Abstract
Prostate cancer (PCa) is recognized as a disease possessing not only great variation in its geographic and racial distribution but also tremendous variation in its potential to cause morbidity and death and it, therefore, ought not to be considered a homogenous disease entity. Morbidity and death from PCa are disproportionately higher in men of African ancestry (MAA) who are generally observed to have more aggressive disease and worse outcomes following treatment compared to men of European ancestry (MEA). The higher rates of PCa among MAA relative to MEA appear to be multifactorial and related to inherent differences in biological aggressiveness; a continued lack of awareness of the disease and methods of prevention; a lower prevalence of screen-detected PCa; comparatively lower access to quality healthcare as well as systemic and institutionalized disparities in the administration of optimal care to MAA in developed countries such as the United States of America where high-quality care is available. Even when access to quality healthcare is assured in equal access settings, it appears that MAA still have worse outcomes after PCa treatment stage-for-stage and grade-for-grade compared to MEA, suggesting that, inherent racial, ethnic and biological differences are paramount in predicting poor outcomes. This review has explored the different contributing factors to the current disparities in PCa incidence and mortality rates with emphasis on the incongruence in how research has been conducted in understanding the disease towards developing therapies.
Keywords: men of African ancestry, men of European ancestry, Caucasian, Black, White
1. Introduction
The paradigm of cancer disparities is well established1 and encapsulates differences in cancer outcomes such as, genetic and biological factors, health care access and socioeconomic factors among others (FIG 1) across population groups. It is reported by the CDC’s Office of Minority Health Equity2 that while life expectancy and overall health have improved for most Americans in recent years from cancer, this trend is not experienced by all Americans. Furthermore, in a review by3, African Americans irrespective of gender are reported to face considerable disparity in cancer incidence and mortality rates as African American men have a 25% higher incidence and 43% higher mortality rate for all cancers combined in comparison to African American women4, 5.
Figure 1:
Showing risk factors associated with cancer disparities.
This review will focus on prostate cancer (PCa), one of the most diagnosed malignancies among men globally. PCa is the leading cause of cancer deaths in men worldwide. In general MAA carry the highest burden of PCa worldwide and the disease appears to be more aggessive in comparison to other racial groups leading to higher mortality rates. The disease is the second leading cause of cancer-related deaths in the USA6 while the Caribbean has the highest age-standardized PCa mortality in the world7. The quest for seeking to make sense of and addressing the current disparity in PCa incidence and mortality rates among men of African ancestry (MAA) cannot be done in isolation of a comprehensive examination of this variable disease for which diagnosis and prognosis are not that lucid. Indeed, tackling the solution of minimizing such a disparity has to engage a concerted effort from public and private sectors, having shown that socioeconomic status (SES) through modifiable risk factors can contribute to a patient succumbing to PCa mortality. Notwithstanding, this review not only highlights the disparity of PCa incidence and mortality rates but the disparity in research geared to understanding the disease towards developing therapies for MAA compared to men of European ancestry (MEA). Fairly recent data indicates a decline in PCa incidence, yet, the overall PCa-related mortality continues to rise among MAA8.
2. Prostate Cancer
2.1. The disease
Clinical suspicion of the presence of PCa can be assessed by the detection of an abnormal feeling prostate during the digital rectal examination (DRE) and/or abnormal prostate specific antigen (PSA). PCa is typically diagnosed with the aid of a trans-rectal ultrasound-guided prostate biopsy. Standard prostate biopsy protocols involve pre-procedural antibiotics that are known to penetrate prostatic tissue, given to reduce the likelihood of acute bacterial prostatitis caused by the inevitable inoculation of coliforms (gram negative bacteria) into the prostate during trans-rectal biopsy.
The dilemma in evaluating and treating patients with PCa has always been to accurately and precisely risk stratify the patient with respect to the potential for disease progression and death so that a patient who is destined to run a relatively innocuous course and can be left alone while aggressively treating those fated to die from the disease thereby hopefully averting this outcome. It is now recognized that significant harms have been done by the over-detection and over-treatment of men with innocuous forms of PCa over the last several decades9, 10. This has been caused by the indiscriminate application of prostate-specific antigen (PSA)-based screening to populations of men at low risk of death from PCa. This prompted the United States Preventive Services Task Force (USPSTF) in 2012 to recommend against the use of PSA-based screening in all men11. In making this recommendation the USPSTF failed to employ a more nuanced approach which took into consideration the disproportionately higher risk of death of MAA from PCa. MAA possess a greater likelihood of deriving more benefits than harms from PSA-based screening when compared to men of European ancestry (MEA).
Being of African ancestry is one of only three established risk factors for PCa, the other two being a family history and advancing age. PCa mortality is highest in the Caribbean region where most countries are comprised predominantly of persons of African ancestry12, 13. African-American men are almost 2.5 times as likely to die from PCa than their Caucasian-American counterparts14. MAA are disproportionately at a higher risk of death from PCa compared to their Caucasian counterparts- stage for stage and this is related to not only differences in biologic aggressiveness but also to systematic under-treatment resulting from institutionalized racism in the approach to treating PCa15. Significant barriers exist to accessing quality care, continued unawareness of the disease and its risk factors, and unequal distribution of risk factors for poor outcomes such as obesity, cigarette smoking and sedentary lifestyle15, 16.
We now recognize that PSA-based screening for PCa is an effective and efficacious method of preventing death from PCa, but it ought to be applied selectively to populations of men at high risk of morbidity and death from the disease who are most likely to derive maximum benefit from treatment of screen-detected cancers while minimizing harms. Populations of MAA are inherently at highest risk of death from PCa and therefore theoretically stand to benefit the most from screening. It is prudent to focus on preventive approaches on this high-risk group. While primary prevention in terms of modification of diet and lifestyle may have some merit, it really is in secondary prevention or PSA-based screening that an immediate impact on PCa mortality will be seen.
Men belonging to populations characterized by high risk of death from PCa such as Afro-Caribbean populations are recommended to start PSA-based screening at age 40 years. In Jamaica, for instance, the Jamaica Urological Society in partnership with the Jamaica Cancer Society recommends PSA-based screening for PCa beginning at age 40 years and the latter entity actively screens men who walk into its office13. Two thirds of urologists belonging to the Caribbean Urological Association support PSA-based screening in Afro-Caribbean men and recommend that Caribbean-specific guidelines should be drafted17. In Afro-Caribbean and African countries, far too many men present with features of advanced or metastatic PCa, an incurable stage of the disease which contributes to the high mortality rate in the Caribbean and sub-Saharan Africa. Features of locally-advanced or metastatic PCa include lower urinary tract symptoms (LUTS), urinary retention, haematuria, lower limb oedema, anorexia, weight loss and bony pain, usually arising from the lower back or pelvis.
Late presentation of the disease may be related to ignorance surrounding the disease, particularly its asymptomatic nature when organ-confined; financial constraints; lack of insurance coverage; fear of the DRE; fear of a diagnosis of cancer or the potential adverse functional outcomes resulting from treatment such as urinary incontinence and erectile dysfunction; a false perception of manhood, fatalism surrounding the disease and its treatment, a false perception that men don’t die from the disease but with it, lack of specialists, and a mal-distribution of the availability of specialist urological care in which urologists concentrate in the cities but are not present in rural areas. In countries like Jamaica with very mountainous terrain, geographical barriers to accessing care may also play a role.
PCa is recognized as a disease possessing great variation in its potential to cause morbidity and death and therefore it cannot be thought of as a homogenous disease entity. This wide variation in the natural history or phenotypic behaviour of PCa is partially predicated on underlying differences in genotype which confers its biologic aggressive potential. Genotypic differences in PCa are correlated with race and it has inconsistently been found that MAA have poorer outcomes when stage and grade of the disease as well as socioeconomic factors are controlled, suggesting that the genotypes of PCa in MAA are associated with worse outcomes18. A further challenge is that a patient’s underlying genotype is not static but may be modified epigenetically or because of genetic instability as the disease progresses19.
2.2. The disease in men of African ancestry
With the current advances in technology for early detection and treatment, there has been a steady decline in PCa incidence as well as mortality among MAA that is particularly evident in the United States20. In contrast, studies show that PCa incidence is increasing for MAA in Africa and the Caribbean21–24. Disparities in incidence and mortality is most evident between Black and White men20, 22, 25. In the USA, for example, despite the declining rates, PCa incidence and mortality rates have consistently remained highest among MAA in contrast to other racial groups20, 26. Similar disparities have also been observed between Black and White men in Africa22.
Populations in Asia and Europe were reported to have lower PCa incidence and mortality rates in comparison to MAA populations with the exception of Africa where incidence rates were lower than expected, possibly due to under-reporting20, 26, 27 (Table 1).
Table 1:
Incidence and mortality rates comparing MAA populations and other Populations
| MAD Populations | Age-adjusted rates per 100,000, ASR (World) | |
|---|---|---|
| Incidence | Mortality | |
| Africa | 26.6 | 14.6 |
| Caribbean | 64.2 | 25.4 |
| USA - MAD | 145.9 | 23.2 |
| Other Populations | - | - |
| Europe | 62.1 | 11.3 |
| Asia | 11.5 | 4.5 |
A review of the incidence and mortality rates in PCa among MAA populations worldwide also reveal that there are similarities as well as differences between and within geographical regions of Africa, the Caribbean and the USA. PCa incidence for MAA is highest in the USA and lowest in Africa while mortality rates are highest in the Caribbean and lowest in Africa (Table 1). Within the USA, variations of PCa incidence and mortality for MAA have been reported, whereby highest PCa incidence was observed in Delaware (ASR (W): 183.1 per 100,000) and lowest in South Dakota (ASR (W): 58.1 per 100,000) and PCa mortality was highest in Mississippi (ASR (W): 29.5 per 100,000) and lowest in Hawaii (ASR (W): 11.5 per 100,000)20. Within the African continent, geographical variations in PCa incidence were also observed with the highest incidence reported in South Africa (ASR (W), 68.0 per 100,000) and lowest incidence in Niger (ASR (W), 4.4 per 100,000,)27. PCa mortality rates, were highest in Benin (ASR (W), 36.3 per 100,000) and lowest in Niger (ASR (W), 3.4 per 100,000)26. Similarly, in Caribbean countries, the French territory Guadeloupe was reported to have the highest PCa incidence in the region (ASR (W), 189.1 per 100,000) and the lowest PCa incidence was reported in Guyana (ASR (W), 39.3 per 100,0000)27. Mortality rates in the Caribbean region also varied geographically with Barbados having the highest mortality rates (ASR (W), 48.0 per 100,000) and Puerto Rico having the lowest mortality rates (ASR (W), 12.3 per 100,000)27.
Mortality:Incidence rate ratios (MIR) as seen in FIG 2 provide another perspective of PCa disparities. The majority of countries in Africa and two Caribbean countries, Haiti and Jamaica were reported to have death rates that are more than half or almost equal to that of PCa incidence rates (MIR 0.52–0.79). This suggests that in these countries the rate of death may be similar to the rate of PCa incidence. In contrast, the rates of PCa deaths are half or less than half the rates of PCa incidence in the majority of Caribbean countries, Asia, US, Canada, Europe and some countries in Africa (MIR 0.50–0.10). Developed countries such as Europe, USA and Canada are reported to have mortality:incidence rate ratios less than 0.25. This suggests that patients with PCa are more likely to survive in these countries. Similarly, three French territories (LeReunion, Martinique and Guadeloupe) and one US State (Puerto Rico) although geographically located in developing regions of the Caribbean and Africa also have mortality:incidence rate ratios less than 0.25. These countries have health systems that are similar to their mainland France or the USA and suggest that improvement in health systems infrastructure may contribute to improving disparities in PCa survival.
Figure 2:
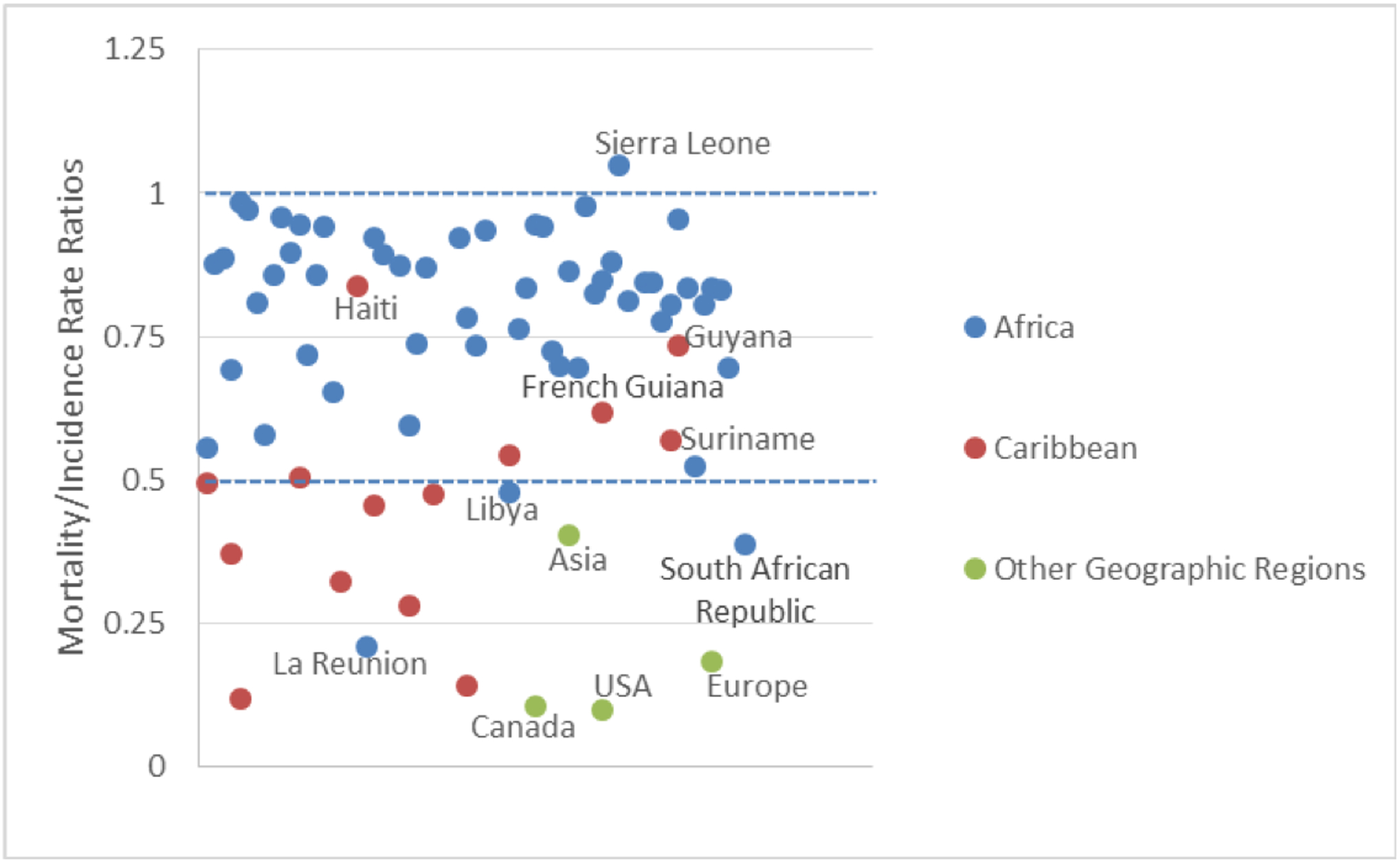
Country-specific Mortality:Incidence Rate Ratios for PCa
The complexity of the geographical patterns of PCa incidence and mortality rates between the USA, Caribbean and Africa, within these regions and also between specific countries in these regions may be explained by multiple factors: including differences in the socioeconomic status; the existence, access or utilization of PCa screening or early detection programs; lack of or limited cancer registration programs and/or differences in health care systems infrastructure. It is also plausible that these factors alone may not fully explain the observed varied geographical patterns of incidence or mortality rates and that behavioral/psychosocial and biological contributors may also be evident and are discussed in subsequent sections of this review.
3. Causes for prostate cancer disparities
3.1. Socio-economic status
While there is increasing evidence that genetic factors may account for the disparities in PCa incidence and mortality rates, socio-economic status (SES) also play a role16, 28. There is no geographical standardized methodology for measuring SES but it is believed that the socio-economic status of an individual, family or community relative to others can be utilized as bellwethers for SES in general.
Other factors such as the timely utilization of diagnostic screening kits, the PSA also provide avenues for assessing potential PCa disparities. In a review by Liu29 it was found that there was greater PSA screening among MEA than MAA in a low SES. Furthermore, it is believed that strategies for PSA screening may benefit from tailoring to the patient’s SES. Interestingly, according to the same review by Liu29, prior to the popularity of the PSA in 1987, there were no reported associations of PCa incidence and SES among varied ethnic groups, but post 1987, PSA screening was more prevalent among the higher SES groups as a result of their access to health care. They also reported that prior to the implementation of PSA based screening, men of a high SES were more likely to engage in healthy lifestyle behaviours. Overall, improved accessibility to health care is linked to men of a higher SES who are more likely to screen for PCa earlier than men from a lower SES which usually results in the latter being more susceptible to impermanence. Self-reported PSA tests were also found to be more common among the highly educated30 which resulted in a higher reported incidence rate of 15–19% of PCa in men having at least a college education compared to men with a high school education or below. Low literacy rates and language barriers in the USA have been associated with advanced PCa and increased mortality rates. Generally, MAA fall in the lower socio-economic quartile in which they experience more advanced and aggressive types of PCa when diagnosed at an earlier age compared to their MEA counterparts.
The PRostate Cancer in Ethnic SubgroupS (PROCESS) study in the United Kingdom investigated whether accessibility to healthcare may be a reason for the correlation observed between race and PCa. Reporting on SES, MAA in the cohort lived in less affluent areas and were inclined to be manual labourers. It was also found that knowledge of PCa among MAA and MEA were commensurate. MEA were aware that age was a risk factor for them while MAA were aware that they had a greater disposition to developing the disease31. Highlighting behavioural attitudes between the two groups, although the main source of referral by all men in the study was the general practitioner, it was found that emergency rooms and other departments in the hospital were more points of referral for MAA than their MEA counterparts. The research group concluded that there was no evidence of inequality in accessing healthcare for MAA in comparison to MEA yet PCa incidence rates were higher in MAA.
More research and data are needed to conclusively show whether SES directly accounts for the disparities observed for PCa among MAA compared to other ethnic groups. Suggestions are that SES is however, linked to other factors such as diet, comorbidity and genetics which may interplay causing the exacerbated disparities being observed. Modifiable risk fators such as diet, smoking, alcohol consumption, physical activity, medication and infections have been attributed to PCa in men of a low SES28. MAA in the USA appear to be at an increased risk for PCa based on their meat consumption32–34. As well, MAA are less inclined to receive more aggressive treatment than MEA16 and so differences in treatment may therefore account for the disparity in the survival rates. These factors are significant barriers in obtaining similar outcomes for MAA with PCa. Therefore, efforts to improve socio-economic factors together with targeted biological approaches may result in the reduction of racial disparities for PCa to be achievable.
3.2. Transatlantic Trade in Africans
The severe population bottleneck caused by the transatlantic trade in Africans may have caused an unnatural selection by favouring survival in persons who were more testosterone responsive. Based on the conditions under which the slaves were transported, slaves possessing greater oxygen-carrying capacity, lean body mass facilitating heat exchange, thick skin, greater sebum production and harder bones (greater bone mineral density), phenotypes all related to testosterone, would have more readily survived the life-threatening journey35. This would cause a population shift towards greater testosterone responsiveness in Africans in the New World compared to West Africans.
The enhanced fitness due to increased testosterone responsiveness would have been consolidated in the New World by the forced interbreeding of slaves from different regions in Africa. Contemporary attributes of African descendants in the New World such as high rates of PCa incidence and mortality as well as sprinting prowess36 are partly attributable to this population shift in testosterone responsiveness35. Examples include the three-fold higher incidence and mortality of PCa in Afro-Trinidadians compared to Indo-Trinidadians37, 38 and, high bone density being associated with PCa in Afro-Caribbean men39.
3.3. Research related barriers
3.3.1. In vitro (cell lines)
It took scientists approximately 44 years from the first documented successful growth of animal cells outside the host organism40 to developing the first human cancer cell line, HeLa41. The presence of the HeLa cells paved the way for all other animal cancer and normal cell lines including noteworthy PCa cancer cell lines, LNCaP, DU-145 and PC-342–44. The presence of these three PCa cell lines were the spring board for an additional 197 sub-cell lines45, 46 of which 95% of them are from MEA as observed from the American Type Culture Collection website. Furthermore, 97% of the PCa cell lines available to researchers globally from main suppliers, ATCC, Sigma and European Collection of Authenticated Cell Cultures (ECACC) are from MEA47.
As mentioned, PCa varies from patient to patient, hence no PCa case is exactly alike48. Given the limited number of available PCa cell lines from MAA, this creates a challenge when using the few available MAA PCa cell lines for research, as there is no such thing as the “perfect cell line.” No single cell line is capable of adequately representing all the characteristics of PCa. Cell lines in general display observable genetic and morphological changes from the host tumor, based on the number of passages they undergo49. Importantly, most cell lines have been grown in vitro for decades which has resulted in genetic drifts and changes at the molecular levels over long periods of time50. Furthermore, cancer cell lines have been known to exhibit a greater growth rate than that of the tumors from which they originate. Through various growth conditions such as monolayer culture as opposed to 3-D tumor environments or microenvironment provided by the extra cellular matrix, cells change over time51. Screening mechanisms might be modelled to identify treatment options based on the characterization specific to a cell line used, but due to molecular changes, results may reflect the genetic changes experienced by the cell line at the point in time49.
As PCa cell lines play a role in drug development, the presence of the 3 popular PCa cell lines (LnCap, DU-145 and PC-3) contributed to the advancement of docetaxel as an anti-PCa drug52. Indeed, clinical research has showed that docetaxel in conjunction with prednisone improves overall survivorship of men with PCa53 and even though the ethnic backgrounds of the subjects were not revealed, research shows that over 90% of patients enrolled in clinical studies overall for PCa research are Caucasian in origin18. The correlation between the ethnic backgrounds of PCa cell lines used in anticancer research and their effectiveness in one race over others needs further delineating. Notwithstanding, over the years in vitro, models have focused on PCa cell lines from MEA towards identifying effective chemotherapy drugs54. It is also observed that MEA experience a decline in mortality rates from PCa while the reverse is observed for MAA55, who are 2.5 times more likely to die from the disease, demonstrate greater cases of recurrences and experience shorter disease-free survival56. It then begs the question as to why is there not a greater push towards broadening the cell line bank with PCa from MAA.?
To date, currently 5 cells lines exist from MAA, expected to represent this widely heterogenous disease. Additionally, all of these are representative of African American and none from the Caribbean, an area with even greater reported mortality rates of PCa among MAA. Of the 5 cell lines, 2 were obtained through viral transformation and immortalization while the remainder were spontaneously transformed. RC-77T/E PCa cell line was established through transfection with recombinant retroviral construct, LXSN-HPV16E6E7 containing the E6 and E7 genes of HPV-16 and a neomycin resistance gene57 while RC165N was established through telomerase (hTERT) immortalization58, 59. Both RC-77T/E and RC165N/E showed androgen dependence and epithelial morphology cells expressed androgen-regulated prostate-specific homobox gene, NKX 3.1, epithelial cell specific cytokeratn 8, androgen receptor (AR), prostate specific antigen (PSA), and p16. MAA PCa 2a and 2b cell lines were derived from a single site bone metastasis in a castration resistant PCa case in an African American male. These cell lines are tumorigenic, expresses AR+ and PSA+ and are models for advance stage PCa52. The other spontaneously transformed PCa cell line, E006AA-Parental (Par), was established as the first cell line of African ethnicity which was from organ confined PCa tumor with a Gleason score of 660. E006AA is an androgen dependent cell line which expresses PSA and Androgen receptors having 26 CAG repeats in exon 160. Furthermore, there is a dispute as to whether E006-AA is actucally prostate which could potentially limit the already limited MAA cell line resourse available to researchers.
Beyond understanding the disease, there is also an importance in identifying the cellular and molecular precursors to PCa. Normal prostate cell lines, BPH1, RWPE-1 and RC165N developed from Benign Prostatic Hyperplasia tissue samples58, 61, 62 provide such an opportunity. Through the utilization of these normal prostate cell lines, a greater understanding of the epithelial markers of PCa and the biological alterations leading up to PCa have been understood. Furthermore, these normal prostate cell lines have been instrumental in comparative studies using several drugs and compounds in assessing their carcinogenic/inhibitory roles in PCa63, 64. There is a greater need for more normal cell lines to understand the multistep progression of carcinogenesis, especially if we are to understand the reasons underlying the higher incidence and mortality rates of PCa in MAA65, 66.
Since the emergence of “omics,” this has triggered an advent of target specific treatment options for cancers as opposed to traditional chemotherapy67. Through focusing on elucidation of molecular composition of tumors and genetic heterogeneity, several target molecules have been identified67, 68. The drawback however, is that target specific treatments can only be used on a small percentage of patients who match the specific profile69. As such there is a need for a panel of PCa cell lines which adequately represent the heterogeneity of the disease69. Through the establishment of multiple PCa in vitro systems, a greater characterization and manipulation of PCa at different stages can take place contributing to the current void in understanding the disparities between MAA and other races46.
3.3.2. Molecular studies
Improved diagnosis and treatment of PCa can be achieved by a personalized approach especially in light of PCa’s extensive variability in behavior coupled with the multitude of treatment options. Technological advances in genetic analyses continue to pave the way for lead biomarkers that may be exploited towards predicting PCa incidence, outcomes and response to therapy70. Technologies such as microarray analyses and next generation sequencing have aided in characterizing genetic mutations in PCa tumor tissue which are subsequently believed to produce individualized bellwethers that can guide clinical decision-making71.
Current methodologies used for diagnosing PCa are: PSA (prostate specific antigen) which can either be administered as PHI (combines total, free and (−2)proPSA) or 4KScore (consists of total PSA, free PSA, intact PSA and klk2); PCA3/DD3; ConfirmMDx (measures the degree of methylation of GSTP1, APC and RASSF1); PCa biopsies and digital rectal examination. Still, PSA screens dominate the PCa diagnostic regime as others are relatively new with ongoing research and have not been fully streamlined across the globe. The advent of PSA screens occurred in 1970 by Ablin et al.,72, and its purification was credited to Stamey et al.,73. As research on PSA was primarily focused on MEA74, regardless of the belief held by the US Preventive Services Task Force (USPSTF) that there are no differences amid the groups (MAA and MEA in America) as it pertains to the ratio of risks to benefits, one cannot dismiss the notion of a lack of optimum PSA diagnostic translatability to both groups especially in light of the 2.5 times higher PCa mortality rates among MAA compared to MEA75, 76. The PSA test continues to pose significant barriers since PCa diagnosis is challenged by the heightened variability in PCa clinical course, as the disease can range from indolent and latent to aggressive and metastatic. An ideal tumour biomarker that can be epigenetic, genetic or proteomic in nature is able to differentiate between patients with PCa from those without and also provide information on the stage of the tumour that guides the clinician on a course of treatment and the patient’s prognosis. On the contrary, while PSA can aid in early detection, it or PSA-like proteins are expressed in extra-prostatic human tissues and surrounding fluids that ultimately limits the specificity of this biomarker. Furthermore, the inability of the PSA protein to distinguish indolent from aggressive PCa tumours have consequently led to over-diagnosis and over-treatment of men with the disease which begs the questions; ‘exactly how many men are saved from PSA screening?’ and ‘are the emotional and physical consequences on the millions of men who are over-diagnosed and over-treated, worth it?’
An improved understanding at the molecular level as to the drivers of PCa is necessary to better diagnose the disease and design ideal treatment regimens. This drive has led to over 40 genome wide association studies (GWAS) executed over the last decade which have identified approximately 170 common variants to the disease77. At the molecular level, these variants can be categorized as hereditary, sporadic or epigenetic, whereby, the latter two can overlap and some noteworthy ones are listed in Table 2 while others are discussed elsewhere77–85.
Table 2:
showing various hereditary, sporadic and epigenetic associations with PCa incidence primarily sourced from Shen et al.,126 and other sources19, 127 can be consulted.
| Hereditary | Sporadic | Epigenetic |
|---|---|---|
| ELAC | TLR4 | NKX3.1 |
| RNASEL | CDKN1B | Myc |
| MSRI | AR | TMPRSS2-ERG |
| NBSI | CYP17 | PTEN |
| CHEK2 | SRD5A2 | Ezhz |
| HOXB13 | Vitamin D receptor | miRNAs |
| HPC1 | CDKN1A | Signalling pathways-Akt/mTOR & MAPK |
| 8q24 & 17q | CDKN1B | Oncogene tyrosin kinase |
| HNF1B | EGFR | Developmental Signalling |
| 17q21 | CAV1 | RASSF1 |
| PCA3 | MSR | |
| 12q24 | NKX3.1 | |
| 2p16 | c-myc | |
| 10911 | PTEN | |
| SPARCL1 on 4q22.1 | RbE-CAD | |
| PTPRR on 12q15 | AR | |
| 10p14 | ANX7LZTSI | |
| Fox P1 | ||
| KAI1 | ||
| GSTP1 | ||
| APC | ||
| ETS P53 |
The common thread observed in these studies is that more than 90% of these investigations are conducted in MEA. As a result, significant strides have been made in the design of serum and urine diagnostic biomarkers, whereby some are currently clinically applicable as mentioned earlier and these were recently reviewed in Sharma et al.,86. Some include i) the noncoding RNA, PCA3 that is believed to be overexpressed in 95% of PCa cases in MEA78, 86, presents in high levels in some men but is unaccompanied by malignancy meanwhile additional studies confirmed an association of this biomarker in Chinese men87, 88; ii) the kallikrein panel (comprises of total PSA, free PSA, intact PSA and human kallikrein-related peptidase 2 (KLK2)) was reported to improve the predictive accuracy of PCa detection over PSA alone with a reduction in the biopsy rate by 362 for every 1000 men with elevated PSA89 and these studies were primarily conducted on MEA83; iii) TMPRSS2: ERG fusion & β-microseminoprotein, identification of the fusion gene in urine was reported to enhance PCa detection by more than 90% specificity and 94% predictive value90, a research that began by Tomlins91 and later a GWAS study reported in Germany92 and across other ethnicities93, confirmed the importance of this biomarker among MEA, conversely, other studies94 did not reveal the ethnicity of the men investigated although given the geographical locale, it is believed they too are primarily of a European ancestry and iv) DNA methylation of genes for example a) Glutathione -S-Transferase-Pi (GSTP1)95 that is involved in DNA detoxification and cell cycle regulation, to the best of our knowledge no GWAS research has been conducted on this epigenetic biomarker but smaller studies conducted in Germany96, Korea97, Italy98 and meta-analysis on MAA in America, the Caribbean and in Africa99 have confirmed an association of this protein to an elevated risk of Pca; b) the promoter region of adenomatous polyposis coli (APC) that is involved in cell apoptosis, migration and adhesions100 have been associated with PCa occurrence among Chinese101, Italians102, Pakistanis103 and other ethnicities104, furthermore, fairly recent meta-analysis101 that covered seven studies (1227 patients for North America and Europe) calculated the pooled hazard ratio to be 1.74 that correlates methylated APC to unfavorable impact on biochemical recurrence of PCa confirming the PCa prognostic potential of this biomarker as well, and; c) the putative tumour suppressor Ras-associated domain family 1A (RASSF1A) whereby meta-analysis conducted on 1123 cases105 primarily in MEA associated the hypermethylation of CpG islands within the promoter region of these genes to PCa105. Research has also demonstrated that PCA3106, TMPRSS2:ERG fusion107 and PTEN108 among others86 possess PCa prognostic potential although investigations are still quite limited in these areas.
As the awareness increases on the current disparities in PCa incidence and mortality rates, recent times bear witness to up and coming research on the underserved MAA population, albeit limited and on relatively small cohorts primarily from America. Some of these have associated biomarkers, PCA3105, 109, TMPRSS2:ERG110, GSTP1, APC and RASSF1111 to PCa presence but more so, the advanced form of the disease. Although some relation of the TMPRSS:ERG fusion gene has been reported to PCa, other research112 has indicated that the fusion gene is twice more prevalent among MEA (49%) compared to MAA (25%) where the latter compared well with Asian men (27%). There are currently no reports on the association of the KLK2 gene to PCa in MAA.
To date, to the best of our knowledge four reported GWAS studies have been conducted with a primary focus on MAA113–116 from 2007–2016, as 10% of all GWAS PCa studies over the last decade. From these investigations: genetic variant/s in a number of chromosomal regions such as, 8q24 (working in conjunction with another variant) was twice more prevalent in MAA compared to MEA114, a novel risk variant, rs7210100 in the 17q21 region was unique to African-Americans, while its functionality is unknown with respect to PCa, it is reported to be associated to the breast cancer susceptibility gene, BRAC1117; a risk variant in 10p14 was most prevalent in Ghanaian men115 while sub-analysis associated SNPs at 5q31.3 with high Gleason score and finally, Rand et al.,116 reported PCa associations to the gene SPARCL1 on 4q22.1 and PTPRR on 12q15. Other studies reported analyses on varied GWAS research with some focus on MAA118–121 while auxiliary research have associated additional variants, primarily on 8q248, 122–125; 109118, 12q24 and 2p16126 chromosomes to PCa occurrence in MAA: In identifying these various risk variants be them hereditary, sporadic or epigenetic in nature, it is believed that unearthing their functionality is the key to enhanced PCa diagnosis, prognosis and theranostics among MAA.
3.3.3. In vivo
The use of in vivo models stemmed from the understanding that cell lines in general cannot replicate all disease mechanisms in the original tumours. To understand these mechanisms, specifically for PCa, preclinical models are needed to reproduce tumor growth conditions that mirror the micro and macro-environment of the tumour in the host. Most PCa in vivo systems are modelled using mice due to the favorable genetic manipulations as compared to the other animal models127, 128 and extensively reviewed in129.
As cell lines are injected in animal models for preclinical assessment, logically, preclinical anti-PCa research would primarily focus on MEA from which most PCa cell lines are derived. The Prostate Cancer Foundation held a Prostate Cancer Models Working Group (PCMWG) Summit in 2007130 to address the lack of applicable preclinical models to study PCa tumorigenesis and to advance propitious prevention and therapeutic strategies. The PCMWG identified several limitations in preclinical research including cell lines, furthermore, it was observed that insufficient models with inadequate molecular and biologic diversity were available to reflect human cancers. Indeed, it is reported that only 5% of drugs investigated at the preclinical level using mice are approved by FDA131. However, it is believed that these statistics could be improved with genetically engineered mouse models (GEMM)132. In addition, GEMM should fast track developing individualized therapies for PCa, bearing in mind that the mouse models encapsulate PCa tumours from varied ethnic backgrounds. The African American PCa cell lines E006AA-Parental (Par), and RC-77T/E cell line were both highly tumorigenic in SCID mice58, 60 while the former was also tumorigenic in athymic nude mice. The presence of these models maybe utilized in the GEMM paving the way for personalized treatments for PCa among MAA bearing in mind the type of cell line E006AA actually is.
3.3.4. Clinical
Clinical treatment of PCa that depends on age, stage of disease, stratified risk-group and patient’s wishes encompasses one or more of the following: radical prostatectomy (RP), radiotherapy, active surveillance, ADT, chemotherapy among other agents. Patients with very-low or low-risk localized PCa may be managed with active surveillance while locally advanced or metastatic PCa may be treated with androgen deprivation therapy (ADT) or chemotherapy.
Normally, RP is the gold-standard for managing localized PCa and is performed by an open retropubic or perineal approach. Studies evaluating the racial disparities on the oncological outcomes after RP are few in number; many including small numbers of MAA, have short follow-up periods and have conflicting results. The oncological outcomes of RP are dependent on several factors and in MAA, aggressive and more advanced disease at diagnosis are significant133. Of note, National Cancer Registry data suggests that MAA are less likely than MEA to have a RP (22.3% versus 29.1%) and are more likely to opt for no treatment (29.1% versus 23.9%)134. This disparity is not clearly understood but may be due to racial or socioeconomic discrimination, MAA mistrust of the healthcare system or more advanced presentation of MAA at diagnosis which precludes curative treatment. Faisal et al reported on 15,993 patients who underwent RP between 1992 and 2013 at a single center135. MAA with very-low risk and low-risk PCa were more likely to have adverse pathological features and increase in Gleason score than MEA. MAA with low-risk prostate cancer were more likely to have biochemical recurrence. The Shared Equal Access Regional Centre (SEARCH) Database Study Group analyzed data from 1665 MAA and 2791 MEA who underwent RP in equal-access hospitals136. At a median follow-up of 8.5 years, on univariate analysis, MAA had higher rates of biochemical recurrence but reduced overall death. On multivariate analysis, black race was not associated with increased risk of aggressive disease recurrence, metastases or PCa- specific death. An international comparison of African-American (AA), Afro-Caribbean from Jamaica and Caucasian-American men with PCa that had undergone RP reported that AA and Afro-Caribbean men have lower 5-year biochemical-free survival rates than Caucasian-American men137. There appears to be no significant differences in quality-of-life outcomes (sexual function, urinary continence) in MAA and MEA undergoing RP138.
Radiotherapy may be delivered via external beam therapy or brachytherapy. Both radiotherapy and RP may be used for patients with low-risk PCa and there is a comparable 10-year survival in patients with localized PCa for both139. Race does not appear to have a significant outcome in MAA who are treated with external beam radiation140, 141. Results of the Radiation Oncology Group trials reported that factors such as: clinical stage, Gleason score, PSA and lymph node status affect overall and disease-specific survival142. In this phase 3 randomized trial, MAA had lower overall and disease-specific survival on univariate analysis. However, on multivariate analysis after adjusting for risk categories and duration of adjuvant ADT, race no longer was associated with outcome. There are conflicting results regarding oncological outcomes in MAA with PCa treated with brachytherapy143, 144.
Active surveillance spares men with low-risk and low-volume PCa (clinically insignificant), that will unlikely progress, of treatment-related morbidity. The surveillance protocol varies between centers; however, it is usually onerous requiring frequent PSA testing, DRE and repeat prostate biopsies at intervals. Since increasing numbers of studies have reported that MAA with very-low risk and low risk PCa have pathological upgrading and upstaging after RP, there are concerns about the suitability of active surveillance in these patients (Gocke MI, Prostate Cancer Pros Diseases, 2017). In addition, MAA with low-risk PCa have higher PCa-specific mortality when treated compared to MEA145. However, MAA continue to be enrolled in active surveillance protocols because there are other studies showing no significant upgrading or upstaging after RP146, 147. Nonetheless, analysis of active surveillance cohorts report that MAA are more likely to have progression and require active treatment148, 149. Further studies are required to evaluate this association since very few studies have been published and these largely have very small numbers of MAA. Interestingly, data from the Surveillance Epidemiology and End-Results Program (SEER)-Medicare database revealed that MAA were less likely than MEA to be placed on active surveillance. Those who were placed on active surveillance more commonly defaulted to watchful waiting after a period of surveillance150. The reasons for lack of adherence to the protocol could be related to the rigid and prolonged nature of follow-up or even socioeconomic reasons.
Androgen deprivation therapy (ADT) may be used in men with locally advanced or metastatic PCa, as an adjuvant to external beam radiation, for treatment of biochemical recurrence after failure of local treatment or in men with localized PCa who refuse curative therapy. ADT was historically initially achieved through surgical castration (bilateral orchiectomy) or chemical castration by using oestrogens151. Oestrogens are no longer used widespread as they are associated with increased thromboembolic risks152. Though surgical castration is associated with poorer health quality of life scores than chemical castration, it is still commonly used for management of metastatic PCa. Common first-line methods of ADT include gonadotropin-releasing hormone (GnRH) agonists, GnRH antagonists and anti-androgens. These agents either ultimately suppress testosterone levels into castrate ranges (<50 ng/dL) or inhibit androgen receptors. Biochemical or clinical response to ADT lasts for a duration of 24–36 months, thereafter patients develop castrate-resistant prostate cancer153. There are several studies that suggest there are no differences in outcomes of MAA with PCa treated with ADT compared to MEA154, 155. However, analysis of data from the Southwestern Oncology Group phase 2 Study in which both MAA and MEA with metastatic PCa were randomized to orchiectomy with or without flutamide revealed that MAA had poorer overall survival than MEA156. This statistically significant result was seen even after adjusting for variables such as patient age, Gleason score, PSA and performance status. Contrastingly, Sassani et al157 evaluated biochemical failure in 681 men from the Southern California Cancer registry (treated in an equal access health care system) treated primarily with ADT, found that MAA had a 34% lower rate of biochemical failure than MEA. The authors theorized that the lower rate of biochemical failure in MAA were due to racial differences in sensitivity of the androgen receptor. The authors however were not able to report on changes in survival.
Chemotherapy is used in men with metastatic castrate-resistant PCa (CRPC) as well as in men with newly diagnosed androgen sensitive prostate cancer. CRPC occurs when there is clinical or biochemical tumor progression despite having castrate levels of testosterone. Median survival in these men is typically less than 2 years. Docetaxel and cabazitaxel, chemotherapeutic agents which lead to apoptosis, have dramatically changed the management and outcome of men with metastatic CRPC by improving survival. In a phase 3 trial comparing 3 weekly docetaxel and prednisone to mitoxantrone and prednisone, there was a significant survival benefit (2.4 months) demonstrated in patients treated with docetaxel158. Petrylak et al159 reported a similar survival benefit of 2 months in a similar subset of men treated with docetaxel and estramustine compared to mitoxantrone and prednisone. This trial consisted of 12% and 15% MAA in both treatment arms, however statistical analysis based on race was not done. Prior to 2004, mitoxantrone and prednisone were the standard of care for these patients for relief of symptoms and palliation but with no survival benefit. Cabazitaxel is currently used as a second-line agent in patients with metastatic CRPC who were previously exposed to docetaxel160. Other than improved survival, chemotherapy also provides a significant decline in PSA, greater tumor response and relief of pain in men with metastatic CRPC. More recently, docetaxel chemotherapy administered for 6 cycles in men with newly diagnosed androgen sensitive PCa prolongs survival for a median 13 months and delays emergence of castrate resistance disease161. As is seen in many other clinical trials, MAA were under-represented contributing to 9% of the study population.
Additional non-chemotherapeutic drugs used in patients with CRPC include: enzalutamide, abiraterone acetate, apalutamide, sipuleucel-T and radium-223. All agents are Food and Drug Administration (FDA)- approved and prolong survival by a median of 3–5 months. Enzalutamide, an androgen receptor signaling inhibitor may be used in men with metastatic or non-metastatic CRPC with or without prior exposure to docetaxel chemotherapy162–164. Abiraterone acetate, an androgen biosynthesis inhibitor which blocks cytochrome p450 c17 (CYP 17) also increases survival in men with metastatic PCa and may be used in these patients with or without prior exposure to docetaxel chemotherapy165. Apalutamide is a competitive inhibitor of the androgen receptor and may be used in non-metastatic and metastatic CRPC166. Sipuleucel-T is a form of immunotherapy for PCa consisting of autologous peripheral blood mononuclear cells. It is administered to men with asymptomatic or minimally symptomatic metastatic CRPC167. Radium- 223 dichloride emits alpha particles which binds to areas of metastases in bone and is cytotoxic in these areas168.
Recent advances in drug treatment seek to discover new agents to treat CRPC that are more effective in prolonging survival than current agents and are more tolerable. Although many of these new agents have been investigated, many are not yet FDA- approved. Interestingly, none of these agents have been tested specifically in large populations of MAA. Some include androgen synthesis inhibitors such as the CYP17 inhibitors, VT-464 (Viamet) and TAK-700 (Orteronel). VT-464 produced a greater suppression of the androgen receptor axis than abiraterone acetate in CRPC cell lines169. The drug is currently being tested in a phase 1/2 trial. Orteronel showed promising results in phase 2 trials with good PSA and testosterone suppression and reduction of circulating tumor cells170. However, the drug showed no improvement in overall survival in men with chemotherapy-naïve metastatic CRPC and was associated with significant toxicity. The drug is therefore no longer being developed for men with metastatic CRPC171. Androgen receptor antagonists, ODM-201(Darolutamide) which appears to be more tolerable than enzalutamide, does not cross the blood-brain barrier and has low risk of seizures. Phase 1 and 2 trials suggest that the drug is effective in men with progressive metastatic CRPC and is currently being investigated in a phase 3 trial (ARAMIS) including men with non-metastatic CRPC172, 173. AZD3514 is an androgen-dependent and independent receptor inhibitor administered orally. Phase 1 trial showed modest tumor activity but this was associated with significant nausea and vomiting174. TOK-001 (Galeterone) is a dual androgen synthesis inhibitor (CYP17 inhibitor), androgen receptor antagonist and down-regulator of androgen receptor expression. Phase 1 and 2 trials show galaterone to be effective and safe in men with metastatic and non-metastatic PCa175.
New promising immunotherapies include pox-based virus vaccines (PROSTVAC-VF), currently being evaluated in a phase 3 trial and Ipilimumab, a human monoclonal antibody administered in combination with radiotherapy176–178. Additional new agents include Cabozantinib or XL-184, a receptor tyrosine kinase inhibitor of c-Met and vascular endothelial growth factor (VEGF) which appears to be beneficial in bone metastases in PCa179. Custirsen or OGX-011 is an oligonucleotide which targets clusterin, which is thought to mediate resistance to docetaxel. Most recent data suggests that the agent does not appear to improve survival in patients treated with docetaxel or cabazitaxel180, 181. Tasquinimod, an oral quinolone with anti-angiogenic properties is associated with improved radiological progression free survival in men with metastatic CRPC182.
4. Overcoming these
The heterogeneity of PCa makes treating the disease a continuous challenge for medical practitioners. Indeed, research findings show an overall decline in death rates from PCa among one group, MEA, more than another group, MAA. Hetergenity of the disease is mutifactoral and takes into account inter alia geographic locale and racial ancestry. Notwithstanding, research shows that the higher incidence and mortality rates among MAA is caused by numerous factors that range from biological to SES. Interestingly, while many instances associate inacessibility to quality healthcare as a contributing factor to elevated mortality rates among MAA with PCa, it is shown that in instances where quality healthcare exist for this group, MAA still have worst outcomes from PCa than MEA. Such findings highlight the signifcant role that biology plays in enhancing one’s understanding of the disease among MAA before treating or curing become possibilities. With this in mind, basic science has to be mobilized at all levels, in vitro and in vivo, whereby access to a wide array of the requisite tools, cell lines, that are used to understand the biological composition of the myriad of PCa tumours from MAA, can be used. These tools in addition to tissue samples from the original tumours can be exploited for WGS, paving the way for identifying biomarkers that can be used to improve PCa diagnoses among MAA while developing theranostics for this group with the disease. What is clear is that these studies have been limited to MEA and this is believed to contribute to the reduction in PCa mortality rates among this group. Moreover, clinical trials are primarily conducted around MEA further concretizing the reason for reduced PCa mortality rates among them. Certainly, tackling the burden of PCa among MAA has to engage relevant stakeholders from public to private sectors to scientists. But importantly to note is that while earlier diagnoses can inadvertently curtail PCa progression among MAA and thus optimize treatment, the advanced stages of the disease are more often associated with the increased mortality rates among them. Hence, more tailored chemotherapies are required that MAA can equally benefit from compared to MEA and this can only be achieved through research at clinical and preclinical levels. An intentioanl shift in the way we conduct research has to incorporate equal representation among all groups with PCa. Social barriers have to be broken down rendering equal access to quality care for all groups.
6. Source of Funding
S. Badal is supported by the National Health Fund (214163), Jamaica and Principal’s New Initiative Fund (15120), UWI, Mona. The funding sources had no involvement in this work.
Footnotes
5 Conflict of interest
The authors disclose no conflict of interest at this time
References
- 1.Nelson A Unequal treatment: confronting racial and ethnic disparities in health care. Journal of the National Medical Association 94, 666–668 (2002). [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. (ed. Division of Cancer Prevention and Control, C.f.D.C.a.P.) (1600 Clifton Road Atlanta, GA 30329–4027 USA: 2018). [Google Scholar]
- 3.Siegel RL & Miller KD Cancer statistics, 2019. 69, 7–34 (2019). [DOI] [PubMed] [Google Scholar]
- 4.Tiwari RC et al. A new method of predicting US and state-level cancer mortality counts for the current calendar year. CA Cancer J Clin 54, 30–40 (2004). [DOI] [PubMed] [Google Scholar]
- 5.Robinson KD, Kimmel EA & Yasko JM Reaching out to the African American community through innovative strategies. Oncol Nurs Forum 22, 1383–91 (1995). [PubMed] [Google Scholar]
- 6.Bhardwaj A et al. Racial disparities in prostate cancer: a molecular perspective. Front Biosci (Landmark Ed) 22, 772–782 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jemal A et al. Global cancer statistics. CA Cancer J Clin 61, 69–90 (2011). [DOI] [PubMed] [Google Scholar]
- 8.Fernandez P, Salie M, du Toit D & van der Merwe A Analysis of Prostate Cancer Susceptibility Variants in South African Men: Replicating Associations on Chromosomes 8q24 and 10q11. Prostate Cancer 2015, 465184 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grossman DC et al. Screening for Prostate Cancer: US Preventive Services Task Force Recommendation Statement. Jama 319, 1901–1913 (2018). [DOI] [PubMed] [Google Scholar]
- 10.Lichtenfeld L Overdiagnosed: Making people sick in the pursuit of health. The Journal of Clinical Investigation 121, 2954–2954 (2011). [Google Scholar]
- 11.Moyer VA Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 157, 120–34 (2012). [DOI] [PubMed] [Google Scholar]
- 12.Brown CR et al. Social determinants of prostate cancer in the Caribbean: a systematic review and meta-analysis. BMC public health 18, 900–900 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aiken WD & Eldemire-Shearer D Prostate cancer in Jamaica and the wider Caribbean: it is time to consider screening. West Indian Med J 61, 90–3 (2012). [PubMed] [Google Scholar]
- 14.Zenka D (Prostate Cancer Foundation, 2012).
- 15.Chornokur G, Dalton K, Borysova ME & Kumar NB Disparities at presentation, diagnosis, treatment, and survival in African American men, affected by prostate cancer. The Prostate 71, 985–997 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng I et al. Socioeconomic status and prostate cancer incidence and mortality rates among the diverse population of California. Cancer causes & control : CCC 20, 1431–1440 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Persaud S & Aiken WD Prostate-specific antigen-based screening in Afro-Caribbean men: a survey of members of the Caribbean Urological Association. Ecancermedicalscience 12, 842 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cancer Genome Atlas Research Network. The Molecular Taxonomy of Primary Prostate Cancer. Cell 163, 1011–25 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koochekpour S Genetic and Epigenetic Changes in Human Prostate Cancer. Iranian Red Crescent Medical Journal 13, 80–98 (2011). [PMC free article] [PubMed] [Google Scholar]
- 20.CDC wonder. in United States Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute (WONDER Online Database., 1600 Clifton Road Atlanta, GA 30329–4027 USA: 2018). [Google Scholar]
- 21.Chokunonga E, Borok MZ, Chirenje ZM, Nyakabau AM & Parkin DM Trends in the incidence of cancer in the black population of Harare, Zimbabwe 1991–2010. Int J Cancer 133, 721–9 (2013). [DOI] [PubMed] [Google Scholar]
- 22.Chokunonga E, Windridge P, Sasieni P, Borok M & Parkin DM Black-white differences in cancer risk in Harare, Zimbabwe, during 1991–2010. Int J Cancer 138, 1416–21 (2016). [DOI] [PubMed] [Google Scholar]
- 23.Somdyala NI, Parkin DM, Sithole N & Bradshaw D Trends in cancer incidence in rural Eastern Cape Province; South Africa, 1998–2012. Int J Cancer 136, E470–4 (2015). [DOI] [PubMed] [Google Scholar]
- 24.Wabinga HR et al. Trends in the incidence of cancer in Kampala, Uganda 1991–2010. Int J Cancer 135, 432–9 (2014). [DOI] [PubMed] [Google Scholar]
- 25.Noone AM et al. (National Cancer Institute. , Bethesda, MD, 2018).
- 26.Bray F et al. (International Agency for Research on Cancer., Lyon, 2017).
- 27.Ferlay J et al. in International Agency for Research on Cancer (ed. GLOBOCAN; ) (Lyon, France, 2018). [Google Scholar]
- 28.Feinstein JS The relationship between socioeconomic status and health: a review of the literature. Milbank Q 71, 279–322 (1993). [PubMed] [Google Scholar]
- 29.Liu L, Cozen W, Bernstein L, Ross RK & Deapen D Changing relationship between socioeconomic status and prostate cancer incidence. J Natl Cancer Inst 93, 705–9 (2001). [DOI] [PubMed] [Google Scholar]
- 30.Steenland K, Rodriguez C, Mondul A, Calle EE & Thun M Prostate cancer incidence and survival in relation to education (United States). Cancer Causes Control 15, 939–45 (2004). [DOI] [PubMed] [Google Scholar]
- 31.Ben-Shlomo Y et al. The risk of prostate cancer amongst black men in the United Kingdom: the PROCESS cohort study. Eur Urol 53, 99–105 (2008). [DOI] [PubMed] [Google Scholar]
- 32.Rodriguez C et al. Meat consumption among Black and White men and risk of prostate cancer in the Cancer Prevention Study II Nutrition Cohort. Cancer Epidemiol Biomarkers Prev 15, 211–6 (2006). [DOI] [PubMed] [Google Scholar]
- 33.Moses KA et al. The impact of sociodemographic factors and PSA screening among low-income Black and White men: data from the Southern Community Cohort Study. Prostate Cancer Prostatic Dis 20, 424–429 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jackson M, Walker S, Simpson C, McFarlane-Anderson N & Bennett F Are food patterns associated with prostate cancer in Jamaican men: a preliminary report. Infect Agent Cancer 4 Suppl 1, S5 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aiken WD Historical determinants of contemporary attributes of African descendants in the Americas: the androgen receptor holds the key. Med Hypotheses 77, 1121–4 (2011). [DOI] [PubMed] [Google Scholar]
- 36.Hamilton B East African running dominance: what is behind it? Br J Sports Med 34, 391–4 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hosein I, Sukhraj R, Goetz L & Rambarran N A Clinicopathological Profile of Prostate Cancer in Trinidad and Tobago. 2016, 2075021 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mungrue K, Moonan S, Mohammed M & Hyatali S Prostate cancer survival in Trinidad: Is PSA a prognostic factor? Canadian Urological Association journal = Journal de l’Association des urologues du Canada 6, E249–E255 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bunker CH et al. High bone density is associated with prostate cancer in older Afro-Caribbean men: Tobago prostate survey. Cancer Causes Control 17, 1083–9 (2006). [DOI] [PubMed] [Google Scholar]
- 40.Harrison R The outgrowth of the nerve fiber as a mode of protoplasmic movement. Journal of Experimental Zoology banner 9, 787–846 (1910). [DOI] [PubMed] [Google Scholar]
- 41.Jones HW Jr., McKusick VA, Harper PS & Wuu KD George Otto Gey. (1899–1970). The HeLa cell and a reappraisal of its origin. Obstet Gynecol 38, 945–9 (1971). [PubMed] [Google Scholar]
- 42.Stone KR, Mickey DD, Wunderli H, Mickey GH & Paulson DF Isolation of a human prostate carcinoma cell line (DU 145). Int J Cancer 21, 274–81 (1978). [DOI] [PubMed] [Google Scholar]
- 43.Horoszewicz JS et al. The LNCaP cell line--a new model for studies on human prostatic carcinoma. Prog Clin Biol Res 37, 115–32 (1980). [PubMed] [Google Scholar]
- 44.Kaighn ME, Narayan KS, Ohnuki Y, Lechner JF & Jones LW Establishment and characterization of a human prostatic carcinoma cell line (PC-3). Invest Urol 17, 16–23 (1979). [PubMed] [Google Scholar]
- 45.Sobel RE & Sadar MD Cell lines used in prostate cancer research: a compendium of old and new lines--part 1. J Urol 173, 342–59 (2005). [DOI] [PubMed] [Google Scholar]
- 46.Koochekpour S et al. Establishment and characterization of a highly tumorigenic African American prostate cancer cell line, E006AA-hT. Int J Biol Sci 10, 834–45 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Badal S, Campbell KS, Valentine H & Ragin C The need for cell lines from diverse ethnic backgrounds for prostate cancer research. Nat Rev Urol (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boyd LK, Mao X & Lu YJ The complexity of prostate cancer: genomic alterations and heterogeneity. Nat Rev Urol 9, 652–64 (2012). [DOI] [PubMed] [Google Scholar]
- 49.Nelson-Rees WA & Flandermeyer RR HeLa cultures defined. Science 191, 96–8 (1976). [DOI] [PubMed] [Google Scholar]
- 50.Torsvik A et al. U-251 revisited: genetic drift and phenotypic consequences of long-term cultures of glioblastoma cells. Cancer Med 3, 812–24 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Okita K & Yamanaka S Induced pluripotent stem cells: opportunities and challenges. Philosophical Transactions of the Royal Society B: Biological Sciences 366, 2198–2207 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Navone NM et al. Establishment of two human prostate cancer cell lines derived from a single bone metastasis. Clinical Cancer Research 3, 2493–2500 (1997). [PubMed] [Google Scholar]
- 53.Tannock IF et al. Docetaxel plus Prednisone or Mitoxantrone plus Prednisone for Advanced Prostate Cancer. New England Journal of Medicine 351, 1502–1512 (2004). [DOI] [PubMed] [Google Scholar]
- 54.NickKholgh B et al. Cell line modeling to study biomarker panel in prostate cancer. Prostate 76, 245–58 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carver BS et al. Aberrant ERG expression cooperates with loss of PTEN to promote cancer progression in the prostate. Nat Genet 41, 619–24 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shavers VL & Brown ML Racial and Ethnic Disparities in the Receipt of Cancer Treatment. JNCI: Journal of the National Cancer Institute 94, 334–357 (2002). [DOI] [PubMed] [Google Scholar]
- 57.Galloway DA & McDougall JK Human papillomaviruses and carcinomas. Adv Virus Res 37, 125–71 (1989). [DOI] [PubMed] [Google Scholar]
- 58.Theodore S et al. Establishment and characterization of a pair of non-malignant and malignant tumor derived cell lines from an African American prostate cancer patient. Int J Oncol 37, 1477–82 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gu Y et al. Phenotypic characterization of telomerase-immortalized primary non-malignant and malignant tumor-derived human prostate epithelial cell lines. Exp Cell Res 312, 831–43 (2006). [DOI] [PubMed] [Google Scholar]
- 60.Koochekpour S et al. Establishment and characterization of a primary androgen-responsive African-American prostate cancer cell line, E006AA. Prostate 60, 141–52 (2004). [DOI] [PubMed] [Google Scholar]
- 61.Hayward SW et al. Establishment and characterization of an immortalized but non-transformed human prostate epithelial cell line: BPH-1. In Vitro Cell Dev Biol Anim 31, 14–24 (1995). [DOI] [PubMed] [Google Scholar]
- 62.Bello D, Webber MM, Kleinman HK, Wartinger DD & Rhim JS Androgen responsive adult human prostatic epithelial cell lines immortalized by human papillomavirus 18. Carcinogenesis 18, 1215–23 (1997). [DOI] [PubMed] [Google Scholar]
- 63.Pennanen P et al. The effects of metformin and simvastatin on the growth of LNCaP and RWPE-1 prostate epithelial cell lines. Eur J Pharmacol 788, 160–167 (2016). [DOI] [PubMed] [Google Scholar]
- 64.Clubbs EA & Bomser JA Basal cell induced differentiation of noncancerous prostate epithelial cells (RWPE-1) by glycitein. Nutr Cancer 61, 390–6 (2009). [DOI] [PubMed] [Google Scholar]
- 65.Mutetwa B et al. Prostate cancer characteristics and survival in males of African Ancestry according to place of birth: data from Brooklyn-New York, Guyana, Tobago and Trinidad. Prostate 70, 1102–9 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kheirandish P & Chinegwundoh F Ethnic differences in prostate cancer. Br J Cancer 105, 481–5 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Casado-Vela J, Gomez del Pulgar T, Cebrian A, Alvarez-Ayerza N & Lacal JC Human urine proteomics: building a list of human urine cancer biomarkers. Expert Rev Proteomics 8, 347–60 (2011). [DOI] [PubMed] [Google Scholar]
- 68.Gillet JP, Varma S & Gottesman MM The clinical relevance of cancer cell lines. J Natl Cancer Inst 105, 452–8 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McDermott U et al. Genomic alterations of anaplastic lymphoma kinase may sensitize tumors to anaplastic lymphoma kinase inhibitors. Cancer Res 68, 3389–95 (2008). [DOI] [PubMed] [Google Scholar]
- 70.Barbieri CE et al. The mutational landscape of prostate cancer. Eur Urol 64, 567–76 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jeronimo C et al. Epigenetics in prostate cancer: biologic and clinical relevance. Eur Urol 60, 753–66 (2011). [DOI] [PubMed] [Google Scholar]
- 72.Ablin R, Soanes W, Bronson P & Witesby E Precipitating antigens of the normal human prostate. J Reprod Fert 22, 573–5 (1970). [DOI] [PubMed] [Google Scholar]
- 73.Stamey TA et al. Prostate-specific antigen as a serum marker for adenocarcinoma of the prostate. N Engl J Med 317, 909–16 (1987). [DOI] [PubMed] [Google Scholar]
- 74.Slomski A USPSTF finds little evidence to support advising PSA screening in any man. Journal of the American Medical Association 306, 2549–2551 (2011). [DOI] [PubMed] [Google Scholar]
- 75.DeSantis C, Siegel R & Jemal A 1–40 (American Cancer Society, Atlanta, GA, 2013).
- 76.National Cancer Institute. (2014).
- 77.Benafif S, Kote-Jarai Z & Eeles RA A review of prostate cancer genome wide association studies (GWAS). Cancer Epidemiol Biomarkers Prev (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen R, Ren S & Sun Y Genome-wide association studies on prostate cancer: the end or the beginning? Protein & Cell 4, 677–686 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hoffmann TJ, Passarelli MN & Graff RE Genome-wide association study of prostate-specific antigen levels identifies novel loci independent of prostate cancer. 8, 14248 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Eeles RA et al. Identification of 23 new prostate cancer susceptibility loci using the iCOGS custom genotyping array. Nat Genet 45, 385–91, 391e1–2 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kerns SL et al. Meta-analysis of Genome Wide Association Studies Identifies Genetic Markers of Late Toxicity Following Radiotherapy for Prostate Cancer. EBioMedicine 10, 150–163 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Eeles R Prostate cancer genome-wide association study from 89,000 men using the OncoArray chip to identify novel prostate cancer susceptibility loci. Journal of Clinical Oncology 34, 1525–1525 (2016). [Google Scholar]
- 83.Sjoberg DD et al. Twenty-year Risk of Prostate Cancer Death by Midlife Prostate-specific Antigen and a Panel of Four Kallikrein Markers in a Large Population-based Cohort of Healthy Men. Eur Urol (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chadha KC et al. New serum biomarkers for prostate cancer diagnosis. Clinical cancer investigation journal 3, 72–79 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Amin Al Olama A et al. Multiple novel prostate cancer susceptibility signals identified by fine-mapping of known risk loci among Europeans. Hum Mol Genet 24, 5589–602 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sharma P, Zargar-Shoshtari K & Pow-Sang JM Biomarkers for prostate cancer: present challenges and future opportunities. Future Science OA 2, FSO72 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen Z et al. Genome-wide association study identifies genetic determinants of urine PCA3 levels in men. Neoplasia 15, 448–53 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chunhua L et al. Clinical Significance of Peripheral Blood PCA3 Gene Expression in Early Diagnosis of Prostate Cancer. Translational Oncology 11, 628–632 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vickers AJ et al. A four-kallikrein panel predicts prostate cancer in men with recent screening: data from the European Randomized Study of Screening for Prostate Cancer, Rotterdam. Clin Cancer Res 16, 3232–9 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hessels D et al. Detection of TMPRSS2-ERG fusion transcripts and prostate cancer antigen 3 in urinary sediments may improve diagnosis of prostate cancer. Clin Cancer Res 13, 5103–8 (2007). [DOI] [PubMed] [Google Scholar]
- 91.Tomlins SA et al. ETS gene fusions in prostate cancer: from discovery to daily clinical practice. Eur Urol 56, 275–86 (2009). [DOI] [PubMed] [Google Scholar]
- 92.Hofer MD et al. Genome-wide linkage analysis of TMPRSS2-ERG fusion in familial prostate cancer. Cancer Res 69, 640–6 (2009). [DOI] [PubMed] [Google Scholar]
- 93.Al Olama AA et al. A meta-analysis of 87,040 individuals identifies 23 new susceptibility loci for prostate cancer. Nature genetics 46, 1103–1109 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tomlins SA et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science 310, 644–8 (2005). [DOI] [PubMed] [Google Scholar]
- 95.Harden SV, Guo Z, Epstein JI & Sidransky D Quantitative GSTP1 methylation clearly distinguishes benign prostatic tissue and limited prostate adenocarcinoma. J Urol 169, 1138–42 (2003). [DOI] [PubMed] [Google Scholar]
- 96.Bastian PJ et al. Molecular biomarker in prostate cancer: the role of CpG island hypermethylation. Eur Urol 46, 698–708 (2004). [DOI] [PubMed] [Google Scholar]
- 97.Yoon H-Y et al. DNA Methylation of GSTP1 in Human Prostate Tissues: Pyrosequencing Analysis. Korean Journal of Urology 53, 200–205 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Martignano F & Gurioli G GSTP1 Methylation and Protein Expression in Prostate Cancer: Diagnostic Implications. 2016, 4358292 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Taioli E et al. Multi-institutional prostate cancer study of genetic susceptibility in populations of African descent. Carcinogenesis 32, 1361–5 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jeronimo C et al. A quantitative promoter methylation profile of prostate cancer. Clin Cancer Res 10, 8472–8 (2004). [DOI] [PubMed] [Google Scholar]
- 101.Wang Y, Fan C, Yu J & Wang X APC methylation predicts biochemical recurrence of patients with prostate cancer: a meta-analysis. International Journal of Clinical and Experimental Medicine 8, 15575–15580 (2015). [PMC free article] [PubMed] [Google Scholar]
- 102.Richiardi L et al. Methylation of APC and GSTP1 in non-neoplastic tissue adjacent to prostate tumour and mortality from prostate cancer. PLoS One 8, e68162 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yaqinuddin A et al. Frequent DNA Hypermethylation at the RASSF1A and APC Gene Loci in Prostate Cancer Patients of Pakistani Origin. ISRN Urol 2013, 627249 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dimberg J et al. Analysis of APC and IGFBP7 promoter gene methylation in Swedish and Vietnamese colorectal cancer patients. Oncol Lett 5, 25–30 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ge Y-Z et al. The association between RASSF1A promoter methylation and prostate cancer: evidence from 19 published studies. Tumor Biology 35, 3881–3890 (2014). [DOI] [PubMed] [Google Scholar]
- 106.Tosoian JJ et al. Accuracy of PCA3 measurement in predicting short-term biopsy progression in an active surveillance program. J Urol 183, 534–8 (2010). [DOI] [PubMed] [Google Scholar]
- 107.Nam RK et al. Expression of the TMPRSS2:ERG fusion gene predicts cancer recurrence after surgery for localised prostate cancer. Br J Cancer 97, 1690–5 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Saal LH et al. Poor prognosis in carcinoma is associated with a gene expression signature of aberrant PTEN tumor suppressor pathway activity. Proc Natl Acad Sci U S A 104, 7564–9 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Adam A et al. The role of the PCA3 assay in predicting prostate biopsy outcome in a South African setting. BJU Int 108, 1728–33 (2011). [DOI] [PubMed] [Google Scholar]
- 110.Feibus AH et al. Clinical performance of PCA3 and TMPRSS2:ERG urinary biomarkers for African American men undergoing prostate biopsy. Journal of Clinical Oncology 33, 91–91 (2015). [DOI] [PubMed] [Google Scholar]
- 111.Van Neste L et al. PD07–04 CLINICAL PERFORMANCE OF AN EPIGENETIC ASSAY TO IDENTIFY OCCULT HIGH-GRADE PROSTATE CANCER IN AFRICAN AMERICAN MEN. The Journal of Urology 197, e128 (2017). [Google Scholar]
- 112.Zhou CK et al. TMPRSS2:ERG Gene Fusions in Prostate Cancer of West African Men and a Meta-Analysis of Racial Differences. Am J Epidemiol 186, 1352–1361 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Haiman CA et al. Genome-wide association study of prostate cancer in men of African ancestry identifies a susceptibility locus at 17q21. Nat Genet 43, 570–3 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gudmundsson J et al. Genome-wide association study identifies a second prostate cancer susceptibility variant at 8q24. Nat Genet 39, 631–7 (2007). [DOI] [PubMed] [Google Scholar]
- 115.Cook MB et al. A genome-wide association study of prostate cancer in West African men. Hum Genet 133, 509–21 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rand KA et al. Whole-exome sequencing of over 4100 men of African ancestry and prostate cancer risk. Hum Mol Genet 25, 371–81 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rosen EM, Fan S & Goldberg ID BRCA1 and prostate cancer. Cancer Invest 19, 396–412 (2001). [DOI] [PubMed] [Google Scholar]
- 118.Hoffmann TJ et al. A large multiethnic genome-wide association study of prostate cancer identifies novel risk variants and substantial ethnic differences. Cancer Discov 5, 878–91 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bensen JT et al. Genetic polymorphism and prostate cancer aggressiveness: a case-only study of 1,536 GWAS and candidate SNPs in African-Americans and European-Americans. Prostate 73, 11–22 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Han Y et al. Generalizability of established prostate cancer risk variants in men of African ancestry. Int J Cancer 136, 1210–7 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Barnholtz-Sloan JS, Raska P, Rebbeck TR & Millikan RC Replication of GWAS “Hits” by Race for Breast and Prostate Cancers in European Americans and African Americans. Front Genet 2, 37 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Aguirre L, Fernandez-Quintela A, Arias N & Portillo MP Resveratrol: anti-obesity mechanisms of action. Molecules 19, 18632–55 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Du Z et al. Genetic risk of prostate cancer in Ugandan men. Prostate 78, 370–376 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chung CC et al. A comprehensive resequence-analysis of 250 kb region of 8q24.21 in men of African ancestry. Prostate 74, 579–89 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yeager M et al. Genome-wide association study of prostate cancer identifies a second risk locus at 8q24. Nat Genet 39, 645–9 (2007). [DOI] [PubMed] [Google Scholar]
- 126.Ledet EM, Sartor O, Rayford W, Bailey-Wilson JE & Mandal DM Suggestive evidence of linkage identified at chromosomes 12q24 and 2p16 in African American prostate cancer families from Louisiana. Prostate 72, 938–47 (2012). [DOI] [PubMed] [Google Scholar]
- 127.Dunning WF PROSTATE CANCER IN THE RAT. Natl Cancer Inst Monogr 12, 351–69 (1963). [PubMed] [Google Scholar]
- 128.Axiak SM & Bigio A Canine prostatic carcinoma. Compend Contin Educ Vet 34, E1–5 (2012). [PubMed] [Google Scholar]
- 129.Valkenburg KC & Pienta KJ Drug discovery in prostate cancer mouse models. Expert opinion on drug discovery 10, 1011–1024 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pienta KJ et al. The current state of preclinical prostate cancer animal models. The Prostate 68, 629–639 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hutchinson L & Kirk R High drug attrition rates--where are we going wrong? Nat Rev Clin Oncol 8, 189–90 (2011). [DOI] [PubMed] [Google Scholar]
- 132.Chen M & Pandolfi PP Preclinical and Coclinical Studies in Prostate Cancer. Cold Spring Harb Perspect Med 8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Moul JW, Douglas TH, McCarthy WF & McLeod DG Black race is an adverse prognostic factor for prostate cancer recurrence following radical prostatectomy in an equal access health care setting. J Urol 155, 1667–73 (1996). [PubMed] [Google Scholar]
- 134.Mettlin CJ, Murphy GP, Cunningham MP & Menck HR The National Cancer Data Base report on race, age, and region variations in prostate cancer treatment. Cancer 80, 1261–6 (1997). [PubMed] [Google Scholar]
- 135.Faisal FA et al. Racial disparities in oncologic outcomes after radical prostatectomy: long-term follow-up. Urology 84, 1434–41 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Freedland SJ et al. Race and risk of metastases and survival after radical prostatectomy: Results from the SEARCH database. Cancer 123, 4199–4206 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ritch CR et al. Pathological outcome and biochemical recurrence-free survival after radical prostatectomy in African-American, Afro-Caribbean (Jamaican) and Caucasian-American men: an international comparison. BJU Int 111, E186–90 (2013). [DOI] [PubMed] [Google Scholar]
- 138.Lee EW, Marien T, Laze J, Agalliu I & Lepor H Comparison of health-related quality-of-life outcomes for African-American and Caucasian-American men after radical prostatectomy. BJU Int 110, 1129–33 (2012). [DOI] [PubMed] [Google Scholar]
- 139.Hamdy FC et al. 10-Year Outcomes after Monitoring, Surgery, or Radiotherapy for Localized Prostate Cancer. N Engl J Med 375, 1415–1424 (2016). [DOI] [PubMed] [Google Scholar]
- 140.Movsas A et al. Do Sociodemographic Factors Influence Outcome in Prostate Cancer Patients Treated With External Beam Radiation Therapy? Am J Clin Oncol 39, 563–567 (2016). [DOI] [PubMed] [Google Scholar]
- 141.Jani AB & Gratzle J Analysis of impact of age and race on biochemical control after radiotherapy in different prostate cancer settings. Urology 66, 124–9 (2005). [DOI] [PubMed] [Google Scholar]
- 142.Roach M 3rd et al. Race and survival of men treated for prostate cancer on radiation therapy oncology group phase III randomized trials. J Urol 169, 245–50 (2003). [DOI] [PubMed] [Google Scholar]
- 143.Shah C et al. Differences in disease presentation, treatment outcomes, and toxicities in African American patients treated with radiation therapy for prostate cancer. Am J Clin Oncol 35, 566–71 (2012). [DOI] [PubMed] [Google Scholar]
- 144.Winkfield KM et al. Race and survival following brachytherapy-based treatment for men with localized or locally advanced adenocarcinoma of the prostate. Int J Radiat Oncol Biol Phys 81, e345–50 (2011). [DOI] [PubMed] [Google Scholar]
- 145.Mahal BA et al. Racial disparities in prostate cancer-specific mortality in men with low-risk prostate cancer. Clin Genitourin Cancer 12, e189–95 (2014). [DOI] [PubMed] [Google Scholar]
- 146.Qi R & Moul J African American Men With Low-Risk Prostate Cancer Are Candidates for Active Surveillance: The Will-Rogers Effect? Am J Mens Health 11, 1765–1771 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Leapman MS et al. Pathological and Biochemical Outcomes among African-American and Caucasian Men with Low Risk Prostate Cancer in the SEARCH Database: Implications for Active Surveillance Candidacy. J Urol 196, 1408–1414 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Iremashvili V, Soloway MS, Rosenberg DL & Manoharan M Clinical and demographic characteristics associated with prostate cancer progression in patients on active surveillance. J Urol 187, 1594–9 (2012). [DOI] [PubMed] [Google Scholar]
- 149.Abern MR et al. Race is associated with discontinuation of active surveillance of low-risk prostate cancer: Results from the Duke Prostate Center. Prostate Cancer and Prostatic Diseases 16, 85–90 (2013). [DOI] [PubMed] [Google Scholar]
- 150.Krishna S et al. Racial Disparities in Active Surveillance for Prostate Cancer. J Urol 197, 342–349 (2017). [DOI] [PubMed] [Google Scholar]
- 151.Huggins C & Hodges CV Studies on prostatic cancer. I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. 1941. J Urol 167, 948–51; discussion 952 (2002). [PubMed] [Google Scholar]
- 152.Cox RL & Crawford ED Estrogens in the treatment of prostate cancer. J Urol 154, 1991–8 (1995). [PubMed] [Google Scholar]
- 153.Karantanos T, Corn PG & Thompson TC Prostate cancer progression after androgen deprivation therapy: mechanisms of castrate resistance and novel therapeutic approaches. Oncogene 32, 5501–11 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Fowler JE, Bigler SA, Renfroe DL & Dabagia MD Prostate specific antigen in black and white men after hormonal therapies for prostate cancer. J Urol 158, 150–4 (1997). [DOI] [PubMed] [Google Scholar]
- 155.McLeod DG et al. Exploratory analysis on the effect of race on clinical outcome in patients with advanced prostate cancer receiving bicalutamide or flutamide, each in combination with LHRH analogues. The Casodex Combination Study Group. Prostate 40, 218–24 (1999). [DOI] [PubMed] [Google Scholar]
- 156.Thompson I et al. Association of African-American ethnic background with survival in men with metastatic prostate cancer. J Natl Cancer Inst 93, 219–25 (2001). [DOI] [PubMed] [Google Scholar]
- 157.Sassani P et al. Black men have lower rates than white men of biochemical failure with primary androgen-deprivation therapy. Perm J 15, 4–8 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tannock IF et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 351, 1502–12 (2004). [DOI] [PubMed] [Google Scholar]
- 159.Petrylak DP et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med 351, 1513–20 (2004). [DOI] [PubMed] [Google Scholar]
- 160.de Bono JS et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet 376, 1147–54 (2010). [DOI] [PubMed] [Google Scholar]
- 161.Sweeney CJ et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N Engl J Med 373, 737–46 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hussain M, Saad F & Sternberg CN Enzalutamide in Castration-Resistant Prostate Cancer. N Engl J Med 379, 1381 (2018). [DOI] [PubMed] [Google Scholar]
- 163.Hussain M et al. Enzalutamide in Men with Nonmetastatic, Castration-Resistant Prostate Cancer. N Engl J Med 378, 2465–2474 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Scher HI et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med 367, 1187–97 (2012). [DOI] [PubMed] [Google Scholar]
- 165.de Bono JS et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med 364, 1995–2005 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Smith MR et al. Apalutamide Treatment and Metastasis-free Survival in Prostate Cancer. N Engl J Med 378, 1408–1418 (2018). [DOI] [PubMed] [Google Scholar]
- 167.Kantoff PW et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 363, 411–22 (2010). [DOI] [PubMed] [Google Scholar]
- 168.Parker C et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med 369, 213–23 (2013). [DOI] [PubMed] [Google Scholar]
- 169.Toren PJ et al. Anticancer activity of a novel selective CYP17A1 inhibitor in preclinical models of castrate-resistant prostate cancer. Mol Cancer Ther 14, 59–69 (2015). [DOI] [PubMed] [Google Scholar]
- 170.Agus DB et al. Safety, efficacy, and pharmacodynamics of the investigational agent orteronel (TAK-700) in metastatic castration-resistant prostate cancer (mCRPC): Updated data from a phase I/II study. Journal of Clinical Oncology 30, 98 (2012). [Google Scholar]
- 171.Saad F et al. Orteronel plus prednisone in patients with chemotherapy-naive metastatic castration-resistant prostate cancer (ELM-PC 4): a double-blind, multicentre, phase 3, randomised, placebo-controlled trial. Lancet Oncol 16, 338–48 (2015). [DOI] [PubMed] [Google Scholar]
- 172.Fizazi K et al. Activity and safety of ODM-201 in patients with progressive metastatic castration-resistant prostate cancer (ARADES): an open-label phase 1 dose-escalation and randomised phase 2 dose expansion trial. Lancet Oncol 15, 975–85 (2014). [DOI] [PubMed] [Google Scholar]
- 173.Matsubara N et al. Phase 1 study of darolutamide (ODM-201): a new-generation androgen receptor antagonist, in Japanese patients with metastatic castration-resistant prostate cancer. Cancer Chemother Pharmacol 80, 1063–1072 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Omlin A et al. AZD3514, an oral selective androgen receptor down-regulator in patients with castration-resistant prostate cancer - results of two parallel first-in-human phase I studies. Invest New Drugs 33, 679–90 (2015). [DOI] [PubMed] [Google Scholar]
- 175.Montgomery B et al. Androgen Receptor Modulation Optimized for Response (ARMOR) Phase I and II Studies: Galeterone for the Treatment of Castration-Resistant Prostate Cancer. Clin Cancer Res 22, 1356–63 (2016). [DOI] [PubMed] [Google Scholar]
- 176.Kantoff PW et al. Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol 28, 1099–105 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Beer TM et al. Randomized, Double-Blind, Phase III Trial of Ipilimumab Versus Placebo in Asymptomatic or Minimally Symptomatic Patients With Metastatic Chemotherapy-Naive Castration-Resistant Prostate Cancer. J Clin Oncol 35, 40–47 (2017). [DOI] [PubMed] [Google Scholar]
- 178.Kwon ED et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184–043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol 15, 700–12 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Smith MR et al. Cabozantinib in chemotherapy-pretreated metastatic castration-resistant prostate cancer: results of a phase II nonrandomized expansion study. J Clin Oncol 32, 3391–9 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Chi KN et al. Custirsen in combination with docetaxel and prednisone for patients with metastatic castration-resistant prostate cancer (SYNERGY trial): a phase 3, multicentre, open-label, randomised trial. Lancet Oncol 18, 473–485 (2017). [DOI] [PubMed] [Google Scholar]
- 181.Beer TM et al. Custirsen (OGX-011) combined with cabazitaxel and prednisone versus cabazitaxel and prednisone alone in patients with metastatic castration-resistant prostate cancer previously treated with docetaxel (AFFINITY): a randomised, open-label, international, phase 3 trial. Lancet Oncol 18, 1532–1542 (2017). [DOI] [PubMed] [Google Scholar]
- 182.Gong P et al. Efficacy of tasquinimod in men with metastatic castration-resistant prostate cancer: A meta-analysis of randomized controlled trials. Medicine (Baltimore) 97, e13204 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Shen MM & Abate-Shen C Molecular genetics of prostate cancer: new prospects for old challenges. Genes Dev 24, 1967–2000 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Baumgart SJ & Haendler B Exploiting Epigenetic Alterations in Prostate Cancer. International Journal of Molecular Sciences 18, 1017 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]


