Abstract
Background
Most current models for predicting survival after resection of colorectal liver metastasis include largest diameter and number of colorectal liver metastases as dichotomous variables, resulting in underestimation of the extent of risk variation and substantial loss of statistical power. The aim of this study was to develop and validate a new prognostic model for patients undergoing liver resection including largest diameter and number of colorectal liver metastases as continuous variables.
Methods
A prognostic model was developed using data from patients who underwent liver resection for colorectal liver metastases at MD Anderson Cancer Center and had RAS mutational data. A Cox proportional hazards model analysis was used to develop a model based on largest colorectal liver metastasis diameter and number of metastases as continuous variables. The model results were shown using contour plots, and validated externally in an international multi-institutional cohort.
Results
A total of 810 patients met the inclusion criteria. Largest colorectal liver metastasis diameter (hazard ratio (HR) 1.11, 95 per cent confidence interval 1.06 to 1.16; P < 0.001), number of colorectal liver metastases (HR 1.06, 1.03 to 1.09; P < 0.001), and RAS mutation status (HR 1.76, 1.42 to 2.18; P < 0.001) were significantly associated with overall survival, together with age, primary lymph node metastasis, and prehepatectomy chemotherapy. The model performed well in the external validation cohort, with predicted overall survival values almost lying within 10 per cent of observed values. Wild-type RAS was associated with better overall survival than RAS mutation even when liver resection was performed for larger and/or multiple colorectal liver metastases.
Conclusion
The contour prognostic model, based on diameter and number of lesions considered as continuous variables along with RAS mutation, predicts overall survival after resection of colorectal liver metastasis.
A prognostic model that incorporated largest diameter and number of colorectal liver metastases as continuous variables along with RAS mutation status performed well in the development cohort and an external cohort. This model permits calculation of a specific survival probability for each patient and may help in clinical decision-making.
Introduction
Liver resection remains the only potentially curative treatment option for patients with colorectal liver metastasis, and reported 5-year overall survival rates after resection are approximately 50 per cent1,2. To predict survival outcomes for patients with colorectal liver metastasis, various prognostic scoring systems based on clinicopathological factors have been proposed3–8. Most of these include largest diameter and number of colorectal liver metastases, factors that are used by clinicians (along with the location of colorectal liver metastasis) to determine oncological and technical resectability. Both parameters are often dichotomized around cut-off values. This approach is perceived to offer the advantage of simplifying statistical analysis, and the clinical interpretation and presentation of results9. However, dichotomization also has disadvantages: underestimation of the extent of risk variation and substantial loss of statistical power compared with use of continuous variables9,10.
Studies reported prognostic models for hepatocellular carcinoma, which were built on the largest diameter and number of tumors as continuous variables for paivertients undergoing l transplant11,12 and undergoing liver resection, trans-arterial chemoembolization, and ablation13. The model for transplant was named the Metroticket model, because the ‘cost’ of travelling further on the contour plot (with larger and more lesions) was decreased post-transplant survival. The term Metroticket was used recently to describe a prognostic model developed for patients undergoing resection of colorectal liver metastasis14. However, instead of predicting overall survival on the basis of largest diameter and number of colorectal liver metastases as independent continuous variables, largest diameter and number of colorectal liver metastases were combined into a Cartesian plane to produce a tumour burden score, representing the distance from the origin of the plane, which was then used to categorize patients into three groups. However, none of the existing prognostic models for patients undergoing resection of colorectal liver metastasis uses largest diameter and number as continuous independent variables. The diameter and number of colorectal liver metastases are always obtained by radiological cross-sectional imaging. When decision-making about the resectability of colorectal liver metastasis was based purely on radiological evidence, approximately 60 per cent of patients with colorectal liver metastasis deemed unresectable by non-specialists were considered to have potentially resectable disease by specialist liver surgeons15. A prognostic model that provides individualized survival probabilities after resection of colorectal liver metastasis based on diameter and number considered as continuous variables might narrow the gap between non-specialists and specialist liver surgeons in decision-making regarding the resectability of colorectal liver metastases.
To address these issues, the primary aim of this study was to develop and validate a new prognostic model predicting survival probability after resection of colorectal liver metastases, including largest diameter and number as continuous variables.
Methods
The study was conducted according to the principles outlined in the TRIPOD checklist16 and the STROBE statement17.
Study population
Patients who underwent initial liver resection of colorectal liver metastasis with curative intent at the University of Texas MD Anderson Cancer Center from January 1998 to March 2017 were identified from a prospectively compiled database. Patients with unknown RAS mutation status were excluded. Demographic and clinicopathological characteristics, and outcomes were collected. The study was approved by the institutional review board at MD Anderson Cancer Center.
Surgical management of colorectal liver metastasis
The institutional approach to surgical management of colorectal liver metastasis has been described previously18. Almost all patients have preoperative chemotherapy, followed by restaging. Colorectal liver metastases are deemed resectable when a hepatectomy can achieve a negative margin while preserving more than 30 per cent of the total estimated liver volume, sparing two contiguous hepatic segments, and maintaining vascular inflow, vascular outflow, and biliary drainage19. Second-line chemotherapy is considered for patients with disease progression or a suboptimal tumour response. For patients with synchronous colorectal liver metastasis and an intact primary tumour, the order of resection (primary tumour first, colorectal liver metastasis first, or simultaneous removal) is decided at a multidisciplinary conference. Patients with an anticipated insufficient future liver remnant are offered preoperative portal vein embolization and staged hepatectomy20. Postoperative chemotherapy is typically administered to complete 12 cycles, including the cycles of preoperative chemotherapy21. After resection, patients have a history taken, physical examination, laboratory evaluation, and axial imaging every 3–4 months for the first 2 years, and every 4–6 months for the next 3 years22.
Definitions
Largest diameter and number of colorectal liver metastases were assessed from radiological images taken just before surgery. Synchronous metastases were defined as metastases diagnosed within 12 months of the primary tumour diagnosis. A positive surgical margin was defined by the presence of tumour cells within 1 mm of the transection line. Tumour category was classified according to the American Joint Committee on Cancer Staging Manual, eighth edition23. Preoperative chemotherapy regimens were recorded and categorized according to whether they included an anti-vascular endothelial growth factor or anti-epidermal growth factor receptor (EGFR) agent.
Somatic gene mutation profiling
RAS status was determined from tumour tissue blocks or slides from the primary colorectal cancer or specimens of colorectal liver metastasis, as described previously24, because the RAS mutation concordance rate between primary tumours and metastases is high (over 90 per cent)25,26. Routine PCR-based primer extension assay was performed to screen for mutations in KRAS codons 12 and 13 in all patients, and for mutations in KRAS codons 61 and 146 and NRAS codons 12, 13, and 61 in the majority of patients27. Single mutations in KRAS and NRAS were analysed together and reported as RAS mutations28.
Prognostic model development
A Cox proportional hazards model analysis was used to derive a prognostic model for overall survival in patients undergoing resection of colorectal liver metastasis based on largest diameter and number of colorectal liver metastases as continuous variables. Cox model results were presented graphically as contour plots showing the joint effect of the model co-variables on survival probability.
External validation of the prognostic model
The prognostic model was validated in an external cohort of patients who underwent resection of colorectal liver metastasis at Catholic University of the Sacred Heart (Rome, Italy), University of Verona (Verona, Italy), or the University of Tokyo (Tokyo, Japan), and met the same inclusion criteria as patients in the development cohort.
Statistical analysis
Categorical variables are presented as numbers with percentages, and continuous variables as median (i.q.r.) unless indicated otherwise. Patients who were lost to follow-up or alive in March 2019 were censored at the date of last known follow-up. A Cox proportional hazards model initially included the following factors: age (continuous variable), sex, primary tumour location, tumour category, primary lymph node metastasis, prehepatectomy carcinoembryonic antigen level (continuous variable), timing of metastasis (synchronous versus metachronous), prehepatectomy chemotherapy, number of colorectal liver metastases (continuous variable), largest diameter of colorectal liver metastasis (continuous variable), surgical margin status (R1 versus R0), and RAS mutation status. A backward elimination process with a threshold P value of 0.050 was used to select variables for the final models. Hazard ratios and 95 per cent confidence intervals were calculated for each factor. The proportional hazards assumption was tested by using Schoenfeld residuals29.
To develop a prognostic model based on largest diameter and number of colorectal liver metastases as continuous variables, published methods were followed12. Largest diameter and number of colorectal liver metastases were modelled as continuous variables using three-knot restricted cubic splines30, and diameter-by-number linear and non-linear interaction terms. Models were simplified by backward selection according to the Akaike information criterion31. Largest diameter of colorectal liver metastasis was plotted on the x-axis and number of colorectal liver metastases on the y-axis in a Cartesian plane11,12. The performance of the final fitted Cox model was assessed by calibration and discrimination. Calibration was performed by comparing the average overall survival probability predicted by the prognostic model with the average overall survival probability estimated by the Kaplan–Meier method after grouping predicted survival by quintile. Discrimination was evaluated using a Harrell’s concordance statistic32. P ≤ 0.050 was considered to indicate statistical significance. Statistical analysis was conducted with SAS® version 9.4 (SAS Institute, Cary, North Carolina, USA).
Results
Of 1131 patients who underwent resection of colorectal liver metastasis at MD Anderson Cancer Center during the study period and had known RAS mutation status, 810 met the inclusion criteria (Fig. S1). Demographic and clinicopathological characteristics are summarized in Table 1. The median largest diameter of colorectal liver metastasis was 2.3 (i.q.r. 1.5–3.7 ) cm. The median number of colorectal liver metastases was 2 (1–4). The rate of prehepatectomy chemotherapy was 88.4 per cent (716 patients). The median duration of follow-up was 5.5 (95 per cent c.i. 5.2 to 5.8) years based on the Kaplan–Meier method. During follow-up, 372 patients died (45.9 per cent) and 619 (76.4 per cent) experienced recurrence.
Table 1.
Demographic and clinicopathological characteristics of patients who underwent resection of colorectal liver metastais
|
No. of patients*
(n = 810) |
|
|---|---|
| Patient factors | |
| Age (years)† | 55 (48–63) |
| Sex ratio (M : F) | 477 : 333 |
| ASA fitness grade ≥ III | 680 (84.0) |
| Primary lesion factors | |
| Location | |
| Colon | 594 (73.3) |
| Rectum | 216 (26.7) |
| Tumour category ≥ T3‡ | 708 (87.4) |
| Lymph node metastasis‡ | 568 (71.9) |
| Liver metastasis factors | |
| Prehepatectomy CEA level (ng/ml)† | 3.6 (1.8–11.9) |
| Synchronous metastasis | 612 (75.6) |
| Prehepatectomy chemotherapy | 716 (88.4) |
| > 6 cycles | 239 (29.5) |
| Oxaliplatin-containing regimen | 591 (73.0) |
| Irinotecan-containing regimen | 166 (20.5) |
| With anti-VEGF agent | 548 (67.7) |
| With anti-EGFR agent | 75 (9.3) |
| Largest diameter of colorectal liver metastasis (cm)† | 2.3 (1.5–3.7; 0.3–15.0) |
| No. of colorectal liver metastases† | 2 (1–4; 1–30) |
| R1 surgical margin | 138 (17.0) |
| Molecular biomarker | |
| RAS mutation | 364 (44.9) |
* With percentages in parentheses unless indicated otherwise;
values are median (i.q.r.; range).
Data not available for tumour category in 14 patients and lymph node metastasis in 20 patients. CEA, carcinoembryonic antigen; VEGF, vascular endothelial growth factor; EGFR, epidermal growth factor receptor.
Predictors of overall survival after resection of colorectal liver metastasis
Multivariable Cox proportional hazards model analysis revealed that largest diameter of colorectal liver metastasis, number of colorectal liver metastases, RAS mutation status, age, primary lymph node metastasis, and prehepatectomy chemotherapy were significantly associated with overall survival (Table 2).
Table 2.
Multivariable Cox proportional hazards model for overall survival after resection of colorectal liver metastasis in 790 patients
| No. of patients | No. of events | Multivariable analysis |
||
|---|---|---|---|---|
| Hazard ratio * | P | |||
| Largest diameter of colorectal liver metastasis (continuous variable) | – | – | 1.11 (1.06, 1.16) | <0.001 |
| No. of colorectal liver metastases (continuous variable) | – | – | 1.06 (1.03, 1.09) | <0.001 |
| RAS mutation status | ||||
| Mutant | 354 | 179 | 1.76 (1.42, 2.18) | <0.001 |
| Wild type | 436 | 178 | 1.00 (reference) | |
| Age (per year, continuous variable) | – | – | 1.02 (1.01, 1.03) | 0.002 |
| Primary lymph node metastasis | ||||
| Positive | 568 | 268 | 1.58 (1.23, 2.03) | <0.001 |
| Negative | 222 | 89 | 1.00 (reference) | |
| Prehepatectomy chemotherapy | ||||
| Yes | 697 | 318 | 1.47 (1.04, 2.08) | 0.031 |
| No | 93 | 39 | 1.00 (reference) | |
Values in parentheses are 95 per cent confidence intervals. Of 810 patients, 790 were included in the analysis because data were not available for lymph node metastasis in 20 patients.
Based on analysis of data from 787 patients with complete data on tumour category and primary lymph node metastasis, the six variables in the table were selected for the final model.
Prognostic model based on largest diameter and number of colorectal liver metastases, and RAS mutation status
On the basis of the results of the multivariable analysis, a prognostic model that incorporated largest diameter and number of colorectal liver metastases, and RAS mutation status as a prognostic factors was developed. Primary lymph node metastasis was not included because this information is not available when colorectal liver metastasis-first and simultaneous approaches are used in the treatment of synchronous colorectal liver metastasis. The use of prehepatectomy chemotherapy was also omitted from the model because this practice varies widely by institution.
A Cox regression model was evaluated for patients with mutant RAS and those with wild-type RAS. Results of models based on largest diameter and number of colorectal liver metastases are shown in Fig. 1. After application of a backward selection procedure, both models included a linear term for largest diameter, a cubic spline for number, and the linear-by-linear interaction diameter-by-number. Fig. 1 shows 5-year overall survival estimates as a function of different values of largest diameter and number of colorectal liver metastases. The contour lines connect points of equal 5-year overall survival probability. The findings were also used to develop a tool based on Excel® (Microsoft, Redmond, Washington, USA) (called the 5-OS calculator for colorectal liver metastasis) in which one enters RAS mutation status, largest diameter of colorectal liver metastasis, and number of colorectal liver metastases, and the 5-year overall survival probability with its 95 per cent confidence interval is provided (Appendix S1). For example, if a patient has three colorectal liver metastases and the largest one measures 5 cm in diameter, the model estimates that the 5-year overall survival rate is 43.0 (95 per cent c.i. 34.1 to 51.6) per cent if the patient has mutant RAS disease, and 49.5 (40.7 to 57.6) per cent if the patient has wild-type RAS disease.
Fig. 1.
Contour plot of 5-year overall survival probability according to largest diameter and number of colorectal liver metastases for patients with mutant RAS and wild-type RAS disease
a Mutant RAS and b wild-type RAS.
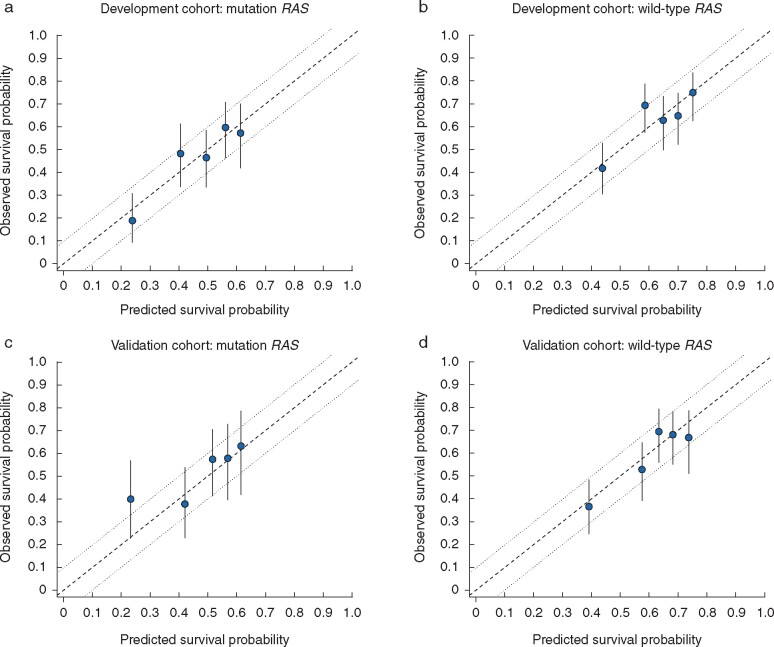
Fig. 2 shows the calibration of observed versus predicted survival probabilities. Observed survival lay within a 10 per cent margin of error around predicted survival in all instances. Harrell’s concordance statistics was 0.629 (s.e. 0.021) in patients with RAS mutation and 0.625 (0.022) in those with wild-type RAS. On the other hand, Harrell’s concordance statistics for the model including the other three risk factors (age, primary lymph node status, and prehepatectomy chemotherapy) was 0.644 (0.021) in patients with RAS mutation and 0.657 (0.021) in patients with wild-type RAS, which showed limited enhancement on model discrimination.
Fig. 2.
Calibration of prognostic model by mutant and wild-type RAS disease in development and external multi-international validation cohorts
Calibration plots for a mutant and b wild-type RAS disease in development cohort, and c mutant and d wild-type RAS disease in validation cohort. Observed overall survival probability was measured by Kaplan–Meier analysis; error bars represent 95 per cent c.i. The dashed line represents the ideal reference line where observed survival corresponds with predicted survival, and the dotted lines indicate the 10 per cent margin of error.
External validation of prognostic model
Of 1091 patients who underwent resection of colorectal liver metastasis at the three institutions during 2006–2018 and had known RAS mutation status, 673 met the inclusion criteria (Fig. S2). Demographic and clinicopathological characteristics of these patients are summarized in Table S1. The rate of RAS mutation was 37.3 per cent (254 patients). The median largest diameter of colorectal liver metastasis was 2.6 (i.q.r. 1.6–4.1) cm. The median number of colorectal liver metastases was 2 (1–4). The rate of prehepatectomy chemotherapy was 63.3 per cent (422 patients) and differed widely by institution: 73.9 per cent (286 of 387) in Catholic University of the Sacred Heart, 66.4 per cent (79 of 119) in University of Verona, and 35.4 per cent (57 of 161) in the University of Tokyo. The characteristics of the external validation cohort were different from those of the development cohort, ensuring the efficacy of the validation. The median duration of follow-up was 4.7 (95 per cent c.i. 4.3 to 5.0) years based on the Kaplan–Meier method. During the follow-up period, 256 patients died (38.0 per cent) and 482 (71.6 per cent) experienced recurrence.
Similar to findings for the development cohort, a multivariable Cox proportional hazards model analysis showed that largest diameter of colorectal liver metastasis, number of colorectal liver metastases, RAS mutation status, age, primary lymph node metastasis, and prehepatectomy chemotherapy were significantly associated with overall survival in the validation cohort (Table S2).
Fig. 2 shows the calibration of observed versus predicted survival probabilities in the validation cohort. Observed survival almost lay within a 10 per cent margin of error around the predicted survival for both mutant RAS and wild-type RAS disease. Harrell's concordance statistic was 0.644 (s.e. 0.028) in patients with RAS mutation and 0.624 (0.026) in those with wild-type RAS.
Discussion
The present prognostic model was able to predict survival probabilities in patients undergoing resection of colorectal liver metastases based on largest diameter and number of colorectal liver metastases along with RAS mutation status. The model used diameter and number of colorectal liver metastases as continuous variables rather than dichotomous variables. RAS wild-type status was associated with better overall survival than mutant RAS status, even if liver resection was performed for larger and/or multiple colorectal liver metastases.
The most important finding of this study is that the impact of diameter and number of colorectal liver metastases on prognosis differed between patients with mutant RAS and those with wild-type RAS disease (Fig. 1). The performance of the model was good, as the observed survival corresponded closely with the predicted survival in the development cohort (Fig. 2), and mostly lay within a 10 per cent margin of error in an external validation cohort that had characteristics different from the development cohort (Fig. 2). The heterogeneity of prehepatectomy chemotherapy use in the external multi-international validation cohort (35.4–73.9 per cent) may confirm the appropriateness of the present model, irrespective of the effect of chemotherapy on diameter of colorectal liver metastases.
The present three-covariable model relies on variables that are widely and readily available, making it a useful tool that can be used by oncologists and surgeons in various clinical settings. An earlier study15 showed that, when decision-making was based purely on radiological evidence, approximately 60 per cent of patients with colorectal liver metastasis deemed unresectable by non-specialists were considered to have potentially resectable disease by specialist liver surgeons. Individualized survival probabilities after resection of colorectal liver metastasis provided by the present model based on diameter and number of colorectal liver metastasis might narrow the gap between non-specialists and specialist liver surgeons in decision-making regarding resectability.
The Cox multivariable proportional hazards model suggested that age, primary lymph node metastasis, and prehepatectomy chemotherapy may improve prediction of survival compared with the three-co-variable model. However, barriers exist to the broad application of primary lymph node status and prehepatectomy chemotherapy. At MD Anderson Cancer Center, colorectal liver metastasis-first or simultaneous approaches were used in approximately half of patents with synchronous colorectal liver metastasis treated between 2005 and 200933. For these patients, it is not known whether primary lymph node metastasis is present before resection of colorectal liver metastasis. With respect to prehepatectomy chemotherapy, it can be difficult to interpret the impact reliably because of the heterogeneity of the chemotherapy protocols employed and the lack of randomization. In addition, the improvement in discriminative ability for the six-co-variable model compared with the three-co-variable model is minimal. A model adding age alone (4-co-variable model) performs slightly better than the three-co-variable model in patients with RAS mutation (Harrell’s concordance statistic 0.630, s.e. 0.021), but slightly worse than the three-co-variable model in patients with wild-type RAS (0.620, s.e. 0.022).
The present model performed better than Fong’s model4 (Harrell’s concordance statistic 0.563), the tumour burden score model13 (0.593), and the Genetic And Morphological Evaluation (GAME) model34 (0.606).
A contour plot is a graphical depiction of a response variable as contours on a two-dimensional plane using two predictor variables plotted on the x- and y-axes. Contour plots are widely used in cartography, astronomy, meteorology, physics, and molecular biology35,36. An earlier study11,12 originally reported the contour prognostic model used here, terming it the Metroticket system for predicting survival in patients undergoing liver transplant for hepatocellular carcinoma. A recently reported prognostic tool for patients undergoing resection of colorectal liver metastasis, referred to as a tumour burden score Metro-ticket model1, is not a survival prediction model based on continuous variables of largest diameter and number of colorectal liver metastases, and is different from the previously reported model and also from the present model. The tumour burden score Metro-ticket model re-categorized the combination of largest diameter and number of colorectal liver metastases into three categories (zone 1, zone 2, and zone 3) using the Pythagorean theorem. Patients in zone 2 of that model (the area represented by 3 ≤ < 9, where CLM is colorectal liver metastasis) have wide-ranging 5-year overall survival probabilities based on the present contour prognostic model. Predicted 5-year overall survival rates for patients in zone 2 of the tumour burden score Metro-ticket model may range from 30 to 70 per cent for patients with wild-type RAS disease and from 30 to 50 per cent for those with mutant RAS disease. In contrast, the present contour prognostic model moves beyond previous systems for predicting prognosis after resection of colorectal liver metastasis, replacing the practice of categorizing patients into risk groups with an individualized risk prediction paradigm. The present contour prognostic model may help patients, oncologists, and surgeons make clinical decisions before and after resection of colorectal liver metastasis.
This study has several limitations. First, it was a retrospective analysis of patients undergoing resection of colorectal liver metastasis. Nonetheless, the performance of the contour prognostic model was validated externally in an international multi-institutional cohort, which differed in terms of demographic and clinical characteristics. Second, assessment of RAS mutation status may not be mandatory in some institutions. However, assessment of RAS mutation status for patients with colorectal cancer is strongly recommended when anti-EGFR therapy is being considered37. Moreover, as the impact of RAS mutation on both prognosis and treatment becomes clearer27,38, it is likely that RAS testing will be performed more widely and routinely to guide medical therapy, especially for patients with metastases.
Funding
This research was supported in part by the National Institutes of Health through MD Anderson Cancer Center Support Grant CA016672.
Supplementary Material
Acknowledgements
The authors thank E. A. Vega for reviewing the data used in the study, R. Haynes for administrative support in preparation of this manuscript, and S. Deming, an employee of the Research Medical Library at MD Anderson Cancer Center, for copy-editing the manuscript.
Disclosure. The authors have no conflict of interest.
Supplementary material
Supplementary material is available at BJS online.
References
- 1. Choti MA, Sitzmann JV, Tiburi MF, Sumetchotimetha W, Rangsin R, Schulick RD. et al. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg 2002;235:759–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Abdalla EK, Vauthey JN, Ellis LM, Ellis V, Pollock R, Broglio KR. et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg 2004;239:818–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nordlinger B, Guiguet M, Vaillant JC, Balladur P, Boudjema K, Bachellier P. et al. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Francaise de Chirurgie. Cancer 1996;77:1254–1262 [PubMed] [Google Scholar]
- 4. Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH.. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 1999;230:309–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Iwatsuki S, Dvorchik I, Madariaga JR, Marsh JW, Dodson F, Bonham AC. et al. Hepatic resection for metastatic colorectal adenocarcinoma: a proposal of a prognostic scoring system. J Am Coll Surg 1999;189:291–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ueno H, Mochizuki H, Hatsuse K, Hase K, Yamamoto T.. Indicators for treatment strategies of colorectal liver metastases. Ann Surg 2000;231:59–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Malik HZ, Prasad KR, Halazun KJ, Aldoori A, Al-Mukhtar A, Gomez D. et al. Preoperative prognostic score for predicting survival after hepatic resection for colorectal liver metastases. Ann Surg 2007;246:806–814 [DOI] [PubMed] [Google Scholar]
- 8. Rees M, Tekkis PP, Welsh FK, O'Rourke T, John TG.. Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg 2008;247:125–135 [DOI] [PubMed] [Google Scholar]
- 9. Royston P, Altman DG, Sauerbrei W.. Dichotomizing continuous predictors in multiple regression: a bad idea. Stat Med 2006;25:127–141 [DOI] [PubMed] [Google Scholar]
- 10. Faraggi D, Simon R.. A simulation study of cross-validation for selecting an optimal cutpoint in univariate survival analysis. Stat Med 1996;15:2203–2213 [DOI] [PubMed] [Google Scholar]
- 11. Mazzaferro V. Results of liver transplantation: with or without Milan criteria? Liver transplantation: official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. Liver Transpl 2007;13:S44–S47 [DOI] [PubMed] [Google Scholar]
- 12. Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L. et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol 2009;10:35–43 [DOI] [PubMed] [Google Scholar]
- 13. Kawaguchi Y, Hasegawa K, Hagiwara Y, De Bellis M, Famularo S, Panettieri E. et al. Effect of Diameter and Number of Hepatocellular Carcinomas on Survival after Resection, Trans-Arterial Chemoembolization, and Ablation. Am J Gastroenterol 2021. in press. [DOI] [PubMed] [Google Scholar]
- 14. Sasaki K, Morioka D, Conci S, Margonis GA, Sawada Y, Ruzzenente A. et al. The tumor burden score: a new ‘Metro-ticket’ prognostic tool for colorectal liver metastases based on tumor size and number of tumors. Ann Surg 2018;267:132–141 [DOI] [PubMed] [Google Scholar]
- 15. Jones RP, Vauthey JN, Adam R, Rees M, Berry D, Jackson R. et al. Effect of specialist decision-making on treatment strategies for colorectal liver metastases. Br J Surg 2012;99:1263–1269 [DOI] [PubMed] [Google Scholar]
- 16. Moons KG, Altman DG, Reitsma JB, Ioannidis JP, Macaskill P, Steyerberg EW. et al. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med 2015;162:W1–W73 [DOI] [PubMed] [Google Scholar]
- 17. Vandenbroucke JP, von Elm E, Altman DG, Gotzsche PC, Mulrow CD, Pocock SJ. et al. ; STROBE Initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Ann Intern Med 2007;147:W163–W194 [DOI] [PubMed] [Google Scholar]
- 18. Kawaguchi Y, Lillemoe HA, Panettieri E, Chun YS, Tzeng CWD, Aloia TA. et al. Conditional recurrence-free survival after resection of colorectal liver metastases: persistent deleterious association with RAS and TP53 co-mutation. J Am Coll Surg 2019;229:286–.e1.–294.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kishi Y, Abdalla EK, Chun YS, Zorzi D, Madoff DC, Wallace MJ. et al. Three hundred and one consecutive extended right hepatectomies: evaluation of outcome based on systematic liver volumetry. Ann Surg 2009;250:540–548 [DOI] [PubMed] [Google Scholar]
- 20. Kawaguchi Y, Lillemoe HA, Vauthey JN.. Dealing with an insufficient future liver remnant: portal vein embolization and two-stage hepatectomy. J Surg Oncol 2019;119:594–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brouquet A, Abdalla EK, Kopetz S, Garrett CR, Overman MJ, Eng C. et al. High survival rate after two-stage resection of advanced colorectal liver metastases: response-based selection and complete resection define outcome. J Clin Oncol 2011;29:1083–1090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kawaguchi Y, Kopetz S, Lillemoe HA, Hwang H, Wang X, Tzeng CD. et al. A new surveillance algorithm after resection of colorectal liver metastases based on changes in recurrence risk and RAS mutation status. J Natl Compr Cancer Netw 2020;18:1500–1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK. et al. The Eighth Edition AJCC Cancer Staging Manual: continuing to build a bridge from a population-based to a more ‘personalized’ approach to cancer staging. CA: Cancer J Clin 2017;67:93–99 [DOI] [PubMed] [Google Scholar]
- 24. Kawaguchi Y, Kopetz S, Newhook TE, De Bellis M, Chun YS, Tzeng CD. et al. Mutation status of RAS, TP53, and SMAD4 is superior to mutation status of RAS alone for predicting prognosis after resection of colorectal liver metastases. Clin Cancer Res 2019;25:5843–5851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cejas P, Lopez-Gomez M, Aguayo C, Madero R, de Castro Carpeno J, Belda-Iniesta C. et al. KRAS mutations in primary colorectal cancer tumors and related metastases: a potential role in prediction of lung metastasis. PloS One 2009;4:e8199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tie J, Lipton L, Desai J, Gibbs P, Jorissen RN, Christie M. et al. KRAS mutation is associated with lung metastasis in patients with curatively resected colorectal cancer. Clin Cancer Res 2011;17:1122–1130 [DOI] [PubMed] [Google Scholar]
- 27. Vauthey JN, Zimmitti G, Kopetz SE, Shindoh J, Chen SS, Andreou A. et al. RAS mutation status predicts survival and patterns of recurrence in patients undergoing hepatectomy for colorectal liver metastases. Ann Surg 2013;258:619–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kawaguchi Y, Newhook TE, Tran Cao HS, Tzeng CD, Chun YS, Aloia TA. et al. Alteration of FBXW7 is associated with worse survival in patients undergoing resection of colorectal liver metastases. J Gastrointest Surg 2021;25:186–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schoenfeld D. Partial residuals for the proportional hazards regression model. Biometrika 1982;69:239–241 [Google Scholar]
- 30. Durrleman S, Simon R.. Flexible regression models with cubic splines. Stat Med 1989;8:551–561 [DOI] [PubMed] [Google Scholar]
- 31. Akaike H. A new look at the statistical model identification. IEEE Trans Automat Contr 1974;19:716–723 [Google Scholar]
- 32. Harrell FE Jr, Lee KL, Mark DB.. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 1996;15:361–387 [DOI] [PubMed] [Google Scholar]
- 33. Brouquet A, Mortenson MM, Vauthey JN, Rodriguez-Bigas MA, Overman MJ, Chang GJ. et al. Surgical strategies for synchronous colorectal liver metastases in 156 consecutive patients: classic, combined or reverse strategy? J Am Coll Surg 2010;210:934–941 [DOI] [PubMed] [Google Scholar]
- 34. Margonis GA, Sasaki K, Gholami S, Kim Y, Andreatos N, Rezaee N. et al. Genetic And Morphological Evaluation (GAME) score for patients with colorectal liver metastases. Br J Surg 2018;105:1210–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Robblee JH, Messinger J, Cinco RM, McFarlane KL, Fernandez C, Pizarro SA. et al. The Mn cluster in the S0 state of the oxygen-evolving complex of photosystem II studied by EXAFS spectroscopy: are there three di-μ-oxo-bridged Mn2 moieties in the tetranuclear Mn complex? J Am Chem Soc 2002;124:7459–7471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yamaji M, Jishage M, Meyer C, Suryawanshi H, Der E, Garzia A. et al. DND1 maintains germline stem cells via recruitment of the CCR4-NOT complex to target mRNAs. Nature 2017;543:568–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sepulveda AR, Hamilton SR, Allegra CJ, Grody W, Cushman-Vokoun AM, Funkhouser WK. et al. Molecular biomarkers for the evaluation of colorectal cancer: guideline from the American Society for Clinical Pathology, College of American Pathologists, Association for Molecular Pathology, and the American Society of Clinical Oncology. J Clin Oncol 2017;35:1453–1486 [DOI] [PubMed] [Google Scholar]
- 38. Chun YS, Passot G, Yamashita S, Nusrat M, Katsonis P, Loree JM. et al. Deleterious effect of RAS and evolutionary high-risk TP53 double mutation in colorectal liver metastases. Ann Surg 2019;269:917–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.