Abstract
Diverse organic compounds, many derived from consumer products, are found in sewage sludge worldwide. Understanding which of these poses the most significant environmental threat following land application can be investigated through a variety of predictive and cell-based toxicological techniques. Non-targeted analysis using High Resolution Mass Spectrometry with predictive estrogenic activity modeling was performed on sewage sludge samples from 12 wastewater treatment plants in California. Diisobutyl phthalate and dextrorphan were predicted to exhibit estrogenic activity and identified in >75% of sludge samples signifying their universal presence and persistence. Additionally, the application of an estrogen-responsive cell bioassays revealed reductions in agonistic activity during mesophilic and thermophilic treatment, but significant antagonism during thermophilic treatment, which warrants further research. Ten nontarget features were identified (metoprolol, fenofibric acid, erythrohydrobupropion, oleic acid, mestranol, 4’-chlorobiphenyl-2,3-diol, medrysone, scillarenin, sudan I, and N,O-didesmethyltramadol) in treatment set samples and are considered to have influenced the in vitro estrogenic activity observed. The combination of predictive and in vitro estrogenicity with nontargeted analysis has led to confirmation of twelve estrogen active contaminants in California sewage sludge and has highlighted the importance of evaluating both agonistic and antagonistic responses when evaluating the bioactivity of complex samples.
Keywords: nontarget analysis, accurate mass LCMS, in vitro bioassays, sewage sludge, biosolids, trace contaminants
Graphical Abstract

1. INTRODUCTION
Consumers are exposed to hundreds of anthropogenic chemicals daily. Many synthetic chemicals have been identified as hormone mimics (agonists) or hormone antagonists, potentially interfering with hormone signaling processes in humans and in wildlife.1,2 Chronic exposure to these endocrine disrupting chemicals (EDCs) has been suggested to contribute to increases in diabetes, obesity, and adult cancers, consistent with up to five times higher rates of breast, pancreatic, endometrial, prostate and kidney cancers.1,3 Exposure to this group of chemicals can also result in adverse effects on reproduction, development, and behavior in wildlife species.4–9
Many compounds used in households persist past their period of intended use, with significant fractions reaching wastewater treatment plants (WWTP). Synthetic estrogens commonly used in chemical contraception, like ethinyl estradiol,10,11 antidepressants like fluoxetine, sertraline, and venflaxine,12,13 and pharmaceuticals like anticholesteremics, antibiotics, and chemotherapeutics14–17 are frequently detected in wastewater treatment plant effluents. Similarly, a number of known estrogenic chemicals (e.g., bisphenol A18) are commonly detected in sewage sludge; over 50% of the 7 billion dry tons of sludge produced annually in the United States is land applied, providing a pathway for environmental transport.19,20 The full spectrum of chemicals that have estrogenic activity and/or can affect the estrogen receptor (ER) signaling pathway and subsequent downstream physiological events remains largely unexplored, however.
Targeted chemical approaches have been used to identify numerous anthropogenic compounds in both WWTP effluent and sludge.21–24 These methods have the advantage of high sensitivity, however, a broader spectrum of compounds can be identified via suspect screening (using high resolution mass spectral libraries) and non-targeted analysis.25,26 Non-targeted techniques have been used to profile the chemical makeup of many environmental samples including household dust,27,28 surface water,29–31 drinking water,32 roadside runoff,33 biological tissue,34 and wastewater.35–38 Non-targeted analysis provides a holistic evaluation of chemicals present without the need for deliberate targets, and therefore promotes the discovery of new and emerging contaminants in environmental samples.39 Non-targeted analysis results in datasets typically comprising thousands of chemical features (defined by mass-to-charge at a given retention time) and identification of each is impractical.40 Careful statistics and modes of prioritization are necessary to select features of importance for further identification efforts28,41 such as Quantitative Structure-Activity Relationships (QSAR) and in vitro bioassay measurements. QSAR is commonly used to predict activity based on structural moieties and has been used in a wide range of applications, including adsorption, distribution, metabolism, elimination and toxicity,42–46 but while QSAR models can be useful tools for predicting the toxicity or biological activity of individual compounds, chemicals in complex mixtures are often influenced by multiple compounds acting concurrently, which is where mechanism-based in vitro bioassays can help identify endocrine active chemical mixtures.47
Numerous cell-based bioassays have been developed and utilized in major high-throughput chemical screening efforts, and many of these take advantage of nuclear receptor signaling pathways to identify and characterize potential endocrine active chemicals. 48,49 In addition to pure chemicals, nuclear receptor bioassays have been widely used to determine the presence of endocrine active chemicals in a diverse set of environmental, biological, commercial consumer products, and food.50–56 The VM7Luc4E2 (previously designated BG1Luc4E2) bioassay takes advantage of a recombinant human breast carcinoma cell line that contains a stably-integrated estrogen receptor-responsive firefly luciferase reporter gene.57,58 These cells respond to estrogenic chemicals with the induction of firefly luciferase in a chemical-specific, ER, time- and concentration-dependent manner52,57,59 These bioassays have been accepted by the Organization for Economic Co-operation and Development (OECD) and have been included in the US Environmental Protection Agency Endocrine Disruptor Screening Program.48,51–53,60
Combining nontargeted analysis with bioassay activity and predictive toxicology modeling have been performed on many substrates.41,49,61 Effects-directed analysis (EDA) and toxicity identification evaluation (TIE) have been used for both monitoring and identification of new bioactive, priority substances in the environment by using measured effects as a method of isolating single compounds responsible for such observations.62–64 Our overall objective is to employ non-targeted high-resolution liquid chromatography mass spectrometry techniques paired with predictive toxicity to identify anthropogenic, estrogenic compounds in sewage sludge and to use in vitro VM7Luc4E2 cell bioassays to better understand the effects of additional sludge treatment on estrogenic activity. More specifically, we aim to (1) identify commonly detected compounds within a defined physicochemical space that are predicted to be estrogen active, and (2) investigate sludges subject to more advanced treatment to determine how additional treatment mechanisms affect measured estrogenic activity and chemical feature abundances. Our main goal is to identify consumer product compounds in sewage sludge that present the most significant environmental barriers to its beneficial reuse as a soil amendment. To the best of our knowledge, this work presents the first nontargeted chemical analyses of sewage sludge with both estrogen agonistic and antagonistic activity, and the first study to evaluate both estrogen agonistic and antagonistic activity of sewage sludge.
2. EXPERIMENTAL METHODS
2.1. SAMPLE PREPARATION AND ACQUISITION
2.1.1. MATERIALS
Isotopically labeled internal standards were purchased from Cambridge Isotope Laboratories (Andover, MA) or Toronto Research Chemicals (North York, ON) and were >98% purity. Solvents and reagents were acquired from Honeywell – Burdick & Jackson (Muskegon, MI), ACROS organics (Morris Plains, NJ), Sigma Aldrich (St. Louis, MO), and Fisher Scientific (Hampton, NH). Deionized water (> 18 MΩ) was acquired using a Milli-Q® Integral 5 Water Purification System. Solid Phase Extraction (SPE) cartridges (Bond Elut Plexa, 45 μm particle size, 500 mg, 6 cc) and PTFE syringe filters (Captiva, 15 mm diameter, 0.2 μm pore size) were purchased from Agilent Technologies (Santa Clara, CA). α-Minimal Essential Medium (α-MEM) and phosphate-buffered saline (PBS) were obtained from InVitrogen, phenol red-free Dulbecco’s MEM and 17β-estradiol (E2) from Sigma Aldrich (St. Louis, MI), dimethylsulfoxide (DMSO) from EMD Chemicals (Burlington, MA), fetal bovine serum (FBS) and charcoal-stripped FBS from Atlanta Biologicals (Atlanta, GA), and L-glutamine and trypsin from Gibco (St. Louis, MI). Luciferin reagent and lysis buffer were obtained from Promega (Madison, WI).
2.1.2. SAMPLE COLLECTION
Twelve California wastewater treatment plants (WWTP) provided sludge samples, and two of these contributed two samples each from before and after mesophilic and thermophilic treatment. The first treatment set contains Class B (S9) and Class A (S13) sewage sludge (as defined by the Code of Federal Regulations 40, part 503- Standards for the Use or Disposal of Sewage Sludge).65 The Class A sample was produced by anaerobic thermophilic digestion (55° C) for 30–40 hours followed by 10 days of mesophilic digestion (40° C). This set will be referred to as the “thermophilic treatment set”. The second treatment set contains samples that spent 15 days under mesophilic digestion conditions (35° C) (S3) followed by 5 years in an open-air, anaerobic digestion pond (ambient) (S2), and will be referred to as the “mesophilic treatment set”.
The WWTPs contributing samples for this study represent various geographical locations and feature diverse influent characteristics and treatment processes. All facilities participated anonymously and have been assigned randomized identifiers. Grab samples of sludge were collected by WWTP staff in clean 1-gallon glass jars and were returned to the lab using overnight shipping in ice-filled coolers. Samples were immediately transferred to a dark room at 4 °C where they were stored until extraction. Samples were prepared and analyzed within a month of receipt. Because the goal of this work was to identify the most persistent and recalcitrant compounds in sludge, we hypothesized that any compounds transformed significantly during storage would likely not be persistent following land application and therefore were not prioritized. Sample descriptors, dry weights, and ash content for the sludge samples can be found in SI-1.
2.1.3. SAMPLE PREPARATION
Sludge extracts were prepared in quadruplicate following procedures validated for trace contaminant analysis of sludge and published previously (SI-4) for identification of compounds within the physicochemical space described by Table 1.46
Table 1.
Physicochemical properties defining chemical space coverage of the sample preparation and analytical method (Black et al, 2019)
| Parameter | Range |
|---|---|
| LogKow | −3.27 – 5.96 |
| Molecular weight (m/z) | 170 – 414 |
| Hydrogen bonding acidity (A) | 0.0 – 1.04 |
| Hydrogen bonding basicity (B) | 0.41 – 1.98 |
| McGowan volume characteristic (V) | 1.38 – 2.72 |
| Polarizability (S) | 0.88 – 2.55 |
| Partitioning coefficient between gas phase and hexadecane (L) | 7.12 – 13.14 |
| Excessive molar refraction (E) | 0.49 – 2.40 |
Sample preparation steps including pH adjustments, solid phase extraction media, and cartridge washing and elution solvents, were carefully selected to reduce matrix interferences and enhance surrogate recoveries. Briefly, a 0.5 g sample was split into two fractions and pH adjusted to 2 and 10. Each fraction was extracted via ultrasonication with MeOH:ACN (1:1 v/v%) followed by a solid phase extraction (SPE) (Agilent BondElut Plexa) clean up. Cartridges were eluted with 12 mL of 5% MTBE in MeOH, concentrated to 1 mL and syringe filtered through a 0.2 μm PTFE filter. Two hundred microliter aliquots were solvent exchanged into dimethyl sulfoxide (DMSO) for VM7Luc4E2 bioassay analysis. Isotopically labeled internal standards (400 ng g−1) were added to all other extracts. Matrix spikes (1,000 ng g−1) were used for quality control (SI-2).
Ash content was determined for four sludge samples (S2, S3, S9, and S13) that were used in a before-and-after treatment analysis. Ash content was determined gravimetrically following the procedures published by Neilsen (2017).66
2.1.5. INSTRUMENTAL ANALYSIS
An Agilent 1260 HPLC pump was used for chromatographic separation with an Agilent Zorbax Eclipse Plus C18 column (2.1 × 100 mm, 1.8 μM). Deionized water with 0.1% (v/v) formic acid (A) and ACN with 0.1% formic acid (v/v) (B) were used as mobile phases for positive electrospray ionization (ESI+), and deionized water with 1 mM ammonium fluoride (A) and ACN (B) were used for negative electrospray ionization (ESI−). The initial gradient was held at 2% B for 1.5 min, followed by a linear increase to 100% B at 16.5 min and held for 4 min. A post-run column equilibration time of 3.0 min was implemented resulting in a total sample run time of 23.5 min. An injection volume of 5 μL was used, the mobile phase flowrate was 350 μL min−1 and the column temperature was maintained at 30 °C for the duration of the run. Mass spectra were acquired using an Agilent 6530 Quadrupole Time-of-Flight (QTOF) mass spectrometer in both data dependent (DDA) and data independent acquisition (DIA) modes. DIA was acquired using the Agilent All-Ions acquisition method with collision cell voltages cycling through 0 eV, 10 eV and 40 eV at a scan speed of 4.5 spectra s−1. DDA was acquired using targeted MS/MS methods with a list of exact mass targets and retention times where collision cell voltages again cycled through 0 eV, 10 eV and, 40 eV once a precursor was isolated. Full scan data (CE 0 eV) was acquired at a rate of 4 spectra s−1 and high energy scans at 6 spectra s−1. Additional LC-QTOF-MS parameters are summarized in SI-4.
2.1.6. VM7Luc4E2 CELL BIOASSAYS FOR ESTROGENIC ACTIVITY
Estrogen-responsive recombinant VM7Luc4E2 cells were grown and maintained in α-MEM containing 10% FBS in a humidified incubator at 37 °C with 5% CO2. For analysis, VM7Luc4E2 cells were transferred into estrogen-free medium (phenol red-free α-MEM containing 10% charcoal-stripped FBS, 1.9% L-glutamine) and incubated for 3 days before plating into white, clear-bottomed 96-well tissue culture plates at a density of 75,000 cells/well. Cells were allowed to attach for 24 h and then were incubated with carrier solvent DMSO (1% final concentration), estradiol (E2, 1 nM), or an aliquot (1 μL) of the indicated extract (in DMSO) for 24 h at 37 °C for agonistic estrogen assays. To measure estrogen antagonism, E2 (1nM) and an aliquot (1μL) of the extract (in DMSO) was added to treatment wells then incubated. All samples and controls were analyzed in triplicate. No cytotoxicity was observed with any chemical or extract treatment. After incubation, cells were rinsed twice with PBS, lysed with Promega cell lysis buffer, and shaken for 20 min at room temperature to allow complete cell lysis. Luciferase activity in each well was measured using an automated microplate luminometer (Anthos Lucy2) in enhanced flash mode with the automatic injection of 50 μL of Promega stabilized luciferase reagent as previously described in detail.60 Luciferase activity (relative light units (RLUs)) of solvent control (DMSO) treated cells was subtracted from that of all treated cells to obtain the final induced luciferase activity of test samples and values were then normalized to luciferase activity obtained with a maximal inducing concentration of E2 (set at 100%). For antagonistic assays, if the luciferase activity of cells incubated with 1 nM E2 and 1 μl of the sample extract (in DMSO) was less than that measured in cells incubated with 1nM E2 and 1 μl of DMSO, assuming the sample extract produced no cell toxicity, then it would be concluded that the sample extract contained an ER antagonist. The coefficients of variation for all replicate treatments (n=3) were less than 20%.
2.2. DATA PROCESSING
2.2.1. FEATURE ALIGNMENT AND PRIORITIZATION
Commonly Detected.
All samples, including instrument blanks and matrix spikes (56 injections in both positive and negative modes) were deconvoluted and aligned using MassHunter Profinder (B.08) following methods previously reported (SI-5).27 Aligned data was imported into Mass Profiler Professional (MPP, 14.9) where a one-way Analysis of Variance (ANOVA) was performed against a blank with asymptotic p-value computation and Benjamini Hochberg False Discovery Rate correction. A frequency filter was applied to eliminate any features (mass-to-charge (m/z) at a retention time (RT)) not present in all technical replicates (n=4) of at least one sample. A Venn diagram tool was used to find features that were present in more than 75% of the sludge samples. All features were required to be present at an abundance greater than 5 times the abundance observed in the blank injections.
Treatment Sets.
Aligned feature abundances were normalized in the four samples making up the treatment sets using each samples’ organic matter content. This removed any bias of feature abundance caused by decreases in overall sludge mass during treatment. Whole sample extracts (constituted in DMSO with no isotopically labelled standard additions) for the four treatment samples were run on the VM7Luc4E2 estrogenic cell bioassays. Features that significantly increased or decreased after treatment were identified via a moderated pairwise t-test and fold change analysis. A corrected p-value cutoff of 0.001 was used with a fold change of 5 and a minimum raw abundance of 100,000 counts to stringently select high abundance features that drastically changed in intensity with treatment. The abundance cutoff was set to 100,000 or greater to identify features that made significant contributions to the overall ion count. Internal standard abundances varied by up to 50% across samples due to varying matrix interferences; therefore, a fold change factor of 5 was used to eliminate any features that had significant changes in abundances solely due to signal interference.
2.2.2. TENTATIVE IDENTIFICAITON
Lists of emerging environmental substances (>40,000 compounds) were downloaded from the Norman Network, entered into the VEGA-QSAR model (version 1.2.4, downloaded from www.vegahub.eu)67, and modeled for Estrogen Receptor Mediated Effect (ERME, EPA-CERAPP model) and Estrogen Receptor Binding Affinity (ERBA, IRFMN model). Compounds that were predicted to be active on one or both of these pathways were screened against prioritized feature lists in ID Browser (Agilent Technologies, version B.08). Designation of a chemical as a positive identification required less than a 5 ppm mass error and a match score >75%. The m/z and retention times of positively identified features from the Norman substance list were used to acquire targeted MS/MS data for in silico fragmentation.68
Targeted MS/MS (tMS/MS) data was imported into MS-DIAL (version 3.40, http://prime.psc.riken.jp/Metabolomics_Software/MS-DIAL/index2.html)69 and in silico fragmentation performed in MS-FINDER (version 3.12, http://prime.psc.riken.jp/Metabolomics_Software/MS-FINDER/index2.html).69–71 The SMILES codes for the top three structural results were used to run the VEGA-QSAR model for ERBA and ERME activity. Only compounds that returned positive results for one or both pathways were investigated further.72 Additional matching parameters are reported in SI-6.
2.2.3. Quality Control and Assurance of in silico Identifications
Twenty-three compounds (SI-2) were fortified in sludge prior to sample preparation and data acquisition. Spectra from these fortified samples were aligned in MS-DIAL and imported into MS-FINDER as described. The correct structure was proposed in one of the top 3 hits 88% (21/23 compounds) of the time, where the top hit was the correct structure 65% of the time. One compound was missed entirely, and one was the 4th ranking identification. Due to the molecular nature of many contaminants of concern (many containing only C, H, N, O elements that result in similar MSMS spectra) and because the top 3 proposed structures are analyzed, false detections are frequent. Manual investigation into each proposed structure resulting from prioritized features is performed and either confirmed or rejected based on expert analyst examination, online MS/MS spectra, or pure chemical reference standards.
With each analytical batch, fortified samples, analytical standards, and blanks were measured and analyzed via the workflows described. Identification of the 21 compounds in fortified samples and standards were used for quality assurance. Furthermore, instrument and method blanks were manually investigated for contamination and internal standards were used to monitor consistent retention times, mass accuracy, and abundances. Due to the complex nature and variation of these samples, we observed up to a 50% variation in internal standard abundances and have structured appropriate fold change analyses thresholds and other statistical filters to account for these discrepancies.
3. RESULTS & DISCUSSION
3.1. NON-TARGET RESULTS FOR COMMONLY DETECTED FEATURES
The 2,334 features (148 ESI−, 2,186 ESI+, SI-7) detected in greater than 75% of sludge samples were screened against the accurate mass list of the 4,954 estrogenic Norman Suspects. The screen resulted in 173 tentative matches (138 in ESI+, 35 in ESI−) whose m/z values were used to trigger targeted MS/MS experiments for in silico fragmentation in MS-FINDER. Potential structures (453 total, 434 in ESI+, 19 in ESI−) were proposed (analyzing the top three structural matches for each feature). Of the 453 potential structures, 84 (76 ESI+, 8 ESI−) were predicted to have estrogenic activity on at least one of the estrogen endpoints used in the VEGA-QSAR model, 31 of which were hypothesized to be of anthropogenic origin and were further investigated in this study. Only estradiol and topanol CA (4,4’,4’’-(1-methylpropanyl-3-ylidene)tris[6-tert-butyl-m-cresol]) were predicted to be active on both endpoints, where 20 of the 31 compounds were predicted to be active on only the ERRBA model, and 9 were predicted to be active on only the ERME endpoint. These 31 compounds consisted of fragrances, antibiotics, synthetic hormones, pain medications, antiproliferative tumor medications, and plasticizer metabolites. Reference standards were commercially available for only 7 of the 31 proposed structures (testosterone propionate, levorphanol, licochalcone A, estradiol, diisobutyl phthalate, monoethylhexyl phthalic acid, and methyl tetradecanoate). MS/MS fragments and retention times of diisobutyl phthalate and levorphanol were used to confirm in silico identifications, while the remaining 5 were rejected with reference standards. In the case of monoethylhexyl phthalic acid (structure rank 1), diisobutyl phthalate was proposed as the second ranked structure and ultimately confirmed only after comparison with a reference standard. Both structures had structure scores >7 and were proposed based on the identification of two fragments (m/z 149.0233 and 57.0704) equally plausible for each structure. Unfortunately, for most of the remaining proposed structures (24), MS/MS spectra were unavailable in the Agilent libraries or the MoNA mass spectral database. MS/MS spectra were available for Irganox 1035 in mzcloud and MoNA, but instrument parameters of the library MS/MS spectra were not similar enough to the instrument conditions used here to draw any further conclusions. For Irganox 1035 and the remaining 23 structures, reference standards were unavailable, so these remain as level 5 identifications with suggested molecular formula assignments (SI-8).72
The identification of diisobutyl phthalate and levorphanol in over 75% of sludge samples analyzed here signifies their widespread use in California. Diisobutyl phthalate is a common plasticizer used in soft plastics like shower curtains, raincoats, food wraps, bowls, car interiors, vinyl fabrics, and floor tiles, and its metabolites have been repeatedly detected in human urine73 and is known to have low removal efficiencies in wastewater treatment.74,75 Levorphanol is a long-lasting opioid pain medication but is often referred to as “the forgotten opioid” after the release of longer-lasting morphine, oxycodone and fentanyl.76–78 Levorphanol, however, is the D-enantiomer of dextrorphan, the human liver metabolite (o-demethylation product) of dextromethorphan (DM, a synthetic codeine analog and the active ingredient in most over-the-counter cough medicines) (Figure 1).79–82 Because the two compounds are optical isomers with the same logKOW, and therefore the same retention time and MS/MS fragments, we cannot distinguish between them using these methods and are unable to confidently differentiate between the isomers.83 Evaluating usage data for each compound strongly suggests this compound is dextrorphan. The Center for Disease Control and Prevention estimated that 37% of adults in the United States took pain medications stronger than morphine, a class which would include levorphanol, whereas the Consumer Healthcare Products Association found that 73% of parents and caregivers administer cough medicine to children or dependents experiencing a cough, and 66% of adults self-administer it.84,85
Figure 1.
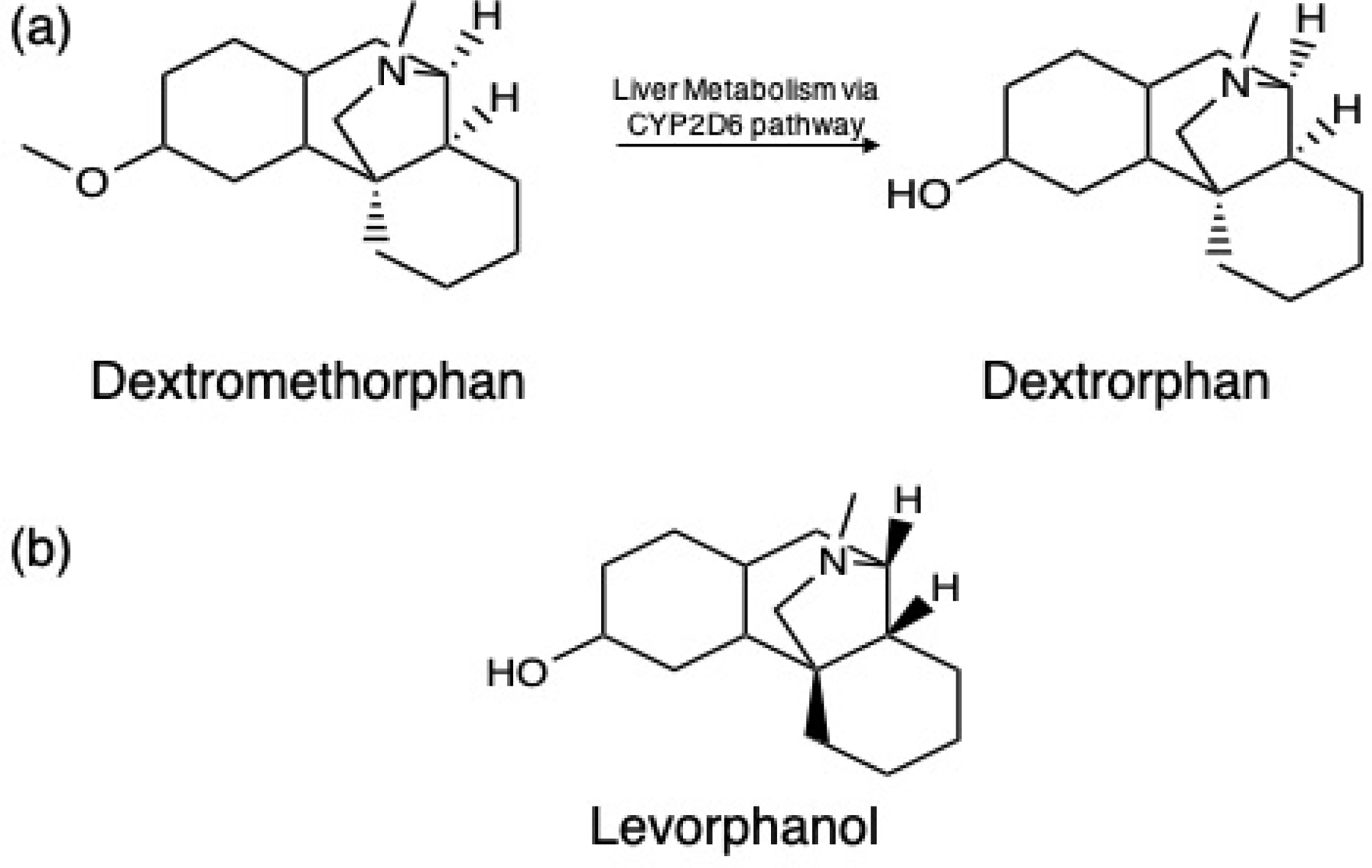
(a) demethylation pathway of dextromethorphan, the primary ingredient found in all cough medicines, via the liver metabolic CYP2D6 pathway into dextrorphan, an optical isomer of levorphanol (b).
3.1.1. HOMOLOGOUS SERIES IDENTIFICATION
Envihomolog (https://www.envihomolog.eawag.ch/)86 was used to identify homologous series present among the commonly detected features to highlight major classes of compounds present. Repeating units between 12 and 100 m/z with a 10 ppm mass accuracy tolerance were examined. Of the 2,334 features present, 27% of features in positive mode, and 3% in negative mode, were identified as part of a homologous series. All homologous series identified had mass differences consistent with alkyl chain series. The only series detected in negative mode, and 11% of the homologous series detected in positive mode, had a mass increment of 28.0313 (-C2H4-)n. Additionally 33% of the positive mode series had a mass increment of 14.157 (-CH2)n, 19% had a mass increment of 42.047 (-C3H6-)n, and 37% had a mass increment of 21.0235, suggesting an alkyl compound (C3H6-)n) with a doubly charged head group. This series likely belongs to a broad range of quaternary ammonium cationic surfactants including poly-dialkyldimethylammonium (poly-DADMACs), poly-alkyltrimethylammonium (poly-ATMACs) and poly-benzylalkyldimethylethylammonium (poly-BACs). These are commonly used as flocculants in wastewater treatment, particularly in sludge dewatering applications, while their monomeric analogues are widely used in disinfectants, antistatics, biocides, personal care products, and cleansers.87–90 Ten quaternary ammonium surfactants downloaded from the EPA’s Comptox Database were modeled using the VEGA-QSAR estrogen prediction endpoints and were all predicted to be inactive (SI-9).
It is made particularly clear in this step, in addition to the relative number of ESI+ and ESI− features isolated during the alignment process, that the sample preparation method used in this work strongly favors cationic species. Previously, many others have found high abundances of anionic surfactants, predominantly nonylphenol polyethoxylates (NPnEOs), in sewage sludge that are not reported here.23,91 Furthermore, NPnEO’s have been shown to mimic the effects of estradiol both in vitro and in vivo and have been evaluated extensively.92–94 We believe this method may have allowed for the identifications of lower abundance, negatively ionized compounds that otherwise may have been suppressed by NPnEOs, linear alkylbenzenesulfonates, or other compounds with a strong affinity for electrospray ionization.
3.2. EFFECTS OF ADDITIONAL TREATMENT ON ESTROGENIC ACTIVITY
3.2.1. VM7Luc4E2 CELL BIOASSAY ACTIVITY
Reduction in estrogenic agonist activity after sludge treatment was observed in both the mesophilic and thermophilic treatment sets (Figure 2). Mesophilic treatment decreased agonist activity from 115% to 90%, whereas a more dramatic reduction was observed in the thermophilic treatment set from 85% to a non-detectable level.
Figure 2.

ER-CALUX activity measured in estradiol equivalents Agonist activity is percent activity relative to 1 nM E2 treatment. Antagonist activity is the observed suppression of 1nM E2 (calculated 1 – observed activity of extract where 1nM E2 was added).
The elimination of agonist activity of the post-thermophilic treatment (PostThermo) sample suggested the presence of antiestrogenic compounds (i.e. ER antagonists). To test this hypothesis, antagonistic VM7Luc4E2 assays were run. The luciferase activity of the PostThermo extract was 19% on the antagonist assay, indicating an 81% suppression of agonist activity (relative to the control), confirming the presence of antiestrogenic compounds. This high degree of antagonistic activity suggests that treatment does not eliminate all detectable estrogenic response, as would have been concluded if only agonistic assays were run.
Evidence of antiestrogenic effects present in waste streams is abundant in the literature, but little has been published on antiestrogenic compounds in sewage sludge, or specific chemicals responsible for antiestrogenic behavior in general.95,96 Only dioxins, polycyclic aromatic hydrocarbons (PAHs),97–100 chlorinated polar compounds,101 polychlorinated biphenyls (PCBs), carbamate pesticides, and indol-3-carbinol derivatives102 have been reported as antiestrogenic compounds. EPA’s ToxCast Dashboard (https://actor.epa.gov/dashboard/, version 2), however, contains experimental antiestrogenicity data obtained using the VM7Luc4E2 antagonism assay (formerly identified as ERa_LUC_BG1) for 739 compounds and these data were downloaded on May 23, 2019 (SI-10) and screened against the mesophilic and thermophilic treatment sets, using the parameters detailed in section 2.2.2. Presence across 75% of technical replicates (n=4) and peak heights five times the height in the blank were required. A total of 77 (13 ESI+, 64 ESI−) compounds were found, 14 were unique to the PostThermo sample, 13 unique to pre-thermophilic treatment (PreThermo), 5 unique to the pre-mesophilic treatment (PreMeso), and 9 unique to PostThermo. Of the 14 compounds unique to PostThermo, MS/MS spectra were available for six: calcifediol, emodin, nitroxoline, acitretin, gestrinone, and tropisetron but ultimately not confirmed. All 14 of these chemical features are assigned as level 5 identifications (SI-11) because it is plausible that the presence of these compounds in PostThermo contribute to its observed antiestrogenic activity even though they remain unidentified.
Knowing the complexity of these samples, it is likely that a mix of agonistic, synergistic, and antagonistic compounds are significant contributors to overall bioassay activity, and further work is needed to better understand the bioactive complexity of these samples.
3.2.2. SIGNIFICANT CHANGES IN CHEMICAL FEATURES WITH ADDITIONAL SLUDGE TREATMENT
A second route to identifying estrogen active compounds that have been removed or amplified by treatment operations was to isolate features with significant increases or decreases (fold change of 5 or greater, p<0.05)) in normalized abundance. This approach supports discovery of compounds that may have a relationship with estrogen activity based on relative abundance.
3.2.2.1. MESOPHILIC TREATMENT SET
506 chemical features decreased in abundance by a fold change of 5 or greater (p < 0.001, abundance > 100,000) within the mesophilic treatment set. Of these, 223 features had accurate masses consistent with predicted estrogen active Norman compounds, resulting in 426 potential structures proposed via in silico fragmentation (top 3 structures per feature, when available). 56 of these compounds were predicted to have estrogenic activity in one or both of the pathways predicted by the VEGA-QSAR model and 17 were believed to be of anthropogenic origin. Reference standards were used to confirm (level 1) the identification of (1) metoprolol, a beta blocker used to treat high blood pressure, angina, and heart failure, (2) fenofibric acid, the active metabolite of a commonly prescribed cholesterol medication, fenofibrate, and (3) erythrohydrobupropion, a metabolite of buproprion, which is prescribed as an antidepressant and smoking cessation aid.103,104 MS/MS spectra from MoNA were used to confirm (level 2a) oleic acid, a naturally occurring omega-3 fatty acid found in oils used for human consumption. Reference standards were unavailable for 4 of the 12 remaining compounds, which also lacked MS/MS spectra in public repositories, so further identification efforts ceased here for ximaosteriod D (MS-FINDER 2nd structure rank), brevicolline (MS-FINDER 3rd structure rank), 4,4-bis((tert)amylperoxy)valeric acid butyl ester (MS-FINDER 1st structure rank), and 2,4-diphenyl-1-butene (MS-FINDER 1st structure rank). These compounds are therefore reported as level 5 identifications, or masses of interest. Reference standards ultimately rejected linoleic acid (MS-FINDER 1st structure), CX-516 (MS-FINDER 2nd structure), cintiapride (MS-FINDER 3rd structure), n-lauroylglycine (MS-FINDER 1st structure), 4-oxo-retinoic acid (MS-FINDER 1st structure), and 4-(1h-imadzaol-1-yl)phenol (MS-FINDER 2nd structure). The remaining 8 compounds without available MS/MS spectra were manually inspected for data quality, resulting in the rejection of two compounds, 4-ketoifosfamide and 6-hydroxy-3,4-dihydro-2(1H)-quinolinone due to the low abundance of their molecular ions.
676 features increased in abundance by a factor of 5 or greater (p < 0.001, abundance > 100,000 counts), 56 were mass-matched with the estrogen-predicted Norman list, and 36 (24 in ESI−, 12 in ESI+) are of anthropogenic origin. Targeted MS/MS was performed on these 36 features and 5 structures were assigned that were active on one or both of the VEGA-QSAR estrogen pathways. Of these 6 structures, 3 are believed to be of anthropogenic origin. The main fragment of mestranol (m/z 96.96) was used to tentatively confirm mestranol acetate. (2-methoxy-4-prop-2-enylphenyl) and 2-[4-(2-methylpropyl)phenyl]propanoate, metabolites of ibuprofen were identified as a single feature, but rejected after investigating the three distinct entities and determining that the plausibility of the retention time with these structures was unlikely. 4’-chlorobiphenyl-2,3-diol was identified, but could not be confirmed or rejected using online MS/MS spectra.
3.2.2.2. THERMOPHILIC TREATMENT SET
Thermophilic treatment decreased the normalized abundance of 576 features by a factor of 5 or greater, 90 of which were consistent with accurate masses of predicted estrogen active compounds from the Norman lists, resulting in 288 proposed structures identified via in silico fragmentation (some features resulted in two or more MS/MS experiments due to retention time shifts, and thus, more than three potential structures per feature sometimes occur). Of these, 26 were predicted to be estrogenic in one or both estrogen pathways predicted by the VEGA-QSAR model, but only 5 of these compounds were believed to be of anthropogenic origin. MS/MS spectra were available for 3 of the 5 anthropogenic compounds (testosterone propinoate, medrysone, and coniferyl alcohol). Reference standards were used to confirm medrysone, a synthetic glucocorticoid used as an anti-inflammatory in ophthalmology, and scillarenin, the active metabolite of proscillarenin used to treat congestive heart failure and cardiac arrhythmia; and used to reject testosterone propinoate, an anabolic steroid used in the treatment of low testosterone. Coniferyl alcohol (MS-FINDER 1st structure) was rejected upon comparison with MS/MS spectra available in MoNA, and gugglusterone was rejected for poor MS/MS data quality due to low abundance of the molecular ion.
1,304 features increased in abundance by a factor of 5 or greater (p < 0.001, abundance > 100,000 counts), 97 were mass-matched with the estrogen-predicted Norman list. MSFINDER assigned 50 plausible structures to these features, 11 were active on one or both of the VEGA estrogen pathways, and 7 were believed to be of anthropogenic origin. Sudan I, and N,O-didesmethyl tramadol were compared to online MSMS spectra in MoNA and confirmed with matching fragment ions. Standards and online MSMS spectra were unavailable for ruspolinone and N,N,O-tridesmethylvenlaflaxine. Ethyl paraben and cinnamic acid were rejected upon comparison with reference standards, and 1,2’-dinaphthylmethane was rejected for its inability to be measured under the instrumental conditions used here.
The results reported here are consistent with past literature. Lorenzen (2004) observed a significant increase in estrogenic activity in anaerobically digested sewage sludge, which is supported by Paterakis (2012) who observed a reduction in estrogens and nonylphenol ethoxylates (NPnEOs) during bench-scale thermophilic, anaerobic digestion.105,106 Holbrook (2002) reported a similar increase, but noted that thermophilic anaerobic digestion increased estrogenic activity more so than mesophilic anaerobic digestion.107 The discrepancy between results published by Holbrook and those reported here likely stem from the high extractability of nonylphenol polyethoxylates (NPnEOs), with Holbrook and others attributing much of the observed estrogenic activity to these compounds.108–111 As discussed here, our sample preparation method focused on analyzing contaminants at a trace level and has excluded the coextraction of NPnEOs and therefore, the estrogenic activity observed in this study is due to other, unidentified contaminants. A review of the scientific literature failed to identify any studies investigating estrogen antagonistic activity of sewage sludge samples, yet, many suggest more bioassay-focused studies to better understand the endocrine disrupting potential of sewage sludge.112
The chemical features whose abundances increased with each treatment suggest (1) they are potentially estrogen antagonistic and correlated with the observed antagonism, (2) they are persistent and are concentrated due to biomass reductions during treatment,107 or (3) these features are transformation products that were formed during treatment. Alternatively, features that decreased with treatment are believed to be degraded or transformed and potentially correlate with decreased agonist activity observed. More work is needed with a larger sample size to correlate features with observed activity and to further confirm individual compounds’ activity contributions with effects directed analyses.
4. IMPLICATIONS OF RESULTS
The debate over safely using human waste-derived material, like sewage sludge, as soil amendments is ongoing. The benefit of recycling nutrients necessary for crop production and avoiding the production of energy intensive synthetic fertilizers can be significant, but best management practices for preventing harmful exposures are still being investigated. The work presented here represents the first nontargeted chemical analysis of sewage sludge, and aimed at better understanding biologically relevant chemicals of concern. Additional work using this technology can provide information to regulators and consumers so that the use of products containing such chemicals can be reduced, making sewage sludge safer and eventually, more widely accepted. Furthermore, the evaluation of both agonistic and antagonistic estrogen receptor bioassays revealed how crucial it is to include both mechanisms when evaluating overall activity. The mesophilic and thermophilic treatment techniques evaluated in this study reduced estrogen active compounds, but increased antagonistic behavior and further work is needed to identify the compounds directly affecting antagonistic activity. Additional studies are critically needed to determine suitable methods that evaluate overall estrogenic activity when both agonists and antagonists are present.
Supplementary Material
Table 2.
Summary of identified compounds in commonly detected and treatment set features.
| Confirmed Compound | Molecular Formula | CASRN | Exact Mass | RT (min) | Confidence Level | Identification |
|---|---|---|---|---|---|---|
| Diisobutyl Phthalate | C16H22O4 | 86–69-5 | 278.1521 | 14.75 | 1 | Commonly Detected (<75%) |
| Dextrorphan* | C17H23NO | 125–73-5 | 257.1721 | 6.71 | 2a | Commonly Detected (<75%) |
| Metoprolol | C15H25NO3 | 51384–51-1 | 267.1834 | 6.88 | 1 | Decreased with mesophilic treatment |
| Fenofibric Acid | C17H15ClO4 | 42017–89-0 | 318.0659 | 12.83 | 1 | Decreased with mesophilic treatment |
| Erythrohydro-bupropion | C13H20ClNO | 99102–04-2 | 241.1233 | 8.87 | 1 | Decreased with mesophilic treatment |
| Oleic Acid | C18H34O2 | 112–80-1 | 282.2559 | 17.95 | 2a | Decreased with mesophilic treatment |
| Mestranol | C21H26O2 | 72–33-3 | 310.1938 | 6.73 | 2a | Increased with mesophilic treatment |
| 4'-chlorobiphenyl-2,3-diol | C12H9ClO2 | 119386–13-9 | 220.0291 | 12.74 | 4 | Increased with mesophilic treatment |
| Medrysone | C22H32O3 | 2668–66-8 | 344.2351 | 11.37 | 1 | Decreased with thermophilic treatment |
| Scillarenin | C24H32O4 | 565–22-5 | 384.2300 | 11.08 | 1 | Decreased with thermophilic treatment |
| Sudan 1 | C16H12N2O | 842–07-9 | 248.0949 | 10.05 | 2a | Increased with thermophilic treatment |
| N,O-didesmethyl tramadol | C14H21NO2 | 138853–73-3 | 235.1572 | 9.61 | 2a | Increased with thermophilic treatment |
Dextrorphan was confirmed using a pure chemical standard of its optical isomer, levorphanol but based on usage statistics, dextrorphan is the more plausible identification.
ACKNOWLEDGEMENT
The authors would like to thank Agilent Technologies, specifically Daniel Cuthbertson and Tarun Anumol for their support and assistance with data processing, and Clayton Bloszies from the West Coast Metabolomics Center at University of California, Davis for his assistance with MS-DIAL and MS-FINDER analysis. We would also like to thank the participating wastewater treatment plants for their cooperation in providing sewage sludge samples and the California Association of Sanitation Agencies for facilitating WWTP volunteers.113
Funding Sources:
Research reported in this publication was supported by the UC Davis Superfund Research Center, National Institutes of Health, NIEHS award (P42ES004699), the National Science Foundation Graduate Research Fellowship Program, the Jena and Michael King Foundation, and John Wick and Peggy Rathmann.
Footnotes
SUPPORTING INFORMATION
Additional sample information, QC spiked native and isotopically labeled compounds, sample preparation, optimized analytical parameters for LC-QTOF-MS, deconvolution and alignment, tentative identification, commonly detected features, ToxCast VM7Luc4E2 antagonist compounds, up- and down- tentatively identified features.
REFERENCES
- 1.Bergman K, Heindel JJ, Jobling S, Kidd KA, Zoeller RT. State of the Science of Endocrine Disrupting Chemicals- 2012: An Assessment of the State of the Science of Endocrine Disruptors.; 2013. doi: 10.1016/j.toxlet.2012.03.020 [DOI] [Google Scholar]
- 2.Snyder S a., Benotti MJ. Endocrine disruptors and pharmaceuticals: Implications for water sustainability. Water Sci Technol. 2010;61:145–154. doi: 10.2166/wst.2010.791 [DOI] [PubMed] [Google Scholar]
- 3.Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, Zoeller RT, Gore AC. Endocrine-disrupting chemicals: An Endocrine Society scientific statement. Endocr Rev. 2009;30(4):293–342. doi: 10.1210/er.2009-0002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kidd KA, Blanchfield PJ, Mills KH, Palace VP, Evans RE, Lazorchak JM, Flick RW. Collapse of a fish population after exposure to a synthetic estrogen. Proc Natl Acad Sci. 2007;104(21):8897–8901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nash JP, Kime DE, Ven LTM Van Der, Wester PW, Tyler CR. Long-term exposure to environmental concentrations of the pharmaceutical ethynylestradiol causes reproductive failure in fish. Environ Heal Perspect Natl Inst Environ Heal Sci. 2004;112(17):1725–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qiu W, Zhao Y, Yang M, Farajzadeh M, Pan C, Wayne NL. Actions of bisphenol A and bisphenol S on the reproductive neuroendocrine system during early development in zebrafish. Endocrinology. 2016;157(2):636–647. doi: 10.1210/en.2015-1785 [DOI] [PubMed] [Google Scholar]
- 7.Vajda AM, Barber LB, Gray JL, Lopez EM, Woodling JD, Norris DO. Reproductive disruption in fish downstream from an estrogenic wastewater effluent. Environ Sci Technol. 2008;42(9):3407–3414. doi: 10.1021/es0720661 [DOI] [PubMed] [Google Scholar]
- 8.Vajda AM, Barber LB, Gray JL, Lopez EM, Bolden AM, Schoenfuss HL, Norris DO. Demasculinization of male fish by wastewater treatment plant effluent. Aquat Toxicol. 2011;103(3–4):213–221. doi: 10.1016/j.aquatox.2011.02.007 [DOI] [PubMed] [Google Scholar]
- 9.Barber LB, Vajda AM, Douville C, Norris DO, Writer JH. Fish endocrine disruption responses to a major wastewater treatment facility upgrade. Environ Sci Technol. 2012;46(4):2121–2131. doi: 10.1021/es202880e [DOI] [PubMed] [Google Scholar]
- 10.Martin J, Santos JL, Aparicio I, Alonso E. Multi-residue method for the analysis of pharmaceutical compounds in sewage sludge, compost and sediments by sonication-assisted extraction and LC determination. J Sep Sci. 2010;33(12):1760–1766. doi: 10.1002/jssc.200900873 [DOI] [PubMed] [Google Scholar]
- 11.Langdon KA, Warne MSTJ, Kookanaz RS. Aquatic hazard assessment for pharmaceuticals, personal care products, and endocrine-disrupting compounds from biosolids-amended land. Integr Environ Assess Manag. 2010;6(4):663–676. doi: 10.1002/ieam.74 [DOI] [PubMed] [Google Scholar]
- 12.Lajeunesse A, Smyth SA, Barclay K, Sauvé S, Gagnon C. Distribution of antidepressant residues in wastewater and biosolids following different treatment processes by municipal wastewater treatment plants in Canada. Water Res. 2012;46(17):5600–5612. doi: 10.1016/j.watres.2012.07.042 [DOI] [PubMed] [Google Scholar]
- 13.Boix C, Ibáñez M, Fabregat-Safont D, Morales E, Pastor L, Sancho JV, Sánchez-Ramírez JE, Hernández F. Behaviour of emerging contaminants in sewage sludge after anaerobic digestion. Chemosphere. 2016;163:296–304. doi: 10.1016/j.chemosphere.2016.07.098 [DOI] [PubMed] [Google Scholar]
- 14.Ternes TA, Joss A, Siegrist H. Peer Reviewed: Scrutinizing Pharmaceuticals and Personal Care Products in Wastewater Treatment. Environ Sci Technol. 2004;38(20):392A–399A. doi: 10.1021/es040639t [DOI] [PubMed] [Google Scholar]
- 15.Roberts PH, Thomas KV. The occurrence of selected pharmaceuticals in wastewater effluent and surface waters of the lower Tyne catchment. Sci Total Environ. 2006;356(1–3):143–153. doi: 10.1016/j.scitotenv.2005.04.031 [DOI] [PubMed] [Google Scholar]
- 16.Singer HP, Wössner AE, McArdell CS, Fenner K. Rapid Screening for Exposure to “non-Target” Pharmaceuticals from Wastewater Effluents by Combining HRMS-Based Suspect Screening and Exposure Modeling. Environ Sci Technol. 2016;50(13):6698–6707. doi: 10.1021/acs.est.5b03332 [DOI] [PubMed] [Google Scholar]
- 17.Jelic A, Gros M, Ginebreda A, Cespedes-Sánchez R, Ventura F, Petrovic M, Barcelo D. Occurrence, partition and removal of pharmaceuticals in sewage water and sludge during wastewater treatment. Water Res. 2011;45(3):1165–1176. doi: 10.1016/j.watres.2010.11.010 [DOI] [PubMed] [Google Scholar]
- 18.Meng XZ, Venkatesan AK, Ni YL, Steele JC, Wu LL, Bignert A, Bergman Å, Halden RU. Organic Contaminants in Chinese Sewage Sludge: A Meta-Analysis of the Literature of the Past 30 Years. Environ Sci Technol. 2016;50(11):5454–5466. doi: 10.1021/acs.est.5b05583 [DOI] [PubMed] [Google Scholar]
- 19.Beecher N, Crawford K, Goldstein N, Lono-Batura M, Dziezyk E. A National Biosolids Regulation, Quality, End Use, and Disposal Survey; 2007. doi: 10.1596/978-0-8213-7581-5 [DOI] [Google Scholar]
- 20.Langdon KA, Warne MSJ, Smernik RJ, Shareef A, Kookana RS. PERSISTENCE OF ESTROGENIC ACTIVITY IN SOILS FOLLOWING LAND. Environ Toxicol Chem. 2014;33(1):26–28. doi: 10.1002/etc.2395 [DOI] [PubMed] [Google Scholar]
- 21.Heidler J, Halden RU. Meta-analysis of mass balances examining chemical fate during wastewater treatment. Environ Sci Technol. 2008;42(17):6324–6332. doi: 10.1021/es703008y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McClellan K, Halden RU. Pharmaceuticals and personal care products in archived U.S. biosolids from the 2001 EPA national sewage sludge survey. Water Res. 2010;44(2):658–668. doi: 10.1016/j.watres.2009.12.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Furlong ET, Gray JL, Quanrud DM, Teske SS, Esposito K, Marine J, Ela WP, Stinson B, Kolpin DW, Phillips PJ. Fate of Estrogenic Compounds During Municipal Sludge Stabilization and Dewatering; 2010. [Google Scholar]
- 24.U.S. EPA. Targeted National Sewage Sludge Survey Sampling and Analysis Technical Report; 2009. doi:EPA-822-R-08–016
- 25.Black GP, Anumol T, Young TM. Analyzing a broader spectrum of endocrine active organic contaminants in sewage sludge with high resolution LC-QTOF-MS suspect screening and QSAR toxicity prediction. Environ Sci Process Impacts. Published online 2019. doi: 10.1039/C9EM00144A [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hollender J, Schymanski EL, Singer HP, Ferguson PL. Nontarget Screening with High Resolution Mass Spectrometry in the Environment: Ready to Go? Environ Sci Technol. 2017;51(20):11505–11512. doi: 10.1021/acs.est.7b02184 [DOI] [PubMed] [Google Scholar]
- 27.Moschet C, Anumol T, Lew BM, Bennett DH, Young TM. Household Dust as a Repository of Chemical Accumulation: New Insights from a Comprehensive High-Resolution Mass Spectrometric Study. Environ Sci Technol. 2018;52(5):2878–2887. doi: 10.1021/acs.est.7b05767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang X, Di Lorenzo RA, Helm PA, Reiner EJ, Howard PH, Muir DCG, Sled JG, Jobst KJ. Compositional space: A guide for environmental chemists on the identification of persistent and bioaccumulative organics using mass spectrometry. Environ Int. 2019;132(May):104808. doi: 10.1016/j.envint.2019.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moschet C, Lew BM, Hasenbein S, Anumol T, Young TM. LC- and GC-QTOF-MS as Complementary Tools for a Comprehensive Micropollutant Analysis in Aquatic Systems. Environ Sci Technol. 2017;51(3):1553–1561. doi: 10.1021/acs.est.6b05352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kern S, Fenner K, Singer HP, Schwarzenbach RP, Hollender J. Identification of Transformation Products of Organic Contaminants in Natural Waters by Computer-Aided Prediction and High-Resolution Mass Spectrometry. Environ Sci Technol. Published online 2009:7039–7046. doi: 10.1021/es901979h [DOI] [PubMed] [Google Scholar]
- 31.Ruff M, Mueller MS, Loos M, Singer HP. Quantitative target and systematic non-target analysis of polar organic micro-pollutants along the river Rhine using high-resolution mass-spectrometry - Identification of unknown sources and compounds. Water Res. 2015;87:145–154. doi: 10.1016/j.watres.2015.09.017 [DOI] [PubMed] [Google Scholar]
- 32.Sultan J, Gabryelski W. Structural identification of highly polar nontarget contaminants in drinking water by ESI-FAIMS-Q-TOF-MS. Anal Chem. 2006;78(9):2905–2917. doi: 10.1021/ac060384x [DOI] [PubMed] [Google Scholar]
- 33.Du B, Lofton JM, Peter KT, Gipe AD, James CA, McIntyre JK, Scholz NL, Baker JE, Kolodziej EP. Development of suspect and non-target screening methods for detection of organic contaminants in highway runoff and fish tissue with high-resolution time-of-flight mass spectrometry. Environ Sci Process Impacts. 2017;19(9):1185–1196. doi: 10.1039/c7em00243b [DOI] [PubMed] [Google Scholar]
- 34.Peter KT, Tian Z, Wu C, Lin P, White S, Du B, McIntyre JK, Scholz NL, Kolodziej EP. Using High-Resolution Mass Spectrometry to Identify Organic Contaminants Linked to Urban Stormwater Mortality Syndrome in Coho Salmon. Environ Sci Technol. 2018;52:10317–10327. doi: 10.1021/acs.est.8b03287 [DOI] [PubMed] [Google Scholar]
- 35.Hug C, Ulrich N, Schulze T, Brack W, Krauss M. Identification of novel micropollutants in wastewater by a combination of suspect and nontarget screening. Environ Pollut. 2014;184:25–32. doi: 10.1016/j.envpol.2013.07.048 [DOI] [PubMed] [Google Scholar]
- 36.Gago-Ferrero P, Schymanski EL, Bletsou AA, Aalizadeh R, Hollender J, Thomaidis NS. Extended Suspect and Non-Target Strategies to Characterize Emerging Polar Organic Contaminants in Raw Wastewater with LC-HRMS/MS. Environ Sci Technol. 2015;49(20):12333–12341. doi: 10.1021/acs.est.5b03454 [DOI] [PubMed] [Google Scholar]
- 37.Schymanski EL, Singer HP, Longrée P, Loos M, Ruff M, Stravs MA, Ripollés Vidal C, Hollender J. Strategies to characterize polar organic contamination in wastewater: Exploring the capability of high resolution mass spectrometry. Environ Sci Technol. 2014;48(3):1811–1818. doi: 10.1021/es4044374 [DOI] [PubMed] [Google Scholar]
- 38.Blum KM, Andersson PL, Renman G, Ahrens L, Gros M, Wiberg K, Haglund P. Non-target screening and prioritization of potentially persistent, bioaccumulating and toxic domestic wastewater contaminants and their removal in on-site and large-scale sewage treatment plants. Sci Total Environ. 2017;575:265–275. doi: 10.1016/j.scitotenv.2016.09.135 [DOI] [PubMed] [Google Scholar]
- 39.Parry E, Young TM. Comparing targeted and non-targeted high-resolution mass spectrometric approaches for assessing advanced oxidation reactor performance. Water Res. 2016;104:72–81. doi: 10.1016/j.watres.2016.07.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sobus JR, Wambaugh JF, Isaacs KK, Williams AJ, McEachran AD, Richard AM, Grulke CM, Ulrich EM, Rager JE, Strynar MJ, Newton SR. Integrating tools for non-targeted analysis research and chemical safety evaluations at the US EPA. J Expo Sci Env Epidemiol. 2018;28(5):411–426. doi: 10.1038/s41370-017-0012-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brack W Effect-directed analysis: A promising tool for the identification of organic toxicants in complex mixtures? Anal Bioanal Chem. 2003;377(3):397–407. doi: 10.1007/s00216-003-2139-z [DOI] [PubMed] [Google Scholar]
- 42.Wignall JA, Muratov E, Sedykh A, Guyton KZ, Tropsha A, Rusyn I, Chiu WA. Conditional Toxicity Value (CTV) Predictor: An In Silico Approach for Generating Quantitative Risk Estimates for Chemicals. Environ Health Perspect. 2018;126(05). doi: 10.1097/BRS.0000000000000726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guo W, Van Langenhove K, Denison MS, Baeyens W, Elskens M, Gao Y. Estrogenic Activity Measurements in Water Using Diffusive Gradients in Thin-Film Coupled with an Estrogen Bioassay. Anal Chem. 2017;89(24):13357–13364. doi: 10.1021/acs.analchem.7b03537 [DOI] [PubMed] [Google Scholar]
- 44.Goldsmith MR, Grulke CM, Brooks RD, Transue TR, Tan YM, Frame A, Egeghy PP, Edwards R, Chang DT, Tornero-Velez R, Isaacs K, Wang A, Johnson J, Holm K, Reich M, Mitchell J, Vallero DA, Philips L, Phillips M, Wambaugh JF, Judson RS, Buckley TJ, Dary CC. Development of a consumer product ingredient database for chemical exposure screening and prioritization. Food Chem Toxicol. 2014;65:269–279. doi: 10.1016/j.fct.2013.12.029 [DOI] [PubMed] [Google Scholar]
- 45.Richard AM. DSSTox web site launch: improving public access to databases for building structure-toxicity prediction models. Preclinica. 2004;2(2):103–108. [Google Scholar]
- 46.Black GP, Young TM, Anumol T. Analyzing a broader spectrum of endocrine active organic contaminants in sewage sludge with High Resolution LC-QTOF-MS suspect screening and QSAR toxicity prediction. Environ Sci Process Impacts. 2019;(21):1099–1114. doi: 10.1039/C9EM00144A [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baston DS, Denison MS. Considerations for potency equivalent calculations in the Ah receptor-based CALUX bioassay: Normalization of superinduction results for improved sample potency estimation. Talanta. 2011;83(5):1415–1421. doi: 10.1016/j.talanta.2010.11.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ceger P, Allen D, Huang R, Xia M, Casey W. Performance of the BG1Luc ER TA method in a qHTS format. ALTEX. 2015;32(4):287–296. doi: 10.14573/altex.1505121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Escher BI, Allinson M, Altenburger R, Bain PA, Balaguer P, Busch W, Crago J, Denslow ND, Dopp E, Hilscherova K, Humpage AR, Kumar A, Grimaldi M, Sumith Jayasinghe B, Jarosova B, Jia A, Makarov S, Maruya KA, Medvedev A, Mehinto AC, Mendez JE, Poulsen A, Prochazka E, Richard J, Schifferli A, Schlenk D, Scholz S, Shiraishi F, Snyder S, Su G, Tang JYM, van der Burg B, van der Linden SC, Werner I, Westerheide SD, Wong CKC, Yang M, Yeung BHY, Zhang X, Leuch FDL. Benchmarking organic micropollutants in wastewater, recycled water and drinking water with in vitro bioassays. Environ Sci Technol. 2014;48(3):1940–1956. doi: 10.1021/es403899t [DOI] [PubMed] [Google Scholar]
- 50.Zhao B, Bohonowych JES, Timme-Laragy A, Jung D, Affatato AA, Rice RH, Di Giulio RT, Denison MS. Common Commercial and Consumer Products Contain Activators of the Aryl Hydrocarbon (Dioxin) Receptor. Nebert D, ed. PLoS One. 2013;8(2):e56860. doi: 10.1371/journal.pone.0056860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bittner GD, Denison MS, Yang CZ, Stoner MA, He G. Chemicals having estrogenic activity can be released from some bisphenol a-free, hard and clear, thermoplastic resins. Environ Heal. 2014;13(1):103. doi: 10.1186/1476-069X-13-103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vandermarken T, De Galan S, Croes K, Van Langenhove K, Vercammen J, Sanctorum H, Denison MS, Goeyens L, Elskens M, Baeyens W. Characterisation and implementation of the ERE-CALUX bioassay on indoor dust samples of kindergartens to assess estrogenic potencies. J Steroid Biochem Mol Biol. 2016;155:182–189. doi: 10.1016/J.JSBMB.2015.01.005 [DOI] [PubMed] [Google Scholar]
- 53.Vandermarken T, Croes K, Van Langenhove K, Boonen I, Servais P, Garcia-Armisen T, Brion N, Denison MS, Goeyens L, Elskens M. Endocrine activity in an urban river system and the biodegradation of estrogen-like endocrine disrupting chemicals through a bio-analytical approach using DRE- and ERE-CALUX bioassays. Chemosphere. 2018;201:540–549. doi: 10.1016/j.chemosphere.2018.03.036 [DOI] [PubMed] [Google Scholar]
- 54.Blavier J, Songulashvili G, Simon C, Penninckx M, Flahaut S, Scippo ML, Debaste F. Assessment of methods of detection of water estrogenicity for their use as monitoring tools in a process of estrogenicity removal. Environ Technol (United Kingdom). 2016;37(24):3104–3119. doi: 10.1080/09593330.2016.1177119 [DOI] [PubMed] [Google Scholar]
- 55.Brennan JC, Denison MS, Holstege DM, Magiatis P, Dallas JL, Gutierrez EG, Soshilov AA, Millam JR. 2,3-cis-2R,3R-(−)-epiafzelechin-3-O-p-coumarate, a novel flavan-3-ol isolated from Fallopia convolvulus seed, is an estrogen receptor agonist in human cell lines. BMC Complement Altern Med. 2013;13(1):133. doi: 10.1186/1472-6882-13-133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Snyder SA, Villeneuve DL, Snyder EM, Giesy JP. Identification and quantification of estrogen receptor agonists in wastewater effluents. Environ Sci Technol. 2001;35(18):3620–3625. doi: 10.1021/es001254n [DOI] [PubMed] [Google Scholar]
- 57.Rogers JM, Denison MS. Recombinant cell bioassays for endocrine disruptors: development of a stably transfected human ovarian cell line for the detection of estrogenic and anti-estrogenic chemicals. In Vitr Mol Toxicol. 2000;13(1):67–82. [PubMed] [Google Scholar]
- 58.NIEHS, (The National Institute of Environmental Health). BG1Luc4E2 Cells Are Being Renamed VM7Luc4E2 Cells; 2016. Accessed April 24, 2019. https://ntp.niehs.nih.gov/iccvam/methods/endocrine/bg1luc/bg1luc-vm7luc-june2016-508.pdf
- 59.Brennan JC, Bassal A, He G, Denison MS. Development of a recombinant human ovarian (BG1) cell line containing estrogen receptor α and β for improved detection of estrogenic/antiestrogenic chemicals. Environ Toxicol Chem. 2016;35(1):91–100. doi: 10.1002/etc.3146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.He G, Zhao J, Brennan JC, Affatato AA, Zhao B, Rice RH, Denison MS. Cell-Based Assays for Identification of Aryl Hydrocarbon Receptor (AhR) Activators. In: Humana Press, Totowa, NJ; 2014:221–235. doi: 10.1007/978-1-62703-742-6_13 [DOI] [Google Scholar]
- 61.Brack W, Escher BI, Müller E, Schmitt-Jansen M, Schulze T, Slobodnik J, Hollert H. Towards a holistic and solution-oriented monitoring of chemical status of European water bodies: how to support the EU strategy for a non-toxic environment? Environ Sci Eur. 2018;30(1):1–11. doi: 10.1186/s12302-018-0161-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Burgess RM, Ho KT, Brack W, Lamoree M. Effects-directed analysis (EDA) and toxicity identification evaluation (TIE): Complementary but different approaches for diagnosing causes of environmental toxicity. Environ Toxicol Chem. 2013;32(9):1935–1945. doi: 10.1002/etc.2299 [DOI] [PubMed] [Google Scholar]
- 63.Brack W, Ait-Aissa S, Burgess RM, Busch W, Creusot N, Di Paolo C, Escher BI, Mark Hewitt L, Hilscherova K, Hollender J, Hollert H, Jonker W, Kool J, Lamoree M, Muschket M, Neumann S, Rostkowski P, Ruttkies C, Schollee J, Schymanski EL, Schulze T, Seiler TB, Tindall AJ, De Aragão Umbuzeiro G, Vrana B, Krauss M. Effect-directed analysis supporting monitoring of aquatic environments - An in-depth overview. Sci Total Environ. 2016;544:1073–1118. doi: 10.1016/j.scitotenv.2015.11.102 [DOI] [PubMed] [Google Scholar]
- 64.Escher BI, Stapleton HM, Schymanski EL. Tackling complex mixtures of chemicals in our changing environment. 2020;392(January):388–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Environmental Protection Agency. 40 PART 503 — Standards for the Use or Disposal of Sewage Sludge; 2005:829–830. https://www.ecfr.gov/cgi-bin/text-idx?SID=9cbee0da87e6ad24d89079204b909fac&mc=true&node=pt40.32.503&rgn=div5#sp40.32.503.b
- 66.Nielsen SS. Food Science Text Series Food Analysis Laboratory Manual. Vol 3. Springer International Publishing; 2017. doi: 10.3329/jbau.v7i1.4985 [DOI] [Google Scholar]
- 67.Benfenati E, Manganaro A, Gini G. VEGA-QSAR: AI inside a platform for predictive toxicology. CEUR Workshop Proc. 2013;1107:21–28. doi: 10.1055/s-0033-1360292 [DOI] [Google Scholar]
- 68.Norman Suspect List Exchange. https://www.norman-network.com/?q=suspect-list-exchange [Google Scholar]
- 69.Tsugawa H, Cajka T, Kind T, Ma Y, Higgins B, Ikeda K, Kanazawa M, Vandergheynst J, Fiehn O, Arita M. MS-DIAL: Data-independent MS/MS deconvolution for comprehensive metabolome analysis. Nat Methods. 2015;12(6):523–526. doi: 10.1038/nmeth.3393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lai Z, Tsugawa H, Wohlgemuth G, Mehta S, Mueller M, Zheng Y, Ogiwara A, Meissen J, Showalter M, Takeuchi K, Kind T, Beal P, Arita M, Fiehn O. Identifying metabolites by integrating metabolome databases with mass spectrometry cheminformatics. Nat Methods. 2018;15(1):53–56. doi: 10.1038/nmeth.4512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tsugawa H, Kind T, Nakabayashi R, Yukihira D, Tanaka W, Cajka T, Saito K, Fiehn O, Arita M. Hydrogen Rearrangement Rules: Computational MS/MS Fragmentation and Structure Elucidation Using MS-FINDER Software. Anal Chem. 2016;88(16):7946–7958. doi: 10.1021/acs.analchem.6b00770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schymanski EL, Jeon J, Gulde R, Fenner K, Ruff M, Singer HP, Hollender J. Identifying small molecules via high resolution mass spectrometry: Communicating confidence. Environ Sci Technol. 2014;48(4):2097–2098. doi: 10.1021/es5002105 [DOI] [PubMed] [Google Scholar]
- 73.Department of Health and Human Services Centers for Disease Control and Prevention. Fourth National Report on Human Exposure to Environmental Chemicals.; 2009.
- 74.Wang Y, Hu W, Cao Z, Fu X, Zhu T. Occurrence of endocrine-disrupting compounds in reclaimed water from Tianjin, China. Anal Bioanal Chem. 2005;383(5):857–863. doi: 10.1007/s00216-005-0082-x [DOI] [PubMed] [Google Scholar]
- 75.Meng XZ, Wang Y, Xiang N, Chen L, Liu Z, Wu B, Dai X, Zhang YH, Xie Z, Ebinghaus R. Flow of sewage sludge-borne phthalate esters (PAEs) from human release to human intake: Implication for risk assessment of sludge applied to soil. Sci Total Environ. 2014;476–477:242–249. doi: 10.1016/j.scitotenv.2014.01.007 [DOI] [PubMed] [Google Scholar]
- 76.Prommer E Levorphanol: The forgotten opioid. Support Care Cancer. 2007;15(3):259–264. doi: 10.1007/s00520-006-0146-2 [DOI] [PubMed] [Google Scholar]
- 77.Gudin J, Fudin J, Nalamachu SR. Levorphanol use: Past, present and future. Postgrad Med. 2016;128(1):46–53. doi: 10.1080/00325481.2016.1128308 [DOI] [PubMed] [Google Scholar]
- 78.McNulty JP. Can Levorphanol be Used Like Methadone for Intractable Refractory Pain? J Palliat Med. 2007;10(2):293–296. doi: 10.1089/jpm.2006.0201 [DOI] [PubMed] [Google Scholar]
- 79.Yu A, Dong H, Lang D, Haining R. Characterization of Dextromethorphan O- and N-Demethylation Catalyzed by Highly Purified Recombinant Human CYP2D6. Drug Metab Diposition. 2001;29(11):1362–1365. [PubMed] [Google Scholar]
- 80.DuBois BN, Mehvar R. UPLC-MS/MS analysis of dextromethorphan-O-demethylation kinetics in rat brain microsomes. J Chromatogr B Anal Technol Biomed Life Sci. 2018;1096(June):66–72. doi: 10.1016/j.jchromb.2018.08.011 [DOI] [PubMed] [Google Scholar]
- 81.Pechnick RN. Comparison of the Effects of Dextromethorphan, Dextrorphan, and Levorphanol on the Hypothalamo-Pituitary-Adrenal Axis. J Pharmacol Exp Ther. 2004;309(2):515–522. doi: 10.1124/jpet.103.060038 [DOI] [PubMed] [Google Scholar]
- 82.Pert CB, Snyder SH. Opiate Receptor: Demonstration in Nervous Tissue. Science (80-). 1973;179(4077):1011–1014. [DOI] [PubMed] [Google Scholar]
- 83.Watson AR, Roberts A. Retrospective Study Identifying Levorphanol Ingestion Using Urine Biomarkers in Health Care Patients. Pain Physician. 2018;21:167–171. www.painphysicianjournal. [PubMed] [Google Scholar]
- 84.Frenk SM, Porter KS, Paulozzi LJ. Prescription Opioid Analgesic Use Among Adults: United States, 1999–2012.; 2015. [PubMed] [Google Scholar]
- 85.Key Findings of a National Survey On Cough Medicine Use; 2007.
- 86.Loos M, Singer H. Nontargeted homologue series extraction from hyphenated high resolution mass spectrometry data. J Cheminform. 2017;9(1):1–11. doi: 10.1186/s13321-017-0197-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Merino F, Rubio S, Pérez-Bendito D. Mixed aggregate-based acid-induced cloud-point extraction and ion-trap liquid chromatography-mass spectrometry for the determination of cationic surfactants in sewage sludge. J Chromatogr A. 2003;998(1–2):143–154. doi: 10.1016/S0021-9673(03)00565-X [DOI] [PubMed] [Google Scholar]
- 88.Kumar K, Adhikary P, Karmakar NC, Gupta S, Singh RP, Krishnamoorthi S. Synthesis, characterization and application of novel cationic and amphoteric flocculants based on amylopectin. Carbohydr Polym. 2015;127:275–281. doi: 10.1016/j.carbpol.2015.03.044 [DOI] [PubMed] [Google Scholar]
- 89.Hennecke D, Bauer A, Herrchen M, Wischerhoff E, Gores F. Cationic polyacrylamide copolymers (PAMs): environmental half life determination in sludge-treated soil. Environ Sci Eur 2018;30(1). doi: 10.1186/s12302-018-0143-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang C, Cui F, Zeng G ming, Jiang M, Yang Z zhu, Yu Z gang, Zhu M ying, Shen L qing. Quaternary ammonium compounds (QACs): A review on occurrence, fate and toxicity in the environment. Sci Total Environ. 2015;518–519:352–362. doi: 10.1016/j.scitotenv.2015.03.007 [DOI] [PubMed] [Google Scholar]
- 91.La Guardia MJ, Hale RC, Harvey E, Matteson Mainor T. Alkylphenol ethoxylate degradation products in land-applied sewage sludge (biosolids). Environ Sci Technol. 2001;35(24):4798–4804. doi: 10.1021/es0109040 [DOI] [PubMed] [Google Scholar]
- 92.White R, Jobling S, Hoare SA, Sumpter JP, Parker MG. Environmentally persistent alkylphenolic compounds are estrogenic. Endocrinology. 1994;135(1):175–182. doi: 10.1210/en.135.1.175 [DOI] [PubMed] [Google Scholar]
- 93.Jobling S, Sumpter JP. Detergent components in sewage effluent are weakly oestrogenic to fish: An in vitro study using rainbow trout (Oncorhynchus mykiss) hepatocytes. Aquat Toxicol. 1993;27(3–4):361–372. doi: 10.1016/0166-445X(93)90064-8 [DOI] [Google Scholar]
- 94.Nimrod AC, Benson WH. Environmental estrogenic effects of alkylphenol ethoxylates. Crit Rev Toxicol. 1996;26(3):335–364. doi: 10.3109/10408449609012527 [DOI] [PubMed] [Google Scholar]
- 95.Tang X, Wu Q, Huang H, Hu H, Li Q. Chemosphere Removal potential of anti-estrogenic activity in secondary effluents by coagulation. Chemosphere. 2013;93(10):2562–2567. doi: 10.1016/j.chemosphere.2013.09.073 [DOI] [PubMed] [Google Scholar]
- 96.Gehrmann L, Bielak H, Behr M, Itzel F, Lyko S, Simon A, Kunze G, Dopp E, Wagner M, Tuerk J. (Anti-) estrogenic and (anti-) androgenic effects in wastewater during advanced treatment : comparison of three in vitro bioassays. Published online 2018:4094–4104. doi: 10.1007/s11356-016-7165-4 [DOI] [PubMed] [Google Scholar]
- 97.Hirose T, Morito K, Kizu R, Toriba A, Hayakawa K, Ogawa S, Inoue S, Muramatsu M, Masamune Y. Estrogenic / Antiestrogenic Activities of Benzo [ a ] pyrene Monohydroxy Derivatives. J Heal Sci 2001;47(6):552–558. [Google Scholar]
- 98.Oh SM, Ryu BT, Chung KH. Identification of Estrogenic and Antiestrogenic Activities of Respirable Diesel Exhaust Particles by Bioassay-directed Fractionation. Arch Pharm Res. 2008;31(1):75–82. doi: 10.1007/s12272-008-1123-8 [DOI] [PubMed] [Google Scholar]
- 99.Fertuck KC, Kumar S, Sikka HC, Matthews JB. Interaction of PAH-related compounds with the a and b isoforms of the estrogen receptor. Toxicol Lett. 2001;121:167–177. [DOI] [PubMed] [Google Scholar]
- 100.Tran DQ, Ide CF, Mclachlan JA, Arnold SF. The Anti-estrogenic Activity of Selected Polynuclear Aromatic Hydrocarbons in Yeast Expressing Human Estrogen Receptor One of the largest classes of chemicals measured in some contaminated ecosystems are polyaromatic hydrocarbons (PAH). PAHs are produce. Biochem Biophys Res Commun. 1996;229:102–108. [DOI] [PubMed] [Google Scholar]
- 101.Wu Q, Hu H. Effect of Chlorination on the Estrogenic / Antiestrogenic Activities of Biologically Treated Wastewater. 2009;43(13):4940–4945. [DOI] [PubMed] [Google Scholar]
- 102.Navas JM, Segner H. Antiestrogenic Activity of Anthropogenic and Natural Chemicals. 1998;5(2):75–82. [DOI] [PubMed] [Google Scholar]
- 103.Boogaerts T, Degreef M, Covaci A, van Nuijs ALN. Development and validation of an analytical procedure to detect spatio-temporal differences in antidepressant use through a wastewater-based approach. Talanta. 2019;200(March):340–349. doi: 10.1016/j.talanta.2019.03.052 [DOI] [PubMed] [Google Scholar]
- 104.Ma L dan, Li J, Li J jun, Liu M, Yan D zhi, Shi W yan, Xu G. Occurrence and source analysis of selected antidepressants and their metabolites in municipal wastewater and receiving surface water. Environ Sci Process Impacts. 2018;20(7):1020–1029. doi: 10.1039/c8em00077h [DOI] [PubMed] [Google Scholar]
- 105.Lorenzen A, Hendel JG, Conn KL, Bittman S, Kwabiah AB, Lazarovitz G, Masse D, Mcallister TA, Topp E. Survey of Hormone Activities in Municipal Biosolids and Animal Manures. Environ Toxicol. 2004;19(3):216–225. doi: 10.1002/tox.20014 [DOI] [PubMed] [Google Scholar]
- 106.Paterakis N, Chiu TY, Koh YKK, Lester JN, McAdam EJ, Scrimshaw MD, Soares A, Cartmell E. The effectiveness of anaerobic digestion in removing estrogens and nonylphenol ethoxylates. J Hazard Mater. 2012;199–200:88–95. doi: 10.1016/j.jhazmat.2011.10.075 [DOI] [PubMed] [Google Scholar]
- 107.Holbrook RD, Novak JT, Grizzard TJ, Love NG. Estrogen receptor agonist fate during wastewater and biosolids treatment processes: A mass balance analysis. Environ Sci Technol. 2002;36(21):4533–4539. doi: 10.1021/es020577b [DOI] [PubMed] [Google Scholar]
- 108.Routledge EJ, SUmpter JP. ESTROGENIC ACTIVITY OF SURFACTANTS AND SOME OF THEIR DEGRADATION PRODUCTS ASSESSED USING A RECOMBINANT YEAST SCREEN. Environ Toxicol Chem. 1996;15(3):241–248. [Google Scholar]
- 109.Hoffman TC, Zitomer DH, McNamara PJ. Pyrolysis of wastewater biosolids significantly reduces estrogenicity. J Hazard Mater. 2016;317:579–584. doi: 10.1016/j.jhazmat.2016.05.088 [DOI] [PubMed] [Google Scholar]
- 110.Janex-Habibi ML, Huyard A, Esperanza M, Bruchet A. Reduction of endocrine disruptor emissions in the environment: The benefit of wastewater treatment. Water Res. 2009;43(6):1565–1576. doi: 10.1016/j.watres.2008.12.051 [DOI] [PubMed] [Google Scholar]
- 111.Giger W, Brunner PH, Schaener C. 4-Nonylphenol in Sewage Sludge: Accumulation of Toxic Metabolites from Nonionic Surfactants. Science (80-). 1984;225(August):623–625. [DOI] [PubMed] [Google Scholar]
- 112.Citulski JA, Farahbakhsh K. Fate of endocrine-active compounds during municipal biosolids treatment: A review. Environ Sci Technol. 2010;44(22):8367–8376. doi: 10.1021/es102403y [DOI] [PubMed] [Google Scholar]
- 113.Bade R, Bijlsma L, Miller TH, Barron LP, Sancho JV, Hernández F. Suspect screening of large numbers of emerging contaminants in environmental waters using artificial neural networks for chromatographic retention time prediction and high resolution mass spectrometry data analysis. Sci Total Environ. 2015;538:934–941. doi: 10.1016/j.scitotenv.2015.08.078 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


