Abstract
Background:
The detection of circulating tumor cells (CTCs) is prognostic during the disease in women with metastatic breast cancer. Integrins are key role players in nearly every step of cancer progression. In this study, we aimed to analyze integrin alpha6beta4 expression on CTCs isolated from blood samples of patients with advanced breast cancer.
Materials and Methods:
In this single-center study, peripheral blood samples from 23 breast cancer patients were obtained and analyzed for the presence of CTCs by EasySep™ Direct Human CTC Enrichment Kit combined with subsequent immunocytochemical staining of anti-cytokeratin and anti-epithelial cell adhesion molecules, and β4 integrin on CTCs. Data were correlated with clinicopathological parameters.
Results:
CTCs were detected in 100% of the patients. The ratio of integrin β4+ CTC was 61% ± 8% of total CTCs. No significant correlation between histopathological parameters and CTC detection was found.
Conclusion:
Our results demonstrated the importance of α6 β4 integrin expression on CTCs in distant metastasis.
Keywords: Circulating tumor cells, epithelial-to-mesenchymal transition, metastatic breast cancer, α6 β4 integrin
Introduction
Integrins are cellular adhesion molecules that serve as receptors for extracellular matrix components. They named integrin because of their ability to integrate signals from the extracellular environment to the inside of the cell.[1,2]
Integrins are key role players in nearly every step of cancer progression, including cancer initiation and proliferation, local invasion and intravasation into vasculature, survival of circulating tumor cells (CTCs), priming of the metastatic niche, extravasation into the secondary site, and metastatic colonization of the new tissue.[2]
α6-Integrin subunit (also known as CD49f) has been known as a stemness marker that has been found on the plasma membrane of many stem cell populations.[3] Previous studies have shown its role in maintaining self-renewal of pluripotent stem cells and breast and glioblastoma cancer stem cells.
Integrin α6 can form heterodimers with either integrin β1 (CD29) or integrin β4 (CD104), functions as a receptor for the various laminins.[4] The tumorigenic role of integrin α6 β4 is mediated by the phosphorylation of the long cytoplasmic tail of integrin B4, which releases integrin α6 β4 from hemidesmosomes, leading to its interaction with growth factor receptors and the induction of growth signaling.[5] Integrin α6 β4 binding to laminin triggers phosphoinositide-3-kinase (PI3K) and RhoA small GTPases activation. Moreover, integrin α6 β4 networks with the epidermal growth factor receptor family to activate signaling pathways involved in tumorigenesis and metastasis, including PI3K, AKT, and MAPK signaling. It has been shown that integrin α6 β4 is released from the hemidesmosomes in epithelial cells and associates with the actin cytoskeleton in tumor progression.[6]
An increased expression of the integrin alpha6beta4 is correlated with a poor prognosis in cancer patients. It has been shown that in several tumor types the a6b4integrin has a role in the expansion capacity of cancer stem cells and signals to sustain self-renewal.[2]
CTCs are the main drivers of cancer recurrence and metastasis. The assessment of CTCs' clinical role in early metastasis prediction, diagnosis, and treatment needs more information about their biology, their roles in cancer dormancy, and immune evasion as well as in therapy resistance. There is not enough knowledge about the CTC functional and biochemical phenotypes in human patients. CTC detection, characterization, and enumeration is a potent tool for the management of each patient with cancer. The investigation of CTCs in cancer patients has recently received extensive attention because of its clinical implications, particularly for precision medicine.
In this study, we aimed to analyze integrin alpha6beta4 expression on CTCs isolated from blood samples of patients with advanced breast cancer.
Materials and Methods
Sample collection and preparation
Twenty-three metastatic breast cancer patients, before chemotherapy at the university hospital, were enrolled in this prospective study. Demographic and clinical data were gathered for each patient. Histopathological analysis was performed in accordance with the eighth edition of the cancer staging manual cancer by a senior specialist in pathology.[7]
Patient blood samples were collected from Omid Cancer referral Hospital and processed at the Applied Physiology Research Center. The study protocol was approved by the NIMAD Institutional ethics Board. All blood samples were collected after obtaining informed consent from patients. In order to isolate CTCs from whole blood, we utilized EasySep™ Direct Human CTC Enrichment Kit (Stemcell Technologies, USA) according to the manufacturer's protocol. CD2, CD14, CD16, CD19, CD45, CD61, CD66b, and Glycophorin A surface markers expressed on hematopoietic cells and platelets are recognized by antibodies cocktail to eliminate the cells other than CTCs. In fact, undesired cells are marked with antibodies and RapidSpheres™ and therefore are removed by an EasySep™ magnet.
In short, EasySep™ antibodies cocktail was added directly to the whole blood (50 μl/1 ml blood) and incubated for 5 min at room temperature. Next, RapidSpheres™ (50 μl/1 ml blood) was added to the sample. The sample was then topped up to double the volume with the suggested medium (phosphate-buffer saline [PBS] containing 2% fetal bovine serum and 1 mM ethylenediaminetetraacetic acid) and gently mixed. The tube was placed into the magnet and incubated for 10 min at room temperature. After picking up the magnet, and pouring the enriched cell suspension into a new tube, RapidSpheres™ (50 μl per 1 ml blood) was applied to the sample and placed into the magnet, and incubated for 10 min at room temperature. Isolated CTCs must transfer into a new tube to continue downstream experiments.
Immunocytochemistry procedure
In order to confirm the purity of isolated cells, we used fluorescence-conjugated antibodies targeting the proteins expressed on CTCs. Cell fixation, permeabilization, and blocking were done before immunostaining process.[8] Rinsing the cells in PBS is required between each steps. First of all, the isolated cells were fixed and permeablized using 4% paraformaldehyde and Tritonx 0.2%, respectively. Next, nonspecific binding sites were blocked using BSA 1% for 1 h at 37°C. Then, the cells were labeled with FITC-epithelial cell adhesion molecules (EpCAM) antibody (1:500 FITC anti-human CD326 Antibody, BioLegend) and β4 integrin antibody (0.5/100 microliter PBS, Anti-Integrin β4 Antibody, clone ASC-8, Sigma-Aldrich) overnight at 4°C. Then the cells were incubated with PE-IgG1 antibody (PE anti-mouse IgG1 Antibody, BioLegend) as a secondary antibody for 40 min at 37°C. Furthermore, in order to label double-stranded DNA and thus visualizing the nuclei, the cells were stained by Hoechst®33342 dye at 37°C for 20 min (5 μg/ml). In addition, the cells were labeled with using pancytokeratin antibody (1:500 Alexa Fluor® 488 anti-Cytokeratin, BioLegend), another CTC marker, following with β4-integrin staining. Images were captured using an inverted fluorescent microscope (Leica Microscope, USA) at ×20 magnification.
Statistical analysis
For statistical analysis, PASW Statistics 18 (SPSS Inc., Chicago, IL, USA) was applied. Descriptive statistics were used to describe patient baseline characteristics. To evaluate a potential association between CTCs and clinicopathological parameters, the Chi-square test was used.
Results
Hundred percent of the patients had CTCs in the bloodstream with at least 1 detectable CTC per 1 ml blood sample with strong EpCAM and cytokeratin immunocytofluorescence [Figures 1 and 2]. The patient's characteristics are summarized in Table 1.
Figure 1.
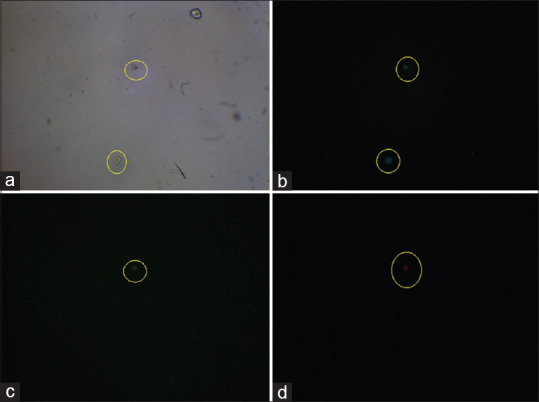
The illustration of circulating tumor cells isolated from whole blood sample of a breast cancer patient. The light microscopy image of isolated circulating tumor cell is represented in panel (a). Captured cells were stained with Hoechst®33342 dye (b), with an anti-epithelial cell adhesion molecules FITC-conjugated antibody showing 1 cell with epithelial cell adhesion molecules + (c), and with an unconjugated β4 integrin antibody and PE anti-mouse IgG1 Antibody (d)
Figure 2.
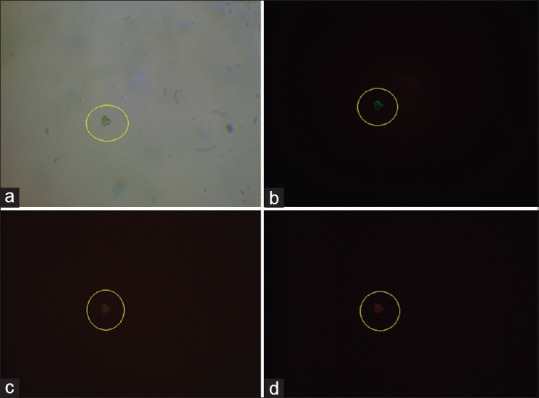
The illustration of circulating tumor cells isolated from whole blood sample of a breast cancer patient. Panel (a) represent light microscopy image of isolated circulating tumor cell. Captured cells were stained with Hoechst®33342 dye (b), with an anti-cytokeratin FITC-conjugated antibody (c), and with an unconjugated β4 integrin antibody and PE anti-mouse IgG1 Antibody (d)
Table 1.
Characteristics of patients
| n | |
|---|---|
| Total number of assessable patients | 23 |
| Age at study entry, years | 63.2±11.3 |
| HER2/neu positive (%) | 12 |
| ER positive (%) | 100 |
| PR positive (%) | 100 |
| Grading (%) | |
| 2 | 56.6 |
| 3 | 43.4 |
| Sites of metastases at study entry (%) | |
| Visceral | 13.1 |
| Bone | 86.9 |
| CTC+ | 100 |
| Integrin β4+ CTCs+/total | 61 |
HER2/neu: Human epidermal growth factor receptor 2, ER: Estrogen receptor, CTCs: Circulating tumor cells, PR: Progesterone receptor
The amount of detected CTCs in different patients varied from 1 to 12 CTCs per 1 ml of whole blood expressing with various expression of β4 on CTCs [Table 1]. There was no significant correlation between histopathological parameters and variation of CTC positivity rate.
Discussion
Our current understanding of breast cancer biology has enabled biologically driven disease stratification and introduced personalized treatment selection that has also impacted survival and quality of life. MBC continues to be considered incurable, however and is treated with palliative intent in spite of increased availability of FDA-approved therapeutic drugs designed to treat specific disease subtypes. Our results add substantially to the prior body of literature because, to our knowledge, this is the first study on integrin α6 β4 expression on CTCs. Studies examining integrin β4 expression in patient-derived tissue, in some cancer types, have obtained conflicting results.[9]
In breast cancer, integrin β4 overexpression is associated with aggressive behavior and poor prognosis. Given the challenges described with immunohistochemistry, gene expression profiling provides an excellent alternative that allows for quantitative assessment of integrin β4 expression. One study used gene expression profiling and immunohistochemistry of tissue microarray sections to demonstrate that integrin β4 is overexpressed in basal-like breast cancer.[10] This finding is particularly notable as basal-like breast cancer is an aggressive subtype that is associated with a notoriously poor prognosis.[1] This integrin “β4 signature” was shown to be a prognostic indicator that could predict both decreased survival and decreased time to recurrence in four breast cancer cohorts.[10] In other studies, integrin β4 mRNA expression was found to positively correlate with nuclear grade and tumor size,[11] and elevated integrin α6 β4 protein expression has been found to associate with decreased survival.[12] Co-expression of integrin α6 β4 and Net1, a RhoA guanine nucleotide exchange factor, has also been associated with decreased distant metastasis-free survival.[13]
In addition to integrin β4 overexpression correlation with nuclear grade and tumor size in breast cancer, there is a correlation between integrin β4 and cervical cancer, head and neck cancer, and pancreatic cancer.[5] The association of ITGB4 with poor prognosis in many cancers is attributed to the ability of integrin α6 β4 to promote malignant behaviors, such as proliferative signaling, the evasion of apoptosis, tissue invasion, and metastasis, and the induction of angiogenesis. However, the relation of ITGB4 expression with prognosis in some tumors and not in others remains unclear.[5] Rescently, Ruan et al. have shown that Integrin β4 has a key role in the cancer cell stemness, and immunologic targeting of integrin β4 is a promising therapeutic strategy.[14]
Conclusion
Our study results showed that tumor integrin β4 participates in mediating early metastatic steps and may have a role in the bone tropism. A focus for further work would be to investigate if integrin β4 positive breast cancer patients with metastases are likely to benefit from inhibition of this receptor.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors are grateful for the support of this research by NIMAD institute. They also acknowledge kind cooperation of Mrs Azam Mosayebi and Saeideh Arab for their commitment to patient data collection.
References
- 1.Stewart RL, O'Connor KL. Clinical significance of the integrin α6β4 in human malignancies. Lab Invest. 2015;95:976–86. doi: 10.1038/labinvest.2015.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cooper J, Giancotti FG. Integrin signaling in cancer: Mechanotransduction, stemness, epithelial plasticity, and therapeutic resistance. Cancer Cell. 2019;35:347–67. doi: 10.1016/j.ccell.2019.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou Z, Qu J, He L, Peng H, Chen P, Zhou Y. α6-Integrin alternative splicing: Distinct cytoplasmic variants in stem cell fate specification and niche interaction. Stem Cell Res Ther. 2018;9:122. doi: 10.1186/s13287-018-0868-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krebsbach PH, Villa-Diaz LG. The role of integrin α6 (CD49f) in stem cells: More than a conserved biomarker. Stem Cells Dev. 2017;26:1090–9. doi: 10.1089/scd.2016.0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li XL, Liu L, Li DD, He YP, Guo LH, Sun LP, et al. Integrin β4 promotes cell invasion and epithelial-mesenchymal transition through the modulation of Slug expression in hepatocellular carcinoma. Sci Rep. 2017;7:40464. doi: 10.1038/srep40464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cruz-Monserrate Z, Qiu S, Evers BM, O'Connor KL. Upregulation and redistribution of integrin alpha6beta4 expression occurs at an early stage in pancreatic adenocarcinoma progression. Mod Pathol. 2007;20:656–67. doi: 10.1038/modpathol.3800782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giuliano AE, Connolly JL, Edge SB, Mittendorf EA, Rugo HS, Solin LJ, et al. Breast cancer-major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67:290–303. doi: 10.3322/caac.21393. [DOI] [PubMed] [Google Scholar]
- 8.Rivera-Báez L, Lohse I, Lin E, Raghavan S, Owen S, Harouaka R, et al. Expansion of circulating tumor cells from patients with locally advanced pancreatic cancer enable patient derived Xenografts and functional studies for personalized medicine. Cancers (Basel) 2020;12:1–18. doi: 10.3390/cancers12041011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raymond K, Kreft M, Song JY, Janssen H, Sonnenberg A. Dual Role of alpha6beta4 integrin in epidermal tumor growth: Tumor-suppressive versus tumor-promoting function. Mol Biol Cell. 2007;18:4210–21. doi: 10.1091/mbc.E06-08-0720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu S, Simin K, Khan A, Mercurio AM. Analysis of integrin beta4 expression in human breast cancer: Association with basal-like tumors and prognostic significance. Clin Cancer Res. 2008;14:1050–8. doi: 10.1158/1078-0432.CCR-07-4116. [DOI] [PubMed] [Google Scholar]
- 11.Diaz LK, Cristofanilli M, Zhou X, Welch KL, Smith TL, Yang Y, et al. Beta4 integrin subunit gene expression correlates with tumor size and nuclear grade in early breast cancer. Mod Pathol. 2005;18:1165–75. doi: 10.1038/modpathol.3800411. [DOI] [PubMed] [Google Scholar]
- 12.Tagliabue E, Ghirelli C, Squicciarini P, Aiello P, Colnaghi MI, Ménard S. Prognostic value of alpha 6 beta 4 integrin expression in breast carcinomas is affected by laminin production from tumor cells. Clin Cancer Res. 1998;4:407–10. [PubMed] [Google Scholar]
- 13.Gilcrease MZ, Kilpatrick SK, Woodward WA, Zhou X, Nicolas MM, Corley LJ, et al. Coexpression of alpha6beta4 integrin and guanine nucleotide exchange factor Net1 identifies node-positive breast cancer patients at high risk for distant metastasis. Cancer Epidemiol Biomarkers Prev. 2009;18:80–6. doi: 10.1158/1055-9965.EPI-08-0842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruan S, Lin M, Zhu Y, Lum L, Thakur A, Jin R, et al. Integrin β4-targeted cancer immunotherapies inhibit tumor growth and decrease metastasis. Cancer Res. 2020;80:771–83. doi: 10.1158/0008-5472.CAN-19-1145. [DOI] [PMC free article] [PubMed] [Google Scholar]


