Abstract
Placebo effects have traditionally involved concealment or deception. However, recent evidence suggests that placebo effects can also be elicited when prescribed transparently as “open-label placebos” (OLPs), and that the pairing of an unconditioned stimulus (eg, opioid analgesic) with a conditioned stimulus (eg, placebo pill) can lead to the conditioned stimulus alone reducing pain. In this randomized control trial, we investigated whether combining conditioning with an OLP (COLP) in the immediate postoperative period could reduce daily opioid use and postsurgical pain among patients recovering from spine surgery. Patients were randomized to COLP or treatment as usual, with both groups receiving unrestricted access to a typical opioid-based postoperative analgesic regimen. The generalized estimating equations method was used to assess the treatment effect of COLP on daily opioid consumption and pain during postoperative period from postoperative day (POD) 1 to POD 17. Patients in the COLP group consumed approximately 30% less daily morphine milligram equivalents compared with patients in the treatment as usual group during POD 1 to 17 (−14.5 daily morphine milligram equivalents; 95% CI: [−26.8, −2.2]). Daily worst pain scores were also lower in the COLP group (21.0 point on the 10-point scale; 95% CI: [−2.0, −0.1]), although a significant difference was not detected in average daily pain between the groups (−0.8 point; 95% CI: [−1.7, 0.2]). These findings suggest that COLP may serve as a potential adjuvant analgesic therapy to decrease opioid consumption in the early postoperative period, without increasing pain.
Keywords: Placebo, Open-label, Conditioning, Postsurgical pain, Opioid
1. Introduction
Placebo treatments evoke clinically meaningful benefits beyond spontaneous remission and regression to the mean.52 Investigations of placebo effects in both randomized controlled trials (RCTs) and experimental pain settings are abundant,30,80,89,90 with placebo treatments generally eliciting moderate effect sizes.50,59,75,96 For example, in one meta-analysis (215 RCTs, 41,392 patients) placebo responses were equivalent to 75% the effect of diverse analgesic interventions.101 Traditionally, studying placebo effects has involved concealment (in RCTs) or deception (in laboratory experiments), which are impractical and ethically problematic for clinical practice. However, open-label placebos (OLPs), which meet the ethical standards of informed consent and transparency,13 have recently demonstrated efficacy in treating a wide variety of symptoms22 and conditions, including cancer-related fatigue,38 irritable bowel syndrome,49 migraines,47 chronic knee osteoarthritis,64 and allergic rhinitis.78 Open-label placebo treatments have also reduced chronic low back pain and disability in 2 RCTs using pills20,58 and one using honest sham injections.4
Another potentially transparent use of placebos is conditioning of placebos, which use the stimulus substitution principle of classical conditioning. The unconditioned stimulus (US: drug) is repeatedly paired with a conditioned stimulus (CS: placebo), resulting in a new learned response where the CS alone can elicit similar responses. Conditioning has a long history in animal research33,36,68,100 and has been applied to laboratory-based human placebo research,79 demonstrating reduced need for analgesia when opioids (US) are paired with placebos (CS).3 Conditioned placebos have shown clinical benefits while allowing a reduction of medication, without increase in morbidity or symptoms, including in psoriasis,2 insomnia,69 allergic rhinitis,31 attention deficit or hyperactivity disorder,77 and immune suppression in renal transplant patients.56 To date, however, there has been less research using conditioning as a “dose-extension” or “partial reinforcement” strategy for pain management.23,50,62
Although traditional deceptive placebos have shown efficacy in reducing postsurgical pain,9 the effects of transparent applications of placebos (eg, OLP and conditioned placebos) have not previously been examined in postsurgical pain management. Surgery is a potent noxious stimulus, posing an important challenge to pain management.54 In particular, spine surgery is associated with significant postoperative pain,55 traditionally, involving management with opioid analgesics, as well as frequent dosing and self-titration of analgesics. However, extensive perioperative and postoperative opioid use has come under increased scrutiny during the opioid epidemic because of its association with an increased risk of persistent use46 and new opioid dependence,16 highlighting the need for adjuvant analgesic therapies to facilitate opioid dose reduction and more rapid postoperative tapering. The frequent dosing of opioid analgesics early after spine surgery provides an ideal opportunity to pair opioids with placebos, thus adding the benefit of a transparent conditioning paradigm to OLP, which we called conditioned OLP (COLP).
Open-label placebo, although showing promise for chronic symptoms in patients with low back pain, has not previously been investigated for management of acute moderate-to-severe pain, and in the context of postsurgical pain management. Our aim was to determine whether COLP would reduce opioid consumption and pain after spine surgery. We hypothesized that patients receiving COLP would exhibit lower opioid consumption and a reduction in pain postoperatively. Given the substantial heterogeneity in the magnitude of placebo responses,72,84,91 another exploratory aim was to examine characteristics associated with greater COLP efficacy. We hypothesized that COLP benefits would vary substantially across patients, potentially interacting with known biopsychosocial modulators of pain.
2. Methods
2.1. Subject recruitment and enrollment
This prospective RCT was approved by the Partners Institutional Review Board and was registered on ClinicalTrials.gov under the study name “Evaluation of Open-Label Conditioned Placebo Analgesia for Postoperative Opioid Reduction Following Spinal Fusion (COLP)” (NCT04574388). Patients scheduled for surgery for degenerative conditions of the spine with a single surgeon were recruited from Brigham and Women’s Hospital preoperative clinic in Boston, MA, between November 2018 and February 2020. Patients aged 18 to 75 years, who were without cognitive impairment or English improficiency that would impair completion of study questionnaires or comprehension of procedures, were eligible for participation.
Patients were approached at their preoperative visit to receive information regarding the study, and if interested in participating, provided informed consent. Key study points discussed with patients during recruitment included (1) definition and explanation of placebo effects, (2) evidence highlighting placebos’ ability to reduce pain in double-blind RCTs, (3) explanation of “open-label” concept (eg, patient knowingly receiving a placebo), (4) introduction of several previous successful OLP studies, noting the absence of any evidence for postsurgical patients specifically, (5) explanation of conditioning paradigm (pairing the OLP pills with their other analgesics), (6) suggestion that COLP treatment may or may not work to reduce their pain or opioid consumption, (7) emphasis that placebo effectiveness was not contingent on belief, and (8) repeated assurances that taking a COLP would in no way restrict their access to other analgesics, including opioids, after surgery (Appendix A, available at http://links.lww.com/PAIN/B257). Patients underwent baseline quantitative sensory testing (QST) in person during their preoperative visit, and demographic, baseline pain, and psychosocial questionnaires were completed through an email link to a secure database (REDCap) preoperatively.
2.2. Assessment of baseline patient pain characteristics
Baseline pain before surgery was assessed using the Brief Pain Inventory (BPI). The BPI contains questions inquiring about current pain, and worst, least, and self-reported average pain within the past 24 hours. The BPI mean is an average of these 4 pain scores. The BPI also contains 7 questions evaluating the impact of pain on general functioning, which are summed to give a functional impact score and then averaged together as BPI interference.88
2.3. Psychosocial assessments
Psychosocial assessment tools were selected based on strong psychometric properties and brevity. The Pain Catastrophizing Scale was used to measure pain-associated with catastrophic thinking (range: 0–52).85 Depressive symptoms (range: 9–40), anxiety (range: 7–35), and sleep disturbance (range: 4–20) were assessed using the Patient Reported Outcome Measurement Information System Short Form (PROMIS-SF).21 The 6 items related to somatization from the Brief Symptom Index 18Somatization Scale were used to measure somatization (range: 5–30).25 The fibromyalgianess scale (range 0–31), adapted from the clinical criteria for fibromyalgia,15,97 was used to characterize widespread pain (indication of number of body areas with pain) and related symptom severity of generalized symptoms (fatigue, etc). The Positive Affect Negative Affect Scale was used to assess positive (range: 0–40) and negative (range: 0–40) affect,94 and preferences for coping strategies were measured using the Coping Strategies Questionnaire (range: 0–84).45,73 The Screener and Opioid Assessment for Patients with Pain (SOAPP-R-8) (range: 0–32) was used to evaluate the risk for developing problems with long-term opioid use.17
2.4. Bedside quantitative sensory testing
After providing informed consent at their preoperative visit, patients underwent brief bedside QST in nonsurgical areas (hands, extensor forearm, and trapezius).
2.4.1. Temporal summation of pain
Using methods from our previous studies81,82 similar to those described by Rolke et al.,76 mechanical pinprick pain was assessed using standardized weighted pinprick applicators. First, a single stimulation of the lowest force (128 mN) pinprick was applied to the dorsal aspect of the index finger between the first and second interphalangeal joints of the left hand while resting the palm facing downward on a flat surface. The subject rated the pain intensity from the mechanical stimulus on a scale of 0 to 10. If pain was rated as 0 to 1, the next highest force probe was tested as a single application. One of 3 designated force (128, 256, and 512 mN) probes was selected: specifically, the lowest force probe to result in a mildly painful sensation (pain score 1–3) with a single application. After a break of at least 10 seconds, a train of 10 stimuli was applied at the same location at a rate of 1 stimulation/second. The subject rated their pain on a scale of 0 to 10 after the first, fifth, and 10th stimuli. This was then repeated on the right index finger, followed by the third finger of each hand, alternating sides of testing. Temporal summation of pain (TSP) was calculated as D (10th-first stimulus) pain rating. This was calculated for each of the 4 finger sites and then averaged.
2.4.2. Pressure pain threshold and tolerance
As in our previous studies,81,82 pressure pain threshold and tolerance were measured using a digital pressure algometer (Wagner FDX, Greenwich, CT) with a flat round transducer, probe area 0.785 cm2. Testing was performed bilaterally on the dorsal aspect of the proximal forearm approximately 3 to 4 cm distal to the elbow crease (extremity site) and over the trapezius muscle at the upper back approximately 2 to 3 cm above the scapular spine, midway between C7 prominence and humeral head (truncal site). To determine pressure pain threshold, pressure was increased at a steady rate of approximately 1 lb/s (0.45 kg/second), with the subject indicating when this pressure first became painful. To determine tolerance, the pressure was further increased, with the subject indicating when the pain from the stimulus was no longer tolerable. Testing was performed bilaterally, alternating between sides and extremity or truncal sites.
2.5. Randomization and blinding
Randomization was performed using the randomization function in REDCap. Once initial QST and baseline questionnaires were completed, patients were automatically randomized to either the COLP or treatment as usual (TAU) group (Fig. 1). Given the transparency inherent in the treatment being tested, neither patients nor research staff were blind to grouping, and group assignment was revealed to patients on the morning of their surgery. Anesthesiologists and surgical teams were not aware of the patient’s group assignment, and any intraoperative medications were administered at the discretion of the anesthesia team. Postoperative analgesic medications were prescribed by the surgical team per typical clinical practice, consisting of oxycodone (5–10 mg) or hydromorphone (1–2 mg), primarily through oral route every 4 to 6 hours as needed, as well as acetaminophen 500 to 1000 mg every 6 to 8 hours.
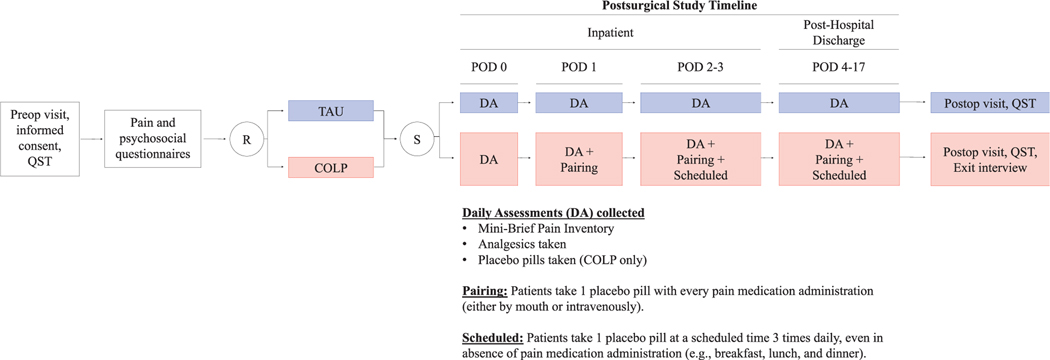
Figure 1.
Patients consenting to participation completed baseline questionnaires and were randomized to either the treatment as usual (TAU) or conditioned open-label placebo (COLP) group. Study staff visited patients in both groups twice daily while inpatient and contacted once daily after hospital discharge to collect daily reports on their analgesics taken and pain scores. Patients in the COLP group also reported how many placebo pills were taken each day. On POD 1, COLP patients began the “pairing” regime by taking one open-label placebo pill each time they took an opioid analgesic. On POD 2, COLP patients were instructed to begin taking 3 daily scheduled placebo pills in addition to the placebo pills paired with each opioid analgesic (“pairing 1 scheduled”). All patients were asked to continue recording daily analgesic consumption and pain scores, with COLP patients also continuing the “pairing 1 scheduled” regime, until their postoperative follow-up appointment (approximately 17 days after surgery). DA, daily assessments; POD, postoperative day; QST, quantitative sensory testing; R, randomization; S, surgery.
2.6. Conditioned open-label placebo group and treatment as usual group
After surgery, once patients were out of the recovery phase and admitted as inpatients on postoperative day 0 (POD 0), study staff visited to assess pain, further explain study procedures and answer any potential questions. It was re-emphasized that being in the study would not limit their access to opioid or other analgesics, which followed the standard as needed dosing schedule prescribed to all patients undergoing this procedure under this single surgical provider. Nursing staff was provided information regarding the research study and was instructed that patient participation should not impact their administration of other medications. Patients in the COLP group were provided with placebo pills, which were small white capsules containing microcrystalline cellulose, in a large, easy open prescription bottle prepared by the BWH Investigational Pharmacy. Patients were instructed to self-administer one COLP pill with all analgesics (whether administered intravenously or orally) and record the pairing in a bedside diary. Conditioning with an open-label placebo initiation took place on either POD 0 or early POD 1, depending on the acute postoperative state and time of transfer to inpatient unit. Beginning on POD 2, participants were further instructed to take 3 scheduled placebo pills every day, at 3 convenient times of their choosing, in addition to pairing placebos with all analgesics. In an attempt to continually link the US (analgesics) to the CS (placebos) and prevent habituation, patients in the COLP group were instructed to continue taking both paired and scheduled OLP pills until their follow-up appointment.
A concerted effort was made to ensure that an equivalent quantity and quality of attention and time was paid to both the TAU and COLP patients. Patients in both groups were visited twice daily (10–15 minutes visits) by both physician and nonphysician study staff during their inpatient hospital stay, and patients in both groups were contacted once daily through phone, text, or email (whatever their preferred contact method) after discharge to collect recorded diary information from the day before. In both groups, topics of conversation with patients during in hospital visits and follow-up visits included pain, use of different analgesics, the recording of pain and analgesic consumption in the daily diary, sleep quality, and recovery, including drain output, physical therapy, plans for discharge, family visits, and overall experience. Specific discussion topics in the COLP group included guidance on taking placebos and re-explanation of the COLP rationale: (1) placebos may induce a physiological response in the brain to decrease pain even without deception (OLP concept), (2) pairing placebos with other pain-reducing medications may strengthen this effect (conditioning concept), and (3) it might not be necessary to believe in the effect for it to work (automatic response concept) (Appendix A, available at http://links.lww.com/PAIN/B257).
Study staff reinforced instructions about study procedures, including recording pain and analgesic data in the study diary towards the end of the inpatient stay. In this way, before discharge, all patients in both groups were trained to use the study diary to record daily mini-BPI data, including pain scores and analgesic use (COLP and TAU groups), and placebo pills taken (COLP group alone) after hospital discharge. Patients were instructed to complete diary entries until postsurgical follow-up appointment or POD 17, and study staff contacted all patients daily through phone, text, or secure email to collect mini-BPI and analgesic utilization data.
2.7. Postsurgical daily pain assessment
A brief version of the BPI (mini-BPI) was used to assess daily pain severity during the postoperative course of the study. The mini-BPI included questions about current pain, worst pain, self-averaged pain, and least pain during the preceding 24 hours, which was collected verbally from participants during PM visits while inpatient. After hospital discharge, the mini-BPI was filled out by the patient in their daily diary and was conveyed to study staff during a daily text, phone call, or secure email link.
2.8. Analgesic consumption
Patients took opioids and other analgesics on an as needed basis. Patients in both groups recorded daily opioid analgesic consumption and pain scores, beginning while inpatient, at which time this was cross-referenced with the medication administration record, and at home in a daily diary until their follow-up appointment. Patients reported the number, type, and dose of opioid analgesics consumed each day. All opioids were converted to daily morphine milligram equivalents (MMEs) (Appendix B, available at http://links.lww.com/PAIN/B257).
2.9. Follow-up appointment
Study staff met all patients to repeat QST at the surgical follow-up appointment, which occurred at a median of 20 days after surgery (range: 11–33 days). At this time, patients in the COLP group, after finishing psychophysical testing, also underwent a semistructured qualitative interview regarding their experience on taking a COLP. The results of these qualitative assessments will be reported in a separate manuscript.
2.10. Statistical analyses
Continuous variables are reported as means with SDs, and categorical variables are reported as counts and percentages. All opioid analgesics were converted to MMEs (Appendix B, available at http://links.lww.com/PAIN/B257). Differences in postoperative opioid consumption and pain scores between COLP and TAU treatment groups were assessed using the general estimating equation (GEE) method with an autoregressive correlation structure, which takes into account the correlation between repeated measurements on the same patient and can accommodate missing data across timepoints (POD 1–17), if this missingness is random. Effect sizes are reported as differences in means through the GEE model beta coefficients (B) and confidence intervals (CIs).
To address the second exploratory aim of identifying variation in COLP effectiveness among individuals, we assessed for interactions between COLP and baseline patient characteristics known to significantly impact pain and opioid consumption. Potential moderators were each tested in separate regression models, with independent variables being the treatment group (COLP vs TAU), the potential moderator, and an interaction term. For continuous moderators, the groups in the interaction terms were defined by values corresponding to the 16th percentile, the median, and the 84th percentile of the distribution of that variable.35
Based on postoperative opioid consumption from a previous cohort of orthopedic postsurgical patients,1 we estimated a mean ± SD postoperative cumulative MME dose over the study period of 200 ± 70 in the TAU group. Using this estimated mean and SD, an a priori power analysis determined that a sample size of 18 patients per group would provide 80% power to detect a 30% difference in opioid use at a two-sided alpha level of 0.05. Statistical analyses were conducted using R version 3.6.2. and IBM- SPSS v26, with the PROCESS macro35 used to assess moderation between treatment groups and outcomes.
3. Results
3.1. Study participants
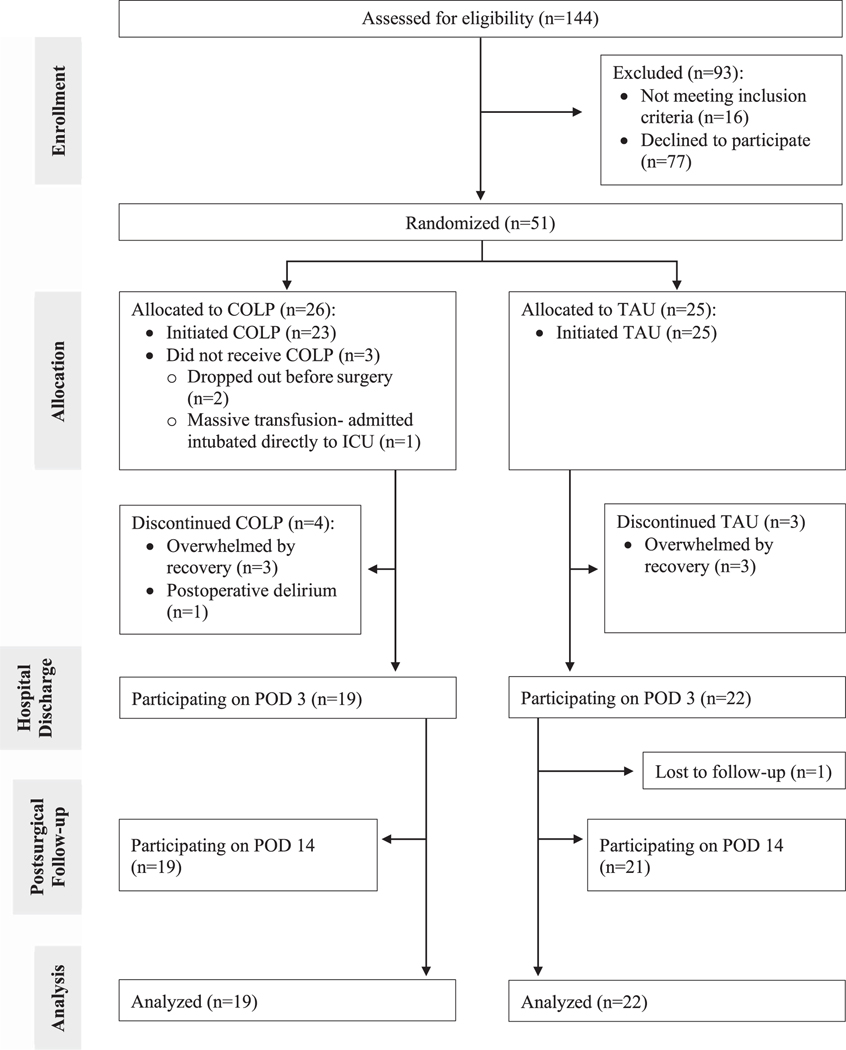
Of the 144 patients assessed for eligibility, 51 provided informed consent, underwent psychophysical testing, completed electronic baseline questionnaires, and were randomized to receive COLP (n = 26) or TAU (n = 25) before surgery (Fig. 2). Before beginning treatment, 2 participants in the COLP group withdrew consent because of anxiety about surgery and recovery, and one participant became ineligible because of prolonged admission to the intensive care unit. After treatment initiation, 4 patients in the COLP group and 3 in the TAU group discontinued study participation in the early postoperative period because of delirium or being overwhelmed by demands of recovery. Ultimately, the final analysis included 19 COLP and 22 TAU patients who provided postoperative data regarding opioid consumption and pain. Assessment of missing pain and opioid data from participants revealed an average of 8.3% missing data on any given assessment day, with data missing from 0 to 6 of 41 participants on any given day, with consistency of missingness over time. There were relatively similar amounts of missingness between treatment groups (COLP: 7.1%; TAU: 9.3%), suggesting a random pattern of missingness that would allow for use of the GEE analysis.
Figure 2.
CONSORT study flow diagram. Patients were approached and recruited from the anesthesia preoperative clinic. Of the 144 patients assessed for eligibility, 51 patients were enrolled and randomized to either the TAU (n = 25) or COLP (n = 26) group. Before beginning COLP treatment, 2 participants withdrew because of anxiety about the upcoming surgery, and one participant became ineligible because of postoperative complications necessitating intensive care unit admission postoperatively. After beginning allocated treatment, 4 patients in the COLP group and 3 in the TAU group withdrew. There were 41 patients included in the final analysis (TAU: n = 22 and COLP: n = 19). COLP, conditioned open-label placebo; POD, postoperative day; TAU, treatment as usual.
3.2. Baseline patient characteristics
Patient demographic, psychosocial, and psychophysical baseline characteristics were similar between groups (Table 1). Importantly, both baseline pain severity (COLP: 5.3 6 2.6; TAU: 5.4 ± 1.4) and preoperative opioid use (COLP:11%; TAU:18%) were similar between groups, with all holding only minimal Prescriptions for as needed oxycodone of the 5 mg denomination.
Table 1.
Patient baseline characteristics for the TAU and COLP groups.
| Baseline characteristics | TAU (n = 22) | COLP (n = 19) |
|---|---|---|
|
| ||
| Demographics | ||
| Age | 61.2 ± 13.0 | 59.1 ± 13.1 |
| Female | 13 (59%) | 7 (37%) |
| Non-Hispanic White | 22 (100%) | 18 (95%) |
| BMI | 31.6 ± 7.8 | 31.5 ± 8.0 |
|
| ||
| Education level | ||
| High school | 1 (5%) | 1 (5%) |
| Technical school | 0 (0%) | 1 (5%) |
| Some college | 4 (18%) | 5 (26%) |
| Associate degree | 3 (14%) | 2 (11%) |
| Bachelor’s degree | 9 (41%) | 3 (16%) |
| Master’s degree | 3 (14%) | 6 (32%) |
| Doctoral degree | 2 (9%) | 1 (5%) |
|
| ||
| Psychosocial measures | ||
| Catastrophizing | 15.1 ± 10.0 | 16.4 ± 14.0 |
| Depression | 14.2 ± 6.3 | 13.3 ± 6.1 |
| Sleep disturbance | 27.9 ± 7.3 | 26.1 ± 7.7 |
| Anxiety | 14.9 ± 6.3 | 16.3 ± 6.1 |
| Somatization | 11.1 ± 3.6 | 11.2 ± 3.4 |
| Positive affect | 31.1 ± 7.8 | 31.2 ± 7.8 |
| Negative affect | 18.5 ± 5.6 | 19.4 ± 7.2 |
|
| ||
| Pain and opioid use | ||
| BPI mean | 5.4 ± 1.4 | 5.3 ± 2.6 |
| BPI interference | 5.6 ± 2.0 | 4.6 ± 2.5 |
| Fibromyalgianess | 11.8 ± 5.0 | 9.4 ± 4.3 |
| Opioid misuse risk (SOAPP) | 3.5 ± 2.4 | 2.9 ± 2.3 |
| Taking opioid medications | 4 (18%) | 2 (11%) |
|
| ||
| Quantitative sensory testing (QST) | ||
| Temporal summation of pain (TSP) | 2.9 ± 1.6 | 2.2 ± 1.8 |
| Forearm threshold | 8.0 ± 3.0 | 9.23 ± 4.7 |
| Forearm tolerance | 12.7 ± 4.7 | 13.5 ± 5.6 |
| Trapezius threshold | 10.9 ± 4.6 | 12.9 ± 4.4 |
| Trapezius tolerance | 15.4 ± 5.0 | 16.3 ± 4.6 |
Data are given in either mean ± SD or n(percent). Treatment groups were balanced across all variables. BMI, body mass index; BPI, Brief Pain Inventory; COLP, conditioned open-label placebo; SOAPP, Screener and Opioid Assessment for Patients with Pain; TAU, treatment as usual
3.3. Surgical and anesthetic treatment
Patient surgical variables are reported in Table 2. Pain was the most frequently reported symptom present at the time of surgery (COLP = 95%; TAU = 96%), with additional symptoms including weakness (COLP = 32%; TAU = 14%), numbness (COLP = 26%; TAU = 14%), and other symptoms (COLP = 5%; TAU = 14%). Eight patients had previous spine surgery (COLP = 21%; TAU = 19%). Surgical procedures involved cervical, thoracic, lumbar, and/or sacral spine, with nearly all involving spinal fusion (COLP = 84%; TAU = 96%). All patients received general anesthesia, including both volatile and IV-based anesthetics for maintenance, with 46% of cases involving intraoperative remifentanil and sufentanil infusions. The average amount of opioids (MME/hr) consumed on the evening of surgery, both while admitted to the post-anesthesia care unit (COLP: 13.3 ± 9.2; TAU: 13.6 ± 11.0) and once transferred to the inpatient floor (COLP: 3.6 ± 2.31; TAU: 4.1 ± 2.7), was similar between groups.
Table 2.
Patient surgical variables for the TAU and COLP groups.
| Surgical variables | TAU (n = 22) | COLP (n = 19) |
|---|---|---|
|
| ||
| No. of levels | 3.1 ± 1.3 | 3.5 ± 1.4 |
|
| ||
| Duration of surgery (min) | 161 ± 49 | 168 ± 53 |
|
| ||
| Blood loss during surgery (mL) | 710 ± 632 | 622 ± 629 |
|
| ||
| Re-operation | 4 (19%) | 4 (21%) |
|
| ||
| Spinal segments involved | ||
| Cervical | 5 (23%) | 6 (32%) |
| Thoracic | 1 (5%) | 1 (5%) |
| Lumbar | 17 (77%) | 13 (68%) |
| Sacral | 1 (5%) | 3 (16%) |
|
| ||
| Surgical aspects involved | ||
| Discectomy | 3 (14%) | 5 (26%) |
| Laminectomy | 20 (91%) | 17 (90%) |
| Fusion | 21 (96%) | 16 (84%) |
| Other | 2 (9%) | 3 (16%) |
|
| ||
| Indication(s) for surgery | ||
| Spinal stenosis | 19 (86%) | 14 (74%) |
| Spondylolisthesis | 13 (59%) | 10 (53%) |
| Herniated disk | 1 (5%) | 2 (11%) |
| Fracture | 3 (14%) | 1 (5%) |
| Other | 9 (41%) | 9 (47%) |
|
| ||
| Symptoms present at time of surgery | ||
| Pain | 21 (96%) | 18 (95%) |
| Weakness | 3 (14%) | 6 (32%) |
| Numbness | 3 (14%) | 5 (26%) |
| Other | 3 (14%) | 1 (5%) |
Data are given in either mean ± SD or n(percent). Treatment groups were balanced across all surgical variables.
COLP, conditioned open-label placebo; TAU, treatment as usual.
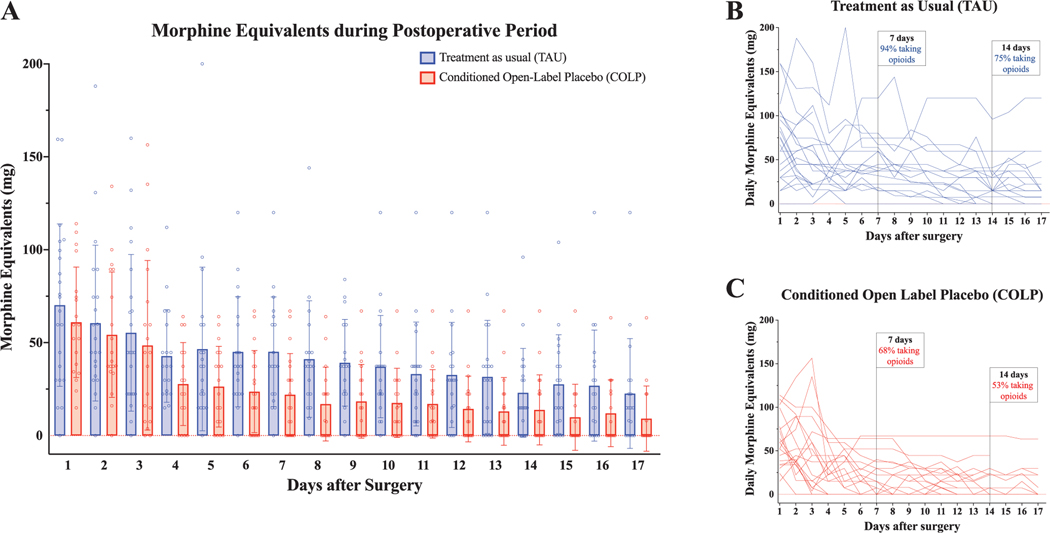
3.4. Postoperative opioid consumption
There was a significant difference in overall opioid consumption between groups on POD 1 to 17, with lower average daily MMEs consumed by patients receiving COLP (B =−15.90, 95% CI: −28.69, −3.10; Wald x2: 5.93, P = 0.015) (Fig. 3A). This represents an overall average 30% reduction in opioid use in the COLP group, although substantial intragroup variability was also observed (Figs. 3B and C). The percentage of patients still taking some opioids on POD 7 was 94% for the TAU group and 68% for the COLP group, and on POD 14 was 75% for the TAU group and 52.6% for the COLP group.
Figure 3.
Daily opioid analgesic consumption in the postoperative period. Daily opioid consumption during the postoperative period was calculated by converting all opioids to morphine milligram equivalents (MMEs) for both inpatient (approximately POD 1–3) and outpatient (approximately POD 4–17) periods. (A) Daily opioid consumption was compared between groups over time. Overall, patients randomized to COLP consumed significantly less opioids daily from POD 1 to POD 17 compared with patients in the TAU group (Wald x2: 5.93, P = 0.015). (B) Timelines of daily opioid consumption for individual patients in the TAU group (EMM: 43.1, 95% CI: [33.4, 52.9]). On POD 7, 94% of patients in the TAU group were taking opioids, with 75% of TAU patients still taking opioids on POD 14. (C) Timelines of daily opioid consumption for individual patients in the COLP group (EMM: 28.6, 95% CI: [21.1, 36.2]). On POD 7, 68% of patients in the COLP group were taking opioids, with 53% of COLP patients still taking opioids on POD 14; CI, confidence interval; COLP, conditioned open-label placebo; EMM, Estimated Marginal Mean; POD, postoperative day; TAU, treatment as usual.
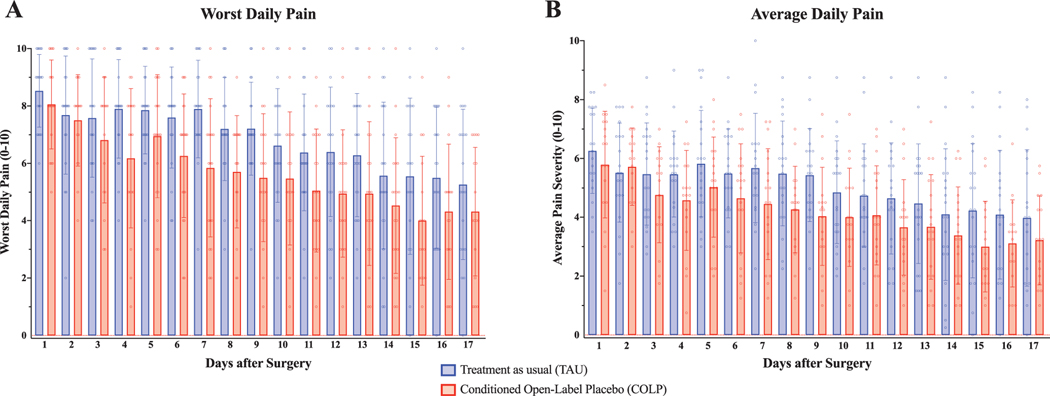
3.5. Postoperative pain
Postoperative daily worst pain and average pain scores are shown in Figure 4. The GEE analysis revealed a significant between-group difference in overall worst pain for POD 1 to 17, with significantly lower values reported among patients receiving COLP (B=−1.03, 95% CI: −1.99, −0.08, Wald x2: 4.50, P = 0.034). There was no significant difference in average pain (BPI mean) between groups (B =−0.70, 95% CI: −1.59, 0.188; Wald x2: 2.39, P = 0.122).
Figure 4.
Daily pain scores in the postoperative period. Patients provided daily reports of their worst pain and average pain experienced in the previous 24 hours. (A) Patients in the COLP group reported significantly lower worst daily pain scores in the postoperative period compared with patients in the TAU group (COLP EMM: 5.9, 95% CI: [5.2, 6.6] vs TAU EMM: 6.9, 95% CI: [6.3, 7.6]; Wald x2: 4.50, P = 0.034). (B) Patients’ average daily pain (BPI mean) experienced in the previous 24 hours was not significantly different between groups (COLP EMM: 4.4, 95% CI: [3.8, 5.0] vs TAU EMM: 5.1, 95% CI: [4.4, 5.7]; Wald x2: 2.39, P = 0.122). BPI, Brief Pain Inventory; CI, confidence interval; COLP, conditioned open-label placebo; EMM, Estimated Marginal Mean; POD, postoperative day; TAU, treatment as usual.
3.6. Exploratory analysis of differential response to conditioning with an open-label placebo among patients
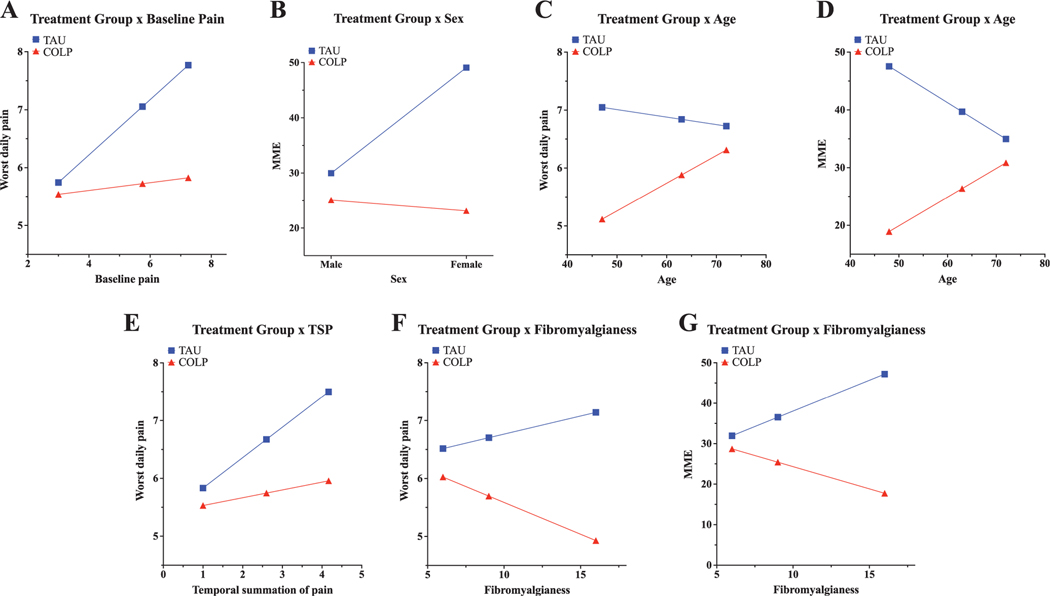
We found a significant moderating effect of baseline pain on treatment, suggesting that the COLP was more effective at lowering postoperative worst daily pain in patients who reported greater baseline pain before surgery (baseline pain 3/10: −0.20 [−0.78, 0.38] vs baseline pain 5.75/10: −1.33 [−1.69, −0.97] vs baseline pain 7.25/10: −1.94 [−2.46, −1.43]) (Fig. 5A, Appendix C, available at http://links.lww.com/PAIN/B257). We also observed a significant interaction between sex and treatment group, with the effects of COLP on postoperative opioid consumption being more pronounced in females compared with males (males: −4.89 [−11.47, 1.70] vs females: −26.0 [−33.22, −18.73]) (Fig. 5B, Appendix D, available at http://links.lww.com/PAIN/B257). Similarly, age moderated the treatment effect on both opioid use and worst pain, with younger patients receiving COLP having lower opioid utilization (age 48: −28.64 [−35.24, −22.04] vs age 63: −13.31 [−18.25, −8.38] vs age 72: −4.12 [−10.67, 2.43]) and lower worst pain (age 47: −1.93 [−2.43, −1.43] vs age 63: −0.96 [−1.33, −0.59] vs age 72: −0.41 [−0.90, 0.08]) than patients receiving TAU (Fig. 5C, Appendix D and 5D, Appendix C, available at http://links.lww.com/PAIN/B257). Baseline TSP, which tests pain amplification after a repeated stimulus, also moderated COLP efficacy, such that between-group differences in worst pain were more pronounced among patients with higher TSP (TSP 1/10: −0.30 [−0.81, 0.20] vs TSP 2.6/10: −0.93 [−1.29, −0.57] vs TSP 4.17/10: −1.54 [−2.03, −1.05]) (Fig. 5E, Appendix C, available at http://links.lww.com/PAIN/B257). Interestingly, baseline fibromyalgianess (FM) moderated treatment effect on both postoperative opioid use (FM 6: −3.25 [−10.06, 3.57] vs FM 9: −11.11 [−16.33, −5.89] vs FM 16: −29.47 [−37.59, −21.35]) and worst pain (FM 6: −0.49 [−0.99, 0.01] vs FM 9: −1.01 [−1.39, −0.62] vs FM 16 −2.21 [−2.81, −1.61]), with COLP efficacy greatest among individuals with higher baseline fibromyalgianess scores (Fig. 5F, Appendix D and Fig. 5G, Appendix C, available at http://links.lww.com/PAIN/B257).
Figure 5.
Exploratory analysis of treatment moderation by baseline patient characteristics. Interactions between treatment and baseline characteristics were assessed using the PROCESS macro in SPSS, which assesses for significant moderation of treatment effect by other variables. (A) Significant treatment x baseline pain interaction, suggesting a greater benefit of COLP among patients with higher reported baseline pain. (B) Significant treatment x sex interaction, with COLP associated with lower opioid consumption predominantly among female patients. (C and D) Significant treatment x age interaction, with COLP associated with decreased pain (C) and opioid consumption (D) among younger patients. (E) Significant treatment x TSP interaction, suggesting a greater benefit of COLP among patients higher baseline TSP. (F and G) Significant treatment x fibromyalgianess interaction, with COLP associated with lower pain (F) and daily opioid consumption (D) among patients with higher baseline fibromyalgianess scores. COLP, conditioned open-label placebo; POD, postoperative day; TAU, treatment as usual; TSP, temporal summation of pain.
4. Discussion
This RCT examined the efficacy of COLP in reducing opioid use and postoperative pain among spinal fusion patients. Compared with TAU, COLP treatment was associated with approximately 30% less postoperative daily opioid consumption and lower worst daily pain scores during the postoperative period (POD 117). Before initiating treatment, early postoperative opioid consumption and pain scores were comparable between groups (Figs. 3 and 4, POD 1 and 2). Beginning around POD 3, we observed reduced consumption of daily opioids and worst pain scores in the COLP group, which seemed to be sustained through follow-up (Fig. 3). There also seemed to be an earlier discontinuation from opioids after surgery in the COLP group, which is of interest in light of the current opioid epidemic. Exploratory moderation analyses suggested greater COLP efficacy among younger patients, women, and patients with higher overall baseline pain severity, TSP, and fibromyalgianess scores. This study is, to the best of our knowledge, the first to examine the efficacy of COLP on postoperative opioid reduction and pain.
The use of placebos in an open fashion48 obviates important ethical concerns about deception,14 which can limit the clinical application of placebo treatment. Other studies have also demonstrated OLP benefits on a variety of symptoms,20,49,53,61 including back pain,20,58 and conditioned-placebo studies have shown successful conditioning of opioid effects in laboratory experiments,3 exhibiting analgesic efficacy in the form of opioid dose reductions.23,62 The current findings suggest that a combination of these techniques (COLP) may facilitate decreased opioid requirements and reduced postoperative pain intensity after spine surgery. Of note, large survey13 and focus group3 studies indicate that participants are amenable to accepting placebo treatments if they are transparently prescribed, with one study showing that 50% to 84% of patients reported willingness to take OLP under the recommendation of their physician.40
Open-label placebo seeks to directly reduce symptoms by introducing a radical paradoxical cognitive and embodied conundrum: physicians transparently prescribe “inert” pills, while suggesting that they might have, in fact, benefits.8,48 Although OLP has sometimes produced medication dose reductions,19,20 symptom amelioration has generally been the primary target. On the other hand, classical conditioning in clinical RCTs has primarily targeted dose reduction (“dose-extension”) as the primary outcome.56 Other successful RCTs have included pairing placebos with corticosteroids in psoriasis,2 zolpidem in insomnia,69 and amphetamines in attention deficit or hyperactivity disorder.77 Such dose-extension methods mitigate extinction processes because active drugs serving as the unconditioned stimuli are interspersed with conditioned stimuli (the placebos). This type of intermittent reinforcement was likely also in play in the current study because the protocol included both pairing and scheduled pills.
With the exception of a recent small feasibility study,62 our RCT is the first that combines OLP and conditioning methodologies. An important limitation of bundling these approaches is that we cannot determine whether their effects were additive, synergistic, or otherwise. Our study is a “proof-of-concept,” and future RCTs comparing COLP vs OLP alone vs conditioning alone would clarify this issue.
Although the underlying mechanisms of COLP have not been fully delineated, neurophysiological studies have produced compelling evidence that placebo effects involve an array of pain-relevant neurotransmitters (eg, endorphins, cannabinoids, and dopamine),28,30 with placebo-evoked activation of specific, pain-relevant areas of the brain (eg, prefrontal cortex, anterior insula, rostral anterior cingulate cortex, and amygdala).90,102 Emergent research also suggests genetic signatures of likelihood to respond may exist.34,93 The question of whether similar mechanisms may operate in COLP requires further research.
One potential mechanism often posited for placebo effects is classical conditioning,5,28 which likely contributed to COLP’s benefits here. Generally, however, the placebo literature suggests that expectation is the dominant factor,57 with expectancies based on previous experiences and verbal suggestions strongly impacting placebo responses.24,71,72 It is clear that when participants in laboratory experiments involving short-term pain are told the placebo will provide pain relief, they are generally more likely to experience placebo analgesia.11,12,47 Furthermore, laboratory experiments have shown that conditioning is more powerful than verbal suggestion alone for inducing placebo effects.5,6,18 Interestingly, the findings of our qualitative research, which will be fully described in a forthcoming paper, largely found that most patients expressed skepticism or uncertainty towards our intervention, making it more likely that any such expectations were nonconscious.43,44 Another theoretical mechanism underpinning OLP is Bayesian brain/prediction coding. In most clinical situations, there is an inherent hope for and uncertainty of a clinical benefit, even in the paradoxical intervention of “nothing.” This uncertainty and hope are thought to have the potential to automatically and nonconsciously shift previous neutrally encoded sensory biases of heightened pain to sensory biases of reduced pain.50,67 Collectively, the extent to which COLP involves conditioning, expectations, Bayesian brain processes, and/or other processes is still to be determined.
Many conditioning methods have been used in human clinical situations. For instance, one study design using conditioning involved pairing an active drug (US) with a gustatory stimulus (CS) plus placebo pills,56 using 50% active medication and 50% placebos randomly arranged in blister packs, to create an intermittent reinforcement paradigm.69 In this study, our goals in finding the best conditioning approach were simplicity and transparency. Thus, the conditioning we used in this study was to simply pair the OLP pill with active analgesic medications taken by the patient. The US was the pain relief provided by these analgesic medications, and the CS was the OLP pill. Once conditioned, subsequent self-administration of this CS on its own (which started with the 33 daily scheduled dosing of OLP on postoperative day 2) might provoke the unconditioned response (ie, analgesia). As ongoing reinforcement, to avoid an extinction of this pairing, patients still also continued to pair OLP with any other analgesics, even after starting to take OLP on a schedule. Another important consideration was the temporal link of pharmacodynamic effect of the opioid compared with the timing of swallowing the OLP pill (which could be considered immediate). When opioids were administered IV, this delay would be insignificant. However, it was more typical for postoperative oxycodone to be administered by mouth, with a delay in full effect of 10 to 20 minutes, which provides a slower, prolonged analgesic profile and less of an euphoric effect. It is unknown what the critical closeness in time is for effective pairing, but it is possible that this relative temporal mismatch may have somewhat decreased the effectiveness of the pairing in this case.
In the era of personalized medicine, it is important to examine which patients benefit most from COLP,10 considering that the magnitudes of placebo and conditioning responses are quite variable.51,72,84,91 Variation in a placebo response has been attributed to patient expectation of clinical benefit,24,84,91,92 extent of conditioning,3,56 individual psychosocial characteristics,24,29,63,83 and baseline variability and uncertainty.7,27,41,87 Previous studies suggest that some patient characteristics are associated with greater placebo efficacy, including female sex60,65,66,99 and younger age.37,74,86,99 These results are consistent with our findings suggesting an interaction of COLP treatment with both sex and age.
In addition, patients scoring high on indices of central sensitization, including TSP and fibromyalgianess, benefitted most from COLP. Temporal summation of pain, a measure of central pain-facilitatory processes, predicts greater acute1,39,82,95 and persistent postsurgical pain.26,32,70,98 Previous findings have also suggested enhanced benefits of nonpharmacologic treatments, such as high-frequency TENS, in back pain in patients who showed the greatest QST-assessed pain sensitivity.42 Similarly, higher fibromyalgianess scores,97 which predict greater persistent postsurgical pain severity and opioid consumption,15 were related to greater COLP benefit. One possible explanation for the observed moderation effects may be that the neural mechanisms by which COLP provides benefits overlap most closely with the neural mechanisms that are indirectly assessed by central sensitization indices, such as TSP and fibromyalgianess. Future studies are needed to specifically investigate whether “central sensitization” phenotypes may respond more favorably to COLP.
4.1. Limitations
Although this study found both a significant main effect of COLP on opioid consumption and worst pain, the sample size was relatively small, and definitive conclusions regarding the efficacy of COLP require a larger RCT. The observed moderating effects of age, sex, fibromyalgianess, and TSP on COLP treatment are exploratory, although these preliminary findings suggest the utility of careful preoperative patient phenotyping in future trials. In addition, participants were recruited at a tertiary referral hospital from a single surgeon and surgical type (spine surgery), potentially limiting the generalizability to other patients and settings. As with any RCT, a self-selection bias by participants willing to volunteer for a nonpharmacological clinical trial may have also been operative. Furthermore, as the trial centered around acute postoperative pain and opioid use, the findings may not be extrapolated to longer-term COLP effects or COLP effects on nonopioid analgesics. Finally, it is unknown to what extent OLP, conditioning, or some interaction/combination was this intervention’s active ingredient.
4.2. Conclusion
The findings suggest that this innovative treatment approach combining conditioning and open-label placebo has the potential to serve as an analgesic adjuvant, potentially reducing pain, lowering opioid requirements, and facilitating earlier opioid tapering in the postoperative period.
Supplementary Material
Acknowledgements
The authors thank all of the patients who took time to participate in this research study.
The conduct of this study was supported by funding from the Foundation for the Study of the Therapeutic Encounter and the Department of Anesthesiology, Perioperative, and Pain Medicine at BWH.
Footnotes
Appendix A. Supplemental digital content
Supplemental digital content associated with this article can be found online at http://links.lww.com/PAIN/B257.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.painjournalonline.com).
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Conflict of interest statement
The authors have no conflicts of interest to declare.
References
- [1].Abrecht CR, Cornelius M, Wu A, Jamison RN, Janfaza D, Urman RD, Campbell C, Smith M, Haythornthwaite J, Edwards RR. Prediction of pain and opioid utilization in the perioperative period in patients undergoing primary knee arthroplasty: psychophysical and psychosocial factors. Pain Med 2019;20:161–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ader R, Mercurio MG, Walton J, James D, Davis M, Ojha V, Kimball AB, Fiorentino D. Conditioned pharmacotherapeutic effects: a preliminary study. Psychosomatic Med 2010;72:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Amanzio M, Benedetti F. Neuropharmacological dissection of placebo analgesia: expectation-activated opioid systems versus conditioning-activated specific subsystems. J Neurosci 1999;19:484–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ashar JK, Gordon A, Lumley M, Geuter S, Flood T, Clark J, Anderson Z, Polisky L, Carlisle J, Dimidjian S, Wager T. Open-label placebo injection for chronic back pain: a randomized controlled trial. In: P3.51, 2nd Official SIPS Conference, Society for Interdisciplinary Placebo Studies (SIPS), 2019. [Google Scholar]
- [5].Bąbel P Classical conditioning as a distinct mechanism of placebo effects. Front Psychiatry 2019;10:449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bajcar EA, Wiercioch-Kuzianik K, Adamczyk WM, Bąbel P. To experience or to be informed? Classical conditioning induces nocebo hyperalgesia even when placebo analgesia is verbally suggested—results of a preliminary study. Pain Med 2020;21:548–60. [DOI] [PubMed] [Google Scholar]
- [7].Ballou S, Beath A, Kaptchuk TJ, Hirsch W, Sommers T, Nee J, Iturrino J, Rangan V, Singh P, Jones M. Factors associated with response to placebo in patients with irritable bowel syndrome and constipation. Clin Gastroenterol Hepatol 2018;16:1738–44. e1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ballou S, Kaptchuk TJ, Hirsch W, Nee J, Iturrino J, Hall KT, Kelley JM, Cheng V, Kirsch I, Jacobson E. Open-label versus double-blind placebo treatment in irritable bowel syndrome: study protocol for a randomized controlled trial. Trials 2017;18:234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Benedetti F, Amanzio M, Maggi G. Potentiation of placebo analgesia by proglumide. Lancet 1995;346:1231. [DOI] [PubMed] [Google Scholar]
- [10].Benedetti F, Piedimonte A, Frisaldi E. How do placebos work? Eur J Psychotraumatol 2018;9:1533370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Benedetti F, Pollo A, Lopiano L, Lanotte M, Vighetti S, Rainero I. Conscious expectation and unconscious conditioning in analgesic, motor, and hormonal placebo/nocebo responses. J Neurosci 2003;23: 4315–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bingel U, Wanigasekera V, Wiech K, Mhuircheartaigh RN, Lee MC, Ploner M, Tracey I. The effect of treatment expectation on drug efficacy: imaging the analgesic benefit of the opioid remifentanil. Sci translational Med 2011;3:70ra14. [DOI] [PubMed] [Google Scholar]
- [13].Blease C, Colloca L, Kaptchuk TJ. Are open-label placebos ethical? Informed consent and ethical equivocations. Bioethics 2016;30: 407–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Blease CR, Bishop FL, Kaptchuk TJ. Informed consent and clinical trials: where is the placebo effect? BMJ 2017;356:j463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Brummett CM, Urquhart AG, Hassett AL, Tsodikov A, Hallstrom BR, Wood NI, Williams DA, Clauw DJ. Characteristics of fibromyalgia independently predict poorer long-term analgesic outcomes following total knee and hip arthroplasty. Arthritis Rheumatol 2015;67:1386–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Brummett CM, Waljee JF, Goesling J, Moser S, Lin P, Englesbe MJ, Bohnert AS, Kheterpal S, Nallamothu BK. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surg 2017;152:e170504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Butler SF, Budman SH, Fernandez KC, Fanciullo GJ, Jamison RN. Cross-validation of a screener to predict opioid misuse in chronic pain patients (SOAPP-R). J Addict Med 2009;3:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Carlino E, Guerra G, Piedimonte A. Placebo effects: from pain to motor performance. Neurosci Lett 2016;632:224–30. [DOI] [PubMed] [Google Scholar]
- [19].Carvalho C The effect of nothing? Time to abandon the concept of placebo [Reply to editor]. PAIN 2017;158:1179. [DOI] [PubMed] [Google Scholar]
- [20].Carvalho C, Caetano JM, Cunha L, Rebouta P, Kaptchuk TJ, Kirsch I. Open-label placebo treatment in chronic low back pain: a randomized controlled trial. PAIN 2016;157:2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, Amtmann D, Bode R, Buysse D, Choi S. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol 2010;63:1179–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Charlesworth JE, Petkovic G, Kelley JM, Hunter M, Onakpoya I, Roberts N, Miller FG, Howick J. Effects of placebos without deception compared with no treatment: a systematic review and meta‐analysis. J Evidence Based Med 2017;10:97–107. [DOI] [PubMed] [Google Scholar]
- [23].Colloca L, Enck P, DeGrazia D. Relieving pain using dose-extending placebos: a scoping review. PAIN 2016;157:1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].De Pascalis V, Chiaradia C, Carotenuto E. The contribution of suggestibility and expectation to placebo analgesia phenomenon in an experimental setting. PAIN 2002;96:393–402. [DOI] [PubMed] [Google Scholar]
- [25].Durá E, Andreu Y, Galdón MJ, Ferrando M, Murgui S, Poveda R, Jimenez Y. Psychological assessment of patients with temporomandibular disorders: confirmatory analysis of the dimensional structure of the Brief Symptoms Inventory 18. J Psychosomatic Res 2006;60:365–70. [DOI] [PubMed] [Google Scholar]
- [26].Edwards RR, Mensing G, Cahalan C, Greenbaum S, Narang S, Belfer I, Schreiber KL, Campbell C, Wasan AD, Jamison RN. Alteration in pain modulation in women with persistent pain after lumpectomy: influence of catastrophizing. J Pain Symptom Manage 2013;46:30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Farrar JT, Troxel AB, Haynes K, Gilron I, Kerns RD, Katz NP, Rappaport BA, Rowbotham MC, Tierney AM, Turk DC. Effect of variability in the 7-day baseline pain diary on the assay sensitivity of neuropathic pain randomized clinical trials: an ACTTION study. PAIN 2014;155:1622–31. [DOI] [PubMed] [Google Scholar]
- [28].Finniss DG, Kaptchuk TJ, Miller F, Benedetti F. Placebo effects: biological, clinical and ethical advances. Lancet 2010;375:686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Geers AL, Helfer SG, Kosbab K, Weiland PE, Landry SJ. Reconsidering the role of personality in placebo effects: dispositional optimism, situational expectations, and the placebo response. J psychosomatic Res 2005;58:121–7. [DOI] [PubMed] [Google Scholar]
- [30].Geuter S, Koban L, Wager TD. The cognitive neuroscience of placebo effects: concepts, predictions, and physiology. Annu Rev Neurosci 2017;40:167–88. [DOI] [PubMed] [Google Scholar]
- [31].Goebel MU, Meykadeh N, Kou W, Schedlowski M, Hengge UR. Behavioral conditioning of antihistamine effects in patients with allergic rhinitis. Psychother Psychosom 2008;77:227–34. [DOI] [PubMed] [Google Scholar]
- [32].Gwilym SE, Oag HC, Tracey I, Carr AJ. Evidence that central sensitisation is present in patients with shoulder impingement syndrome and influences the outcome after surgery. J Bone Joint Surg Br 2011;93:498–502. [DOI] [PubMed] [Google Scholar]
- [33].Hadamitzky M, Lückemann L, Pacheco-López G, Schedlowski M. Pavlovian conditioning of immunological and neuroendocrine functions. Physiol Rev 2020;100:357–405. [DOI] [PubMed] [Google Scholar]
- [34].Hall KT, Loscalzo J, Kaptchuk TJ. Genetics and the placebo effect: the placebome. Trends Molecular Medicine 2015;21:285–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hayes AF. Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. New York, NY: Guilford publications, 2017. [Google Scholar]
- [36].Herrnstein RJ. Placebo effect in the rat. Science 1962;138:677–8. [DOI] [PubMed] [Google Scholar]
- [37].Ho TW, Fan X, Rodgers A, Lines CR, Winner P, Shapiro RE. Age effects on placebo response rates in clinical trials of acute agents for migraine: pooled analysis of rizatriptan trials in adults. Cephalalgia 2009;29: 711–18. [DOI] [PubMed] [Google Scholar]
- [38].Hoenemeyer TW, Kaptchuk TJ, Mehta TS, Fontaine KR. Open-label placebo treatment for cancer-related fatigue: a randomized-controlled clinical trial. Scientific Rep 2018;8:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Hsu YW, Somma J, Hung YC, Tsai PS, Yang CH, Chen CC. Predicting postoperative pain by preoperative pressure pain assessment. Anesthesiology 2005;103:613–18. [DOI] [PubMed] [Google Scholar]
- [40].Hull SC, Colloca L, Avins A, Gordon NP, Somkin CP, Kaptchuk TJ, Miller FG. Patients’ attitudes about the use of placebo treatments: telephone survey. BMJ 2013;347:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Irizarry MC, Webb DJ, Ali Z, Chizh BA, Gold M, Kinrade FJ, Meisner PD, Blum D, Silver MT, Weil JG. Predictors of placebo response in pooled lamotrigine neuropathic pain clinical trials. Clin J Pain 2009;25:469–76. [DOI] [PubMed] [Google Scholar]
- [42].Jamison RN, Wan L, Edwards RR, Mei A, Ross EL. Outcome of a high-frequency transcutaneous electrical nerve stimulator (hfTENS) device for low back pain: a randomized controlled trial. Pain Pract 2019;19: 466–75. [DOI] [PubMed] [Google Scholar]
- [43].Jensen KB, Kaptchuk TJ, Chen X, Kirsch I, Ingvar M, Gollub RL, Kong J. A neural mechanism for nonconscious activation of conditioned placebo and nocebo responses. Cereb Cortex 2015;25:3903–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Jensen KB, Kaptchuk TJ, Kirsch I, Raicek J, Lindstrom KM, Berna C, Gollub RL, Ingvar M, Kong J. Nonconscious activation of placebo and nocebo pain responses. Proc Natl Acad Sci 2012;109:15959–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Jensen MP, Keefe FJ, Lefebvre JC, Romano JM, Turner JA. One-and two-item measures of pain beliefs and coping strategies. PAIN 2003; 104:453–69. [DOI] [PubMed] [Google Scholar]
- [46].Jivraj NK, Scales DC, Gomes T, Bethell J, Hill A, Pinto R, Wijeysundera DN, Wunsch H. Evaluation of opioid discontinuation after non-orthopaedic surgery among chronic opioid users: a population-based cohort study. Br J Anaesth 2020;124:281–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Kam-Hansen S, Jakubowski M, Kelley JM, Kirsch I, Hoaglin DC, Kaptchuk TJ, Burstein R. Labeling of medication and placebo alters the outcome of episodic migraine attacks. Sci translational Med 2014;6: 218ra215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Kaptchuk TJ. Open-label placebo: reflections on a research agenda. Perspect Biol Med 2018;61:311–34. [DOI] [PubMed] [Google Scholar]
- [49].Kaptchuk TJ, Friedlander E, Kelley JM, Sanchez MN, Kokkotou E, Singer JP, Kowalczykowski M, Miller FG, Kirsch I, Lembo AJ. Placebos without deception: a randomized controlled trial in irritable bowel syndrome. PloS One 2010;5:e15591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Kaptchuk TJ, Hemond CC, Miller FG. Placebos in chronic pain: evidence, theory, ethics, and use in clinical practice. BMJ 2020;370: 1668. [DOI] [PubMed] [Google Scholar]
- [51].Kaptchuk TJ, Kelley JM, Deykin A, Wayne PM, Lasagna LC, Epstein IO, Kirsch I, Wechsler ME. Do “placebo responders” exist? Contemp Clin Trials 2008;29:587–95. [DOI] [PubMed] [Google Scholar]
- [52].Kaptchuk TJ, Miller FG. Placebo effects in medicine. N Engl J Med 2015; 373:8–9. [DOI] [PubMed] [Google Scholar]
- [53].Kelley JM, Kaptchuk TJ, Cusin C, Lipkin S, Fava M. Open-label placebo for major depressive disorder: a pilot randomized controlled trial. Psychother Psychosom 2012;81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Kent ML, Tighe PJ, Belfer I, Brennan TJ, Bruehl S, Brummett CM, Buckenmaier CC, Buvanendran A, Cohen RI, Desjardins P. The ACTTION–APS–AAPM Pain Taxonomy (AAAPT) multidimensional approach to classifying acute pain conditions. Pain Med 2017;18: 947–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Kim HJ, Park JH, Kim JW, Kang KT, Chang BS, Lee CK, Yeom JS. Prediction of postoperative pain intensity after lumbar spinal surgery using pain sensitivity and preoperative back pain severity. Pain Med 2014;15:2037–45. [DOI] [PubMed] [Google Scholar]
- [56].Kirchhof J, Petrakova L, Brinkhoff A, Benson S, Schmidt J, Unteroberdörster M, Wilde B, Kaptchuk TJ, Witzke O, Schedlowski M. Learned immunosuppressive placebo responses in renal transplant patients. Proc Natl Acad Sci 2018;115:4223–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Kirsch I Response expectancy as a determinant of experience and behavior. Am Psychol 1985;40:1189. [Google Scholar]
- [58].Kleine-Borgmann J, Schmidt K, Hellmann A, Bingel U. Effects of open-label placebo on pain, functional disability, and spine mobility in patients with chronic back pain: a randomized controlled trial. PAIN 2019;160: 2891–7. [DOI] [PubMed] [Google Scholar]
- [59].Machado GC, Maher CG, Ferreira PH, Pinheiro MB, Lin C-WC, Day RO, McLachlan AJ, Ferreira ML. Efficacy and safety of paracetamol for spinal pain and osteoarthritis: systematic review and meta-analysis of randomised placebo controlled trials. BMJ 2015;350:1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Mallinckrodt CH, Zhang L, Prucka WR, Millen BA. Signal detection and placebo response in schizophrenia: parallels with depression. Psychopharmacol Bull 2010;43:53–72. [PubMed] [Google Scholar]
- [61].Meeuwis SH, Van Middendorp H, Veldhuijzen DS, Van Laarhoven AI, De Houwer J, Lavrijsen AP, Evers AW. Placebo effects of open-label verbal suggestions on itch. Acta Dermato-Venereologica 2018;98:268–74. [DOI] [PubMed] [Google Scholar]
- [62].Morales-Quezada L, Mesia-Toledo I, Estudillo-Guerra A, O’Connor KC, Schneider JC, Sohn DJ, Crandell DM, Kaptchuk T, Zafonte R. Conditioning open-label placebo: a pilot pharmacobehavioral approach for opioid dose reduction and pain control. Pain Rep 2020; 5:828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Morton DL, Watson A, El-Deredy W, Jones AK. Reproducibility of placebo analgesia: effect of dispositional optimism. PAIN 2009;146: 194–8. [DOI] [PubMed] [Google Scholar]
- [64].Olliges ES, Haile A, Malhis M, Funke A, Karin M. Open-label placebos for elderly patients with chronic knee pain: Effects on pain, functionality, and quality of life, 2nd Official SIPS Conference, Society for Interdisciplinary Placebo Studies (SIPS), 7 – 9 July 2019 in Leiden, the Netherlands. [Google Scholar]
- [65].Olson EM, Akintola T, Phillips J, Blasini M, Haycock NR, Martinez PE, Greenspan JD, Dorsey SG, Wang Y, Colloca L. Effects of sex on placebo effects in chronic pain participants: a cross-sectional study. PAIN 2021; 162:531–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Ondo WG, Hossain MM, Gordon MF, Reess J. Predictors of placebo response in restless legs syndrome studies. Neurology 81:193–4, 2013. [DOI] [PubMed] [Google Scholar]
- [67].Ongaro G, Kaptchuk TJ. Symptom perception, placebo effects, and the Bayesian brain. PAIN 2019;160:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Pavlov IP: Conditioned reflexes: An investigation of the physiological activity of the cerebral cortex. Anrep GV, editors. London: Oxford University Press, 1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Perlis M, Grandner M, Zee J, Bremer E, Whinnery J, Barilla H, Andalia P, Gehrman P, Morales K, Thase M. Durability of treatment response to zolpidem with three different maintenance regimens: a preliminary study. Sleep Med 2015;16:1160–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Petersen KK, Simonsen O, Laursen MB, Arendt-Nielsen L. The role of preoperative radiologic severity, sensory testing, and temporal summation on chronic postoperative pain following total knee arthroplasty. Clin J Pain 2018;34:193–7. [DOI] [PubMed] [Google Scholar]
- [71].Pollo A, Carlino E, Benedetti F. Placebo mechanisms across different conditions: from the clinical setting to physical performance. Philosophical Trans R Soc B 2011;366:1790–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Price DD, Milling LS, Kirsch I, Duff A, Montgomery GH, Nicholls SS. An analysis of factors that contribute to the magnitude of placebo analgesia in an experimental paradigm. PAIN 1999;83:147–56. [DOI] [PubMed] [Google Scholar]
- [73].Reid GJ, Gilbert CA, McGrath PJ. The pain coping questionnaire: preliminary validation. PAIN 1998;76:83–96. [DOI] [PubMed] [Google Scholar]
- [74].Rheims S, Cucherat M, Arzimanoglou A, Ryvlin P. Greater response to placebo in children than in adults: a systematic review and meta-analysis in drug-resistant partial epilepsy. Plos Med 2008;5:e166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Roelofs PD, Deyo RA, Koes BW, Scholten RJ, van Tulder MW. Nonsteroidal anti-inflammatory drugs for low back pain. Cochrane database Syst Rev 2008;33:1766–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Rolke R, Baron R, Maier Ca, Tölle T, Treede R-D, Beyer A, Binder A, Birbaumer N, Birklein F, Bötefür I. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): standardized protocol and reference values. PAIN 2006;123:231–43. [DOI] [PubMed] [Google Scholar]
- [77].Sandler AD, Glesne CE, Bodfish JW. Conditioned placebo dose reduction: a new treatment in ADHD? J Dev Behav Pediatr JDBP 2010;31:369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Schaefer M, Harke R, Denke C. Open-label placebos improve symptoms in allergic rhinitis: A randomized controlled trial. Psychotherapy and Psychosomatics 2016;83:373–74. [DOI] [PubMed] [Google Scholar]
- [79].Schedlowski M, Enck P, Rief W, Bingel U. Neuro-bio-behavioral mechanisms of placebo and nocebo responses: implications for clinical trials and clinical practice. Pharmacol Rev 2015;67:697–730. [DOI] [PubMed] [Google Scholar]
- [80].Schneider T, Luethi J, Mauermann E, Bandschapp O, Ruppen W. Pain response to open label placebo in induced acute pain in healthy adult males. Anesthesiology 2020;132:571–80. [DOI] [PubMed] [Google Scholar]
- [81].Schreiber KL, Martel MO, Shnol H, Shaffer JR, Greco C, Viray N, Taylor LN, McLaughlin M, Brufsky A, Ahrendt G, Bovbjerg D, Edwards RR, Belfer I. Persistent pain in postmastectomy patients: comparison of psychophysical, medical, surgical, and psychosocial characteristics between patients with and without pain. PAIN 2013;154:660–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Schreiber KL, Zinboonyahgoon N, Xu X, Spivey T, King T, Dominici L, Partridge A, Golshan M, Strichartz G, Edwards RR. Preoperative psychosocial and psychophysical phenotypes as predictors of acute pain outcomes after breast surgery. J Pain 2019;20:540–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Schweinhardt P, Seminowicz DA, Jaeger E, Duncan GH, Bushnell MC. The anatomy of the mesolimbic reward system: a link between personality and the placebo analgesic response. J Neurosci 2009;29:4882–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Scott DJ, Stohler CS, Egnatuk CM, Wang H, Koeppe RA, Zubieta J-K. Individual differences in reward responding explain placebo-induced expectations and effects. Neuron 2007;55:325–36. [DOI] [PubMed] [Google Scholar]
- [85].Sullivan MJ, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess 1995;7:524. [Google Scholar]
- [86].Sun H, Bastings E, Temeck J, Smith PB, Men A, Tandon V, Murphy D, Rodriguez W. Migraine therapeutics in adolescents: a systematic analysis and historic perspectives of triptan trials in adolescents. JAMA Pediatr 2013;167:243–9. [DOI] [PubMed] [Google Scholar]
- [87].Talley N, Locke G, Lahr B, Zinsmeister A, Cohard‐Radice M, D’elia T, Tack J, Earnest D. Predictors of the placebo response in functional dyspepsia. Aliment Pharmacol Ther 2006;23:923–36. [DOI] [PubMed] [Google Scholar]
- [88].Tan G, Jensen MP, Thornby JI, Shanti BF. Validation of the brief pain inventory for chronic nonmalignant pain. J Pain 2004;5:133–7. [DOI] [PubMed] [Google Scholar]
- [89].Tracey I Getting the pain you expect: mechanisms of placebo, nocebo and reappraisal effects in humans. Nat Med 2010;16:1277–83. [DOI] [PubMed] [Google Scholar]
- [90].Wager TD, Atlas LY. The neuroscience of placebo effects: connecting context, learning and health. Nat Rev Neurosci 2015;16:403–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Wager TD, Atlas LY, Leotti LA, Rilling JK. Predicting individual differences in placebo analgesia: contributions of brain activity during anticipation and pain experience. J Neurosci 2011;31:439–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, Kosslyn SM, Rose RM, Cohen JD. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science 2004;303:1162–7. [DOI] [PubMed] [Google Scholar]
- [93].Wang R-S, Hall KT, Giulianini F, Passow D, Kaptchuk TJ, Loscalzo J. Network analysis of the genomic basis of the placebo effect. JCI insight 2017;2:e93911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Personal Soc Psychol 1988;54:1063. [DOI] [PubMed] [Google Scholar]
- [95].Werner MU, Mjobo HN, Nielsen PR, Rudin A. Prediction of postoperative pain: a systematic review of predictive experimental pain studies. Anesthesiology 2010;112:1494–502. [DOI] [PubMed] [Google Scholar]
- [96].Williams CM, Maher CG, Latimer J, McLachlan AJ, Hancock MJ, Day RO, Lin C-WC. Efficacy of paracetamol for acute low-back pain: a double-blind, randomised controlled trial. Lancet 2014;384:1586–96. [DOI] [PubMed] [Google Scholar]
- [97].Wolfe F, Clauw DJ, Fitzcharles M-A, Goldenberg DL, Häuser W, Katz RS, Mease P, Russell AS, Russell IJ, Winfield JB. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: a modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia. J Rheumatol 2011;38:1113–22. [DOI] [PubMed] [Google Scholar]
- [98].Yarnitsky D, Crispel Y, Eisenberg E, Granovsky Y, Ben-Nun A, Sprecher E, Best LA, Granot M. Prediction of chronic post-operative pain: preoperative DNIC testing identifies patients at risk. PAIN 2008;138:22–8. [DOI] [PubMed] [Google Scholar]
- [99].Yildiz A, Vieta E, Tohen M, Baldessarini RJ. Factors modifying drug and placebo responses in randomized trials for bipolar mania. Int J Neuropsychopharmacol 2011;14:863–75. [DOI] [PubMed] [Google Scholar]
- [100].Zeng Y, Hu D, Yang W, Hayashinaka E, Wada Y, Watanabe Y, Zeng Q, Cui Y. A voxel-based analysis of neurobiological mechanisms in placebo analgesia in rats. Neuroimage 2018;178:602–12. [DOI] [PubMed] [Google Scholar]
- [101].Zou K, Wong J, Abdullah N, Chen X, Smith T, Doherty M, Zhang W. Examination of overall treatment effect and the proportion attributable to contextual effect in osteoarthritis: meta-analysis of randomised controlled trials. Ann Rheum Dis 2016;75:1964–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Zunhammer M, Bingel U, Wager TD. Placebo effects on the neurologic pain signature: a meta-analysis of individual participant functional magnetic resonance imaging data. JAMA Neurol 2018;75:1321–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.