡ |
| Men | 760 | 1.28 (1.19–1.38)‡ | 1.32 (1.23–1.40)‡ | 1.27 (1.19–1.36)‡ | 1.24 (1.17–1.31)‡ |
| P | 0.95 | 0.020 | 0.11 | 0.0013 | |
| Stroke | |||||
| Women | 169 | 1.44 (1.24–1.67)‡ | 1.67 (1.44–1.94)‡ | 1.56 (1.34–1.81)‡ | 1.62 (1.40–1.88)‡ |
| Men | 289 | 1.42 (1.26–1.60)‡ | 1.51 (1.36–1.67)‡ | 1.45 (1.30–1.61)‡ | 1.35 (1.24–1.47)‡ |
| P | 0.98 | 0.30 | 0.52 | 0.045 | |
| Cardiac | |||||
| Women | 144 | 1.05 (0.87–1.26) | 1.47 (1.24–1.74)‡ | 1.35 (1.14–1.61)‡ | 1.45 (1.24–1.70)‡ |
| Men | 465 | 1.24 (1.13–1.37)‡ | 1.24 (1.14–1.35)‡ | 1.22 (1.11–1.33)‡ | 1.19 (1.11–1.29)‡ |
| P | 0.41 | 0.10 | 0.35 | 0.034 | |
| Coronary | |||||
| Women | 86 | 1.15 (0.91–1.46) | 1.51 (1.22–1.88)‡ | 1.46 (1.17–1.81)‡ | 1.44 (1.17–1.77)‡ |
| Men | 357 | 1.15 (1.02–1.28)* | 1.18 (1.07–1.30)‡ | 1.19 (1.07–1.31)‡ | 1.12 (1.03–1.23)† |
| P | 0.86 | 0.12 | 0.21 | 0.090 |
P indicates the significance of the sex difference in the HRs. No. refers to the number of fatal and nonfatal events. See the footnote to Table 2 for details.
Significance of the HRs:
P<0.05
P<0.01
P<0.001.
The absolute 10-year risk of death, a composite cardiovascular end point, a fatal or nonfatal stroke, or a fatal or nonfatal cardiac event in relation to the 24-hour and nighttime systolic BPs appear in Figure 3 and online-only Figure I. The continuous-risk functions were fitted by Cox regression with adjustment for cohort, age, body mass index, smoking and drinking, serum total cholesterol, a history of cardiovascular disease, the presence of diabetes mellitus, and antihypertensive drug treatment at baseline. To illustrate the fit of the continuous risk function, Figures 3 and I also include the HRs expressing the risk by quintiles of the BP distributions. Absolute risk was lower in women than in men, but the increase in risk with BP was slightly or significantly steeper in women than men.
Figure 3.
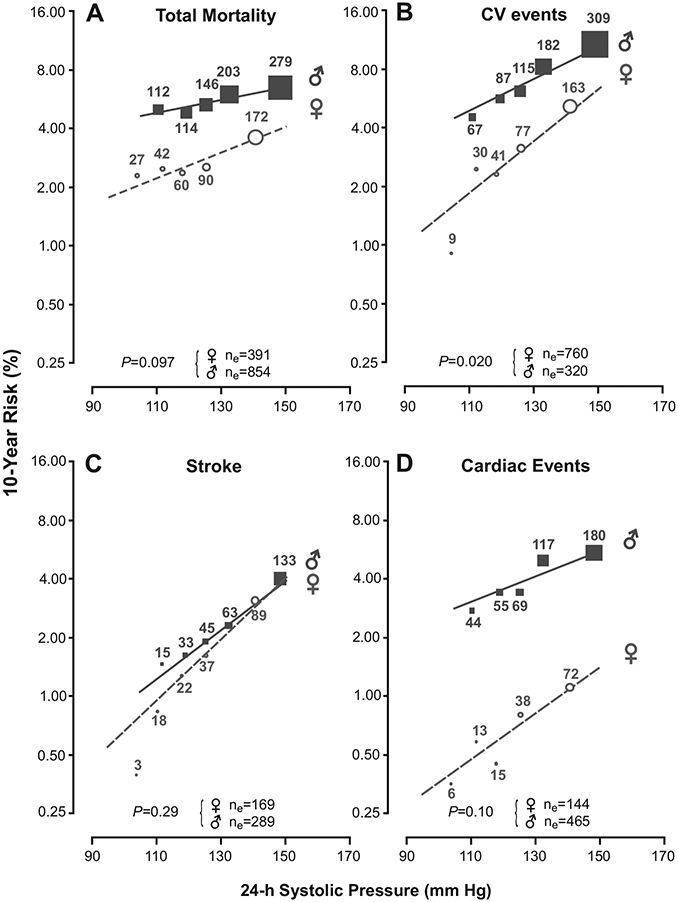
Absolute 10-year risk of death (A), a composite cardiovascular (CV) end point (B), a fatal or nonfatal stroke (C), or a fatal or nonfatal cardiac event (D) in relation to the 24-hour systolic BP. The continuous risk functions cover the 5th to 95th percentile interval of the 24-hour systolic BP and were fitted by Cox regression with adjustment for cohort, age, body mass index, smoking and drinking, serum total cholesterol, history of cardiovascular disease, presence of diabetes mellitus, and antihypertensive drug treatment at baseline. Circles (women) and squares (men) represent the multivariable-adjusted HRs in quintiles of the distribution of the 24-hour systolic BP and have a size proportional to the inverse of the variance of the HR. The number of events in each quintile is given next to each circle or square; ne is the total number of events by disease category and sex. The probability values for interaction were derived from multivariable-adjusted Cox models as given in Tables 2 and 3.
Number of Prevented Events
Estimates of the number of end points potentially prevented by a 1-SD decrease in systolic BP on 24-hour or nighttime measurement appear in Figure 4. Because women experienced fewer events than did men, we expressed the number of preventable events as a percentage of the total number in either sex. The proportion of potentially preventable events was higher in women than in men for the composite cardiovascular end point (35.9% vs 24.2%; P=0.018) in relation to the 24-hour systolic BP, for all-cause mortality (23.1% vs 12.3%; P=0.021), and for all cardiovascular (35.1% vs 19.4%; P=0.001), cerebrovascular (38.3% vs 25.9%; P=0.043), and cardiac (31.0% vs 16.0%; P=0.027) events in relation to systolic BP at night.
Figure 4.
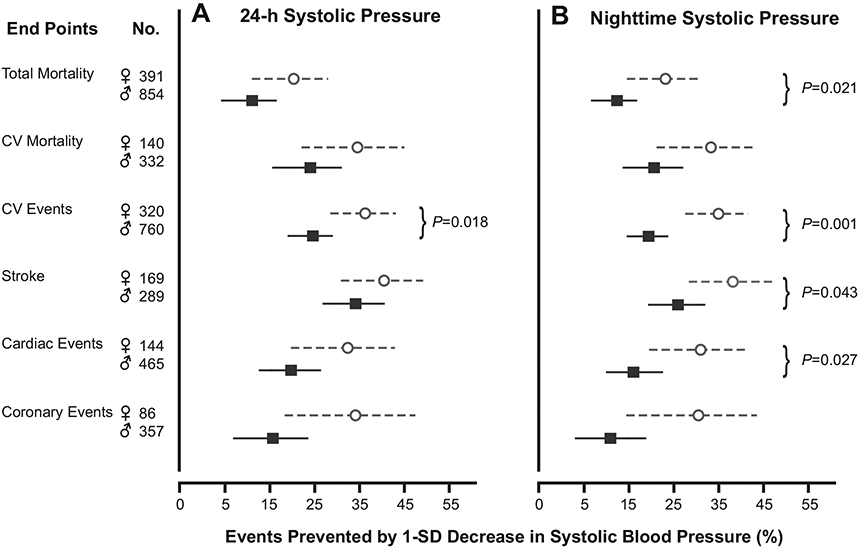
Changes in the incidence of mortality and cardiovascular (CV) events that would be associated with a 1-SD decrease in the 24-hour systolic BP (A) or in the nighttime BP (B) in women (circles) and men (squares). Estimates were derived from the multivariable-adjusted Cox models presented in Tables 2 and 3 and the observed number of each end point. Probability values indicate significant sex differences.
Sensitivity Analyses
In sensitivity analyses, we excluded 1 cohort at a time (Tables III and IV available online only at http://hyper.ahajournals.org), and we stratified all participants according to baseline characteristics (online-only Tables V and VI). With 1 cohort excluded, all HRs expressing the risk associated with systolic BP were larger in women than in men, although because of the lower number of subjects in the analysis, not all HRs remained significant. The analyses stratified according to baseline characteristics, in general, showed slightly or significantly higher HRs in women than in men except for total mortality below age 50, in subjects with cardiovascular disease at baseline, and except for the composite cardiovascular end point in South American and Asian participants.
Discussion
The key finding of our current meta-analysis of individual data is that although absolute risk was lower in women than in men, the increase in risk with the 24-hour and nighttime BPs was steeper in women than in men. The proportion of events potentially preventable by BP lowering was therefore higher in women than in men for the composite cardiovascular end point in relation to the 24-hour systolic BP, for all fatal plus nonfatal end points, and for fatal plus nonfatal cerebrovascular and cardiac events in relation to systolic BP at night.
We did a PubMed search using the key words “women” AND “blood pressure” AND “risk.” Of the 49 “hits,” we selected 5 articles,21-25 all based on population studies. Already in 1969,21 the Framingham investigators noticed that after 14 years of follow-up, the incidence of coronary heart disease was lower in women than in men (5.9% vs 14.2%). Subsequent population studies confirmed that women are at lower risk of angina pectoris,21 myocardial infarction,21-24 stroke,24 and cardiovascular complications,25 but few studies reported detailed comparisons of relative and absolute risk between the sexes. None of the 5 reviewed studies21-25 addressed the association between risk and BP on ambulatory measurement.
In the Reykjavik Study,23 absolute risk was lower in women than in men: 7.3% versus 19.1%. In multivariable-adjusted analyses, the HRs relating the risk of myocardial infarction to office systolic BP were 1.013 (95% CI, 1.009 to 1.017) in women and 1.010 (95% CI, 1.007 to 1.013) in men; for a 20-mm Hg increase in systolic BP, as in the current study, these estimates would translate into values of 1.29 and 1.22, respectively. Because the Icelandic investigators did not report significance for the sex interaction term in the multivariable analyses,23 we used a normal approximation to estimate the sex difference in the adjusted HRs. The z statistic was 1.18 (P=0.24). The Rotterdam Study included 6004 women and men age 55 years or more.22 The authors did not state the number of women and men included in their analyses but reported that there was no evidence for a sex difference in the association of systolic or diastolic BP with the risk of myocardial infarction (P for interaction ≥0.44). The Japanese Arteriosclerosis Longitudinal Study Group24 performed a meta-analysis involving 27 163 women and 21 061 men. The standardized HRs relating stroke and myocardial infarction to systolic BP were 1.46 (95% CI, 1.35 to 1.58) and 1.25 (95% CI, 0.99 to 1.58) in women and 1.51 (95% CI, 1.41 to 1.63) and 1.23 (95% CI, 1.06 to 1.44) in men. With the normal approximation to compute the significance of the sex difference, the z values were 0.62 (P=0.54) for stroke and −0.11 (P=0.91) for myocardial infarction. In Singaporean women and men with the metabolic syndrome,25 the incidence of cardiovascular complications was 3.7 events per 1000 person-years in 108 women (4 events) and 15.9 events per 1000 person-years in 136 men (19 events). However, the HRs describing the associations of cardiovascular complications with BP were not reported.
In keeping with our previous findings,4,26 nighttime compared with daytime BP was a stronger predictor of outcome. Why relative risk increased more with nighttime BP in women than in men remains to be elucidated. In the International Database of the Ambulatory Blood Pressure,27 after adjustment for age and other significant covariables, the nocturnal fall in systolic BP was smaller in 3590 women than in 3730 men (15.1 vs 16.7 mm Hg) and women had a greater night-to-day ratio of systolic BP (0.883 vs 0.875). With similar adjustments applied in the current database, we confirmed the curvilinear association of the nocturnal BP fall and the night-to-day ratio with age (online-only Figure II), but we did not find a significant difference between the sexes in the nocturnal fall in systolic BP (women vs men, 17.9 vs 18.0 mm Hg; P=0.75) or in the systolic night-to-day BP ratio (0.862 vs 0.866, P=0.12; Table II). These previous27 and current observations exclude the hypothesis that sex-specific diurnal patterns in BP might explain the higher HRs associated with the nighttime systolic BP in women compared with men. We did not have information on the menopausal state of women at baseline or follow-up. However, the evidence currently available suggests that the cardiovascular effects usually attributed to menopause are a consequence of aging rather than of a change in the hormonal environment.28
The present study must be interpreted within the context of its potential limitations. First, BP was measured under differing conditions in the cohorts. However, in all but 1 cohort,9 BP was measured in the sitting position, and in all cohorts, the average of the first 2 measurements was used for analysis. In addition, all of the centers implemented rigorous quality-control programs for BP measurement. Second, BP was only measured at baseline. It needs to be confirmed that that our current results hold true when BP collected during follow-up would be accounted for. The IDACO consortium is currently collecting follow-up measurements of the conventional and ambulatory BPs. Unfortunately, these data are not yet available. However, use of BP-lowering drugs after enrolment can only have weakened the prognostic significance of the BP at baseline. On the plus side, our study is the first to address sex-specific differences in the association between outcome and BP based on ambulatory monitoring. Other strong points of our study are the large sample, including populations from Europe, Asia, and South America, and the large number of events.
Perspectives
In line with our current findings, most epidemiologic studies21-25 are concordant in showing that women experience cardiovascular complications at an older age and at a lower rate than do men. Although in Europe29 and elsewhere in the world women have a higher life expectancy than men do, men consistently report a higher proportion of healthy life years, when compared with women. In our current study population, 74.7% of hypertensive women and 87.4% of hypertensive men were either untreated or uncontrolled at baseline. Against this background, what our current study highlights is the large proportion of events potentially preventable in hypertensive women by BP-lowering treatment. Although absolute risk is lower in women than in men, the proportion of preventable cardiovascular complication is from 30% to 100% higher in women than in men. The lower absolute risk in women should therefore not be considered an excuse for therapeutic laxity. Women and their healthcare providers should be aware of this and request a wider use of ambulatory BP measurement to diagnose and take control of BP. This approach will help women live a longer life with higher quality.
Supplementary Material
Acknowledgments
We gratefully acknowledge the expert assistance of Sandra Covens and Ya Zhu (Studies Coordinating Centre, Leuven, Belgium). The IDACO investigators are listed in Reference 6.
Sources of Funding
The European Union (grants IC15-CT98-0329-EPOGH, LSHM-CT-2006-037093, and HEALTH-F4-2007-201550), the Fonds voor Wetenschappelijk Onderzoek Vlaanderen, Ministry of the Flemish Community, Brussels, Belgium (grants G.0575.06 and G.0734.09), and the Katholieke Universiteit Leuven (grants OT/00/25 and OT/05/49) gave support to the Studies Coordinating Centre in Leuven. The European Union (grants LSHM-CT-2006-037093 and HEALTH-F4-2007-201550) also supported the research groups in Shanghai, Kraków, Padova, and Novosibirsk. The Bilateral Scientific and Technological Collaboration between China and Flanders (grant BIL02/10) supported the fellowship of Y.L. in Leuven. The Danish Heart Foundation (grant 01-2-9-9A-22914) and the Lund-beck Fonden (grant R32-A2740) supported the studies in Copenhagen. The Ministries of Education, Culture, Sports, Science, and Technology (grants 15790293, 16590433, 17790381, 18390192, 18590587, 19590929, and 19790423) and of Health, Labor, and Welfare (H17-Kenkou-007, H18 Junkankitou [Seishuu]-Ippan-012 and H20-Junkankitou[Seishuu]-Ippan-009, 013), a grant-in-aid from the Japanese Society for the Promotion of Science (16.54041, 18.54042, 19.7152, 20.7198, 20.7477, and 20.54043), the Japan Atherosclerosis Prevention Fund, the Uehara Memorial Foundation, and the Takeda Medical Research Foundation supported research in Japan. The National Natural Science Foundation of China (grants 30871360 and 30871081), Beijing, China, and the Shanghai Commissions of Science and Technology (grant 07JC14047 and the “Rising Star” program 06QA14043) and Education (grant 07ZZ32 and the “Dawn” project) supported the JingNing study in China.
Footnotes
Disclosures
None.
Contributor Information
José Boggia, Centro de Nefrología and Departamento de Fisiopatología, Hospital de Clínicas, Universidad de la República, Montevideo, Uruguay; Studies Coordinating Centre, Division of Hypertension and Cardiovascular Rehabilitation, Department of Cardiovascular Diseases, University of Leuven, Leuven, Belgium.
Lutgarde Thijs, Studies Coordinating Centre, Division of Hypertension and Cardiovascular Rehabilitation, Department of Cardiovascular Diseases, University of Leuven, Leuven, Belgium.
Tine W. Hansen, Research Center for Prevention and Health and Department of Clinical Physiology, Nuclear Medicine and PET, Copenhagen University Hospital, Faculty of Health Sciences, Rigshospitalet, Copenhagen, Denmark
Yan Li, Center for Epidemiological Studies and Clinical Trials, Center for Vascular Evaluation, Ruijin Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, China.
Masahiro Kikuya, Tohoku University Graduate School of Pharmaceutical Science and Medicine, Sendai, Japan.
Kristina Björklund-Bodegård, Section of Geriatrics, Department of Public Health and Caring, Sciences, Uppsala University, Uppsala, Sweden.
Tom Richart, Studies Coordinating Centre, Division of Hypertension and Cardiovascular Rehabilitation, Department of Cardiovascular Diseases, University of Leuven, Leuven, Belgium; Department of Epidemiology, Maastricht University, Maastricht, The Netherlands.
Takayoshi Ohkubo, Tohoku University Graduate School of Pharmaceutical Science and Medicine, Sendai, Japan; Shiga University School of Medical Science, Otsu, Japan.
Jørgen Jeppesen, Copenhagen University Hospital, Copenhagen, Denmark.
Christian Torp-Pedersen, Copenhagen University Hospital, Copenhagen, Denmark.
Eamon Dolan, Cambridge University Hospitals, Addenbrook’s Hospital, Cambridge, United Kingdom.
Tatiana Kuznetsova, Studies Coordinating Centre, Division of Hypertension and Cardiovascular Rehabilitation, Department of Cardiovascular Diseases, University of Leuven, Leuven, Belgium; Institute of Internal Medicine, Novosibirsk, Russian Federation.
Valérie Tikhonoff, Department of Clinical and Experimental Medicine, University of Padova, Padova, Italy.
Sofia Malyutina, Institute of Internal Medicine, Novosibirsk, Russian Federation.
Edoardo Casiglia, Department of Clinical and Experimental Medicine, University of Padova, Padova, Italy.
Yuri Nikitin, Institute of Internal Medicine, Novosibirsk, Russian Federation.
Lars Lind, Section of Geriatrics, Department of Public Health and Caring, Sciences, Uppsala University, Uppsala, Sweden.
Gladys Maestre, Laboratorio de Neurociencias, Universidad del Zulia, Maracaibo, Venezuela.
Edgardo Sandoya, Asociación Española Primera de Socorros Mutuos, Montevideo, Uruguay.
Kalina Kawecka-Jaszcz, First Department of Cardiology and Hypertension, Jagiellonian University Medical College, Kraków, Poland.
Yutaka Imai, Tohoku University Graduate School of Pharmaceutical Science and Medicine, Sendai, Japan.
Jiguang Wang, Center for Epidemiological Studies and Clinical Trials, Ruijin Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, China.
Hans Ibsen, Aarhus University and Division of Cardiology, Holbak Hospital, Holbak, Denmark.
Eoin O’Brien, Conway Institute of Biomolecular and Biomedical Research University College Dublin, Dublin, Ireland.
Jan A. Staessen, Studies Coordinating Centre, Division of Hypertension and Cardiovascular Rehabilitation, Department of Cardiovascular Diseases, University of Leuven, Leuven, Belgium; Department of Epidemiology, Maastricht University, Maastricht, The Netherlands
References
- 1.Mosca L, Appel LJ, Benjamin EJ, Berra K, Chandra-Strobos N, Fabunmi RP, Grady D, Haan CK, Hayes SN, Judelson DR, Keenan NL, McBride P, Oparil S, Ouyang P, Oz MC, Mendelsohn ME, Pasternak RC, Pinn VW, Robertson RM, Schenck-Gustafsson K, Sila CA, Smith SC Jr, Sopko G, Taylor AL, Walsh BW, Wenger NK, Williams CL. Evidence-based guidelines for cardiovascular disease prevention in women. Circulation. 2004;109:672–693. [DOI] [PubMed] [Google Scholar]
- 2.Wenger NK. You’ve come a long way, baby: cardiovascular health and disease in women: problems and prospects. Circulation. 2004;109:558–560. [DOI] [PubMed] [Google Scholar]
- 3.Clement DL, De Buyzere ML, De Bacquer DA, de Leeuw PW, Duprez DA, Fagard RH, Gheeraert PJ, Missault LH, Braun JJ, Six RO, Van der Niepen P, O’Brien E, for the Office versus Ambulatory Pressure Study investigators. Prognostic value of ambulatory blood-pressure recordings in patients with treated hypertension. N Engl J Med. 2003;348:2407–2415. [DOI] [PubMed] [Google Scholar]
- 4.Dolan E, Stanton A, Thijs L, Hinedi K, Atkins N, McClory S, Den Hond E, McCormack P, Staessen JA, O’Brien E. Superiority of ambulatory over clinic blood pressure measurement in predicting mortality: the Dublin Outcome Study. Hypertension. 2005;46:156–161. [DOI] [PubMed] [Google Scholar]
- 5.Hansen TW, Kikuya M, Thijs L, Björklund-Bodegård K, Kuznetsova T, Ohkubo T, Richart T, Torp-Pedersen C, Lind L, Jeppesen J, Ibsen H, Imai Y, Staessen JA, on behalf of the IDACO Investigators. Prognostic superiority of daytime ambulatory over conventional blood pressure in four populations: a meta-analysis of 7030 individuals. J Hypertens. 2007;25:1554–1564. [DOI] [PubMed] [Google Scholar]
- 6.Thijs L, Hansen TW, Kikuya M, Björklund-Bodegård K, Li Y, Dolan E, Tikhonoff V, Sleidlerová J, Kuznetsova T, Stolarz K, Bianchi M, Richart T, Casiglia E, Malyutina S, Filipovský J, Kawecka-Jaszcz K, Nikitin Y, Ohkubo T, Sandoya E, Wang JG, Torp-Pedersen C, Lind L, Ibsen H, Imai Y, Staessen JA, on behalf of the IDACO Investigators. The International Database of Ambulatory blood pressure in relation to Cardiovascular Outcome (IDACO): protocol and research perspectives. Blood Press Monit. 2007;12:255–262. [DOI] [PubMed] [Google Scholar]
- 7.Hansen TW, Jeppesen J, Rasmussen S, Ibsen H, Torp-Pedersen C. Ambulatory blood pressure and risk of cardiovascular disease: a population based study. Am J Hypertens. 2006;19:243–259. [DOI] [PubMed] [Google Scholar]
- 8.Staessen JA, Bieniaszewski L, O’Brien ET, Imai Y, Fagard R. An epidemiological approach to ambulatory blood pressure monitoring: the Belgian population study. Blood Press Monit. 1996;1:13–26. [PubMed] [Google Scholar]
- 9.Ingelsson E, Björklund K, Lind L, Ärnlöv J, Sundström J. Diurnal blood pressure pattern and risk of congestive heart failure. J Am Med Assoc. 2006;295:2859–2866. [DOI] [PubMed] [Google Scholar]
- 10.Kuznetsova T, Malyutina S, Pello E, Thijs L, Nikitin Y, Staessen JA. Ambulatory blood pressure of adults in Novosibirsk, Russia: interim report on a population study. Blood Press Monit. 2000;5:291–296. [DOI] [PubMed] [Google Scholar]
- 11.Kuznetsova T, Staessen JA, Kawecka-Jaszcz K, Babeanu S, Casiglia E, Filipovský J, Nachev C, Nikitin Y, Peleská J, O’Brien E, on behalf of the EPOGH Investigators. Quality control of the blood pressure phenotype in the European Project on Genes in Hypertension. Blood Press Monit. 2002;7:215–224. [DOI] [PubMed] [Google Scholar]
- 12.Ohkubo T, Hozawa A, Yamaguchi J, Kikuya M, Ohmori K, Michimata M, Matsubara M, Hashimoto J, Hoshi H, Araki T, Tsuji I, Satoh H, Hisamichi S, Imai Y. Prognostic significance of the nocturnal decline in blood pressure in individuals with and without high 24-h blood pressure: the Ohasama study. J Hypertens. 2002;20:2183–2189. [DOI] [PubMed] [Google Scholar]
- 13.Li Y, Wang JG, Gao HF, Nawrot T, Wang GL, Qian YS, Staessen JA, Zhu DL. Are published characteristics of the ambulatory blood pressure generalizable to rural Chinese? the JingNing population study. Blood Press Monit. 2005;10:125–134. [DOI] [PubMed] [Google Scholar]
- 14.Schettini C, Bianchi M, Nieto F, Sandoya E, Senra H, Hypertension Working Group. Ambulatory blood pressure: normality and comparison with other measurements. Hypertension. 1999;34(pt 2):818–825. [DOI] [PubMed] [Google Scholar]
- 15.O’Brien E, Murphy J, Tyndall A, Atkins N, Mee F, McCarthy G, Staessen J, Cox J, O’Malley K. Twenty-four-hour ambulatory blood pressure in men and women aged 17 to 80 years: the Allied Irish Bank Study. J Hypertens. 1991;9:355–360. [DOI] [PubMed] [Google Scholar]
- 16.Staessen J, Amery A, Fagard R. Editorial review: isolated systolic hypertension. J Hypertens. 1990;8:393–405. [DOI] [PubMed] [Google Scholar]
- 17.Franklin SS, Larson MG, Khan SA, Wong ND, Leip EP, Kannel WB, Levy D. Does the relation of blood pressure to coronary heart disease change with aging? the Framingham Heart Study. Circulation. 2001;103:1245–1249. [DOI] [PubMed] [Google Scholar]
- 18.Inoue R, Ohkubo T, Kikuya M, Metoki H, Asayama K, Obara T, Hoshi H, Hashimoto J, Totsune K, Satoh H, Kondo Y, Imai Y. Predicting stroke using 4 ambulatory blood pressure monitoring-derived blood pressure indices: the Ohasama Study Hypertension. 2006;48:877–882. [DOI] [PubMed] [Google Scholar]
- 19.Staessen JA, Bieniaszewski L, Brosens I, Fagard R. The epidemiology of menopause and its association with cardiovascular disease. In: Messerli FH, Aepfelbacher FC, eds. Hypertension in Postmenopausal Women. New York: Marcel Dekkers Inc; 1996:43–78. [Google Scholar]
- 20.Pocock S, Clayton TC, Altman DG. Survival plot of time-to-event outcomes in clinical trials. Lancet. 2002;359:1686–1689. [DOI] [PubMed] [Google Scholar]
- 21.Kannel WB, Schwartz MJ, McNamara PM. Blood pressure and risk of cardiovascular disease: the Framingham Heart Study. Chest. 1969;56: 43–51. [DOI] [PubMed] [Google Scholar]
- 22.van den Hoogen PCW, van Popele NM, Feskens EJM, van der Kuip DAM, Grobbee DE, Hofman A, Witteman JCM. Blood pressure and risk of myocardial infarction in elderly men and women: the Rotterdam Study. J Hypertens. 1999;17:1373–1378. [DOI] [PubMed] [Google Scholar]
- 23.Jónsdóttir LS, Sigfússon N, Guðnason V, Sigvaldason H, Thorgeirsson G. Do lipids, blood pressure, diabetes, and smoking confer equal risk of myocardial infarction in women as men? the Reykjavik Study. J Cardiovasc Risk. 2002;9:67–76. [PubMed] [Google Scholar]
- 24.Miura K, Nakagawa H, Ohashi Y, Harada A, Taguri M, Kushiro T, Takahashi A, Nishinaga M, Soejima H, Ueshima H, for the Japan Arteriosclerosis Longitudinal Study (JALS) Group. Four blood pressure indexes and the risk of stroke and myocardial infarction in Japanese men and women: a meta-analysis of 16 cohort studies. Circulation. 2009;119:1892–1898. [DOI] [PubMed] [Google Scholar]
- 25.Mak KH, Ma S, Heng D, Tan CE, Tai ES, Topol EJ, Chew SK. Impact of sex, metabolic syndrome, and diabetes mellitus on cardiovascular events. Am J Cardiol. 2007;100:227–233. [DOI] [PubMed] [Google Scholar]
- 26.Boggia J, Li Y, Thijs L, Hansen TW, Kikuya M, Björklund-Bodegård K, Richart T, Ohkubo T, Kuznetsova T, Torp-Pedersen C, Lind L, Ibsen H, Imai Y, Wang JG, Sandoya E, O’Brien E, Staessen JA, on behalf of the International Database on Ambulatory blood pressure monitoring in relation to Cardiovascular Outcomes (IDACO) investigators. Prognostic accuracy of day versus night ambulatory blood pressure: a cohort study. Lancet. 2007;370:1219–1229. [DOI] [PubMed] [Google Scholar]
- 27.Staessen JA, Bieniaszewski L, O’Brien E, Gosse P, Hayashi H, Imai Y, Kawasaki T, Otsuka K, Palatini P, Thijs L, Fagard R, on behalf of the ‘Ad Hoc’ Working Group. Nocturnal blood pressure fall on ambulatory monitoring in a large international database. Hypertension. 1997;29:30–39. [DOI] [PubMed] [Google Scholar]
- 28.Casiglia E, Tikhonoff V, Caffi S, Bascelli A, Schiavon L, Guidotti F, Saugo M, Giacomazzo M, Martini B, Mazza A, D’Este D, Pessina AC. Menopause does not affect blood pressure and risk profile, and menopausal women do not become similar to men. J Hypertens. 2008;26: 1983–1992. [DOI] [PubMed] [Google Scholar]
- 29.European Union. The Life of Women and Men in Europe. A Statistical Portrait. Brussels, Belgium: European Communities; 2010. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


