Abstract
Idiopathic granulomatous mastitis (IGM) is a rare benign breast condition with a course that is often rapidly progressive and slow to resolve. There is no consensus on management, especially during pregnancy and lactation. A 30-year-old at 33 weeks presented with mastalgia, induration and galactorrhoea in the left breast. There was no improvement with antibiotics. Initial workup was negative, and a core needle biopsy showed findings consistent with the diagnosis of IGM. She was treated with steroids antepartum. She was co-managed by rheumatology and her obstetrician/breastfeeding medicine specialist postpartum. She was treated with azathioprine, breastfed exclusively for 6 months and continued breastfeeding through the first year. A multidisciplinary team approach is crucial in diagnosing, treating, and facilitating successful breastfeeding in patients with IGM.
Keywords: obstetrics, gynaecology and fertility, pregnancy, dermatological, infant nutrition (including breastfeeding)
Background
Idiopathic granulomatous mastitis (IGM) is a rare benign breast condition with a course that is often rapidly progressive and slow to resolve. It was first described in 1972 and primarily occurs in women of childbearing age.1 2 This condition presents a unique diagnostic dilemma as symptoms can be very non-specific and mimic other common breast conditions. This often results in a delay in diagnosis.2 The most commonly reported symptoms are breast mass, mastalgia, erythema and axillary lymphadenopathy.2–4 The exact aetiology of IGM is unclear, but it is known to be associated with autoimmune disorders, breast trauma, contraceptive use and alpha-1 antitrypsin deficiency.4–6 Other aetiologies, such as hyperprolactinaemia leading to excessive milk production, milk stasis and a T-cell-mediated inflammatory response as well as Corynebacterium infection, have been described.5 7 IGM can be a chronic disease, sometimes a disfiguring disease with a high recurrence rate.1–4 Various treatment options have been proposed and used, including surgical management and medical treatment with steroids.1–8 While there has been a recent shift to support the use of steroids over surgical intervention due to its effectiveness, the optimal treatment choice and duration for affected patients is unknown. There is also no consensus on management, especially during pregnancy and lactation. This case illustrates how a multidisciplinary approach, particularly including a breastfeeding medicine specialist in the management of IGM during pregnancy and postpartum, can facilitate a successful breastfeeding experience.
Case presentation
A 33-year-old gravida 3 para 1 with a longstanding history of eczema presented to her obstetric clinic visit at 33 weeks’ gestation with left breast pain and unilateral galactorrhoea for 3 weeks. She was afebrile. On examination, the left breast was erythematous and tender to palpation. No distinct mass was appreciated and there was severe induration. Dicloxacillin was initiated for presumed mastitis.
Investigations
Within a day of starting dicloxacillin, she developed joint pain and erythematous plaques along her left elbow, left wrist and right heel. The decision was made to discontinue dicloxacillin in favour of clindamycin. Due to persistent symptoms despite treatment, a general surgeon with specialisation in breast pathology was consulted. A mammogram and breast ultrasound revealed findings consistent with mastitis, including dilated fluid-filled ducts and hypervascularity of overlying skin. Enlarged left axillary lymphadenopathy was noted, but no discrete mass was seen.
Diagnosis
Fine needle aspiration showed acute inflammation with no growth on culture. Blood cultures for acid fast bacilli and fungi were negative. Antinuclear antibody testing was also negative. Mastalgia and mastitis persisted in spite of antibiotics (dicloxacillin 500 mg four times a day for 2 days and oral clindamycin 300 mg four times a day for 7 days). An ultrasound-guided breast core biopsy was performed, and histological review revealed chronic granulomatous inflammation, neutrophilic and eosinophilic inflammation consistent with the diagnosis of IGM.
Treatment
Along with her breast symptoms, the patient continued to develop progressive joint pain and the erythematous plaques had spread to her abdomen, posterior calves and arms. Dermatology was consulted, and the diagnosis of concurrent erythema nodosum was made. She was also evaluated by rheumatology and prednisone was recommended to treat both the IGM and her other symptoms. Prednisone 20 mg once daily was initiated at 35 weeks’ gestation and continued for the remainder of the pregnancy. The erythema nodosum and polyarthritis responded well to steroid therapy. Her breast symptoms showed minimal improvement, however, and she subsequently developed multiple draining fistulas on her left breast. At 37 weeks, the recommendation was to continue steroid therapy with a plan to treat with methotrexate postpartum.
Outcome and follow-up
She had a normal spontaneous vaginal delivery at 39 weeks, and due to multiple draining fistulas on her left breast, she was started on a course of trimethoprim–sulfamethoxazole for bacterial mastitis prophylaxis. Given her strong desire to breastfeed, methotrexate was not used. She initiated breastfeeding from her right breast. Due to the severity of her symptoms, breastfeeding from her left breast was not encouraged, and instead, hand expression for comfort was recommended. Her treatment options were reconsidered by a multidisciplinary team that included rheumatology, breast surgery and her obstetrician/breastfeeding medicine specialist. The decision was made to treat with azathioprine, and prednisone was tapered to 2.5 mg once a day slowly over 4 weeks, then discontinued. After the prednisone taper, azathioprine 50 mg two times a day was started. At 8 weeks postpartum, the patient was breastfeeding exclusively. She was able to breastfeed directly from the right breast and she bottlefed expressed milk from the left breast. There was minimal improvement in the induration of her left breast. She still had multiple residual draining fistulas on her left breast, with new ones forming on a regular basis (figures 1 and 2). The patient managed drainage from the fistulas with breast pads, and found them minimally bothersome. At 3 months postpartum, her breast continued to improve, she had minimal residual induration, but still had some persistent draining fistulas. She chose to discontinue azathioprine because it was unclear if the improvement was due to the medication. After discontinuation of azathioprine, she developed mild symptoms on the contralateral breast with induration, but no fistula development. At 7 months postpartum, her breast examination was normal except for a 2 cm firm area. The patient reported breastfeeding exclusively for 6 months, and breastfeeding continued throughout the first year.
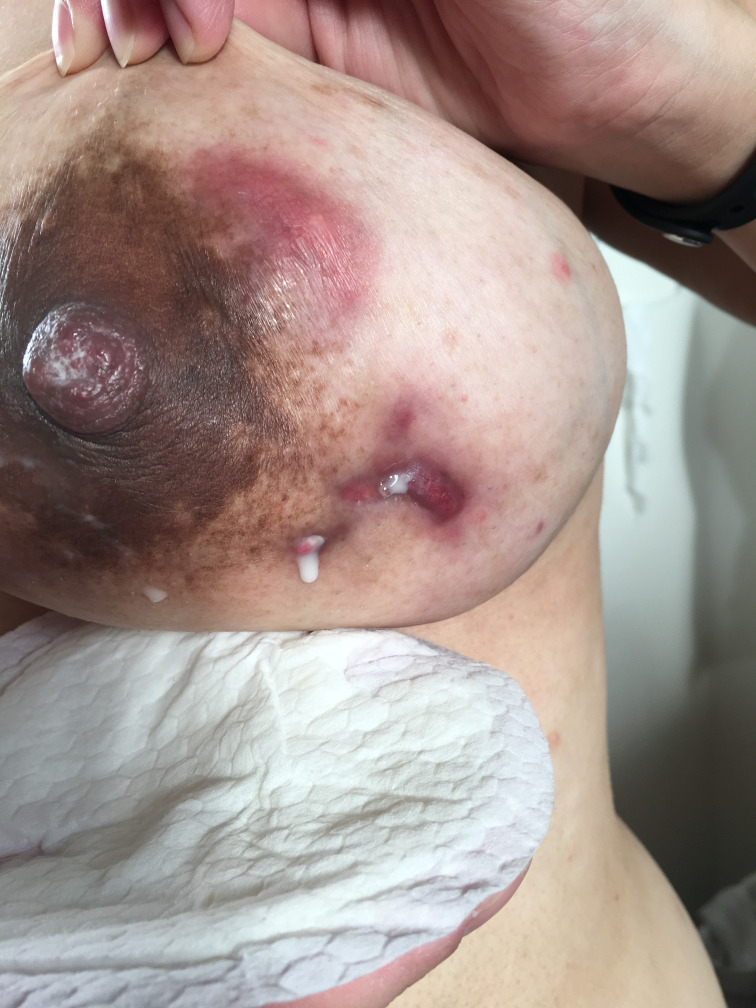
Figure 1.
Draining fistula on the left breast postpartum.
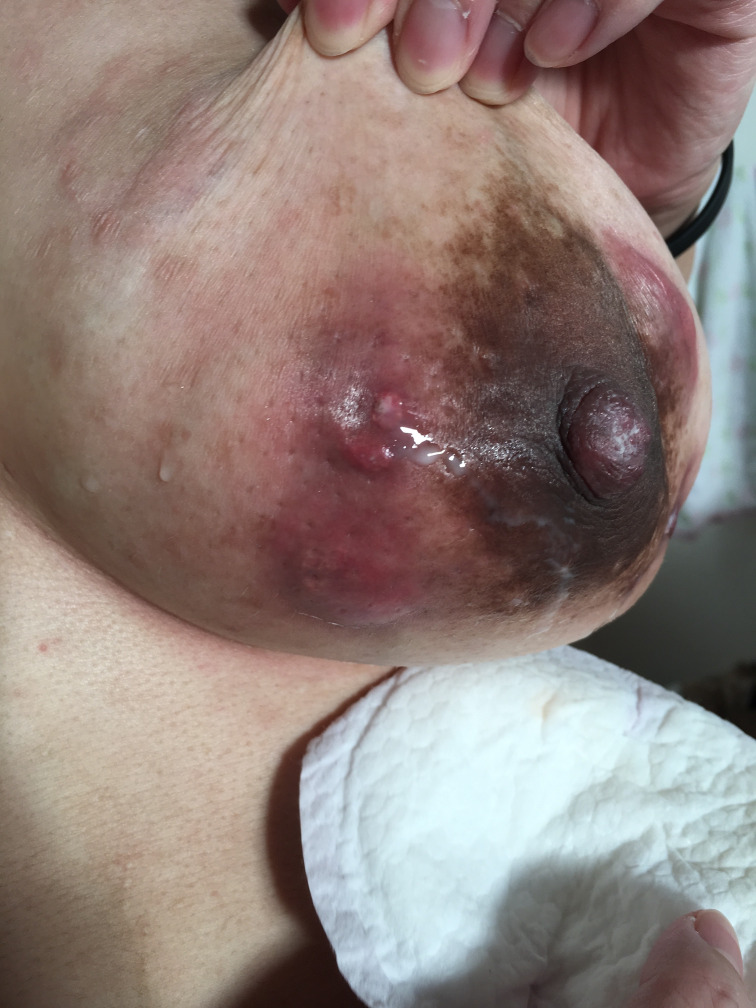
Figure 2.
Draining fistula of the lateral breast postpartum.
Four years since her diagnosis of IGM, the residual scars at the sites of the fistulas remain, but the patient reports no additional long-term complications (figures 3 and 4).
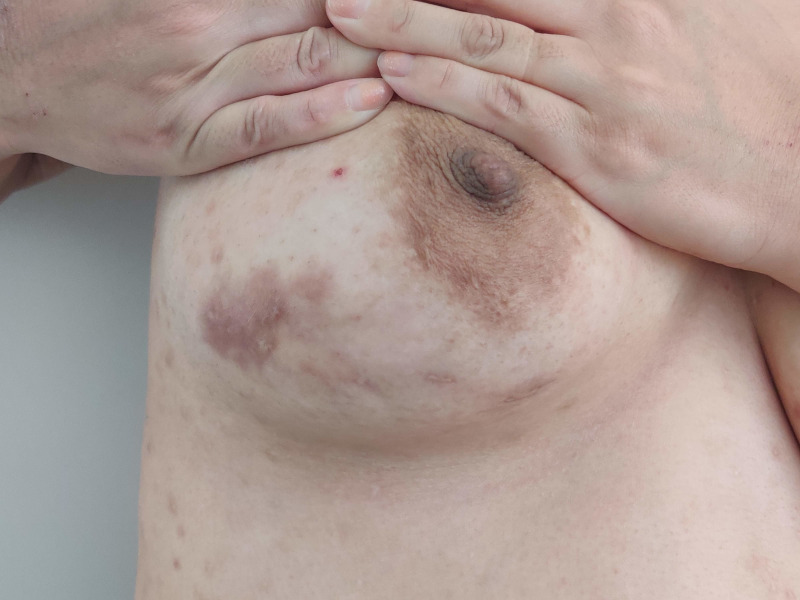
Figure 3.
Residual scar on the lateral aspect of the left breast 4 years after the diagnosis and management of idiopathic granulomatous mastitis.
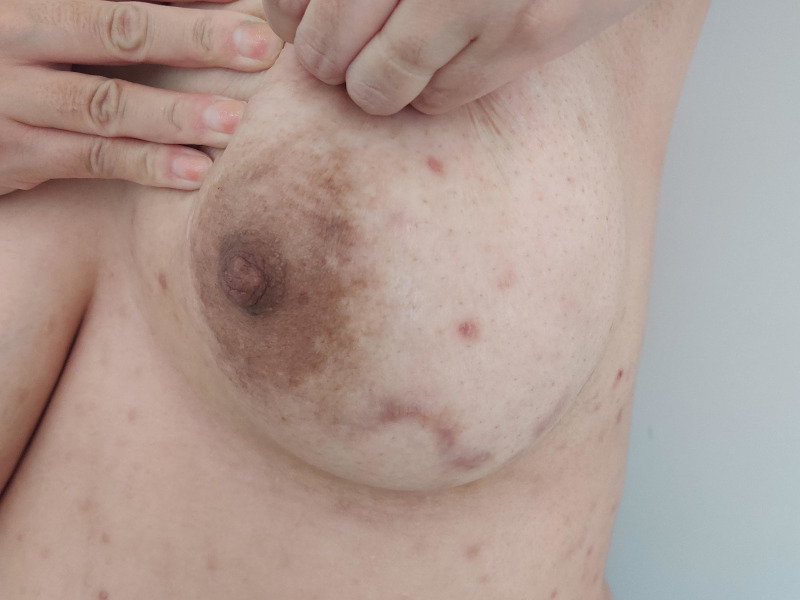
Figure 4.
Scar on the inferior part of the left breast after the resolution of idiopathic granulomatous mastitis.
Discussion
IGM is a rare, inflammatory breast condition with unclear aetiology. It was first described by Kessler and Wolloch in 1972.1 It occurs primarily in women of childbearing age and can mimic two common breast conditions, namely breast abscess and breast malignancy.2 The most common presenting symptoms include a firm breast mass, breast tenderness, erythema, nipple retraction and axillary adenopathy.2 3 While it is typically diagnosed in women of childbearing age, cases have been reported in patients at extremes of age without a recent history of childbirth or breastfeeding. Two such scenarios include cases in an 11-year-old girl and an 83-year-old woman.3 4 The precise aetiology of IGM is yet to be elucidated. It has been associated with autoimmune disorders, breast trauma, contraceptive use, alpha-1 antitrypsin deficiency and hyperprolactinaemia.4–6 High levels of prolactin may have a role in promoting inflammatory response in the breast, leading to excess milk production and possible accumulation within the mammary lobules. These static secretions may become infected or infiltrate the perilobular stroma leading to T-cell-mediated immune response and formation of granulomas.7 An association with Corynebacterium infection has also been reported.5 To lend further credence to the autoimmune association, extramammary manifestations of autoimmune processes frequently reported with IGM include erythema nodosum and arthritis, both of which were seen in our patient.8 9
The exact prevalence of IGM is unknown. Baslaim et al reported in a cohort of 1106 patients with benign breast disease, over 10 years, histologically confirmed diagnosis of IGM accounted for 1.8%.2 It has been reported in all races with a higher occurrence in Asian women and women of Hispanic descent.2 The most common clinical manifestation of IGM is a tender, firm unilateral breast mass. Other findings that have been reported include erythema, galactorrhoea, eschars, skin ulcerations and axillary lymphadenopathy. Nipple retraction and peau d’orange appearance of the breast due to granulomatous inflammatory changes may be seen mimicking breast carcinoma.2 IGM can be chronic and affected patients can develop sterile abscesses and disfigurement. Fistulas or draining sinus tracts to the skin have been reported and this was seen in our patient.10
Imaging modalities used in the evaluation of these symptoms include ultrasound, mammography and MRI. These modalities may help further define the characteristics of the findings observed on physical examination. Findings on ultrasound include a hypoechoic heterogenous mass or masses with irregular margins, skin thickening, parenchymal oedema, fluid collection and axillary lymphadenopathy. Ultrasound is also helpful for documenting the presence of sinus tracts.10 Mammographic findings include focal or regional asymmetry, and trabecular thickening. Calcifications are rare, but have been reported.11 While helpful, these imaging modalities have limitations and cannot differentiate between IGM and malignancy. Therefore, the diagnosis relies on histological evaluation. The characteristic findings include the presence of non-caseating granulomas, epithelioid histiocytes in a background rich in neutrophils, lymphocytes and plasma cells. An absence of malignant cells is also an important finding.2
Due to the rarity of the condition and non-specific nature of presenting symptoms, making the diagnosis of IGM is challenging and often delayed. It requires meticulous assessment of histopathological specimens and is a diagnosis of exclusion. Often the consideration for IGM arises when patients are treated for the more common diagnoses of mastitis or breast abscess and show no improvement or even worsening disease. While postpartum mastitis is a common diagnosis with a reported incidence of 2%–33%, antepartum mastitis is far less common.12 The first two cases were reported in 1986, with only a few more reported since then.13 Atypical presentation of mastitis should prompt a broader differential. Other granulomatous conditions should also be considered in the differential diagnosis, including sarcoidosis, tuberculosis, syphilis, Wegener’s granulomatosis and polyarteritis nodosa.11
The clinical course of IGM is highly variable, and reported cases have ranged from self-limiting to refractory or recurrent requiring surgical resection.3 14 It is often characterised by rapid progression and slow resolution.2 A retrospective case series of 18 patients with histological diagnosis of IGM over a span of 25 years showed that regardless of therapeutic intervention, the condition takes about 6–12 months to resolve completely.6
There is currently no definitive consensus on the optimal management for IGM. Treatment options include expectant management, medical and surgical therapies. One case series of eight patients managed expectantly, reported a 50% resolution rate and no cases of recurrence.3 The most commonly used medications include corticosteroids and immunosuppressive agents, such as methotrexate and azathioprine. Antibiotic therapy is controversial in the treatment of IGM. A trial of antibiotics may be warranted during the diagnostic evaluation or if patients develop culture positive abscesses. However, in true cases of IGM, antibiotics appear to have little role in management.2 6 15 Corticosteroid therapy was first proposed in 1980,16 and since then, successful treatment of IGM with high-dose oral steroids has been reported.2 17 Glucocorticoids, specifically prednisone and prednisolone, are the most commonly used steroids in the treatment of IGM.8 9 14 16 Doses of prednisone ranging from 10–60 mg/day have been reported, with the majority of cases using 60 mg.8 9 16 A prospective series of 49 women with IGM reported in 44 of 49 women treated with prednisone, 80% had complete resolution of disease in a median time of 159 days.17 In another study of a cohort of 48 patients, they found steroid to be treatment effective in the treatment of IGM.18 Steroids are considered compatible with breastfeeding due to low excretion into breast milk. As expected with high-dose steroids, adverse effects, including the development of Cushing-like syndrome and abnormal glucose tolerance, have been reported in some cases.19 In addition, the use of intralesional steroid injections with promising results have been reported.20 21 One such report was a retrospective study that evaluated 15 patients with IGM who were managed with intralesional triamcinolone injections with or without oral steroid administration. Triamcinolone 40 mg mixed with 2% lidocaine was injected into the IGM lesion under ultrasound guidance once every 1 or 2 weeks and was repeated until the resolution of symptoms and ultrasonographic findings. They found all lesions responded to the injections and the mean complete remission time was 115±75.9 days. They reported no complications related to the steroid injections.21 There are more ongoing clinical trials evaluating the use of intralesional steroids in this population. In scenarios where patients do not respond to steroid treatment or develop adverse effects, immunosuppressive agents are often the next step.22 The use of thiopurines, such as azathioprine during pregnancy, has increased notably in patients with inflammatory bowel disease (IBD).23 Two systematic reviews evaluating the perinatal outcomes with thiopurine exposure during pregnancy found that congenital abnormalities, miscarriage and low birth weight infants were not increased when compared with non-exposed subjects. However, they did find a slight increase in the incidence of preterm birth,24 this could be related to underlying medical conditions. In infants breastfed by mothers treated with thiopurines, the metabolite concentrations were found to be almost undetectable in breast milk.25 Another study evaluating the risk of infection in babies breastfed by mothers treated with azathioprine did not reveal an increased risk.26
The teratogenicity of methotrexate in pregnancy is well documented.27 28 Its use is also contraindicated in breastfeeding as it is excreted into breast milk and may concentrate in neonatal tissues.29 The potential effects in the neonate include neutropenia, immunosuppression and increased risk for malignancy.29 30
Surgical management using wide excisions or, in rare cases, mastectomies, depending on the extent of the disease have been reported.14 However, surgical excision is associated with delayed wound healing, fistulas and disfigurement.3 Recurrence rates after surgical excision range from 16% to 50%.31
There is little information available on breastfeeding with a diagnosis of IGM, perhaps due to the discomfort and chronic nature of the disease. However, in cases where patients desire to breastfeed and are motivated like our patient, it is important to involve a multidisciplinary team and explore treatment options that support this goal.
Learning points.
Idiopathic granulomatous mastitis (IGM) should be considered in atypical presentation of mastitis, such as in the antepartum period or in sterile abscesses.
IGM requires careful assessment of histopathological specimens and often is a diagnosis of exclusion.
Management is complex and regardless of therapeutic intervention, reported duration to resolution ranges from 6 to 12 months.
Limited data is available regarding the diagnosis and breastfeeding perhaps due to discomfort and chronic nature of the condition.
This case illustrates how a multidisciplinary approach, particularly including a breastfeeding medicine specialist in the management of IGM during pregnancy and postpartum, can facilitate a successful breastfeeding experience.
Footnotes
Contributors: AMA, primary author, participated in the planning of the manuscript, performing the literature review and writing the initial and final drafts of the manuscript. AJ participated in the planning of the manuscript, literature review and revising all versions of the manuscript. SC obtained patient consent, obtained pictures, provided detailed timeline of the case, revised the manuscript, managed the patient and liaised with supervising consultants
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer-reviewed.
Ethics statements
Patient consent for publication
Obtained.
References
- 1.Kessler E, Wolloch Y. Granulomatous mastitis: a lesion clinically simulating carcinoma. Am J Clin Pathol 1972;58:642–6. 10.1093/ajcp/58.6.642 [DOI] [PubMed] [Google Scholar]
- 2.Baslaim MM, Khayat HA, Al-Amoudi SA. Idiopathic granulomatous mastitis: a heterogeneous disease with variable clinical presentation. World J Surg 2007;31:1677–81. 10.1007/s00268-007-9116-1 [DOI] [PubMed] [Google Scholar]
- 3.Lai ECH, Chan WC, Ma TKF, et al. The role of conservative treatment in idiopathic granulomatous mastitis. Breast J 2005;11:454–6. 10.1111/j.1075-122X.2005.00127.x [DOI] [PubMed] [Google Scholar]
- 4.Bani-Hani KE, Yaghan RJ, Matalka II, et al. Idiopathic granulomatous mastitis: time to avoid unnecessary mastectomies. Breast J 2004;10:318–22. 10.1111/j.1075-122X.2004.21336.x [DOI] [PubMed] [Google Scholar]
- 5.Taylor GB, Paviour SD, Musaad S. A clinicopathological review of 34 cases of inflammatory breast disease showing an association between corynebacteria infection and granulomatous mastitis. Pathology 2003;35:109–19. [PubMed] [Google Scholar]
- 6.Al-Khaffaf B, Knox F, Bundred NJ. Idiopathic granulomatous mastitis: a 25-year experience. J Am Coll Surg 2008;206:269–73. 10.1016/j.jamcollsurg.2007.07.041 [DOI] [PubMed] [Google Scholar]
- 7.Nikolaev A, Blake CN, Carlson DL. Association between hyperprolactinemia and granulomatous mastitis. Breast J 2016;22:224–31. 10.1111/tbj.12552 [DOI] [PubMed] [Google Scholar]
- 8.Salesi M, Karimifar M, Salimi F, et al. A case of granulomatous mastitis with erythema nodosum and arthritis. Rheumatol Int 2011;31:1093–5. 10.1007/s00296-009-1273-0 [DOI] [PubMed] [Google Scholar]
- 9.Kalaycı Tuğçe Özlem, Koruyucu MB, Apaydın M, et al. Idiopathic granulomatous mastitis associated with erythema nodosum. Balkan Med J 2016;33:228–31. 10.5152/balkanmedj.2015.150089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kocaoglu M, Somuncu I, Ors F, et al. Imaging findings in idiopathic granulomatous mastitis. A review with emphasis on magnetic resonance imaging. J Comput Assist Tomogr 2004;28:635–41. 10.1097/01.rct.0000131927.82761.40 [DOI] [PubMed] [Google Scholar]
- 11.Boufettal H, Essodegui F, Noun M, et al. Idiopathic granulomatous mastitis: a report of twenty cases. Diagn Interv Imaging 2012;93:586–96. 10.1016/j.diii.2012.04.028 [DOI] [PubMed] [Google Scholar]
- 12.Malik S, Patil VA, Korday CS, et al. Antepartum mastitis: a rare occurrence. J Hum Lact 2015;31:367–70. 10.1177/0890334415585983 [DOI] [PubMed] [Google Scholar]
- 13.Wong MK, Smith CV, Phelan JP. Antepartum mastitis. A report of two cases. J Reprod Med 1986;31:511–3. [PubMed] [Google Scholar]
- 14.Freeman CM, Xia BT, Wilson GC, et al. Idiopathic granulomatous mastitis: a diagnostic and therapeutic challenge. Am J Surg 2017;214:701–6. 10.1016/j.amjsurg.2017.07.002 [DOI] [PubMed] [Google Scholar]
- 15.Wilson JP, Massoll N, Marshall J, et al. Idiopathic granulomatous mastitis: in search of a therapeutic paradigm. Am Surg 2007;73:798–802. 10.1177/000313480707300813 [DOI] [PubMed] [Google Scholar]
- 16.DeHertogh DA, Rossof AH, Harris AA, et al. Prednisone management of granulomatous mastitis. N Engl J Med 1980;303:799–800. 10.1056/NEJM198010023031406 [DOI] [PubMed] [Google Scholar]
- 17.Pandey TS, Mackinnon JC, Bressler L, et al. Idiopathic granulomatous mastitis--a prospective study of 49 women and treatment outcomes with steroid therapy. Breast J 2014;20:258–66. 10.1111/tbj.12263 [DOI] [PubMed] [Google Scholar]
- 18.Mahmodlou R, Dadkhah N, Abbasi F, et al. Idiopathic granulomatous mastitis: dilemmas in diagnosis and treatment. Electron Physician 2017;9:5375–9. 10.19082/5375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Donn W, Rebbeck P, Wilson C, et al. Idiopathic granulomatous mastitis. A report of three cases and review of the literature. Arch Pathol Lab Med 1994;118:822–5. [PubMed] [Google Scholar]
- 20.Tang A, Dominguez DA, Edquilang JK, et al. Granulomatous mastitis: comparison of novel treatment of steroid injection and current management. J Surg Res 2020;254:300–5. 10.1016/j.jss.2020.04.018 [DOI] [PubMed] [Google Scholar]
- 21.Byung Seup Kim BYK. Usefulness of ultrasound-guided intralesional steroid injection in management of idiopathic granulomatous mastitis. Journal of Surgical Ultrasound 2016;3. [Google Scholar]
- 22.Schmajuk G, Genovese MC. First report of idiopathic granulomatous mastitis treated with methotrexate monotherapy. J Rheumatol 2009;36:1559–60. 10.3899/jrheum.090091 [DOI] [PubMed] [Google Scholar]
- 23.Peyrin-Biroulet L, Oussalah A, Roblin X, et al. The use of azathioprine in Crohn's disease during pregnancy and in the post-operative setting: a worldwide survey of experts. Aliment Pharmacol Ther 2011;33:707–13. 10.1111/j.1365-2036.2011.04577.x [DOI] [PubMed] [Google Scholar]
- 24.Akbari M, Shah S, Velayos FS, et al. Systematic review and meta-analysis on the effects of thiopurines on birth outcomes from female and male patients with inflammatory bowel disease. Inflamm Bowel Dis 2013;19:15–22. 10.1002/ibd.22948 [DOI] [PubMed] [Google Scholar]
- 25.Gardiner SJ, Gearry RB, Roberts RL, et al. Exposure to thiopurine drugs through breast milk is low based on metabolite concentrations in mother-infant pairs. Br J Clin Pharmacol 2006;62:453–6. 10.1111/j.1365-2125.2006.02639.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Angelberger S, Reinisch W, Messerschmidt A, et al. Long-term follow-up of babies exposed to azathioprine in utero and via breastfeeding. J Crohns Colitis 2011;5:95–100. 10.1016/j.crohns.2010.10.005 [DOI] [PubMed] [Google Scholar]
- 27.Lloyd ME, Carr M, McElhatton P, et al. The effects of methotrexate on pregnancy, fertility and lactation. QJM 1999;92:551–63. 10.1093/qjmed/92.10.551 [DOI] [PubMed] [Google Scholar]
- 28.Milunsky A, Graef JW, Gaynor MF. Methotrexate-induced congenital malformations. J Pediatr 1968;72:790–5. 10.1016/S0022-3476(68)80430-5 [DOI] [PubMed] [Google Scholar]
- 29.Johns DG, Rutherford LD, Leighton PC, et al. Secretion of methotrexate into human milk. Am J Obstet Gynecol 1972;112:978–80. 10.1016/0002-9378(72)90824-1 [DOI] [PubMed] [Google Scholar]
- 30.Nielsen OH, Maxwell C, Hendel J. IBD medications during pregnancy and lactation. Nat Rev Gastroenterol Hepatol 2014;11:116–27. 10.1038/nrgastro.2013.135 [DOI] [PubMed] [Google Scholar]
- 31.Asoglu O, Ozmen V, Karanlik H, et al. Feasibility of surgical management in patients with granulomatous mastitis. Breast J 2005;11:108–14. 10.1111/j.1075-122X.2005.21576.x [DOI] [PubMed] [Google Scholar]