Abstract
Background/Aim: A novel human coronavirus, named SARS-COV-2, has recently caused thousands of deaths all around the world. Endoplasmic reticulum (ER) stress plays an important role in the development of diseases. Patients and Methods: We aimed to to investigate the relationship between ER stress markers in patients infected with SARS-COV-2 and patients with pneumonia. A total of 9 patients (4 patients diagnosed with pneumonia and 5 patients diagnosed with SARS-COV-2 infection) who admitted to the emergency Department with symptoms of pneumonia and SARS-COV-2 were included in the study. A total of 18 healthy individuals without any known chronic or acute disease and drug use were included as the healthy control group. Serum human glucose regulated protein 78 (GRP78), serum human C/EBP homologous protein (CHOP) and serum human phospho extracellular signal regulated kinase (PERK) levels were measured using enzyme-linked immunosorbent assay (ELISA). Results: GRP78 levels were found to be significantly higher in SARS-COV-2 positive cases compared to individuals in other groups. Serum GRP-78 level median value was statistically significantly higher in SARS-COV-2-positive group compared to the other groups (p=0.0003). Serum PERK level was statistically significantly higher in SARS-COV-2-positive pneumonia cases (p=0.046). Conclusion: An association was shown between GRP78 and SARS-COV-2 infection. Although a small number of patients was investigated, these results will be important and guide future treatments of SARS-COV-2.
Keywords: Endoplasmic reticulum stress, SARS-COV-2, pneumonia, GRP78
Coronaviruses are spherical or pleomorphic in shape with a mean diameter of 80-120 nm. They have heavily glycosylated trimeric spike (S) proteins on their surface (1). Coronavirus infection starts with receptor binding via the S protein. The interaction between the host cell surface receptor and the S1 subunit is the major determinant of the tropism of coronaviruses (2,3). Upon receptor binding of S1, a conformational change is triggered in the S2 subunit, exposing its hidden fusion peptide for insertion into the cellular membrane.
Endoplasmic reticulum (ER) is the organelle where the synthesis, folding, maturation and transport of proteins, calcium storage and lipid biosynthesis of the cell processes take place. ER stress is defined as the result of ER protein folding capacity, resulting in wrongly folded or unfolded protein accumulation (4). Excessive synthesis of secretion proteins, mutations in proteins involved in protein folding, abnormal changes in the amount of Ca+2 in ER and viral infections are some of the factors that cause protein accumulation in ER (5,6). The cell activates three mechanisms to eliminate ER stress caused by an imbalance between the amount of unfolded proteins in ER and the capacity of the cellular mechanism that handles this amount. Firstly, protein synthesis and translocation of proteins to ER are decreased, and the amount of protein entering ER is reduced with a temporary adaptation. Secondly; unfolded protein response (UPR) is activated. Therefore, an increase in the capacity of the ER will occur to deal with unfolded proteins. Thirdly, if homeostasis cannot be restored, a cell death response occurs to protect the organism from cells that display unfolded proteins (7). In order to prevent ER stress in the cell, proteins formed as a result of stress, unfolded or misfolded are destroyed by proteosomes in the cytoplasm. This mechanism is called ER-related decay, endoplasmic-reticulum-associated protein degradation (ERAD) (8,9).
If the endoplasmic reticulum stress cannot be corrected by the ERAD mechanism, the UPR path must be activated in order to prevent ER stress in the cell and create ER homeostasis again (5,6). UPR activates three important signaling pathways initiated by localized stress sensors in ER such as pancreatic ER kinase (PKR), pancreatic-like ER kinase (PERK), inositol-requiring enzyme 1 (IRE1) and activating transcription factor 6 (ATF6) (10,11).
Inflammatory processes lead to oxidative stress and oxidative stress leads to release of toxic oxygen metabolites (12,13). After initiating endoplasmic reticulum stress through various activators, UPR is activated. After UPR activation, glucose regulating protein 78 (GRP78) and other chaperone protein levels increase. The PERK protein is activated by autophosphorylation and protein synthesis is suppressed to reduce ER stress. Autophosphorylation of PERK protein plays an important role in providing homeostasis in ER stress (14).
The GRP78 or binding immunoglobulin protein (BiP) is the master chaperone protein of the UPR (when unfolded or misfolded proteins accumulate) (15-17). Under reasonable conditions, GRP78 is found in the lumen of the ER and inactivates three enzymes responsible for cell death or differentiation. These enzymes are ATF6, PERK, and IRE1 (18). Above a threshold of accumulated unfolded proteins, GRP78 releases ATF6, PERK, and IRE1, leading to their activation. Inhibition of protein synthesis and enhancement of refolding is the end result of the enzymes’ activation (18,19). Overexpression of GRP78 is also initiated upon cell stress, which increases the chance for GRP78 to escape ER retention and translocate to the cell membrane. Once translocated to the cell membrane, GRP78 is susceptible to virus recognition by its substrate-binding domain (SBD), and can mediate the virus entry in the cell (18).
C/EBP homologous protein (CHOP) is a pro-apoptotic protein, whose expression is regulated by the PERK-activating transcription factor 4 (ATF4), the ATF6, and the inositol-requiring enzyme 1 (IRE1) pathways. CHOP protein plays a role especially in apoptosis (20). Thus, the aim of our study was to investigate the relationship between, ER stress markers in patients with pnuemonia and patients infected with SARS-COV-2.
Patients and Methods
Study population and data collection. A total of 9 patients (4 patients diagnosed with pneumonia and 5 diagnosed and confirmed with SARS-COV-2 infection) who admitted to the emergency department of the Pamukkale University Hospital with symptoms of pneumonia and SARS-COV-2 infection were included in the study. A total of 18 healthy individuals without any known chronic or acute disease and drug use were included as healthy control group. Informed consent was waived, and researchers analyzed only deidentified (anonymized) data.
Specimen collection and testing. Blood samples and biochemical parameters. Blood samples were drawn from the antecubital vein of each individual at the time of their attendance to the Emergency Clinic for biochemical analysis. Blood samples were collected to test tubes containing ethylenediamine-tetraacetic acid, test tubes containing citrate and test tubes without anticoagulant were centrifuged within 20 min after collection. Serum and plasma samples obtained by centrifugation at 4,000×g for 15 min were utilized for biochemical analysis. Two ml of each serum sample were stored at –80˚C in eppendorf tubes for analysis.
Measurement of CHOP, PERK and GRP78 levels. Serum GRP78 levels were measured using a commercially available enzyme-linked immunosorbent assay (ELISA) kit, (SL2048Hu; SunLong Biotech, Hangzhou, Zhejiang, PR China), according to the manufacturer’s protocol. The detection rate of this kit is 16 pg/ml. Serum CHOP levels were measured using a commercially available ELISA kit (SL2631Hu; Sunlong Biotech, Hangzhou, Zhejiang, PR China), according to the manufacturer’s protocol. The detection rate of this kit is 6 pg/ml. Serum PERK levels were measured using a commercially available ELISA kit (MyBioSource.com, MBS014568, USA), according to the manufacturer’s protocol. The detection rate of this kit is 18.75 pg/ml (10).
Data analysis. The statistical Package for Social Science program (SPSS for Windows, version 17.0; SPSS, Chicago, IL, USA) was used for analyzing differences between three groups. The Chi-square test was used to compare gender distribution of the groups. When parametric test assumptions were not provided, the Kruskal Wallis test was used to compare between three independent groups. Mann-Whitney U-test was used to compare independent subgroup differences. All the analyses were evaluated statistically significant at p<0.05.
Results
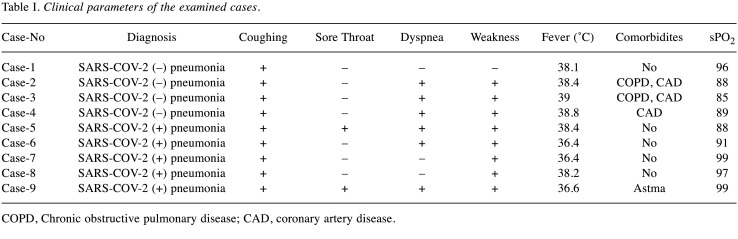
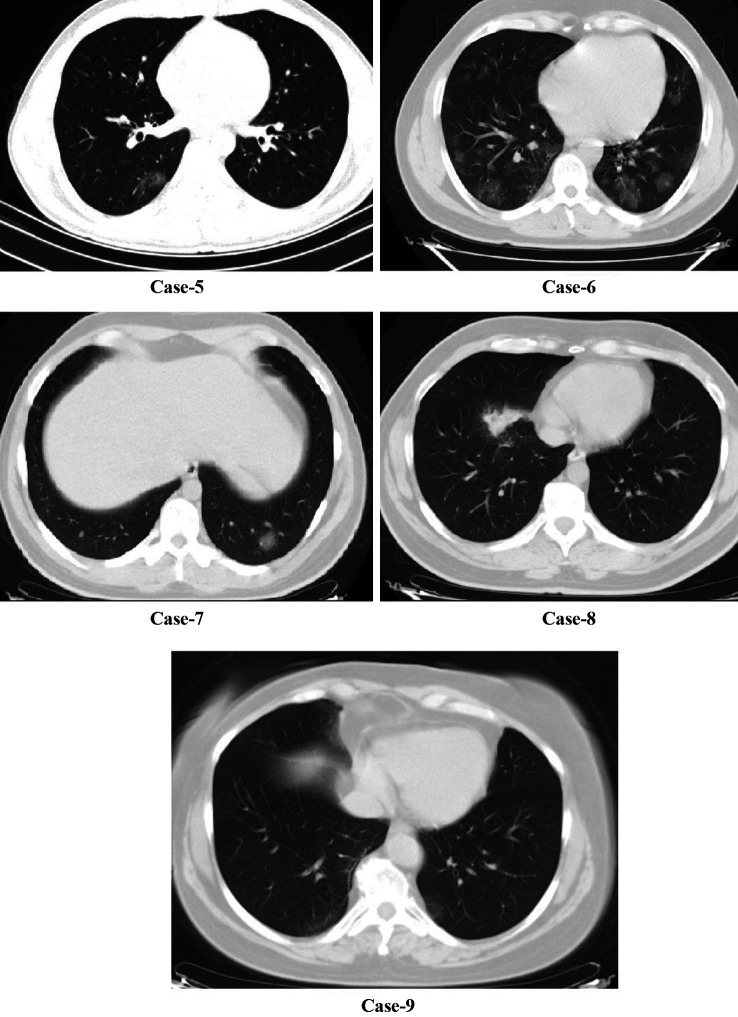
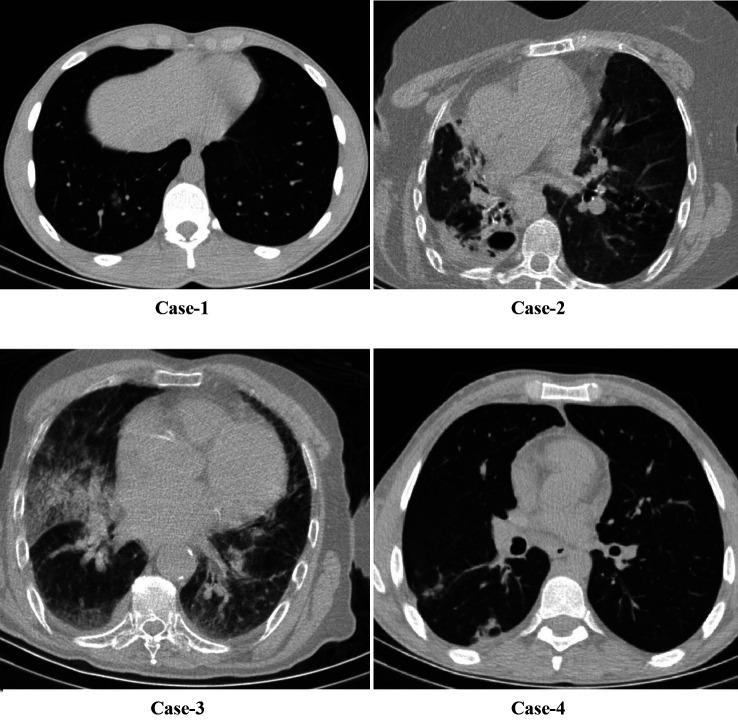
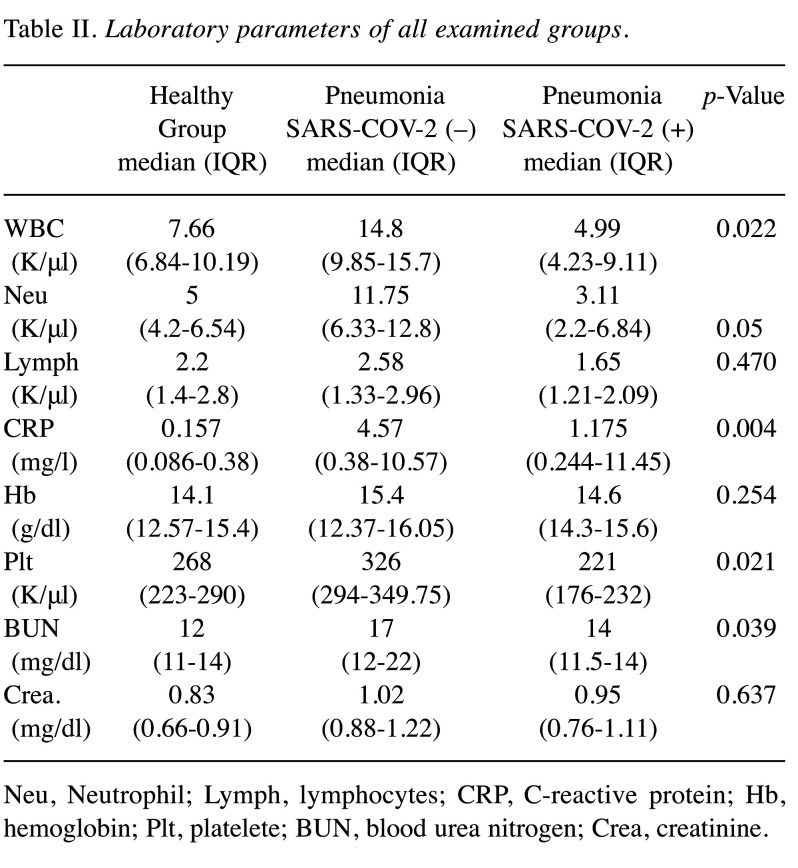
All patients had cough complaints. A total of 8 (88.8%) patients had weakness, 6 (66.6%) patients had dyspnea, and 2 (22%) had sore throat. Body temperature was above 38˚C in all SARS-COV-2-negative pneumonia patients, but body temperature was above 38˚C in 2 patients with SARS-COV-2-positive pneumonia (Table I). Age and gender distribution of all groups were similar (p=0.313 and p=0.443). Considering CT images, only 1 of SARS-COV-2-positive patients had bilateral multiple ground glass opacities. The other patients only a ground-glass appearance at one point (Figure 1). In SARS-COV-2-negative pneumonia cases, 2 patients had multilober ground glass areas, while 2 patients had ground glass areas in one lobe (Figure 2). In the SARS-COV-2-positive pneumonia group, white blood cell (WBC) and platelet levels were found statistically significantly lower (p=0.022 and p=0.004). Blood platelet level and serum CRP level median value were significantly higher in SARS-COV-2-negative pneumonia cases (p=0.039) (Table II).
Table I. Clinical parameters of the examined cases.
COPD, Chronic obstructive pulmonary disease; CAD, coronary artery disease.
Figure 1. Radiological images of SARS-COV-2-positive pneumonia cases.
Figure 2. Radiological images of SARS-COV-2 -negative pneumonia cases.
Table II. Laboratory parameters of all examined groups.
Neu, Neutrophil; Lymph, lymphocytes; CRP, C-reactive protein; Hb, hemoglobin; Plt, platelete; BUN, blood urea nitrogen; Crea, creatinine.
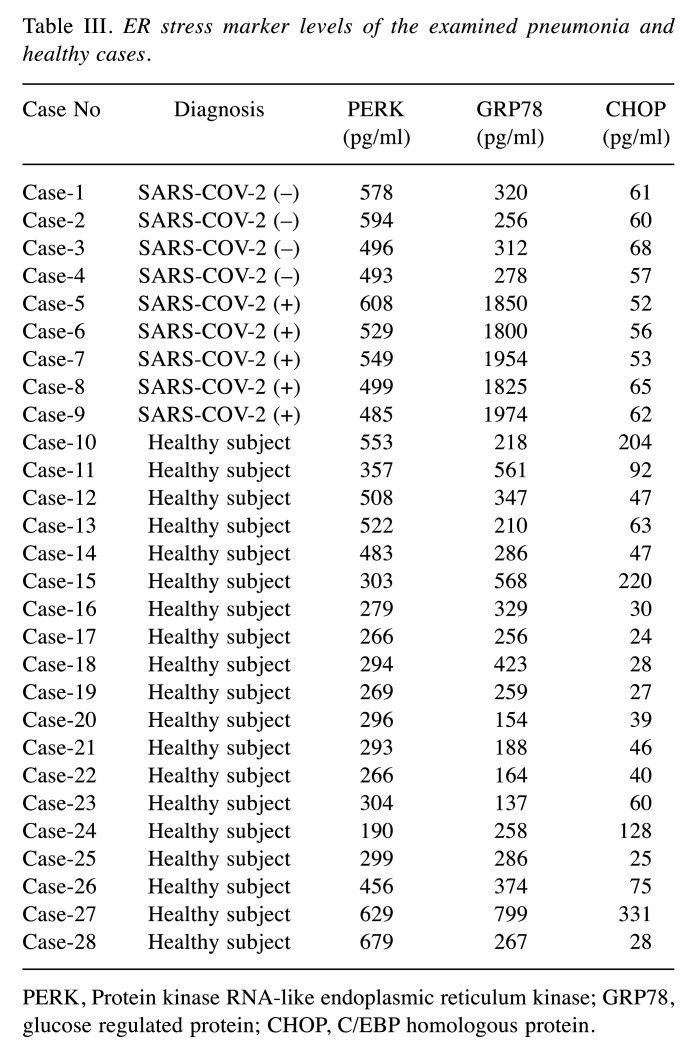
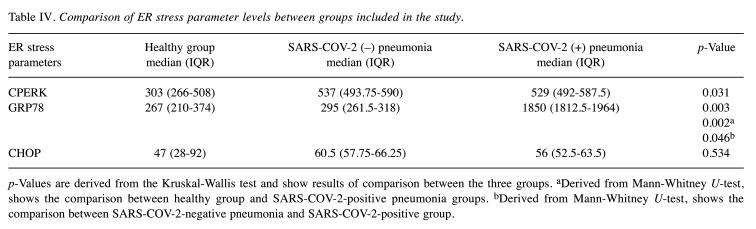
Considering serum ER stress markers, GRP78 levels were found to be significantly higher in SARS-COV-2-positive cases compared to individuals in the other groups (Table III). Serum GRP-78 level median value was statistically significantly higher in the SARS-COV-2-positive group compared to the SARS-COV-2 negative and control groups (p=0.0003). Serum PERK levels were statistically significantly higher in SARS-COV-2-positive pneumonia cases (p=0.046) compared to the control group (Table IV).
Table III. ER stress marker levels of the examined pneumonia and healthy cases.
PERK, Protein kinase RNA-like endoplasmic reticulum kinase; GRP78, glucose regulated protein; CHOP, C/EBP homologous protein.
Table IV. Comparison of ER stress parameter levels between groups included in the study.
p-Values are derived from the Kruskal-Wallis test and show results of comparison between the three groups. aDerived from Mann-Whitney U-test, shows the comparison between healthy group and SARS-COV-2-positive pneumonia groups. bDerived from Mann-Whitney U-test, shows the comparison between SARS-COV-2-negative pneumonia and SARS-COV-2-positive group.
Discussion
Treatment of diseases has been an important issue throughout history. For this reason, protection from diseases, diagnosis and treatment have gained importance in parallel with the developments in scientific methods. It is important to reveal the underlying causes of diseases and the effectiveness of the treatments to be applied.
Errors that occur during the maturation of proteins as a result of malfunctions in ER normal function cause the accumulation of proteins in the organelle. Accumulation of proteins triggers ER stress. It has been found that ER stress and response pathways related to ER stress are active in the pathogenesis of diseases (21). As it is known maintaining cellular homeostasis is important for cell survival (22). Viral virulence is determined by successful entrance, replication in the host cell, and release of mature virion. ER stress may arise from the exploitation of the ER membrane, accumulation of misfolded proteins, depletion of the ER membrane during virion release.
Many positive-strand RNA viruses cause the rearrangement of host intracellular membrane compartments that house replication complexes. ER, trans-Golgi, or lysosomes are the likely origin of virally induced membranes (23). In a in vitro study, it was determined that expression of the coronovirus spike proteins induce ER stress and also trigger the immune response (24). In a recent study, molecular docking results suggests the possible recognition of the SARS-COV-2 spike by the cell-surface GRP78 in the cell stress (25).
Herein in our case control study, higher serum GRP78 concentrations were found in the SARS-COV-2 infected patients compared to patients with pneumonia and the control group. No significant differences were found in serum CHOP concentrations between all the groups. Since CHOP is a protein that plays a role in apoptosis mechanism rather than acute inflammatory processes (21), it was not found at different levels in both SARS-COV-2 (+) and SARS-COV-2 (–) pneumonia cases compared to the healthy group. If the issue was a chronic inflammatory process and prolonged ER stress, we could also expect a high CHOP concentration.
As expected, there are certain limitations to our study. There is no clear literature data on levels of serum ER stress markers serum level at the time of symptom onset, which was different from symptoms in patients presenting with SARS-COV-2 infection. An association was shown between GRP78 and SARS-COV-2 infection. Although a small number of patients was investigated, these results will be important and guide future treatments of SARS-COV-2.
Conflicts of Interest
The Authors declare that they have no conflicts of interest.
Authors’ Contributions
Conception: Aylin Koseler. Study design: Ramazan Sabirli, Aylin Koseler. Funding: Aylin Koseler. Materials: Tarik Goren and Ibrahim Turkcuer. Data collection and processing: Tarik Goren, Özgür Kurt. Literature Review: Ramazan Sabirli, Ibrahim Turkcuer and Tarik Goren. Composition: Ramazan Sabirli and Aylin Koseler. Clinical Review: Ibrahim Turkcuer, Özgür Kurt.
References
- 1.Masters PS. The molecular biology of coronaviruses. Adv Virus Res. 2006;66:193–292. doi: 10.1016/S0065-3527(06)66005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuo L, Godeke GJ, Raamsman MJ, Masters PS, Rottier PJ. Retargeting of coronavirus by substitution of the spike glycoprotein ectodomain: crossing the host cell species barrier. J Virol. 2000;74(3):1393–1406. doi: 10.1128/jvi.741393-1406.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamada Y, Liu XB, Fang SG, Tay FP, Liu DX. Acquisition of cell-cell fusion activity by amino acid substitutions in spike protein determines the infectivity of a coronavirus in cultured cells. PLoS One. 2009;4(7):e6130. doi: 10.1371/journal.pone.0006130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cnop M, Foufelle F, Velloso LA. Endoplasmic reticulum stress, obesity and diabetes. Trends Mol Med. 2012;18(1):59–68. doi: 10.1016/j.molmed.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 5.Zhang K, Kaufman RJ. Signaling the unfolded protein response from the endoplasmic reticulum. J Biol Chem. 2004;279(25):25935–25938. doi: 10.1074/jbc.R400008200. [DOI] [PubMed] [Google Scholar]
- 6.Kincaid MM, Cooper AA. ERADicate ER stress or die trying. Antioxid Redox Signal. 2007;9(12):2373–2387. doi: 10.1089/ars.2007.1817.. [DOI] [PubMed] [Google Scholar]
- 7.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8(7):519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 8.Nath D, Shadan S. The ubiquitin system. Nature. 2009;458(7237):421. doi: 10.1038/458421a. [DOI] [PubMed] [Google Scholar]
- 9.Nishikawa S, Brodsky JL, Nakatsukasa K. Roles of molecular chaperones in endoplasmic reticulum (ER) quality control and ER-associated degradation (ERAD) J Biochem. 2005;137(5):551–555. doi: 10.1093/jb/mvi068. [DOI] [PubMed] [Google Scholar]
- 10.Lai E, Teodoro T, Volchuk A. Endoplasmic reticulum stress: signaling the unfolded protein response. Physiology (Bethesda) 2007;22:193–201. doi: 10.1152/physiol.00050.2006. [DOI] [PubMed] [Google Scholar]
- 11.Nakka VP, Prakash-Babu P, Vemuganti R. Crosstalk between endoplasmic reticulum stress, oxidative stress, and autophagy: Potential therapeutic targets for acute CNS injuries. Mol Neurobiol. 2016;53(1):532–544. doi: 10.1007/s12035-014-9029-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Riehle C, Bauersachs J. Key inflammatory mechanisms underlying heart failure. Herz. 2019;44(2):96–106. doi: 10.1007/s00059-019-4785-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keith M, Geranmayegan A, Sole MJ, Kurian R, Robinson A, Omran AS, Jeejeebhoy KN. Increased oxidative stress in patients with congestive heart failure. J Am Coll Cardiol. 1998;31(6):1352–1356. doi: 10.1016/S0735-1097(98)00101-6. [DOI] [PubMed] [Google Scholar]
- 14.Liu X, Kwak D, Lu Z, Xu X, Fassett J, Wang H, Wei Y, Cavener DR, Hu X, Hall J, Bache RJ, Chen Y. Endoplasmic reticulum stress sensor protein kinase R-like endoplasmic reticulum kinase (PERK) protects against pressure overload-induced heart failure and lung remodeling. Hypertension. 2014;64(4):738–744. doi: 10.1161/HYPERTENSIONAHA.114.03811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee AS. The ER chaperone and signaling regulator GRP78/BiP as a monitor of endoplasmic reticulum stress. Methods. 2005;35(4):373–381. doi: 10.1016/j.ymeth.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 16.Li J, Lee AS. Stress induction of GRP78/BiP and its role in cancer. Curr Mol Med. 2006;6(1):45–54. doi: 10.2174/156652406775574523. [DOI] [PubMed] [Google Scholar]
- 17.Rao RV, Peel A, Logvinova A, del Rio G, Hermel E, Yokota T, Goldsmith PC, Ellerby LM, Ellerby HM, Bredesen DE. Coupling endoplasmic reticulum stress to the cell death program: role of the ER chaperone GRP78. FEBS Lett. 2002;514(2-3):122–128. doi: 10.1016/s0014-5793(02)02289-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ibrahim IM, Abdelmalek DH, Elfiky AA. GRP78: A cell's response to stress. Life Sci. 2019;226:156–163. doi: 10.1016/j.lfs.2019.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shen J, Chen X, Hendershot L, Prywes R. ER stress regulation of ATF6 localization by dissociation of BiP/GRP78 binding and unmasking of Golgi localization signals. Devel Cell. 2002;3(1):99–111. doi: 10.1016/s1534-5807(02)00203-4. [DOI] [PubMed] [Google Scholar]
- 20.Li Y, Guo Y, Tang J, Jiang J, Chen Z. New insights into the roles of CHOP-induced apoptosis in ER stress. Acta Biochim Biophys Sin (Shanghai) 2014;46(8):629–640. doi: 10.1093/abbs/gmu048. [DOI] [PubMed] [Google Scholar]
- 21.Sabirli R, Koseler A, Mansur N, Zeytunluoglu A, Tukenmez Sabirli G, Turkcuer I, Kilic ID. Predictive value of endoplasmic reticulum stress markers in low ejection fractional heart failure. In Vivo. 2019;33(5):1581–1592. doi: 10.21873/invivo.11640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levine B, Klionsky DJ. Development byself digestion: molecular mechanisms and biological functions of autophagy. Dev Cell. 2004;6(4):463–477. doi: 10.1016/s1534-5807(04)00099-1. [DOI] [PubMed] [Google Scholar]
- 23.Miller S, Krijnse-Locker J. Modification of intracellular membrane structures for virus replication. Nat Rev Microbiol. 2008;6(5):363–374. doi: 10.1038/nrmicro1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Versteeg GA, van de Nes PS, Bredenbeek PJ, Spaan WJ. The coronavirus spike protein ınduces endoplasmic reticulum stress and upregulation of ıntracellular chemokine mRNA concentrations. J Virol. 2007;81(20):10981–10990. doi: 10.1128/JVI.01033-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ibrahim IM, Abdelmalek DH, Elshahat ME, Elfiky AA. COVID-19 Spikespike-host cell receptor GRP78 binding site prediction. J Infect. 2020 doi: 10.1016/j.jinf.2020.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]