Abstract
The phenotypic heterogeneity and functional diversity of macrophages confer on them complexed roles in the development and progression of kidney diseases. After kidney injury, bone marrow-derived monocytes are rapidly recruited to the glomerulus and tubulointerstitium. They are activated and differentiated on site into pro-inflammatory M1 macrophages, which initiate Th1-type adaptive immune responses and damage normal tissues. In contrast, anti-inflammatory M2 macrophages induce Th2-type immune responses, secrete large amounts of TGF-β and anti-inflammatory cytokines, transform into αSMA+ myofibroblasts in injured kidney, inhibit immune responses, and promote wound healing and tissue fibrosis. Previous studies on the role of macrophages in kidney fibrosis were mainly focused on inflammation-associated injury and injury repair. Apart from macrophage-secreted profibrotic cytokines, such as TGF-β, evidence for a direct contribution of macrophages to kidney fibrosis is lacking. However, under inflammatory conditions, Wnt ligands are derived mainly from macrophages and Wnt signaling is central in the network of multiple profibrotic pathways. Largely underinvestigated are the direct contribution of macrophages to profibrotic signaling pathways, macrophage phenotypic heterogeneity and functional diversity in relation to kidney fibrosis, and on their cross-talk with other cells in profibrotic signaling networks that cause fibrosis. Here we aim to provide an overview on the roles of macrophage phenotypic and functional diversity in their contribution to pro-fibrotic signaling pathways, and on the therapeutic potential of targeting macrophages for the treatment of kidney fibrosis.
Keywords: macrophages, fibrosis, signaling pathways, TGF-β, Wnt
Introduction
Kidney fibrosis is an inevitable outcome of all progressive chronic kidney diseases (CKD), including hypertensive, diabetic, and vascular nephropathy. Chronic inflammation is a direct cause of kidney injury. Chronic inflammation leads to excessive kidney repair and consequent kidney fibrosis and thereby failure of kidney function. Macrophages have long been known to be master players in inflammatory kidney diseases and to be associated with the development of kidney fibrosis in CKD. However, evidence for a direct contribution of macrophages to kidney fibrosis is lacking. Here we summarize the biological and pathological functions of macrophages polarized during the course of disease progression and their role in the development of kidney fibrosis in CKD, in particular, their contribution to profibrotic signaling networks.
Macrophage as a Master Player in Kidney Fibrosis
Macrophages are an important part of the mononuclear phagocyte system comprising monocytes, macrophages, and dendritic cells (Viehmann et al., 2018). Mouse F4/80 or human epidermal growth factor module-containing mucin-like receptor 1 (EMR1) are considered to be signature markers of macrophage (Khazen et al., 2005). Macrophages are primarily responsible for pathogen clearance and the repair of injured tissues (Rosenberger and Finlay, 2003; Das et al., 2015). They are multifunctional cells with great phenotypic plasticity serving at the frontier of innate immune defenses. Kidney macrophages include long-lived tissue-resident macrophages and macrophages derived from circulating monocytes of bone marrow origin (Tang et al., 2019). With functional diversity depending on the local microenvironment, macrophages play a critical role in inflammatory kidney disease (Wang and Harris, 2011).
Kidney fibrosis develops in a milieu of inflammatory cell infiltration, mesenchymal cell proliferation and activation, and progressive deposition of extracellular matrix (ECM), leading to scar formation (fibrosis) that destroys the parenchymal structure of kidney and causes progressive loss of kidney function. Observations from human CKD and experimental CKD models have shown that tubulointerstitial fibrosis is an essential feature of chronic kidney failure, and the degree of macrophage infiltration is directly associated with the severity of fibrosis (Yu et al., 2010). Accumulation of kidney macrophages correlates with severity of kidney injury and kidney fibrosis in human and experimental diabetic nephropathy (Chow et al., 2004) and also in other classically non-inflammatory kidney diseases. The infiltration of monocytes expressing chemokine (C-C motif) receptor 2 (CCR2) leads to kidney inflammation and fibrosis in murine chronic obstructive nephropathy (Braga et al., 2018). Kidney macrophage numbers and chemokine (C-C motif) ligand 2 (CCL2) levels correlate significantly with the progression of interstitial fibrosis in human CKD (Eardley et al., 2008). Moreover, selective depletion of macrophages reduces kidney fibrosis (Furuta et al., 1993). These studies support a role for macrophages in genesis and progression of kidney inflammation and fibrosis.
In CKD, macrophages polarize to various phenotypes in response to complex microenvironmental stimuli in diseased kidneys. Macrophages of different phenotypes secrete a variety of growth factors, cytokines, proteins, and enzymes which contribute to or mitigate fibrosis (Eddy and Neilson, 2006). Macrophages produce profibrotic mediators including TGF-β, Wingless and Int-1 (Wnt), platelet-derived growth factor (PDGF), tumor necrosis factor α (TNF-α), hepatocyte growth factor (HGF), connective tissue growth factor (CTGF), angiotensin converting enzyme (ACE), angiotensin I (Ang I) and II (Ang II), plasminogen activators, plasminogen activator inhibitor-1 (PAI-1), tissue inhibitor of metalloproteinases (TIMP), collagen, fibronectin, thrombospondin, coagulation factors, reactive oxygen species, and endothelin. They can also produce mediators that protect against kidney fibrosis including collagenases, matrix metalloproteinase 12 (MMP-12), nitric oxide, and bone morphogenic protein-7 (BMP-7) (Eddy, 2011). Macrophages of various phenotypes are therefore responsible for several key processes in progressive fibro-inflammatory kidney disease, including initiation of inflammatory damage, resolution of inflammation, phagocytotic clearance of debris after inflammation, tissue repair, remodeling of fibrotic tissue, and excessive repair leading to irreversible kidney fibrosis. Thus, macrophages play very complex roles in kidney fibrosis (Ricardo et al., 2008; Shen et al., 2014; Table 1).
TABLE 1.
Macrophage phenotypes, stimuli, Secreted products, and functions.
PAMPs, pathogen-associated molecular patterns; DAMPs, danger- associated molecular patterns; FGF2, basic fibroblast growth factor; LPS, lipopolysaccharide; TLR, toll-like receptors; TNF, tumor necrosis factor; iNOS, inducible nitric oxide synthase; MINCLE, macrophage-inducible C-type lectin; Arg1, arginase-1; MCHII, major histocompatibility complex (MHC) class II; MR, mannose receptor; IGF-1, insulin like growth factor; IRF, interferon-related factor; IL-1R, IL-1 receptor; TGF-β, transforming growth factor-β; Wnt, Wingless and Int-1; RAS, renin angiotensin system.
Macrophage Contributions to Kidney Fibrosis Via Inflammation
Inflammation, starting from recruitment and activation of macrophages, is considered to be a key factor behind fibrotic diseases (Cao et al., 2015). Macrophages are rapidly recruited to the glomerulus or tubulointerstitium to initiate innate immune responses and play important defensive as well as destructive roles in kidney injury. Ongoing kidney damage can cause continuing macrophage infiltration in a vicious cycle that leads to destruction of the normal kidney tissue structure and irreversible tissue fibrosis. Although it is widely believed that glomerular and interstitial macrophages are closely associated with development of kidney fibrosis, they also play beneficial roles in stromal remodeling during tissue repair (Ricardo et al., 2008; Alikhan and Ricardo, 2013). It is important to understand the complex roles of macrophages in kidney inflammation and fibrosis.
Inflammatory Role of M1 Macrophages
The ability of macrophages to play complex roles in kidney diseases is explained by their phenotypic heterogeneity and functional diversity (Anders and Ryu, 2011). Macrophages are activated and differentiated under specific microenvironmental conditions into two broad phenotypes, namely classically activated macrophages (CAM or M1) and alternatively activated macrophages (AAM or M2) (Figure 1). However, the concept of M1 and M2 macrophage phenotypes was mostly derived from in vitro observations of cultured macrophages. Such distinct M1 and M2 macrophage phenotypes are not consistent with in vivo observations, where M1 and M2 markers can co-exist on same macrophage (Wang et al., 2014). We use the terms of M1 and M2 macrophage phenotypes in this review for the convenience in citing respective studies and for description of functionally different macrophages. The existence of such heterogeneous phenotypes is explained by the cellular plasticity of circulating monocytes and macrophages in response to different stimuli. There is compelling evidence that the major factor determining kidney injury versus tissue restoration is the activation state of macrophages within local tissues rather than the degree of macrophage infiltration (Ricardo et al., 2008).
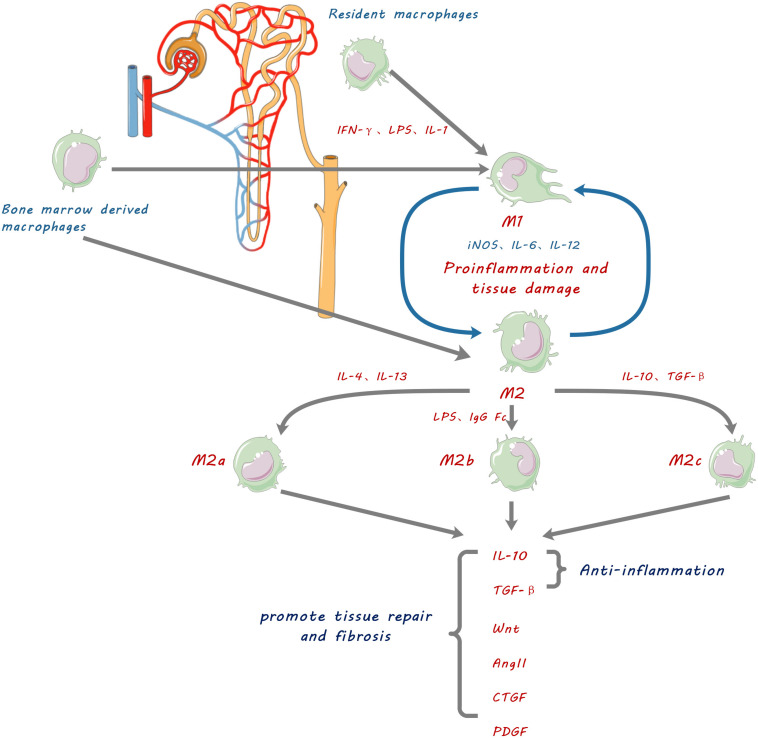
FIGURE 1.
Schematic diagram showing macrophage contribution to kidney fibrosis. Kidney resident macrophages and infiltrating bone marrow-derived macrophages are stimulated by inflammatory factors such as IFN-γ, lipopolysaccharide (LPS), and IL-1, to be polarized into pro-inflammatory M1 macrophages which promote inflammation and tissue damage by releasing IL-6, 12 and inducible nitric oxide synthase (iNOS). They can also be polarized into three functionally different phenotypes of anti-inflammatory and reparative M2 macrophages by anti-inflammatory cytokines IL-4, IL-10, IL-13, and TGF-β. M2 macrophages resolve inflammation, promote tissue repair and cause fibrosis by secretion of anti-inflammatory cytokines and tissue repair mediators including IL-10, transforming growth factor.(TGF-β), Wingless and Int-1 (Wnts), Angiotensin II (Ang II), connective tissue growth factor (CTGF), and platelet-derived growth factor (PDGF).
Circulating monocytes are recruited by cytokines and chemoattractants within the pathogenic microenvironment of diseased kidneys. They adhere to activated endothelial surfaces, infiltrate into interstitial and/or glomerular compartments, and differentiate into pro-inflammatory M1 macrophages (Tang et al., 2019). M1 macrophages can be polarized by pathogen-related molecular patterns (PAMPs) such as lipopolysaccharides (LPS), alarmins such as S100A9 and IL-1α, and pro-inflammatory cytokines such as tumor necrosis factor α (TNF-α) (Kalish et al., 2015; Venturin et al., 2016). Polarized M1 macrophages highly express major histocompatibility complex (MHC) class II and co-stimulating molecule CD86 and initiate Th1 type adaptive immune responses, resulting in cytotoxicity and more effective killing of bacteria, intracellular pathogens and tumor cells (Lv et al., 2017; Tang et al., 2019). Concurrently, M1 macrophages secrete a series of pro-inflammatory factors (including IL-1, IL-6, IL-12, TNF-α), chemokines (such as IL-8), activated oxygen species, and nitric oxide (NO) which promote inflammation and damage of normal tissues (Inoue, 2017; Tang et al., 2019).
In the early stage of kidney ischemia-reperfusion injury (IRI) in rats, macrophages are M1 in phenotype and highly express iNOS (Huen and Cantley, 2015). Depletion of macrophages at this stage by liposome clodronate significantly attenuated kidney injury, accompanied with decreased expression of inflammatory and profibrotic cytokines (Ko et al., 2008). Similarly, miR-30c-5p agomir which directly inhibits Interferon regulatory factor 1 (IRF1) reduced kidney ischemic injury by reducing M1 macrophages and increasing of M2 macrophages, and by reducing inflammatory cytokine TNF-α and increasing anti-inflammatory cytokines IL-4 and IL-10 (Zhang et al., 2019; Guo et al., 2020). In contrast, transfusion of IFN- induced M1 macrophages following acute kidney IRI increased tubulointerstitial fibrosis and functional impairment (Lee et al., 2011).
Apart from inflammatory cytokines, activated macrophages also secrete matrix metalloproteinases (MMPs), including abundant MMP- 1, −3, −7, −9, −10, −12, −14, and −25 with less abundant MMP-2, 3, 8, 10, 11, 12 (Huang et al., 2012). Those MMPs contribute not only to degradation of extracellular matrix, but also to inflammatory injury in kidney (Kunugi et al., 2011). Macrophage-derived MMP-9 has been shown to contribute to kidney fibrosis through induction of profibrotic changes in tubular epithelial cells (Tan et al., 2010) and recruitment of macrophages via proteolytic activation of osteopontin (Tan et al., 2013). More importantly, MMP-mediated proteolytic releasing and activation of TGF-β bound to extracellular matrix (Karsdal et al., 2002) may directly contribute to kidney fibrosis and indirectly through induction of profibrotic M2 macrophages.
In addition to promotion of inflammation and tissue damage, pro-inflammatory M1 macrophages were found also to be capable of switching to anti-inflammatory and reparative M2 macrophages (Arnold et al., 2007). Thus, the classification of M1 and M2 macrophage phenotypes may well represent but oversimplify the plastic functional status of macrophages at different stages of disease progression.
Anti-inflammatory and Pro-fibrotic Roles of M2 Macrophages
Alternatively activated macrophages, M2 macrophages, can be defined from in vitro experiments into three functional subtypes according to their activation stimuli and functions: M2a, M2b, and M2c (Tang et al., 2019; Figure 1). M2a macrophages are typically induced by IL-4 and IL-13 (Zhang et al., 2017); M2b macrophages are induced by immune complexes, LPS, IgG Fc receptor ligands, and CD40 (Lisi et al., 2014); M2c macrophages are induced by IL-10 and TGF-β or glucocorticoids (Kim et al., 2015). Those phenotypic definitions of anti-inflammatory M2 macrophages are used for the convenience in description of their respective functions.
The subtypes of M2 macrophages are thought to suppress immune responses and promote tissue repair, but with different and sometimes controversial functions (Mantovani et al., 2004). M2a macrophages, highly express the marker arginase 1 (Arg-1), produce a large amounts of anti-inflammatory IL-10 and IL-1 receptor antagonist (IL-1ra), and inhibit secretion of pro-inflammatory cytokines (IL-12, IL-1, TNF-α) and production of NO, thereby exerting anti-inflammatory and immunosuppressive functions. M2b macrophages specifically up-regulate IL-10 and down-regulate IL-12, and induce T cells to secrete IL-4, which in turn promotes B cells to produce antibodies, and induce anti-inflammatory Th2 immune responses. M2c macrophages secrete large amounts of IL-10 and TGF-β, suppress inflammatory immune responses, and promote wound healing and tissue fibrosis (Tang et al., 2017, 2019). Supporting evidence includes that reduced infiltration of macrophages (mainly M2) in murine models of kidney disease can prevent progressive interstitial collagen deposition and inhibit kidney fibrosis (Kim et al., 2015). Furthermore, the adoptive transfer of M2c macrophages rather than M1 macrophages reversed the beneficial effects of macrophage depletion in kidney fibrosis (Tang et al., 2019). In the unilateral ureteral obstruction (UUO) model, depletion of macrophages from day 4 significantly reduced kidney fibrosis, while the adoptive transfer of M2 macrophages promoted the accumulation of αSMA+ cells and kidney fibrosis (Shen et al., 2014). In a rat model of anti-glomerular basement membrane disease, inhibition of M2 macrophage infiltration by inhibitor of the macrophage-specific c-fms receptor at days 14–35 resulted in a significant reduction in both glomerular sclerosis and interstitial fibrosis (Han et al., 2013). Consistent with findings from experimental animal models, the number of M2 macrophages expressing CD206 and/or CD163 is associated with kidney interstitial fibrosis and tubular atrophy in human kidney diseases such as diabetic nephropathy, IgA nephropathy, and in kidney transplants (Wu et al., 2020). Together these findings indicate that M2 macrophage polarization and infiltration can promote kidney fibrosis and progression of kidney disease. However, in acute or non-persistent kidney injuries such as acute tubular necrosis (ATN), M2 macrophages were mainly anti-inflammatory and promoted epithelial healing and rapid regeneration of intact tubules (Anders and Ryu, 2011).
M2 macrophages were thought to promote kidney fibrosis via secretion of TGF-β1 which is well-known to cause fibrosis; larger quantities of TGF-β1 were detected in M2 macrophages than in myofibroblasts in the UUO model (Shen et al., 2014). However, macrophage-specific deletion of TGF-β1 failed to prevent renal fibrosis after severe ischemia-reperfusion or obstructive injury (Huen et al., 2013). In contrast, selective deletion of TGF-β receptor II (TβRII) in monocytes/macrophages promoted kidney fibrosis by enhancing renal macrophage infiltration (Chung et al., 2018). These controversial findings suggested that it would be too simplistic to conclude or disprove profibrotic roles of macrophage TGF-β1 by selective depletion of either TGF-β1 or its receptor (TβRII) alone, given that TGF-β1 is also the most potent anti-inflammatory factor secreted by M2 macrophages (Ricardo et al., 2008), and inflammation is unarguably the initial cause of kidney fibrosis (Tang et al., 2019). We found that by alteration of TGF-β1 signaling in bone marrow-derived macrophages via shifting β-catenin binding from TCF to Foxo1 using β-catenin/TCF inhibitor ICG-001, the anti-inflammatory function of TGF-β1 was enhanced by increased production of anti-inflammatory IL-10 and reduced production of IL-6 and TNF-α in the bone marrow-derived macrophages. Concurrently the pro-fibrotic effect of TGF-β1 was abolished by significant reduction of GFP (+) F4/80 (+) α-SMA (+) bone marrow-derived macrophages undergoing macrophage-myofibroblast transformation (MMT) (Wang et al., 2017) and thereby kidney fibrosis was reduced in the murine model of unilateral ureteral obstruction (UUO) (Yang et al., 2019).
In addition to TGF-β1, M2 macrophage polarization is also tightly regulated by the Wnt pathway. Wnt5a can enhance TGF-β-induced macrophage M2 polarization and the expression of Yes-associated protein (Yap)/transcriptional coactivator with PDZ-binding motif (Taz) to promote kidney fibrosis (Feng et al., 2018a). The Wnt ligand Wnt3a induces the polarization of M2 macrophages by enhancing IL-4 or TGF-β1 (Feng et al., 2018b). Conditional deletion of Wnt3a in bone marrow cells lessens the accumulation of macrophages and the polarization of M2, and reduces kidney fibrosis in the murine UUO model (Feng et al., 2018b).
Bone Marrow Macrophage Contribution to Kidney Fibrosis
Bone marrow-derived monocytes are recruited to the kidney after injury. They constitute a large proportion of interstitial infiltrating macrophages (Tang et al., 2019) and play a major role in progression of kidney fibrosis as they polarize to macrophages of various phenotypes (Conway et al., 2020). Bone marrow-derived macrophages can differentiate into α-SMA+ myofibroblasts in injured kidney, via MMT (Ikezumi et al., 2015; Wang et al., 2017). Flow cytometric analysis found that most CD45+ leukocytes isolated from obstructed kidneys expressed both collagen I and α-SMA (Chen et al., 2011). The CD45+ cells in these fibrotic kidneys are infiltrating monocytes derived from bone marrow. They have undergone MMT and transdifferentiated into collagen-producing myofibroblasts within the microenvironment of the damaged kidney, driven by TGF-β1 (Nikolic-Paterson et al., 2014) secreted by M2 macrophages (Shen et al., 2014). In vitro TGF-β1 drove transdifferentiation of cultured macrophages into collagen-secreting α-SMA+ myofibroblasts (Pilling and Gomer, 2012). Cells expressing macrophage marker CD68 and myofibroblast marker α-SMA+ have been identified in the kidney of patients with active fibrosis (Meng et al., 2016b). Nikolic-Paterson et al. (2014) found evidence of MMT in human kidney disease with active fibrosis using confocal microscopy, and showed that the severity of kidney fibrosis correlated with the number of MMT cells co-expressing α-SMA and CD68. In addition to TGF-β1, chemokine receptor CXCR6 contributes to recruitment of bone marrow-derived fibroblast precursors (Xia et al., 2014), while IL-4 and IL-13 activated Jak3/STAT6 signaling stimulates bone marrow–derived fibroblast MMT in the UUO model of kidney fibrosis (Yan et al., 2015; Liang et al., 2017).
The contribution of bone marrow-derived macrophages to kidney fibrosis is also supported by the observation that down-regulation of CCR2 expression reduced recruitment and activation of myeloid derived macrophages and alleviated kidney fibrosis in UUO model (Jiang et al., 2019). Production of chemokine CXCL16 by kidney tubular epithelial cells is necessary for recruitment of myeloid derived CD45+ col I+ α-SMA+ cells and development of kidney fibrosis in UUO model (Chen et al., 2011; Nikolic-Paterson et al., 2014).
Macrophage Contribution to Profibrotic Signaling Pathways
Kidney fibrosis is the direct result of activation of fibroblasts and accumulation of myofibroblasts, driven by multiple profibrotic signaling pathways (Kuppe et al., 2021). Profibrotic changes in other cells, including mesenchymal transition of tubular epithelial cells (EMT) (Zheng et al., 2009; Tan et al., 2010; Qiao et al., 2018; Yang et al., 2020; Rao et al., 2021) and endothelial cells (EndoMT) (Zeisberg et al., 2008; Li et al., 2010; LeBleu et al., 2013; Zhao et al., 2016), also contribute to the activation of fibroblasts and kidney fibrosis, but may not directly transform into myofibroblasts (Kuppe et al., 2021).
Wnt/β-Catenin Signaling Pathway
The Wnt/β-catenin signaling pathway is activated in various kidney diseases, contributing to the development and progression of kidney fibrosis (Zhou et al., 2020). Wnt/β-catenin signaling is an evolutionarily conserved pathway involved in embryonic development, tissue homeostasis, and organ injury repair (Ng et al., 2019; Perugorria et al., 2019). Wnt ligands are a large family of secreted glycoproteins and fundamentally indispensable for transduction of the Wnt signaling pathway (Nie et al., 2020).
Wnt/β-catenin signaling in kidney disease is versatile; transient activation of Wnt/β-catenin signaling induces repair and regeneration during acute kidney injury, but sustained (uncontrolled) Wnt/β-catenin activation promotes kidney fibrosis (Schunk et al., 2021). Lin et al. (2010) found that Wnt7b secreted by macrophages facilitates kidney regeneration through directing epithelial cell-cycle progression and basement membrane repair; kidney injury repair was substantially retarded after macrophage specific deletion of Wnt7b.
In kidney, Wnt5a promotes fibrosis by stimulating Yap/Taz-mediated macrophage polarization in both UUO and IRI models (Feng et al., 2018a). Wnt3a can also promote M2 macrophage polarization induced by IL-4 or TGF-β1, following Wnt/β-catenin signaling activation, and in turn accelerate macrophage proliferation and accumulation, giving rise to kidney fibrosis (Cosin-Roger et al., 2019; Figure 2).
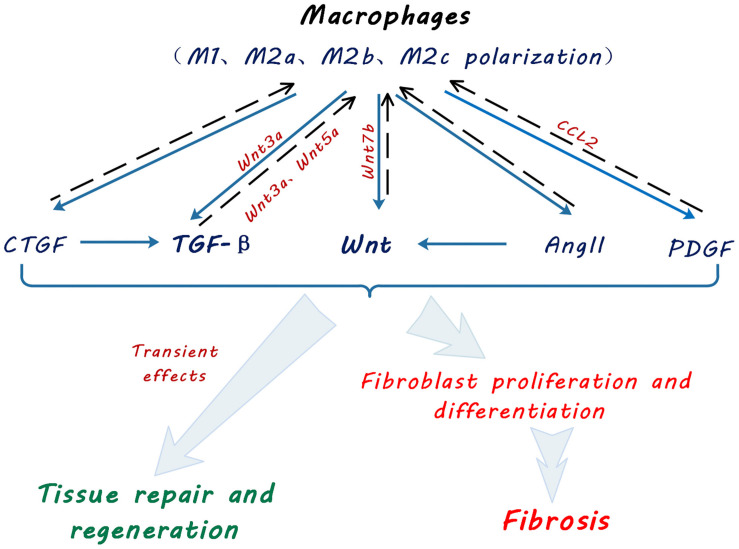
FIGURE 2.
Schematic diagram showing macrophage contribution to profibrotic signaling pathways. Macrophages promote tissue repair, regeneration and fibroblast activation and myofibroblast proliferation via multiple signaling pathways by secretion of transforming growth factor beta (TGF-β), Wingless and Int-1 (Wnts), angiotensin II (Ang II), connective tissue growth factor (CTGF) and platelet-derived growth factor (PDGF). Solid arrows indicate secretion of cytokines and growth factors by macrophages. Broken line arrows indicate autocrine effects of macrophage-secreted cytokines and growth factors on macrophage functional polarization. Light blue arrows indicate effects of macrophage secreted cytokines and growth factors on kidney tissue repair and fibrosis.
Studies of fibrosis in other organs also demonstrated macrophage contribution to the Wnt/β-catenin pathway. After myocardial infarction in mice, macrophages within the area of infarction exhibited an increase in expression of non-canonical Wnt ligands Wnt5a and Wnt11 (Palevski et al., 2017). The activated Wnt/β-catenin signal promoted cardiac fibrosis by inducing the transition of endothelial cells and epicardial cells to a mesenchymal state, fibroblast differentiation into myofibroblasts and collagen production (Palevski et al., 2017). In a murine model of intestinal fibrosis, CD16+ macrophages expressed high levels of Wnt6, inducing intestinal fibrosis (Salvador et al., 2018). M2 macrophage release of Wnt7a promoted myofibroblast differentiation of lung resident mesenchymal stem cells, leading to lung fibrosis (Hou et al., 2018).
TGF-β Signaling Pathway
TGF-β is a well-known inducer of kidney fibrosis. While secretion of anti-inflammatory TGF-β by M2 macrophages contributes to resolution of inflammation, it also mediates kidney injury repair and causes kidney fibrosis when in excess. The mechanism by which macrophages promote kidney fibrosis through the TGF-β signaling has been extensively investigated. M1 macrophages can be reprogrammed into alternately activated M2 macrophages by anti-inflammatory cytokine stimulation (IL-10 or colony-stimulating factor 1) or upon their phagocytotic ingestion of apoptotic cells. M2 macrophages promote and coordinate the regeneration of kidney tubular cells and maintain the integrity of the kidney tubules after injury (Rogers et al., 2014). During tissue repair, M2b and M2c macrophages are mainly responsible for immunosuppression, matrix remodeling and wound healing once tissue damage has been resolved (Tang et al., 2019). In contrast, uncontrolled kidney inflammation triggers M2a macrophage polarization in the injured kidney through IL-4 and IL-13, promoting increased TGF-β1 production and kidney fibrosis (Pan et al., 2015). M2 macrophages exert anti-inflammatory effects and promote kidney fibrosis through tissue repair by producing a large amount of TGF-β1 in the UUO model (Eddy, 2005).
Renin-Angiotensin System (RAS), PDGF and CTGF Signaling Pathways
In addition to Wnt and TGF-β, macrophages are also identified as a source of components of the renin-angiotensin system (RAS), including renin, angiotensin converting enzyme (ACE), Ang I and Ang II, AT1 and AT2 receptors (Okamura et al., 1999). The RAS is known to cause kidney fibrosis through Wnt/β-catenin signaling (Miao et al., 2019; Figure 2). Other pro-fibrotic mediators such as PDGF and CTGF were also found to be produced by macrophages (Cicha et al., 2005; Eitner et al., 2008).
Integrin/ILK and Notch Signaling Pathways
Apart from direct secretion of pro-fibrotic mediators, macrophages produce matrix metalloproteinases (MMP), which not only contribute to tissue remodeling after injury, but also activate other pro-fibrotic signaling pathways such as Integrin/ILK (Zheng et al., 2009, 2016; Tan et al., 2010) and Notch (Zhao et al., 2016).
Activation and Proliferation of Myofibroblasts by Crosstalk Between Profibrotic Signaling Pathways
Activation and proliferation of myofibroblasts is a central and complex event in development of kidney fibrosis. It involves multiple signaling pathways activated by profibrotic mediators from the fibro-inflammatory microenvironment of the injured kidney. Macrophages are unarguably a major source of those mediators (Table 1). A profibrotic signaling network including TGF-β/Smad, Wnt/β-catenin, the renin- angiotensin system (RAS) and Integrin/ILK pathways cross-talk and synchronize to promote kidney fibrosis (Figure 3).
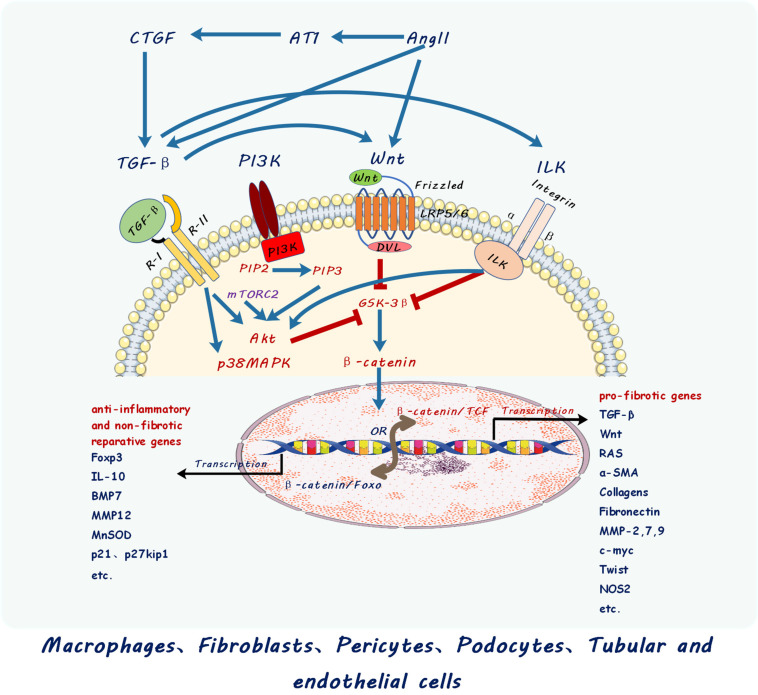
FIGURE 3.
Schematic diagram showing cross-talk between different signaling pathways including those of transforming growth factor beta (TGF-β), Wingless and Int-1 (Wnts), Renin Angiotensin System (RAS), Integrin linked kinases (ILK), connective tissue growth factor (CTGF), and PI3K–mTOR. Multiple signaling pathways cross-talk and converge at β-catenin nuclear translocation and binding with different transcription factors to activate different target genes in macrophage and kidney cells described. PI3K, Phosphatidylinositol 3 kinase; Akt, Ak strain protein kinase B; mTOR, mammalian target of rapamycin; PIP2, phosphatidylinositol (4,5)-trisphosphate; PIP3, phosphatidylinositol (3,4,5)-trisphosphate; mTORC2, mTOR complex 2 including mTOR, Rictor, GβL, Sin1, PRR5/Protor-1, and DEPTOR; Ang II, Angiotensin II; AT1, Angiotensin receptor 1.
Wnt/β-catenin signaling is a key player in kidney fibrosis contributing to activation of fibroblasts into myofibroblasts and consequent excessive extracellular matrix production. Upon binding of Wnt ligands to its receptor Frizzled (Fz) and transmembrane receptor LRP5/6, dishevelled (dvl) protein in the cytoplasm is phosphorylated and activated to bind to Axin to antagonize GSK3β, which prevents β-catenin signaling by degradation of cytosolic β-catenin via phosphorylation and ubiquitination machinery (Tan et al., 2014; Wang Y. et al., 2018). Inhibition of GSK3β by Wnt ligands results in β-catenin nucleus translocation followed by transcriptional activation of Wnt target genes when β-catenin complexes TCF/LEF, the transcription binding partners of β-catenin (Zhou et al., 2012; Guo et al., 2019). This canonical Wnt/β-catenin signaling pathway activates a transcriptome of profibrotic inducers such as Snail/Slug, and fibrotic genes such as α smooth muscle actin (α-SMA), collagen, fibronectin and other extracellular matrix genes involved in fibroblast activation and extracellular matrix production. Importantly, Wnt/β-catenin signaling is not acting alone during the development of kidney fibrosis.
TGF-β released by M2 macrophage is also one of the most important contributors to kidney fibrosis. TGF-β signals through both Smad-dependent and Smad-independent pathways. TGF-β binds to TGF-β receptor II which sequentially complexes with TGF-β receptor I. TGF-β receptor II binding to receptor I then leads to receptor I phosphorylation of Smad2/3 which translocate into the nucleus with co-Smad4 to activate profibrotic gene transcription in kidney myfibroblasts (Meng et al., 2016a). In addition to Smad-dependent signaling in activating profibrotic genes in myofibroblasts, TGF-β also promotes β-catenin nuclear translocation through phosphorylation of β-catenin Tyr-654 and dephosphorylation of β-catenin Ser-37 and Thr-41. Furthermore, Smad-independent activation of Akt and p38 MAP kinase (Wang et al., 2011; Zhou et al., 2012; Tan et al., 2014) also subsequently inhibit GSK3β, thereby promoting β-catenin nuclear translocation and activation of Wnt/β-catenin signaling.
The renin-angiotensin system (RAS) is also known to cause hypertension and fibrosis in CKD (Floege, 2015). Macrophage secretion of RAS components [renin, angiotensin converting enzyme (ACE), Ang I and Ang II] promote synthesis and release of profibrotic factors TGF-β, CTGF, PDGF, ET1 (Wang M. et al., 2018; Zhou et al., 2020) and is a direct target of the Wnt/β-catenin pathway, causing kidney injury, and fibrosis. Reciprocally, blockade of Wnt/β-catenin by inhibition of β-catenin/TCF signaling also blocks RAS and consequent hypertension and kidney fibrosis in CKD (Floege, 2015).
Integrin/ILK are known to contribute to both glomerular and interstitial fibrosis in diseased kidneys (Liu, 2010; Zheng et al., 2016). The underlying mechanism for ILK in causing fibrotic signaling involves its direct or indirect (via activation of Akt) inhibition of GSK3β in facilitating β-catenin nuclear translocation and activation of Wnt/β-catenin signaling (Liu, 2010). We found that proximal tubular cell upregulation of ILK via the compensatory increase of α3 integrin worsened kidney fibrosis in the UUO model in proximal tubular specific E-cadherin knockout mice (Zheng et al., 2016). Importantly, ILK is downstream of TGF-β mediation of both glomerular and tubulointerstitial fibrosis in kidneys (Li et al., 2009; Kang et al., 2010). Our study demonstrated that autophagy links TGF-β/Smad signaling with β-catenin through the pY654-beta-catenin/p-Smad2/ILK pathway (Pang et al., 2016).
mTOR activation has been identified in macrophages and myofibroblasts in kidney fibrosis (Chen et al., 2012). mTORC1 activation in podocytes led to the development of glomerular crescents contributing to fibrosis of glomeruli in both experimental and human glomerulonephritis (Mao et al., 2014). mTORC2 is activated by TGF-β to transduce profibrotic signaling through mTOR activation of PI3K-Akt (Li et al., 2015) which subsequently inactivates GSK3β to facilitate β-catenin nuclear translocation and thereby activate β-catenin/TCF in the Wnt/β-catenin pathway. Macrophage polarization has been shown to be controlled by the PI3K-Akt-mTOR pathway; increased mTORC1 activity promoted M1 macrophage polarization and reduced M2 macrophage polarization (Weichhart et al., 2015). mTOR activation was observed in myofibroblasts and macrophages and inhibition of mTOR pathway by rapamycin ameliorated kidney fibrosis (Chen et al., 2012). Both TGF-β and ILK activate PI3K-Akt and thus cross-talk with mTOR, whereas mTORC2 activation of PI3K-Akt also links with the Wnt/β-catenin pathway via PI3K-Akt inhibition of GSK3β (Ching and Hansel, 2010).
Together multiple signaling pathways (TGF-β, Wnt, ILK, RAS, mTOR, etc.) interact via activation of β-catenin in the initiation and progression of kidney fibrosis. The functional status of β-catenin determines the activity of these signaling pathways and the progression or regression of kidney fibrosis. Studies from us and others demonstrated the key role for β-catenin/TCF in mediating profibrotic signaling of multiple pathways (Liu, 2010; Qiao et al., 2018). Importantly, we found that shifting β-catenin binding from TCF toward Foxo in both macrophages and kidney tubular cells by inhibition of β-catenin/TCF redirected TGF-β signaling from pro-fibrotic to anti-inflammatory, protected against kidney fibrosis and promoted epithelial repair in UUO and IRI models (Qiao et al., 2018; Rao et al., 2019, 2021; Yang et al., 2019).
Targeting Macrophages as a Treatment for Kidney Fibrosis
Anti-inflammatory and reparative properties of macrophages (Lin et al., 2010; Urbina and Singla, 2014; Ratnayake et al., 2021) argue for their therapeutic application. We have shown that ex vivo programmed M2 macrophages protect against inflammation and kidney injury in experimental models of inflammatory renal disease (Wang et al., 2007; Cao et al., 2010, 2011). Jung et al. (2012) found that infusion of IL-10 overexpressing macrophages protected ischemia injury in an IRI model. Adoptive transfer of genetically modified macrophages expressing heme-oxygenase-1 (HO-1) protected kidney function in mice with IRI (Ferenbach et al., 2010). Netrin-1-induced M2 macrophages suppressed inflammation and protected against kidney injury in IRI mice (Ranganathan et al., 2013).
However, the phenotypic instability of those M2 macrophages remains as a challenge (Cao et al., 2014). To overcome the hurdle of phenotypic instability, adenovirus vector NGAL (Neutrophil gelatinase-associated lipocalin-2) was used to stabilize phenotype of injected M2 macrophages which reduced inflammation and fibrosis in UUO model. While protection by anti-inflammatory M2 macrophages has been reported increasingly, the profibrotic effects of M2 macrophages remain largely unaddressed as another hurdle for their therapeutic application; M2 macrophages secrete large amounts of TGF-β which not only suppresses inflammation but also promotes kidney fibrosis (Kim et al., 2015).
Depletion of inflammatory M1 macrophages does not protect against kidney fibrosis, while depletion of anti-inflammatory and reparative M2 macrophages can reduce kidney fibrosis (Shen et al., 2014). Thus, although inflammation is an important driver of fibrosis, other non-inflammatory profibrotic pathways are activated by anti-inflammatory and tissue reparative cytokines from M2 macrophages such as TGF-β, Wnt, Ang II, CTGF, and PDGF. Moreover, the results of these macrophage depletion studies are consistent with the fact that M1 and M2 macrophages represent different and sometimes co-existing functional phenotypes of the same population. They polarize across their life span according to stimuli within the microenvironment in which they reside during the progression kidney diseases.
Opposing roles of phenotypically distinct macrophages suggested that targeting macrophages of different phenotypes may not be practical in developing therapeutic treatment for fibrotic diseases (Cao et al., 2014). More importantly, precise targeting of functionally different macrophages with opposing roles requires a better understanding of downstream signaling events and the diverse functions of multi-functional cytokines, such as TGF-β1 (Qiao et al., 2018), which although profibrotic contributes to suppression of inflammation and to tissue repair in kidney (Tang et al., 2019).
Instead of targeting specific functional phenotypes of macrophages, targeting a central factor in multiple profibrotic signaling pathways in macrophages is likely to be a more effective strategy for treating kidney diseases. Indeed, we found in the UUO model that inhibition of β-catenin/TCF promotes β-catenin/Foxo in the Wnt and TGF-β signaling pathways of bone marrow-derived macrophages (Yang et al., 2019). Importantly, redirection of β-catenin binding from TCF to Foxo resulted in reduction of inflammatory cytokines produced by bone marrow-derived macrophages, altered the fate of MMT macrophages and protected against kidney fibrosis (Yang et al., 2019).
Conclusion
Macrophages are master regulators of inflammation and kidney fibrosis. Monocytes and macrophages are recruited and activated in response to chemoattractants and stimuli released after kidney injury. Macrophage plasticity adds complexity to their central roles in kidney fibrosis. After kidney injury, macrophages polarize into various phenotypes in response to alteration of the microenvironment in kidney disease. M1 pro-inflammatory macrophages clear infection but also cause kidney injury; M2 anti-inflammatory macrophages contribute to resolution of inflammation and kidney repair yet cause kidney fibrosis (Tang et al., 2019). Functionally distinct macrophage phenotypes contribute to the fibro-inflammatory microenvironment by abundant secretion of inflammatory and anti-inflammatory cytokines, mediators of tissue repair including TGF-β, Wnt ligands, PDGF, CTGF as well as all components of RAS. Those tissue repair mediators are also key inducers of kidney fibrosis when secreted in excess and maintained at higher levels in the chronic inflammatory milieu of kidney disease. Profibrotic mediators activate a profibrotic signaling network by cross-talking among multiple signaling pathways including TGF-β, Wnts, RAS, intergin/ILK, mTOR. Importantly, multiple pro-fibrotic signaling pathways all converge at activation of β-catenin/TCF, making β-catenin/TCF a key target for prevention of kidney fibrosis. Switching β-catenin/TCF to β-catenin/Foxo redirects signaling from profibrotic to anti-inflammatory and protects against kidney fibrosis. Targeting macrophages has long been proposed as a treatment for fibro-inflammatory kidney diseases. However, the phenotypic plasticity and conflicting roles of M2 macrophages are major hurdles for their therapeutic application. Recently we have identified the β-catenin/TCF/Foxo axis as a key determinant of the signaling direction of multiple profibrotic pathways. Thus, targeting macrophage signaling pathway via the β-catenin/TCF/Foxo axis may provide a new promising strategy for the treatment of kidney fibrosis in chronic kidney diseases.
Author Contributions
JXu, JXie, DH, and GZ contributed to conception and design of the study. XW wrote the first draft of the manuscript. GZ, JC, JXu, DH, and JXie wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Alikhan M., Ricardo S. (2013). Mononuclear phagocyte system in kidney disease and repair. Nephrology 18 81–91. 10.1111/nep.12014 [DOI] [PubMed] [Google Scholar]
- Anders H. J., Ryu M. (2011). Renal microenvironments and macrophage phenotypes determine progression or resolution of renal inflammation and fibrosis. Kidney Int. 80 915–925. 10.1038/ki.2011.217 [DOI] [PubMed] [Google Scholar]
- Arnold L., Henry A., Poron F., Baba-Amer Y., van Rooijen N., Plonquet A., et al. (2007). Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J. Exp. Med. 204 1057–1069. 10.1084/jem.20070075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchini R., Roth-Walter F., Ohradanova-Repic A., Flicker S., Hufnagl K., Fischer M. B., et al. (2019). IgG4 drives M2a macrophages to a regulatory M2b-like phenotype: potential implication in immune tolerance. Allergy 74 483–494. 10.1111/all.13635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal A., Ehlers S., Lauber J., Buer J., Lange C., Goldmann T., et al. (2006). The wingless homolog WNT5A and its receptor Frizzled-5 regulate inflammatory responses of human mononuclear cells induced by microbial stimulation. Blood 108 965–973. 10.1182/blood-2005-12-5046 [DOI] [PubMed] [Google Scholar]
- Braga T., Correa-Costa M., Silva R., Cruz M., Hiyane M., da Silva J., et al. (2018). CCR2 contributes to the recruitment of monocytes and leads to kidney inflammation and fibrosis development. Inflammopharmacology 26 403–411. 10.1007/s10787-017-0317-4 [DOI] [PubMed] [Google Scholar]
- Cao Q., Harris D., Wang Y. (2015). Macrophages in kidney injury, inflammation, and fibrosis. Physiology (Bethesda, Md) 30 183–194. 10.1152/physiol.00046.2014 [DOI] [PubMed] [Google Scholar]
- Cao Q., Wang C., Zheng D., Wang Y., Lee V. W., Wang Y. M., et al. (2011). IL-25 induces M2 macrophages and reduces renal injury in proteinuric kidney disease. J. Am. Soc. Nephrol. 22 1229–1239. 10.1681/asn.2010070693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Q., Wang Y., Zheng D., Sun Y., Wang C., Wang X. M., et al. (2014). Failed renoprotection by alternatively activated bone marrow macrophages is due to a proliferation-dependent phenotype switch in vivo. Kidney Int. 85 794–806. 10.1038/ki.2013.341 [DOI] [PubMed] [Google Scholar]
- Cao Q., Wang Y., Zheng D., Sun Y., Wang Y., Lee V. W., et al. (2010). IL-10/TGF-beta-modified macrophages induce regulatory T cells and protect against adriamycin nephrosis. J. Am. Soc. Nephrol. 21 933–942. 10.1681/asn.2009060592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Chen H., Wang C., Peng Y., Sun L., Liu H., et al. (2012). Rapamycin ameliorates kidney fibrosis by inhibiting the activation of mTOR signaling in interstitial macrophages and myofibroblasts. PLoS One 7:e33626. 10.1371/journal.pone.0033626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Lin S., Chen J., He L., Dong F., Xu J., et al. (2011). CXCL16 recruits bone marrow-derived fibroblast precursors in renal fibrosis. J. Am. Soc. Nephrol. JASN 22 1876–1886. 10.1681/asn.2010080881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Wen Z., Xu W., Xiong S. (2013). Granulin exacerbates lupus nephritis via enhancing macrophage M2b polarization. PLoS One 2013, 8:e65542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching C. B., Hansel D. E. (2010). Expanding therapeutic targets in bladder cancer: the PI3K/Akt/mTOR pathway. Laboratory Investigat. 90 1406–1414. 10.1038/labinvest.2010.133 [DOI] [PubMed] [Google Scholar]
- Chow F., Ozols E., Nikolic-Paterson D., Atkins R., Tesch G. (2004). Macrophages in mouse type 2 diabetic nephropathy: correlation with diabetic state and progressive renal injury. Kidney Int. 65 116–128. 10.1111/j.1523-1755.2004.00367.x [DOI] [PubMed] [Google Scholar]
- Chung S., Overstreet J. M., Li Y., Wang Y., Niu A., Wang S., et al. (2018). TGF-β promotes fibrosis after severe acute kidney injury by enhancing renal macrophage infiltration. JCI Insight 3:e123563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicha I., Yilmaz A., Klein M., Raithel D., Brigstock D. R., Daniel W. G., et al. (2005). Connective tissue growth factor is overexpressed in complicated atherosclerotic plaques and induces mononuclear cell chemotaxis in vitro. Arterioscler. Thromb. Vasc. Biol. 25 1008–1013. 10.1161/01.atv.0000162173.27682.7b [DOI] [PubMed] [Google Scholar]
- Conway B. R., O’Sullivan E. D., Cairns C., O’Sullivan J., Simpson D. J., Salzano A., et al. (2020). Kidney single-cell atlas reveals myeloid heterogeneity in progression and regression of kidney disease. J. Am. Soc. Nephrol. 31 2833–2854. 10.1681/asn.2020060806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosin-Roger J., Ortiz-Masià M., Barrachina M. (2019). Macrophages as an emerging source of Wnt ligands: relevance in mucosal integrity. Front. Immunol. 10:2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A., Sinha M., Datta S., Abas M., Chaffee S., Sen C. K., et al. (2015). Monocyte and macrophage plasticity in tissue repair and regeneration. Am. J. Pathol. 185 2596–2606. 10.1016/j.ajpath.2015.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eardley K. S., Kubal C., Zehnder D., Quinkler M., Lepenies J., Savage C. O., et al. (2008). The role of capillary density, macrophage infiltration and interstitial scarring in the pathogenesis of human chronic kidney disease. Kidney Int. 74 495–504. [DOI] [PubMed] [Google Scholar]
- Eddy A. (2005). Progression in chronic kidney disease. Adv. Chronic Kidney Dis. 12 353–365. 10.1053/j.ackd.2005.07.011 [DOI] [PubMed] [Google Scholar]
- Eddy A., Neilson E. (2006). Chronic kidney disease progression. J. Am. Soc. Nephrol. JASN 17 2964–2966. [DOI] [PubMed] [Google Scholar]
- Eddy A. A. (2011). Overview of the cellular and molecular basis of kidney fibrosis. Kidney Int. Suppl. 2014 2–8. 10.1038/kisup.2014.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eitner F., Bücher E., van Roeyen C., Kunter U., Rong S., Seikrit C., et al. (2008). PDGF-C is a proinflammatory cytokine that mediates renal interstitial fibrosis. J. Am. Soc. Nephrol. 19 281–289. 10.1681/asn.2007030290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y., Liang Y., Zhu X., Wang M., Gui Y., Lu Q., et al. (2018a). The signaling protein Wnt5a promotes TGFβ1-mediated macrophage polarization and kidney fibrosis by inducing the transcriptional regulators Yap/Taz. J. Biol. Chem. 293 19290–19302. 10.1074/jbc.ra118.005457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y., Ren J., Gui Y., Wei W., Shu B., Lu Q., et al. (2018b). βWnt/-catenin-promoted macrophage alternative activation contributes to kidney fibrosis. J. Am. Soc. Nephrol. JASN 29 182–193. 10.1681/asn.2017040391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferenbach D. A., Ramdas V., Spencer N., Marson L., Anegon I., Hughes J., et al. (2010). Macrophages expressing heme oxygenase-1 improve renal function in ischemia/reperfusion injury. Mol. Ther. 18 1706–1713. 10.1038/mt.2010.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floege J. (2015). Antagonism of canonical Wnt/beta-catenin signaling: taking RAS blockade to the next level? J. Am. Soc. Nephrol. 26 3–5. 10.1681/asn.2014060567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta T., Saito T., Ootaka T., Soma J., Obara K., Abe K., et al. (1993). The role of macrophages in diabetic glomerulosclerosis. Am. J. Kidney Dis. Offi. J. Natl. Kidney Found. 21 480–485. 10.1016/s0272-6386(12)80393-3 [DOI] [PubMed] [Google Scholar]
- Guo Q., Zhong W., Duan A., Sun G., Cui W., Zhuang X., et al. (2019). Protective or deleterious role of Wnt/beta-catenin signaling in diabetic nephropathy: an unresolved issue. Pharmacol. Res. 144 151–157. 10.1016/j.phrs.2019.03.022 [DOI] [PubMed] [Google Scholar]
- Guo Q., Zhu X., Wei R., Zhao L., Zhang Z., Yin X., et al. (2020). miR-130b-3p regulates M1 macrophage polarization via targeting IRF. J. Cell. Physiol. 236 2008–2022. 10.1002/jcp.29987 [DOI] [PubMed] [Google Scholar]
- Han Y., Ma F., Tesch G., Manthey C., Nikolic-Paterson D. (2013). Role of macrophages in the fibrotic phase of rat crescentic glomerulonephritis. Am. J. Physiol. Renal. Physiol. 304 F1043–F1053. [DOI] [PubMed] [Google Scholar]
- Hou J., Shi J., Chen L., Lv Z., Chen X., Cao H., et al. (2018). M2 macrophages promote myofibroblast differentiation of LR-MSCs and are associated with pulmonary fibrogenesis. Cell Commun. Sign. CCS 16:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W.-C., Sala-Newby G. B., Susana A., Johnson J. L., Newby A. C. (2012). Classical macrophage activation up-regulates several matrix metalloproteinases through mitogen activated protein kinases and nuclear factor-κB. PLoS One 7:e42507. 10.1371/journal.pone.0042507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huen S., Cantley L. (2015). Macrophage-mediated injury and repair after ischemic kidney injury. Pediatr. Nephrol. 30 199–209. 10.1007/s00467-013-2726-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huen S. C., Moeckel G. W., Cantley L. G. (2013). Macrophage-specific deletion of transforming growth factor-β1 does not prevent renal fibrosis after severe ischemia-reperfusion or obstructive injury. Am. J. Physiol. Renal. Physiol. 305 F477–F484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikezumi Y., Suzuki T., Yamada T., Hasegawa H., Kaneko U., Hara M., et al. (2015). Alternatively activated macrophages in the pathogenesis of chronic kidney allograft injury. Pediatr. Nephrol. 30 1007–1017. 10.1007/s00467-014-3023-0 [DOI] [PubMed] [Google Scholar]
- Inoue T. (2017). M1 macrophage triggered by mincle leads to a deterioration of acute kidney injury. Kidney Int. 91 526–529. 10.1016/j.kint.2016.11.026 [DOI] [PubMed] [Google Scholar]
- Jiang Y., Wang Y., Ma P., An D., Zhao J., Liang S., et al. (2019). Myeloid-specific targeting of Notch ameliorates murine renal fibrosis via reduced infiltration and activation of bone marrow-derived macrophage. Protein Cell 10 196–210. 10.1007/s13238-018-0527-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung M., Sola A., Hughes J., Kluth D. C., Vinuesa E., Viñas J. L., et al. (2012). Infusion of IL-10-expressing cells protects against renal ischemia through induction of lipocalin-2. Kidney Int. 81 969–982. 10.1038/ki.2011.446 [DOI] [PubMed] [Google Scholar]
- Kadowaki T., Yasui Y., Takahashi Y., Kohchi C., Soma G., Inagawa H. (2009). Comparative immunological analysis of innate immunity activation after oral administration of wheat fermented extract to teleost fish. Anticancer Res. 29 4871–4877. [PubMed] [Google Scholar]
- Kalish S., Lyamina S., Usanova E., Manukhina E., Larionov N., Malyshev I. (2015). Macrophages reprogrammed in vitro towards the M1 phenotype and activated with LPS extend lifespan of mice with ehrlich ascites carcinoma. Med. Sci. Monitor Basic Res. 21 226–234. 10.12659/msmbr.895563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y. S., Li Y., Dai C., Kiss L. P., Wu C., Liu Y. (2010). Inhibition of integrin-linked kinase blocks podocyte epithelial–mesenchymal transition and ameliorates proteinuria. Kidney Int. 78 363–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsdal M. A., Larsen L., Engsig M. T., Lou H., Ferreras M., Lochter A., et al. (2002). Matrix metalloproteinase-dependent activation of latent transforming growth factor-beta controls the conversion of osteoblasts into osteocytes by blocking osteoblast apoptosis. J. Biol. Chem. 277 44061–44067. 10.1074/jbc.m207205200 [DOI] [PubMed] [Google Scholar]
- Khazen W., M’Bika J. P., Tomkiewicz C., Benelli C., Chany C., Achour A., et al. (2005). Expression of macrophage-selective markers in human and rodent adipocytes. FEBS Lett. 579 5631–5634. 10.1016/j.febslet.2005.09.032 [DOI] [PubMed] [Google Scholar]
- Kim M. G., Kim S. C., Ko Y. S., Lee H. Y., Jo S. K., Cho W. (2015). The role of M2 macrophages in the progression of chronic kidney disease following acute kidney injury. PLoS One 10:e0143961. 10.1371/journal.pone.0143961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko G., Boo C., Jo S., Cho W., Kim H. (2008). Macrophages contribute to the development of renal fibrosis following ischaemia/reperfusion-induced acute kidney injury. Nephrol. Dialy. Trans. Offi. Publ. Eur. Dialy. Trans. Assoc. Eur. Renal Assoc. 23 842–852. 10.1093/ndt/gfm694 [DOI] [PubMed] [Google Scholar]
- Kunugi S., Shimizu A., Kuwahara N., Du X., Takahashi M., Terasaki Y., et al. (2011). Inhibition of matrix metalloproteinases reduces ischemia-reperfusion acute kidney injury. Laboratory Investigat. 91 170–180. 10.1038/labinvest.2010.174 [DOI] [PubMed] [Google Scholar]
- Kuppe C., Ibrahim M. M., Kranz J., Zhang X., Ziegler S., Perales-Patón J., et al. (2021). Decoding myofibroblast origins in human kidney fibrosis. Nature 589 281–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBleu V. S., Taduri G., O’Connell J., Teng Y., Cooke V. G., Woda C., et al. (2013). Origin and function of myofibroblasts in kidney fibrosis. Nat. Med. 19 1047–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Huen S., Nishio H., Nishio S., Lee H., Choi B., et al. (2011). Distinct macrophage phenotypes contribute to kidney injury and repair. J. Am. Soc. Nephrol. JASN 22 317–326. 10.1681/asn.2009060615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Qu X., Yao J., Caruana G., Ricardo S. D., Yamamoto Y., et al. (2010). Blockade of endothelial-mesenchymal transition by a smad3 inhibitor delays the early development of streptozotocin-induced diabetic nephropathy. Diabetes 59 2612–2624. 10.2337/db09-1631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Ren J., Liu X., Jiang L., He W., Yuan W., et al. (2015). Rictor/mTORC2 signaling mediates TGFβ1-induced fibroblast activation and kidney fibrosis. Kidney Int. 88 515–527. 10.1038/ki.2015.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Tan X., Dai C., Stolz D. B., Wang D., Liu Y. (2009). Inhibition of integrin-linked kinase attenuates renal interstitial fibrosis. J. Am. Soc. Nephrol. 20 1907–1918. 10.1681/asn.2008090930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H., Zhang Z., Yan J., Wang Y., Hu Z., Mitch W. E., et al. (2017). The IL-4 receptor α has a critical role in bone marrow-derived fibroblast activation and renal fibrosis. Kidney Int. 92 1433–1443. 10.1016/j.kint.2017.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S., Li B., Rao S., Yeo E., Hudson T., Nowlin B., et al. (2010). Macrophage Wnt7b is critical for kidney repair and regeneration. Proc. Natl. Acad. Sci. U.S.A. 107 4194–4199. 10.1073/pnas.0912228107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisi L., Stigliano E., Lauriola L., Navarra P., Dello Russo C. (2014). Proinflammatory-activated glioma cells induce a switch in microglial polarization and activation status, from a predominant M2b phenotype to a mixture of M1 and M2a/B polarized cells. ASN Neuro 6 171–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. (2010). New insights into epithelial-mesenchymal transition in kidney fibrosis. J. Am. Soc. Nephrol. JASN 21 212–222. 10.1681/asn.2008121226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J., Cao Q., Zheng D., Sun Y., Wang C., Yu X., et al. (2013). Discrete functions of M2a and M2c macrophage subsets determine their relative efficacy in treating chronic kidney disease. Kidney Int. 84 745–755. 10.1038/ki.2013.135 [DOI] [PubMed] [Google Scholar]
- Lv L., Tang P., Li C., You Y., Li J., Huang X., et al. (2017). The pattern recognition receptor, Mincle, is essential for maintaining the M1 macrophage phenotype in acute renal inflammation. Kidney Int. 91 587–602. 10.1016/j.kint.2016.10.020 [DOI] [PubMed] [Google Scholar]
- Mantovani A., Sica A., Sozzani S., Allavena P., Vecchi A., Locati M. (2004). The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 25 677–686. 10.1016/j.it.2004.09.015 [DOI] [PubMed] [Google Scholar]
- Mao J., Zeng Z., Xu Z., Li J., Jiang L., Fang Y., et al. (2014). Mammalian target of rapamycin complex 1 activation in podocytes promotes cellular crescent formation. Am. J. Physiol. Renal Physiol. 307 F1023–F1032. [DOI] [PubMed] [Google Scholar]
- Meng X., Wang S., Huang X., Yang C., Xiao J., Zhang Y., et al. (2016b). Inflammatory macrophages can transdifferentiate into myofibroblasts during renal fibrosis. Cell Death Dis. 7:e2495. 10.1038/cddis.2016.402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X. M., Nikolic-Paterson D. J., Lan H. Y. (2016a). TGF-β: the master regulator of fibrosis. Nat. Rev. Nephrol. 12 325–338. 10.1038/nrneph.2016.48 [DOI] [PubMed] [Google Scholar]
- Meng X. M., Tang P. M., Li J., Lan H. Y. (2015). Macrophage phenotype in kidney injury and repair. Kidney Dis. 1 138–146. 10.1159/000431214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao J., Liu J., Niu J., Zhang Y., Shen W., Luo C., et al. (2019). Wnt/β-catenin/RAS signaling mediates age-related renal fibrosis and is associated with mitochondrial dysfunction. Aging Cell 18:e13004-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng L., Kaur P., Bunnag N., Suresh J., Sung I., Tan Q., et al. (2019). WNT signaling in disease. Cells 8:826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie X., Liu H., Liu L., Wang Y. D., Chen W. D. (2020). Emerging roles of Wnt ligands in human colorectal cancer. Front. Oncol. 10:1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolic-Paterson D., Wang S., Lan H. (2014). Macrophages promote renal fibrosis through direct and indirect mechanisms. Kidney Int. Suppl. 4 34–38. 10.1038/kisup.2014.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura A., Rakugi H., Ohishi M., Yanagitani Y., Takiuchi S., Moriguchi K., et al. (1999). Upregulation of renin-angiotensin system during differentiation of monocytes to macrophages. J. Hypertens 17 537–545. 10.1097/00004872-199917040-00012 [DOI] [PubMed] [Google Scholar]
- Palevski D., Levin-Kotler L. P., Kain D., Naftali-Shani N., Landa N., Ben-Mordechai T., et al. (2017). Loss of macrophage Wnt secretion improves remodeling and function after myocardial infarction in mice. J. Am. Heart Assoc. 6:e004387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan B., Liu G., Jiang Z., Zheng D. (2015). Regulation of renal fibrosis by macrophage polarization. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 35 1062–1069. 10.1159/000373932 [DOI] [PubMed] [Google Scholar]
- Pang M., Wang H., Rao P., Zhao Y., Xie J., Cao Q., et al. (2016). Autophagy links beta-catenin and smad signaling to promote epithelial-mesenchymal transition via upregulation of integrin linked kinase. Int. J. Biochem. Cell. Biol. 76 123–134. 10.1016/j.biocel.2016.05.010 [DOI] [PubMed] [Google Scholar]
- Perugorria M., Olaizola P., Labiano I., Esparza-Baquer A., Marzioni M., Marin J., et al. (2019). Wnt-β-catenin signalling in liver development, health and disease. Nat. Rev. Gastroenterol. Hepatol. 16 121–136. 10.1111/j.1365-2184.2012.00806.x [DOI] [PubMed] [Google Scholar]
- Philipp D., Suhr L., Wahlers T., Choi Y.-H., Paunel-Görgülü A. (2018). Preconditioning of bone marrow-derived mesenchymal stem cells highly strengthens their potential to promote IL-6-dependent M2b polarization. Stem. Cell Res. Ther. 9:286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilling D., Gomer R. (2012). Differentiation of circulating monocytes into fibroblast-like cells. Methods Mol. Biol. 904 191–206. 10.1007/978-1-61779-943-3_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao X., Rao P., Zhang Y., Liu L., Pang M., Wang H., et al. (2018). Redirecting TGF-beta Signaling through the beta-catenin/foxo complex prevents kidney fibrosis. J. Am. Soc. Nephrol. 29 557–570. 10.1681/asn.2016121362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganathan P. V., Jayakumar C., Ramesh G. (2013). Netrin-1-treated macrophages protect the kidney against ischemia-reperfusion injury and suppress inflammation by inducing M2 polarization. Am. J. Physiol. Renal Physiol. 304 F948–F957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao P., Pang M., Qiao X., Yu H., Wang H., Yang Y., et al. (2019). Promotion of beta-catenin/Foxo1 signaling ameliorates renal interstitial fibrosis. Lab Invest 99 1689–1701. 10.1038/s41374-019-0276-z [DOI] [PubMed] [Google Scholar]
- Rao P., Qiao X., Hua W., Hu M., Tahan M., Chen T., et al. (2021). Promotion of β-catenin/Foxo signaling mediates epithelial repair in kidney injury. Am. J. Pathol. 191 993–1009. 10.1016/j.ajpath.2021.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratnayake D., Nguyen P. D., Rossello F. J., Wimmer V. C., Tan J. L., Galvis L. A., et al. (2021). Macrophages provide a transient muscle stem cell niche via NAMPT secretion. Nature 591 281–287. 10.1038/s41586-021-03199-7 [DOI] [PubMed] [Google Scholar]
- Ricardo S., van Goor H., Eddy A. (2008). Macrophage diversity in renal injury and repair. J. Clin. Investigat. 118 3522–3530. 10.1172/jci36150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers N., Ferenbach D., Isenberg J., Thomson A., Hughes J. (2014). Dendritic cells and macrophages in the kidney: a spectrum of good and evil. Nat. Rev. Nephrol. 10 625–643. 10.1038/nrneph.2014.170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberger C. M., Finlay B. B. (2003). Phagocyte sabotage: disruption of macrophage signalling by bacterial pathogens. Nat. Rev. Mol. Cell Biol. 4 385–396. 10.1038/nrm1104 [DOI] [PubMed] [Google Scholar]
- Salvador P., Macías-Ceja D., Gisbert-Ferrándiz L., Hernández C., Bernardo D., Alós R., et al. (2018). CD16+ macrophages mediate fibrosis in inflammatory bowel disease. J. Crohn’s Colitis 12 589–599. 10.1093/ecco-jcc/jjx185 [DOI] [PubMed] [Google Scholar]
- Schunk S. J., Floege J., Fliser D., Speer T. (2021). WNT–β-catenin signalling a versatile player in kidney injury and repair. Nat. Rev. Nephrol. 17 172–184. 10.1038/s41581-020-00343-w [DOI] [PubMed] [Google Scholar]
- Shen B., Liu X., Fan Y., Qiu J. (2014). Macrophages regulate renal fibrosis through modulating TGFβ superfamily signaling. Inflammation 37 2076–2084. 10.1007/s10753-014-9941-y [DOI] [PubMed] [Google Scholar]
- Tan R., Zhou D., Zhou L., Liu Y. (2014). Wnt/β-catenin signaling and kidney fibrosis. Kidney Int. Suppl. 4 84–90. 10.1038/kisup.2014.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan T. K., Zheng G., Hsu T. T., Lee S. R., Zhang J., Zhao Y., et al. (2013). Matrix metalloproteinase-9 of tubular and macrophage origin contributes to the pathogenesis of renal fibrosis via macrophage recruitment through osteopontin cleavage. Lab Invest 93 434–449. 10.1038/labinvest.2013.3 [DOI] [PubMed] [Google Scholar]
- Tan T. K., Zheng G., Hsu T. T., Wang Y., Lee V. W., Tian X., et al. (2010). Macrophage matrix metalloproteinase-9 mediates epithelial-mesenchymal transition in vitro in murine renal tubular cells. Am. J. Pathol. 176 1256–1270. 10.2353/ajpath.2010.090188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang L., Zhang H., Wang C., Li H., Zhang Q., Bai J. (2017). M2A and M2C macrophage subsets ameliorate inflammation and fibroproliferation in acute lung injury through interleukin 10 pathway. Shock 48 119–129. 10.1097/shk.0000000000000820 [DOI] [PubMed] [Google Scholar]
- Tang P. M., Nikolic-Paterson D. J., Lan H. Y. (2019). Macrophages: versatile players in renal inflammation and fibrosis. Nat. Rev. Nephrol. 15 144–158. 10.1038/s41581-019-0110-2 [DOI] [PubMed] [Google Scholar]
- Tseng W., Tsai M., Chen N., Tarng D. (2020). Trichostatin a alleviates renal interstitial fibrosis through modulation of the M2 macrophage subpopulation. Int. J. Mol. Sci. 21:5966. 10.3390/ijms21175966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich C., Seibert E., Heine G. H., Fliser D., Girndt M. (2011). Monocyte angiotensin converting enzyme expression may be associated with atherosclerosis rather than arteriosclerosis in hemodialysis patients. Clin. J. Am. Soc. Nephrol. 6 505–511. 10.2215/cjn.06870810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbina P., Singla D. K. (2014). BMP-7 attenuates adverse cardiac remodeling mediated through M2 macrophages in prediabetic cardiomyopathy. Am. J. Physiol. Heart Circ. Physiol. 307 H762–H772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venturin G., Chiku V., Silva K., de Almeida B., de Lima V. (2016). M1 polarization and the effect of PGE on TNF-α production by lymph node cells from dogs with visceral leishmaniasis. Parasite Immunol. 38 698–704. 10.1111/pim.12353 [DOI] [PubMed] [Google Scholar]
- Viehmann S. F., Böhner A. M. C., Kurts C., Brähler S. (2018). The multifaceted role of the renal mononuclear phagocyte system. Cell Immunol. 330 97–104. 10.1016/j.cellimm.2018.04.009 [DOI] [PubMed] [Google Scholar]
- Wang D., Dai C., Li Y., Liu Y. (2011). Canonical Wnt/β-catenin signaling mediates transforming growth factor-β1-driven podocyte injury and proteinuria. Kidney Int. 80 1159–1169. 10.1038/ki.2011.255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Melton D. W., Porter L., Sarwar Z. U., McManus L. M., Shireman P. K. (2014). Altered macrophage phenotype transition impairs skeletal muscle regeneration. Am. J. Pathol. 184 1167–1184. 10.1016/j.ajpath.2013.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. X., Zhang S. X., Wu H. J., Rong X. L., Guo J. (2019). M2b macrophage polarization and its roles in diseases. J. Leukocyte Biol. 106 345–358. 10.1002/jlb.3ru1018-378rr [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Chen D., Chen L., Cao G., Zhao H., Liu D., et al. (2018). Novel inhibitors of the cellular renin-angiotensin system components, poricoic acids, target Smad3 phosphorylation and Wnt/β-catenin pathway against renal fibrosis. Br. J. Pharmacol. 175 2689–2708. 10.1111/bph.14333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Harris D. C. (2011). Macrophages in renal disease. J. Am. Soc. Nephrol. 22 21–27. 10.1681/asn.2010030269 [DOI] [PubMed] [Google Scholar]
- Wang Y., Jiang H., Pan J., Huang X., Wang Y., Huang H., et al. (2017). Macrophage-to-myofibroblast transition contributes to interstitial fibrosis in chronic renal allograft injury. J. Am. Soc. Nephrol. JASN 28 2053–2067. 10.1681/asn.2016050573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Wang Y. P., Zheng G., Lee V. W., Ouyang L., Chang D. H., et al. (2007). Ex vivo programmed macrophages ameliorate experimental chronic inflammatory renal disease. Kidney Int. 72 290–299. 10.1038/sj.ki.5002275 [DOI] [PubMed] [Google Scholar]
- Wang Y., Zhou C., Liu Y. (2018). Wnt signaling in kidney development and disease. Prog. Mol. Biol. Trans. Sci. 153 181–207. 10.1016/bs.pmbts.2017.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weichhart T., Hengstschläger M., Linke M. (2015). Regulation of innate immune cell function by mTOR. Nat. Rev. Immunol. 15 599–614. 10.1038/nri3901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong D. W. L., Yiu W. H., Chan K. W., Li Y., Li B., Lok S. W. Y., et al. (2018). Activated renal tubular Wnt/β-catenin signaling triggers renal inflammation during overload proteinuria. Kidney Int. 93 1367–1383. 10.1016/j.kint.2017.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S., Li M., Xu F., Li G., Han B., He X., et al. (2020). Fibrinogen-like protein 2 deficiency aggravates renal fibrosis by facilitating macrophage polarization. Biomed. Pharmacother. 130:110468. 10.1016/j.biopha.2020.110468 [DOI] [PubMed] [Google Scholar]
- Xia Y., Yan J., Jin X., Entman M. L., Wang Y. (2014). The chemokine receptor CXCR6 contributes to recruitment of bone marrow-derived fibroblast precursors in renal fibrosis. Kidney Int. 86 327–337. 10.1038/ki.2014.64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J., Zhang Z., Yang J., Mitch W. E., Wang Y. (2015). JAK3/STAT6 stimulates bone marrow-derived fibroblast activation in renal fibrosis. J. Am. Soc. Nephrol. JASN 26 3060–3071. 10.1681/asn.2014070717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Feng X., Liu X., Wang Y., Hu M., Cao Q., et al. (2019). Fate alteration of bone marrow-derived macrophages ameliorates kidney fibrosis in murine model of unilateral ureteral obstruction. Nephrol. Dialy. Trans. Offi. Publi. Eur. Dialy. Trans. Assoc. Eur. Renal Assoc. 34 1657–1668. 10.1093/ndt/gfy381 [DOI] [PubMed] [Google Scholar]
- Yang Y., Nankivell B. J., Hua W., Rao P., Ren X., Yu H., et al. (2020). Renal tubular cell binding of beta-catenin to TCF1 versus FoxO1 is associated with chronic interstitial fibrosis in transplanted kidneys. Am. J. Trans. 21 727–739. 10.1111/ajt.16287 [DOI] [PubMed] [Google Scholar]
- Yu F., Wu L., Tan Y., Li L., Wang C., Wang W., et al. (2010). Tubulointerstitial lesions of patients with lupus nephritis classified by the 2003 international society of nephrology and renal pathology society system. Kidney Int. 77 820–829. 10.1038/ki.2010.13 [DOI] [PubMed] [Google Scholar]
- Zeisberg E. M., Potenta S. E., Sugimoto H., Zeisberg M., Kalluri R. (2008). Fibroblasts in kidney fibrosis emerge via endothelial-to-mesenchymal transition. J. Am. Soc. Nephrol. 19 2282–2287. 10.1681/asn.2008050513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Yu S., Zheng B., Liu D., Wan F., Ma Y., et al. (2019). miR-30c-5p reduces renal ischemia-reperfusion involving macrophage. Med. Sci. Monitor Int. Med. J. Exp. Clin. Res. 25 4362–4369. 10.12659/msm.914579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M., Wang X., Wang Y., Niu A., Wang S., Zou C., et al. (2017). IL-4/IL-13-mediated polarization of renal macrophages/dendritic cells to an M2a phenotype is essential for recovery from acute kidney injury. Kidney Int. 91 375–386. 10.1016/j.kint.2016.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Qiao X., Wang L., Tan T. K., Zhao H., Zhang Y., et al. (2016). Matrix metalloproteinase 9 induces endothelial-mesenchymal transition via Notch activation in human kidney glomerular endothelial cells. BMC Cell Biol. 17:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng G., Lyons J. G., Tan T. K., Wang Y., Hsu T. T., Min D., et al. (2009). Disruption of E-cadherin by matrix metalloproteinase directly mediates epithelial-mesenchymal transition downstream of transforming growth factor-beta1 in renal tubular epithelial cells. Am. J. Pathol. 175 580–591. 10.2353/ajpath.2009.080983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng G., Zhang J., Zhao H., Wang H., Pang M., Qiao X., et al. (2016). alpha3 integrin of cell-cell contact mediates kidney fibrosis by integrin-linked kinase in proximal tubular E-cadherin deficient mice. Am. J. Pathol. 186 1847–1860. 10.1016/j.ajpath.2016.03.015 [DOI] [PubMed] [Google Scholar]
- Zhou B., Liu Y., Kahn M., Ann D. K., Han A., Wang H., et al. (2012). Interactions between beta-catenin and transforming growth factor-beta signaling pathways mediate epithelial-mesenchymal transition and are dependent on the transcriptional co-activator cAMP-response element-binding protein (CREB)-binding protein (CBP). J. Biol. Chem. 287 7026–7038. 10.1074/jbc.m111.276311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G., Li J., Zeng T., Yang P., Li A. (2020). The regulation effect of WNT-RAS signaling in hypothalamic paraventricular nucleus on renal fibrosis. J. Nephrol. 33 289–297. 10.1007/s40620-019-00637-8 [DOI] [PMC free article] [PubMed] [Google Scholar]