Abstract
Objective
To estimate the effectiveness of the inactivated whole virus vaccine, CoronaVac (Sinovac Biotech), against symptomatic covid-19 in the elderly population of São Paulo state, Brazil during widespread circulation of the gamma variant.
Design
Test negative case-control study.
Setting
Community testing for covid-19 in São Paulo state, Brazil.
Participants
43 774 adults aged ≥70 years who were residents of São Paulo state and underwent reverse transcription polymerase chain reaction (RT-PCR) testing for SARS-CoV-2 from 17 January to 29 April 2021. 26 433 cases with symptomatic covid-19 and 17 622 test negative controls with covid-19 symptoms were formed into 13 283 matched sets, one case with to up to five controls, according to age, sex, self-reported race, municipality of residence, previous covid-19 status, and date of RT-PCR test (±3 days).
Intervention
Vaccination with a two dose regimen of CoronaVac.
Main outcome measures
RT-PCR confirmed symptomatic covid-19 and associated hospital admissions and deaths.
Results
Adjusted vaccine effectiveness against symptomatic covid-19 was 24.7% (95% confidence interval 14.7% to 33.4%) at 0-13 days and 46.8% (38.7% to 53.8%) at ≥14 days after the second dose. Adjusted vaccine effectiveness against hospital admissions was 55.5% (46.5% to 62.9%) and against deaths was 61.2% (48.9% to 70.5%) at ≥14 days after the second dose. Vaccine effectiveness ≥14 days after the second dose was highest for the youngest age group (70-74 years)—59.0% (43.7% to 70.2%) against symptomatic disease, 77.6% (62.5% to 86.7%) against hospital admissions, and 83.9% (59.2% to 93.7%) against deaths—and declined with increasing age.
Conclusions
Vaccination with CoronaVac was associated with a reduction in symptomatic covid-19, hospital admissions, and deaths in adults aged ≥70 years in a setting with extensive transmission of the gamma variant. Vaccine protection was, however, low until completion of the two dose regimen, and vaccine effectiveness was observe to decline with increasing age among this elderly population.
Introduction
As of early July 2021 the covid-19 pandemic has been responsible for 3.9 million deaths worldwide,1 with a disproportionately high mortality and morbidity among elderly people.2 A key question is whether the authorised covid-19 vaccines are effective in elderly people, who might have impaired immune responses3 4 and are underrepresented in randomised controlled trials.5 6 7 mRNA and adenovirus vector based vaccines have been shown to be effective against covid-19 in elderly people,8 9 but evidence on the effectiveness of inactivated vaccines in this population is limited.7 10 11 12
CoronaVac (Sinovac Biotech), an inactivated whole virus vaccine, has been approved by 32 countries and jurisdictions10 and been implemented as part of mass vaccination campaigns in low and middle income countries, many of which are experiencing covid-19 epidemics as a result of the emergence of SARS-CoV-2 variants of concern. Estimates from randomised controlled trials of vaccine efficacy against symptomatic covid-19 of a two dose CoronaVac regimen in healthcare workers and the general population have varied (51% to 84%).5 7 10 The World Health Organization’s Emergency Use Listing (EUL) procedure approved the use of CoronaVac in early June 2021 but identified an evidence gap for the effectiveness of this vaccine in adults aged ≥60 years.11 The WHO EUL cited an observational study in Chile,10 12 which found that the adjusted effectiveness of CoronaVac among adults aged ≥60 years at 14 days or more after the second dose was 66.6%. During the study period, the gamma variant of concern was detected in 28.6% of SARS-CoV-2 genomes in Chile.12 Furthermore, randomised controlled trials and observational studies have not investigated whether CoronaVac provides important protection after the first dose or in the setting of widespread transmission of variants of concern.5 10 11
Brazil has experienced one of the world’s highest covid-19 burdens during the pandemic, with more than 18 million people affected and 526 000 deaths reported as of early July 2021.1 13 Variants of concern, and in particular the gamma variant, have played an important role in the recent epidemic wave in Brazil, which began in early 2021.14 15 16 The gamma variant, which was first detected in Manaus, shows increased transmissibility,16 has accrued mutations associated with decreased in vitro seroneutralisation,17 18 19 has a possible association with increased disease severity,20 21 and, at present, accounts for most of the SARS-CoV-2 isolates genotyped in Brazil from 1 January 2021.14 22 In the setting of a large epidemic associated with the gamma variant in São Paulo, the most populous state in Brazil, we conducted a matched, test negative23 case-control study to evaluate the real world effectiveness of CoronaVac against symptomatic covid-19 and severe clinical outcomes in people aged ≥70 years.
Methods
Study setting
The State of São Paulo (23°3′S, 46°4′W) has 645 municipalities and 46 million inhabitants, 3.23 million of whom are aged ≥70 years.24 The state experienced three successive waves of covid-19, during which 2 997 282 cases (cumulative incidence rate: 6475 per 100 000 population) and 100 649 deaths (cumulative mortality: 217 per 100 000 population) have been reported as of 9 May 2021 (fig 1, supplementary figure 1).25 The state secretary of health of Sao Paulo initiated a covid-19 vaccination campaign for the general population on 17 January 2021 according to an age based prioritisation strategy (fig 1) and is administering a two dose regimen of CoronaVac with a two to four week interval between doses, and a two dose regimen of ChAdOx1 nCoV-19 (Oxford-AstraZeneca), with a 12 week interval.26 As of 29 April 2021, 8.63 million doses (5.16 million first doses and 3.47 million second doses) of CoronaVac had been administered and 2.06 million doses (1.99 million first doses and 0.07 million second doses) of ChAdOx1 nCoV-19.
Fig 1.
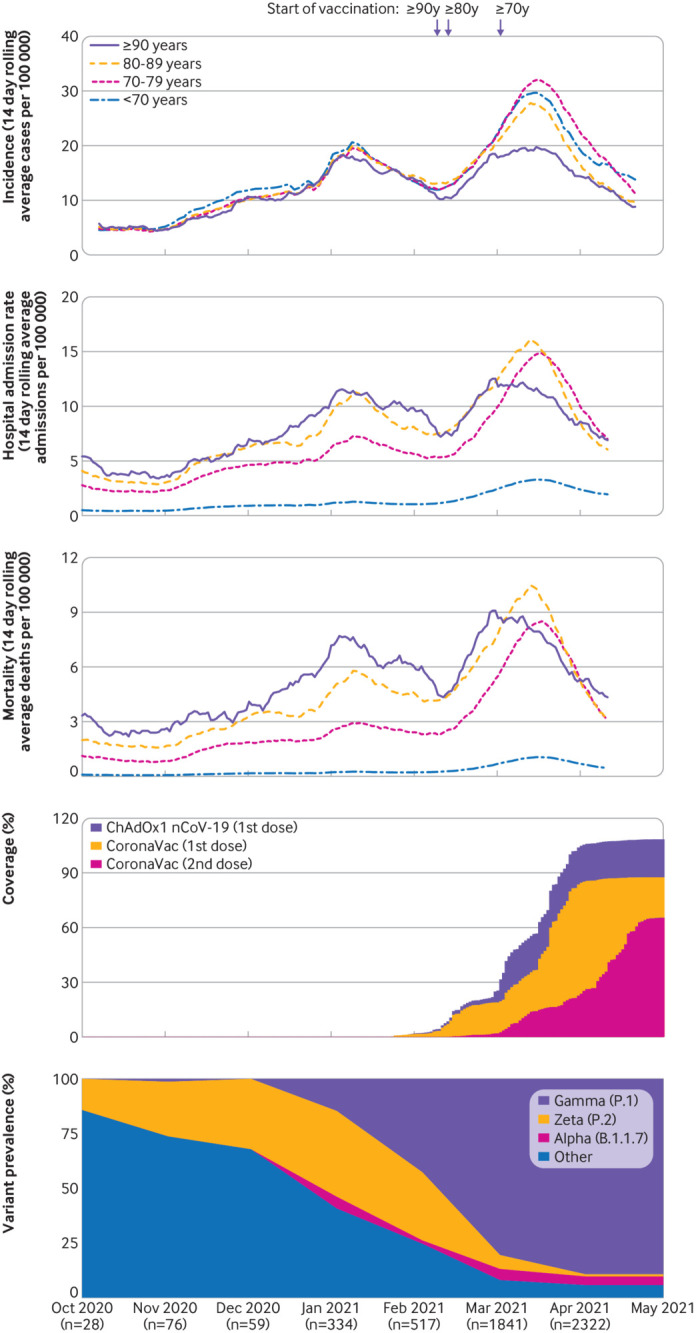
Incidence of reported covid-19, vaccination coverage, and prevalence of SARS-CoV-2 variants of concern from 1 October 2020 to 29 April 2021 in São Paulo state, Brazil. Panels A-C show the 14 day rolling average of daily age group specific incidence of reported covid-19 cases, hospital admission rate, and mortality (events per 100 000 population). Panel D shows daily cumulative vaccination coverage in people aged ≥70 years. Population estimates for age groups were obtained from national projections for 2020.24 Panel E shows the monthly prevalence of SARS-CoV-2 variants among genotyped isolates in the GISAID (global initiative on sharing avian influenza data) database (extraction on 20 June 2021).22 Vertical bars show dates that adults aged ≥90, 80-89, and 70-79 years in the general population became eligible for vaccination
Study design
We conducted a matched test negative case-control study to estimate the effectiveness of CoronaVac in reducing the odds of reverse transcription polymerase chain reaction (RT-PCR) confirmed symptomatic covid-19 in adults aged ≥70 years from São Paulo state from 17 January 2021 (the start of covid-19 vaccination) to 29 April 2021. Test negative design studies have provided estimates of vaccine effectiveness in concordance with those obtained from randomised controlled trials,27 28 and such studies have been used extensively to evaluate vaccines against respiratory infections,29 including covid-19.8 23 30 We chose the test negative design because of the feasibility of accessing information on people who were tested for SARS-CoV-2 through the São Paulo state surveillance systems and because of the opportunity to control for potential biases, such as healthcare seeking behaviour and access to testing.23 The study population was adults aged ≥70 years who had a residential address in São Paulo state, underwent SARS-CoV-2 RT-PCR testing during the study period, and had complete and consistent information between data sources on age, sex, residence, and on vaccination and testing status and dates. We matched test negative controls with covid-19 symptoms to covid-19 cases by date of testing (±3 days) to address potential sources of bias that might vary during the course of an epidemic, as well as by participant characteristics of age, sex, self-reported race, municipality of residence, and previous covid-19 status.
In the protocol, we prespecified power thresholds for conducting analyses on the effectiveness of CoronaVac and ChAdOx1 nCoV-19. These thresholds were achieved for CoronaVac but not for ChAdOx1 nCoV-19 because of lower rates of ChAdOx1 nCov-19 administered in the population during the study period. We therefore restricted the evaluation of vaccine effectiveness to CoronaVac.
Amendment of protocol
The study design and statistical analysis plan were specified in advance of extracting information from data sources and are described in a publicly available protocol (https://github.com/juliocroda/VebraCOVID-19) and the supplementary file. We made two major changes to the original protocol: we added the analysis for hospital admissions and deaths inside the framework of a test negative design before submission to peer review (post hoc analysis), and after submission, as suggested by peer reviewers, we changed the matching procedure of the main analysis from one case matched to one control without replacement, to one case matched with up to five controls with replacement of controls between cases (unbalanced design); and we added two other sensitivity analyses for the matching procedure.
Data sources
We obtained individual level information on personal characteristics, comorbidities, SARS-CoV-2 testing, and covid-19 vaccination during the study period by extracting information on 6 May 2021 from the state secretary of health of Sao Paulo laboratory testing registry (GAL), the national surveillance databases for covid-19-like illnesses (e-SUS) and severe acute respiratory illness (SIVEP-Gripe), and the state secretary of health of Sao Paulo vaccination registry (Vacina Já). All people living in Brazil are eligible for testing and have access to the public health system. RT-PCR tests are performed by trained healthcare professionals following standard protocols. Notification of people with suspected covid-19, SARS-CoV-2 test results, and suspected deaths with covid-19 is compulsory in Brazil. Supplementary table 1 provides additional information on data sources. The information technology bureau of the São Paulo state government linked individual level records from the four databases using CPF (Cadastro de Pessoas Físicas) numbers (Brazilian citizens’ unique identifier code) and provided anonymised datasets. The genotyping of all isolated SARS-CoV-2 in São Paulo state was not possible and these data are not available in the surveillance systems used in this study. We retrieved information on SARS-CoV-2 variants from genotyped isolates from São Paulo state deposited in the global initiative on sharing avian influenza data (GISAID) database.22
Selection of cases and matched controls
We selected cases from the study population who had covid-19 symptoms, defined as a covid-19-like illness, a positive SARS-CoV-2 RT-PCR test result from a respiratory sample that was collected within 10 days after the onset of symptoms, and did not have a positive RT-PCR test result in the preceding 90 days. We selected controls from the study population who had a covid-19-like illness, a negative SARS-CoV-2 RT-PCR test result from a respiratory sample that was collected within 10 days after the onset of symptoms,23 and no positive RT-PCR test result in the previous 90 days during the study period, or in the subsequent 14 days. Cases and controls were excluded if they received the ChAdOx1 nCoV-19 vaccine before sample collection for RT-PCR testing. We defined covid-19-like illness as the presence of one or more reported covid-19 related symptoms.31
One case was matched with to up to five test negative controls according to RT-PCR sample collection date (±3 days), age category (five year age bands, eg, 70-74, 75-79 years), sex, municipality of residence, self-reported race (defined as brown, black, yellow, white, or indigenous),32 and previous symptomatic events that were reported to the surveillance systems31 between 1 February 2020 and 16 January 2021, as a proxy for previous SARS-CoV-2 infection. Matching factors were chosen from variables that were associated with vaccination coverage or timing, and with risk of SARS-CoV-2 infection or healthcare access (see protocol in supplementary file).23 After identification of each case, we randomly chose up to five controls in an unbalanced design from the set of all eligible matching controls, allowing for replacement of controls between cases (main analysis). We conducted three sensitivity analyses, varying two features of the matching while keeping the same matching factors. In the first analysis we matched one case to one random control without replacement of controls (original analysis in the protocol); in the second analysis we matched one case to one random control, allowing for replacement of controls between cases; and in the third analysis we matched one case to two random controls, allowing for replacement of controls between cases.
Statistical analysis
We estimated the effectiveness of CoronaVac against symptomatic covid-19 in the 0-13 days and ≥14 days after the second dose and ≥14 days after the first dose. Furthermore, we estimated the effectiveness of a single dose 0-13 days after the first dose, when the vaccine has shown no or limited effectiveness.5 33 34 An association during this period might serve as an indicator of unmeasured confounding in the effectiveness estimate.35 36 We also expanded our bias indicator by evaluating the 0-13 days after the first dose as 0-6 days and 7-13 days.36 The reference group for vaccination status was those who had not received a first vaccine dose before the date of sample collection.
Conditional logistic regression was used to estimate the odds ratio of vaccination among cases and controls: 1−odds ratio provided an estimate of vaccine effectiveness under the assumptions of a test negative design.37 We included age and covid-19 associated comorbidities (cardiovascular, renal, neurological, haematological, and hepatic, diabetes, chronic respiratory disorder, obesity, or immunosuppression) as covariates in the model. Because age is a strong determinant of covid-19 outcomes, we adjusted for age after matching by age bands to control for potential residual confounding.38 Non-linearity for age was evaluated using restricted cubic splines and we chose the most parsimonious model comparing nested models with a likelihood ratio test. To evaluate potential residual confounding by time varying factors that might not be dealt with by the matching criteria, we also conducted a post hoc sensitivity analysis that incorporated the calendar date of RT-PCR sample collection in the model.
In a post hoc analysis we estimated vaccine effectiveness against covid-19 associated hospital admission and death. To account for the competing event of dying before being admitted to hospital, we estimated vaccine effectiveness for the composite outcome of hospital admission or death, or both in a sensitivity analysis. In these separate analyses, we selected matched pairs in which the case had the secondary outcome of interest.39 40 We fit the same conditional logistic regression model as for the primary outcome.
We conducted a prespecified analysis of vaccine effectiveness among age subgroups for the primary and secondary outcomes but could not perform analyses stratified by previous covid-19 documented infection because of small numbers. The age subgroups were prespecified and followed the five year age categories used for matching, with age groups older than 80 collapsed into a single group. Additional post hoc analyses were performed of vaccine effectiveness for the primary outcome for subgroups stratified by sex, number of chronic comorbidities (none versus at least one), the two most common chronic comorbidities (cardiovascular disease and diabetes), and region of residence (Grande São Paulo health region versus others). Interaction terms were incorporated into the model to evaluate the association of each subgroup of interest with vaccine effectiveness ≥14 days after the second dose.
Power calculation
Our protocol specified that we would conduct proposed analyses after achieving ≥80% power to identify a vaccine effectiveness of 40% against symptomatic covid-19 for ≥14 days after the second dose of CoronaVac compared with not receiving a vaccine dose. The power was simulated fitting conditional logistic regressions on 1000 simulated datasets. After extracting information from the surveillance databases on 6 May 2021 and generating matched case-control pairs, we determined that the power of the study was 99.9% and proceeded to conduct the prespecified analyses. We did not perform an analysis for ChAdOx1 nCoV-19 because the simulated power was 31% to identify a vaccine effectiveness of 40% for ≥28 days after the first dose of ChAdOx1 nCoV-19 compared with not receiving a vaccine dose.
All analyses were done in R, version 4.0.2.
Patient and public involvement
Because this study used routine surveillance data sources and there was no direct funding, no members of the public or patients were directly involved. Nevertheless, we did speak to patients about the study and the outcomes to be evaluated, and we asked a member of the public to read our manuscript and provide inputs for its interpretation. No members of the public or patients were involved in writing up the results.
Results
São Paulo state experienced three covid-19 epidemic waves, with a peak incidence in July 2020 for the first wave and in January 2021 for the second wave (supplementary figure 1) and March 2021 for the third wave (fig 1). The second wave was preceded in November 2020 by an increase in the prevalence of the zeta variant among genotyped isolates from São Paulo state deposited into the GISAID database (fig 1). The third wave was preceded in January 2021 by an increase in the prevalence of the gamma variant among genotyped isolates (fig 1). The gamma variant replaced other SARS-CoV-2 variants22 and accounted for 78.4% (3834/4887) of the genotyped isolates that were reported in GISAID during the study period and 85.5% (3584/4192) of genotyped isolates that were reported between 1 March and 29 April 2021, when the majority of discordant case-control sets were identified (supplementary figure 2). The vaccination campaign, initiated on 17 January 2021, achieved an estimated coverage of roughly 85% for the first CoronaVac dose (2.82 million) and 65% for the second dose (2.10 million) among adults aged ≥70 years by 29 April 2021 (fig 1). After initiation of the vaccination campaign and during the third epidemic wave, the incidence of covid-19 increased and peaked in late March in all age groups except for those aged ≥90 years (fig 1).
Study population
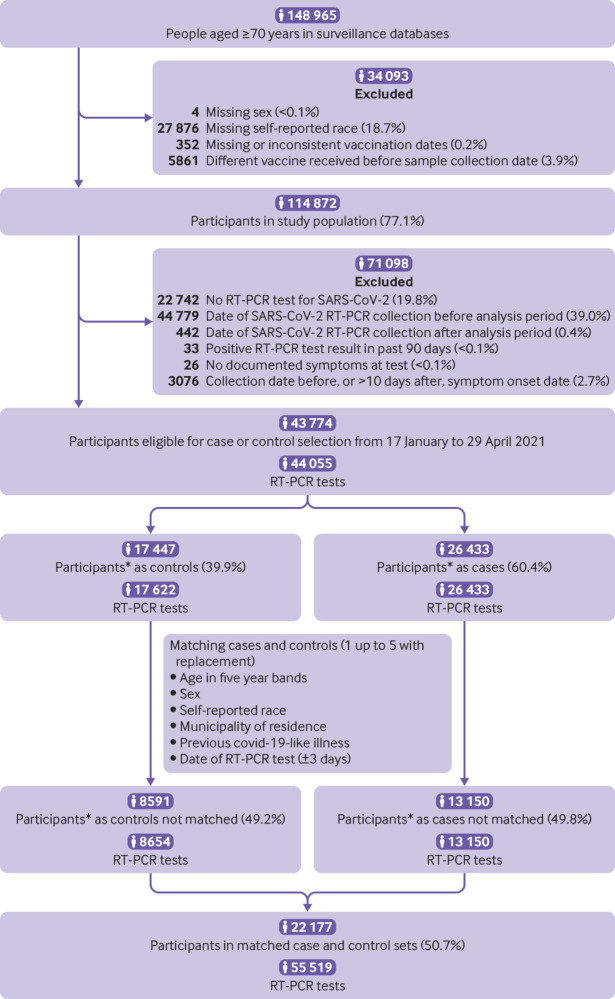
From 17 January 2021 to 29 April 2021, the rate of RT-PCR testing for SARS-CoV-2 in those age ≥70 years in São Paulo state was 25 per 1000 persons. Among 43 774 adults eligible for study inclusion (fig 2), 22 177 (50.7%) who provided 55 519 RT-PCR test results were included in matched case and control sets as follows: 3881 pairs matched 1:1, 1963 pairs matched 1:2, 1044 pairs matched 1:3, 678 pairs matched 1:4, and 5717 pairs matched 1:5. Overall, 6223 participants contributed more than one time as controls and 18 participants contributed as both control and case. Table 1 shows the characteristics of eligible participants with positive and negative RT-PCR test results, and selected cases and matched controls. Matched characteristics appear unbalanced because of the variable matching procedure. A higher proportion of cases than controls had reported comorbidities. Most of the discordant sets, based on vaccination status, were selected after 14 March 2021 (supplementary figure 2). For cases and controls who completed the two dose vaccine regimen, the intervals between doses were similar (mean 30 v 25 days). Likewise, the intervals between vaccine doses and RT-PCR testing were similarly distributed for cases and controls (table 1 and supplementary figure 3). Supplementary table 2 shows the distribution of matched sets according to the vaccination status of cases and controls at the time of RT-PCR testing. Supplementary tables 3 and 4 show the characteristics of the matched case and control sets selected for the analysis of secondary outcomes of hospital admission (n=30 308) and death (n=14 624).
Fig 2.
Flowchart of study population from surveillance databases, and selection of matched cases and controls. *Some participants contributed as controls and cases, and matching allowed for replacement of controls between cases. RT-PCR=reverse transcription polymerase chain reaction
Table 1.
Characteristics of adults aged ≥70 years in São Paulo state, Brazil, who were eligible for matching and selected into case test negative pairs. Values are numbers (percentages) unless stated otherwise
| Characteristics | Eligible cases and controls | Matched sets | |||
|---|---|---|---|---|---|
| Test negative (n=17 622)* | Test positive (n=26 433)* | Controls (n=42 236)* | Cases (n=13 283)* | ||
| Mean (SD) age (years) | 77.53 (6.8) | 76.71 (6.2) | 75.69 (5.44) | 75.90 (5.64) | |
| Age groups (years): | |||||
| 70-79 | 12 123 (68.8) | 19 673 (74.4) | 34134 (80.8) | 10543 (79.4) | |
| 80-89 | 4301 (24.4) | 5437 (20.6) | 7045 (16.7) | 2311 (17.4) | |
| ≥90 | 1198 (6.8) | 1323 (5.0) | 1057 (2.5) | 429 (3.2) | |
| Men | 7689 (43.6) | 12 431 (47.0) | 18610 (44.1) | 5919 (44.6) | |
| Self-reported race†: | |||||
| White/branca | 13 415 (76.1) | 19 796 (74.9) | 34603 (81.9) | 10803 (81.3) | |
| Brown/pardo | 3192 (18.1) | 4983 (18.9) | 6797 (16.1) | 2115 (15.9) | |
| Black/preta | 785 (4.5) | 1258 (4.8) | 727 (1.7) | 287 (2.2) | |
| Yellow/amarela | 226 (1.3) | 390 (1.5) | 109 (0.3) | 78 (0.6) | |
| Indigenous/Indígena | 4 (0.0) | 6 (0.0) | - | - | |
| Residence in Grande São Paul health region | 12 381 (70.3) | 16 538 (62.6) | 14368 (34.0) | 6113 (46.0) | |
| Reported No of comorbidities‡: | |||||
| 0 | 10 027 (56.9) | 12 668 (47.9) | 23961 (56.7) | 5886 (44.3) | |
| 1 or 2 | 6984 (39.6) | 12 548 (47.5) | 16626 (39.4) | 6713 (50.5) | |
| ≥3 | 611 (3.5) | 1217 (4.6) | 1649 (3.9) | 684 (5.1) | |
| Cardiovascular disease | 5293 (30.0) | 10 079 (38.1) | 12563 (29.7) | 5482 (41.3) | |
| Diabetes | 3233 (18.3) | 6533 (24.7) | 8269 (19.6) | 3578 (26.9) | |
| Past exposure to SARS-CoV-2§ | |||||
| Previous symptomatic events notified to surveillance systems¶ | 685 (3.9) | 354 (1.3) | 47 (0.1) | 37 (0.3) | |
| Positive SARS-CoV-2 test result** | 66 (0.4) | 13 (0.0) | 1 (0.0) | 4 (0.0) | |
| Median (interquartile range) interval between symptoms onset and RT-PCR testing (days) | 3 (2-5) | 4 (2-6) | 3 (1-5) | 4 (2-6) | |
| Hospital admissions | 4524/17 484 (25.9) | 12 987/26 221 (49.5) | 11 020/41 980 (26.3) | 7043/13 175 (53.5) | |
| Deaths | 1594/16 710 (9.5) | 7054/24 508 (28.8) | 4072/40 134 (10.1) | 3549/12 251 (29.0) | |
| Median (interquartile range) interval between symptoms onset and hospital admission (days) | 3 (2-6) | 7 (4-10) | 4 (2-7) | 7 (4-10) | |
| Median (interquartile range) interval between symptoms onset and deaths (days) | 8 (4-13) | 14 (9-21) | 8 (4-16) | 15 (10-22) | |
| Vaccination status: | |||||
| Not vaccinated | 11 986 (68.0) | 17 233 (65.2) | 27994 (66.3) | 8989 (67.7) | |
| Single dose, within 0-13 days | 1446 (8.2) | 2976 (11.3) | 4873 (11.5) | 1565 (11.8) | |
| Single dose, ≥14 days | 1797 (10.2) | 3312 (12.5) | 4631 (11.0) | 1489 (11.2) | |
| Two doses, within 0-13 days | 1041 (5.9) | 1533 (5.8) | 2445 (5.8) | 700 (5.3) | |
| Two doses, ≥14 days | 1352 (7.7) | 1379 (5.2) | 2293 (5.4) | 540 (4.1) | |
| Mean (SD) interval between 1st and 2nd dose (days) | 25 (6) | 30 (12) | 25 (6) | 30 (12) | |
| Mean (SD) interval between 1st dose and RT-PCR testing (days) | 28 (19) | 23 (16) | 22 (17) | 21 (16) | |
| Mean (SD) interval between second dose and RT-PCR testing (days) | 20 (15) | 17 (14) | 17 (14) | 16 (14) | |
RT-PCR=reverse transcription polymerase chain reaction.
Numbers refer to RT-PCR tests and represent 43 774 people for the eligible cases and controls and 22 177 people in matched cases and controls.
Race/skin colour as defined by the Brazilian national census bureau (Instituto Nacional de Geografia e Estatísticas).32
Comorbidities included cardiovascular, renal, neurological, haematological, or hepatic conditions, diabetes, chronic respiratory disorder, obesity, or immunosuppression.
Before start of study on 17 January 2021 and after systematic surveillance was implemented on 1 February 2020.
Reported illness with covid-19 associated symptoms in eSUS and SIVEP-Gripe databases.
Defined as a positive SARS-CoV-2 RT-PCR or antigen detection test result.
Vaccine effectiveness against symptomatic covid-19
The adjusted effectiveness against symptomatic covid-19 was 24.7% (95% confidence interval 14.7% to 33.4%) at 0-13 days and 46.8% (38.7% to 53.8%) at ≥14 days after the second dose (table 2). No statistically significant change was identified in the odds of covid-19 in the 0-13 days after the first dose, which serves as a potential bias indicator. The bias indicator was similar 0-6 days and 7-13 days after the first dose (supplementary table 5). In the sensitivity analysis including calendar date of testing as a covariate, vaccine effectiveness after the second dose was 25.1% (15.2% to 33.8%) at 0-13 days and 47.1% (39.1% to 54.1%) at ≥14 days.
Table 2.
Effectiveness of CoronaVac (Sinovac Biotech) against symptomatic covid-19, hospital admissions, and deaths in adults aged ≥70 years in São Paulo state, Brazil
| Unadjusted analysis | Adjusted analysis* | ||||||
|---|---|---|---|---|---|---|---|
| Odds ratio (95% CI) | Vaccine effectiveness, % (95% CI) | P value | Odds ratio (95% CI) | Vaccine effectiveness, % (95% CI) | P value | ||
| Symptomatic covid-19 (n=55 519) | |||||||
| One dose: | |||||||
| 0-13 days v unvaccinated† | 1.02 (0.94 to 1.10) | −1.7 (−10.4 to 6.2) | 0.68 | 1.01 (0.93 to 1.09) | −0.8 (−9.4 to 7.2) | 0.86 | |
| ≥14 days v unvaccinated† | 0.88 (0.80 to 0.97) | 11.9 (3.1 to 19.9) | 0.01 | 0.88 (0.79 to 0.96) | 12.5 (3.7 to 20.6) | 0.01 | |
| Two doses: | |||||||
| 0-13 days v unvaccinated† | 0.77 (0.68 to 0.87) | 23.5 (13.5 to 32.3) | <0.001 | 0.75 (0.67 to 0.85) | 24.7 (14.7 to 33.4) | <0.001 | |
| ≥14 days v unvaccinated† | 0.54 (0.47 to 0.62) | 45.8 (37.7 to 52.9) | <0.001 | 0.53 (0.46 to 0.61) | 46.8 (38.7 to 53.8) | <0.001 | |
| Hospital admissions associated with covid-19 (n=30 308) | |||||||
| One dose: | |||||||
| 0-13 days v unvaccinated† | 0.98 (0.89 to 1.09) | 1.6 (−9.3 to 11.5) | 0.76 | 0.93 (0.84 to 1.04) | 6.6 (−4.3 to 16.3) | 0.23 | |
| ≥14 days v unvaccinated† | 0.87 (0.77 to 0.99) | 12.6 (1.3 to 22.6) | 0.03 | 0.83 (0.73 to 0.94) | 16.9 (5.7 to 26.8) | 0.004 | |
| Two doses: | |||||||
| 0-13 days v unvaccinated† | 0.66 (0.56 to 0.77) | 34.4 (23.1 to 44.1) | <0.001 | 0.61 (0.52 to 0.72) | 39.1 (28.0 to 48.5) | <0.001 | |
| ≥14 days v unvaccinated† | 0.48 (0.40 to 0.57) | 51.9 (42.6 to 59.7) | <0.001 | 0.45 (0.37 to 0.54) | 55.5 (46.5 to 62.9) | <0.001 | |
| Deaths associated with covid-19 (n=14 624) | |||||||
| One dose: | |||||||
| 0-13 days v unvaccinated† | 0.90 (0.78 to 1.04) | 10 (−4.2 to 22.2) | 0.16 | 0.87 (0.74 to 1.02) | 13.1 (−1.5 to 25.6) | 0.08 | |
| ≥14 days v unvaccinated† | 0.75 (0.63 to 0.89) | 25.1 (11.2 to 36.9) | 0.001 | 0.69 (0.58 to 0.82) | 31.2 (17.6 to 42.5) | <0.001 | |
| Two doses: | |||||||
| 0-13 days v unvaccinated† | 0.56 (0.44 to 0.70) | 44.3 (29.6 to 55.9) | <0.001 | 0.51 (0.40 to 0.66) | 48.9 (34.4 to 60.1) | <0.001 | |
| ≥14 days v unvaccinated† | 0.43 (0.33 to 0.56) | 57.1 (44.3 to 67) | <0.001 | 0.39 (0.30 to 0.51) | 61.2 (48.9 to 70.5) | <0.001 | |
Adjusted for age (linear term for symptomatic covid-19 and restricted cubic spline for hospital admissions and deaths) and number of comorbidities (0, 1 or 2, ≥3).
At date of index sample collection for cases and controls.
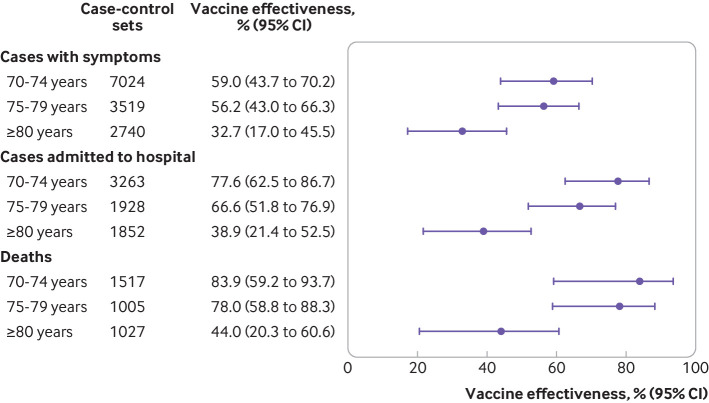
Vaccine effectiveness against symptomatic covid-19 was observed to decline with increasing age ≥14 days after the second dose and was 59.0% (43.7% to 70.2%) among those aged 70-74 years, 56.2% (43.0% to 66.3%) among those aged 75-79 years, and 32.7% (17.0% to 45.5%) among those aged ≥80 years (P=0.007 for interaction; figure 3, supplementary table 6). Vaccine effectiveness against symptomatic covid-19 did not differ among subgroups defined by sex, presence of comorbidities, reported cardiovascular disease, or regions of residence. Participants with reported diabetes, however, had lower protection than those without reported diabetes (vaccine effectiveness 32.6% v 50.5%, P=0.008 for interaction) ≥14 days after the second dose (supplementary table 7 and supplementary figure 4).
Fig 3.
Adjusted vaccine effectiveness ≥14 days after the second dose of CoronaVac (Sinovac Biotech) for subgroups of adults aged ≥70 years. Estimates of vaccine effectiveness were obtained from a conditional logistic regression model that included covariates of age and number of comorbidities and incorporated an interaction term between the category of interest and the period ≥14 days after the second dose
Vaccine effectiveness against covid-19 associated hospital admissions
The adjusted effectiveness against hospital admission was 39.1% (28.0% to 48.5%) at 0-13 days and 55.5% (46.5% to 62.9%) at ≥14 days after the second dose (table 2). No statistically significant reduction was observed in the odds of covid-19 in the periods after one dose, and the bias indicator effectiveness was close to zero (supplementary table 5).
Vaccine effectiveness against hospital admission was observed to decline with increasing age ≥14 days after the second dose and was 77.6% (62.5% to 86.7%) among those aged 70-74 years, 66.6% (51.8% to 76.9%) among those aged 75-79 years, and 38.9% (21.4% to 52.5%) among those aged ≥80 years (P<0.001 for interaction; fig 3, supplementary table 6).
Vaccine effectiveness against deaths with covid-19
The adjusted effectiveness against deaths with covid-19 was 31.2% (17.6% to 42.5%) ≥14 days after the first dose, 48.9% (34.4% to 60.1%) 0-13 days after the second dose, and 61.2% (48.9% to 70.5%) ≥14 days after the second dose (table 2). The bias indicator was close to zero 0-13 days after the first dose, and 0-6 days and 7-13 days after the first dose (supplementary table 5).
Vaccine effectiveness against deaths was observed to decline with increasing age ≥14 days after the second dose and was 83.9% (59.2% to 93.7%) among those aged 70-74 years, 78.0% (58.8% to 88.3%) among those aged 75-79 years, and 44.0% (20.3% to 60.6%) among those aged ≥80 years (P=0.001 for interaction; fig 3, supplementary table 6).
The adjusted effectiveness for the composite outcome hospital admissions or deaths, or both, was 39.2% (28.3% to 48.4%) 0-13 days after the second dose, and 55.4% (46.5% to 62.8%) ≥14 days after the second dose (supplementary table 8).
Sensitivity analyses for the matching procedure
Overall, 13 150 cases (49.7%) could not be matched with a potential control. Thus, 30.1% of cases (7950 case-control pairs) could be matched in the first sensitivity analysis (1:1 without replacement), 50.3% of cases (13 283 case-control pairs) in the second sensitivity analysis (1:1, allowing for replacement of controls), and 35.6% of cases (9402 case-control pairs) in the third sensitivity analysis (1:2, allowing for replacement of controls). Supplementary table 9 shows the characteristics of the population in these three matching analyses. Overall, vaccine effectiveness was comparable to the findings of the main analysis, with varying precision. Vaccine effectiveness against symptomatic covid-19 was 41.6% (26.9% to 53.3%) in the first sensitivity analysis (n=15 900), 48.6% (38.9% to 56.8%) in the second sensitivity analysis (n=26 566), and 47.8% (38.2% to 56.0%) in the third sensitivity analysis (n=28 206; supplementary tables 10-13). The same pattern of vaccine effectiveness observed in the main analysis when stratified by age and for severe outcomes was observed in the three sensitivity analyses (supplementary tables 10 and 14-16).
Discussion
In this test negative case-control study we found that the effectiveness of a two dose schedule of CoronaVac in the real world was 47% against symptomatic covid-19, 56% against covid-19 associated hospital admissions, and 61% against covid-19 associated deaths among those aged ≥70 years during a gamma variant associated epidemic in Brazil. Furthermore, we have addressed several evidence gaps for the use of CoronaVac: vaccination showed an effectiveness against covid-19, including associated severe outcomes, in the setting of widespread transmission of the gamma variant, which was similar to that found in the Brazilian randomised controlled trial conducted before the emergence of the gamma variant5; a single dose of CoronaVac was associated with low protection against symptomatic covid-19 or hospital admission; and vaccine effectiveness was observed to decline with increasing age among adults aged ≥70 years.
Research in context
A key evidence gap, as raised in the WHO EUL for CoronaVac,11 has been the effectiveness of this vaccine in the elderly population, because this age group was not well represented in Brazilian and Turkish randomised controlled trials.5 7 10 11 We found that two doses of CoronaVac administered at an average interval of four weeks had an overall effectiveness against symptomatic covid-19 of 47% (39% to 54%) in a population with a mean age of 76 years. This estimate is lower than the efficacy of 84% (95% confidence interval 65% to 92%) reported in the Turkish trial, with a participant median age of 45 years and two week dosing interval7; and comparable to the efficacy of 51% (95% confidence interval 36% to 62%) from the Brazilian trial in healthcare workers, with a participant mean age of 39 years and two week dosing interval.5 Additionally, a cohort study in Chile reported an effectiveness for CoronaVac of 66.6% (95% confidence interval 65.4% to 67.8%) in those aged ≥60 years. It is not clear whether the observed differences are related to the age distribution, dosing interval, risk of infection in the community, or the presence of the gamma variant of concern, which was not prevalent during the trials’ follow-up periods and was responsible for only 28.6% of genotyped infections in Chile during the study period.10 12
Among elderly people in our study, we observed a statistically significant decline in vaccine effectiveness against symptomatic covid-19 with increasing age, from 59.0% (43.7% to 70.2%) in those aged 70-74 year to 32.7% (17.0% to 45.5%) in those aged ≥80 years. These findings parallel real world evidence for the BNT162b2 mRNA vaccine, which showed reduced effectiveness in residents of long term care facilities in Denmark,41 skilled nursing facilities in the USA,42 and the general population aged ≥70 years in Finland43 and ≥80 years in Israel.44 As well as having a slower immune response and lower peak of neutralising antibodies than younger populations, elderly people seem to have faster decay of antibody titres.4 Together, these findings suggest that specific vaccines or vaccination schedules might be required to effectively vaccinate the very elderly (≥80 years) population against covid-19.
Vaccine effectiveness was greater against severe outcomes than against symptomatic covid-19 in all age subgroups among elderly people. This finding, consistent with the findings from randomised controlled trials and observational studies for multiple covid-19 vaccines and across settings,5 6 9 10 12 suggests that vaccination will reduce morbidity and mortality among elderly people even if effectiveness at preventing infections is reduced. The direct comparison of the effectiveness against hospital admission with other vaccines and between countries is not straightforward, because hospital admission depends on admission triage policies, which change according to age and hospital bed availability. Therefore, someone older than 80 years with symptomatic covid-19 has a higher likelihood of being admitted compared with younger patients even if the disease is not severe, and this likelihood varies between public and private facilities and whether the health system is overwhelmed.13 Thus, we cannot generalise our findings for protection against hospital admission without considering this context. We evaluated vaccine effectiveness at the individual level, not accounting for the indirect effect and the total effect from the vaccination campaign. A preliminary aggregated analysis using weekly times series of covid-19 deaths in Brazil found a relative decrease in mortality among those aged ≥70 years compared with all ages after vaccination with CoronaVac and ChAdOx1 nCov-19,45 suggesting a discernible impact of vaccination on mortality at the population level. Additional investigation is required to determine the duration of protection conferred by CoronaVac.7 19 23
The absence of demonstrable effectiveness of CoronaVac until completion of the two dose regimen has profound implications for use of this vaccine in response to an epidemic. In contrast with covid-19 vaccines that confer protection after the first dose,9 46 CoronaVac showed low effectiveness until after the second dose (more than four weeks after the first dose).19 Our findings suggest that in countries where CoronaVac supplies are constrained and there is high SARS-CoV-2 transmission, vaccination should prioritise completion of the two dose regimen among the highest risk populations and avoid being expanded to broader segments of the population for whom provisions for a second dose have not been secured.
Our study did not directly address the question of whether vaccination with CoronaVac is effective against gamma variant associated covid-19 because we had no data on whether the analysed cases were related to the gamma variant. However, 91.0% (5054/5551) of the discordant sets in this matched case-control study were selected from 1 March to 29 April 2021, when the gamma variant accounted for 85% of the genotyped isolates during surveillance in São Paulo state. A test negative study in Canada evaluated adults aged ≥70 years and estimated an adjusted vaccine effectiveness of single dose mRNA vaccines of 61% (95% confidence interval 45% to 72%) against the gamma variant of concern compared with 72% (58% to 81%) for non-variants of concern.47 Although further studies are required to determine the effectiveness of CoronaVac against the gamma variant and additional variants of concern, our findings provide supportive evidence for the use of CoronaVac in countries in South America that are experiencing epidemics due to extensive spread of the gamma variant22 and are using CoronaVac as part of a mass vaccination campaign in response to the epidemic.
Strengths and limitations of this study
This study has several strengths, which include the large sample size and geospatial coverage, comprising the State of São Paulo with 46 million inhabitants distributed across 645 municipalities. We implemented a prespecified publicly available protocol, which is in accordance with the recent WHO guideline for evaluation of covid-19 vaccine effectiveness.23 Using a test negative design, we have dealt with biases that affect observational studies on vaccine effectiveness, such as health seeking behaviour and access. Additionally, after matching and adjustment, the bias indicator association between recent vaccination with a single dose 0-13 days before sample collection was close to null, suggesting that the underlying risk of testing positive for SARS-CoV-2 did not differ between vaccinated and unvaccinated people.8 35 36 48 Finally, we performed three sensitivity analyses for the matching procedure, which yielded comparable estimates to those of the main analysis, resulting in increased precision and showing the robustness of our vaccine effectiveness estimation.
Our study had limitations. We could not assess the influence of a previous SARS-CoV-2 infection on vaccine effectiveness because passive surveillance identified too few people with a positive RT-PCR or rapid antigen test result before the study period. Before the start of the vaccination campaign, the estimated seroprevalence of covid-19 in inhabitants aged ≥60 years in the capital of São Paulo state was 19.9% (14.9% to 29.9%) in January 2021.49 Our estimates of vaccine effectiveness might therefore be subject to downward bias, as unvaccinated people were at lower risk of reinfection. We attempted to exclude false negative RT-PCR test results by excluding as controls those with a subsequent positive test result within 14 days after the initial test and including only tests performed within 10 days of symptom onset.23 However, we cannot rule out some level of misclassification, although it is likely to be non-differential and thus would bias the estimate towards the null. In addition, we restricted our study population to elderly people because they were a priority group for vaccination and received the majority of CoronaVac doses during the initial stages of the vaccination campaign in Brazil; as a result, it was not possible to compare the effectiveness of CoronaVac between older and younger populations directly. Our analyses were also limited by the lack of more refined covariates, such as frailty, chronic illness status, and nursing home residence status, which could influence vaccine effectiveness in very elderly people and in itself would not be addressed by age and reported comorbidities. Finally, we cannot exclude the possibility of time varying changes in behaviour, non-drug interventions, or testing practices among participants. We tried to control for these by matching on time of RT-PCR testing (±3 days),21 geography (ie, municipality of residence), and self-reported race, which is strongly associated with socioeconomic position in Brazil. When we tried to further adjust for unmeasured confounding by adjusting for the day of year, estimates of vaccine effectiveness remained similar.
Conclusion
This study found that a two dose schedule of CoronaVac was 47% effective in preventing symptomatic covid-19, with higher effectiveness against severe clinical outcomes, among elderly people aged ≥70 years in a setting with extensive transmission of the gamma variant. The delayed onset of vaccine mediated protection, however, underscores the need to prioritise vaccine supplies and maximise the number of people who complete the two dose schedule, when CoronaVac is used as part of a mass vaccination campaign that is implemented in response to a covid-19 epidemic.
What is already known on this topic
Estimates of effectiveness of the inactivated whole virus vaccine, CoronaVac (Sinovac Biotech), against symptomatic covid-19 in randomised controlled trials have varied (51% to 84%)
Current evidence is limited on whether CoronaVac is effective against covid-19 associated severe disease or death, or in the setting of extensive circulation of the gamma variant
More evidence is needed for the real world effectiveness of CoronaVac and other inactivated vaccines among elderly people, a population that has been underrepresented in trials of these vaccines
What this study adds
A two dose regimen of CoronaVac was associated with 47% protection against symptomatic covid-19, 56% against hospital admissions, and 61% against deaths among adults aged ≥70 years in the setting of widespread transmission of the gamma variant
Protection is low until ≥14 days after the second dose of CoronaVac
The effectiveness of CoronaVac was observed to decline with increasing age in the elderly population
Acknowledgments
We thank the Pan American Health Organization for its support and the São Paulo state for making the databases available for analysis.
Web extra.
Extra material supplied by authors
Supplementary information: additional tables 1-16 and figures 1-4
Contributors: All authors conceived the study. OTR and MDTH contributed equally as first authors and JRA, DATC, AIK, and JC contributed equally as senior authors. OTR, MDTH, and MD completed analyses with guidance from JRA, DATC, AIK, and JC. MSST, OFPP, OTR, and MDTH curated and validated the data. OTR and MDTH wrote the first draft of the manuscript. TLD, RCP, OFPP, EFMV, MA, RS, JCG, and WNA provided supervision. All authors contributed to, and approved, the final manuscript. JC is the guarantor. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: No external funding was provided for this study. JC is supported by the Oswaldo Cruz Foundation (Edital Covid-19 – resposta rápida: 48111668950485). OTR is funded by a Sara Borrell fellowship (CD19/00110) from the Instituto de Salud Carlos III. OTR acknowledges support from the Spanish Ministry of Science and Innovation through the Centro de Excelencia Severo Ochoa 2019-2023 programme and from the Generalitat de Catalunya through the Centres de Recerca de Catalunya (CERCA) programme. These institutions had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The health secretary of State of São Paulo and information technology bureau of the São Paulo state government reviewed the data and findings of the study, but the academic authors retained editorial control.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
The lead author (the manuscript’s guarantor) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as originally planned have been explained.
Dissemination to participants and related patient and public communities: The findings of this study will be disseminated through presentations to the São Paulo state health department and the national immunisation programme, as well as the World Health Organization. Blogs, press releases, and social media dissemination will also be prepared for public dissemination.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Ethical approval
This study was approved by the ethical committee for research of Federal University of Mato Grosso do Sul (CAAE: 43289221.5.0000.0021).
Data availability statement
Deidentified databases as well as the R codes will be deposited in the repository https://github.com/juliocroda/VebraCOVID-19 on publication of this article.
References
- 1.Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis 2020;20:533-4. 10.1016/S1473-3099(20)30120-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Driscoll M, Ribeiro Dos Santos G, Wang L, et al. Age-specific mortality and immunity patterns of SARS-CoV-2. Nature 2021;590:140-5. 10.1038/s41586-020-2918-0 [DOI] [PubMed] [Google Scholar]
- 3.Bajaj V, Gadi N, Spihlman AP, Wu SC, Choi CH, Moulton VR. Aging, Immunity, and COVID-19: How Age Influences the Host Immune Response to Coronavirus Infections? Front Physiol 2021;11:571416. 10.3389/fphys.2020.571416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collier DA, Ferreira IATM, Kotagiri P, et al. Age-related immune response heterogeneity to SARS-CoV-2 vaccine BNT162b2. Nature 2021; published online June 30. DOI: 10.1038/s41586-021-03739-1. [DOI] [PMC free article] [PubMed]
- 5.Palacios R, Batista AP, Albuquerque CSN, et al. Efficacy and Safety of a COVID-19 Inactivated Vaccine in Healthcare Professionals in Brazil: The PROFISCOV Study. SSRN Journal2021. DOI: 10.2139/ssrn.3822780. [DOI]
- 6.Voysey M, Clemens SAC, Madhi SA, et al. Oxford COVID Vaccine Trial Group . Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021;397:99-111. 10.1016/S0140-6736(20)32661-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanriover MD, Doğanay HL, Akova M, et al. CoronaVac Study Group . Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet 2021;398:213-22. 10.1016/S0140-6736(21)01429-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ 2021;373:n1088. 10.1136/bmj.n1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dagan N, Barda N, Kepten E, et al. BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Mass Vaccination Setting. N Engl J Med 2021;384:1412-23. 10.1056/NEJMoa2101765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strategic Advisory Group of Experts on Immunization - SAGE (WHO). Evidence Assessment: Sinovac/CoronaVac COVID-19 vaccine. Report from 29/04/2021. https://cdn.who.int/media/docs/default-source/immunization/sage/2021/april/5_sage29apr2021_critical-evidence_sinovac.pdf (accessed May 26, 2021).
- 11.Strategic Advisory Group of Experts on Immunization - SAGE (WHO). Interim recommendations for use of the inactivated COVID-19 vaccine, CoronaVac, developed by Sinovac. 2021; published online May 24. https://apps.who.int/iris/bitstream/handle/10665/341454/WHO-2019-nCoV-vaccines-SAGE-recommendation-Sinovac-CoronaVac-2021.1-eng.pdf.
- 12.Jara A, Undurraga EA, González C, et al. Effectiveness of an Inactivated SARS-CoV-2 Vaccine in Chile. N Engl J Med 2021. 10.1056/NEJMoa2107715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ranzani OT, Bastos LSL, Gelli JGM, et al. Characterisation of the first 250,000 hospital admissions for COVID-19 in Brazil: a retrospective analysis of nationwide data. Lancet Respir Med 2021;9:407-18. 10.1016/S2213-2600(20)30560-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lamarca AP, de Almeida LGP, Francisco R da S, et al. Genomic surveillance of SARS-CoV-2 tracks early interstate transmission of P.1 lineage and diversification within P.2 clade in Brazil. medRxiv, 2021. 10.1101/2021.03.21.21253418 [DOI] [PMC free article] [PubMed]
- 15.Naveca FG, Nascimento V, de Souza VC, et al. COVID-19 in Amazonas, Brazil, was driven by the persistence of endemic lineages and P.1 emergence. Nat Med 2021;27:1230-8. 10.1038/s41591-021-01378-7. [DOI] [PubMed] [Google Scholar]
- 16.Faria NR, Mellan TA, Whittaker C, et al. Genomics and epidemiology of the P.1 SARS-CoV-2 lineage in Manaus, Brazil. Science 2021;372:815-21. 10.1126/science.abh2644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jangra S, Ye C, Rathnasinghe R, et al. Personalized Virology Initiative study group . SARS-CoV-2 spike E484K mutation reduces antibody neutralisation. Lancet Microbe 2021;2:e283-4. 10.1016/S2666-5247(21)00068-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoffmann M, Arora P, Groß R, et al. SARS-CoV-2 variants B.1.351 and P.1 escape from neutralizing antibodies. Cell 2021;184:2384-2393.e12. 10.1016/j.cell.2021.03.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Souza WM, Amorim MR, Sesti-Costa R, et al. Neutralisation of SARS-CoV-2 lineage P.1 by antibodies elicited through natural SARS-CoV-2 infection or vaccination with an inactivated SARS-CoV-2 vaccine: an immunological study. Lancet Microbe 2021;•••. 10.1016/S2666-5247(21)00129-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freitas ARR, Beckedorff OA, Cavalcanti LP de G, et al. The emergence of novel SARS-CoV-2 variant P.1 in Amazonas (Brazil) was temporally associated with a change in the age and sex profile of COVID-19 mortality: A population based ecological study. The Lancet Regional Health - Americas 2021; 10.1016/j.lana.2021.100021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bastos LS, Ranzani OT, Souza TML, Hamacher S, Bozza FA. COVID-19 hospital admissions: Brazil’s first and second waves compared. Lancet Respir Med 2021;9:e82-3. 10.1016/S2213-2600(21)00287-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.GISAID - hCov19 Variants. https://www.gisaid.org/hcov19-variants/ (accessed June 20, 2021).
- 23.Patel MK, Bergeri I, Bresee JS, et al. Evaluation of post-introduction COVID-19 vaccine effectiveness: Summary of interim guidance of the World Health Organization. Vaccine 2021;39:4013-24. 10.1016/j.vaccine.2021.05.099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Freire FHM de A, Gonzaga MR. Gomes MMF. Projeções populacionais por sexo e idade para pequenas áreas no Brasil. RELAP 2019;14:124-49 10.31406/relap2020.v14.i1.n26.6. [DOI] [Google Scholar]
- 25.SEADE Foundation - São Paulo State. CORONAVIRUS - CASOS EM SP. Fundação SEADE. https://www.seade.gov.br/coronavirus/ (accessed May 9, 2021).
- 26.Brazilian Ministry of Health. Campanha Nacional de Vacinação contra a Covid-19. Décimo oitavo informe técnico. https://www.conasems.org.br/wp-content/uploads/2021/04/20a-distribuic%CC%A7a%CC%83o_Campanha_Nacional_de_Vacinacao_contra_a_Covid_19.pdf (accessed May 25, 2021).
- 27.De Serres G, Skowronski DM, Wu XW, Ambrose CS. The test-negative design: validity, accuracy and precision of vaccine efficacy estimates compared to the gold standard of randomised placebo-controlled clinical trials. Euro Surveill 2013;18:20585. 10.2807/1560-7917.ES2013.18.37.20585. [DOI] [PubMed] [Google Scholar]
- 28.Schwartz LM, Halloran ME, Rowhani-Rahbar A, Neuzil KM, Victor JC. Rotavirus vaccine effectiveness in low-income settings: An evaluation of the test-negative design. Vaccine 2017;35:184-90. 10.1016/j.vaccine.2016.10.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jackson ML, Chung JR, Jackson LA, et al. Influenza Vaccine Effectiveness in the United States during the 2015-2016 Season. N Engl J Med 2017;377:534-43. 10.1056/NEJMoa1700153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of Covid-19 Vaccines against the B.1.617.2 (Delta) Variant. N Engl J Med 2021;385:585-94. 10.1056/NEJMoa2108891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brazilian Ministry of Health. Epidemiologic Surveillance Guide. National Emergency of Public Health Concern due to the COVID-19 disease. https://www.conasems.org.br/wp-content/uploads/2021/03/Guia-de-vigila%CC%82ncia-epidemiolo%CC%81gica-da-covid_19_15.03_2021.pdf (accessed May 25, 2021).
- 32.Brazilian Institute of Geography and Statistics - IBGE. Ethnic and race characteristics of the population. Classifications and identities. 2013. https://biblioteca.ibge.gov.br/visualizacao/livros/liv63405.pdf (accessed May 24, 2021).
- 33.Bueno SM, Abarca K, González PA, et al. Interim report: Safety and immunogenicity of an inactivated vaccine against SARS-CoV-2 in healthy chilean adults in a phase 3 clinical trial. medRxiv, 2021. 10.1101/2021.03.31.21254494 [DOI]
- 34.Wu Z, Hu Y, Xu M, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy adults aged 60 years and older: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis 2021;21:803-12. 10.1016/S1473-3099(20)30987-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hitchings MDT, Lewnard JA, Dean NE, et al. Use of recently vaccinated individuals to detect bias in test-negative case-control studies of COVID-19 vaccine effectiveness. medRxiv, 2021. 10.1101/2021.06.23.21259415 [DOI] [PMC free article] [PubMed]
- 36.Lewnard JA, Patel MM, Jewell NP, et al. Theoretical Framework for Retrospective Studies of the Effectiveness of SARS-CoV-2 Vaccines. Epidemiology 2021;32:508-17. 10.1097/EDE.0000000000001366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sullivan SG, Tchetgen Tchetgen EJ, Cowling BJ. Theoretical Basis of the Test-Negative Study Design for Assessment of Influenza Vaccine Effectiveness. Am J Epidemiol 2016;184:345-53. 10.1093/aje/kww064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pearce N. Analysis of matched case-control studies. BMJ 2016;352:i969. 10.1136/bmj.i969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Andrejko KL, Pry J, Myers JF, et al. California COVID-19 Case-Control Study Team . Prevention of COVID-19 by mRNA-based vaccines within the general population of California. Clin Infect Dis 2021. 10.1093/cid/ciab640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crowe E, Pandeya N, Brotherton JML, et al. Effectiveness of quadrivalent human papillomavirus vaccine for the prevention of cervical abnormalities: case-control study nested within a population based screening programme in Australia. BMJ 2014;348:g1458. 10.1136/bmj.g1458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moustsen-Helms IR, Emborg H-D, Nielsen J, et al. Vaccine effectiveness after 1st and 2nd dose of the BNT162b2 mRNA Covid-19 Vaccine in long-term care facility residents and healthcare workers – a Danish cohort study. medRxiv, 2021 10.1101/2021.03.08.21252200 [DOI]
- 42.Britton A, Jacobs Slifka KM, Edens C, et al. Effectiveness of the Pfizer-BioNTech COVID-19 Vaccine Among Residents of Two Skilled Nursing Facilities Experiencing COVID-19 Outbreaks - Connecticut, December 2020-February 2021. MMWR Morb Mortal Wkly Rep 2021;70:396-401. 10.15585/mmwr.mm7011e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baum U, Poukka E, Palmu AA, Salo H, Lehtonen TO, Leino T. Effectiveness of vaccination against SARS-CoV-2 infection and Covid-19 hospitalization among Finnish elderly and chronically ill – An interim analysis of a nationwide cohort study. medRxiv, 2021 10.1101/2021.06.21.21258686 [DOI] [PMC free article] [PubMed]
- 44.Yelin I, Katz R, Herzel E, et al. Associations of the BNT162b2 COVID-19 vaccine effectiveness with patient age and comorbidities. medRxiv, 2021. DOI: 10.1101/2021.03.16.21253686. [DOI]
- 45.Victora PC, Castro PMC, Gurzenda S, Medeiros AC, França GVA, Barros PAJD. Estimating the early impact of vaccination against COVID-19 on deaths among elderly people in Brazil: Analyses of routinely-collected data on vaccine coverage and mortality. EClinicalMedicine 2021;36:101036. 10.1016/j.eclinm.2021.101036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Voysey M, Costa Clemens SA, Madhi SA, et al. Oxford COVID Vaccine Trial Group . Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet 2021;397:881-91. 10.1016/S0140-6736(21)00432-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Skowronski DM, Setayeshgar S, Zou M, et al. Single-dose mRNA vaccine effectiveness against SARS-CoV-2, including Alpha and Gamma variants: a test-negative design in adults 70 years and older in British Columbia, Canada. Clin Infect Dis 2021;ciab616. 10.1093/cid/ciab616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hitchings MDT, Ranzani OT, Torres MSS, et al. Effectiveness of CoronaVac among healthcare workers in the setting of high SARS-CoV-2 Gamma variant transmission in Manaus, Brazil: A test-negative case-control study. The Lancet Regional Health - Americas 2021; 100025. 10.1016/j.lana.2021.100025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.SoroEpi Group. SoroEpi MSP study in São Paulo Capital - Phase 5 results. https://www.monitoramentocovid19.org (accessed May 17, 2021).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information: additional tables 1-16 and figures 1-4
Data Availability Statement
Deidentified databases as well as the R codes will be deposited in the repository https://github.com/juliocroda/VebraCOVID-19 on publication of this article.