Abstract
Objective:
To describe oncologic outcomes after using acute normovolemic hemodilution (ANH) to reduce requirement for allogenic red blood cell transfusions (ABT) in patients undergoing primary debulking surgery (PDS) for advanced ovarian cancer.
Methods:
We performed a post-hoc analysis of a recent prospective trial investigating the safety and feasibility of ANH during PDS for advanced ovarian cancer. We report long-term survival outcomes. We compared demographics, clinicopathological characteristics, survival outcomes in this cohort of Stage IIIB-IVB high-grade serous ovarian cancer patients undergoing ANH (ANH group), with a retrospective cohort of all other patients (standard group) undergoing PDS during the same time period (01/2012–04/2017). Standard statistical tests were used.
Results:
There were no demographic or clinicopathological differences between ANH (n=33) and standard groups (n=360), except for higher median age at diagnosis (57 vs. 62 years, respectively; p=0.044) and shorter operative time (357 vs. 446 min, respectively; p<0.001) in the standard group. Cytoreductive outcomes (ANH vs. standard): 0 mm, 69.7 vs. 63.9%; gross residual disease (RD) ≤1cm, 21.2 vs. 26.9%; >1cm, 9.1 vs. 9.2% (p=0.78). RD after PDS was the only independent factor associated with worse progression-free survival (PFS) on multivariable analysis (p<0.001). Patients with BRCA mutations trended towards improved PFS (p=0.057). Significant factors for overall survival (OS) on multivariable analysis: preoperative CA125 (p=0.004), ascites (p=0.018), RD after PDS (p=0.04), BRCA mutation status (p<0.001). After adjustment for potential confounders, ANH was not independently associated with PFS or OS [PFS: HR 0.928 (0.618–1.395); p=0.721; OS: HR 0.588 (95%CI: 0.317–1.092); p=0.093].
Conclusions:
ANH is an innovative approach in intraoperative management. It was previously proven to decrease need for ABT while maintaining the ability to achieve complete gross resection and associated benefits.
Keywords: Allogenic blood transfusion, Acute normovolemic hemodilution, Primary debulking surgery, Ovarian cancer, Cytoreductive surgery
INTRODUCTION
Cytoreductive surgery, whether performed before chemotherapy as primary debulking surgery (PDS) or after neoadjuvant chemotherapy as interval debulking surgery (IDS), is a well-established cornerstone in the primary treatment of advanced ovarian, tubal, and primary peritoneal cancer [1]. These lengthy procedures require extensive surgical exploration, often including removal of multiple organs, and are frequently accompanied by substantial blood loss necessitating perioperative allogenic blood transfusion (ABT) [1, 2]. Although the risk of infection has declined with improved donor screening, other risks persist, including immunosuppression, transfusion reactions, cardiovascular events, acute kidney injury, and higher postoperative morbidity/mortality [3, 4]. Recognizing these potential drawbacks, as well as the decreased availability of allogenic blood products (due to increased fragility of the national blood supply in recent years) [5, 6], researchers have focused on developing new and innovative blood-saving techniques to minimize transfusion of allogenic blood products.
Acute normovolemic hemodilution (ANH) has been successfully performed since the 1970s, mainly in the field of cardiac surgery. It has been proven to reduce the need for ABT by diluting surgical blood loss, resulting in an overall decreased loss of red blood cells (RBC) [7].
We recently published the results of a prospective trial evaluating the safety and efficacy of ANH in reducing the requirement for allogenic RBC transfusions in patients undergoing PDS for advanced ovarian cancer [8]. The utilization of ANH led to a reduced rate of ABT versus historic controls, without increasing perioperative complications. Based on these promising short-term results, the objective of the current study was to assess the long-term oncologic outcomes of patients enrolled in this trial compared with historic controls.
METHODS
This study was approved by the Institutional Review Board at Memorial Sloan Kettering Cancer Center (MSKCC). We analyzed two cohorts.
The first cohort (ANH group) used patient data extracted from the recently conducted prospective single-center study, noted above [8]. In this trial, 41 patients undergoing PDS for advanced ovarian cancer underwent ANH at the time of primary cytoreductive surgery. ANH was performed as previously described [8]. Intraoperative blood withdrawal was performed to a target hemoglobin of 8.0 g/dL. A standardized transfusion protocol, using autologous and then allogenic blood, was applied intraoperatively and throughout hospitalization, according to institutional guidelines.
The second cohort (standard group) consisted of all 360 women with stage IIIB-IVB ovarian, tubal, and primary peritoneal high-grade serous cancer (HGSC) (hereafter referred to as ovarian cancer) who underwent PDS in the same study period (January 2012-April 2017).
Electronic medical records were reviewed to abstract patient demographics, clinicopathological characteristics, and survival data. Complete gross resection (CGR) was defined as no visible disease upon completion of surgery. Optimal and suboptimal surgical debulking were defined as residual disease (RD) of 0.1–1cm and >1cm in tumor diameter, respectively.
We compared survival outcomes between the ANH and standard groups as a post-hoc analysis. In order to create a homogenous cohort we included only patients with high-grade serous histology and FIGO stage IIIB-IVB disease. Eight of the original 41 ANH patients were therefore excluded from our survival analysis on the basis of histology: four had clear-cell carcinoma, three had low-grade histology, and one had high-grade endometrial carcinoma on final pathologic analysis. In the standard group, we included all 360 women with high-grade serous stage IIIB-IVB ovarian cancer who underwent PDS in the same study period. The two treatment groups (ANH vs. standard) were compared using Fisher’s Exact test for categorical variables and the Wilcoxon Rank-Sum test for continuous variables.
Progression-free survival (PFS) and overall survival (OS) were calculated from the date of PDS until first relapse or death, whichever came first (for PFS), or until time of death (for OS). Patients who did not experience the event of interest by the end of the study were censored at the time of the last follow-up. Median PFS and OS as well as 5-year PFS/OS rates were estimated using Kaplan-Meier methods, and compared between treatment groups using the logrank test for categorical variables and the Wald test based on Cox Proportional Hazards (CoxPH) regression for continuous variables. A multivariable CoxPH model for PFS and OS was built, based on the findings of univariable analyses (p≤0.05). Statistical analyses were performed using SAS 9.4.
RESULTS
Clinical and pathologic features of the cohort
All 393 patients who underwent PDS for advanced ovarian HGSC from January 2012 to April 2017 at MSKCC were included in this study. Demographic and clinicopathological features are detailed in Table 1. Thirty-three women (8%) were enrolled in the prospective trial assessing ANH at time of surgery (ANH group), and the remaining 360 (92%) received standard perioperative care during the same time period (standard group). There were no demographic or clinicopathological differences between the two cohorts, except for a significantly higher median age at diagnosis (62 yrs vs. 57 yrs; p=0.044) and a shorter operative time (357 vs. 446 min, respectively; p<0.001) in the standard group. However, the proportion of patients 65 years of age or older was similar between the two groups (p=0.579). In 23 patients (69.7%) in the ANH cohort, a CGR was achieved;in the remaining patients, 7 (21.2%) had RD <1cm, and 3 (9.1%) RD >1cm. In the standard group, CGR, optimal, and suboptimal rates were 63.9%, 26.9% and 9.2%, respectively. There were no statistical differences between the two groups (ANH vs. standard) with respect to RD status after PDS (p=0.78).
Table 1:
Demographic and clinicopathological findings by treatment groups
| Variables | All | Standard | ANH | p-value* |
|---|---|---|---|---|
| Whole cohort (n) | 393 | 360 | 33 | |
|
| ||||
| Age at diagnosis (years) | ||||
| Median (range) | 62 (33–91) | 62 (34–91) | 57 (33–72) | 0.044 |
| Age<65 | 241 (61.3%) | 219 (60.8%) | 22 (66.7%) | 0.579 |
| Age>=65 | 152 (38.7%) | 141 (39.2%) | 11 (33.3%) | |
|
| ||||
| Preoperative albumin (g/dl) | ||||
| Median (range) | 4.2 (2.3–5) | 4.2 (2.3–5) | 4.2 (3–4.8) | 0.616 |
| Low (<4.0) | 119 (30.4%) | 112 (31.2%) | 7 (21.2%) | 0.322 |
| High (≥4.0) | 273 (69.6%) | 247 (68.8%) | 26 (78.8%) | |
|
| ||||
| Preoperative CA125 (U/ml) | ||||
| Median (range) | 394 (3–19140) | 382 (3–19140) | 508 (39–12774) | 0.333 |
| <500 | 216 (55.4%) | 200 (56%) | 16 (48.5%) | 0.466 |
| ≥500 | 174 (44.6%) | 157 (44%) | 17 (51.5%) | |
|
| ||||
| Preoperative hemoglobin (g/dl) | ||||
| Median (range) | 12.4 (8.8–15.3) | 12.4 (8.8–15.3) | 12.5 (10.1–14.3) | 0.736 |
|
| ||||
| Residual disease status | ||||
| CGR | 253 (64.4%) | 230 (63.9%) | 23 (69.7%) | 0.78 |
| 0.1–1cm | 104 (26.5%) | 97 (26.9%) | 7 (21.2%) | |
| >1cm | 36 (9.2%) | 33 (9.2%) | 3 (9.1%) | |
|
| ||||
| Ascites (ml) | ||||
| Median (range) | 250 (0–9800) | 200 (0–9800) | 300 (0–6000) | 0.163 |
| <500 | 216 (55%) | 198 (55%) | 18 (54.5%) | 1 |
| ≥500 | 177 (45%) | 162 (45%) | 15 (45.5%) | |
|
| ||||
| EBL (ml) | ||||
| Median (range) | 750 (10–8150) | 725 (10–8150) | 1000 (150–2700) | 0.057 |
|
| ||||
| Operative time (min) | ||||
| Median (range) | 369 (67–835) | 357 (67–835) | 446 (168–645) | <0.001 |
|
| ||||
| Length of hospital stay (d) | ||||
| Median (range) | 8 (1–45) | 8 (1–45) | 8 (5–28) | 0.159 |
|
| ||||
| Stage | ||||
| III | 272 (69.2%) | 251 (69.7%) | 21 (63.6%) | 0.555 |
| IV | 121 (30.8%) | 109 (30.3%) | 12 (36.4%) | |
|
| ||||
| mBRCA | ||||
| Negative | 248 (63.1%) | 222 (61.7%) | 26 (78.8%) | 0.182 |
| BRCA1/BRCA2 | 99 (25.2%) | 94 (26.1%) | 5 (15.2%) | |
| Not Tested | 46 (11.7%) | 44 (12.2%) | 2 (6.1%) | |
|
| ||||
| Year of PDS | ||||
| 2012 | 85 (21.6%) | 75 (20.8%) | 10 (30.3%) | 0.33 |
| 2013 | 72 (18.3%) | 66 (18.3%) | 6 (18.2%) | |
| 2014 | 64 (16.3%) | 59 (16.4%) | 5 (15.2%) | |
| 2015 | 58 (14.8%) | 57 (15.8%) | 1 (3%) | |
| 2016 | 86 (21.9%) | 78 (21.7%) | 8 (24.2%) | |
| 2017 | 28 (7.1%) | 25 (6.9%) | 3 (9.1%) | |
p-value obtained using Fisher’s Exact test or Wilcoxon Rank-Sum test
ANH, acute normovolemic hemodilution; CGR, complete gross resection; EBL, estimated blood loss; mBRCA, mutated BRCA status; PDS, primary debulking surgery
Survival outcomes
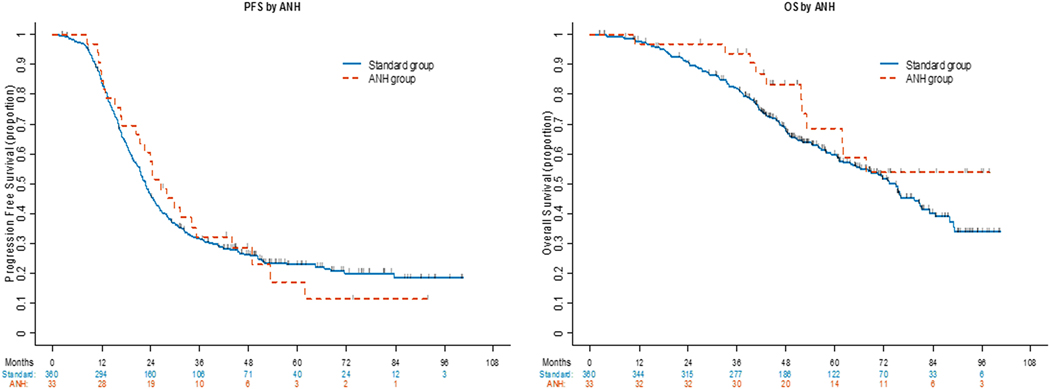
The median follow-up for all survivors was 55.3 months (range: 2.5–100.7) and was not statistically different between groups [ANH: 62.2 months (95%CI: 27.3–97.9) vs. standard: 55.3 months (95%CI: 2.5–100.7); p=0.39], respectively. At the time of analysis, 294 patients had recurred (26 in the ANH group, 268 in the standard group). The median PFS was 23 months (95%CI: 21.4–24.9) for the entire cohort, with a 5-year PFS rate of 23% (95%CI: 18–27%) (Supplementary Table S1). PFS was similar between the two groups [ANH: 26.4 months (20.5–35.2) vs. standard: 22.8 months (21.3–24.6); p=0.825] (Figure 1 and Supplementary Table S1).
Figure 1.
PFS and OS stratified by acute normovolemic hemodilution (ANH)
On multivariate analysis (Table 2), only gross RD after cytoreduction was independently associated with an increased risk of recurrence (0.1–1cm RD vs. CGR: HR 1.398 (95%CI: 1.075–1.819), RD >1cm vs. CGR: HR 2.544 (95%CI: 1.744–3.712); p<0.001). Patients with deleterious BRCA1/2 mutations (BRCAmut) trended towards longer PFS versus those with BRCA wild-type (wt)[BRCAwt vs. BRCAmut: HR 1.406 (95%CI: 1.061–1.864), BRCAwt vs. not tested: HR 1.383 (95%CI: 0.904–2.117); p=0.057]. The use of ANH had neither a beneficial nor detrimental effect on PFS [HR 0.928 (95%CI: 0.618–1.395); p=0.721].
Table 2:
Multivariable analysis of progression-free survival (n=393; events=294) and overall survival (n=390; events=166)
| PFS | OS | |||||
|---|---|---|---|---|---|---|
| Variable | HR | 95%CI | p-value | HR | 95%CI | p-value |
| ANH: Yes vs. No | 0.92 8 | 0.618–1.395 | 0.721 | 0.58 8 | 0.317–1.092 | 0.093 |
| Age at diagnosis: ≥65yr vs <65yr | - | - | 1.032 | 0.749–1.423 | 0.846 | |
| Preoperative CA125: ≥500 U/ml vs <500 U/ml | - | - | 0.592 | 0.415–0.845 | 0.004 | |
| Ascites: ≥500ml vs <500ml | - | - | 1.528 | 1.076–2.17 | 0.018 | |
| Residual disease status | <0.001 | 0.04 | ||||
| 0.1–1cm vs CGR | 1.398 | 1.075–1.819 | 1.41 4 | 1.001–1.998 | ||
| >1cm vs CGR | 2.544 | 1.744–3.712 | 1.742 | 1.05–2.892 | ||
| mBRCA | 0.057 | <0.001 | ||||
| Negative vs BRCA1/BRCA2 | 1.406 | 1.061–1.864 | 2.058 | 1.309–3.237 | ||
| Not Tested vs BRCA1/BRCA2 | 1.383 | 0.904–2.117 | 5.37 | 3.042–9.48 | ||
ANH, acute normovolemic hemodilution; PFS, progression-free survival; OS, overall survival; yr, years; HR, hazard ratio; CGR, complete gross resection; mBRCA, mutated BRCA status
In the entire cohort (n=393), there were a total of 167 deaths (11 in the ANH group, 156 in the standard group) at the time of analysis, with a median OS not reached in the ANH group and 74.9 months (65.2–81) in the standard group (Supplementary Table S2). Survival was similar between the two groups (p=0.146), with 5-year OS rates of 68.7% (95%CI: 46–83.3%) and 59.8% (95%CI: 53.9–65.2%) for the ANH and standard groups, respectively (Figure 1 and Supplementary Table S2).
After adjustment for potential confounders, ascites ≥500ml [HR 1.528 (95%CI: 1.076–2.17); p=0.018], gross RD after PDS [0.1–1cm RD vs. CGR: HR 1.414 (95%CI: 1.001–1.998), >1cm RD vs. CGR: HR 1.742 (95%CI: 1.05–2.892); p=0.04], and BRCAmut status [BRCAwt vs. BRCAmut: HR 2.058 (95%CI: 1.309–3.237), BRCAwt vs. not tested: HR 5.37 (95%CI: 3.042–9.48); p<0.001] were independently associated with worse OS (Table 2). Utilization of ANH was not statistically significant to predict OS differences [HR 0.588 (95%CI: 0.317–1.092); p=0.093].
DISCUSSION
ANH has previously been shown to be an effective blood conservation technique with minimal adverse events, especially in patients undergoing major surgery entailing presumed high blood loss (>1000ml) [9, 10]. A recent meta-analysis by Barile et al. investigating the use of ANH in cardiac surgery, which included 29 randomized controlled trials, observed a significantly decreased ABT rate in the ANH group versus the control group (42.1% vs. 56.1%; p≤0.0001) [11].
The initial experience with ANH in patients undergoing PDS for advanced ovarian cancer was previously reported by our group [8]. The application of ANH reduced allogenic RBC transfusion rates by 32% compared with historic controls, without increasing perioperative complications. These positive findings were recently supported by a retrospective study from Japan. Saito et al. evaluated the efficacy of ANH for reducing perioperative ABT in 586 patients with gynecological cancer. The results of their study demonstrated a significantly lower perioperative ABT transfusion rate in the ANH group compared with the non-ANH group (3.5% vs. 11.8%; p<0.001) [12].
In the present study, we demonstrate that ANH did not have any detrimental effects on long-term oncologic outcomes; in fact, there was a trend towards improvement in PFS and OS with the use of ANH. In addition, use of ANH did not appear to affect the completeness of cytoreduction, as a CGR was achieved in over 60% of cases in both groups.
Our findings support the hypothesis that ANH is an effective intervention for reducing perioperative RBC transfusion in women undergoing PDS, without exerting a negative impact on perioperative complications and long-term survival. A growing body of scientific evidence suggests that blood transfusions may be associated with increased disease progression, as well as morbidity and mortality, in cancer patients [13, 14]. A potential explanation for the negative effects on short and long-term survival might be the known immunosuppressive effect of allogenic blood [4, 15]. However, it remains unclear if factors influencing the need for blood transfusion have a greater bearing on prognosis than the transfusion of blood itself. Preoperative patient optimization and attention to hemostatic techniques should be employed for patients undergoing ovarian cancer debulking and a restrictive blood transfusion regimen is recommended, only aimed at transfusion when necessary while maintaining a euvolemic state. The use of ANH might be an additional effective treatment approach to avoid these transfusion-related immunomodulations. In women with gynecologic malignancies, large retrospective studies have demonstrated an association between perioperative ABT and increased surgical wound infections, more postoperative venous thromboembolic events, longer hospital stay, higher recurrence rates, and increased mortality. In our study, only one patient in the ANH group developed a postoperative DVT, which was successfully treated with subcutaneous enoxaparin injections. Otherwise no postoperative venous thrombotic event (VTE) occurred. We could also not detect a high rate of surgical site infections in ANH patients (n=3/33, 9%), which represents a similar rate as in previously publications (12%) [16]. It is important to mention that the length of stay was not negatively affected by the use of ANH. The purported drawbacks of ANH, such as greater likelihood of kidney injury or increased rate of cardiovascular events, were not confirmed in our study or in previous studies [17–19]. No postoperative cardiac complication and no major pulmonary complication occurred intra- or postoperatively in patients receiving ANH.
ANH has primarily been investigated as a way to reduce the need for perioperative ABT in patients undergoing cardiac surgery. To our knowledge, only one prior study evaluating the long-term effects of ANH in cancer patients has been published to date. Correa-Gallego et al. investigated the effect of ANH on survival in patients from a randomized trial evaluating ANH during major hepatectomy for metastatic colorectal cancer (n=90) [20]. Similar to our findings, the authors found no detrimental impact of ANH on recurrence-free survival (p=0.3) or OS (p=0.9). Larger prospective randomized studies with adequate power are clearly needed to confirm our findings, and to determine the role of ANH in this patient population.
Interestingly, in this study preoperative CA125 values higher than 500 U/mL were independently associated with enhanced OS, a finding that has been previously described [21]. While we previously reported that higher preoperative CA125 levels are associated with a higher tumor burden and therefore impact the ability to achieve optimal or complete gross tumor resection [22, 23], tumors that generate higher levels of CA125 may have different tumor biology and may therefore respond differently to postoperative treatment, resulting in improved survival rates. Further investigation of this topic is warranted, and will certainly require larger case series.
The current study has several limitations. First, although it was extracted from a prospective trial with predefined inclusion and exclusion criteria, the ANH cohort carries a risk of selection bias and the results are not generalizable to all patients undergoing primary cytoreduction. One obvious limitation to ANH is the difficulty of performing it in anemic patients. Anemia is a common finding in patients with advanced ovarian cancer and has been described in 15% of women undergoing PDS [24]. While these patients might become eligible for ANH after preoperative anemia management (i.e., intravenous iron supplementation), this might delay surgery for 2–4 weeks. Second, the application of ANH adds complexity to intraoperative management because it is labor-intensive and time-consuming, resulting in prolonged operative times (357 vs. 446 min, respectively; p<0.001). The median hemodilution time was 50 min (n=33, range 19–165 min). These drawbacks must be weighed against the potential benefits of ANH, and may explain why this effective blood conservation technique has not been widely adopted into clinical practice. In the past two decades other methods of blood conservation (i.e., antifibrinolytics, cell-saver) [25–27] and improved surgical techniques [1] resulting in lower blood loss have been developed. ANH has previously been shown to be particularly effective in surgeries associated with major blood loss (>1000ml) [9, 10]. Therefore, further research, using preoperative biomarkers or imaging, is warranted to identify the subgroup of patients at highest risk for major blood loss during PDS. Future studies should investigate the benefit of ANH at time of PDS for advanced ovarian cancer in larger prospective trials, with patients randomized to either ANH or standard of care.
In conclusion, ANH is an innovative approach to intraoperative management. It significantly reduces the requirement for allogenic RBC transfusions in patients undergoing PDS for advanced ovarian cancer, without any associated increase in perioperative complications or negative impact on long-term outcomes.
Supplementary Material
Supplementary Table 1: Univariable analysis of progression-free survival (PFS)
Supplementary Table 2: Univariable analysis of overall survival (OS)
Highlights.
In PDS with high intraoperative blood loss, use of ANH prevents the need for allogenic blood transfusions
The application of ANH does not preclude maximal surgical efforts, with achievement of high complete gross resection rates
ANH is not associated with any detrimental impact on long-term oncologic outcomes
Acknowledgments
Funding: This study was funded in part through the NIH/NCI Support Grant P30 CA008748.
Footnotes
Disclosures: ET reports personal fees from Merck and personal fees from Astrazenica, outside the submitted work. AI reports personal fees from Mylan, outside the submitted work. REO reports personal fees from Clovis and personal fees from Tesaro, outside the submitted work. NRA reports grants from Stryker/Novadaq, Olympus, and GRAIL, outside the submitted work. KLR reports other from Intuitive Surgical Inc., outside the submitted work. AMA reports other for Merck and other from Pacira, outside the submitted work. DSC reports personal fees from Bovie Medical Co., personal fees from Verthermia Inc. (now Apyx Medical Corp.), personal fees from C Surgeries, perfonal fees from Biom ‘Up, other from Intuitive Surgical Inc., and other from TransEnterix Inc., outside the submitted work.
Conflicts of Interest: None declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Chi DS, et al. , Improved progression-free and overall survival in advanced ovarian cancer as a result of a change in surgical paradigm. Gynecologic Oncology, 2009. 114(1): p. 26–31. [DOI] [PubMed] [Google Scholar]
- 2.Zivanovic O, et al. , Advanced cytoreductive surgery: American perspective. 2009. 114(2): p. S3–S9. [DOI] [PubMed] [Google Scholar]
- 3.Delaney M, et al. , Transfusion reactions: prevention, diagnosis, and treatment. The Lancet, 2016. 388(10061): p. 2825–2836. [DOI] [PubMed] [Google Scholar]
- 4.Cata J, et al. , Inflammatory response, immunosuppression, and cancer recurrence after perioperative blood transfusions. British journal of anaesthesia, 2013. 110(5): p. 690–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whitaker B, et al. , Trends in United States blood collection and transfusion: results from the 2013 AABB Blood Collection, Utilization, and Patient Blood Management Survey. Transfusion, 2016. 56(9): p. 2173–2183. [DOI] [PubMed] [Google Scholar]
- 6.Chung K-W, et al. , Declining blood collection and utilization in the United States. Transfusion, 2016. 56(9): p. 2184–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grant MC, Resar LM, and Frank SM, The efficacy and utility of acute normovolemic hemodilution. 2015, LWW. [DOI] [PubMed] [Google Scholar]
- 8.Tanner EJ, et al. , A prospective trial of acute normovolemic hemodilution in patients undergoing primary cytoreductive surgery for advanced ovarian cancer. Gynecologic Oncology, 2018. 151(3): p. 433–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanders G, et al. , Prospective randomized controlled trial of acute normovolaemic haemodilution in major gastrointestinal surgery. British Journal of Anaesthesia, 2004. 93(6): p. 775–781. [DOI] [PubMed] [Google Scholar]
- 10.Jarnagin WR, et al. , A prospective randomized trial of acute normovolemic hemodilution compared to standard intraoperative management in patients undergoing major hepatic resection. Annals of surgery, 2008. 248(3): p. 360–369. [DOI] [PubMed] [Google Scholar]
- 11.Barile L, et al. , Acute Normovolemic Hemodilution Reduces Allogeneic Red Blood Cell Transfusion in Cardiac Surgery: A Systematic Review and Meta-analysis of Randomized Trials. 2017. 124(3): p. 743–752. [DOI] [PubMed] [Google Scholar]
- 12.Saito J, et al. , The efficacy of acute normovolemic hemodilution for preventing perioperative allogeneic blood transfusion in gynecological cancer patients. 2019. 60: p. 42. [DOI] [PubMed] [Google Scholar]
- 13.De Almeida JP, et al. , Transfusion requirements in surgical oncology patientsa prospective, randomized controlled trial. 2015. 122(1): p. 29–38. [DOI] [PubMed] [Google Scholar]
- 14.Caro JJ, et al. , Anemia as an independent prognostic factor for survival in patients with cancer: a systematic, quantitative review. 2001. 91(12): p. 2214–2221. [PubMed] [Google Scholar]
- 15.Goubran H, et al. , Transfusion-related immunomodulation and cancer. Transfusion and Apheresis Science, 2017. 56(3): p. 336–340. [DOI] [PubMed] [Google Scholar]
- 16.Schiavone MB, et al. , Surgical site infection reduction bundle in patients with gynecologic cancer undergoing colon surgery. Gynecologic oncology, 2017. 147(1): p. 115–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balasubramanian V, et al. , Myocardial oxygen balance during acute normovolemic hemodilution: A novel compartmental modeling approach. Computers in biology and medicine, 2019. 105: p. 16–26. [DOI] [PubMed] [Google Scholar]
- 18.Mikami N, et al. , Acute normovolemic hemodilution and acute kidney injury after open abdominal cancer surgery. Journal of Clinical Anesthesia, 2020. 61: p. 109657. [DOI] [PubMed] [Google Scholar]
- 19.Zhou Z.-f., et al. , Mild volume acute normovolemic hemodilution is associated with lower intraoperative transfusion and postoperative pulmonary infection in patients undergoing cardiac surgery -- a retrospective, propensity matching study. BMC Anesthesiology, 2017. 17(1): p. 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Correa-Gallego C, et al. , Perioperative complications influence recurrence and survival after resection of hepatic colorectal metastases. Annals of Surgical Oncology, 2013. 20(8): p. 2477–2484. [DOI] [PubMed] [Google Scholar]
- 21.Morales-Vásquez F, et al. , High levels of pretreatment CA125 are associated to improved survival in high grade serous ovarian carcinoma. Journal of Ovarian Research, 2016. 9(1): p. 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suidan RS, et al. , A multicenter prospective trial evaluating the ability of preoperative computed tomography scan and serum CA-125 to predict suboptimal cytoreduction at primary debulking surgery for advanced ovarian, fallopian tube, and peritoneal cancer. Gynecologic oncology, 2014. 134(3): p. 455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chi DS, et al. , The ability of preoperative serum CA-125 to predict optimal primary tumor cytoreduction in stage III epithelial ovarian carcinoma. Gynecologic oncology, 2000. 77(2): p. 227–231. [DOI] [PubMed] [Google Scholar]
- 24.O’Shea A, et al. , Neoadjuvant chemotherapy is associated with more anemia and perioperative blood transfusions than primary debulking surgery in women with advanced stage ovarian cancer. Gynecologic Oncology, 2018. 150(1): p. 19–22. [DOI] [PubMed] [Google Scholar]
- 25.Laupacis A. and D.f. Fergusson, Erythropoietin to minimize perioperative blood transfusion: a systematic review of randomized trials. Tranfusion Medicine, 1998. 8(4): p. 309–317. [DOI] [PubMed] [Google Scholar]
- 26.Henry DA, et al. , Anti‐fibrinolytic use for minimising perioperative allogeneic blood transfusion. Cochrane database of systematic reviews, 2011(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carless P, et al. , Cell salvage for minimising perioperative allogeneic blood transfusion Cochrane Database of Systematic Reviews Issue 3. Available from: wwwthecochranelibrary.com/SpringboardWebApp/userfilesl/ccoch/flle/CDOOISSSpdf [Accessed April 2011, 2010. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1: Univariable analysis of progression-free survival (PFS)
Supplementary Table 2: Univariable analysis of overall survival (OS)