Abstract
Background
Antibiotic stewardship in the pretravel care of older adults is important to effectively treat infections while minimizing harm from side effects and unnecessary antibiotic use. The objective of this study was to compare the characteristics, risk behaviors, infectious diseases, and antibiotic use between older (≥60 years) and younger (18–59 years) travelers.
Methods
TravMil is a prospective, observational cohort of United States (US) Department of Defense beneficiaries traveling outside the continental US for ≤6.5 months. For this analysis, we included adults enrolled pretravel between January 2010 and August 2018 and excluded active duty personnel on deployment. Pre and post-travel surveys captured trip characteristics, exposures, illnesses, and antibiotic use.
Results
A total of 1742 travelers were analyzed: 747 (42.9%) were aged ≥60 years and 995 (57.1%) were aged 18–59 years. Older travelers were less likely to engage in high-risk dietary behaviors and experience travelers’ diarrhea than younger travelers (18.2% vs 22.9%; P < .05). Influenza-like illness (12.5%) and febrile illness (3.4%) occurred less frequently in the older cohort. Antibiotic use for self-treatment was common in both age groups (25.7% vs 26.7%) and often inappropriate, for example, for treatment of occasional loose stool or mild travelers’ diarrhea (67.0% [67/100] in older adults vs 57.6% [83/144] in younger adults; P < .05), and influenza-like illness (63.4% [64/101] vs 58.6% [68/116], respectively; P < .05).
Conclusions
Older travelers were less likely to engage in high-risk behaviors and experience travelers’ diarrhea, and both age groups experienced mild, self-limited infections. Inappropriate use of antibiotics was common, suggesting that antimicrobial stewardship should be emphasized at pretravel counseling with international travelers.
Keywords: antibacterial agents, diarrhea, travel, travelers’ diarrhea
Older travelers (≥60 years) are less likely to engage in high-risk dietary behaviors and experience travelers’ diarrhea than younger travelers (18–59 years). Antibiotics are often used inappropriately for mild or self-limited diarrhea and influenza-like illnesses during travel.
International travel among older adults has increased in popularity over the last decade [1]. Twenty-nine percent of travelers seen for a pretravel consultation in the Global TravEpiNet Consortium were >50 years of age, and 9% were >65 years [2]. Despite accounting for a substantial proportion of travelers to low and low-middle income countries, notable gaps exist in our understanding of aging and health risks during travel. Older travelers are suspected to be at a higher risk for travel-related infections due to immunosenescence, resulting in a diminished immune response to vaccines, increased susceptibility to infections, and a more severe clinical course [3, 4].
Despite these age-related physiological changes, advanced age has not been definitively associated with increased adverse travel-related events. A survey of Israeli travelers revealed that those >60 years of age had a lower risk of common travel-related illnesses such as acute diarrhea and febrile illness (FI) compared to young adults [5]. Data from ill travelers presenting to the GeoSentinel Network clinics indicates that while the proportionate morbidity due to certain infections (eg, rickettsiosis, lower respiratory tract infections) is higher in older travelers, the proportionate morbidity of acute diarrhea and flu-like illness is higher in younger travelers [6].
Furthermore, it is well known that advanced age is independently associated with various medication-related side effects, due to pharmacokinetic changes associated with age (eg, decreased renal function), drug–drug interactions, and comorbidities leading to physiological changes [7, 8]. Accordingly, besides acquisition of a travel-related illness, elderly travelers also are at an elevated risk for adverse effects from prophylactic or self-treatment regimens administered during travel. However, there is a lack of data on the use of medications for self-treatment among older travelers, their effectiveness in shortening the duration of symptoms, and the incidence of adverse effects. Additional studies are needed to inform evidence-based guidelines for pretravel counseling and the assessment of travel-related illnesses in older travelers [9, 10].
The objective of this study was to compare the trip characteristics, risk behaviors, illnesses, and antibiotic use between older (≥60 years) and younger (18–59 years) travelers, using a prospective, observational cohort of United States (US) Department of Defense (DoD) beneficiaries (ie, active duty military and US Public Health Service personnel, military retirees, employees of the Department of State, and military-dependent family members) traveling outside the continental US for ≤6.5 months. We hypothesized that older adults would be more compliant with preventive measures and have a lower risk of travel-related infections but would use antibiotics more frequently than younger travelers.
METHODS
TravMil (Deployment and Travel-Related Infectious Disease Risk Assessment, Outcomes, and Prevention Strategies Among Department of Defense Beneficiaries) is a prospective, observational cohort study of US DoD beneficiaries traveling outside the continental US for ≤6.5 months. The study is approved by the Uniformed Services University Institutional Review Board (Bethesda, Maryland). Consenting individuals are enrolled pretravel at 6 military travel clinics (Walter Reed National Military Medical Center, Bethesda, Maryland; Brooke Army Medical Center, San Antonio, Texas; Naval Medical Center, Portsmouth, Virginia; Madigan Army Medical Center, Tacoma, Washington; Naval Medical Center, San Diego, California; and Landstuhl Regional Medical Center, Landstuhl, Germany) and in the predeployment setting. Travelers with itineraries limited to regions with low risk of travel-related infections (ie, Western or Northern Europe, Canada, or New Zealand) are excluded. For this current analysis, we included adults enrolled at the pretravel visit between January 2010 and August 2018 who completed a post-travel survey. Active duty personnel traveling for deployment or military exercises were excluded due to the inherent differences in predeployment and pretravel care. For example, antibiotics for traveler’s diarrhea (TD) self-treatment are not routinely prescribed at the predeployment visit since deployed personnel are evaluated by a medical corpsman for illnesses during deployment. Active duty personnel traveling for purposes other than deployment (eg, vacation, military business) were included.
At the pretravel visit, travel medicine physicians provide counseling and prescriptions for TD, malaria chemoprophylaxis, and immunizations, but the counseling is not standardized for the study. Pretravel prescriptions (ie, antibiotics and loperamide for TD and malaria chemoprophylaxis) are abstracted from the medical records. Enrollees complete a paper-based pretravel survey recording their demographics, targeted medical history (ie, psychiatric diagnoses [depression, bipolar disorder, or schizophrenia], diabetes mellitus, inflammatory bowel disease, irritable bowel syndrome [IBS], seizures, or immunosuppressive disease), and planned use of acid reflux medication during travel and travel characteristics. Travelers selected their reasons for travel from a list of common categories, from which they were able to choose >1 option. Travelers completed a post-travel survey within 2 months of return from travel. The survey was available in electronic (sent via email) or paper form, and patients self-reported information regarding high-risk exposures, compliance with malaria chemoprophylaxis, nonspecific symptoms, and episodes of diarrhea, influenza-like illness (ILI), or FI, including severity and treatment. Enrollees were also sent a separate survey specifically inquiring about symptoms related to IBS 3 months following return from their travel destinations.
Travelers’ diarrhea was defined as ≥3 loose or liquid stools during a 24-hour period, or 2 loose or liquid stools during a 24-hour period and the addition of either nausea, vomiting, abdominal pain, fever, or bloody stool. Mild TD was defined as acute watery diarrhea with normal level of activity or function Moderate or severe TD was defined as acute watery diarrhea with partial or complete incapacitation, dysentery, or febrile diarrhea. Loose or liquid bowel movements that did not meet TD criteria were defined as loose stools. ILI was defined as subjective fever associated with either sore throat or cough. FI was defined as subjective fever not associated with diarrhea or ILI. In ILI and FI, the symptom severity was graded from mild (symptoms present but allowed for normal level of activity/function), moderate (symptoms caused decreased level of activity/function), or severe (symptoms caused inability to participate in activities). The modified Rome III criteria were used to classify participants with IBS in pretravel and follow-up surveys [11]. Partial or noncompliance with malaria chemoprophylaxis was defined as missing ≥2 doses of weekly (eg, mefloquine) or ≥3 doses of daily (eg, atovaquone-proguanil) antimalarials in a row.
Pearson χ 2 or Fisher exact tests were performed for univariate analysis of categorical variables, and Mann-Whitney U test was performed for continuous variables. Incidence rates between the 2 age groups were compared using the log-rank test. Statistical analysis was conducted using SAS version 9.4 software (SAS Institute, Cary, North Carolina).
RESULTS
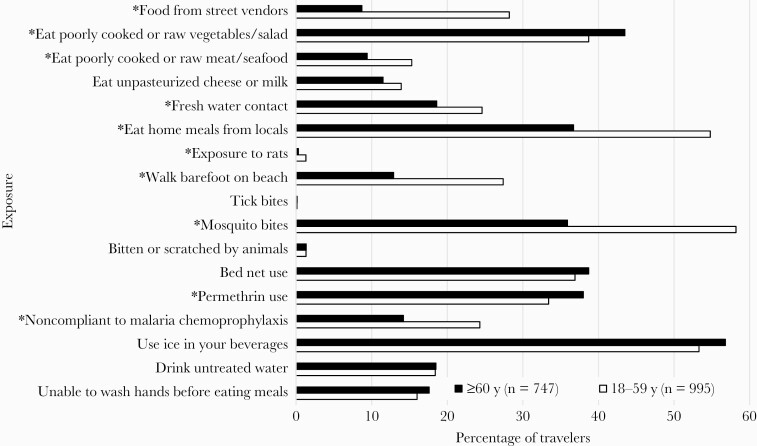
A total of 4070 adults were enrolled in TravMil during the study period, of whom 2328 were excluded from analysis due to trip cancellations (n = 216), deployment (n = 1618), or not completing a post-travel survey (n = 494). Of the 1742 who met inclusion criteria, 42.9% (n = 747) were ≥60 years of age, with a median age of 68 years (interquartile range [IQR], 64–72 years), and 57.1% (n = 995 travelers) were between 18 and 59 years of age, with a median age of 42 years (IQR, 29–52 years). Demographic and travel characteristics differed significantly between the 2 age groups (Table 1). Older adults were more likely to be white, male, and retired military service members and to schedule a pretravel visit at least 2 weeks prior to their departure date. The travel destinations and median length of travel were similar between both cohorts. Older adults were more likely to be compliant with malaria chemoprophylaxis and to avoid high-risk dietary behaviors and other exposures when compared to younger travelers (Figure 1). The median duration between return from travel and completion of the post-travel survey was 20 days (IQR, 9–28 days).
Table 1.
Demographic and Trip Characteristics of Older (≥60 Years) and Younger (18–59 Years) Travelers
| Characteristic | Age ≥60 y (n = 747) |
Age 18–59 y (n = 995) |
P Value |
|---|---|---|---|
| Age, y, median (IQR) | 68 (64–72) | 42 (29–52) | <.0001 |
| Sex | .033 | ||
| Male | 387 (51.8) | 464 (46.3) | |
| Female | 360 (48.2) | 531 (53.4) | |
| Racea | <.0001 | ||
| White | 601 (80.8) | 636 (64.0) | |
| Black | 31 (4.2) | 171 (17.2) | |
| Asian | 78 (10.5) | 119 (12.0) | |
| Other | 21 (2.8) | 41 (4.1) | |
| Multiple | 13 (1.7) | 27 (2.7) | |
| Beneficiary statusb | <.0001 | ||
| Active duty | 3 (<1) | 293 (29.5) | |
| Retired military | 406 (54.5) | 217 (21.9) | |
| Family of active duty or retired military | 322 (43.2) | 417 (42.0) | |
| Multiple status options checked | 14 (1.9) | 65 (6.6) | |
| Used PPI during travel | 51 (6.8) | 55 (5.5) | .261 |
| Diabetes mellitusc | 93 (12.5) | 38 (3.8) | <.0001 |
| Depression, bipolar disorder, or schizophreniad | 56 (7.5) | 84 (8.5) | .470 |
| Duration of travel, d, median (IQR) | 18 (13–27) | 15 (10–23) | <.0001 |
| Interval between pretravel visit and departure date ≤14 d | 151 (20.2) | 300 (30.2) | <.0001 |
| Purpose of travele | <.0001 | ||
| Multipurpose | 163 (21.8) | 342 (34.4) | |
| Military business | 6 (<1) | 48 (4.8) | |
| Pleasure/vacation | 480 (64.3) | 292 (29.3) | |
| Visiting friends and relatives | 23 (3.1) | 80 (8.0) | |
| Civilian business | 7 (<1) | 25 (1.4) | |
| Teaching/study | 6 (<1) | 30 (3.0) | |
| Providing medical support | 2 (<1) | 16 (1.6) | |
| Missionary work | 53 (7.1) | 144 (14.5) | |
| Adventure | 5 (<1) | 4 (<1) | |
| Adoption | … | 5 (<1) | |
| Region of travel | <.0001 | ||
| Africa | 197 (26.4) | 310 (31.2) | |
| South America, Central America, Caribbean | 182 (24.4) | 331 (33.3) | |
| Southeast Asia, East and North Asia, Oceania | 198 (26.5) | 224 (22.5) | |
| South Central and West Asia | 61 (8.2) | 81 (8.1) | |
| Europe | 26 (3.5) | 2 (<1) | |
| Multiple destinations | 83 (11.1) | 46 (4.6) | |
| Excluded region | … | 1 (<1) | |
| Accommodationsf | <.0001 | ||
| High-risk (hotel without air conditioning, private residence, outdoor camping, dormitory, or multiple accommodations) | 511 (69.4) | 589 (62.6) | |
| Cruise ship | 48 (6.5) | 11 (1.2) | |
| Other | 177 (24.1) | 341 (36.2) |
Data are presented as No. (%) unless otherwise indicated. P values were calculated using χ 2 or Fisher exact test for categorical values and Wilcoxon-Mann-Whitney test for continuous variables. All analysis was done with SAS version 9.4 software.
Abbreviations: IQR, interquartile range; PPI, proton pump inhibitor.
aFour subjects’ race was entered as unknown or missing (3 in the older group and 1 subject in the younger group).
bBeneficiary status was unknown for 2 subjects in the older age group and 3 subjects in the younger age group.
cDiabetes mellitus history was missing for 2 subjects in each of the age groups.
dHistory of depression, bipolar disorder, or schizophrenia was missing in 1 subject in the older age group and 2 subjects in the younger age group.
eCalculated the overall P value by regrouping the subjects for multipurpose and single-trip purpose; 1 older-age subject did not provide the travel purpose.
fSixty-five subjects were missing accommodation information (11 subjects in the older age group and 54 subjects in the younger age group). Other accommodations were: hotel with air conditioning, military, safari lodge, did not stay overnight, other.
Figure 1.
Comparison of high-risk behaviors and personal protective measures between older (≥60 years) and younger (18–59 years) travelers. The denominator for “noncompliant to malaria chemoprophylaxis” is the number of subjects who were prescribed malaria chemoprophylaxis during travel (≥60 years, n = 451; 18–59 years, n = 668). P values were calculated using χ 2 or Fisher exact test for categorical values. *P < .05.
Overall, the incidence of nonspecific symptoms and travel-related infectious disease syndromes in the cohort was low (Table 2). Insomnia (5.2%) and headache (5.6%) were the most frequent nonspecific symptoms reported in older adults. The proportion of older travelers reporting TD was significantly lower than younger adults (18.2% [incidence rate, 5.1 cases/100 person-weeks] vs 22.9% [incidence rate, 7.3 cases/100 person-weeks]; P < .05), while the proportions of reported ILI (13.5% vs 11.7%) and FI (3.0% vs 3.8%) were similar in the 2 cohorts. Most travel-related illness in older adults were mild. The median duration of TD was 2 days and 56.3% of TD cases reported mild watery diarrhea with no impact on daily activities. The incidence of IBS post-travel was low in both age cohorts. Similarly, 75.3% of older travelers with an ILI and 50.0% of those with an FI reported mild illness without impact on daily activities.
Table 2.
Illnesses Cases, Antibiotic Use, and Nonspecific Symptoms Reported by Travelers
| Symptom | Cases | Age ≥60 y (n = 747) |
Age 18–59 y (n = 995) |
P Value |
|---|---|---|---|---|
| Loose stools | Cases (n = 52) | 24 (3.2) | 28 (2.8) | .623 |
| Incidence rate, cases/100 person-weeks (95% CI) | 0.9 (.6–1.4) | 0.9 (.6–1.3) | .881a | |
| Duration of illness, d, median (IQR) | n = 17, 1 (1–3) | n = 23, 2 (1–3) | .657 | |
| Antibiotic use | 13 (54.2) | 11 (39.3) | .283 | |
| Travelers’ diarrhea | Case (n = 364) | 136 (18.2) | 228 (22.9) | .017 |
| Mild (n = 192) | 76 (56.3) | 116 (51.3) | .360 | |
| Moderate or severe (n = 169) | 59 (43.7) | 110 (48.7) | ||
| Acute watery diarrhea | 45 (76.3) | 64 (58.2) | .019 | |
| Febrile TD | 11 (18.6) | 30 (27.3) | .212 | |
| Dysentery | 3 (5.1) | 16 (14.6) | .064 | |
| Incidence rate, cases/100 person-weeks (95% CI) | 5.1 (4.3–6.1) | 7.3 (6.4–8.3) | <.0001a | |
| Duration of illness, d, median (IQR) | n = 135, 2 (1–3) | n = 224, 2 (1–4) | .004 | |
| Antibiotic use (n = 248) | ||||
| Mild TD | 54 (71.1) | 72 (62.1) | .200 | |
| Moderate or severe TD | 47 (79.7) | 75 (68.2) | .112 | |
| Influenza-like illness | Case (n = 217) | 101 (13.5) | 116 (11.7) | .244 |
| Mild (n = 140) | 76 (75.3) | 64 (55.2) | .002 | |
| Moderate or severe (n = 77) | 25 (24.8) | 52 (44.8) | ||
| Incidence rate, cases/100 person-weeks (95% CI) | 3.8 (3.1–4.6) | 3.7 (3.1–4.5) | .750a | |
| Duration of illness, d, median (IQR) | n = 99, 4 (3–7) | n = 114, 3 (2–5) | .006 | |
| Antibiotic use | 64 (63.4) | 68 (58.6) | .475 | |
| Febrile illness | Case (n = 60) | 22 (3.0) | 38 (3.8) | .322 |
| Mild (n = 22) | 11 (50.0) | 11 (29.0) | .103 | |
| Moderate and severe (n = 38) | 11 (50.0) | 27 (71.0) | ||
| Incidence rate, cases/100 person-weeks (95% CI) | 0.8 (.6–1.3) | 1.2 (.9–1.7) | .104a | |
| Duration of illness, d, median (IQR) | n = 21, 4 (2–8) | n = 34, 3 (1–5) | .128 | |
| Antibiotic use | 7 (31.8) | 13 (34.2) | .850 | |
| Reported symptoms | Skin rash | 12 (1.6) | 42 (4.2) | .002 |
| Sunburn | 24 (3.2) | 101 (10.2) | <.0001 | |
| Itchy skin | 23 (3.1) | 38 (3.8) | .406 | |
| Vomiting | 6 (0.8) | 25 (2.5) | .008 | |
| Nausea | 25 (3.4) | 84 (8.4) | <.0001 | |
| Stomach ache | 30 (4.0) | 103 (10.4) | <.0001 | |
| Headache | 42 (5.6) | 130 (13.1) | <.0001 | |
| Strange dreams | 22 (2.9) | 59 (5.9) | .003 | |
| Insomnia | 39 (5.2) | 104 (10.5) | <.0001 | |
| Dizziness | 26 (3.5) | 66 (6.6) | .004 | |
| Unsteadiness | 21 (2.8) | 30 (3.0) | .803 | |
| Visual disturbances | 5 (0.7) | 10 (1.0) | .453 | |
| Irritable bowel syndrome | IBS prior to travel | 26 (3.5) | 47 (4.7) | .200 |
| IBS at 3 mo after returnb | 5/433 (1.2) | 13/444 (2.9) | .064 | |
| Sought health care during travel | 23 (2.9) | 34 (3.2) | .748 | |
| No. of travelers who used antibiotics during travel | 192 (25.7) | 266 (26.7) | .629 |
Data are presented as No. (%) unless otherwise indicated. P values were calculated using χ 2 or Fisher exact test for categorical values and Wilcoxon-Mann-Whitney test for continuous variables. All analysis was done with SAS version 9.4 software.
Abbreviations: CI, confidence interval; IBS, irritable bowel syndrome; IQR, interquartile range; TD, travelers’ diarrhea.
aP values of the incidence rate comparison between 2 age groups were calculated using log-rank test.
bDenominator is the number of travelers who were negative for IBS pretravel and completed the 3-month post-travel IBS survey.
Antibiotics were prescribed for TD self-treatment in 88.6% (n = 662) of older travelers and 84.2% (n = 838) of younger travelers at the pretravel visit. Overall, 25.7% of older and 26.7% of younger travelers reported using antibiotics for self-treatment. A significant proportion of older and younger travelers used antibiotics inappropriately. Per expert treatment guidelines [12], antibiotics and adjunctive loperamide are indicated for treatment of moderate or severe TD, but antibiotics are not indicated for loose stools or mild watery diarrhea that do not interfere with daily activity. Among travelers who reported loose stool or mild diarrhea, 67 of 100 (67.0%) of older travelers and 83/144 (57.6%) of younger adults took antibiotics. Among cases of moderate or severe TD, 47 of 59 (79.7%) of older adults and 75 of 110 (68.2%) of younger adults took antibiotics alone and approximately one-third took antibiotics and loperamide in both age groups. Although there are no specific guidelines for treatment of ILI during travel, the Centers for Disease Control and Prevention treatment guidelines [13] for upper respiratory tract infections recommend against the use of antibiotics unless there is significant worsening of symptoms (eg high-grade fever, purulent sputum) for >3–4 days or persistent symptoms for >10 days. Although most older adults with ILI (75.3%) reported a mild illness with a median duration of symptoms of 4 days, 64 of 101 (63.4%) took an antibiotic. A similar trend was observed in younger travelers. FIs were infrequent, mild, and self-limited in both cohorts, and approximately one-third of older and younger travelers with an FI took antibiotics. Less than 5.0% of older and young travelers sought health care for travel-related illnesses.
Travelers most frequently received a prescription for either a fluoroquinolone or macrolide antibiotic at their pretravel visits (Table 3). Fluoroquinolones were the most commonly prescribed antibiotic in both cohorts (61.9% vs 69.9%), followed by azithromycin (36.1% vs 24.8%).
Table 3.
Class of Pretravel Antibiotics Prescribed Between Older (≥60 Years) and Younger (18–59 Years) Travelers
| Antibiotica | Age ≥60 y (n = 662) | Age 18–59 y (n = 838) |
|---|---|---|
| Azithromycin | 239 (36.1) | 208 (24.8) |
| Ciprofloxacin | 387 (58.5) | 557 (66.5) |
| Levofloxacin | 23 (3.5) | 29 (3.5) |
| Rifaximin | 4 (0.6) | 1 (0.1) |
| Otherb | 9 (1.4) | 43 (5.1) |
Data are presented as No. (%).
aForty-three subjects (23 older and 20 younger travelers) were prescribed >1 antibiotic.
bIncludes all prescriptions other than azithromycin, ciprofloxacin, levofloxacin, rifaximin, and trimethoprim-sulfamethoxazole that were prescribed at the pretravel visit survey.
DISCUSSION
Older adults are considered to be more susceptible to travel-related infections, but there is a lack of evidence from large, prospective studies evaluating the trip characteristics, behaviors, and illnesses experienced by older travelers to inform an appropriate risk assessment at the pretravel visit. In this observational cohort of 1742 travelers, we compared the travel characteristics of older (≥60 years) and younger (18–59 years) DoD beneficiaries. Older travelers were less likely to engage in activities or have exposures associated with travel-related infections, and they did not have an increased risk of TD, ILI, or FI compared to younger travelers. This suggests that collectively older adults, possibly aware of their own physiological age-related changes, take extra precautions to mitigate any adverse travel outcomes. Our findings are consistent with a prior study of approximately 400 Israeli travelers by Alon et al [5], which reported a lower rate of travel-related illnesses in older travelers (18.8% vs 34.0%), also attributed to increased compliance with preventive measures. Other studies have suggested that the longer duration of travel in younger travelers may also increase the risk of illness [14, 15], although both cohorts had a similar duration of travel in our study.
The incidence of TD and ILI observed in the overall cohort was significantly lower than incidence estimates from travelers in other large cohorts. For example, diarrhea was reported by 33.0% of 628 adult travelers (median age, 47 years [IQR, 19–83 years]) in the Boston Area Travel Medicine Network cohort, and respiratory symptoms were reported by approximately 23.0% [16]. This may be explained in part by the use of standardized criteria for diagnosis of TD and ILI in the TravMil cohort rather than the individual nonspecific symptoms, such as diarrhea, which can result in an overestimation of disease burden. In addition, differences in the proportion of travelers going to intermediate vs high-risk TD destinations, and improvements in food and sanitation in parts of the developing world frequented by tourists over the last decade may also account for differences in the incidence of infections between studies.
A significant proportion of infections experienced during travel were mild and self-limited. Fifty-six percent of TD cases, 75.3% of ILI cases, and 50.0% of FI cases in older adults did not report an impact on their daily activities. Older adults had numerically lower rates of moderate or severe infections compared to younger travelers, although this was only statistically significant for ILI. The mild nature of infections in older adults may also be reflective of our study design and patient population. For example, the GeoSentinel cohort consists of travelers evaluated at medical clinics for travel-related illnesses and thus has a greater proportion of high-acuity infections. A study from the GeoSentinel network reported that severe Plasmodium falciparum malaria and lower respiratory tract infections (ie, bronchitis and pneumonia) were more frequent in ill travelers over the age of 60 years [17]. In contrast, the TravMil cohort enrolls travelers seen at pretravel clinics who have free access to medical care and are likely to have a lower risk profile than adults who do not seek pretravel care.
Unwarranted antibiotic use was pervasive in both cohorts. Older travelers used antibiotics for loose stools (54.2%), mild TD (62.1%), and ILI (63.0%), contrary to published guidelines that discourage the use of antibiotics when a bacterial infection is unlikely. Overuse of standby antibiotic has been previously described: in a study by Vilkman et al, travelers carrying antibiotics used them at significantly higher rates for milder and moderate TD than noncarriers (38.0% vs 4.0%) [18]. Perhaps travelers, out of an abundance of caution, preemptively take antibiotics with mild symptoms for precautionary measures, and unknowingly are increasing their risk of antibiotic-related harm [19]. We did not have an adequate sample size or endpoints to determine if antibiotic use was associated with a significantly higher incidence of nonspecific symptoms in older adults (eg, gastrointestinal symptoms or dizziness with macrolides or quinolones).
There is growing evidence demonstrating increased gut colonization by extended-spectrum β-lactamase–producing Enterobacteriaceae (ESBL-E), particularly in international travelers. In a study by Kantele et al, 47.0% of travelers who developed diarrhea during travel to South Asia were colonized with ESBL-E and the proportion increased to 80.0% with antibiotic treatment [20]. Additional studies have also suggested that the use of fluoroquinolone antibiotics (the most prescribed antibiotic in our analysis) can also facilitate the development of fluoroquinolone resistance in ESBL-E organisms [21]. Our finding underscores the need for educating travelers at the pretravel visit regarding the appropriate indications for self-treatment with antibiotics, including the provision of handouts or cell phone applications that can be used as a quick reference guide during travel [22, 23]. Interestingly, the Global TravEpiNet Consortium reported a decline in the proportion of pretravel consultations with an antibiotic prescription for TD self-treatment from 92.0% in 2009 to 70.0% in 2018, attributed in part to the US Food and Drug Administration warning on fluroquinolones in 2016 and the International Society of Travel Medicine guidelines regarding TD self-treatment in 2017 [24]. Prescribing practices must balance the risk of antibiotic overuse with the risk of moderate or severe TD in regions without access to appropriate medical care and the potential for treatment with substandard or counterfeit antimicrobials. Forty-four percent of TD cases among older adults were moderate or severe, necessitating the use of antibiotics and loperamide for effective treatment. Additional studies are needed to inform the risk assessment and mitigation strategies at the pretravel visit and to promote antibiotic stewardship.
There are limitations to our study. Our study population consisted of DoD beneficiaries presenting to a specialized travel clinic for pretravel care and is therefore not representative of most travelers who do not seek pretravel care or are evaluated by their primary care physicians. Older DoD beneficiaries presenting to travel medicine clinics have access to medical care for management of comorbidities, and may be less likely to participate in risky behaviors, thus experiencing a lower incidence and milder severity of infections. Recall bias is a potential concern in retrospective surveys, although most travel durations were <1 month and 75% of subjects completed the post-travel survey within a month of return. We excluded active duty personnel on deployment due to differences in pretravel prescriptions and treatment of infections during travel, which could potentially skew our incidence estimates in the younger cohort. Finally, we did not evaluate for noninfectious illnesses and comorbidities such as heart disease that are of significant concern in older travelers.
In conclusion, our findings suggest that travelers aged ≥60 years presenting for pretravel care are less likely to engage in high-risk behaviors during travel than younger adults and have a similar incidence of TD, ILI, and FI. In addition, most infections experienced by older and young travelers are mild/self-limited and are inappropriately treated with antibiotics. Specific instructions regarding the indications for self-treatment of TD and ILI, which can be easily accessed during travel, should be provided at the pretravel visit to promote judicious use of antibiotics. With increasing global antibiotic resistance, attention to antimicrobial stewardship practices is critical in the international traveler. Additional studies are needed to guide risk assessment and antibiotic prescription practices at the pretravel visit.
Notes
Author contributions. Concept and design: V. H. C., T. L., J. F., D. T. Acquisition, analysis, or interpretation of data: V. H. C., D. H. T., G. U., T. L., A. G., J. F. Drafting of the manuscript: V. H. C., T. L. Statistical analysis: K. T. Critical revision of the manuscript for important intellectual content: All authors.
Acknowledgments. We thank the Infectious Disease Clinical Research Program TravMil study team of clinical coordinators, laboratory technicians, data managers, clinical site managers, and administrative support personnel for their contributions to this project.
Patient consent statement. All participants in this study provided written informed consent. This study was approved by the Uniformed Services University Institutional Review Board (Bethesda, Maryland).
Copyright statement. Some authors are service members of the US government. This work was prepared as part of their official duties. Title 17 United States Code (U.S.C.) §105 provides that “Copyright protection under this title is not available for any work of the United States Government.” Title 17 U.S.C. §101 defines a US government work as a work prepared by a military service member or employee of the US government as part of that person’s official duties.
Disclaimer. The contents of this publication are the sole responsibility of the author(s) and do not necessarily reflect the views, opinions, or policies of the Uniformed Services University of the Health Sciences (USUHS), the Department of Defense (DoD), the Departments of the Army, Navy, or Air Force, Brooke Army Medical Center, the US Army Medical Department, the US Army Office of the Surgeon General, Landstuhl Regional Medical Center, Walter Reed National Military Medical Center, Madigan Army Medical Center, Naval Medical Center Portsmouth, or the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc. Mention of trade names, commercial products, or organizations does not imply endorsement by the US government. The investigators have adhered to the policies for protection of human subjects as prescribed in 45 Code of Federal Regulations 46. The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Financial support. This study (IDCRP-037) was supported by the Infectious Disease Clinical Research Program, a DoD program executed by the USUHS through a cooperative agreement with the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc. This project has been funded in whole, or in part, with federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health (Interagency Agreement Y1-AI-5072).
Potential conflicts of interest. All authors: No reported conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Alén E, Nieves L, De Carlos P. Profiling the segments of senior tourists throughout motivation and travel characteristics. Curr Issues Tour 2015; 20:1454–69. [Google Scholar]
- 2.LaRocque RC, Sowmya RR, Lee J, et al. Global TravEpiNet: a national consortium of clinics providing care to international travelers—analysis of demographic characteristics, travel destinations, and pretravel healthcare of high-risk US international travelers, 2009–2011. Clin Infect Dis 2011; 54:455–62. [DOI] [PubMed] [Google Scholar]
- 3.Lord JM. The effect of ageing of the immune system on vaccination responses. Hum Vaccin Immunother 2013; 9:1364–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wei HY, Shu PY, Hung MN. Characteristics and risk factors for fatality in patients with dengue hemorrhagic fever, Taiwan, 2014. Am J Trop Med Hyg 2016; 95:322–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alon D, Shitrit P, Chowers M. Risk behaviors and spectrum of diseases among elderly travelers: a comparison of younger and older adults. J Travel Med 2010; 17:250–5. [DOI] [PubMed] [Google Scholar]
- 6.Gautret P, Gaudart J, Leder K, et al. ; GeoSentinel Surveillance Network. Travel-associated illness in older adults (>60 y). J Travel Med 2012; 19:169–77. [DOI] [PubMed] [Google Scholar]
- 7.Faulkner CM, Cox HL, Williamson JC. Unique aspects of antimicrobial use in older adults. Clin Infect Dis 2005; 40:997–1004. [DOI] [PubMed] [Google Scholar]
- 8.Suh KN, Flaherty GT, et al. The older traveler. In: Keystone J, Kozarsky P, Connor BA, et al., eds. Travel Medicine. 4th edn. London: Elsevier; 2019; 247–53. [Google Scholar]
- 9.Lee TK, Hutter JN, Masel J, et al. Guidelines for the prevention of travel-associated illness in older adults. Trop Dis Travel Med Vaccines 2017; 3:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flaherty GT, Rossanese A, Steffen R.et al. A golden age of travel: advancing the interests of older travellers. J Travel Med 2018; 25. doi:10.1093/jtm/tay088. [DOI] [PubMed] [Google Scholar]
- 11.Drossman DA. The functional gastrointestinal disorders and the Rome III process. Gastroenterology 2006; 130:1377–90. [DOI] [PubMed] [Google Scholar]
- 12.Riddle MS, Connor BA, Beeching NJ, et al. Guidelines for the prevention and treatment of travelers’ diarrhea: a graded expert panel report. J Travel Med 2017; 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. Adult treatment recommendations.2017. www.cdc.gov/antibiotic-use/community/for-hcp/outpatient-hcp/adult-treatment-rec.html. Accessed 28 October 2020.
- 14.Rack J, Wichmann O, Kamara B, et al. Risk and spectrum of diseases in travelers to popular tourist destinations. J Travel Med 2005; 12:248–53. [DOI] [PubMed] [Google Scholar]
- 15.Hill DR. Health problems in a large cohort of Americans traveling to developing countries. J Travel Med 2006; 7:259–66. [DOI] [PubMed] [Google Scholar]
- 16.Chen LH, Han PV, Wilson ME, et al. Self-reported illness among Boston-area international travelers: a prospective study. Travel Med Infect Dis 2016; 14:604–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Checkley AM, Smith A, Smith V, et al. Risk factors for mortality from imported falciparum malaria in the United Kingdom over 20 years: an observational study. BMJ 2012; 344:e2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vilkman K, Lääveri T, Pakkanen SH, Kantele A. Stand-by antibiotics encourage unwarranted use of antibiotics for travelers’ diarrhea: a prospective study. Travel Med Infect Dis 2019; 27:64–71. [DOI] [PubMed] [Google Scholar]
- 19.Field TS, Gurwitz JH, Harrold LR, et al. Risk factors for adverse drug events among older adults in the ambulatory setting. J Am Geriatr Soc 2004; 52:1349–54. [DOI] [PubMed] [Google Scholar]
- 20.Kantele A, Laaveri T, Mero S, et al. Antimicrobials increase travelers’ risk of colonization by extended-spectrum beta lactamase-producing Enterobacteriaceae. Clin Infect Dis 2015; 60:837–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kantele A, Mero S, Kirveskari J, Lääveri T. Fluoroquinolone antibiotic users select fluoroquinolone-resistant ESBL-producing Enterobacteriaceae (ESBL-PE)—data of a prospective traveller study. Travel Med Infect Dis 2017; 16:23–30. [DOI] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention. Travelers’ diarrhea.2019. wwwnc.cdc.gov/travel/page/travelers-diarrhea. Accessed 30 October 2020.
- 23.Unbound Medicine. CDC Yellow Book (1.4) (mobile app).2021. https://apps.apple.com/us/app/cdc-yellow-book/id1235766820. Accessed 30 October 2020.
- 24.Gandhi AR, Rao SR, Chen LH, et al. Prescribing patterns of antibiotics for the self-treatment of travelers’ diarrhea in global TravEpiNet, 2009-2018. Open Forum Infect Dis 2020; 7:ofaa376. [DOI] [PMC free article] [PubMed] [Google Scholar]