Abstract
Induced pluripotent stem cells (iPSCs) have emerged as a key component of cardiac tissue engineering, enabling studies of cardiovascular disease mechanisms, drug responses, and developmental processes in human 3D tissue models assembled from isogenic cells. Since the very first engineered heart tissues were introduced more than two decades ago, a wide array of iPSC-derived cardiac spheroids, organoids, and heart-on-a-chip models have been developed incorporating the latest available technologies and materials. In this review, we will first outline the fundamental biological building blocks required to form a functional unit of cardiac muscle, including iPSC-derived cells differentiated by soluble factors (e.g., small molecules), extracellular matrix scaffolds, and exogenous biophysical maturation cues. We will then summarize the different fabrication approaches and strategies employed to reconstruct the heart in vitro at varying scales and geometries. Finally, we will discuss how these platforms, with continued improvements in scalability and tissue maturity, can contribute to both basic cardiovascular research and clinical applications in the future.
Keywords: Cardiovascular tissue engineering, iPSC, Cardiac organoids, Engineered heart tissue, 3D bioprinting
1. Introduction
The human heart is among the most structurally intricate and functionally complex organs of the body, composed of several distinct cell types of varying developmental origin. Seminal discoveries in the field of cardiac development over the years have enabled the identification of a vast number of genetic, biochemical, and biophysical factors that are spatiotemporally orchestrated to form a mature heart [1–3]. These studies have provided a molecular roadmap for engineers to follow in their efforts to build in vitro cardiac muscle tissue [4,5], particularly in the differentiation of human iPSCs into various cardiovascular cell types. More than two decades since the earliest iteration of engineered heart tissue (EHT) was built using chicken embryonic [6] and rat neonatal [7] cardiomyocytes, iPSC-derived cells have now emerged as the predominant cell source for cardiac tissue engineering (cTE). The unique versatility of iPSCs, combined with advancements in engineered biomaterials and organ-on-a-chip technology, has yielded a wide array of in vitro cardiac tissue models, ranging from spheroids and organoids to transplantable cardiac patches and 3D-bioprinted hearts.
This review aims to first outline the fundamental components required to construct human cardiac muscle, and to summarize the various engineering strategies used to assemble the building blocks into organized 3D tissue. We will then briefly outline the various applications of cTE, from 3D disease modeling to drug development. Finally, we will examine the common challenges and limitations of current methods and discuss the future outlook of cTE.
2. The building blocks
While cardiomyocytes occupy up to 70–85% of the mammalian adult heart’s total volume, they constitute just 20–30% of the total population of cells [8,9]. The remaining 70–80% are composed of non-myocyte cell types [8] including endothelial cells, vascular smooth muscle cells, cardiac fibroblasts, and resident immune cells (e.g., macrophages), all of which interact multilaterally with each other by complex paracrine and/or contact-based signaling. In the native myocardium, these diverse cell types are embedded within a robust 3-dimensional (3D) extracellular matrix (ECM) network, which not only provides mechanical integrity to the overall tissue, but also harbors structural and biochemical signals that facilitate anisotropic alignment and organization of cells that are critical for cell-cell electromechanical coupling and efficient force propagation. Importantly, native heart tissue is extensively vascularized with blood vessels of varying scales to meet the high energetic demands of densely packed, contractile cardiomyocytes. The highly specialized in vivo cardiac microenvironment therefore provides an intricate combination of structural and biochemical signals that promote maturation and function at the molecular, cellular, and tissue levels.
The principal goal of cTE as a field is to recapitulate the physiological tissue environment to enable investigation of cardiac muscle function and biology under both normal and pathological conditions. While strategies and applications of cTE have been wide-ranging, three common components are deemed critical (and often indispensable) for engineering a functional unit of human 3D cardiac muscle that faithfully reproduces key features of the adult myocardium: (i) human iPSCs or iPSC-derived cardiovascular cells differentiated by soluble factors (e.g., small molecules), (ii) an extracellular matrix (ECM) scaffold, and (iii) one or more forms of biophysical maturation stimuli (e.g., mechanical and/or electrical). In this section, we will summarize the most common ‘building blocks’ of cTE and outline the various approaches and strategies used to assemble them into functional heart muscle (Fig. 1A).
Fig. 1.
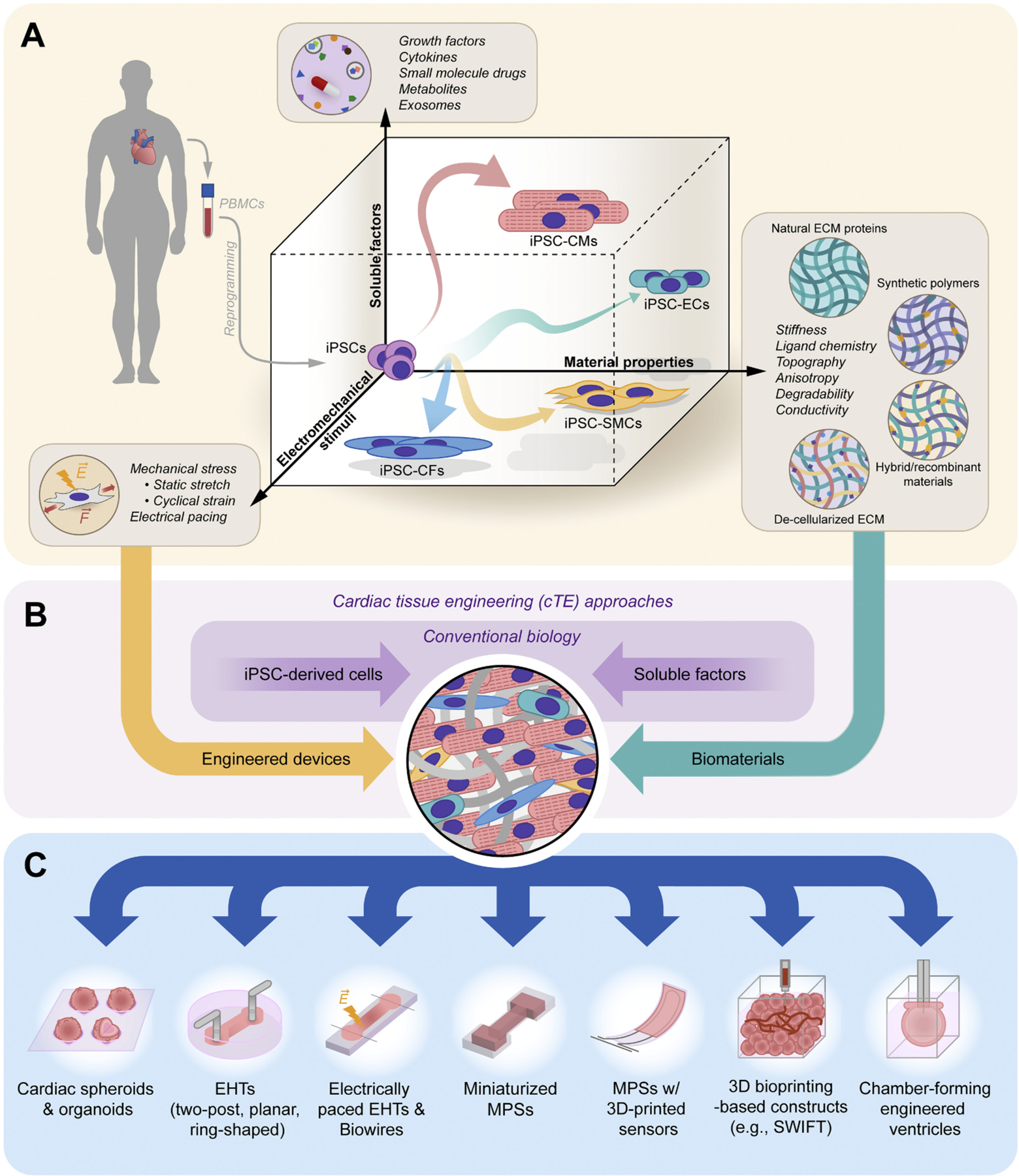
Cardiac tissue engineering using iPSCs. (A) The directed differentiation of patient-specific iPSCs into various cardiovascular lineages (e.g., iPSC-CMs, iPSC-ECs, iPSC-CFs, and iPSC-vSMCs) and the subsequent maturation of differentiated cells to near-adult states require coordinated signals derived from not only soluble (biochemical) factors, but also the material properties of surrounding ECM as well as exogenous biophysical stimuli. (B) While conventional culture methods rely solely on the addition of soluble factors on rigid 2D plastic, tissue engineering approaches bring together diverse devices, tools, and biomaterials to provide cells with the appropriate microenvironmental cues to generate physiological 3D tissue. (C) Example categories of cTE models reported in recent years, ranging from spheroids to chamber-forming engineered ventricles.
2.1. iPSC-derived cells: differentiation by soluble factors
The ability to reprogram adult somatic cells into iPSCs [10,11] has transformed biomedical research by enabling: improved modeling of diseases and developmental processes, development of novel regenerative therapies, human cell-based drug screening platforms, and, importantly, engineering of tissues and organoid models that can synergize with all of the above [12,13]. At the core of these new opportunities lies the ability, by definition, to direct the differentiation of iPSCs into various lineages and functional cell types, including cardiovascular cells that are the focus of this review (Fig. 1A).
Differentiation of iPSCs into beating cardiomyocytes (iPSC-CMs) has in many ways been at the forefront of iPSC biology, providing some of the earliest examples of successful and robust directed differentiation [14–16]. Today, the most common protocols involve biphasic modulation of canonical Wnt signaling [17,18], wherein early activation promotes cardiogenic mesoderm specification and subsequent inhibition steers towards cardiac progenitor differentiation. Regulation of the Wnt pathway is typically achieved through sequential addition of well-defined small molecules (e.g., GSK3 inhibitor CHIR99201) [14,16]. Upon cardiac progenitor differentiation, the cells are subsequently subjected to metabolic selection (e.g., by glucose starvation), which can yield up to >95% pure populations of robustly beating CMs at manageable cost [19,20].
In addition to iPSC-CMs, differentiation protocols for the three other major cardiovascular cell types (endothelial cells, iPSC-ECs; cardiac fibroblasts, iPSC-CFs; and vascular smooth muscle cells, iPSC-vSMCs) have been developed in recent years. De novo derivation of iPSC-ECs, for example, can be achieved by first inducing cardiogenic mesoderm formation (e.g., by GSK3 inhibition) [21,22], followed by addition of VEGF [23–25] and subsequent magnetic-associated cell sorting. High-purity CD31+/VE-cadherin+ iPSC-ECs can also be differentiated without cell sorting, by generating distinct cardiovascular progenitor cells that give rise to both CMs and ECs from iPSCs [26]. The resulting iPSC-ECs exhibit characteristic transcription profiles, cobblestone-like morphologies, as well as functional features (e.g., tube formation) of bona fide ECs, and are increasingly used to replace traditional cell lines (e.g., HUVECs) for cTE applications. The same mesodermal progenitors formed by GSK3 inhibition can also be steered towards the epicardial fate [27–29] and subsequently differentiated into either iPSC-vSMCs by treatment with growth factors PDGF-BB and TGFB1 [23,28,30,31], or into iPSC-CFs by treatment with FGF2 [32,33]. While the purity and maturity of the resulting iPSC-vSMCs and iPSC-CFs remain suboptimal, they have been shown to closely resemble primary vSMCs and CFs in their respective morphologies, transcriptomes, functional properties, and secretory profiles.
The primary advantage of being able to generate various cardiovascular cell types in addition to iPSC-CMs is that more physiological constructs can be made, as demonstrated by the improved structural and functional maturity of multi-cell type microtissues [34]. Multi-cellularity further enables researchers to investigate pathogenic mechanisms and drug responses in a cell-type specific manner, and provides a versatile toolbox with which intercellular communication mechanisms (e.g., paracrine or contact-mediated) can be examined using isogenic lines. While the functional benefits of incorporating multiple cell types have long been known (e.g., from co-cultured cardiac constructs [35]), the ability to incorporate isogenic cell lines greatly reduces the potential undesired effects of confounding factors including species-specificity and cell line dependence. One common challenge for such approaches, however, is that most iPSC-derived cells are functionally immature and exhibit fetal-like features. There exists an apparent maturation limit with conventional small molecule-based differentiation protocols, which can only be overcome with exposure to the appropriate structural [36–39], mechanical [40,41], and/or electrical cues [42] that more closely mimic the physiological tissue microenvironment. It is the overarching goal of many cTE studies to overcome this very limitation, by integrating engineered biomaterials, various microfabricated devices, and electrical circuits with conventional 2D approaches (Fig. 1B).
2.2. Biomaterial scaffold
The cardiac ECM makes up 10–35% of the heart’s proteome [43] and is a critical component that not only provides structural integrity, but also plays key roles in a wide range of genetic and metabolic diseases, immune regulation, and responses to acute and chronic injury. Over the past 15 years, a wide portfolio of ECM-mimicking biomaterials has been designed for cTE, with varying structural, mechanical, and biochemical properties. In the following sections, we briefly summarize the most widely used natural, synthetic, and hybrid biomaterials in cTE.
2.2.1. Natural materials
Materials derived from native cardiac ECM are often the most logical choice for engineering cardiac muscle, given that they closely mimic, by definition, the physiological environment and are biocompatible with little to no immunogenicity. Among the various cardiac ECM components used in cTE, the most common are collagens and collagen-based hydrogels. Approximately 75–90% of the cardiac ECM is composed of fibrillar collagens [44], which form well-aligned anisotropic bundles of fibers that surround and parallel elongated adult CMs. The composition, morphology, and abundance of fibrillar collagens are known to dynamically regulate cardiac function in response to injury [45–48]. The expression of collagen-I in the myocardium also increases exponentially during embryonic development [49,50] and is tightly coupled to the heart’s contractile function and maturation [51], suggesting key roles in regulation of CM alignment, maturation, and function. In addition to mere structural support, collagen-I thus provides critical biochemical and mechanical cues for resident cells including CMs.
One practical advantage of collagen-based gels is that fibrillar collagens are highly conserved across species and can be readily isolated from various animal tissues at substantial quantities. Given their superior biocompatibility and tunable crosslinking/bundling [52,53], collagens and collagen-based scaffolds also provide important functional benefits in that they provide an abundance of native heart-like ligands to embedded cells and promote cell-mediated remodeling of tissue architecture (e.g., alignment) and compaction [35,54]. However, given the low solubility of collagen at neutral pH and its weak mechanical properties, collagens are often combined with synthetic polymers or with other naturally sourced proteins including fibrin, fibronectin, hyaluronic acid, Matrigel, alginate, silk, and chitosan. Fibrin gels have been a particularly popular choice for early EHTs and implantable cardiac patches owing to their ease of use, toughness, and versatility. Alginate has also been widely used due to its simple gelation method and tunable mechanical properties [55], which have shown to improve survival of cells in injectable cardiac implants [56] and presumably provide important stiffness-driven CM maturation cues (as suggested by 2D studies [40,41]). While these biopolymers themselves in the absence of collagens are not necessarily accurate representations of native cardiac ECM, they can provide an initial support in which co-cultured fibroblasts can secrete cell-derived native ECM.
To better recapitulate the physiological ECM, many researchers have also taken a top-down approach by directly decellularizing tissue from both human and animal heart samples [57,58]. The major advantage of decellularized ECM (dECM) is that it largely preserves the native structure and composition of the matrix which, at least in theory, provides the closest representation of the actual tissue microenvironment. Methods employing such dECM have been explored for different purposes including enhancement of CM maturation, disease modeling, and engineering transplantable cardiac patches. However, most decellularization methods rely on surfactants that can damage proteins and strip the ECM of critical growth factors, cytokines, and other matrix-bound components. Inefficient removal of such surfactants results in cytotoxicity, and even with thorough washing, it remains a challenge to properly re-cellularize the dECM with multiple cell types at their physiological locations within the tissue. New strategies are needed to overcome these limitations in re-cellularization and seeding efficiency.
2.2.2. Synthetic biomaterials
While synthetic biomaterials generally do not support cardiac tissue development better than natural hydrogels, they can offer practical advantages as researchers have tighter control over their biochemical, mechanical, and structural properties. To date, various synthetic polymers (e.g., polyethylene glycol, polycaprolactone, poly(lactic acid)) have been employed to build 3D cardiac scaffolds for in vitro disease models or implantable therapeutic patches. Most synthetic polymers lack native bioactivity and require additional processing for ligand functionalization, but many are considered non-toxic, biodegradable, and mechanically robust, which make them an attractive choice for designing scaffolds that can be gradually remodeled by resident cells and cell-derived ECM over time. Importantly, synthetic polymers can be tailored to have specific functional properties and structures such as anisotropically aligned scaffolds (e.g., by electrospinning [59], two-photon initiated polymerization [60], and laser ablation [61]), which have shown to facilitate elongation, uniaxial alignment, and contractile maturation of CMs. One area that is particularly promising is the development of electrically conductive polymers for implantable cardiac patches [62–64]. While these mechanically tunable, electrically conductive polymers are relatively new and have yet to be tested with multiple cardiac cell types in vivo, they have great potential for improving electromechanical coupling and integration with host tissue.
2.2.3. Hybrid/recombinant materials
Hybrid biomaterials and recombinant hydrogels offer a reasonable balance between natural and synthetic materials, as they can faithfully recapitulate the biochemistry and mechanics of the tissue environment without compromising tailorability or biocompatibility. Given their well-rounded properties, hybrid biomaterials and recombinant hydrogels have attracted significant attention in the cTE field in recent years. Injectable biodegradable hybrid scaffolds [65,66], for example, have been developed to enhance repair and regeneration of damaged myocardium. PEG–fibrinogen hybrids designed to generate scaffolds of moderate stiffness (G’ ~ 350 Pa) [65] were seen to accelerate cardiogenic differentiation in 3D muscle tissues compared to conventional gelatin-based systems, demonstrating the utility of mechanically tunable hybrid materials. Given the broad range of materials that can be mixed combinatorically for such natural-synthetic hybrids, systematic studies are needed to evaluate the effects of varying ECM composition (e.g., [54,67,68]) on cell differentiation, tissue maturation, and implantation.
In addition to hybrid materials, recombinant hydrogels offer unique opportunities for tissue engineering and regenerative medicine. Elastic methacrylated tropoelastin hydrogels, for example, have been synthesized recombinantly [69] to generate a micropatterned elastic support that promotes CM spreading, alignment, and contractility. Similarly, elastin-like polypeptides [70,71] and resilin-like polypeptides [72] have been developed either as standalone hydrogels or recombinant-synthetic hybrids with tunable mechanical properties and ligand densities. Modulating the crosslinking density of elastin-like hydrogels, for example, has been shown to regulate the contractility and electrical pacing efficiency of hESC-CMs in 3D cultures [70]. Continued optimization of such recombinant biomaterials, combined with rigorous evaluation of their safety and efficacy in vivo, will likely provide new avenues for addressing translational needs currently unmet with traditional biomaterials.
2.3. Mechanical and electrical stimuli
Enhancing iPSC-CM maturity to near adult CM levels has been a long-standing challenge in cTE, and researchers have developed a variety of strategies to improve tissue maturation and function in vitro. Early studies have focused primarily on biomimetic materials (see previous sections), either with or without a rigid/static support system (e.g., casting molds [73], silicone membranes [74], and Velcro frames [75]). More recently, however, it has become increasingly clear that externally applied mechanical and electrical stimuli also play key roles in inducing further maturation of cells and tissue. Exogenous stimulation has been achieved by various methods involving one or more of the following: (i) an elastic support that provides resistance against each contraction (e.g., flexible pillars [76,77], deformable elastic suspensions [78,79], or silicone frames [80]), (ii) mechanical stretching devices [81,82], and (iii) electrical training systems [83].
Mechanical load is among the most important factors that regulate cardiac development, maturation, and aging [84,85]. The heart’s inherent mechano-sensitivity is evident not only at the single-CM level [40,41,86], but also at the tissue and organ levels [51]. Various cTE platforms developed in recent years have thus focused on application of auxotonic mechanical strain to mimic the forces that CMs contract against in the myocardium [78,87,88]. The precise mechanisms by which passive mechanical load facilitates CM maturation remain incompletely understood, but are thought to involve promotion of cell alignment, force-mediated sarcomere organization [51,89,90], mechanosensitive activation of CM transcription programs [91], and perhaps a metabolic switch induced by increased contractile work [92,93].
As an electrically active organ, the heart and its resident CMs can also be stimulated exogenously by electrical currents to trigger, synchronize, and/or pace contractions. Electrical stimulation has long been known to be a key regulator of hPSC-CM maturation [42,78,94,95], and the cTE field has capitalized on these findings to develop various electrical stimulation systems to promote cardiac tissue maturation in vitro. Early work in this area demonstrated, for example, that electrical stimulation in neonatal rat CMs in collagen/Matrigel scaffolds enhances synchronization of contractions, cell-cell alignment, and ultrastructural organization [42]. A diverse array of stimulation protocols as well as devices and bioreactors have since been developed [96–99] to enhance excitation-contraction coupling in engineered cardiac tissue constructs. Such electrical pacing platforms complement the effects of 3D architectural cues and external mechanical loading, providing a set of orthogonal stimuli that can create synergy in cardiac tissue formation and maturation.
More recently, researchers have developed and refined protocols that combine both mechanical and electrical stimulation for cTE [78,99,100]. For example, combined electromechanical stimulation of hPSC-CM-based tissues was shown to result in greater force generation, a positive Frank-Starling effect, and improved force-frequency relationship [100]. These effects were correlated with the expression of RYR2 and SERCA2, suggesting that combined conditioning can facilitate maturation at both the transcriptional and functional levels. A recent study also demonstrated that enhanced tissue maturation can be achieved by electrical conditioning of early-stage iPSC-CMs with increasing intensity over time against elastic pillars [99]. Intensity training for 4 weeks resulted in adult-like transcriptional and metabolic profiles, improved organization of subcellular architecture (e.g., sarcomere length), formation of transverse tubules (t-tubules), a positive force–frequency relationship, and enhanced calcium handling. While the protocol still does not fully recapitulate the electromechanical properties of human adult myocardium, physical conditioning was necessary for modeling physiological responses to drugs and pathological hyper-trophy. Upon further validation and optimization, similar electromechanical stimulation approaches will likely become increasingly popular in the cTE field for unmasking cardiac disease and drug responses in vitro that otherwise cannot be observed in 2D monolayer cultures.
3. Engineering strategies and approaches
Three fundamentally distinct strategies are commonly used to engineer cardiac tissues: (i) those that rely primarily on self-assembly of cells and ECM, (ii) those that employ specialized devices and tools to exogenously promote formation of heart-like, macro-level tissue structure, and (iii) top-down 3D printing-based approaches that enable maximal architectural control (Fig. 1C). The various strategies have unique advantages and limitations in terms of their scalability, biological complexity, and clinical applicability, which are outlined in the following sections.
3.1. Cardiac spheroids and organoids
Spheroids and organoids are prime examples of tissue constructs that rely almost entirely on the self-assembly of cells. Lack of strict terminology often results in confusion in terms of what should be classified as a spheroid versus an organoid, but several characteristic features have been used to distinguish the two. Spheroids broadly refer to small, simple, and usually scaffold-free 3D aggregates of differentiated or primary adult cells that give rise to primitive tissue-like structures. Organoids, like spheroids, are also often spherical in nature, but in most cases incorporate multiple organ-specific cell types derived from stem cells or progenitors, recapitulating at least one key organ function with some level of lineage-dependent spatial organization. Organoids in general are therefore structurally and functionally more complex than spheroids, and better recapitulate the in vivo microenvironment. Both spheroids and organoids are typically formed by promoting cellular aggregation on micropatterned surfaces, in low adhesion microwells/pits, or in hanging droplet format.
One practical advantage of cardiac spheroids and organoids is that they can provide a 3D microenvironment for cells without significantly compromising throughput and scalability [101]. Spheroid/organoid fabrication requires few manual steps and relatively low cell numbers (thousands to tens of thousands of cells), which can allow for simultaneous characterization of hundreds to thousands of replicates in array format. To date, various cardiac spheroid and organoid systems have been used for: (i) drug screening [102,103], (ii) investigation of cardiac developmental processes [104], (iii) in vitro modeling of myocardial infarction and response to injury [105,106], (iv) assessment of the impact of environmental stressors (e.g., iron overload [107]), (v) elucidation of intercellular crosstalk mechanisms [34,108], and (vi) investigation of coordinated development of multiple tissues [109,110]. More recently, efforts are being geared towards developing protocols for generating constructs that form chamber-like structures [111], with inner cavities lined by a putative endocardial-like layer [112,113]. While it remains to be seen whether the cavities are indeed bona fide cardiac chambers and whether they can be generated reproducibly, such chamber-forming cardiac organoids will likely have a transformative impact on cTE research going forward, with rigorous validation and elucidation of mechanisms.
3.2. Engineered heart tissue and heart-on-a-chip models
While cardiac spheroids and organoids require low cell numbers and can be readily leveraged for higher throughput studies, researchers have limited control over the structure of the tissue as well as the functional assays that can be used to characterize them. EHTs and miniaturized microphysiological systems (MPS) provide a set of attractive complementary strategies, by incorporating custom devices, training systems, and engineered biomaterials to allow for increased architectural control (e.g., tissue size, shape, cell/fiber alignment) as well as versatility in terms of compatible assays (e.g., using built-in magnetoresistive force sensors that can assess contractile function in real-time [114]).
A wide array of tissue models has been designed in various geometries and formats over the past two decades [5]. Examples introduced in recent years include intensity-trained, hanging two-post mini EHT systems [99], cardiac biowires [97,98], microwires [115], patches [116], ring-shaped EHTs [117,118], and chamber-forming ventricle models [119]. Many of these EHT models can incorporate genetically encoded calcium and voltage sensors, along with optical mapping, to enable simultaneous analyses of electrophysiological properties, calcium dynamics, and contractile force [120]. However, a common limitation for many compact EHTs is inefficient nutrient supply, which can in some cases result in formation of an ischemic core [121]. A diameter of ~100 μm has generally been considered to be the maximal thickness for such EHTs, although a lack of hypoxia markers at the core has suggested that tissue density, rather than thickness per se, is likely the major determinant of core ischemia and necrosis [122]. New strategies are being developed nevertheless to enhance nutrient delivery (e.g., by continuous rocking [80]) and vascularization/perfusion to enable formation of thicker, more densely packed, and scalable EHTs suitable for both basic and clinical applications.
Miniaturized MPSs [76,123] can circumvent the issue of limited diffusion given their small dimensions and microfluidic systems which facilitate supply of culture media and washout of cell debris. While some MPSs do not allow for direct force measurements, one practical advantage is that most MPS platforms are more imaging-compatible compared to traditional EHTs. Continued advancements in MPS technology, particularly in automation and in the development of parallel functional assays, will likely be key to striking a balance between throughput and biological complexity in cTE.
3.3. 3D bioprinting-based methods
3D printing offers a fundamentally distinct, top-down approach to cTE, enabling arbitrary control over macro-scale tissue architecture and cellular distribution. Most 3D printing studies in the cTE field to date have focused on fabrication of largely acellular scaffolds and molds [124] onto which cells can be subsequently seeded. Recently, however, 3D printing has also been used to build instrumented cardiac MPS devices, with piezo-resistive, high-conductance soft materials that allow for integration of strain sensors within aligned micro-grooves that promote anisotropic cell organization. The embedded sensors provide an elegant and generalizable system for non-invasive, electronic measurements of tissue-generated contractile forces, enabling quantitative analyses of drug responses over extended durations (>4 weeks) [125].
In addition to scaffolds and custom MPS devices, direct deposition of cells or cell aggregates by advanced scaffold-free 3D bioprinting technology are also being widely explored [126,127]. To date, such 3D bioprinting techniques have yielded various cardiac tissue constructs including heart valves, blood vessels, and the myocardium. Recently, a versatile technology that enables direct embedding of hydrogel inks within a sacrificial, thermoresponsive support hydrogel was developed to bioprint all of the abovementioned components of the human heart, from intricate capillaries to the full intact organ [128]. Embedded bioprinting has also been used for ‘bottom-up’ approaches, enabling the assembly of highly perfusable cardiac tissues by direct printing of vascular channels into a living 3D matrix of densely packed iPSC-derived cardiac organoids [129]. The technique enables rapid construction of patient-specific, perfusable tissues of arbitrary volume and shape at therapeutic scales, opening up avenues previously unexplored by traditional cTE methods. While compaction of the resulting tissue remains an issue, similar 3D bioprinting technologies with improved fusion and integration of printed organoids are expected to be developed in the near future for various applications.
4. Applications of cardiac tissue engineering
Engineered human cardiac tissue derived from iPSCs offer unique opportunities for both basic and clinical research (Fig. 2) by bridging the gap between conventional 2D monolayers and animal models [130]. The most common applications of cTE include: (i) drug screening and development, (ii) evaluation of cardiotoxicity of environmental pollutants and stressors, (iii) disease modeling, and (iv) regenerative therapy.
Fig. 2.
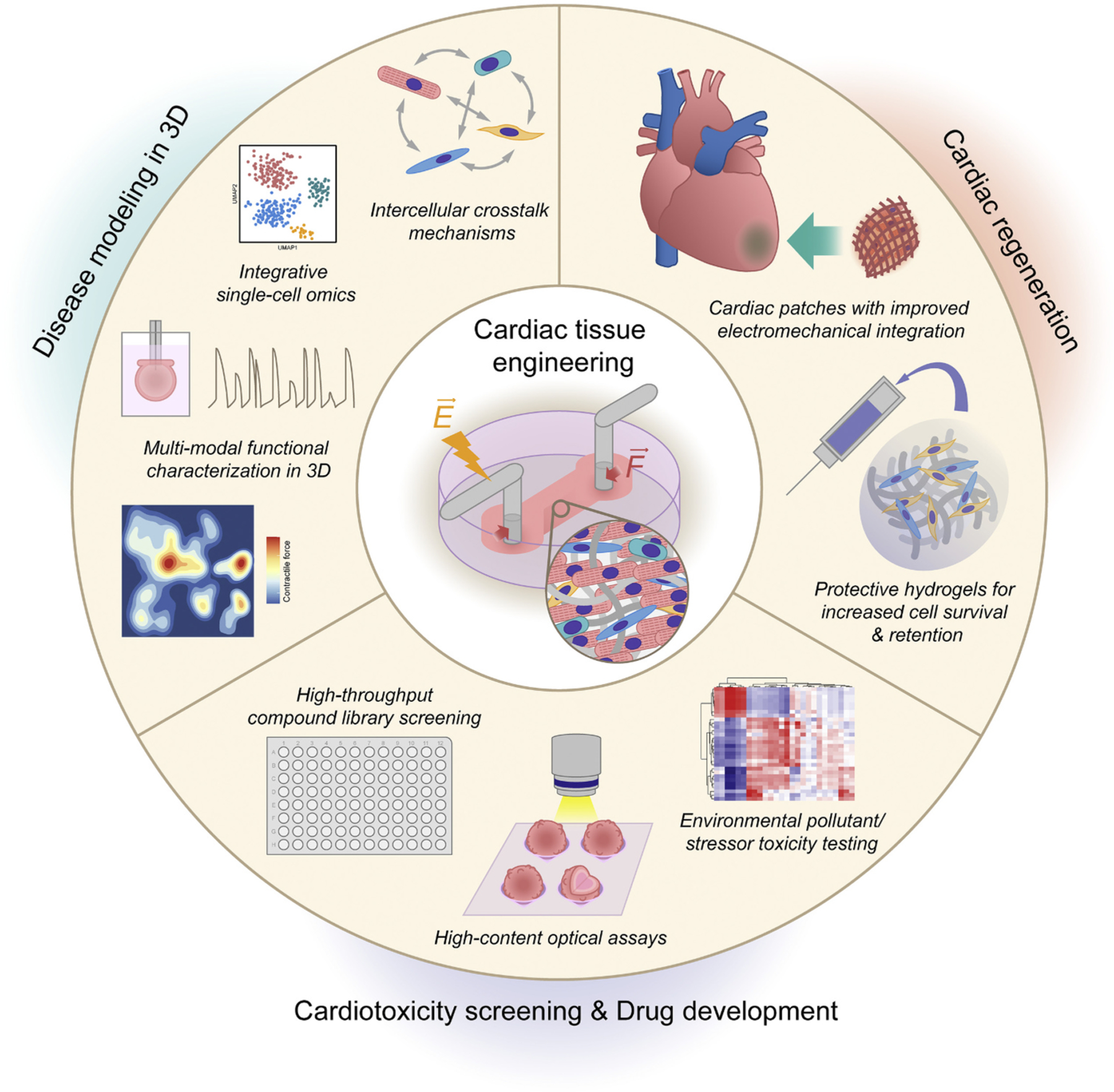
Applications of cardiac tissue engineering. Engineered human cardiac tissue derived from iPSCs offer unique opportunities for both basic and clinical research by bridging the gap between conventional 2D monolayers and animal models. Continued improvements in the scalability and physiological maturation of engineered cardiac tissues will enable: (i) complex disease modeling in a dish to unmask pathological phenotypes that are otherwise difficult to observe in 2D models, (ii) high-throughput systematic investigation of tissue-level responses to drugs and environmental pollutants/stressors, and (iii) novel cardiac regenerative therapies with improved cell retention and electromechanical integration.
4.1. Cardiotoxicity screening and drug development
Testing drugs in human iPSC-derived engineered hearts have several advantages over testing in standard 2D cultures, given that they provide a more physiological in vitro model and that they allow functional characterization under defined conditions (e.g., mechanical load, electrical pacing). Studies have indeed shown that engineered cardiac microtissue exhibit less variability and hypersensitivity to drugs compared to monolayers with the same cellular composition [123]. To date, various in vitro tissue models have been used to assess the effects of inotropic compounds at specific concentrations [131,132], and for broader systematic screening of larger panels of drugs at varying doses [77,102,133,134]. Recent efforts have also been geared towards performing comparative analyses of drug responses across multiple control human PSC-lines in EHT format [134]. While the effects of the drugs tested were qualitatively comparable across 10 different lines, the study revealed widely varying baseline contractile force, kinetics, and rate among the cohort tested. The findings underscore the importance of cell line variability and emphasize the need to better understand the effects of genetic background on tissue formation.
In addition to testing the effects of pharmacological compounds, EHT models are also increasingly being used to assess the cardiotoxicity of a broad range of environmental pollutants and stressors with implications for cardiac health and physiology. For example, normal iPSC-derived cardiac spheroids have been used to model secondary iron-overload cardiomyopathy, recapitulating key clinical phenotypes including defective calcium handling and electrophysiological properties [107]. Given that iPSC-derived cells have served as a popular model system for studying the effects of many environmental factors (e.g., e-cigarettes, radiation, particulate matter, and even microgravity [135]), extending these findings to iPSC-derived 3D tissue models will be the logical next step in characterizing their health effects.
4.2. Tissue-level disease modeling
For similar reasons mentioned above, engineered 3D heart tissues are increasingly replacing 2D monolayers for modeling of complex diseases. Patient-specific cardiac tissues have been used, for example, to model genetic diseases such as the mitochondrial cardiomyopathy, Barth syndrome [136], and PRKAG2 cardiomyopathy [137]. 3D tissues can be particularly powerful in that they can unmask or accentuate disease phenotypes that are otherwise unobserved in standard 2D cultures. Indeed, cardiac microtissues beating against flexible silicone posts revealed contractility defects that were undetectable in titin (TTN) variant-carrying iPSC-CMs cultured in 2D [138]. As is the case with drug testing, however, such studies face steep challenges regarding cell line variability, especially when the functional consequences of genetic variants are subtle and difficult to observe even at the tissue level. These issues should be addressed by routine inclusion of genome-edited isogenic controls and sufficient cell lines to account for variable genetic backgrounds.
4.3. Engineered tissues for cardiac repair
Due to the limited proliferative capacity of adult cardiomyocytes, remuscularization of the injured myocardium has been a long-standing challenge in the cardiovascular field. Various regenerative strategies have been developed with the goal of exogenously promoting cardiac repair following injury, including direct intramyocardial injection of stem cells, differentiated CMs, and cell-free patches [139–141]. Many of these approaches have demonstrated therapeutic potential in large animal studies and are striving towards the clinic, but have been largely limited by low cell retention rates and inefficient integration into host tissue. Engineering approaches to date have thus focused on addressing these challenges by constructing transplantable hydrogel patches or stacked cell sheets that can improve survival, stimulate endogenous tissue remodeling, and facilitate functional integration [79,142–144]. Epicardial transplantation of such engineered constructs [145] or injection of cell-protective biomaterials [146] holds great promise for cardiac regeneration, providing potential therapeutic advantages over traditional cell-based therapies in cell survival and retention.
5. Current limitations and challenges
Although iPSC-derived 3D tissue models have proven to be useful platforms for a variety of applications, the predictive ability of engineered heart constructs remains limited [147]. Three major challenges are currently shared by the cTE community. First, the vast majority of engineered cardiac tissues reported to date are poorly vascularized (if at all), and in some cases form a necrotic core due to limited diffusion of nutrients and oxygen. This can be problematic for long term in vitro modeling of diseases as well as drug responses, but can also be consequential when it comes to tissue transplantation due to limited tissue thickness, poor retention rates, and inefficient integration. Several strategies have been developed to address these issues, including fabrication of a 3D microchannel ‘AngioChip’ scaffold that supports the assembly of vascularized, millimeter-thick cardiac tissues [148]. Perfusable microvascular constructs using micropatterned hESC-ECs have also been developed recently to enhance vascular remodeling and density upon implantation [149]. Continued improvements in such designs, as well as additional strategies for vascularization of engineered hearts (e.g., by direct 3D bioprinting of vasculature [129] or microfluidic systems [150,151]) will be key to the next generation of cTE.
Secondly, despite substantial progress in recent years, engineered constructs still remain structurally and functionally immature compared to native adult myocardium. More can always be done to enhance the maturity of engineered tissues, but a common underlying challenge for cTE is that it is often unclear what minimal essential features (transcriptomic, morphological, metabolic, and functional) are sufficient to model a specific disease phenotype or drug response. Establishing such standards for each target disease or drug response will be an important task for researchers in the cTE field going forward.
Finally, given the limited proliferative capacity of CMs, generating large, clinically relevant numbers of iPSC-CMs required for cTE models remains an elusive challenge. Innovative methods for efficient, massive expansion of iPSC-CMs [152–154] as well as cardiac spheroids/organoids [153] will likely be key to improving the scalability of cTE models and accelerating their clinical application.
6. Conclusion and outlook
Significant progress has been made in the cTE field over the past two decades, incorporating the newest available technologies in iPSC differentiation, engineered biomaterials, and organ-on-chip systems. A wide array of engineered tissue constructs — at varying scales and degrees of biological complexity — have demonstrated the promise of cTE in disease modeling, drug screening, and regenerative cardiology. Although significant challenges remain to be addressed, ongoing efforts in tissue vascularization, maturation, transplant integration, and improved scalability are likely to open new avenues in cardiovascular research. Advancements in these areas are particularly exciting with the emergence of new technology and analytical tools — from single-cell sequencing [155] to 3D force mapping techniques — which are expected to create synergy with increasingly physiological in vitro models. We anticipate that continued improvements in cTE designs will enable, in the foreseeable future, integration of patient iPSC-derived engineered heart microsystems with other organ-on-chip platforms, ultimately enabling systems-level analyses of diseases and drug responses in the age of data-driven precision medicine.
Acknowledgements
This work is supported by National Institutes of Health grants F32 HL152483 (S.C.); R01 HL126527, R01 HL130020, R01 HL146690, and UH3 TR002588 (J.C.W.); and R21 HL138042, R01 HL142718, R01 HL151997 (S.C.H.); and the National Science Foundation grants CBET 2033302 and DMR 1808415 (S.C.H.). Due to space limitation, we apologize in advance for not being able to include all references on this topic.
References
- [1].Heisenberg CP, Bellaiche Y, Forces in tissue morphogenesis and patterning, Cell 153 (2013) 948–962. [DOI] [PubMed] [Google Scholar]
- [2].Srivastava D, Olson EN, A genetic blueprint for cardiac development, Nature 407 (2000) 221–226. [DOI] [PubMed] [Google Scholar]
- [3].Epstein JA, Franklin h. Epstein lecture. Cardiac development and implications for heart disease, N. Engl. J. Med 363 (2010) 1638–1647. [DOI] [PubMed] [Google Scholar]
- [4].Ogle BM, Bursac N, Domian I, Huang NF, Menasche P, Murry CE, et al. , Distilling complexity to advance cardiac tissue engineering, Sci. Transl. Med 8 (2016), 342ps13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Weinberger F, Mannhardt I, Eschenhagen T, Engineering cardiac muscle tissue: a maturating field of research, Circ. Res 120 (2017) 1487–1500. [DOI] [PubMed] [Google Scholar]
- [6].Eschenhagen T, Fink C, Remmers U, Scholz H, Wattchow J, Weil J, et al. , Three-dimensional reconstitution of embryonic cardiomyocytes in a collagen matrix: a new heart muscle model system, FASEB J. 11 (1997) 683–694. [DOI] [PubMed] [Google Scholar]
- [7].Zimmermann WH, Fink C, Kralisch D, Remmers U, Weil J, Eschenhagen T, Three-dimensional engineered heart tissue from neonatal rat cardiac myocytes, Biotechnol. Bioeng 68 (2000) 106–114. [PubMed] [Google Scholar]
- [8].Bergmann O, Zdunek S, Felker A, Salehpour M, Alkass K, Bernard S, et al. , Dynamics of cell generation and turnover in the human heart, Cell 161 (2015) 1566–1575. [DOI] [PubMed] [Google Scholar]
- [9].Zhou P, Pu WT, Recounting cardiac cellular composition, Circ. Res 118 (2016) 368–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. , Induction of pluripotent stem cells from adult human fibroblasts by defined factors, Cell 131 (2007) 861–872. [DOI] [PubMed] [Google Scholar]
- [11].Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, et al. , Induced pluripotent stem cell lines derived from human somatic cells, Science 318 (2007) 1917–1920. [DOI] [PubMed] [Google Scholar]
- [12].Chen IY, Matsa E, Wu JC, Induced pluripotent stem cells: at the heart of cardiovascular precision medicine, Nat. Rev. Cardiol 13 (2016) 333–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Liu C, Oikonomopoulos A, Sayed N, Wu JC, Modeling human diseases with induced pluripotent stem cells: from 2D to 3D and beyond, Development 145 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Burridge PW, Matsa E, Shukla P, Lin ZC, Churko JM, Ebert AD, et al. , Chemically defined generation of human cardiomyocytes, Nat. Methods 11 (2014) 855–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lian X, Zhang J, Azarin SM, Zhu K, Hazeltine LB, Bao X, et al. , Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating wnt/beta-catenin signaling under fully defined conditions, Nat. Protoc 8 (2013) 162–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lian X, Hsiao C, Wilson G, Zhu K, Hazeltine LB, Azarin SM, et al. , Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical wnt signaling, Proc. Natl. Acad. Sci. U.S.A 109 (2012) E1848–E1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ueno S, Weidinger G, Osugi T, Kohn AD, Golob JL, Pabon L, et al. , Biphasic role for wnt/beta-catenin signaling in cardiac specification in zebrafish and embryonic stem cells, Proc. Natl. Acad. Sci. U.S.A 104 (2007) 9685–9690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Marvin MJ, Di Rocco G, Gardiner A, Bush SM, Lassar AB, Inhibition of wnt activity induces heart formation from posterior mesoderm, Genes Dev. 15 (2001) 316–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Burridge PW, Keller G, Gold JD, Wu JC, Production of de novo cardiomyocytes: human pluripotent stem cell differentiation and direct reprogramming, Cell Stem Cell 10 (2012) 16–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Mummery CL, Zhang J, Ng ES, Elliott DA, Elefanty AG, Kamp TJ, Differentiation of human embryonic stem cells and induced pluripotent stem cells to cardiomyocytes: a methods overview, Circ. Res 111 (2012) 344–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lian X, Bao X, Al-Ahmad A, Liu J, Wu Y, Dong W, et al. , Efficient differentiation of human pluripotent stem cells to endothelial progenitors via small-molecule activation of wnt signaling, Stem Cell Rep. 3 (2014) 804–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Orlova VV, Drabsch Y, Freund C, Petrus-Reurer S, van den Hil FE, Muenthaisong S, et al. , Functionality of endothelial cells and pericytes from human pluripotent stem cells demonstrated in cultured vascular plexus and zebrafish xenografts, Arterioscler. Thromb. Vasc. Biol 34 (2014) 177–186. [DOI] [PubMed] [Google Scholar]
- [23].Patsch C, Challet-Meylan L, Thoma EC, Urich E, Heckel T, O’Sullivan JF, et al. , Generation of vascular endothelial and smooth muscle cells from human pluripotent stem cells, Nat. Cell Biol 17 (2015) 994–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sayed N, Wong WT, Ospino F, Meng S, Lee J, Jha A, et al. , Transdifferentiation of human fibroblasts to endothelial cells: role of innate immunity, Circulation 131 (2015) 300–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Williams IM, Wu JC, Generation of endothelial cells from human pluripotent stem cells, Arterioscler. Thromb. Vasc. Biol 39 (2019) 1317–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Palpant NJ, Pabon L, Friedman CE, Roberts M, Hadland B, Zaunbrecher RJ, et al. , Generating high-purity cardiac and endothelial derivatives from patterned mesoderm using human pluripotent stem cells, Nat. Protoc 12 (2017) 15–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Bao X, Lian X, Qian T, Bhute VJ, Han T, Palecek SP, Directed differentiation and long-term maintenance of epicardial cells derived from human pluripotent stem cells under fully defined conditions, Nat. Protoc 12 (2017) 1890–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Iyer D, Gambardella L, Bernard WG, Serrano F, Mascetti VL, Pedersen RA, et al. , Robust derivation of epicardium and its differentiated smooth muscle cell progeny from human pluripotent stem cells, Development 142 (2015) 1528–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Witty AD, Mihic A, Tam RY, Fisher SA, Mikryukov A, Shoichet MS, et al. , Generation of the epicardial lineage from human pluripotent stem cells, Nat. Biotechnol 32 (2014) 1026–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Cheung C, Bernardo AS, Trotter MW, Pedersen RA, Sinha S, Generation of human vascular smooth muscle subtypes provides insight into embryological origin-dependent disease susceptibility, Nat. Biotechnol 30 (2012) 165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Shen M, Quertermous T, Fischbein MP, Wu JC, Generation of vascular smooth muscle cells from induced pluripotent stem cells: methods, applications, and considerations, Circ. Res 128 (2021) 670–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Zhang H, Tian L, Shen M, Tu C, Wu H, Gu M, et al. , Generation of quiescent cardiac fibroblasts from human induced pluripotent stem cells for in vitro modeling of cardiac fibrosis, Circ. Res 125 (2019) 552–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Zhang J, Tao R, Campbell KF, Carvalho JL, Ruiz EC, Kim GC, et al. , Functional cardiac fibroblasts derived from human pluripotent stem cells via second heart field progenitors, Nat. Commun 10 (2019) 2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Giacomelli E, Meraviglia V, Campostrini G, Cochrane A, Cao X, van Helden RWJ, et al. , Human-iPSC-derived cardiac stromal cells enhance maturation in 3D cardiac microtissues and reveal non-cardiomyocyte contributions to heart disease, Cell Stem Cell 26 (2020), 862–79 e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Roberts MA, Tran D, Coulombe KL, Razumova M, Regnier M, Murry CE, et al. , Stromal cells in dense collagen promote cardiomyocyte and microvascular patterning in engineered human heart tissue, Tissue Eng. A 22 (2016) 633–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ribeiro AJS, Schwab O, Mandegar MA, Ang YS, Conklin BR, Srivastava D, et al. , Multi-imaging method to assay the contractile mechanical output of micropatterned human ipsc-derived cardiac myocytes, Circ. Res 120 (2017) 1572–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kim DH, Lipke EA, Kim P, Cheong R, Thompson S, Delannoy M, et al. , Nanoscale cues regulate the structure and function of macroscopic cardiac tissue constructs, Proc. Natl. Acad. Sci. U.S.A 107 (2010) 565–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Carson D, Hnilova M, Yang X, Nemeth CL, Tsui JH, Smith AS, et al. , Nanotopography-induced structural anisotropy and sarcomere development in human cardiomyocytes derived from induced pluripotent stem cells, ACS Appl. Mater. Interfaces 8 (2016) 21923–21932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ribeiro AJ, Ang YS, Fu JD, Rivas RN, Mohamed TM, Higgs GC, et al. , Contractility of single cardiomyocytes differentiated from pluripotent stem cells depends on physiological shape and substrate stiffness, Proc. Natl. Acad. Sci. U.S.A 112 (2015) 12705–12710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Jacot JG, McCulloch AD, Omens JH, Substrate stiffness affects the functional maturation of neonatal rat ventricular myocytes, Biophys. J 95 (2008) 3479–3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Engler AJ, Carag-Krieger C, Johnson CP, Raab M, Tang HY, Speicher DW, et al. , Embryonic cardiomyocytes beat best on a matrix with heart-like elasticity: scar-like rigidity inhibits beating, J. Cell Sci 121 (2008) 3794–3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Radisic M, Park H, Shing H, Consi T, Schoen FJ, Langer R, et al. , Functional assembly of engineered myocardium by electrical stimulation of cardiac myocytes cultured on scaffolds, Proc. Natl. Acad. Sci. U.S.A 101 (2004) 18129–18134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Doll S, Dressen M, Geyer PE, Itzhak DN, Braun C, Doppler SA, et al. , Region and cell-type resolved quantitative proteomic map of the human heart, Nat. Commun 8 (2017) 1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Neuman RE, Logan MA, The determination of collagen and elastin in tissues, J. Biol. Chem 186 (1950) 549–556. [PubMed] [Google Scholar]
- [45].Cho S, Paik DT, Wu JC, An extracellular matrix paradox in myocardial scar formation, Signal Transduct. Target. Ther 5 (2020) 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Yokota T, McCourt J, Ma F, Ren S, Li S, Kim TH, et al. , Type v collagen in scar tissue regulates the size of scar after heart injury, Cell 182 (2020), 545–62 e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Frangogiannis NG, The extracellular matrix in myocardial injury, repair, and remodeling, J. Clin. Invest 127 (2017) 1600–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Dai W, Wold LE, Dow JS, Kloner RA, Thickening of the infarcted wall by collagen injection improves left ventricular function in rats: a novel approach to preserve cardiac function after myocardial infarction, J. Am. Coll. Cardiol 46 (2005) 714–719. [DOI] [PubMed] [Google Scholar]
- [49].Cho S, Irianto J, Discher DE, Mechanosensing by the nucleus: from pathways to scaling relationships, J. Cell Biol 216 (2017) 305–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Cho S, Vashisth M, Abbas A, Majkut S, Vogel K, Xia Y, et al. , Mechanosensing by the lamina protects against nuclear rupture, DNA damage, and cell-cycle arrest, Dev. Cell 49 (2019), 920–35 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Majkut S, Idema T, Swift J, Krieger C, Liu A, Discher DE, Heart-specific stiffening in early embryos parallels matrix and myosin expression to optimize beating, Curr. Biol 23 (2013) 2434–2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Ivanovska IL, Swift J, Spinler K, Dingal D, Cho S, Discher DE, Cross-linked matrix rigidity and soluble retinoids synergize in nuclear lamina regulation of stem cell differentiation, Mol. Biol. Cell 28 (2017) 2010–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Ma B, Wang X, Wu C, Chang J, Crosslinking strategies for preparation of extracellular matrix-derived cardiovascular scaffolds, Regen. Biomater 1 (2014) 81–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Kaiser NJ, Kant RJ, Minor AJ, Coulombe KLK, Optimizing blended collagen-fibrin hydrogels for cardiac tissue engineering with human ipsc-derived cardiomyocytes, ACS Biomater. Sci. Eng 5 (2019) 887–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Levit RD, Landazuri N, Phelps EA, Brown ME, Garcia AJ, Davis ME, et al. , Cellular encapsulation enhances cardiac repair, J. Am. Heart Assoc 2 (2013), e000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Landa N, Miller L, Feinberg MS, Holbova R, Shachar M, Freeman I, et al. , Effect of injectable alginate implant on cardiac remodeling and function after recent and old infarcts in rat, Circulation 117 (2008) 1388–1396. [DOI] [PubMed] [Google Scholar]
- [57].Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI, et al. , Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart, Nat. Med 14 (2008) 213–221. [DOI] [PubMed] [Google Scholar]
- [58].Pati F, Jang J, Ha DH, Won Kim S, Rhie JW, Shim JH, et al. , Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink, Nat. Commun 5 (2014) 3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Castilho M, Feyen D, Flandes-Iparraguirre M, Hochleitner G, Groll J, Doevendans PAF, et al. , Melt electrospinning writing of polyhydroxymethylglycolide-co-epsilon-caprolactone-based scaffolds for cardiac tissue engineering, Adv. Healthc. Mater 6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Ma Z, Koo S, Finnegan MA, Loskill P, Huebsch N, Marks NC, et al. , Three-dimensional filamentous human diseased cardiac tissue model, Biomaterials 35 (2014) 1367–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Engelmayr GC Jr., Cheng, Bettinger CJ, Borenstein JT, Langer R, Freed LE, Accordion-like honeycombs for tissue engineering of cardiac anisotropy, Nat. Mater 7 (2008) 1003–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Kapnisi M, Mansfield C, Marijon C, Guex AG, Perbellini F, Bardi I, et al. , Auxetic cardiac patches with tunable mechanical and conductive properties toward treating myocardial infarction, Adv. Funct. Mater 28 (2018) 1800618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Mawad D, Mansfield C, Lauto A, Perbellini F, Nelson GW, Tonkin J, et al. , A conducting polymer with enhanced electronic stability applied in cardiac models, Sci. Adv 2 (2016), e1601007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Mihic A, Cui Z, Wu J, Vlacic G, Miyagi Y, Li SH, et al. , A conductive polymer hydrogel supports cell electrical signaling and improves cardiac function after implantation into myocardial infarct, Circulation 132 (2015) 772–784. [DOI] [PubMed] [Google Scholar]
- [65].Bearzi C, Gargioli C, Baci D, Fortunato O, Shapira-Schweitzer K, Kossover O, et al. , PLGF-MMP9-engineered iPS cells supported on a PEG-fibrinogen hydrogel scaffold possess an enhanced capacity to repair damaged myocardium, Cell Death Dis. 5 (2014), e1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Xu G, Wang X, Deng C, Teng X, Suuronen EJ, Shen Z, et al. , Injectable biodegradable hybrid hydrogels based on thiolated collagen and oligo(acryloyl carbonate)-poly(ethylene glycol)-oligo(acryloyl carbonate) copolymer for functional cardiac regeneration, Acta Biomater. 15 (2015) 55–64. [DOI] [PubMed] [Google Scholar]
- [67].Hou L, Kim JJ, Wanjare M, Patlolla B, Coller J, Natu V, et al. , Combinatorial extracellular matrix microenvironments for probing endothelial differentiation of human pluripotent stem cells, Sci. Rep 7 (2017) 6551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Jung JP, Hu D, Domian IJ, Ogle BM, An integrated statistical model for enhanced murine cardiomyocyte differentiation via optimized engagement of 3d extracellular matrices, Sci. Rep 5 (2015) 18705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Annabi N, Tsang K, Mithieux SM, Nikkhah M, Ameri A, Khademhosseini A, et al. , Highly elastic micropatterned hydrogel for engineering functional cardiac tissue, Adv. Funct. Mater 23 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Chung C, Anderson E, Pera RR, Pruitt BL, Heilshorn SC, Hydrogel crosslinking density regulates temporal contractility of human embryonic stem cell-derived cardiomyocytes in 3d cultures, Soft Matter 8 (2012) 10141–10148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Wang H, Cai L, Paul A, Enejder A, Heilshorn SC, Hybrid elastin-like polypeptide-polyethylene glycol (ELP-PEG) hydrogels with improved transparency and independent control of matrix mechanics and cell ligand density, Biomacromolecules 15 (2014) 3421–3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].McGann CL, Levenson EA, Kiick KL, Resilin-based hybrid hydrogels for cardiovascular tissue engineering, Macromolecules 214 (2013) 203–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Zimmermann WH, Schneiderbanger K, Schubert P, Didie M, Munzel F, Heubach JF, et al. , Tissue engineering of a differentiated cardiac muscle construct, Circ. Res 90 (2002) 223–230. [DOI] [PubMed] [Google Scholar]
- [74].de Lange WJ, Hegge LF, Grimes AC, Tong CW, Brost TM, Moss RL, et al. , Neonatal mouse-derived engineered cardiac tissue: a novel model system for studying genetic heart disease, Circ. Res 109 (2011) 8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Bian W, Liau B, Badie N, Bursac N, Mesoscopic hydrogel molding to control the 3d geometry of bioartificial muscle tissues, Nat. Protoc 4 (2009) 1522–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Boudou T, Legant WR, Mu A, Borochin MA, Thavandiran N, Radisic M, et al. , A microfabricated platform to measure and manipulate the mechanics of engineered cardiac microtissues, Tissue Eng Part A 18 (2012) 910–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Hansen A, Eder A, Bonstrup M, Flato M, Mewe M, Schaaf S, et al. , Development of a drug screening platform based on engineered heart tissue, Circ. Res 107 (2010) 35–44. [DOI] [PubMed] [Google Scholar]
- [78].Godier-Furnemont AF, Tiburcy M, Wagner E, Dewenter M, Lammle S, El-Armouche A, et al. , Physiologic force-frequency response in engineered heart muscle by electromechanical stimulation, Biomaterials 60 (2015) 82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Zimmermann WH, Melnychenko I, Wasmeier G, Didie M, Naito H, Nixdorff U, et al. , Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts, Nat. Med 12 (2006) 452–458. [DOI] [PubMed] [Google Scholar]
- [80].Jackman CP, Carlson AL, Bursac N, Dynamic culture yields engineered myocardium with near-adult functional output, Biomaterials 111 (2016) 66–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Abilez OJ, Tzatzalos E, Yang H, Zhao MT, Jung G, Zollner AM, et al. , Passive stretch induces structural and functional maturation of engineered heart muscle as predicted by computational modeling, Stem Cells 36 (2018) 265–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Fink C, Ergun S, Kralisch D, Remmers U, Weil J, Eschenhagen T, Chronic stretch of engineered heart tissue induces hypertrophy and functional improvement, FASEB J. 14 (2000) 669–679. [DOI] [PubMed] [Google Scholar]
- [83].Ronaldson-Bouchard K, Yeager K, Teles D, Chen T, Ma S, Song L, et al. , Engineering of human cardiac muscle electromechanically matured to an adult-like phenotype, Nat. Protoc 14 (2019) 2781–2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Chiou KK, Rocks JW, Chen CY, Cho S, Merkus KE, Rajaratnam A, et al. , Mechanical signaling coordinates the embryonic heartbeat, Proc. Natl. Acad. Sci. U.S.A 113 (2016) 8939–8944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Sessions AO, Engler AJ, Mechanical regulation of cardiac aging in model systems, Circ. Res 118 (2016) 1553–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Yahalom-Ronen Y, Rajchman D, Sarig R, Geiger B, Tzahor E, Reduced matrix rigidity promotes neonatal cardiomyocyte dedifferentiation, proliferation and clonal expansion, Elife 4 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Liaw NY, Zimmermann WH, Mechanical stimulation in the engineering of heart muscle, Adv. Drug Deliv. Rev 96 (2016) 156–160. [DOI] [PubMed] [Google Scholar]
- [88].Ulmer BM, Stoehr A, Schulze ML, Patel S, Gucek M, Mannhardt I, et al. , Contractile work contributes to maturation of energy metabolism in hiPSC-derived cardiomyocytes, Stem Cell Rep. 10 (2018) 834–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Smith L, Cho S, Discher DE, Mechanosensing of matrix by stem cells: from matrix heterogeneity, contractility, and the nucleus in pore-migration to cardiogenesis and muscle stem cells in vivo, Semin. Cell Dev. Biol 71 (2017) 84–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Chopra A, Kutys ML, Zhang K, Polacheck WJ, Sheng CC, Luu RJ, et al. , Force generation via beta-cardiac myosin, titin, and alpha-actinin drives cardiac sarcomere assembly from cell-matrix adhesions, Dev. Cell 44 (2018), 87–96 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Saucerman JJ, Tan PM, Buchholz KS, McCulloch AD, Omens JH, Mechanical regulation of gene expression in cardiac myocytes and fibroblasts, Nat. Rev. Cardiol 16 (2019) 361–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Li Y, Lang J, Ye Z, Wang M, Yang Y, Guo X, et al. , Effect of substrate stiffness on redox state of single cardiomyocyte: a scanning electrochemical microscopy study, Anal. Chem 92 (2020) 4771–4779. [DOI] [PubMed] [Google Scholar]
- [93].Pasqualini FS, Nesmith AP, Horton RE, Sheehy SP, Parker KK, Mechanotransduction and metabolism in cardiomyocyte microdomains, Biomed. Res. Int 2016 (2016) 4081638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Hirt MN, Boeddinghaus J, Mitchell A, Schaaf S, Bornchen C, Muller C, et al. , Functional improvement and maturation of rat and human engineered heart tissue by chronic electrical stimulation, J. Mol. Cell. Cardiol 74 (2014) 151–161. [DOI] [PubMed] [Google Scholar]
- [95].Tandon N, Cannizzaro C, Chao PH, Maidhof R, Marsano A, Au HT, et al. , Electrical stimulation systems for cardiac tissue engineering, Nat. Protoc 4 (2009) 155–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Nunes SS, Miklas JW, Liu J, Aschar-Sobbi R, Xiao Y, Zhang B, et al. , Biowire: a platform for maturation of human pluripotent stem cell-derived cardiomyocytes, Nat. Methods 10 (2013) 781–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Zhao Y, Rafatian N, Feric NT, Cox BJ, Aschar-Sobbi R, Wang EY, et al. , A platform for generation of chamber-specific cardiac tissues and disease modeling, Cell 176 (2019), 913–27 e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Xiao Y, Zhang B, Liu H, Miklas JW, Gagliardi M, Pahnke A, et al. , Microfabricated perfusable cardiac biowire: a platform that mimics native cardiac bundle, Lab Chip 14 (2014) 869–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Ronaldson-Bouchard K, Ma SP, Yeager K, Chen T, Song L, Sirabella D, et al. , Advanced maturation of human cardiac tissue grown from pluripotent stem cells, Nature 556 (2018) 239–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Ruan JL, Tulloch NL, Razumova MV, Saiget M, Muskheli V, Pabon L, et al. , Mechanical stress conditioning and electrical stimulation promote contractility and force maturation of induced pluripotent stem cell-derived human cardiac tissue, Circulation 134 (2016) 1557–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Brandenberg N, Hoehnel S, Kuttler F, Homicsko K, Ceroni C, Ringel T, et al. , High-throughput automated organoid culture via stem-cell aggregation in microcavity arrays, Nat. Biomed. Eng 4 (2020) 863–874. [DOI] [PubMed] [Google Scholar]
- [102].Archer CR, Sargeant R, Basak J, Pilling J, Barnes JR, Pointon A, Characterization and validation of a human 3D cardiac microtissue for the assessment of changes in cardiac pathology, Sci. Rep 8 (2018) 10160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Mills RJ, Parker BL, Quaife-Ryan GA, Voges HK, Needham EJ, Bornot A, et al. , Drug screening in human psc-cardiac organoids identifies pro-proliferative compounds acting via the mevalonate pathway, Cell Stem Cell 24 (2019), 895–907 e6. [DOI] [PubMed] [Google Scholar]
- [104].Wilson KD, Ameen M, Guo H, Abilez OJ, Tian L, Mumbach MR, Diecke S, Qin X, Liu Y, Yang H, Ma N, Gaddam S, Cunningham NJ, Gu M, Neofytou E, Prado M, Hildebrandt TB, Karakikes I, Chang HY, Wu JC, et al. , Endogenous retrovirus-derived lncRNA BANCR promotes cardiomyocyte migration in humans and non-human primates, Dev. Cell 54 (2020) 694–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Voges HK, Mills RJ, Elliott DA, Parton RG, Porrello ER, Hudson JE, Development of a human cardiac organoid injury model reveals innate regenerative potential, Development 144 (2017) 1118–1127. [DOI] [PubMed] [Google Scholar]
- [106].Richards DJ, Li Y, Kerr CM, Yao J, Beeson GC, Coyle RC, et al. , Human cardiac organoids for the modelling of myocardial infarction and drug cardiotoxicity, Nat. Biomed. Eng 4 (2020) 446–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Rhee JW, Yi H, Thomas D, Lam CK, Belbachir N, Tian L, et al. , Modeling secondary iron overload cardiomyopathy with human induced pluripotent stem cell-derived cardiomyocytes, Cell Rep. 32 (2020) 107886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Giacomelli E, Bellin M, Sala L, van Meer BJ, Tertoolen LG, Orlova VV, et al. , Three-dimensional cardiac microtissues composed of cardiomyocytes and endothelial cells co-differentiated from human pluripotent stem cells, Development 144 (2017) 1008–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Drakhlis L, Biswanath S, Farr CM, Lupanow V, Teske J, Ritzenhoff K, et al. , Human heart-forming organoids recapitulate early heart and foregut development, Nat. Biotechnol (2021). https://pubmed.ncbi.nlm.nih.gov/33558697/ (online ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Rossi G, Broguiere N, Miyamoto M, Boni A, Guiet R, Girgin M, et al. , Capturing cardiogenesis in gastruloids, Cell Stem Cell 28 (2) (2020) 230–240 e6. https://pubmed.ncbi.nlm.nih.gov/33176168/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Ma Z, Wang J, Loskill P, Huebsch N, Koo S, Svedlund FL, et al. , Self-organizing human cardiac microchambers mediated by geometric confinement, Nat. Commun 6 (2015) 7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Israeli Y, Gabalski M, Ball K, Wasserman A, Zou J, Ni G, et al. , Generation of heart organoids modeling early human cardiac development under defined conditions, bioRxiv (2020), 2020.06.25.171611. [Google Scholar]
- [113].Hofbauer P, Jahnel S, Papai N, Giesshammer M, Penc M, Tavernini K, et al. , Cardioids reveal self-organizing principles of human cardiogenesis, bioRxiv (2020), 2020.07.06.189431. [DOI] [PubMed] [Google Scholar]
- [114].Bielawski KS, Leonard A, Bhandari S, Murry CE, Sniadecki NJ, Real-time force and frequency analysis of engineered human heart tissue derived from induced pluripotent stem cells using magnetic sensing, Tissue Eng. C Methods 22 (2016) 932–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Thavandiran N, Dubois N, Mikryukov A, Masse S, Beca B, Simmons CA, et al. , Design and formulation of functional pluripotent stem cell-derived cardiac microtissues, Proc. Natl. Acad. Sci. U.S.A 110 (2013) E4698–E4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Zhang D, Shadrin IY, Lam J, Xian HQ, Snodgrass HR, Bursac N, Tissue-engineered cardiac patch for advanced functional maturation of human ESC-derived cardiomyocytes, Biomaterials 34 (2013) 5813–5820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Tiburcy M, Hudson JE, Balfanz P, Schlick S, Meyer T, Chang Liao ML, et al. , Defined engineered human myocardium with advanced maturation for applications in heart failure modeling and repair, Circulation 135 (2017) 1832–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Goldfracht I, Protze S, Shiti A, Setter N, Gruber A, Shaheen N, et al. , Generating ring-shaped engineered heart tissues from ventricular and atrial human pluripotent stem cell-derived cardiomyocytes, Nat. Commun 11 (2020) 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].MacQueen LA, Sheehy SP, Chantre CO, Zimmerman JF, Pasqualini FS, Liu X, et al. , A tissue-engineered scale model of the heart ventricle, Nat. Biomed. Eng 2 (2018) 930–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Goldfracht I, Efraim Y, Shinnawi R, Kovalev E, Huber I, Gepstein A, et al. , Engineered heart tissue models from hiPSC-derived cardiomyocytes and cardiac ECM for disease modeling and drug testing applications, Acta Biomater. 92 (2019) 145–159. [DOI] [PubMed] [Google Scholar]
- [121].Yang H, Shao N, Holstrom A, Zhao X, Chour T, Chen H, Itzhaki I, Wu H, Ameen M, Cunningham NJ, Tu C, Zhao MT, Tarantal AF, Abilez OJ, Wu JC, et al. , Transcriptome analysis of non-human Primate induced pluripotent stem cell-derived cardiomyocytes in 2D monolayer culture versus 3D engineered heart tissue, Cardiovasc. Res (2020). https://pubmed.ncbi.nlm.nih.gov/33002105/ (online ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Tiburcy M, Didie M, Boy O, Christalla P, Doker S, Naito H, et al. , Terminal differentiation, advanced organotypic maturation, and modeling of hypertrophic growth in engineered heart tissue, Circ. Res 109 (2011) 1105–1114. [DOI] [PubMed] [Google Scholar]
- [123].Huebsch N, Loskill P, Deveshwar N, Spencer CI, Judge LM, Mandegar MA, et al. , Miniaturized ips-cell-derived cardiac muscles for physiologically relevant drug response analyses, Sci. Rep 6 (2016) 24726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Gao L, Kupfer ME, Jung JP, Yang L, Zhang P, Da Sie Y, et al. , Myocardial tissue engineering with cells derived from human-induced pluripotent stem cells and a native-like, high-resolution, 3-dimensionally printed scaffold, Circ. Res 120 (2017) 1318–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Lind JU, Busbee TA, Valentine AD, Pasqualini FS, Yuan H, Yadid M, et al. , Instrumented cardiac microphysiological devices via multimaterial three-dimensional printing, Nat. Mater 16 (2017) 303–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Borovjagin AV, Ogle BM, Berry JL, Zhang J, From microscale devices to 3d printing: advances in fabrication of 3d cardiovascular tissues, Circ. Res 120 (2017) 150–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Gaetani R, Doevendans PA, Metz CH, Alblas J, Messina E, Giacomello A, et al. , Cardiac tissue engineering using tissue printing technology and human cardiac progenitor cells, Biomaterials 33 (2012) 1782–1790. [DOI] [PubMed] [Google Scholar]
- [128].Lee A, Hudson AR, Shiwarski DJ, Tashman JW, Hinton TJ, Yerneni S, et al. , 3D bioprinting of collagen to rebuild components of the human heart, Science 365 (2019) 482–487. [DOI] [PubMed] [Google Scholar]
- [129].Skylar-Scott MA, Uzel SGM, Nam LL, Ahrens JH, Truby RL, Damaraju S, et al. , Biomanufacturing of organ-specific tissues with high cellular density and embedded vascular channels, Sci. Adv 5 (2019) eaaw2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Wnorowski A, Yang H, Wu JC, Progress, obstacles, and limitations in the use of stem cells in organ-on-a-chip models, Adv. Drug Deliv. Rev 140 (2019) 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Schaaf S, Shibamiya A, Mewe M, Eder A, Stohr A, Hirt MN, et al. , Human engineered heart tissue as a versatile tool in basic research and preclinical toxicology, PLoS One 6 (2011), e26397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Kensah G, Roa Lara A, Dahlmann J, Zweigerdt R, Schwanke K, Hegermann J, et al. , Murine and human pluripotent stem cell-derived cardiac bodies form contractile myocardial tissue in vitro, Eur. Heart J 34 (2013) 1134–1146. [DOI] [PubMed] [Google Scholar]
- [133].Eder A, Hansen A, Uebeler J, Schulze T, Neuber C, Schaaf S, et al. , Effects of proarrhythmic drugs on relaxation time and beating pattern in rat engineered heart tissue, Basic Res. Cardiol 109 (2014) 436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Mannhardt I, Saleem U, Mosqueira D, Loos MF, Ulmer BM, Lemoine MD, et al. , Comparison of 10 control hpsc lines for drug screening in an engineered heart tissue format, Stem Cell Rep. 15 (2020) 983–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Wnorowski A, Sharma A, Chen H, Wu H, Shao NY, Sayed N, et al. , Effects of spaceflight on human induced pluripotent stem cell-derived cardiomyocyte structure and function, Stem Cell Rep. 13 (2019) 960–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Wang G, McCain ML, Yang L, He A, Pasqualini FS, Agarwal A, et al. , Modeling the mitochondrial cardiomyopathy of Barth syndrome with induced pluripotent stem cell and heart-on-chip technologies, Nat. Med 20 (2014) 616–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Hinson JT, Chopra A, Lowe A, Sheng CC, Gupta RM, Kuppusamy R, et al. , Integrative analysis of PRKAG2 cardiomyopathy iPSC and microtissue models identifies ampk as a regulator of metabolism, survival, and fibrosis, Cell Rep. 17 (2016) 3292–3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Hinson JT, Chopra A, Nafissi N, Polacheck WJ, Benson CC, Swist S, et al. , Heart disease. Titin mutations in ips cells define sarcomere insufficiency as a cause of dilated cardiomyopathy, Science 349 (2015) 982–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Hashimoto H, Olson EN, Bassel-Duby R, Therapeutic approaches for cardiac regeneration and repair, Nat. Rev. Cardiol 15 (2018) 585–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Coulombe KL, Bajpai VK, Andreadis ST, Murry CE, Heart regeneration with engineered myocardial tissue, Annu. Rev. Biomed. Eng 16 (2014) 1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Chien KR, Frisen J, Fritsche-Danielson R, Melton DA, Murry CE, Weissman IL, Regenerating the field of cardiovascular cell therapy, Nat. Biotechnol 37 (2019) 232–237. [DOI] [PubMed] [Google Scholar]
- [142].Riegler J, Gillich A, Shen Q, Gold JD, Wu JC, Cardiac tissue slice transplantation as a model to assess tissue-engineered graft thickness, survival, and function, Circulation 130 (2014) S77–S86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Riegler J, Tiburcy M, Ebert A, Tzatzalos E, Raaz U, Abilez OJ, et al. , Human engineered heart muscles engraft and survive long term in a rodent myocardial infarction model, Circ. Res 117 (2015) 720–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Wnorowski A, Wu JC, 3-dimensionally printed, native-like scaffolds for myocardial tissue engineering, Circ. Res 120 (2017) 1224–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Weinberger F, Breckwoldt K, Pecha S, Kelly A, Geertz B, Starbatty J, et al. , Cardiac repair in guinea pigs with human engineered heart tissue from induced pluripotent stem cells, Sci. Transl. Med 8 (2016), 363ra148. [DOI] [PubMed] [Google Scholar]
- [146].Marquardt LM, Doulames VM, Wang AT, Dubbin K, Suhar RA, Kratochvil MJ, et al. , Designer, injectable gels to prevent transplanted schwann cell loss during spinal cord injury therapy, Sci. Adv 6 (2020) eaaz1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Huang NF, Serpooshan V, Morris VB, Sayed N, Pardon G, Abilez OJ, et al. , Big bottlenecks in cardiovascular tissue engineering, Commun. Biol 1 (2018) 199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Zhang B, Montgomery M, Chamberlain MD, Ogawa S, Korolj A, Pahnke A, et al. , Biodegradable scaffold with built-in vasculature for organ-on-a-chip engineering and direct surgical anastomosis, Nat. Mater 15 (2016) 669–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Redd MA, Zeinstra N, Qin W, Wei W, Martinson A, Wang Y, et al. , Patterned human microvascular grafts enable rapid vascularization and increase perfusion in infarcted rat hearts, Nat. Commun 10 (2019) 584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Bettinger CJ, Weinberg EJ, Kulig KM, Vacanti JP, Wang Y, Borenstein JT, et al. , Three-dimensional microfluidic tissue-engineering scaffolds using a flexible biodegradable polymer, Adv. Mater 18 (2005) 165–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Sakaguchi K, Shimizu T, Horaguchi S, Sekine H, Yamato M, Umezu M, et al. , In vitro engineering of vascularized tissue surrogates, Sci. Rep 3 (2013) 1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Buikema JW, Lee S, Goodyer WR, Maas RG, Chirikian O, Li G, et al. , Wnt activation and reduced cell-cell contact synergistically induce massive expansion of functional human ipsc-derived cardiomyocytes, Cell Stem Cell 27 (2020), 50–63 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Chen VC, Ye J, Shukla P, Hua G, Chen D, Lin Z, et al. , Development of a scalable suspension culture for cardiac differentiation from human pluripotent stem cells, Stem Cell Res. 15 (2015) 365–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Tohyama S, Fujita J, Fujita C, Yamaguchi M, Kanaami S, Ohno R, et al. , Efficient large-scale 2D culture system for human induced pluripotent stem cells and differentiated cardiomyocytes, Stem Cell Rep. 9 (2017) 1406–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Paik DT, Cho S, Tian L, Chang HY, Wu JC, Single-cell RNA sequencing in cardiovascular development, disease and medicine, Nat. Rev. Cardiol 17 (2020) 457–473. [DOI] [PMC free article] [PubMed] [Google Scholar]


