Abstract
A 75-year-old man presented with shortness of breath and somnolence and was found to have urosepsis. Blood and urine cultures subsequently grew multidrug-resistant (MDR) Klebsiella pneumoniae (Kp) with the New Delhi metallo-β-lactamase gene. The patient was treated successfully with plazomicin and meropenem/vaborbactam combination therapy. The course was complicated by acute kidney injury temporarily requiring haemodialysis, gastrointestinal bleed requiring multiple transfusions and hospital readmission with blood cultures again positive with MDR Kp. Plazomicin drug levels were persistently high during treatment, suggesting that therapeutic drug monitoring may be needed to safely use this drug in patients with severe renal dysfunction. This case marks the first use of plazomicin for bacteraemia in the literature outside of a clinical trial and demonstrates its safe and effective use in a patient with advanced renal disease, and provides important insights about dosing and therapeutic drug monitoring considerations in this patient population.
Keywords: urinary tract infections, acute renal failure, infections, safety
Background
With the increasing prevalence of multidrug-resistant (MDR) Enterobacteriaceae, particularly within the population of long-term residents of skilled nursing facilities, novel antimicrobial agents with a limited track record of use in clinical care, such as plazomicin, are critical elements in the medical management of these highly antimicrobial resistant infections. Due to the population included in clinical trials, little is known about how to administer these novel agents in patients with limited renal function. Here, we report a case of MDR Enterobacteriaceae bacteraemia in a patient with Acute Kidney Injury Network-defined stage 3 acute renal failure requiring intermittent haemodialysis (HD) and septic shock who was successfully treated with line removal and plazomicin.1 The case provides important insights about how this medication may be dosed and administered in this population, for which there are no published data to guide treatment decisions.
Case presentation
A 75-year-old male resident of a long-term care facility with a history of multiple medical problems presented to an outside community hospital with shortness of breath and somnolence. His medical history was significant for a chronic indwelling urethral catheter, recurrent urinary tract infections (UTIs) resulting in high rates of antimicrobial exposure (most recent hospitalisation was 5 weeks earlier for Enterobacter urosepsis, which was treated with piperacillin/tazobactam and ceftriaxone), chronic kidney disease, bilateral knee arthroplasties with a prolonged postoperative course and admission to a subacute rehabilitation facility, oxygen-dependent chronic obstructive pulmonary disease (COPD), heart failure with preserved ejection fraction, atrial fibrillation, type 2 diabetes mellitus requiring insulin and an allergy to doxycycline. At the time of his presentation, he was somnolent and vital signs were remarkable for temperature 101.8°F, blood pressure 81/49 mm Hg, respiratory rate 32 breaths/min and oxygen saturation 94% on 4 L nasal cannula. Admission labs were notable for white cell count of 0.2 109/L, haemoglobin of 89 g/L, creatinine of 2.3 mg/dL (prior baseline 1.5 mg/dL), estimated glomerular filtration rate (eGFR) 29.3 mL/min/1.72 m2, blood urea nitrogen of 53 mg/dL, procalcitonin of 0.87 µg/L and lactate of 2.3 mmol/L (see online supplemental file 1 for normal lab values). A nasopharyngeal swab for SARS-CoV-2 PCR was negative.
bcr-2021-243609supp001.pdf (94.2KB, pdf)
In the emergency department of the outside hospital, the patient was diagnosed with urosepsis, started on broad-spectrum antimicrobial therapy with ceftazidime and vancomycin, and vasopressors were initiated for blood pressure support. He was admitted to the medical intensive care unit (MICU) where his antibiotic regimen was changed to gentamicin, levofloxacin and piperacillin–tazobactam, per hospital septic shock protocol. The aetiology of his dyspnoea at the time of his initial presentation was attributed by his providers to multiple factors, but driven primarily by acutely decompensated heart failure with reduced ejection fraction, underlying COPD, requiring 3 L of nasal cannula oxygen at baseline and pulmonary hypertension. His dyspnoea subsequently improved with diuresis and treatment of his underlying infection.
On hospital day 1, due to continued positive blood cultures, cefepime was started and gentamicin and levofloxacin were discontinued. On hospital day 2 (41 hours after initial collection), admission blood cultures were positive for MDR Klebsiella pneumoniae (Kp); urine cultures subsequently grew the same organism. Susceptibility testing results prompted initiation of ceftolozone/tazobactam and discontinuation of piperacillin–tazobactam and cefepime. Repeat blood cultures from hospital days 1–4 continued to be positive for Kp, prompting the addition of tigecycline on day 5 due to persistent bacteraemia. On day 8, ceftolozone/tazobactam was discontinued after additional susceptibility testing revealed the isolate was highly antibiotic resistant to this β-lactam/β-lactamase combination. The isolate was resistant to all antibiotics tested except for intermediate susceptibility to meropenem/vaborbactam and fully susceptible to plazomicin and colistin. Given these susceptibility results, plazomicin was initiated on day 8 (see table 1 for results of susceptibility testing).
Table 1.
Antibiotic susceptibility test results
| Antibiotic susceptibility test results | ||
| Klebsiella pneumoniae | ||
| Antibiotic | Susceptibility | Interpretation |
| Amikacin | >32 | R |
| Ampicillin | >16 | R |
| Cefoxitin | >16 | R |
| Cefazolin | >16 | R |
| Tobramycin | >8 | R |
| Trimethoprim/sulfamethoxazole | >2/38 | R |
| Gentamicin | >8 | R |
| Ceftazidime | >16 | R |
| Cefepime | >16 | R |
| Levofloxacin | >8 | R |
| Ciprofloxacin | >4 | R |
| Nitrofurantoin | 128 | R |
| Ceftriaxone | >32 | R |
| Sulbactam/ampicillin | >16/8 | R |
| Cefuroxime | >16 | R |
| Piperacillin/tazobactam | 64 | I |
| Ertapenem | >1 | R |
| Meropenem | >8 | R |
| Colistin | 0.125 | S |
| Eravacycline | 1.5 | Presumed R* |
| Omadacycline | >32 | R |
| Plazomicin | 0.5 | S |
| Ceftazidime/avibactam | >256 | R |
| Doripenem | 4 | R |
| Meropenem/vaborbactam | 8 | I |
| IMP-Type Metallo-β-Lactamase | Not detected | |
| VIM--Type Metallo-β-Lactamase | Not detected | |
| New Delhi Metallo-β-Lactamase | Detected | |
| Klebsiella pneumoniae carbapenemase | Not detected | |
| OXA48 (Carbapenem-hydrolysing oxacillinase) | Not detected | |
*MIC cut-off values not available for eravacycline.
Blood cultures drawn on hospital days 5–7 and 9–11 were negative. Plazomicin was continued for an initial 13-day course and tigecycline was continued for a 22-day course (see figure 1 for complete antibiotic timeline). Susceptibility testing was not available for tigecycline, but the strain was found to be resistant to omadacycline (see table 1).
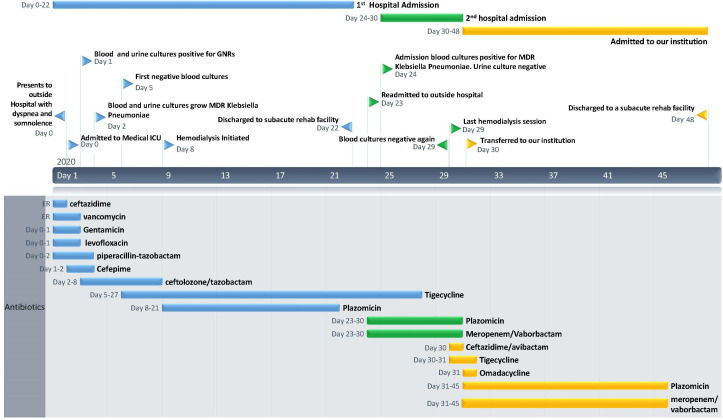
Figure 1.
Timeline denoting hospital course with major events and the timing of all antibiotic therapies. ER, emergency room; GNRs, gram-negative rods; ICU, intensive care unit; MDR, multidrug resistant.
The patient’s first hospital course was complicated by acute kidney injury (AKI) requiring initiation of HD on day 8, the same day that plazomicin was started. A temporary HD catheter was placed on hospital day 8, followed by placement of a tunnelled HD catheter on day 14, both HD accesslines were placed after repeated blood cultures revealed no microbiological growth. HD was continued on an approximate schedule of 3 days/week. The patient’s weight on day 8 prior to dialysis was 103.7 kg, significantly above his preadmission baseline weight of 85.3 kg. On day 8, he also developed acute chronic anaemia, with a haemoglobin decline to 69 g/L, which was attributed to a gastrointestinal source, given that the patient had several witnessed dark stools most consistent with melaena and potentially exacerbated by chronic kidney disease. As part of the work-up for anaemia and melaena, the patient underwent oesophagogastroduodenoscopy, which found a 7 mm gastric ulcer with an underlying visible blood vessel, which was felt to be the likely source of bleeding. He required 10 transfusions of packed red blood cells during his entire treatment course.
Manufacturer guidelines recommend a plazomicin trough of <3 µg/mL, drawn 30 min prior to the second dose and optimal maximum serum concentration (Cmax) 51.0 (±26.7) µg/mL.2 Although no guidelines exist for the dosing of plazomicin while on renal replacement therapy, the patient was dosed at 10 mg/kg every 48 hours while on HD, which was based on the manufacturer recommendations for patients with creatinine clearance 15–30 mL/min. The patient’s GFR was calculated on a daily basis using serum creatinine, age and weight in the Modification of Diet in Renal Disease Study equation for GFR. After initiation of dialysis, the patient’s GFR could no longer be reliably measured and thus plazomicin levels were monitored instead, although real-time adjustments based on serum drug levels were not feasible. After initiation of plazomicin on day 8, levels were checked on day 9 and found to be 9.4 µg/mL. The patient continued on plazomicin dosed every 48 hours. Plazomicin level repeated on day 16 was 27.8 µg/mL and on day 37 was 29 µg/mL. Plazomicin level testing was not available in house and required a turnaround time of 5–7 days, which did not allow for point-of-care adjustments during his treatment course. The patient did not receive dialysis on either day 9 or day 16 (please see online supplemental file 2 for dialysis prescription).
bcr-2021-243609supp002.pdf (170.7KB, pdf)
The patient was discharged to a subacute rehab facility on day 22 with a tunnelled HD catheter and peripherally inserted central catheter (PICC). One day later (day 23 after initial presentation), the patient was readmitted to the outside hospital with shortness of breath attributed to volume overload. Admission blood cultures were again positive for the same MDR Kp; a urine culture on the same day was negative. On day 24, plazomicin was reinitiated and meropenem/vaborbactam was started due to concern for developing plazomicin resistance. Blood cultures on day 27 were again positive for Kp. The patient’s PICC was removed and cultured on day 29. On day 30, the patient was transferred to our institution. Blood cultures from days 29–33 revealed no growth.
Overnight on day 30, due to lack of availability of plazomicin and meropenem/vaborbactam at our institution and at regional academic medical centres, the patient was started on ceftazidime/avibactam and tigecycline. HD was discontinued due to stable renal function and the decision to remove the tunnelled HD catheter as a likely ongoing source of infection. On day 31, the tunnelled HD catheter was removed and sent for culture, and the patient was transitioned to omadacycline and tigecycline, as plazomicin and meropenem/vaborbactam were initially unavailable despite outreach to multiple facilities to identify doses of these medications. Later that evening, doses of both meropenem/vaborbactam and plazomicin became available and these were reinitiated on the evening of day 31. Repeat testing of the isolate at our institution was notable for presence of the New Delhi metallo-β-lactamase (NDM) gene and resistance to omadacycline. During his admission to our facility, colistin was considered as a viable alternative to plazomicin, but was non-formulary at our institution and not readily available. Since the patient had previously been started on plazomicin at the other institution, it was easier to obtain and this was selected for ongoing therapy. The clinicians at the other institution were unavailable to comment on the decision-making process regarding the initial selection of plazomicin.
Plazomicin and meropenem/vaborbactam combination therapy was continued for a full 14 days after removal of his tunnelled HD line (days 24–45) without subsequent recurrence of his bacteraemia during a 6-month period following his treatment.
The patient’s creatinine was 2.26 mg/dL on day 30 following HD earlier that day and stabilised to 4.9 mg/dL by day 37. His daily urine output increased from 500 mL to approximately 2000 mL and he did not require dialysis during the remainder of his hospital course. The patient was discharged to a nursing home on day 48 from initial presentation.
Outcome and follow-up
Our patient had a complicated course requiring repeated hospitalisations and complex antibiotic therapy and removal of central lines to clear his infection. Plazomicin has been demonstrated to act synergistically with other antibiotics against MDR Enterobacteriaceae infections.3 4 The patient was accordingly initiated on tigecycline and then meropenem/vaborbactam due to concern for developing resistance to plazomicin; tigecycline susceptibilities are not available, but the isolate was tested and resistant to omadacycline. We hypothesise that the patient’s initial treatment failure was due to the presence of two central lines that subsequently became infected due to placement prior to completion of a full treatment course for his initial bacteraemia.
The patient’s renal function acutely declined due to AKI and required HD beginning on day 8. Plazomicin, which was begun on day 8 after initiation of HD, could have contributed to the continued decline in renal function. At this time, however, the patient was in the MICU requiring vasopressors, and thus septic shock and acute decompensated heart failure were felt to be the more likely aetiologies of the acute renal failure, as the progressive renal failure predated the initiation of the aminoglycoside antimicrobial. Additionally, the patient’s creatinine stabilised off dialysis, with appropriate urine production while on plazomicin from days 30 through 45. At the time of discharge, the patient was dialysis independent, but his kidney function remained severely impaired as evidenced by his creatinine of 4.9 mg/dL (eGFR 11.6 mL/min/1.72 m2). The patient had several dialysis free weeks which allowed him to have a line holiday while he completed treatment for his bacteraemia, but ultimately required reinitiating HD in the outpatient setting and remains dialysis dependent today.
Discussion
Here, we report a case of a long-term resident of a skilled nursing facility with severe acute chronic renal disease requiring HD, admitted to our facility with an NDM-type Kp bloodstream infection with a near-pan resistance profile (table 1). NDM is a carbapenemase that confers the ability to hydrolyse most penicillins, cephalosporins and carbapenems and which plazomicin, a novel aminoglycoside antibiotic, is known to be active against.5–7 The strain had intermediate susceptibility to meropenem/vaborbactam and was fully susceptible to plazomicin and colistin. Due to the resistance profile and limited treatment options, this infection was managed with a combination of plazomicin and tigecycline and then meropenem/vaborbactam, although there are no data to guide the dosing and therapeutic drug monitoring of plazomicin in patients with advanced renal disease.8 Notably, recent reports from the Massachusetts Department of Public Health suggest that NDM strains are increasing in prevalence throughout the state, and there have been several healthcare-associated clusters recently reported.9 10 Residents of long-term care facilities may be at particularly high risk both of similarly resistant infections and have high rates of advanced kidney disease; thus, our case is unlikely to be unique and our experience using plazomicin in this population may help with the management of future patients with similar treatment dilemmas.
Plazomicin is a new semisynthetic aminoglycoside developed to combat infections caused by MDR Enterobacteriaceae. Currently, there are no recommendations for how to dose plazomicin in patients with creatinine clearance less than 15 cc/min, or in patients undergoing HD. Plazomicin is Food and Drug Administration approved for adults with complicated UTIs and pyelonephritis caused by susceptible organisms who have few or no alternative therapy options.11 The efficacy of plazomicin in treating bloodstream infections was evaluated in the Combating Antibiotic Resistant Enterobacteriaceae (CARE) trial, an open-label study that compared plazomicin and colistin in combination with tigecycline or meropenem. Although this study observed a mortality benefit with plazomicin, its limited size (39 participants) prevented formal hypothesis testing, and plazomicin was not approved for bloodstream infections.8 Further, dosing recommendations for patients with severely impaired renal function were not made based on the results of this trial.
Side effects of plazomicin include nephrotoxicity, otoxicity, gastrointestinal upset including nausea, vomiting and diarrhoea (including Clostridioides difficile-associated diarrhoea), headaches and hypotension. With the exception of nephrotoxicity as discussed elsewhere, the patient did not experience any of these adverse effects. Plazomicin, which is approximately 12% protein bound, is primarily renally cleared, thus the serum half-life is highly dependent on renal function.12 Similar to other aminoglycosides, plazomicin is known to be nephrotoxic. The EPIC trials found that patients receiving plazomicin experienced a small increase in serum creatinine over those who received meropenem and were slightly more likely to have adverse renal outcomes such as AKI and renal failure.13 However, the CARE trial found that fewer patients in the plazomicin group were associated with a clinically meaningful increase in creatinine compared with the colistin treatment group.8 Indeed, a pooled analysis by Tang and Lai found that plazomicin was not associated with an increased risk of nephrotoxicity relative to comparators.14 These studies were conducted in patients with normal renal function, unlike our patient, but because the limited data available suggest that colistin causes more nephrotoxicity than plazomicin, and due to limited availability of colistin, our patient was treated with plazomicin.
Our findings suggest that consideration should be given to monitoring drug levels when administering plazomicin to HD patients given the potentially unpredictable drug levels; although a level drawn after HD was not available, the consistently high levels found in our patient suggest that the drug may not be cleared during HD, and that dosing based on levels in patients with advanced kidney disease may be required to ensure patient safety and limit drug toxicity. Turnaround time for plazomicin levels (>5 days) was a barrier to using this strategy to guide therapeutic drug monitoring and dosing decisions. Our experience with managing plazomicin in an HD patient may be useful for others who have limited options for treating other patients with highly resistant infections and advanced renal disease and suggests that if utilisation of plazomicin continues to increase, access to more rapid turnaround times for drug levels to guide treatment in real time will be needed.
Learning points.
With the increasing prevalence of multidrug-resistant Enterobacteriaceae, novel agents such as plazomicin are critical to advancing clinical care.
This case marks the first reported use of plazomicin for bacteraemia in the literature outside of a clinical trial and demonstrates its safe and effective use for a bloodstream infection in a patient with advanced renal disease.
For highly resistant organisms in patients with advanced renal disease, plazomicin may represent a safer alternative to colistin.
Dosing of plazomicin in patients with severe renal impairment requires monitoring of therapeutic drug levels. In our patient, levels were well above the therapeutic target even though doses were spaced 48 hours apart and this may have partially contributed to his acute kidney injury. Turnaround time for serum drug levels was a barrier to using this strategy to guide real-time treatment decisions.
Future studies will be needed to determine plazomicin’s effectiveness for bacterial infections other than complicated urinary tract infections.
Footnotes
Contributors: WE and WB-E conceived of the project. WE conducted a literature review, collected data and took the lead in writing the manuscript. WB-E, RGBB and DBS assisted with the data analysis and interpretation. All authors provided critical feedback and helped shape the analysis and manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Obtained.
References
- 1.Mehta RL, Kellum JA, Shah SV, et al. Acute kidney injury network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007;11:R31. 10.1186/cc5713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cipla USA, Inc . ZEMDRI (Plazomicin) prescribing information. FDA. Available: www.accessdata.fda.gov/drugsatfda_docs/label/2018/210303orig1s000lbl.pdf
- 3.Thwaites M, Hall D, Stoneburner A, et al. Activity of plazomicin in combination with other antibiotics against multidrug-resistant Enterobacteriaceae. Diagn Microbiol Infect Dis 2018;92:338–45. 10.1016/j.diagmicrobio.2018.07.006 [DOI] [PubMed] [Google Scholar]
- 4.García-Salguero C, Rodríguez-Avial I, Picazo JJ, et al. Can plazomicin alone or in combination be a therapeutic option against carbapenem-resistant Acinetobacter baumannii? Antimicrob Agents Chemother 2015;59:5959–66. 10.1128/AAC.00873-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Snyder BM, Montague BT, Anandan S, et al. Risk factors and epidemiologic predictors of blood stream infections with New Delhi Metallo-b-lactamase (NDM-1) producing Enterobacteriaceae. Epidemiol Infect 2019;147:e137:1–9. 10.1017/S0950268819000256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zmarlicka MT, Nailor MD, Nicolau DP. Impact of the New Delhi metallo-beta-lactamase on beta-lactam antibiotics. Infect Drug Resist 2015;8:297–309. 10.2147/IDR.S39186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Serio AW, Keepers T, Krause KM. Plazomicin is active against metallo-β-lactamase-producing Enterobacteriaceae. Open Forum Infect Dis 2019;6:ofz123. 10.1093/ofid/ofz123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McKinnell JA, Dwyer JP, Talbot GH, et al. Plazomicin for infections caused by carbapenem-resistant Enterobacteriaceae. N Engl J Med 2019;380:791–3. 10.1056/NEJMc1807634 [DOI] [PubMed] [Google Scholar]
- 9.Pecora N, Zhao X, Nudel K, et al. Diverse Vectors and Mechanisms Spread New Delhi Metallo-β-Lactamases among Carbapenem-Resistant Enterobacteriaceae in the Greater Boston Area. Antimicrob Agents Chemother 2019;63:e02040–18. 10.1128/AAC.02040-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown C. Carbapenemase-Producing organism (CPO) activity in Massachusetts. The Commonwealth of Massachusetts Department of Public Health Memo, 2019. [Google Scholar]
- 11.Wagenlehner FME, Cloutier DJ, Komirenko AS, et al. Once-daily plazomicin for complicated urinary tract infections. N Engl J Med 2019;380:729–40. 10.1056/NEJMoa1801467 [DOI] [PubMed] [Google Scholar]
- 12.Karaiskos I, Souli M, Giamarellou H. Plazomicin: an investigational therapy for the treatment of urinary tract infections. Expert Opin Investig Drugs 2015;24:1501–11. 10.1517/13543784.2015.1095180 [DOI] [PubMed] [Google Scholar]
- 13.Saravolatz LD, Stein GE. Plazomicin: a new aminoglycoside. Clin Infect Dis 2020;70:704–9. 10.1093/cid/ciz640 [DOI] [PubMed] [Google Scholar]
- 14.Tang H-J, Lai C-C. Plazomicin-associated nephrotoxicity. Clin Infect Dis 2020;71:1130–1. 10.1093/cid/ciz1064 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bcr-2021-243609supp001.pdf (94.2KB, pdf)
bcr-2021-243609supp002.pdf (170.7KB, pdf)