Abstract
Background/Aim: Coronavirus-19 (COVID-19) pandemic outbreak is currently having a huge impact on medical resource allocation. Breast Cancer (BC) patients are concerned both with BC treatment and COVID-19. This study aimed to estimate the impact of anxiety among patients, caused by the spreading of COVID-19. Patients and Methods: Between the 16th of January and the 20th of March 2020, we retrospectively enrolled 160 patients. Eighty-two patients with a suspected breast lesion (SBL) were divided into two groups: PRE-COVID-19-SBL and POST-COVID-19-SBL. Seventy-eight BC patients were divided into PRE-COVID-19-BC and POST-COVID-19-BC. Patient characteristics including age, marital status, SBL/BC diameter, personal and family history of BC, clinical stage and molecular subtype were recorded. Procedure Refusal (PR) and Surgical Refusal (SR) were also recorded with their reason. Results: BC and SBL analysis showed no difference in pre-treatment characteristics (p>0.05). Both POST-COVID-19-SBL and POST-COVID-19-BC groups showed higher rates of PR and SR (p=0.0208, p=0.0065 respectively). Infection risk represented primary reason for refusal among POST-COVID-19 patients. Conclusion: COVID-19-related anxiety could affect patients’ decision-making process.
Keywords: COVID-19, breast cancer, SARS-SOV-2, decisionmaking process, surgery refusal, anxiety, surgery
Since December 2019, the novel coronavirus (SARS-COV-2) has emerged as a highly contagious human pathogen. On March 20, 2020 more than 234000 cases were confirmed world-wide, with more than 9800 registered deaths (1). Following the initial outbreak in the Chinese Hubei Province on March 11, WHO has labelled the latest coronavirus disease COVID-19 (caused by SARS-COV-2) as a pandemic. The reported fatality rate is 4.2% globally (1,2). Human to human transmission occurs through direct contact or air droplets (2) placing health care providers at a high risk due to the close proximity to potentially infected patients (3).
Preliminary data of nationwide analysis in China demonstrated cancer as a risk factor for developing severe complications/disease course among COVID-19 patients (4,5). Although further studies are required in order to accurately estimate the risk (6,7) among patients who underwent chemotherapy or surgery in the months prior to the outbreak, the risk of developing severe conditions seems considerably higher compared to the general population (6). Breast Cancer (BC) is the most common neoplasm worldwide representing the primary cause of death due to neoplasms in Italy (5). A report published in 2017 stated that more than 50% of BC patients were older than 60 years (8). Therefore, due to the higher risk of adverse events in older patients, underlined in the preliminary data (9), it is essential to evaluate the risk of COVID-19 infection among these frail BC patients (10). Furthermore, during the COVID-19 outbreak, hospital resources are reallocated from elective and semi-elective procedures to meet the needs of COVID-19 patients in critical conditions (11). The subsequent scarcity of resources could potentially delay diagnostic evaluations and treatment of BC patients. Moreover, patients’ anxiety regarding COVID-19 plays a role in treatment timings; at the early stages of the outbreak, many patients had asked for immediate surgery due to the risk of treatment delay. Conversely, some BC patients have refused or delayed treatments like surgery as much as possible.
We hypothesize that the COVID-19 outbreak has led to an increase in procedures and surgical refusal (PR and SR). The purpose of our study was to assess the effect of BC patients’ anxiety caused by the fear of COVID-19 on their decision-making process regarding treatment. Our analysis aimed to evaluate the potential number of BC patients who will suffer in case of delayed preoperative BC assessment and BC surgery.
Patients and Methods
Study design. This study was designed as monocentric and retrospective. The institutional review of Policlinico Tor Vergata waived the need for a formal approval because of the retrospective descriptive design.
Population. All female patients who visited the Breast Unit of Tor Vergata University Hospital were examined. From the 18th of January to the 20th of March 2020 we analysed 303 consecutive patients who were admitted to our ambulatory facilities. Prior to their first visit, all our patients routinely sign an informed consent for clinical practice data analysis. Among this population, 160 patients satisfied the inclusion criteria (BI-RADS≥4 or Proven BC). Two different study groups were subsequently extracted: Suspicious Breast Lesion (SBL) Study Group (n=82) and Breast Cancer (BC) Study Group (n=78). Therefore, 143 patients were excluded from the study.
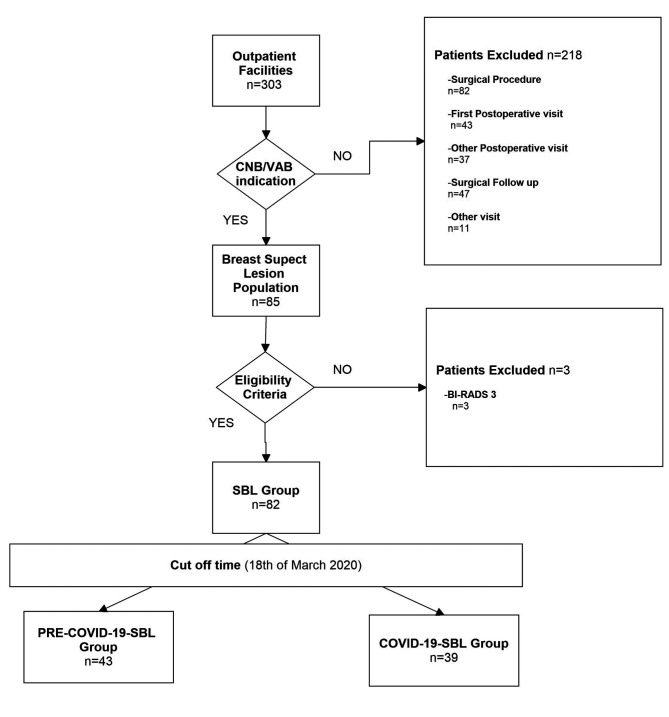
Suspicious breast lesion study group (n=82). A total of 82 patients admitted to percutaneous procedure [as core needle biopsy/vacuum assisted biopsy (CNB/VAB)] for new SBL were selected for this analysis. The main inclusion criterion for retrospective enrolment was a complete previous imaging evaluation of the breast as well as new SBL classified as BI-RADS ≥4, according to breast imaging reports and data system lexicon (BI-RADS) (12), after revaluation by our Breast Expert Radiologists (13). Moreover, when indicated, our patients went through an 8-gauge Vacuum assisted biopsy to reduce the need for further treatment in case of benign lesions (14-16). SBL patients were divided into PRE-COVID-19-SBL and POST-COVID-19-SBL groups, according to the date of visit (43 and 39 patients, respectively). February 18, 2020 was set as the cut-off day, when the first Italian, non-imported case of COVID-19 had been registered (17). Figure 1 describes the distribution of the SBL Study Group.
Figure 1. SBL study population.
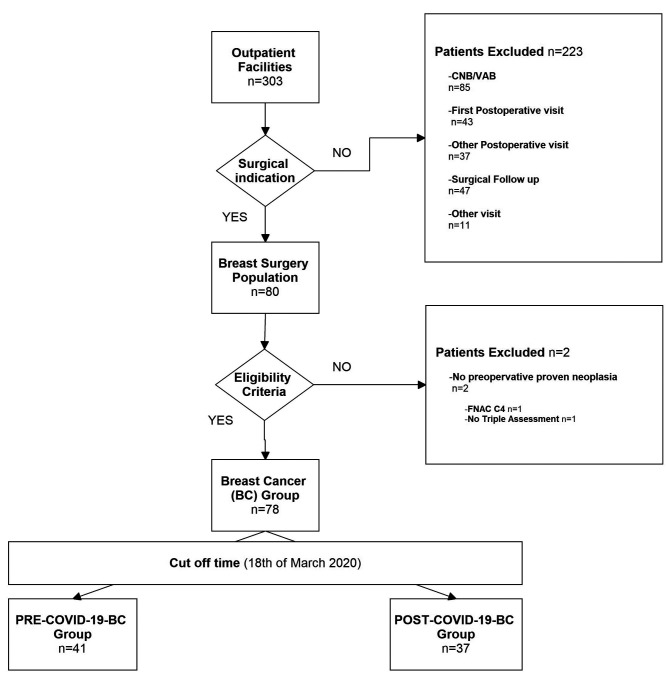
Breast cancer study group (n=78). A total of 78 consecutive patients indicated for BC surgical procedure were included in the analysis. The main eligibility criterion was non metastatic anatomopathologically proven breast cancer (Tis-T4; N0-3; M0). Patients were divided into PRE-COVID-19-BC and POST-COVID-19-BC groups, according to the date of visit (41 and 37 patients, respectively), in line with the aforementioned cut-off (17), as shown in Figure 2.
Figure 2. BC study population.
Data collection. Retrospective data collection from the clinical notes of both groups included age, personal and family history of BC as well as marital status. The impact of these variables on the probability of surgical refusal (SR) have already been analyzed in literature (18-21). Moreover, during the COVID-19 outbreak, all patients were interviewed via telephone in order to evaluate information regarding infection risk prior to the visit, as required by our facilities.
All the mammographic (MMG) and sonographic (US) images were reviewed on a Picture Archiving and Communication System (PACS) workstation (Carestream, Genova, Italy). Additional variables of the different study groups were included in the analysis.
Suspicious breast lesion study group (n=82). Among the SBL study group (n=82), each lesion was categorized according to BI-RADS lexicon and the registered maximum diameter. Procedure Refusal (PR) rate of percutaneous procedure was appraised from our clinical records. Patients were able to refuse prescribed procedures in two ways – during outpatient visit by signing a paper or via telephone (with subsequent email confirmation). Records of PR and the stated reason (if available) were registered. As guided by our Facilities, patients refusing two different appointments were then placed on a standby list.
Breast cancer study group (n=78). Among the BC group (n=78), the “Neoadjuvant chemotherapy administration” variable was added to dataset as a potentially confounding factor. Imaging data were used to define clinical stage based on the recommendations from AJCC 2018 (edition VIII) for TMN classification. Due to the small sample size, clinical stages were categorized as a dichotomous variable, either Early Breast Cancer (EBC) or Local Advance Breast Cancer (LABC), according to NCCN guidelines (22).
Data regarding the BC group, obtained from preoperative biopsy (CNB/VAB) or fine needle aspiration cytology (FNAC), were collected. For patients who underwent CNB/VAB (61 cases; 80.77%), data from pathological examinations such as expression of ER, PR and Ki67 receptors were expressed as a percentage of positive cells in specimens examined by immunohistochemistry. Overexpression of the Her2 gene (HER2 SCORE) was identified by IHC or by FISH, as indicated by the recommendations of the 2018 ASCO/CAP. All patients were divided into the following subgroups in concordance with the classification of intrinsic subtypes recommended by the San Gallen International Expert Consensus Report of 2017. Due to the small sample size, clinical intrinsic subgroups were treated as a dichotomous variable: luminal (LUM) and non-luminal (NLUM). LUM group consists of Luminal A, Luminal B+, Luminal B– patients and NLUM of Her2 type and Triple Negative, respectively.
Surgery refusal (SR) rate was assessed from our clinical records as described above.
Statistical analysis. All data were submitted into the EXCEL datasheet (Microsoft, Washington, DC, USA). Previously known variables from the literature that may affect patients’ decision-making process were integrated into the analysis. For continuous variables, means and ranges were calculated. A t-test was used to determine whether there are significant differences between the means of the two groups’ confounding variables. Categorical data were recoded as numbers and percentages. Analysis was performed using the Fisher’s exact test. The variables of our interest (PR and SR) took on values of either 0 when patient refused procedure or surgery, or 1 when accepted. Fisher’s exact test was used to assess the impact of COVID-19 on these variables. Variables with assigned p-values <0.05 were considered statistically significant. All the statistical analysis was performed in SPSS statistical package version 23.0 (SPSS Inc., Chicago, IL, USA).
Results
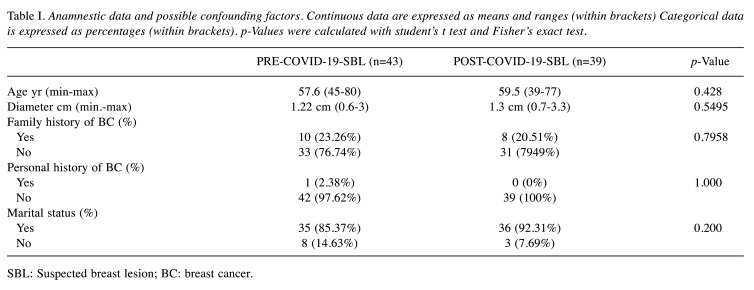
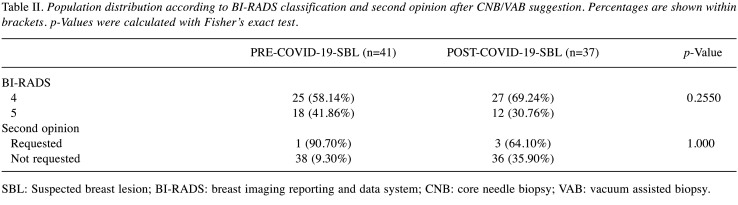
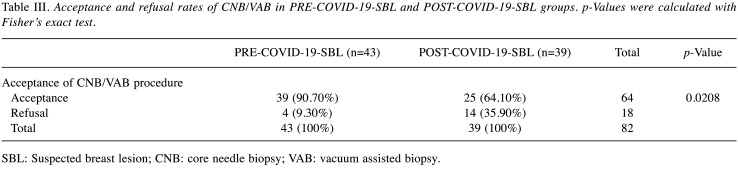
SBL group analysis. Among the 82 patients who underwent surgical evaluation for SBL, no significant statistical differences were found between PRE-COVID-19-SBL and POST-COVID-19-SBL groups regarding personal and anamnestic data like age, diameter of SBL, personal and family history of BC. Table I summarizes the findings showing homogeneity in both groups regarding these potential confounding factors (18-21). Grouping population according to BI-RADS lexicon showed no statistically significant differences between the two different periods of analysis. A total of 30 patients (36.59%) were classified as BI-RADS 5 (Table II). Statistically significant difference was found in the PR rate between the PRE-COVID-19 and POST-COVID-19 periods (p=0.0065) (Table III). The following data were obtained from the analysis of the PR reasons. In the PRE-COVID-19 group, 2 patients (50%) asking for a second opinion, have decided to undergo biopsies in another facility, 1 patient (25%) decided not to undergo CNB/VAB. And 1 additional case had no recorded reason. Differential distribution of PR rate was observed among POST-COVID-19-SBL patients: 3 patients (21.42%) requested a second opinion, 3 patients mentioned COVID-19 in the refusal form and 8 patients did not provide any reason or the data was missing. As reported above, 5 patients preferred to receive a second opinion and refused to undergo the procedure at our facility (2 and 3 patients in the PRE-COVID-19 and COVID-19 period, respectively). In order to evaluate the influence of a second opinion on PR rate between the groups, further analysis was performed and no statistically significant difference was found among the two SBL groups, as displayed in the Table II (p=0.3432).
Table I. Anamnestic data and possible confounding factors. Continuous data are expressed as means and ranges (within brackets) Categorical data is expressed as percentages (within brackets). p-Values were calculated with student’s t test and Fisher’s exact test.
SBL: Suspected breast lesion; BC: breast cancer.
Table II. Population distribution according to BI-RADS classification and second opinion after CNB/VAB suggestion. Percentages are shown within brackets. p-Values were calculated with Fisher’s exact test.
SBL: Suspected breast lesion; BI-RADS: breast imaging reporting and data system; CNB: core needle biopsy; VAB: vacuum assisted biopsy.
Table III. Acceptance and refusal rates of CNB/VAB in PRE-COVID-19-SBL and POST-COVID-19-SBL groups. p-Values were calculated with Fisher’s exact test.
SBL: Suspected breast lesion; CNB: core needle biopsy; VAB: vacuum assisted biopsy.
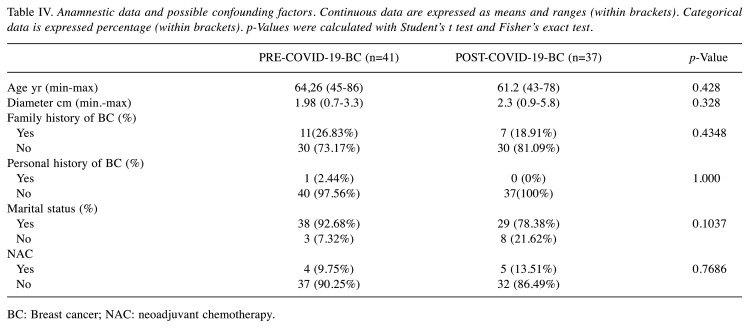
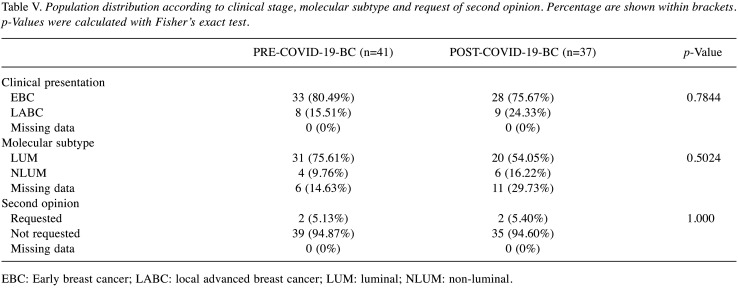
BC group analysis. A total of 78 different patients were enrolled in the analysis. The confounding factors mentioned above are enlisted in Table IV. Marital status, Clinical Stage, personal and family history of breast cancer were randomly distributed between the two groups. Furthermore, clinical presentation did not differ between the two groups and 21.7% of BC group experienced LABC (Table V). Interestingly, there was no SR among patients who underwent NAC, underlying the commitment of NAC patients to the adherence of the multidisciplinary treatment despite the COVID-19 outbreak. According to Molecular subtype (LUM/NLUM), PRE-COVID-19-BC and POST-COVID-19-BC didn’t show any statistically significant difference (p=0.5024). All of the aforementioned factors were evaluated in order to highlight any confounding factors that may have impaired our analysis.
Table IV. Anamnestic data and possible confounding factors. Continuous data are expressed as means and ranges (within brackets). Categorical data is expressed percentage (within brackets). p-Values were calculated with Student’s t test and Fisher’s exact test.
BC: Breast cancer; NAC: neoadjuvant chemotherapy.
Table V. Population distribution according to clinical stage, molecular subtype and request of second opinion. Percentage are shown within brackets. p-Values were calculated with Fisher’s exact test.
EBC: Early breast cancer; LABC: local advanced breast cancer; LUM: luminal; NLUM: non-luminal.
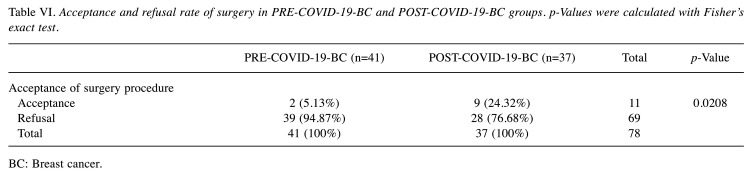
Regarding the primary aim of the study, SR distribution demonstrated statistically significant difference between PRE-COVID-19-BC and POST-COVID-19-BC groups (p=0.0208) (Table VI). Among the PRE-COVID-19-BC group, 3 patients (4.87%) refused surgery. All patients decided to seek a second opinion and to undergo surgery in other facilities. Conversely, a different distribution was demonstrated among the POST-COVID-19 BC group. Two out of 9 (24.32%) patients asked for an external second opinion. The remaining 7 patients specified COVID-19 in the written refusal. Similar to the previous group, we decided to assess the influence of a second opinion on refusals or delays of surgical treatments, no statistically significant difference was found (p=1.000).
Table VI. Acceptance and refusal rate of surgery in PRE-COVID-19-BC and POST-COVID-19-BC groups. p-Values were calculated with Fisher’s exact test.
BC: Breast cancer.
Moreover, during data analysis we found that 30 patients (76%) of the POST-COVID-19-BC underwent CNB/VAB in our facility during the PRE-COVID-19 period. Consequently, we decided to evaluate whether COVID-19 had affected their decision-making. Five of these patients (16.6%) refused surgery after CNB/VAB in the previous months, none reported asking for a second opinion as a reason for refusal.
Discussion
COVID-19 pandemic is currently the primary health concern worldwide. Nowadays, Europe represents the epicentre of virus outbreak. Italy is confronting a grave situation with the number of deaths surpassing China’s. The Italian national health system (NHS) is facing one of the toughest challenges since its foundation (11). In order to provide adequate and equal treatment across the country, we believe there is a need for specific guidelines to be issued by every College of specialists through the outbreak, as already published by the Italian College of Anaesthesiologists (23) and several foreign Colleges (24-29).
Even in normal times, fear and anxiety play a major role in the course of patients’ disease. About 0.64% of BC patients refuse surgical treatment according to SEER database (19,21). Personal and socioeconomic conditions were linked to surgical treatment refusal, including higher age at diagnosis, female gender, Ethnicity, type of insurance, LABC (stage II and III BC), non-triple-negative breast cancer, residence areas with a low percentage of high school diplomas (21). In a large retrospective study, refusal of surgery had a detrimental effect on survival with 2.42 times higher risk of mortality (19). Moreover, cultural issues may play a major role in SR rate, in fact, while western countries show similar SR rates [Switzerland and Canada: 1.3% and 1.2% respectively (18,20)], developing countries face other difficulties, like low level of breast cancer awareness, fear of mastectomy and prior trial of non-orthodox treatment (traditional medicine) (30,31).
Understanding why patients refuse treatment is of a paramount importance for enhancing the ability to recognize those likely to refuse surgical strategies and addressing their concerns (19). The data of this study demonstrated that fear of COVID-19 contagion could potentially have a great impact on treatment refusal by patients presenting with a new SBL or even BC.
COVID-19-related-anxiety is a reasonable reaction to the novel outbreak, but two main problems could arise from this temporary situation. Firstly, BC treatment delays and screening programs abatements will eventually lead to an increase in LABC rate over a prolonged period, worsening the clinical outcomes of our patients. Today, having no reliable data regarding the end of the epidemic, the delay of all oncological treatments until the end of the outbreak is not realistic for BC patients. Moreover, comparison between well-known long term outcomes of BC and the outcomes of COVID-19 patients is still missing (32,33). Second, undiagnosed COVID-19 patients, symptomatic or not, could potentially be in contact with BC patients amidst the health system reorganization. Surgeons should take advantage of techniques such as awake surgery (34-36), oncoplastic techniques (37-39), and prepectoral reconstruction (40-42) in order to shorten hospitalization and surgery recovery (34,36,39). Our study has some limitations such as its retrospective design. Therefore, couldn’t allow the evaluation of other possible confounding factors as the above-mentioned residency area. We believe that a monocentric design may have partially overcome this bias as 85% of the study population resides in the district of our Institution. Moreover, COVID-19-like symptomatic patients were excluded a priori from the study due to the COVID-19 telephonic triage. This mechanism could lead to a selection bias. However, the decision not to include symptomatic patients was made in order to evaluate the anxiety caused by COVID-19 in patients together with its influence on patients’ decision-making process. An additional limitation of our study was the short period of analysis, further studies should evaluate how COVID-19 outbreak would influence long-term acceptance rate in these patients, addressing the consequences of treatment delay on clinical outcomes.
Despite all these limitations, our work demonstrated that fear of COVID-19 contagion could impair correct clinical management with higher rates of PR and SR among patients with SBL or even BC. Despite these limitations and in our opinion, this analysis is useful to underline not only the influence of COVID-19 on patients’ decision making, but also the impact of other acute emerging health issues on patients’ decisions. In order to minimize the delay of BC treatment during outbreaks, BC MDT should triage patients and schedule surgical procedures aiming to optimize the allocation of the limited resources to urgent cases during higher peaks. Surgical oncologists should always notify patients regarding the detrimental effect of refusal and delay of multidisciplinary treatment on the long-term clinical outcomes. Moreover, psychological support should be enhanced during outbreaks. Management of BC surgery during the COVID-19 pandemic requires further investigation and will be the subject of further study by our research group.
Conflicts of Interest
The Authors declare no conflicts of interest regarding this study.
Authors’ Contributions
Study conception and design: Vanni Gianluca, Buonomo Oreste Claudio, Materazzo Marco. Acquisition of data: Rho Maurizio, Ingallinella Sara, Maria Cotesta; Analysis of data: Makarova Anna, Materazzo Marco, Santori Francesca. Interpretation of data: Vanni Gianluca, Pellicciaro Marco, Materazzo Marco. Article draft: Vanni Gianluca, Materazzo Marco, Caspi Jonathan; Critical revision: Pistolese Chiara Adriana. Critical Revision of Literature: Pistolese Chiara Adriana.
References
- 1.Situation Report-60 SITUATION IN NUMBERS total (new) cases in last 24 hours WHO RISK ASSESSMENT Global Level Very High. Available at: https://www.who.int/docs/defaultsource/coronaviruse/situation-reports/20200320-sitrep-60-covid-19.pdf?sfvrsn=8894045a_2. [Last accessed March 21, 2020]
- 2.Chan JFW, Yuan S, Kok KH, To KKW, Chu H, Yang J, Xing F, Liu J, Yip CCY, Poon RWS, Tsoi HW, Lo SKF, Chan KH, Poon VKM, Chan WM, Ip JD, Cai JP, Cheng VCC, Chen H, Hui CKM, Yuen KY. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong TW, Lee CK, Tam W, Lau JTF, Yu TS, Lui SF, Chan PKS, Li Y, Bresee JS, Sung JJY, Parashar UD. Cluster of SARS among medical students exposed to single patient, Hong Kong. Emerg Infect Dis. 2004;10:269–276. doi: 10.3201/eid1002.030452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiang F, Deng L, Zhang L, Cai Y, Cheung CW, Xia Z. Review of the clinical characteristics of Coronavirus Disease 2019 (COVID-19) J Gen Intern. 2020;Med:1–5. doi: 10.1007/s11606-020-05762-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, Znaor A, Bray F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941–1953. doi: 10.1002/ijc.31937. [DOI] [PubMed] [Google Scholar]
- 6.Liang W, Guan W, Chen R, Wang W, Li J, Xu K, Li C, Ai Q, Lu W, Liang H, Li S, He J. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xia Y, Jin R, Zhao J, Li W, Shen H. Risk of COVID-19 for cancer patients. Lancet Oncol. 2020 doi: 10.1016/S1470-2045(20)30150-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inwald EC, Ortmann O, Koller M, Zeman F, Hofstädter F, Evert M, Brockhoff G, Klinkhammer-Schalke M. Screening-relevant age threshold of 70years and older is a stronger determinant for the choice of adjuvant treatment in breast cancer patients than tumor biology. Breast Cancer Res Treat. 2017;163:119–130. doi: 10.1007/s10549-017-4151-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, Ren R, Leung KSM, Lau EHY, Wong JY, Xing X, Xiang N, Wu Y, Li C, Chen Q, Li D, Liu T, Zhao J, Liu M, Tu W, Chen C, Jin L, Yang R, Wang Q, Zhou S, Wang R, Liu H, Luo Y, Liu Y, Shao G, Li H, Tao Z, Yang Y, Deng Z, Liu B, Ma Z, Zhang Y, Shi G, Lam TTY, Wu JT, Gao GF, Cowling BJ, Yang B, Leung GM, Feng Z. Early transmission dynamics in Wuhan, China, of Novel Coronavirus–Infected Pneumonia. N Engl J Med. 2020 doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vanni G, Materazzo M, Pellicciaro M, Morando L, Portarena I, Anenoma L, D’angelillo MR, Barbarino R, Chiaravalloti A, Meucci R, Perretta T, Deiana C, Orsaria P, Capsi J, Pistolese CA, Buonomo OC. Does age matter? Estimating risks of locoregional recurrence after breast-conservative surgery. In Vivo. 2020;34:1125–1132. doi: 10.21873/invivo.11884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosenbaum L. Facing Covid-19 in Italy - ethics, logistics, and therapeutics on the epidemic’s front line. N Engl J. 2020;Med:NEJMp2005492. doi: 10.1056/NEJMp2005492. [DOI] [PubMed] [Google Scholar]
- 12.American College of Radiology. BI-RADS Committee: ACR BIRADS atlas: breast imaging reporting and data system. American College of Radiology. 2013.
- 13.Pistolese CA, Lamacchia F, Tosti D, Anemona L, Ricci F, Censi M, Materazzo M, Vanni G, Collura A, DI Giuliano F, Perretta T, Buonomo OC. Reducing the number of unnecessary percutaneous biopsies: the role of second opinion by expert breast center radiologists. Anticancer Res. 2020;40:939–950. doi: 10.21873/anticanres.14027. [DOI] [PubMed] [Google Scholar]
- 14.Rageth CJ, O’flynn EAM, Pinker K, Kubik-Huch RA, Mundinger A, Decker T, Tausch C, Dammann F, Baltzer PA, Fallenberg EM, Foschini MP, Dellas S, Knauer M, Malhaire C, Sonnenschein M, Boos A, Morris E, Varga Z. Second International Consensus Conference on lesions of uncertain malignant potential in the breast (B3 lesions) Breast Cancer Res Treat. 2019;174:279–296. doi: 10.1007/s10549-018-05071-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orsaria P, Grasso A, Carino R, Caredda E, Sammarra M, Altomare C, Rabitti C, Gullotta G, Perrone G, Pantano F, Buonomo OC, Altomare V. Heterogeneous risk profiles among B3 breast lesions of uncertain malignant potential. Tumori J. 2019;106:115–125. doi: 10.1177/0300891619868301. [DOI] [PubMed] [Google Scholar]
- 16.Perretta T, Lamacchia F, Ferrari D, Beninati E, DI Tosto F, DE Stasio V, Meucci R, DI Stefano C, Buonomo OC, Vanni G, Pistolese CA. Evaluation of ultrasound-guided 8-gauge vacuum-assisted excision system for the removal of US-detectable breast lesions. Anticancer Res. 2020;40:1719–1729. doi: 10.21873/anticanres.14125. [DOI] [PubMed] [Google Scholar]
- 17.Ministry of Health Covid-19 - Situazione in Italia. Available at: http://www.salute.gov.it/portale/nuovocoronavirus/dettaglioContenutiNuovoCoronavirus.jsp?lingua=italiano&id=5351&area=nuovoCoronavirus&menu=vuoto. [Last accessed March 21, 2020]
- 18.Joseph K, Vrouwe S, Kamruzzaman A, Balbaid A, Fenton D, Berendt R, Yu E, Tai P. Outcome analysis of breast cancer patients who declined evidence-based treatment. World J Surg Oncol. 2012;10:1–5. doi: 10.1186/1477-7819-10-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaitanidis A, Alevizakos M, Tsalikidis C, Tsaroucha A, Simopoulos C, Pitiakoudis M. Refusal of cancer-directed surgery by breast cancer patients: risk factors and survival outcomes. Clin Breast Cancer. 2018;18:e469–e476. doi: 10.1016/j.clbc.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 20.Verkooijen HM, Fioretta GM, Rapiti E, Bonnefoi H, Vlastos G, Kurtz J, Schaefer P, Sappino AP, Schubert H, Bouchardy C. Patients’ refusal of surgery strongly impairs breast cancer survival. Ann Surg. 2005;242 doi: 10.1097/01.sla.0000171305.31703.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Restrepo DJ, Sisti A, Boczar D, Huayllani MT, Fishe J, Gabriel E, McLaughlin SA, Bagaria S, Spaulding A, Rinker BD, Forte AJ. Characteristics of breast cancer patients who refuse surgery. Anticancer Res. 2019;39:4941–4945. doi: 10.21873/anticanres.13682. [DOI] [PubMed] [Google Scholar]
- 22.Lurie RH, Anderson BO, Abraham J, Aft R, Agnese D, Allison KH, Blair SL, Burstein HJ, Dang C, Elias AD, Giordano SH, Goetz MP, Goldstein LJ, Isakoff SJ, Krishnamurthy J, Lyons J, Kelly Marcom P, Matro J, Mayer IA, Moran MS, Mortimer J, O RM, Patel SA, Pierce LJ, Rugo HS, Sitapati A, Lisa Smith K, Lou Smith M, Soliman H, Stringer-Reasor EM, Telli ML, Ward JH, Young JS, Rashmi Kumar N, Burns J. NCCN Guidelines Version 3.2020 Breast Cancer. 2020 [Google Scholar]
- 23.Vergano M, Bertolini G, Giannini A, Gristina G, Livigni S, Mistraletti G, Petrini F. Clinical Ethics Recommendations for the Allocation of Intensive Care Treatments in exceptional, resource-limited circumstances, 2020. Available at: http://www.siaarti.it/SiteAssets/News/COVID19 - documentiSIAARTI/SIAARTI - Covid-19 - Clinical EthicsReccomendations.pdf. [Last accessed March 21, 2020]
- 24.Chen Y, Peng J. Treatment strategy for gastrointestinal tumor under the outbreak of novel coronavirus pneumonia in China. Zhonghua Wei Chang Wai Ke Za Zhi. 2020;23:I–IV. doi: 10.3760/cma.j.issn.1671-0274.2020.02.001. [DOI] [PubMed] [Google Scholar]
- 25.Zhao L, Zhang L, Liu JW, Yang ZF, Shen WZ, Li XR. The treatment proposal for the patients with breast diseases in the central epidemic area of 2019 coronavirus disease. Zhonghua Wai Ke Za Zhi. 2020;58:E005. doi: 10.3760/cma.j.cn112139-20200221-00116. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, Xu JM. Medical diagnosis and treatment strategies for malignant tumors of the digestive system during the outbreak of novel coronavirus pneumonia. Zhonghua Zhong Liu Za Zhi. 2020;42:E005. doi: 10.3760/cma.j.cn112152-20200227-00141. [DOI] [PubMed] [Google Scholar]
- 27.Wu F, Song Y, Zeng H, Ye F, Rong W, Wang L, Wu J. Discussion on diagnosis and treatment of hepatobiliary malignancies during the outbreak of novel coronavirus pneumonia. Zhonghua Zhong Liu Za Zhi. 2020;42:E004. doi: 10.3760/cma.j.cn112152-20200227-00137. [DOI] [PubMed] [Google Scholar]
- 28.Liu B, Ma F, Wang J, Fan Y, Mo H, Xu B. Health management of breast cancer patients outside the hospital during the outbreak of 2019 novel coronavirus disease. Zhonghua Zhong Liu Za Zhi. 2020;42:E002. doi: 10.3760/cma.j.cn112152-20200221-00110.. [DOI] [PubMed] [Google Scholar]
- 29.Society of Surgical Oncology Resource for Management Options of Breast Cancer During COVID-19, 2020. Available at: https://www.surgonc.org/wp-content/uploads/2020/03/Breast-Resource-during-COVID-19-3.30.20.pdf. [Last accessed April 6, 2020]
- 30.Martei YM, Vanderpuye V, Jones BA. Fear of mastectomy associated with delayed breast cancer presentation among Ghanaian women. Oncologist. 2018;23:1446–1452. doi: 10.1634/theoncologist.2017-0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tetteh DA, Faulkner SL. Sociocultural factors and breast cancer in sub-Saharan Africa: Implications for diagnosis and management. Women’s Heal. 2016;12:147–156. doi: 10.2217/whe.15.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buonomo OC, Caredda E, Portarena I, Vanni G, Orlandi A, Bagni C, Petrella G, Palombi L, Orsaria P. New insights into the metastatic behavior after breast cancer surgery, according to well-established clinicopathological variables and molecular subtypes. PLoS One. 2017;12:e0184680. doi: 10.1371/journal.pone.0184680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Orsaria P, Caredda E, Genova F, Materazzo M, Capuano I, Vanni G, Granai AV, de Majo A, Portarena I, Sileri P, Petrella G, Palombi L, Buonomo OC. Additional nodal disease prediction in breast cancer with sentinel lymph node metastasis based on clinicopathological features. Anticancer Res. 2018;38:2109–2117. doi: 10.21873/anticanres.12451. [DOI] [PubMed] [Google Scholar]
- 34.Vanni G, Materazzo M, Perretta T, Meucci R, Anemona L, Buonomo C, Dauri M, Granai AV, Rho M, Ingallinella S, Tacconi F, Ambrogi V. Impact of awake breast cancer surgery on postoperative lymphocyte responses. In Vivo. 2019;33(6):1879–1884. doi: 10.21873/invivo.11681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vanni G, Tacconi F, Sellitri F, Ambrogi V, Mineo TC, Pompeo E. Impact of awake videothoracoscopic surgery on postoperative lymphocyte responses. Ann Thorac Surg. 2010;90:973–978. doi: 10.1016/j.athoracsur.2010.04.070.. [DOI] [PubMed] [Google Scholar]
- 36.Mineo TC, Sellitri F, Vanni G, Gallina FT, Ambrogi V. Immunological and inflammatory impact of non-intubated lung metastasectomy. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18071466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pukancsik D, Kelemen P, Újhelyi M, Kovács E, Udvarhelyi N, Mészáros N, Kenessey I, Kovács T, Kásler M, Mátrai Z. Objective decision making between conventional and oncoplastic breast-conserving surgery or mastectomy: An aesthetic and functional prospective cohort study. Eur J Surg Oncol. 2017;43:303–310. doi: 10.1016/j.ejso.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 38.Calì Cassi L, Vanni G, Petrella G, Orsaria P, Pistolese C, Lo Russo G, Innocenti M, Buonomo O. Comparative study of oncoplastic versus non-oncoplastic breast conserving surgery in a group of 211 breast cancer patients. Eur Rev Med Pharmacol Sci. 2016;20:2950–2954. [PubMed] [Google Scholar]
- 39.Calì Cassi L, Biffoli F, Francesconi D, Petrella G, Buonomo O. Anesthesia and analgesia in breast surgery: the benefits of peripheral nerve block. Eur Rev Med Pharmacol Sci. 2017;21:1341–1345. [PubMed] [Google Scholar]
- 40.Buonomo OC, Morando L, Materazzo M, Vanni G, Pistilli G, Palla L, Di Pasquali C, Petrella G. Comparison of round smooth and shaped micro-textured implants in terms of quality of life and aesthetic outcomes in women undergoing breast reconstruction: a single-centre prospective study. Updates Surg. 2020 doi: 10.1007/s13304-020-00721-w. [DOI] [PubMed] [Google Scholar]
- 41.Buonomo OC, Varvaras D, Montuori M, Vanni G, Venditti D, Elia S, Santurro L, Granai AV, Petrella G, Rossi P. One-stage immediate implant-based breast reconstruction, using biological matrices after conservative mastectomies: Preliminary experience of the University Hospital of Tor Vergata, Rome. Chir. 2015;28:221–226. [Google Scholar]
- 42.Rho M, Materazzo M, Antonelli A, Ingallinella S, Portarena I, De Majo A, Orsaria P, Vanni G, Petrella G, Buonomo OC. Breast reconstruction following neoadjuvant chemotherapy (NAC) The Breast. 2019;44:S116–S117. doi: 10.1016/S0960-9776(19)30392-3. [DOI] [Google Scholar]