Abstract
Adequate drinking water is essential to maintain acceptable production levels in beef cattle operations. In the context of global climate change, the water scarcity forecasted for the future is a growing concern and it would determine an increase in the use of poorer quality water by the agricultural sector in many parts of the world. However, consumption of high-salt water by cattle has consequences often overlooked. A meta-analysis was carried out to assess the impact of utilizing high-salt water on dry matter (DMI) and water intake (WI), and performance in beef cattle. The dataset was collected from 25 studies, which were conducted between 1960 and 2020. Within the dataset, the water quality was divided into three categories according to the ratio of sulfates (SO4) or sodium chloride (NaCl) to total dissolved solids (TDS): 1) TDS = all studies included (average SO4:TDS = 0.4); 2) NaCl = considered studies in which water salinity was dominated by NaCl (average SO4:TDS = 0.1); and 3) SO4 = considered studies in which water salinity was dominated by SO4 (average SO4:TDS = 0.8). Results showed that DMI and WI were negatively affected by high-salt water consumption, although the magnitude of the effect is dependent on the type of salt dissolved in the water. There was a quadratic effect (P < 0.01) for the WI vs. TDS, WI vs. NaCl, DMI vs. TDS, and DMI vs. NaCl, and a linear effect (P < 0.01) for WI vs. SO4 and WI vs. SO4. Average daily gain (ADG) and feed efficiency (FE) were quadratically (P < 0.01) affected by high-salt water, respectively. This study revealed significant negative effects of high-salt water drinking on beef cattle WI, DMI, and performance. However, the negative effects are exacerbated when cattle drink high-sulfate water when compared with high-chloride water. To the best of our knowledge, this is the first approach to evaluate animal response to high-salt water consumption and could be included in the development of future beef cattle models to account for the impact of water quality on intake and performance. In addition, this meta-analysis highlights the need for research on management strategies to mitigate the negative effects of high-salt water in cattle.
Keywords: intake, meta-analysis, performance, ruminant, sulfate, water quality
Introduction
It is predicted that in the coming years the world population will increase significantly (FAO, 2011), leading to an increase in food demand of nearly 70% over the next 40 yr (Molden et al., 2007). To sustain this growth, the finite natural resources (water and land) that support primary food production are going to be under increasing pressure. In this scenario, the agricultural sector must face the challenge of increasing food production with resources that are increasingly scarce and vulnerable to the processes of degradation and climate change (Behnassi et al., 2014; Jiménez Cisneros et al., 2014).
Ruminants play a vital role in meeting the demand for food, due to their ability to transform resources of low nutritional value (e.g., forage and agroindustrial byproducts) into animal protein of high biological value for humans. However, even though livestock products provide a third of the protein consumed around the world, it is at the cost of using a third of the water that agriculture uses worldwide (Herrero et al., 2009).
In the future, animal production sustainability will depend to a large extent on the efficient administration of water resources due to global climate change increases water scarcity in quantity and quality (Legesse et al., 2017). Consequently, with an increase in the demand for animal products, improving the water productivity for livestock use is crucial (Descheemaeker et al., 2010).
After oxygen, water is considered the most important nutrient for the growth, digestion, metabolism, lactation, and reproduction of ruminants (NASEM, 2016). In mammals, about 99% of all the body’s molecules are composed of water (Macfarlane and Howard, 1972). Since food and water consumption are directly related (Utley et al., 1970; Bond et al., 1975), adequate supply of good quality water is essential to avoid detrimental effects on animal health and productivity (Meyer et al., 2004; Kume et al., 2010). However, the water scarcity forecasted for the future would determine an increase in the use of low-quality water (e.g., high-salt water) for livestock production.
Water quality is a general term used to describe the physical, bacteriological, and chemical properties that determine the aptitude of a drinking water source for a given purpose. In the case of water intended for animal consumption, excess salts (mainly chlorides and sulfates [SO4]) often limit animal production in different parts of the world. At present, the effort directed to the development of basic research for the generation of knowledge on ways and mechanisms to allow a more efficient use of high salt water is scarce, possibly due to the fact that it has traditionally been considered a resource of little value, easily available and renewable (Brew et al., 2011; NASEM, 2016).
In the context of the background described, the objective of this review was to conduct a meta-analysis of the relationships among types and increasing level of dissolved salts in drinking water and voluntary feed and water intake as well as animal performance in beef cattle. To the best of our knowledge, this study represents the first attempt to review and generalize the effects of high-salt water on intake and performance in beef cattle by a meta-analytical evaluation of current published research.
Materials and Methods
Data collection
We conducted a systematic scientific literature search to collect a dataset for evaluating the effects of high-salt water consumption on dry matter intake (DMI), water intake (WI), average daily gain (ADG), and feed efficiency (FE) in beef cattle. The search was limited to in vivo studies with cattle. The studies were identified using electronic research databases of published literature (Scopus, Google Scholar, and Science Direct). Examples of keywords used in combination were “cattle,” “ruminant,” “steer,” “cow,” “saline water,” “salt water,” “sulfate,” “sulfur,” “sodium chloride,” “total dissolved solid,” “intake,” “performance,” and “gain.” The criteria for each study to be incorporated in the dataset were that at least one of the variables concerned was reported along with a detailed description of animal, diet formulation, water quality, and statistical design. When it was not reported, composition of diet ingredients was estimated from NASEM (2016). A total of 25 studies published from 1960 to 2020 were selected for this meta-analysis (Supplementary Appendix). Details of the specific studies are presented in Table 1.
Table 1.
General review of the studies used in the meta-analysis to evaluate the effects of high-salt water in the performance of beef cattle
| Study | Country | Experimental design | Diet composition | Forage level in diet, % | Water Source1 | Water Quality, g/L2 |
Category | Breed | Initial BW3, kg |
|---|---|---|---|---|---|---|---|---|---|
| Weeth et al., 1960 | USA | Change—Over | Chopped alfalfa hay | 100 | A | 0.1 to 20.0 | Heifer | Hereford | 218 |
| Weeth and Haverland, 1961 | USA | Change—Over | Chopped alfalfa hay | 100 | A | 12.6 to 17.6 | Heifer | Hereford | 185 |
| Weeth and Lesperance, 1965 | USA | Change—Over | Grass hay | 100 | A | 0.1 to 10.0 | Heifer | Hereford | 232 |
| Weeth et al., 1968 | USA | Latin square | Grass hay | 100 | A | 0.2 to 6.7 | Heifer | Hereford | 220 to 253 |
| Weeth and Hunter, 1971 | USA | Latin square | Mixed grass hay | 100 | A | 0.1 to 6.0 | Heifer | Hereford | 256 |
| Weeth and Capps, 1972 | USA | Latin square | Mixed grass hay | 100 | A | 0.1 to 2.8 | Heifer | Hereford | 240 |
| Digesti and Weeth, 1976 | USA | Split-plot | Grass hay, protein pellet | 86 | A | 0.4 to 2.8 | Heifer | Hereford × Angus | 165 |
| Dixon and Milligan, 1983 | Canada | Latin square | Mature grass hay | 100 | A | 1.5 to 10.0 | Steer | Charolais crossbred | 309 to 343 |
| Saul and Flinn, 1985 | Australia | Completely ranzomized | Pasture hay | 100 | A | 0.2 to 11.0 | Heifer | Hereford | 223 |
| Ray, 1989 | USA | Completely ranzomized | Ground alfalfa hay, dry-rolled wheat, molasses | 50 to 100 | A | 1.3 to 6.0 | Steer | Crossbred | 170 |
| Katting et al., 1992 | USA | Split plot | Alfalfa hay, mature grass hay | 100 | A | 0.4 to 2.3 | Steer | Holstein | 234 |
| Sager and Casagrande, 1998 | Argentina | Completely ranzomized | Alfalfa hay, grass hay | 100 | N | 1.6 to 5.5 | Calf | Angus | 106 |
| Loneragan et al., 2001 | USA | Randomized block | Steam-flaked corn, alfalfa haylage, wheat midds, corn silage | 17 | N | 0.1 to 2.0 | Steer | Crossbred | 304 |
| Patterson et al., 2002 | USA | Randomized block | Grass hay, wheat middlings | 53 | N | 1 to 6.2 | Steer | Crossbred | 317 |
| Patterson et al., 2003 | USA | Randomized block | Grass hay, wheat middlings | 55 | N | 1.2 to 7.3 | Steer | Crossbred | 290 |
| Zimmerman, 2003 | Canada | Latin square | Orchardgrass hay | 100 | A | 0 to 2.9 | Heifer | Angus | 372 |
| Ward and Patterson, 2004 | USA | Randomized block | Grass hay, wheat middlings | 55 | A | 0.4 to 3.8 | Steer | Crossbred | 334 |
| Johnson et al., 2004 | USA | Randomized block | Warm-season shortgrasses, cool-season midgrasses | 100 | N | 1.0 to 7.3 | Steer | Crossbred | 285 |
| Grout et al., 2006 | Canada | Latin square | Orchardgrass hay | 100 | A | 1.8 to 4.8 | Heifer | Angus | 345 |
| Cammack et al., 2010 | USA | Randomized block | Chopped crested wheatgrass, wheat middlings pellet | 50 | A | 0.6 to 3.6 | Steer | Crossbred | 318 |
| Sexson et al., 2010 | USA | Completely ranzomized | Corn silage, wheat silage, steam flaked corn, alfalfa hay | 10 | N | 0.8 to 2.5 | Steer | Crossbred | 339 |
| Visscher et al., 2013 | Germany | Latin square | Hay cobs, hay, barley, soybean meal | 70 | A | 0.1 to 10.0 | Bull | Holstein-Friesian | 219 |
| López et al., 2014 | Argentina | Change—Over | Tropical grass hay, soybean meal | 75 to 100 | A | 0.8 to 6.5 | Steer | Braford crossbred | 375 |
| Alves et al., 2017 | Brazil | Completely randomized | Buffel grass hay, soybean meal, ground maize | 50 | A | 0.6 to 8.3 | Heifer | Red Sindhi | 200 |
| Penner et al., 2020 | Canada | Randomized block | Barley silage, barley grain, wheat screening pellet, pea straw | 63 | A | 0.8 to 5.6 | Heifer | Hereford crossbred | 445 |
1A, water source artificially prepared by adding salt (NaCl, MgCl2, CaCl2, Na2SO4, K2SO4, MgSO4) to tap water; N, natural water (ground, well or dam) source.
2TDS, total dissolved solids.
3BW, body weight.
There was a wide range of management, season, cattle characteristics (breed, body weight [BW], and sex), diet formulation, and water quality used among the studies within the dataset. However, only water quality was considered for the meta-analysis because of the reduced numbers of treatments for any other variable of classification. The number of means used in the meta-analysis was not the same for each response variable because not all studies reported all variables (Table 2). Within the dataset, the water quality was divided into three categories according to the ratio of SO4 or sodium chloride (NaCl) to total dissolved solids (TDS): 1) TDS = all studies included (average SO4:TDS = 0.4); 2) NaCl = considered studies in which water salinity was predominantly dominated by NaCl (average SO4:TDS = 0.1); and 3) SO4 = considered studies in which water salinity was predominantly dominated by SO4 (average SO4:TDS = 0.8).
Table 2.
Summary statistics of dry matter (DMI) and water (WI) intake, average daily gain (ADG), and feed efficiency (FE) data used in the meta-analysis to evaluate the effects of high-salt water in the performance of beef cattle
| Item1 | Mean | SD2 | Minimum | Maximum | n 3 |
|---|---|---|---|---|---|
| DMI, g/kg BW0,75 | 97.4 | 27.1 | 30.4 | 148.3 | 83 |
| TDS | 4.3 | 4.2 | 0.1 | 20.1 | |
| SO4:TDS | 0.4 | 0.4 | 0.0 | 1.0 | |
| Animal BW, kg | 261.4 | 85.1 | 106.0 | 494.0 | |
| DMI, g/kg BW0,75 | 92.0 | 24.8 | 30.4 | 145.7 | 47 |
| NaCl | 5.4 | 5.0 | 0.1 | 20.1 | |
| SO4:TDS | 0.1 | 0.1 | 0.0 | 0.4 | |
| Animal BW, kg | 252.4 | 87.7 | 165.0 | 494.0 | |
| DMI, g/kg BW0,75 | 104.5 | 28.6 | 33.0 | 148.3 | 36 |
| SO4 | 3.0 | 2.2 | 0.1 | 7.3 | |
| SO4:TDS | 0.7 | 0.2 | 0.4 | 1.0 | |
| Animal BW, kg | 290.0 | 96.2 | 106.0 | 494.0 | |
| WI, g/kg BW0,75 | 465.7 | 153.7 | 156.0 | 904.6 | 105 |
| TDS | 4.1 | 4.0 | 0.0 | 20.1 | |
| SO4:TDS | 0.4 | 0.4 | 0.0 | 1.0 | |
| Animal BW, kg | 271.1 | 81.8 | 106.0 | 494.0 | |
| WI, g/kg BW0,75 | 480.8 | 165.9 | 171.8 | 904.6 | 53 |
| NaCl | 5.2 | 5.0 | 0.0 | 20.1 | |
| SO4:TDS | 0.1 | 0.1 | 0.0 | 0.4 | |
| Animal BW, kg | 263.8 | 83.6 | 165.0 | 494.0 | |
| WI, g/kg BW0,75 | 450.3 | 140.0 | 156.0 | 722.9 | 52 |
| SO4 | 3.0 | 2.1 | 0.0 | 7.3 | |
| SO4:TDS | 0.8 | 50.2 | 0.4 | 1.0 | |
| Animal BW, kg | 298.3 | 89.3 | 106.0 | 494.0 | |
| ADG, g/kg BW0,75 | 10.1 | 11.1 | −43.0 | 29.7 | 59 |
| TDS | 4.8 | 4.6 | 0.1 | 20.1 | |
| SO4:TDS | 0.4 | 0.4 | 0.0 | 1.0 | |
| Animal BW, kg | 267.2 | 80.8 | 165.0 | 494.0 | |
| FE, kg gain/kg DMI | 0.1 | 0.2 | −1.4 | 0.2 | 56 |
| TDS | 4.3 | 4.1 | 0.1 | 20.1 | |
| SO4:TDS | 0.5 | 0.4 | 0.0 | 1.0 | |
| Animal BW, kg | 270.3 | 82.4 | 165.0 | 494.0 |
1TDS, total dissolved solids; SO4:TDS, ratio of sulfates to total dissolved solids; BW, body weight.
2SD, standard deviation.
3n, number of data points included.
Prior to the analysis, feed and water intake data reported in the studies as kg/d, L/d, and % of BW were converted to be expressed as a common unit (g/kg BW0.75). The concentration of salt in the water was expressed as g/L of TDS. In some studies, FE (kg gain/ kg DMI) were calculated as the ratio between BW gain and dry matter intake.
Statistical analyses
All data were analyzed by the Mixed procedure of SAS (SAS Inst. Inc., Cary, NC), using the meta-analytical techniques recommended by St-Pierre (2001) and Sauvant et al. (2008). The following full model was used for the analysis:
where yij is the response variable for the jth observation of the ith study, B 0 is the overall intercept, B 1 is the overall regression coefficient of Y on TDS, B 2 is the overall quadratic regression coefficient of Y on TDS, TDSij is the total dissolved solids in water for the jth observation of the ith study, β i is the random effect of study on the regression coefficient of Y on TDS, si is the random effect of the ith study, and ε ij is the residual error. Thus, B 0 + B 1TDSij + B 2TDS2ij is the fixed effect part of the model, and β iTDSij + si + ε ij is the random effect part of the model. The variance–covariance matrix of the random factors was declared as variance components.
For all statistical analyses, the number of replicates (animals or pens) in each experiment was used to weight the means (Jayanegara et al., 2012). The WEIGHT statement of PROC MIXED was used to include the weighting variable in the model.
To determine the best fit for the relationship between each dependent variable and TDS, SO4 or NaCl concentration, the bias-corrected Akaike’s information (AICC) and Bayesian’s information criteria (BIC) were used. The equations with the smallest AICC and BIC were chosen to describe the relationship between the variables. Another criterion used to select models was the significance of the parameters. For graphical representation of meta-analysis results, observations were adjusted to take into account the random effect of study before being plotted, as suggested by St-Pierre (2001).
Results and Discussion
A description of the dataset utilized in the analysis is shown in Table 2. Water quality reported covered a broad range of TDS, well above the maximum thresholds recommended (Table 3). Figure 1 shows the frequency distribution of TDS and SO4 contents for the meta-analytical dataset. Although a high frequency of observations for TDS and SO4 contents is in the range of 0.5 to 6 and 0 to 1, respectively, the distribution of the levels suggests that the dataset used allows representing the potential response on intake and animal performance to high levels of salt in water commonly found in beef cattle systems.
Table 3.
Maximum recommended values for total dissolved solids (TDS), sodium chloride (NaCl), and sulfates (SO4) in beef cattle drinking water
| Item | g/L | Comments |
|---|---|---|
| TDS | 7.0 | These waters should not be fed to cattle. Health problems and/or poor production will results |
| > 10.0 | Unsafe. Should not be used as a source of water | |
| NaCl | 7.0–10.0 | Water intake increased. Reduced animal growth |
| 20.0 | Toxic. Severe anorexia, weight loss, lethargy and physical collapse | |
| SO4 | 2.5 | Cattle can tolerate these water for short periods. Performance of grazing cattle may not be affected |
| > 4.0 | Unsafe. Not recommended for use under any conditions |
1Adapted from NRC (2005) and NASEM (2016).
Figure 1.
Frequency plot shows the distribution of (a) total dissolved solids (TDS) and (b) sulfates (SO4) contents in water in the dataset. The dashed lines indicate the maximum safe concentration of TDS and SO4 for all classes of beef cattle (NASEM, 2016). n = number of data points included; SD = standard deviation.
The chemical characteristics of the diets are shown in Table 4. There were large differences between minimum and maximum values of neutral detergent fiber (NDF) and crude protein (CP) concentration, which is explained by the forage-based diets used in most studies. However, only two studies reported a level of forage inclusion below 50% of the total diet DM.
Table 4.
Summary statistics of dietary composition for dry matter intake (DMI), water intake (WI), average daily gain (ADG), and feed efficiency (FE) data used in the meta-analysis to evaluate the effects of high-salt water in the performance of beef cattle
| Item1 | Mean | SD2 | Minimum | Maximum | n 3 |
|---|---|---|---|---|---|
| DMI | 26 | ||||
| DM, % | 87.59 | 9.6 | 48.90 | 94.90 | |
| CP, % | 12.92 | 2.9 | 7.00 | 17.00 | |
| NDF, % | 54.26 | 20.6 | 13.97 | 81.80 | |
| WI | 32 | ||||
| DM, % | 85.95 | 14.7 | 25.00 | 96.20 | |
| CP, % | 12.73 | 2.8 | 7.00 | 17.00 | |
| NDF, % | 55.46 | 18.8 | 13.97 | 81.80 | |
| ADG | 16 | ||||
| DM, % | 82.01 | 19.0 | 25.00 | 94.90 | |
| CP, % | 13.85 | 1.9 | 10.58 | 17.00 | |
| NDF, % | 45.30 | 19.2 | 13.97 | 67.60 | |
| FE | 16 | ||||
| DM, % | 86.07 | 11.4 | 48.90 | 94.90 | |
| CP, % | 13.74 | 2.0 | 10.58 | 17.00 | |
| NDF, % | 44.38 | 19.0 | 13.97 | 67.60 |
1DM, dry matter; CP, crude protein; NDF, neutral detergent fiber.
2SD, standard deviation.
3n, number of data points included.
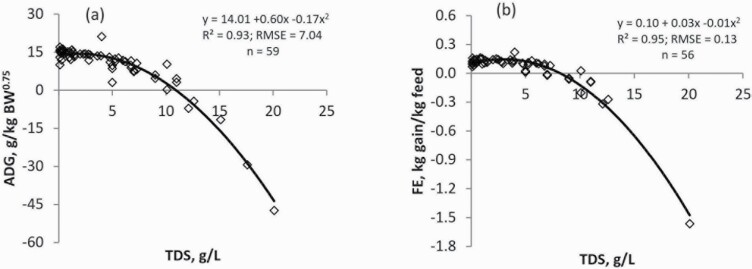
Figures 2 and 3 show the adjusted observations and Table 5 shows the equations for evaluating the effects of high-salt water consumption on the analyzed response variables. All equations were selected based on their goodness of fit and showed a quadratic effect (P < 0.01), with the exception of DMI vs. SO4, WI vs. SO4, which showed a significant linear effect (P < 0.01).
Figure 2.
Influence of high-salt water on dry matter intake (DMI) and water intake (WI) in beef cattle using equations from the meta-analytical results: (a) DMI vs. TDS, (b) DMI vs. NaCl, (c) DMI vs. SO4, (d) WI vs. TDS, (e) WI vs. NaCl, and (f) WI vs. SO4. TDS = total dissolved solids, NaCl= sodium chloride, SO4 = sulfates.
Figure 3.
Influence of high-salt water on average daily gain (ADG) and feed efficiency (FE) in beef cattle using equations from the meta-analytical results: (a) ADG vs. TDS and (b) FE vs TDS. TDS = total dissolved solids.
Table 5.
Terms and significance for each equation that best fit dry matter intake (DMI) and water intake (WI), average daily gain (ADG), and feed efficiency (FE) from total dissolved solids (TDS) in drinking water in beef cattle
| Itema | Parameter estimatesb | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intercept | SEintercept | P-value | X | SEx | P-value | X2 | SEx2 | P-value | AICC | BIC | RMSE | R 2 | |
| Equations for DMI, g/kg BW0.75 | |||||||||||||
| DMI vs. TDS | 110.82 | 5.17 | <0.01 | −0.48 | 0.78 | 0.54 | −0.21 | 0.05 | <0.01 | 688.0 | 689.8 | 22.37 | 0.75 |
| DMI vs. NaCl | 105.67 | 7.10 | <0.01 | 0.85 | 0.85 | 0.35 | −0.28 | 0.05 | <0.01 | 383.7 | 384.5 | 18.80 | 0.86 |
| DMI vs. SO4 | 118.29 | 6.71 | <0.01 | −4.68 | 1.22 | <0.01 | 303.4 | 304.1 | 24.80 | 0.52 | |||
| Equations for WI, g/kg BW0.75 | |||||||||||||
| WI vs. TDS | 474.53 | 29.51 | <0.01 | 12.53 | 8.38 | 0.15 | −1.75 | 0.66 | 0.01 | 1322.3 | 1325.7 | 226.49 | 0.33 |
| WI vs. NaCl | 441.09 | 40.14 | <0.01 | 34.44 | 10.95 | <0.01 | −2.36 | 0.68 | 0.01 | 674.0 | 675.5 | 288.10 | 0.26 |
| WI vs. SO4 | 545.08 | 36.20 | <0.01 | −25.81 | 6.23 | <0.01 | 616.1 | 617.4 | 162.10 | 0.42 | |||
| Equation for ADG, g/kg BW0.75 | |||||||||||||
| ADG vs. TDS | 14.01 | 1.81 | <0.01 | 0.60 | 0.38 | 0.14 | −0.17 | 0.03 | <0.01 | 375.2 | 376.9 | 7.04 | 0.93 |
| Equation for FE, kg gain/kg BW0.75 | |||||||||||||
| FE vs. TDS | 0.10 | 0.02 | <0.01 | 0.03 | 0.01 | <0.01 | −0.01 | <0.01 | <0.01 | −88.4 | −86.8 | 0.13 | 0.95 |
1TDS, total dissolved solid; NaCl, sodium chloride; SO4, sulfates.
2SE, standard error; AICC, corrected Akaike information criterion; BIC, Bayesian information criterion; RMSE, root mean square error; R2, coefficient of determination.
Although this meta-analysis was conducted to summarize the impact of providing high-salt water on beef cattle performance, it is important to note that WI and DMI are influenced by several factors (i.e., BW, physiological state, diet composition, and environmental conditions). Moreover, the relatively short exposure periods to high-salt water (less than 140 d) of all the analyzed studies are another factor to consider. Longer exposure periods can generate the development of adaptation processes and/or other physiological effects (e.g., secondary mineral deficiencies), which might alter the observed responses of beef cattle consuming high-salt water.
Voluntary feed intake and water quality
Meta-analyses results indicated that DMI was negatively affected by the ingestion of large amounts of salt through drinking water (DMI vs. TDS; P < 0.01), although the magnitude of the effect is dependent on the type of salt dissolved in the water. The quadratic effect (P < 0.01) of DMI vs. NaCl showed that DMI reaches a maximum value (e.g., 106.34 g DM/kg BW0.75 for a steer of 250 kg BW) at a TDS concentration of 1.5 g/L, decreasing at greater TDS concentrations. Contrarily, the linear effect (P < 0.01) of the DMI vs. SO4 showed that DMI decreases from the least to the greatest TDS concentrations. Goodness of fit was not improved (based on AICC and BIC values) when the quadratic effect was included in the equation for DMI vs. SO4.
It is widely recognized that feed intake determines nutrients’ availability to meet the metabolic requirements, thus representing one of the main factors influencing animal production (Mertens, 1994; Allen, 1996; Roche et al., 2008). Crucially, DMI seems to be the first and most affected variable when cattle drink high-salt water, especially with high SO4 concentration. This meta-analysis revealed that water within the range of 6 to 8 g/L TDS reduces DMI by 10% to 15% (DMI vs TDS model), but if SO4 is the main salt dissolved in the water, only 3 g/L are needed to decrease DMI to the same magnitude (DMI vs. SO4 model). Reductions greater than 30% in DMI are expected when TDS concentration increases above 12 g/L. The mechanisms by which salts (and especially SO4) in drinking water affect feed intake have not been completely elucidated yet. However, it is known that a systemic hypertonicity can affect the brain feeding centers that regulate consumption in ruminants (Burgos et al., 2000; Masters et al., 2005; Allen, 2014). In addition, some authors have suggested that hydrogen sulfide (H2S) produced by sulfate-reducing bacteria adversely affects ruminal motility, increasing retention time and ruminal digestibility (Bird, 1972; Kandylis, 1984; Drewnoski et al., 2014) and decreasing feed intake (Loneragan et al., 2001; Uwituze et al., 2011; Drewnoski and Hansen, 2013). Oxidative damage could also explain decreases in DMI when beef cattle drink high-sulfate water, because H2S can induce toxicity by increasing the hepatocyte formation of reactive oxygen species (Truong et al., 2006). Kessler et al. (2013) reported changes in hepatic gene expression involved in inflammatory and immune responses in steers drinking high-sulfate water (3.6 g/L SO4). These authors observed reductions of 9% in DMI compared with control treatment. These results are in agreement with the hepatic oxidation theory, which suggests that food intake is regulated by signals carried from the liver to brain (Allen et al., 2009). In summary, the understanding of how high-salt water consumption affects the complex and multifactorial mechanisms that regulate feed intake is still elusive, but it is critical to develop management decisions to improve DMI when cattle are drinking high-salt water.
Voluntary water intake and water quality
Water intake was affected by water quality similarly to DMI. There was a quadratic effect (P < 0.01) for the WI vs. TDS and WI vs. NaCl, and a linear effect (P < 0.01) for the WI vs. SO4.
The effects of salt on WI had higher variability compared to DMI and were determined by the type of salt in the drinking water. For example, in the range of 6 to 8 g/L TDS, voluntary WI might be increased up to 25% or decreased up to 30% depending on whether soluble salt in the water are mainly chlorides (i.e., NaCl) or SO4 (i.e., Na2SO4), respectively. Although it is known that water and feed consumption are normally strongly related (Ahlberg et al., 2018; Zanetti et al., 2019), some authors have reported that in certain circumstances this relationship can be affected (Arias and Mader, 2011; Sexson et al., 2012). For example, DMI normally decreases in response to increasing levels of salt in the drinking water, whereas WI varies according to the type of salt (Grout et al., 2006); therefore, the relationship between WI and DMI appears to be weak and inconsistent when cattle drink high-salt water. Similar to DMI, the mechanisms by which salts affect WI are not fully understood. However, SO4 salts could reduce the hedonic characteristics of water due to its bitter taste, affecting water consumption (Weeth and Capps, 1972; Digesti and Weeth, 1976; Grout et al., 2006). Goatcher and Church (1970) concluded that cattle are more sensitive to bitter than salty flavors, which may help us to explain why SO4 salts are more rejected at lower concentrations than chloride salts (Weeth and Hunter, 1971). Additionally, SO4 salts may affect WI through H2S toxicity, in a similar way as already discussed for DMI. Sodium chloride, on the other hand, is less toxic than SO4 salts (NRC, 2005; Wright, 2007); thus, a common response observed in cattle drinking high-sodium chloride water is an increase in water consumption and urine output, the later enhancing salt excretion and contributing to maintain osmotic balance (Wilson and Dudzinski, 1973; Katting et al., 1992). The kidney plays a significant role in the process of maintaining water homeostasis (Sands, 2009). The increased WI of cattle drinking high-sodium chloride water may be explained by an elevation in the osmotic pressure of the body water (Fitzsimons, 1998; Lazartigues et al., 2008). In a literature review on the central control of osmotic homeostasis in mammals, Bourque (2008) provided evidence that extracellular fluid hyperosmolality promotes WI mediated by osmoreceptors located at the periphery and in the hypothalamus. However, there is a maximum concentration of salt that can be tolerated in drinking water, which depends largely on the animal’s ability to concentrate urine (Kay, 1997). It has been documented that cattle can tolerate up to 15 g of sodium chloride per liter of drinking water (Weeth et al., 1960). Concentrations above the tolerance threshold outweigh the homeostatic regulatory mechanisms, causing a decrease in WI, preventing excessive salt load in the body. In summary, high-salt WI regulation is a complex function mediated by physiological mechanisms with critical roles on preventing toxicity and maintaining body water homeostasis.
Animal performance and water quality
The meta-analysis of the data showed that water quality negatively affected ADG and FE of cattle. A quadratic (P < 0.01) effect was observed for both relationships (ADG vs. TDS and FE vs. TDS, respectively). According to the equation ADG vs. TDS, the maximum ADG would be achieved within the range 1.5 to 3.0 g/L of TDS in drinking water. Concentrations of TDS greater than 6 g/L produce a marked reduction in ADG. Similarly, the best FE would be within the range 2.0 to 4.0 g/L of TDS, strongly worsening as TDS concentrations increase thereafter. Thus, when TDS concentrations raise above 6 g/L, a decrease in FE greater than 20% would be expected.
As expected, the ADG was positively related to DMI (NASEM, 2016). Based on the negative effects of high-salt water on DMI, decreased performance should be expected in animals drinking high-salt water compared with those under the same production conditions but drinking high quality water. Moreover, the meta-analysis suggests that high-salt water could cause a more negative impact on ADG and FE than in DMI. In ruminants, there is evidence that increases in salt intake decrease energy efficiency (Arieli et al., 1989), due to increases in heat production (Rumpler and Johnson, 1987). It has been indicated that movement of ions (Na+/K+) through membranes accounts for about 20%–30% of the basal energy expenditure of animals (Baldwin et al., 1980; Milligan et al., 1985). High levels of NaCl in the body fluid compartments of sheep increase the maintenance energy expenditure, reducing the productive parameters of wethers (De Waal et al., 1989). Similar results have been reported for other mammal species. For instance, Osaka et al. (2001) reported an increase in whole body energy expenditure in rats due to changes in plasma osmolality following intestinal infusion of a hypertonic solution. Similarly, Coelho et al. (2006) observed increases in energy expenditure and decreases in body weight in rats fed high-salt diets. On the other hand, the ruminal H2S gas produced as a consequence of high-sulfur intake, mainly on high-concentrate diets, can block cytochrome C oxidase, negatively altering the electron transport chain in mitochondria (Beauchamp et al., 1984). Because the mitochondria produce 90% of the cellular ATP, any damage on its metabolism leads to inefficient futile cycles in the cells and ATP depletion, reducing FE (Bottje and Carstens, 2009). Therefore, maintaining systemic osmotic balance is an energy costly process that can potentially reduce animal performance. Future research is needed for a better understanding of the metabolism of energy at cellular level under high-salt load in the body fluid compartments of ruminants.
Conclusion
The meta-analysis revealed the overall negative effects of high-salt water on water intake, dry matter, and performance on beef cattle. The effects are more negative when cattle drink high-sulfate water than when they drink high-chloride water. Moreover, the meta-analysis points out that high-salt water could cause a more negative impact on average daily gain and feed efficiency than on dry matter intake. On the other hand, the meta-analysis highlights knowledge gaps and research needs, to have a better understanding of the effect of high-salt water at different levels of organization (cell, tissue, organ, and organ systems). This knowledge is essential for developing management strategies to alleviate the negative effects of high-salt water on cattle and other livestock species. Moreover, since models of global climate change predict shortages in both water quantity and quality in the near future, major technological progress should be achieved to improve high-salt water use efficiency for sustainable livestock production systems, particularly in regions with increasing scarcity and variability in natural resources. Finally, to the best of our knowledge, this is the first approach that assesses the effect of water quality on feed and water intake as well as animal performance. The relationships described in this meta-analytical evaluation could be the first step for the development of future mechanistic models to predict animal response in beef cattle drinking water with increasing levels and different types of salt concentration.
Supplementary Material
Glossary
Abbreviations
- ADG
average daily gain
- AICC
bias-corrected Akaike’s information criterion
- BIC
Bayesian’s information criterion
- BW
body weight
- CP
crude protein
- DM
dry matter
- DMI
dry matter intake
- FE
feed efficiency
- H2S
hydrogen sulfide
- NaCl
sodium chloride
- NDF
neutral detergent fiber
- RMSE
root mean square error
- SO4
sulfates
- TDS
total dissolved solids
- WI
water intake
Conflict of interest statement
The authors declare no real or perceived conflicts of interest.
Literature Cited
- Ahlberg, C. M., Allwardt K., Broocks A., Bruno K., McPhillips L., Taylor A., Krehbiel C. R., Calvo-Lorenzo M. S., Richards C. J., Place S. E., . et al. 2018. Environmental effects on water intake and water intake prediction in growing beef cattle. J. Anim. Sci. 96:4368–4384. doi: 10.1093/jas/sky267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen, M. S. 1996. Physical Constraints on voluntary intake of forage by ruminants. J. Anim. Sci. 74:3063–3075. doi: 10.2527/1996.74123063x [DOI] [PubMed] [Google Scholar]
- Allen, M. S. 2014. Drives and limits to feed intake in ruminants. Anim. Prod. Sci. 54:1513–1524. doi: 10.1071/AN14478 [DOI] [Google Scholar]
- Allen, M. S., Bradford B. J., and Oba M.. . 2009. Board-invited review: The hepatic oxidation theory of the control of feed intake and its application to ruminants. J. Anim. Sci. 87:3317–3334. doi: 10.2527/jas.2009-1779 [DOI] [PubMed] [Google Scholar]
- Arias, R. A., and Mader T. L.. . 2011. Environmental factors affecting daily water intake on cattle finished in feedlots. J. Anim. Sci. 89:245–251. doi: 10.2527/jas.2010-3014 [DOI] [PubMed] [Google Scholar]
- Arieli, A., Naim E., Benjamin R. W., and Pasternak D.. . 1989. The effect of feeding saltbush and sodium chloride on energy metabolism in sheep. Anim. Prod. 49:451–457. doi: 10.1017/S0003356100032657 [DOI] [Google Scholar]
- Baldwin, R. L., Smith N. E., Taylor J., and Sharp M.. . 1980. Manipulating metabolic parameters to improve growth rate and milk secretion. J. Anim. Sci. 51:1416–1428. doi: 10.2527/jas1981.5161416x [DOI] [PubMed] [Google Scholar]
- Beauchamp, R. O.Jr., Bus J. S., Popp J. A., Boreiko C. J., and Andgjelkovich D. A.. . 1984. A critical review of the literature on hydrogen sulfide. Crit. Rev. Toxicol. 13:25–97. doi: 10.3109/10408448409029321 [DOI] [PubMed] [Google Scholar]
- Behnassi, M., Shahid S. A., and Mintz-Habib N.. . 2014. Science, policy and politics of modern agricultural system: Global Context to Local Dynamics of Sustainable Agriculture. Springer Dordrecht; p. 223–237. doi: 10.1007/978-94-007-7957-0_15 [DOI] [Google Scholar]
- Bird, P. R. 1972. Sulphur metabolism and excretion studies in rumiants: X. Sulphide toxicity in sheep. Aust. J. Biol. Sci. 25:1087–1098. doi: 10.1071/BI9721087 [DOI] [PubMed] [Google Scholar]
- Bond, J., Rumsey T. S., and Weinland B. T.. . 1975. Effect of deprivation and reintroduction of feed and water on the feed and water intake behavior of beef cattle. J. Anim. Sci. 43:873–878. doi: 10.2527/jas1976.434873x [DOI] [Google Scholar]
- Bottje, W. G., and Carstens G. E.. . 2009. Association of mitochondrial function and feed efficiency in poultry and livestock species. J. Anim. Sci. 87(14 Suppl):E48–E63. doi: 10.2527/jas.2008-1379 [DOI] [PubMed] [Google Scholar]
- Bourque, C. W. 2008. Central mechanisms of osmosensation and systemic osmoregulation. Nat. Rev. Neurosci. 9:519–531. doi: 10.1038/nrn2400 [DOI] [PubMed] [Google Scholar]
- Brew, M. N., Myer R. O., Hersom M. J., Carter J. N., Elzo M. A., Hansen G. R., Riley D. G.. . 2011. Water intake and factors affecting water intake of growing beef cattle. Livest. Sci. 140:297–300. doi: 10.1016/j.livsci.2011.03.030 [DOI] [Google Scholar]
- Burgos, M. S., Langhans W., and Senn M.. . 2000. Role of rumen fluid hypertonicity in the dehydration-induced hypophagia of cows. Physiol. Behav. 71:423–430. doi: 10.1016/s0031-9384(00)00357-7 [DOI] [PubMed] [Google Scholar]
- Coelho, M. S., Passadore M. D., Gasparetti A. L., Bibancos T., Prada P. O., Furukawa L. L., Furukawa L. N. S., Fukui R. T., Casarini D. E., Saad M. J. A., . et al. 2006. High- or low-salt diet from weaning to adulhood: Effect on body weight, food intake and energy balance in rats. Nut. Metab. & Cardio. Diseases 16:148–155. doi: 10.1016/j.numecd.2005.09.001 [DOI] [PubMed] [Google Scholar]
- De Waal, H. O., Baard M. A., and Engels E. A. N.. . 1989. Effects of sodium chloride on sheep. 2. Voluntary feed intake and changes in certain rumen parameters of young Merino wethers grazing native pasture. S. Afr. J. Anim. Sci. 19:34–42. [Google Scholar]
- Descheemaeker, K., Amede T., and Haileslassie A.. . 2010. Improving water productivity in mixed crop-livestock farming system of sub-Saharan Africa. Agr. Water Manag. 97:579–586. doi: 10.1016/j.agwat.2009.11.012 [DOI] [Google Scholar]
- Digesti, R. D., and Weeth H. J.. . 1976. A defensible maximum for inorganic sulfate in drinking water of cattle. J. Anim. Sci. 42:1498–1502. doi: 10.2527/jas1976.4261498x [DOI] [PubMed] [Google Scholar]
- Drewnoski, M. E., and Hansen S. L.. . 2013. Effect of delaying the feeding of high sulfur until 28 days after adaptation to finishing diet on cattle intake, gain, and ruminal hydrogen sulfide concentrations. Livest. Sci. 155:230–235. doi: 10.1016/j.livsci.2013.05.014 [DOI] [Google Scholar]
- Drewnoski, M. E., Poge D. J., and Hansen S. L.. . 2014. High-sulphur in beef cattle diets: A review. J. Anim. Sci. 92:3763–3780. doi: 10.2527/jas.2013-7242 [DOI] [PubMed] [Google Scholar]
- Fitzsimons, J. T. 1998. Angiotensin, thirst and sodium appetite. Physiol. Rev. 78:583–686. doi: 10.1152/physrev.1998.78.3.583 [DOI] [PubMed] [Google Scholar]
- Food and Agriculture Organization of the United Nations (FAO). 2011. World livestock 2011—Livestock in food security. Rome, Italy: FAO. [Google Scholar]
- Goatcher, W. D., and Church D. C.. . 1970. Taste responses in ruminants. IV. Reactions of pygmy goats, normal goats, sheep and cattle to acetic acid and quinine hydrochloride. J. Anim. Sci. 31:373–382. doi: 10.2527/jas1970.312373x [DOI] [PubMed] [Google Scholar]
- Grout, A. S., Veira D. M., Weary D. M., von Keyserlingk M. A., and Fraser D.. . 2006. Differential effects of sodium and magnesium sulfate on water consumption by beef cattle. J. Anim. Sci. 84:1252–1258. doi: 10.2527/2006.8451252x [DOI] [PubMed] [Google Scholar]
- Herrero, M., Thornton P. K., Gerber P., and Reid R. S.. . 2009. Livestock, livelihoods and the environment: understanding the trade-offs. Curr. Opin. Environ. Sust. 1:111–120. doi: 10.1016/j.cosust.2009.10.003 [DOI] [Google Scholar]
- Jayanegara, A., Leiber F., and Kreuzer M.. . 2012. Meta-analysis of the relationship between dietary level and methane formation in ruminants from in vivo and in vitro experiment. J. Anim. Physiol. Anim. Nutr. 96:365–375. doi: 10.1111/j.1439-0396.2011.01172.x [DOI] [PubMed] [Google Scholar]
- Jiménez Cisneros, B. E., Oki T., Arnell N. W., Benito G., Cogley J. G., Döll P., Jiang T., and Mwakalila S. S.. . 2014. Freshwater resources. In: Field, C. B., Barros V. R., Dokken D. J., Mach K. J., Mastrandrea M. D., Bilir T. E., Chatterjee M., Ebi K. L., Estrada Y. O., Genova R. C., et al. , editors. Climate change 2014: Impacts, adaptation, and vulnerability. Part A: Global and sectoral aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, UK, and New York, NY: Cambridge Univ. Press. p. 229–269. [Google Scholar]
- Kandylis, K. 1984. Toxicology of sulfur in ruminants: review. J. Dairy Sci. 67:2179–2187. doi: 10.3168/jds.S0022-0302(84)81564-7 [DOI] [PubMed] [Google Scholar]
- Katting, R. M., Pordomingo A. J., Schneberger A. G., Duff G. C., and Wallace J. D.. . 1992. Influence of saline water on intake, digesta kinetics, and serum profiles of steers. J. Range Manage. 45:514–518. [Google Scholar]
- Kay, R. N. B. 1997. Responses of African livestock and wild herbivores to drought. J. Arid Env. 37:683–694. doi: 10.1006/jare.1997.0299 [DOI] [Google Scholar]
- Kessler, K. L., Olson K. C., Wright C. L., Austin K. J., McInnerney K., Johnson P. S., Cockrum R. R., Jons A. M., and Cammack K. M.. . 2013. Effects of high-sulphur water on hepatic gene expression of steers fed fibre-based diets. J. Anim. Physiol. Anim. Nutr. (Berl). 97:838–845. doi: 10.1111/j.1439-0396.2012.01327.x [DOI] [PubMed] [Google Scholar]
- Kume, S., Nonaka K., Oshita T., and Kozakai T.. . 2010. Evaluation of drinking water intake, feed water intake and total water intake in dry and lactating cows fed silages. Livest. Sci. 128:46–51. doi: 10.1016/j.livsci.2009.10.012 [DOI] [Google Scholar]
- Lazartigues, E., Sinnayah P., Augoyard G., Gharib C., Johnson A. K., and Davisson R. L.. . 2008. Enhanced water and salt intake in transgenic mice brain-restricted overexpression of angiotensin (AT1) receptors. Am. J. Physiol. Regul. Integr. Comp. Physiol. 295:1539–1545. doi: 10.1152/ajpregu.00751.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legesse, G., Ominski K. H., Beauchemin K. A., Pfister S., Martel M., McGeough E. J., Hoekstra A. Y., Kroebel R., Cordeiro M. R. C., and McAllister T. A.. . 2017. BOARD-INVITED REVIEW: quantifying water use in ruminant production. J. Anim. Sci. 95:2001–2018. doi: 10.2527/jas.2017.1439 [DOI] [PubMed] [Google Scholar]
- Loneragan, G. H., Wagner J. J., Gould D. H., Garry F. B., and Thoren M. A.. . 2001. Effects of water sulfate concentration on performance, water intake, and carcass characteristics of feedlot steers. J. Anim. Sci. 79:2941–2948. doi: 10.2527/2001.79122941x [DOI] [PubMed] [Google Scholar]
- Macfarlane, W. V., and Howard B.. . 1972. Comparative water and energy economy of wild and domestic animals. Symp. Zool. Soc. Lond. 31:261–296. [Google Scholar]
- Masters, D. G., Rintoul A. J., Dynes R. A., Pearce K. L., and Norman H. C.. . 2005. Feed intake and production in sheep diets high in sodium and potassium. Aust. J. Agri. Res. 56:427–434. doi: 10.1071/AR04280 [DOI] [Google Scholar]
- Mertens, D. R. 1994. Regulation of forage intake. In: Fahey G. C. Jr., editor. Forage quality, evaluation, and utilization. Madison, WI: Am. Soc. Agron., Crop Sci. Soc. Am., and Soil Sci. Soc. Am; p. 450–493. [Google Scholar]
- Meyer, U., Everinghoff M., Gädeken D., and Flachowsky G.. . 2004. Investigation on the water intake of lactating dairy cows. Livest. Prod. Sci. 90:117–121. doi: 10.1016/j.livprodsci.2004.03.005 [DOI] [Google Scholar]
- Milligan, L. P., and McBride B. W.. . 1985. Energy costs of ion pumping by animal tissues. J. Nutr. 115:1374–1382. doi: 10.1093/jn/115.10.1374 [DOI] [PubMed] [Google Scholar]
- Molden, D., Frenken K., Barker R., de Fraiture C., Mati B., Svendsen M., Sadoff C., and Finlayson C. M.. . 2007. Trends in water and agricultural development. In: Water for Food, Water for Life: A Comprehensive Assessment of Water Management in Agriculture. London, UK: Earthscan, and Colombo, Sri Lanka: International Water Management Institutez;p. 57–89. [Google Scholar]
- NASEM. 2016. Nutrient requirements of beef cattle. 8th rev. ed. Washington, DC: Natl. Acad. Press. doi: 10.17226/19014 [DOI] [Google Scholar]
- NRC. 2005. Mineral Tolerance of Animals. 2nd rev. ed. Washington: Natl. Acad. Press. [Google Scholar]
- Osaka, T., Kobayashi A., and Inoue S.. . 2001. Thermogenesis induced by osmotic stimulation of the intestines in the rat. J. Physiol. 532(Pt 1):261–269. doi: 10.1111/j.1469-7793.2001.0261g.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche, J. R., Blache D., Kay J. K., Miller D. R., Sheahan A. J., and Miller D. W.. . 2008. Neuroendocrine and physiological regulation of intake with particular reference to domesticated ruminant animals. Nutr. Res. Rev. 21:207–234. doi: 10.1017/S0954422408138744 [DOI] [PubMed] [Google Scholar]
- Rumpler, W. V., and Johnson D. E.. . 1987. The effect of high cation levels in diet with and without ionophores on in vivo methanogenesis in steers. In Energy Metabolism in Farm Animals. Ed. P. W. Moe, H. F. Tyrrell and P. J. Reynolds. [Google Scholar]
- Sands, J. M., and Layton H. E.. . 2009. The physiology of urinary concentration: an update. Semin. Nephrol. 29:178–195. doi: 10.1016/j.semnephrol.2009.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauvant, D., Schmidely P., Daudin J. J., and St-Pierre N. R.. . 2008. Meta-analyses of experimental data in animal nutrition. Animal 2:1203–1214. doi: 10.1017/S1751731108002280 [DOI] [PubMed] [Google Scholar]
- Sexson, J. L., Wagner J. J., Engle T. E., and Eickhoff J.. . 2012. Predicting water intake by yearling feedlot steers. J. Anim. Sci. 90:1920–1928. doi: 10.2527/jas.2011-4307 [DOI] [PubMed] [Google Scholar]
- St-Pierre, N. R. 2001. Invited review: Integrating quantitative findings from multiple studies using mixed model methodology. J. Dairy Sci. 84:741–755. doi: 10.3168/jds.S0022-0302(01)74530-4 [DOI] [PubMed] [Google Scholar]
- Truong, D. H., Eghbal M. A., Hindmarsh W., Roth S. H., and O’Brien P. J.. . 2006. Molecular mechanisms of hydrogen sulfide toxicity. Drug Metab. Rev. 38:733–744. doi: 10.1080/03602530600959607 [DOI] [PubMed] [Google Scholar]
- Utley, P. R., Bradley N. W., and Boling J. A.. . 1970. Effect of restricted water intake on feed intake, nutrient digestibility and nitrogen metabolism in steers. J. Anim. Sci. 31:130–135. doi: 10.2527/jas1970.311130x [DOI] [PubMed] [Google Scholar]
- Uwituze, S., Parsons G. L., Karges K. K., Gibson M. L., Hollis L. C., Higgins J. J., and Drouillard J. S.. . 2011. Effects of distillers grains with high sulfur concentration on ruminal fermentation and digestibility of finishing diets. J. Anim. Sci. 89:2817–2828. doi: 10.2527/jas.2010-3401 [DOI] [PubMed] [Google Scholar]
- Weeth, H. J., and Capps D. L.. . 1972. Tolerance of growing cattle for sulfate-water. J. Anim. Sci. 34:256–260. doi: 10.2527/jas1972.342256x [DOI] [PubMed] [Google Scholar]
- Weeth, H. J., Haverland L. H., and Cassard D. W.. . 1960. Consumption of sodium chloride water by heifers. J. Anim. Sci. 19:845–851. doi: 10.2527/jas1960.193845x [DOI] [Google Scholar]
- Weeth, H. J., and Hunter J. E.. . 1971. Drinking of sulfate-water by cattle. J. Anim. Sci. 32:277–281. doi: 10.2527/jas1971.322277x [DOI] [PubMed] [Google Scholar]
- Wilson, A. D., and Dudzinski M. L.. . 1973. Influence of the concentration and volume of saline water on the food intake of sheep, and on their excretion of sodium and water in urine and faeces. Aust. J. Agric. Res. 24:245–256. doi: 10.1071/AR9730245 [DOI] [Google Scholar]
- Wright, C. L. 2007. Management of water quality for beef cattle. Vet. Clin. North Am. Food Anim. Pract. 23:91–103. doi: 10.1016/j.cvfa.2006.12.002 [DOI] [PubMed] [Google Scholar]
- Zanetti, D., Prados L. F., Menezes A. C. B., Silva B. C., Pacheco M. V. C., Silva F. A. S., Costa e Silva L. F., Detmann E., Engle T. E., Valadares Filho S. C.. . 2019. Prediction of water intake to Bos indicus beef cattle raised under tropical conditions. J. Anim. Sci. 97:1364–1374. doi: 10.1093/jas/skz003 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.