Abstract
Introduction
Coronavirus disease 2019 (COVID-19) has emerged as a global pandemic since its outbreak in Wuhan, China. It is an urgent task to prevent and treat COVID-19 effectively early. In China’s experience combating the COVID-19 pandemic, Chinese herbal medicine (CHM) has played an indispensable role. A large number of epidemiological investigations have shown that mild to moderate COVID-19 accounts for the largest proportion of cases. It is of great importance to treat such COVID-19 cases, which can help control epidemic progression. Many trials have shown that CHM combined with conventional therapy in the treatment of mild to moderate COVID-19 was superior to conventional therapy alone. This review was designed to evaluate the add-on effect of CHM in the treatment of mild to moderate COVID-19.
Methods
Eight electronic databases including PubMed, EMBASE, Cochrane Central Register of Controlled Trials, the Clinical Trials.gov website, China National Knowledge Infrastructure (CNKI), China Science and Technology Journal Database (VIP), Wanfang Database and China Biology Medicine (CBM) were searched from December 2019 to March 2021 without language restrictions. Two reviewers searched and selected studies, and extracted data according to inclusion and exclusion criteria independently. Cochrane Risk of Bias (ROB) tool was used to assess the methodological quality of the included RCTs. Review Manager 5.3.0 software was used for statistical analysis.
Results
Twelve eligible RCTs including 1393 participants were included in this meta-analysis. Our meta-analyses found that lung CT parameters [RR = 1.26, 95% CI (1.15, 1.38), P<0.00001] and the clinical cure rate [RR = 1.26, 95%CI (1.16, 1.38), P<0.00001] of CHM combined with conventional therapy in the treatment of mild to moderate COVID-19 were better than those of conventional therapy. The rate of conversion to severe cases [RR = 0.48, 95%CI (0.32, 0.73), P = 0.0005], TCM symptom score of fever [MD = -0.62, 95%CI (-0.79, -0.45), P<0.00001], cough cases [RR = 1.43, 95%CI (1.16, 1.75), P = 0.0006], TCM symptom score of cough[MD = -1.07, 95%CI (-1.29, -0.85), P<0.00001], TCM symptom score of fatigue[MD = -0.66, 95%CI (-1.05, -0.28), P = 0.0007], and CRP[MD = -5.46, 95%CI (-8.19, -2.72), P<0.0001] of combination therapy was significantly lower than that of conventional therapy. The WBC count was significantly higher than that of conventional therapy[MD = 0.38, 95%CI (0.31, 0.44), P<0.00001]. Our meta-analysis results were robust through sensitivity analysis.
Conclusion
Chinese herbal medicine combined with conventional therapy may be effective and safe in the treatment of mild to moderate COVID-19. More high-quality RCTs are needed in the future.
Introduction
Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has emerged as a global pandemic since its outbreak in Wuhan, China, in December 2019 [1]. As of March 25, 2021, more than 124.21 million confirmed cases and more than 2.73 million deaths had been reported globally [2]. Unfortunately, confirmed cases continue to rise due to rapid spread. Thus, it is an urgent task to prevent and treat COVID-19 effectively early.
To date, the pandemic in China has been gradually controlled due to strong government measures, early detection, early quarantine, and early treatment with conventional Western therapy and Chinese herbal medicine (CHM) [3, 4]. CHM is a special medicine used in the prevention and treatment of diseases and is composed of plant medicine, animal medicine, and mineral medicine [5]. In China’s experience combating the COVID-19 pandemic, CHM has played an indispensable role, and a CHM therapeutic schedule was included in the guidelines on the treatment of COVID-19 [4, 6]. A large number of epidemiological investigations have shown that mild to moderate COVID-19 accounts for the largest proportion of cases [7]. It is of great importance to treat such COVID-19 cases, which can help control epidemic progression. The current conventional therapy recommendations for mild to moderate COVID-19 are mainly antiviral and symptomatic support treatment [6]. The recommended antiviral drugs are interferon, ribavirin, lopinavir-ritonavir, and chloroquine phosphate [6]. However, most of the recommended antiviral drugs used to treat mild to moderate COVID-19 are currently based on previous treatments for severe acute respiratory syndrome (SARS) and influenza A, and uncertainties regarding the efficacy and side effects of these antiviral drugs remain problematic [8]. Many trials have shown that CHM combined with conventional therapy in the treatment of mild to moderate COVID-19 was superior to conventional therapy alone in improving clinical efficacy, clinical symptoms, and anti-inflammatory effects while causing fewer adverse drug events [9, 10].
Presently, there is no systematic evaluation report on the efficacy of CHM combined with conventional therapy in the treatment of mild to moderate COVID-19. Therefore, we performed a systematic review and meta-analysis of trials that tested the add-on effect of CHM in the treatment of mild to moderate COVID-19.
Methods
The protocol for our review has been registered on the International Prospective Register of Systematic Reviews (PROSPERO) with the registration number CRD42020213528. This review was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [11].
Eligibility criteria
Inclusion and exclusion criteria
The diagnostic criteria of mild to moderate COVID-19 refer to the " Diagnosis and Treatment Guideline for COVID-19 (Trial 8th Edition) " [6]. Mild COVID-19 is defined as mild clinical symptoms (such as low fever, mild fatigue, impairment of smell and taste, etc.) with no radiographic evidence of pneumonia [6]. Moderate COVID-19 is defined as having fever, respiratory symptoms, and imaging manifestations of pneumonia [6].
Inclusion criteria: (1) Study design: only randomized controlled trials (RCTs). (2) Participants: adult patients (aged≥18 years) with an established diagnosis of mild to moderate COVID-19. (3) Interventions: the treatment group was treated with a combination of CHM and conventional therapy. The administration of CHM was limited to oral administration. Patients in the control group were treated with conventional therapy. (4) Outcomes: a. clinical efficacy (e.g. lung computed tomography (CT), clinical cure rate, rate of conversion to severe cases, viral nucleic acid testing), b. clinical symptoms (e.g. fever, cough, fatigue), c. inflammatory biomarkers (e.g. white blood cell (WBC) count, lymphocyte (LYM) count, C-reactive protein (CRP)), d. adverse drug events (e.g. nausea and vomit, diarrhea, liver damage).
Exclusion criteria: (1) Patients with suspected diagnosis of COVID-19; (2) Retrospective studies, observational studies, repeated data studies, and cross-over studies.
Search strategy
Eight electronic databases including PubMed, EMBASE, Cochrane Central Register of Controlled Trials, the Clinical Trials.gov website, China National Knowledge Infrastructure (CNKI), China Science and Technology Journal Database (VIP), Wanfang Database and China Biology Medicine (CBM) were searched from December 2019 to March 2021 without language restrictions. The search terms included “coronavirus disease 2019”, “COVID-19”, “novel coronavirus pneumonia”, “SARS-CoV-2”, “2019-nCoV”, “traditional Chinese medicine”, “Chinese herbal medicine”, “Chinese herb”, “Chinese herb therapy”, “Chinese herbal formulas”, “clinical trial”, “randomized controlled trial”, “randomised controlled trial”, and “lin chuang yan jiu”. Potential eligible trials were obtained by searching the reference lists of reviews and meta-analyses. We also contacted with study authors for more information.
The PubMed search strategy is as follows. Search: ((((((coronavirus disease 2019) OR (COVID-19)) OR (novel coronavirus pneumonia)) OR (SARS-CoV-2)) OR (2019-nCoV)) AND (((((traditional Chinese medicine) OR (Chinese herbal medicine)) OR (Chinese herb)) OR (Chinese herb therapy)) OR (Chinese herbal formulas))) AND ((((clinical trial) OR (randomized controlled trial)) OR (randomised controlled trial)) OR (lin chuang yan jiu)).
Study selection and data extraction
Two reviewers (XQD and LPS) read the title, abstract, and full text, and selected the qualified trials according to the eligibility criteria independently. A pre-designed test form in duplicate was used for extracting the following information: basic characteristics (e.g. the title, first authors’ name, publication date), participant characteristics (e.g. age, gender, sample size), intervention details (e.g. description of interventions, description of controls, dose, route of oral administration, duration of treatment), and outcome measures, as well as any adverse events. Reviewers (XQD and LPS) cross-checked the data. Any differences of opinion among the primary reviewers were resolved by a third reviewer (WFC). All reviewers were unbiased and had no conflicting interests.
Assessment of methodological quality
Two reviewers (XQD and LPS) assessed the methodological quality by using the Cochrane Collaboration’s tool [12]. Seven items of risk of bias (ROB) were evaluated as below: random sequence generation, allocation concealment, blinding (patient, investigator and assessor), incomplete outcome data addressed, free of selective reporting, and other biases. Each item of ROB was assessed to be low ROB, high ROB, or unclear ROB. Additionally, any disagreements of ROB were resolved by consultation with the third reviewer (WFC).
Meta-analyses
Review Manager 5.3.0 software (The Cochrane Collaboration, Copenhagen, Denmark) was used for quantitative analysis. The relative risk (RR) was adopted for dichotomous variables. Mean difference (MD) or standard mean difference (SMD) were calculated for continuous variables. Confidence intervals (CIs) were set as 95% with P < 0.05 considered as a statistically significant difference. Heterogeneity was assessed with the χ2 test and the I2 statistical value. When the P≥0.10 or I2 ≤50%, a fixed-effect model was adopted. Otherwise, a random-effect model was applied. Subgroup analyses were carried out according to treatment duration. Sensitivity analyses were performed by leave-one-out method [13]. Funnel plot analysis was conducted to evaluate the reporting bias for outcome measures with more than 10 RCTs [14].
Results
Eligible studies
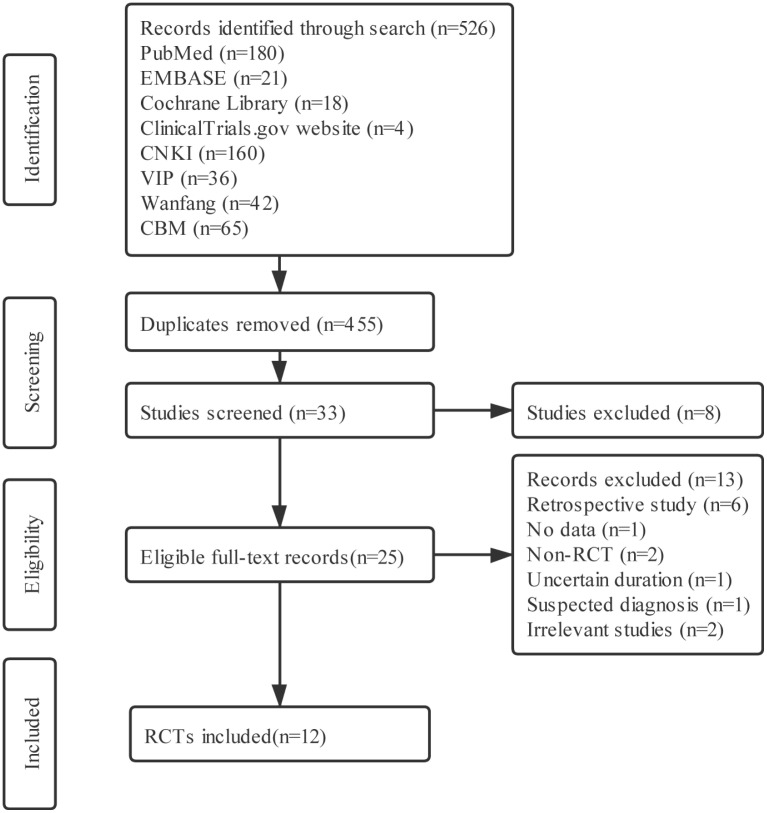
The flow diagram of study selection and identification is showed in (Fig 1). A total of 526 related citations were initially retrieved. Twelve eligible RCTs were included in meta-analysis according to the inclusion and exclusion criteria [15–26]. One RCT was published online in English [19], and the rest were reported online in Chinese.
Fig 1. The flow diagram of study selection and identification.
The characteristics of included RCTs are listed in (Table 1). Twelve RCTs enrolling 1393 participants were included in this meta-analysis. All twelve RCTs were conducted in China in 2020. In all the studies included, the patients in control group received conventional therapy while patients in treatment group received combination therapy of CHM and conventional therapy. The treatment duration varied from 5 to 15 days. Among the twelve RCTs [15–26], three were multi-centered trials [18, 19, 22] and the remaining nine were single-centered trials.
Table 1. The characteristics of included RCTs.
| First author | Type of COVID-19 | Sample size (M/F) | Age (yrs) | Intervention | Control | Duration | Outcome measures |
|---|---|---|---|---|---|---|---|
| Duan C [15] | mild | T:82(39/43) C:41(23/18) | T:51.99±13.88 C:50.29±13.17 | Jinhua Qinggan granule and conventional therapy | Conventional therapy | 5 days | ⑤+⑦ |
| Fu [16] | mild/ moderate | T:32(17/15) C:33(19/14) | T:43.26±7.15 C:43.68±6.45 | Toujie Quwen granule and conventional therapy | Conventional therapy | 10 days | ①+②+③+⑤+⑥+⑦ |
| Fu XX [17] | moderate | T:37(19/18) C:36(19/17) | T:45.26±7.25 C:44.68±7.45 | Toujie Quwen granule and conventional therapy | Conventional therapy | 15 days | ②+③+⑤+⑥+⑦ |
| Hu F [18] | moderate | T:100(49/51) C:100(55/45) | T:47.00±14.06 C:49.28±11.14 | Jinyinhua oral liquid and conventional therapy | Conventional therapy | 10 days | ①+③+④+⑦ |
| Hu K [19] | mild/ moderate | T:142(79/63) C:142(71/71) | T:50.4±15.2 C:51.8±14.8 | Lianhua Qingwen capsule and conventional therapy | Conventional therapy | 14 days | ①+②+③+④+⑤+⑦ |
| Qiu M [20] | moderate | T:25(13/12) C:25(14/11) | T:53.35±18.35 C:51.32±14.62 | Maxing Xuanfei Jiedu Decoction and conventional therapy | Conventional therapy | 10 days | ①+③+⑤ |
| Sun HM [21] | mild/ moderate | T:32(17/15) C:25(11/14) | T:45.4±14.10 C:42.0±11.70 | Lianhua Qingke granule and conventional therapy | Conventional therapy | 14days | ①+③+⑤ |
| Yang MB [22] | moderate | T:26(16/10) C:23(9/14) | T:50.35±13.37 C:47.17±16.57 | Reyanning mixture and conventional therapy | Conventional therapy | 7 days | ③+④+⑤+⑥+⑦ |
| Yu P [23] | mild/ moderate | T:147(82/65) C:148(89/59) | T:48.27±9.56 C:47.25±8.67 | Lianhua Qingwen granule and conventional therapy | Conventional therapy | 7 days | ①+②+③+⑤+⑥+⑦ |
| Zhang CT [24] | moderate | T:22(9/13) C:23(10/13) | T:53.7±3.5 C: 55.6±4.2 | Jiawei Dayuan Decoction and conventional therapy | Conventional therapy | 7 days | ①+⑤+⑥+⑦ |
| Zhang YL [25] | moderate | T:80(50/30) C:40(23/17) | T:53.4±13.70 C:52.0±14.10 | Jinyinhua oral liquid and conventional therapy | Conventional therapy | 10 days | ③+⑤+⑦ |
| Zhou WM [26] | moderate | T:52(32/20) C:52(28/24) | T:52.47±10.99 C:51.11±9.87 | diammonium glycyrrhizinate and conventional therapy | Conventional therapy | 14 days | ②+⑥+⑦ |
①: Lung CT; ②: Clinical cure rate; ③: Rate of conversion to severe cases; ④: Virus nucleic acid testing; ⑤: Clinical symptoms; ⑥: Inflammatory biomarkers; ⑦: Adverse events.
Assessment of methodological quality
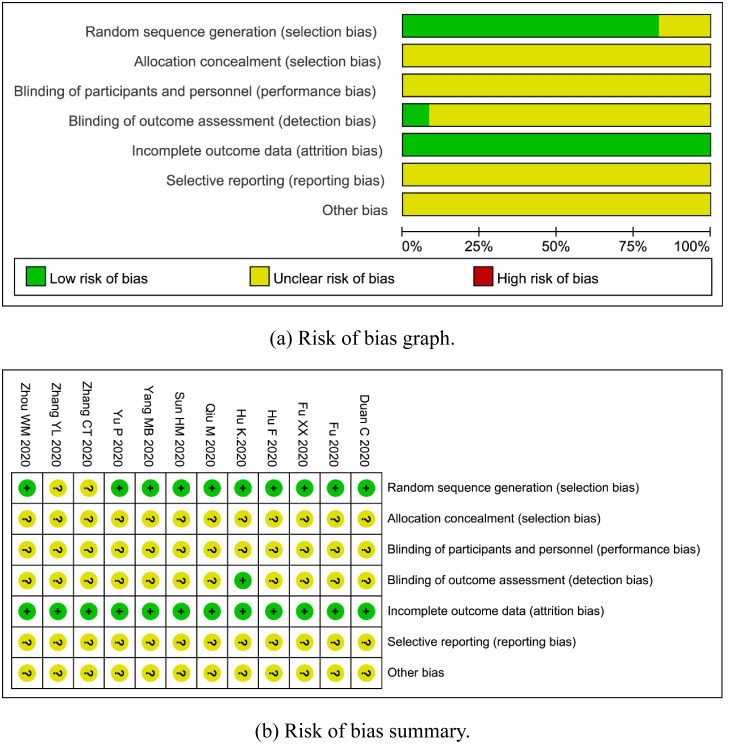
The results of risk of bias assessment are shown in (Fig 2a) and (Fig 2b). In general, the quality of methodology included in this review was not high. Most of the RCTs did not clearly state detection bias, and all of them did not explicitly report allocation concealment, performance bias, and reporting bias.
Fig 2. Assessment of methodological quality.
(a) Risk of bias graph. (b) Risk of bias summary.
Description of CHM
The components of CHM are listed in (Table 2). Nine oral CHM were used in this review, including Jinhua Qinggan granule [15], Toujie Quwen granule [16, 17], Jinyinhua oral liquid [18, 25], Lianhua Qingwen capsule (granule) [19, 23], Maxing Xuanfei Jiedu Decoction [20], Lianhua Qingke granule [21], Reyanning mixture [22], Jiawei Dayuan Decoction [24], diammonium glycyrrhizinate [26].
Table 2. The components of CHM.
| References | CHM | Components |
|---|---|---|
| Duan C [15] | Jinhua Qinggan granule | Jinyinhua 10g, Shigao 10g, Mahuang (processed with honey) 10g, Kuxingren (stir-frying) 10g, Huangqin 10g, Lianqiao 10g, Zhebeimu 10g, Zhimu 10g, Niubangzi 10g, Qinghao 10g, Bohe 10g, Gancao10g |
| Fu [16] | Toujie Quwen granule | Lianqiao 30g, Shancigu 20g, Jinyinhua 15g, Huangqin 10g, Daqingye 10g, Chaihu 5g, Qinghao 10g, Chantui 10g, Qianhu 5g, Chuanbeimu 10g, Zhebeimu 10g, Wumei 30g, Xuanshen 10g, Huangqi 45g, Fuling 30g, Taizishen 15g |
| Fu XX [17] | Toujie Quwen granule | Lianqiao 30g, Shancigu 20g, Jinyinhua 15g, Huangqin 10g, Daqingye 10g, Chaihu 5g, Qinghao 10g, Chantui 10g, Qianhu 5g, Chuanbeimu 10g, Zhebeimu 10g, Wumei 30g, Xuanshen 10g, Huangqi 45g, Fuling 30g, Taizishen 15g |
| Hu F [18] | Jinyinhua oral liquid | Jinyinhua 5.4g |
| Hu K [19] | Lianhua Qingwen capsule | Lianqiao, Jinyinhua, Mahuang (stir-frying), Kuxingren (stir-frying), Shigao, Banlangen, Guanzhong, Yuxingcao, Huoxiang, Dahuang, Hongjingtian, Bohe, Gancao |
| Qiu M [20] | Maxing Xuanfei Jiedu Decoction | Mahuang 9g, Kuxingren 12g, Shigao 15~30g, Zhebeimu 12g, Chantui 10g, Jiangchan 15g, Jianghuang 12g, Jiegeng 12g, Zhiqiao 12g, Caoguo 9g, Caodoukou 12g |
| Sun HM [21] | Lianhua Qingke granule | Mahuang, Sangbaipi, Kuxingren (stir-frying), Lianqiao, mountain honeysuckle, Dahuang |
| Yang MB [22] | Reyanning mixture | Pugongying, Huzhang, Baijiang Herba cum Radice, Banzhilian |
| Yu P [23] | Lianhua Qingwen granule | Lianqiao, Jinyinhua, Mahuang (stir-frying), Kuxingren (stir-frying), Shigao, Banlangen, Guanzhong, Yuxingcao, Huoxiang, Dahuang, Hongjingtian, Bohe, Gancao |
| Zhang CT [24] | Jiawei Dayuan Decoction | Mahuang (stir-frying) 10g, Xingren 15g, crude gypsum 20g, trichosanthes bark 20g, Dahuang (Stir-fry with yellow rice wine) 6g, Tinglizi 10g, Taoren 10g, Caoguo 6g, Binglang 10g, Cangzhu 10g |
| Zhang YL [25] | Jinyinhua oral liquid | Jinyinhua 5.4g |
| Zhou WM [26] | diamine glycyrrhizinate | diamine glycyrrhizinate |
The frequency of each Chinese herb in this meta-analysis was also summarized manually. The top 3 ranked Chinese herbs were honeysuckle (58.33%) [15–19, 23, 25], forsythia (50.00%) [15–17, 19, 21, 23], and ephedra (50.00%) [15, 19–21, 23, 24].
Four dosage formulations of oral CHM were included, including granule [15–17, 21, 23, 24], oral liquid [18, 22, 25], capsule [19, 26], and decoction [20]. The most commonly used dosage formulation was granule (50.00%) [15–17, 21, 23, 24].
Efficacy and safety assessment
Clinical efficacy
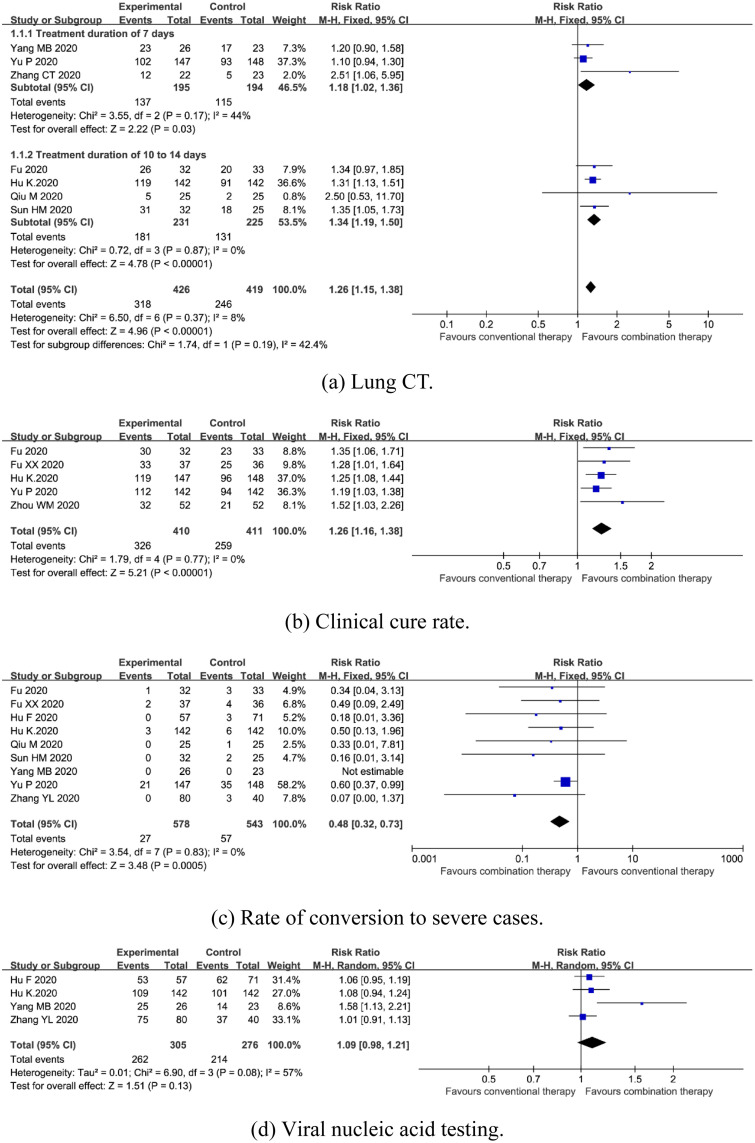
Lung CT. The evaluation criteria for a lung CT refer to the COVID-19 Guidelines for Imaging Assisted Diagnosis [27]. Lung CT can evaluate the curative effect through the parameters basic absorption, improvement, no change, and aggravation. If the lesion range disappears ≥70%, it indicates basic absorption. If the lesion range disappeared ≥30%, it indicates improvement. If there was no change in the lesion range, it indicates no change. If the extent of the lesion increased by ≥30%, it indicates aggravation. The effectiveness of therapy based on lung CT = (basic absorption cases + improvement cases)/total cases × 100%. Seven trials enrolling 845 patients mentioned lung CT [16, 19–24]. A fixed-effects model was used due to no significant heterogeneity (I2 = 8%, P = 0.37). Meta-analysis revealed that combination therapy could significantly improve lung CT [RR = 1.26, 95%CI (1.15, 1.38), P<0.00001] (Fig 3a). Subgroup analysis showed that there was a significant difference between subgroups with 7 days of treatment duration (P = 0.03) and 10 to 14 days of treatment duration (P<0.00001) (Fig 3a).
Fig 3.
Forest plot of the effects of combination therapy for outcomes of (a) lung CT, (b) clinical cure rate, (c) rate of conversion to severe cases, (d) viral nucleic acid testing.
Clinical cure rate. Clinical cure standards refer to Guiding Principles for Clinical Research of New Chinese Materia Medica [28]. Therapeutic effects are classified as effective, improved, and ineffective. If the TCM symptom score is reduced by more than 70%, it suggests effectiveness. If the TCM symptom score is reduced by more than 30%, it represents improved symptoms. If the TCM symptom score is reduced by less than 30%, it represents ineffective treatment. Clinical cure rate = (effective cases + improved cases)/total cases × 100%. Five trials enrolling 821 participants reported clinical cure rate [16, 17, 19, 22, 26]. A fixed-effects model was used due to no significant heterogeneity (I2 = 0%, P = 0.77). The outcome indicated clinical cure rate in combination therapy was higher than conventional therapy [RR = 1.26, 95%CI (1.16, 1.38), P<0.00001] (Fig 3b).
Rate of conversion to severe cases. Nine trials enrolling 1121 patients reported rate of conversion to severe cases [16–23, 25]. A fixed-effects model was used due to no significant heterogeneity (I2 = 0%, P = 0.83). The results showed that combination therapy could significantly reduce rate of conversion to severe cases [RR = 0.48, 95%CI (0.32, 0.73), P = 0.0005] (Fig 3c).
Viral nucleic acid testing. Negative rate of viral nucleic acid testing = (negative cases at the end of the trial − negative cases before the trial)/total cases × 100%. Four trials enrolling 581 patients reported viral nucleic acid testing [18–19, 22, 25]. A random-effects model was used due to the significant heterogeneity (I2 = 57%, P = 0.08). Meta-analyses revealed no statistical difference in viral nucleic acid testing [RR = 1.09, 95%CI (0.98, 1.21), P = 0.13] (Fig 3d).
Clinical symptoms
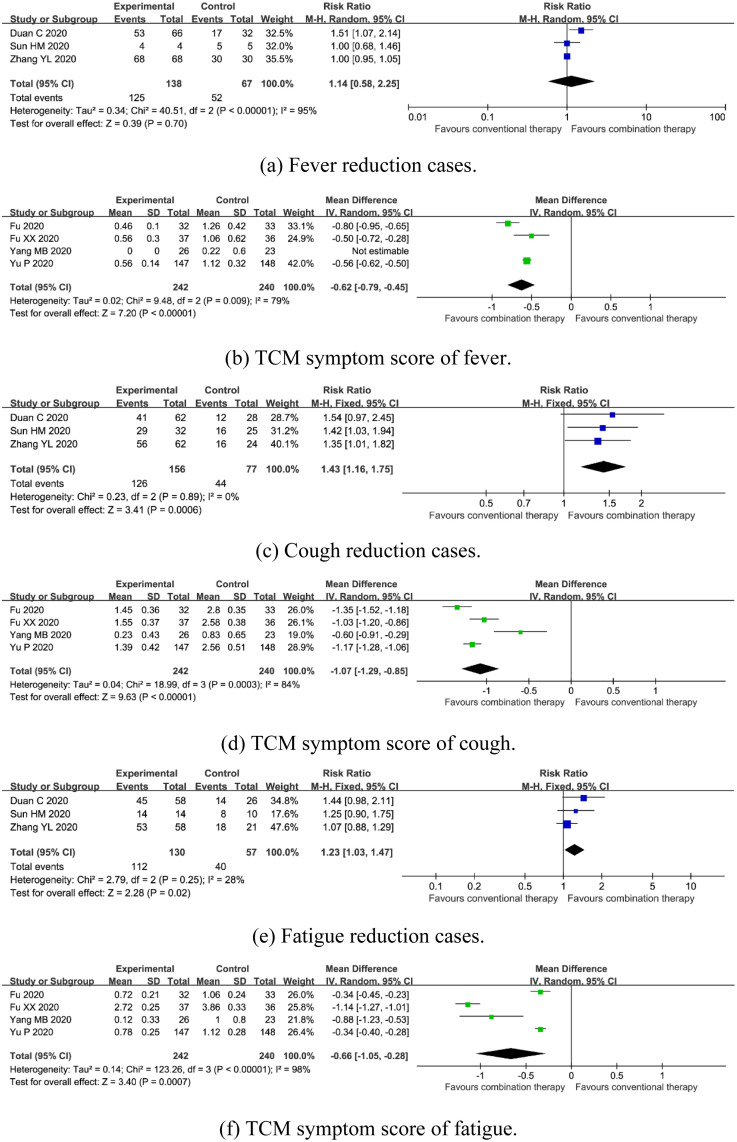
Fever. Three trials enrolling 205 patients mentioned fever reduction cases [15, 21, 25]. A random-effects model was used due to the significant heterogeneity (I2 = 95%, P<0.00001). Meta-analysis showed that there was no statistical difference on fever reduction cases [RR = 1.14, 95%CI (0.58, 2.25), P = 0.70] (Fig 4a). Four trials involved 482 participants reported TCM symptom score of fever [16, 17, 22, 23]. A random-effects model was used due to the significant heterogeneity (I2 = 79%, P = 0.009). The pooled result showed that combination therapy could result in a significant reduction in TCM symptom score of fever [MD = -0.62, 95%CI (-0.79, -0.45), P<0.00001] (Fig 4b).
Fig 4.
Forest plot of the effects of combination therapy for outcomes of (a) fever reduction cases, (b) TCM symptom score of fever, (c) cough reduction cases, (d) TCM symptom score of cough, (e) fatigue reduction cases, (f) TCM symptom score of fatigue.
Cough. Three trials enrolling 205 patients mentioned cough reduction cases [15, 21, 25]. A fixed-effects model was used due to no significant heterogeneity (I2 = 0%, P = 0.89). Meta-analyses revealed that combination therapy could significantly reduce cough cases [RR = 1.43, 95%CI (1.16, 1.75), P = 0.0006] (Fig 4c). Four trials enrolling 482 participants reported TCM symptom score of fever [16, 17, 22, 23]. A random-effects model was used due to the significant heterogeneity (I2 = 84%, P = 0.0003). The pooled estimate found combination therapy decreased TCM symptom score of cough [MD = -1.07, 95%CI (-1.29, -0.85), P<0.00001] (Fig 4d).
Fatigue. Three trials enrolling 205 patients mentioned fatigue reduction cases [15, 21, 25]. A fixed-effects model was used due to no significant heterogeneity (I2 = 28%, P = 0.25). Meta-analysis showed that combination therapy could significantly reduce fatigue cases [RR = 1.23, 95%CI (1.03, 1.47), P = 0.02] (Fig 4e). Four trials enrolling 482 participants reported TCM symptom score of fever [16, 17, 22, 23]. A random-effects model was used due to the significant heterogeneity (I2 = 98%, P<0.00001). The pooled result found combination therapy decreased TCM symptom score of fatigue [MD = -0.66, 95%CI (-1.05, -0.28), P = 0.0007] (Fig 4f).
Inflammatory biomarkers
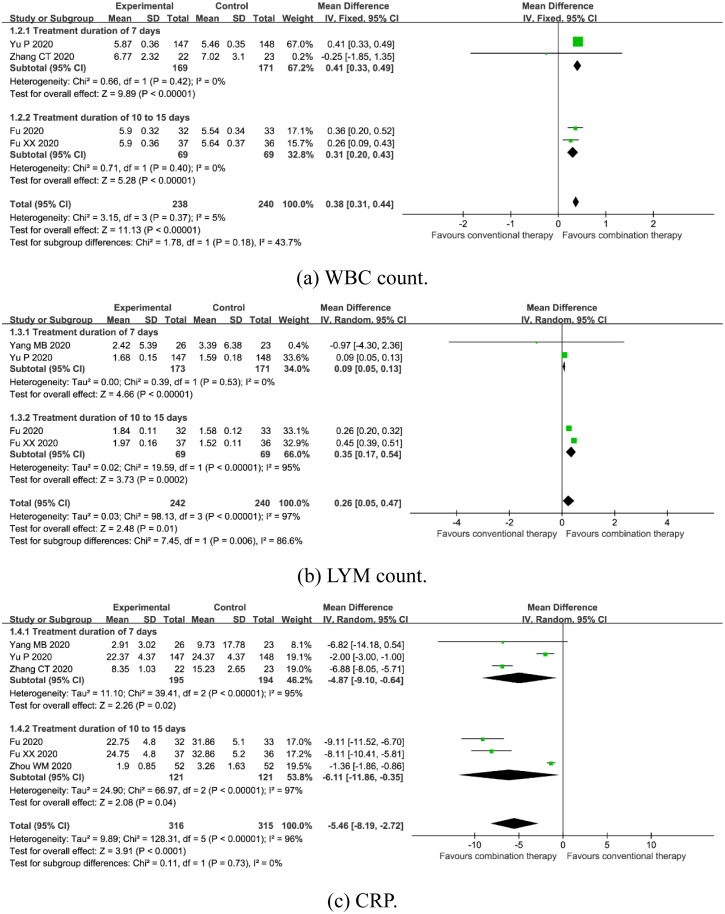
WBC count. Four trials enrolling 478 participants mentioned WBC count [16, 17, 23, 24]. A fixed-effects model was used due to no significant heterogeneity (I2 = 5%, P = 0.37). Meta-analysis revealed that combination therapy could significantly increase WBC count [MD = 0.38, 95%CI (0.31, 0.44), P<0.00001] (Fig 5a). Subgroup analysis showed that there was a significant difference between subgroups with 7 days of treatment duration (P<0.00001) and 10 to 15 days of treatment duration (P<0.00001) (Fig 5a).
Fig 5.
Forest plot of the effects of combination therapy for outcomes of (a) WBC count, (b) LYM count, (c) CRP.
LYM count. Four trials enrolling 482 participants reported LYM count [16, 17, 22, 23]. A random-effects model was used due to the significant heterogeneity (I2 = 97%, P<0.00001). The pooled estimate found combination therapy increased LYM count [MD = 0.26, 95%CI (0.05, 0.47), P = 0.01] (Fig 5b). Subgroup analysis showed that there was a significant difference between subgroups with 7 days of treatment duration (P<0.00001) and 10 to 15 days of treatment duration (P = 0.0002) (Fig 5b).
CRP. Six trials enrolling 631 participants reported CRP [16, 17, 22–24, 26]. A random-effects model was used due to the significant heterogeneity (I2 = 96%, P<0.00001). The pooled result found combination therapy decreased CRP [MD = -5.46, 95%CI (-8.19, -2.72), P<0.0001] (Fig 5c). Subgroup analysis showed that there was a significant difference between subgroups with 7 days of treatment duration (P = 0.02) and 10 to 15 days of treatment duration (P = 0.04) (Fig 5c).
Adverse drug events
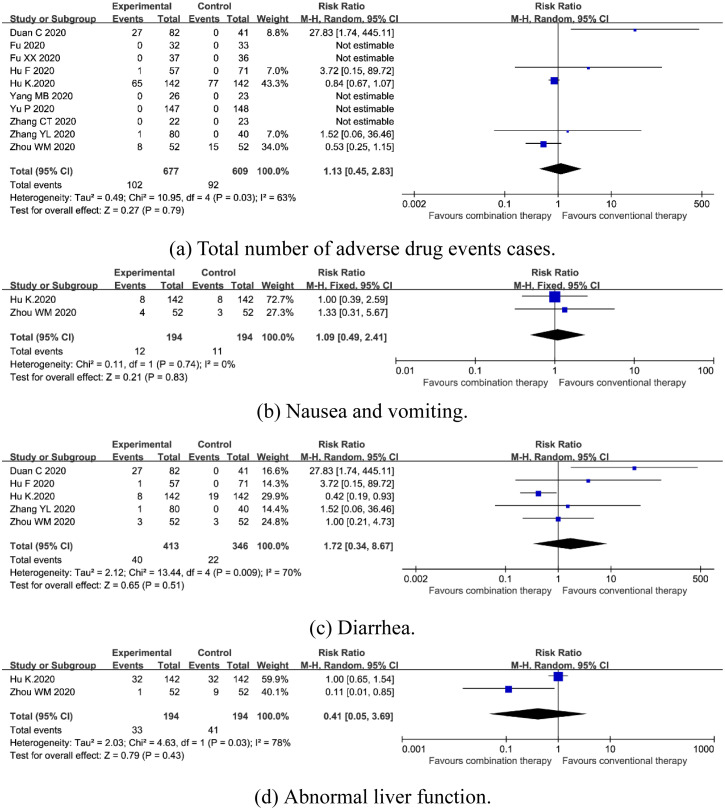
Total number of adverse drug events cases. Ten trials enrolling 1286 participants reported total number of adverse drug events cases [15–19, 22–26]. A random-effects model was used due to the significant heterogeneity (I2 = 63%, P = 0.03). Meta-analyses revealed no statistical difference in total number of adverse drug events cases [RR = 1.13, 95%CI (0.45, 2.83), P = 0.79] (Fig 6a).
Fig 6.
Forest plot of the safety of combination therapy for outcomes of (a) total number of adverse drug events cases, (b) nausea and vomiting, (c) diarrhea, (d) abnormal liver function.
Nausea and vomiting. Two trials enrolling 388 participants reported nausea and vomiting [19, 26]. A fixed-effects model was used due to no significant heterogeneity (I2 = 0%, P = 0.74). Subgroup analysis suggested no statistical difference in nausea and vomiting [RR = 1.09, 95%CI (0.49, 2.41), P = 0.83] (Fig 6b).
Diarrhea. Five trials enrolling 759 participants reported total number of adverse drug events cases [15, 18, 19, 25, 26]. A random-effects model was used due to the significant heterogeneity (I2 = 70%, P = 0.009). Subgroup analysis showed no statistical difference in diarrhea [RR = 1.72, 95%CI (0.34, 8.67), P = 0.51] (Fig 6c).
Abnormal liver function. Two trials enrolling 388 participants reported total number of adverse drug events cases [19, 26]. A random-effects model was used due to the significant heterogeneity (I2 = 78%, P = 0.03). Subgroup analysis revealed no statistical difference in abnormal liver function [RR = 0.41, 95%CI (0.05, 3.69), P = 0.43] (Fig 6d).
One trial reported that there were 8 cases of poor appetite, 1 case of headache, and 8 cases of renal dysfunction in combination therapy group [19].
Sensitivity analysis
Sensitivity analysis showed that there was a small change in the effect amount, and a significant difference in lung CT, clinical cure rate, rate of conversion to severe cases, TCM symptom score of fever, cough reduction cases, TCM symptom score of cough, TCM symptom score of fatigue, WBC count, and CRP, which indicated the above meta-analysis results to be robust.
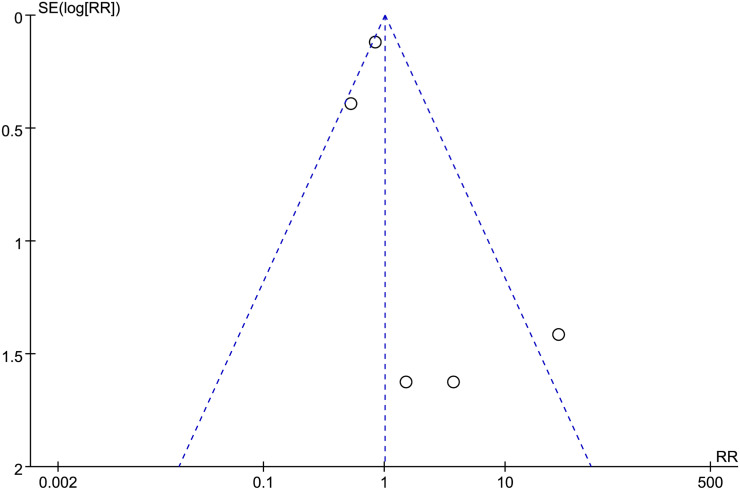
Publication bias
In our study, ten trials reported adverse drug events [15–19, 22–26]. Among them, five trials reported that no adverse drug events were observed [16, 17, 22–24]. The funnel plot was used to analyze the reported adverse events trials to explore the bias (Fig 7). The funnel plot is symmetrical, indicating no obvious deviation.
Fig 7. Adverse drug events trials.
Discussion
The clinical classification of COVID-19 is mild, moderate, severe, and critical [7]. Severe COVID-19 is more likely to have serious complications, such as shock, acute respiratory distress syndrome (ARDS), arrhythmia, and acute heart injury [29, 30], all of which significantly increase the difficulty and cost of treatment. Therefore, it is of great significance to prevent COVID-19 from developing from mild or moderate to severe. In our study, it was found that compared with conventional therapy alone, mild to moderate COVID-19 patients treated with combination therapy of CHM and conventional therapy had more benefit. Similar studies have shown that CHM has positive effects in COVID-19 patients [31–33]. Facing such a severe COVID-19 epidemic, Western countries should pay attention to the therapeutic effect of CHM for COVID-19.
According to the theory of traditional Chinese medicine (TCM), epidemic disease refers to an acute infectious disease characterized by sudden onset, rapid transmission, dangerous conditions, and strong infectivity after feeling pestilence evil [34]. COVID-19 is an "epidemic disease" of TCM in light of its incidence mode and epidemic trend [7]. The pathogenesis of mild to moderate COVID-19 is dampness-heat or cold-dampness obstructing the lung [7]. Therefore, CHM, with the effect of clearing heat, eliminating dampness, resolving phlegm, and dispersing cold, is widely used [7]. In the included studies, nine different oral CHM were used, including Lianhua Qingwen capsules and granules, Toujie Quwen granules, Jinyinhua oral liquids, diammonium glycyrrhizinate, etc. Lianhua Qingwen capsules originate from classical Chinese herbal formulas and can decrease influenza A virus (H1N1) replication, lung lesions, and inflammation [35]. Additionally, Lianhua Qingwen capsules may reduce lung injury and help eliminate SARS-CoV-2 infection by regulating Akt1 [36]. One study has shown that Toujie Quwen granules may have therapeutic effects on COVID-19 by regulating SARS-CoV-2 infection, immune and inflammation-related targets, and pathways [37]. Diammonium glycyrrhizinate is used as a hepatic protector and is the main component of licorice root extracts [38]. Diammonium glycyrrhizinate can decrease serum ALT and AST levels, improve histological damage, downregulate inflammatory cytokines, and inhibit the apoptosis of T lymphocytes in the thymus [38].
Among the nine oral CHM, the most frequently used Chinese herb was honeysuckle, followed by forsythia and ephedra. Honeysuckle and forsythia have the function of clearing heat toxicity and dispersing wind heat in the theory of TCM [5]. Honeysuckle polysaccharide is an active component of honeysuckle that can regulate nonspecific immunity [39], inhibit the expression of the inflammatory factors TNF-α and IL-1β [40], and inhibit a variety of viruses [41]. Phillyrin is an active component of forsythia that has antiviral and anti-inflammatory activities [42, 43]. Ephedra has the function of dissipating cold and diffusing the lung to calm panting in TCM theory [5]. Ephedrine is an active component of ephedra that can increase the production of the anti-inflammatory cytokine IL-10, reduce the production of the proinflammatory cytokines TNF-α and IL-12 [44], and play an antiviral role by inhibiting viral replication [45].
Mild to moderate COVID-19 patients treated with combination therapy of CHM and conventional therapy had better outcomes in parameters including clinical efficacy, clinical symptoms, and inflammatory response. Our study found that compared with conventional therapy alone, combination therapy could improve the scores of symptoms such as fever, cough, and fatigue and reduce cough cases. Combination therapy could increase WBC count and decrease CRP. This is related to the fact that CHM can improve the host immune response and downregulate inflammatory cytokines [35, 38, 46]. Immunopathological changes, including relatively lower levels of WBCs and LYMs and markedly higher levels of CRP and inflammatory cytokines, are correlated with COVID-19 severity [47, 48]. Immune suppression and inflammatory injury are also important drivers of COVID-19 progression [49]. Cytokine storm is a hyperproduction of inflammatory cytokines, which can lead to ARDS aggravation and widespread tissue damage resulting in acute lung injury, multiorgan failure and death [50, 51]. Targeting cytokines during the management of COVID-19 patients could improve survival rates [51]. In our study, we also found that combination therapy had a better effect on improving lung CT parameters, promoting the clinical cure rate, and reducing the rate of conversion to severe cases.
Due to different formulations and unclear compositions, CHM has many unknown factors to be solved. In our study, we found that CHM formulations used in the combination therapy group were different, and the quality of herbal intervention was unclear. CHM is likely to require a standard treatment. In addition, the quality of herbal formulas should be monitored through standardization. In this way, the best evidence can be systematically summarized to better provide an evidence-based basis for TCM decision-making. CHM treatment, which is based on individualized assessment, can be affected by different diet practices and weather, resulting in its difficulty of use in Western countries. Therefore, we think it is necessary for Western countries to hire TCM experts to participate in the treatment of COVID-19. Safety issues should be a concern when CHM is used for COVID-19. In our study, we found that most of the included trials reported adverse drug events. Combination therapy did not increase adverse drug events. The funnel plot of adverse drug events indicated no obvious deviation.
However, it was a common problem that most of the included trials had poor methodological design and that the merger statistical analysis of some outcomes had unexplained heterogeneity. More high-quality trials are needed in the future. Despite the poor methodology and unexplained heterogeneity, our findings are very valuable and timely in view of the lack of specific drugs approved for COVID-19.
Limitations
Despite the usefulness of our findings, this review also has several limitations that could be improved upon in future studies. First, most of the included trials had deficiencies in methodology design, including hidden allocation and inadequate reporting of blind methods. Second, the composition, dosage, and frequency of CHM were different in the treatment groups. Third, the multicenter trials were lacking. In addition, the duration of the included trials ranged from 5 to 15 days. Therefore, it is necessary to design more high-quality trials with a multicenter, larger sample size, and longer follow-up to better observe the efficacy and possible adverse events of CHM combined with conventional therapy in the treatment of mild to moderate COVID-19.
Conclusion
Chinese herbal medicine combined with conventional therapy could be effective and safe in the treatment of mild to moderate COVID-19. Combination therapy can improve the clinical cure rate, main clinical symptoms, imaging and laboratory indexes, and reduce the rate of conversion to severe cases. However, because COVID-19 is a sudden disease, it is difficult to carry out double-blind clinical trials, which leads to insufficient methodology in the existing related trials. Therefore, more high-quality trials are needed to evaluate the efficacy and safety of Chinese herbal medicine combined with conventional therapy in the treatment of adults with mild to moderate COVID-19 in the future.
Supporting information
(DOC)
Data Availability
All relevant data are within the paper and its Supporting information files.
Funding Statement
This work was supported by the National Natural Science Foundation of China (No.81573860), Chongqing Medical University Postdoctoral Foundation (No. R11004), and Chongqing Postdoctoral Special Foundation (Yuren Social Office [2020] No. 379). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Sattler A, Angermair S, Stockmann H, et al. SARS-CoV-2-specific T cell responses and correlations with COVID-19 patient predisposition [J]. J Clin Invest, 2020,130(12):6477–6489. doi: 10.1172/JCI140965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. WHO Coronavirus (COVID-19) Dashboard.2021 [EB/OL]. https://covid19.who.int/ (accessed Mar 26, 2021).
- 3.You C, Deng Y, Hu W, et al. Estimation of the time-varying reproduction number of COVID-19 outbreak in China [J]. Int J Hyg Environ Health, 2020,228:113555. doi: 10.1016/j.ijheh.2020.113555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ni L, Chen L, Huang X, et al. Combating COVID-19 with integrated traditional Chinese and Western medicine in China [J]. Acta Pharm Sin B, 2020,10(7):1149–1162. doi: 10.1016/j.apsb.2020.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gao XM. Traditional Chinese Pharmacy (advanced series of traditional Chinese medicine) [M]. Beijing: People’s Health Publishing House, 2000. [Google Scholar]
- 6.Diagnosis and Treatment Guideline for COVID-19 (Trial 8th Edition) [J]. Chin J Viral Dis, 2020,10(05):321–328. [Google Scholar]
- 7.Wu ZY, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019(COVID-19) outbreak in China [J]. J Am Med Assoc, 2020, 323(13): 1239–1242. [DOI] [PubMed] [Google Scholar]
- 8.Tian J, Yan S, Wang H, et al. Hanshiyi Formula, a medicine for Sars-CoV2 infection in China, reduced the proportion of mild and moderate COVID-19 patients turning to severe status: A cohort study [J]. Pharmacol Res, 2020,161:105127. doi: 10.1016/j.phrs.2020.105127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang XY, Lv L, Zhou YL, et al. Efficacy and safety of Xiyanping injection in the treatment of COVID-19: A multicenter, prospective, open-label and randomized controlled trial [J/OL]. Phytother Res: 7141[2021-05-12]. https://pubmed.ncbi.nlm.nih.gov/33979464/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xin S, Cheng X, Zhu B, et al. Clinical retrospective study on the efficacy of Qingfei Paidu decoction combined with Western medicine for COVID-19 treatment [J]. Biomed Pharmacother, 2020,129:110500. doi: 10.1016/j.biopha.2020.110500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement [J]. Ann Intern Med, 2009,151(4):264–W64. [DOI] [PubMed] [Google Scholar]
- 12.Higgins JPT, Green S. Cochrane Reviewers’ Handbook 5.3.0 [updated March 2014], Review Manager (RevMan) [Computer program]. Version 5.3.0. www.cochrane-handbook.org
- 13.Panahi Y, Hosseini MS, Khalili N, et al. Antioxidant and anti-inflammatory effects of curcuminoid-piperine combination in subjects with metabolic syndrome: A randomized controlled trial and an updated meta-analysis [J]. Clin Nutr, 2015,34(6):1101–1108. doi: 10.1016/j.clnu.2014.12.019 [DOI] [PubMed] [Google Scholar]
- 14.Liu M, Gao Y, Yuan Y, et al. Efficacy and safety of integrated traditional Chinese and western medicine for corona virus disease 2019 (COVID-19): a systematic review and meta-analysis [J]. Pharmacol Res, 2020, 158:104896. doi: 10.1016/j.phrs.2020.104896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duan C, Xia GW, Zheng CJ, et al. Clinical observation on Jinhua Qinggan granule combined with conventional western medicine therapy in treating mild cases of coronavirus disease 2019 [J]. J Tradit Chin Med, 2020,61(17):1473–1477. [Google Scholar]
- 16.Fu XX, Lin LP, Tan XX. Clinical observation on effect of Toujie Quwen granules in treatment of COVID-19 [J]. Chin J Exp Tradit Med Formul, 2020,26(12):44–48. [Google Scholar]
- 17.Fu XX, Lin LP, Tan XX. Clinical study on 37 case of COVID- 19 treated with integrated traditional Chinese and western medicine [J]. Tradit Chin Drug Res Clin Pharmacol, 2020,31(05):600–604. [Google Scholar]
- 18.Hu F, Guo AH, Huang L, et al. Multi-center clinical observation of Jinyinhua oral liquid combined with western medicine in treatment of moderate COVID-19 [J/OL]. J Tradit Chin Med:1–6[2020-11-4]. http://kns.cnki.net/kcms/detail/11.2166.R.20200819.0837.002.html.32227761 [Google Scholar]
- 19.Hu K, Guan WJ, Bi Y, et al. Efficacy and safety of Lianhuaqingwen capsules, a repurposed Chinese herb, in patients with coronavirus disease 2019: A multicenter, prospective, randomized controlled trial [J/OL]. Phytomedicine: 153242[2020-05-16]. https://pubmed.ncbi.nlm.nih.gov/32425361/ doi: 10.1016/j.phymed.2020.153242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qiu M, Li QT, Zhu DP, et al. Efficacy observation of Maxing Xuanfei Jiedu decoction on moderate COVID-19 patients [J]. J Emerg Tradit Chin Med,2020,29(07):1129–1130+1132. [Google Scholar]
- 21.Sun HM, Xu F, Zhang L, et al. Study on clinical efficacy of Lianhua Qingke granule in treatment of mild and ordinary COVID-19 [J]. Chin J Exp Tradit Med Formul,2020,26(14):29–34. [Google Scholar]
- 22.Yang MB, Dang SS, Haung S, et al. Multi-center clinical observation of Reyanning mixture in treatment of COVID-19 [J]. Chin J Exp Tradit Med Formul,2020,26(14):7–12. [Google Scholar]
- 23.Yu P, Li YZ, Wan SB, et al. Clinical efficacy of Lianhua Qingwen granule combined with abidol in treating coronavirus disease 2019 [J]. Chin Pharm J,2020,55(12):1042–1045. [Google Scholar]
- 24.Zhang CT, Yang Y, You FM, et al. Clinical study on COVID-19 from the perspective of "Yidujiashi" theory [J]. Pharmacol Clin Chin Materia Medica,2020,36(02):43–45. [Google Scholar]
- 25.Zhang YL, Lei L, Xu Y, et al. Clinical efficacy of Jinyinhua oral liquid in the treatment of 80 patients with coronavirus disease 2019 [J]. Chin Pharm,2020,29(09):23–26. [Google Scholar]
- 26.Zhou WM, Zhao FM, Li BL, et al. Clinical value of diammonium glycyrrhizinate in treatment of COVID-19 [J]. Chin J Virol,2020,36(02):160–164. [Google Scholar]
- 27.Professional Committee of infection and inflammation radiology of Chinese Research Hospital Association. Guidelines for Imaging Assisted Diagnosis of COVID-19 [J]. Chin J Med Imaging Technol,2020, 36(3):1–11. [Google Scholar]
- 28.Ministry of Health of the People’s Republic of China. Guiding Principles for Clinical Research of New Chinese Materia Medica [M]. Beijing: China Medical Science and Technology Press, 2002:186. [Google Scholar]
- 29.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study [J]. Lancet, 2020,395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang DW, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China [J]. JAMA, 2020, 323(11): 1061–1069. doi: 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiong X, Wang P, Su K, et al. Chinese herbal medicine for coronavirus disease 2019: A systematic review and meta-analysis [J]. Pharmacol Res, 2020, 160:105056. doi: 10.1016/j.phrs.2020.105056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeng M, Li L, Wu Z. Traditional Chinese medicine Lianhua Qingwen treating corona virus disease 2019(COVID-19): Meta-analysis of randomized controlled trials [J]. PLoS One, 2020, 15(9): e0238828. doi: 10.1371/journal.pone.0238828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ang L, Song E, Lee HW, et al. Herbal medicine for the treatment of coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis of randomized controlled trials [J]. J Clin Med, 2020,9(5):1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang J. Warm Diseases [M]. Beijing: People’s Health Publishing House, 2006. [Google Scholar]
- 35.Gao D, Niu M, Wei SZ, et al. Identification of a pharmacological biomarker for the bioassay-based quality control of a thirteen-component TCM formula (Lianhua Qingwen) used in treating influenza A virus (H1N1) infection [J]. Front Pharmacol, 2020,11:746. doi: 10.3389/fphar.2020.00746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xia QD, Xun Y, Lu JL, et al. Network pharmacology and molecular docking analyses on Lianhua Qingwen capsule indicate Akt1 is a potential target to treat and prevent COVID-19 [J]. Cell Prolif, 2020,53(12):e12949. doi: 10.1111/cpr.12949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ye M, Luo G, Ye D, et al. Network pharmacology, molecular docking integrated surface plasmon resonance technology reveals the mechanism of Toujie Quwen Granules against coronavirus disease 2019 pneumonia [J/OL]. Phytomedicine: 153401 [2020-10-28]. https://pubmed.ncbi.nlm.nih.gov/33191068/ doi: 10.1016/j.phymed.2020.153401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gao M, Li X, He L, et al. Diammonium glycyrrhizinate mitigates liver injury via inhibiting proliferation of NKT cells and promoting proliferation of tregs [J]. Drug Des Devel Ther,2019,13:3579–3589. doi: 10.2147/DDDT.S220030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou XN, Dong Q, Kan XZ, et al. Immunomodulatory activity of a novel polysaccharide from Lonicera japonica immunosuppressed mice induced by cyclophosphamide [J]. PloS one,2018,13(10):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bai XY, Chai YW, Shi WL, et al. Lonicera japonica poly-saccharides attenuate ovalbumin-induced allergic rhinitis by regulation of Th17 cells in BALB/c mice [J]. J Functional Foods,2020,65:103758. [Google Scholar]
- 41.Wang J, Hou L, Chen YQ, et al. Extraction and purification of the Lonicera japonica polysaccharide and its antiviral activity in vitro [J]. Chin J Hosp Pharm, 2018,38(8): 810–812. [Google Scholar]
- 42.Qu XY, Li QJ, Zhang HM, et al. Protective effects of phillyrin against influenza a virus in vivo [J]. Arch Pharm Res, 2016, 39(7):998–1005.29. doi: 10.1007/s12272-016-0775-z [DOI] [PubMed] [Google Scholar]
- 43.Jiang Q, Chen J, Long X, et al. Phillyrin protects mice from traumatic brain injury by inhibiting the inflammation of microglia via PPARγ signaling pathway [J]. Int Immunopharmacol, 2020,79:106083. doi: 10.1016/j.intimp.2019.106083 [DOI] [PubMed] [Google Scholar]
- 44.He W, Ma J, Chen Y, et al. Ephedrine hydrochloride protects mice from staphylococcus aureus-induced peritonitis [J]. Am J Transl Res, 2018,10(3):670–683. [PMC free article] [PubMed] [Google Scholar]
- 45.Wei W, Du H, Shao C, et al. Screening of antiviral components of Ma Huang Tang and investigation on the ephedra alkaloids efficacy on influenza virus type A [J]. Front Pharmacol, 2019,10:961. doi: 10.3389/fphar.2019.00961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sun CY, Sun YL, Li XM. The role of Chinese medicine in COVID-19 pneumonia: A systematic review and meta-analysis [J]. Am J Emerg Med, 2020,38(10):2153–2159. doi: 10.1016/j.ajem.2020.06.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ganji A, Farahani I, Khansarinejad B, et al. Increased expression of CD8 marker on T-cells in COVID-19 patients [J]. Blood Cells Mol Dis, 2020,83:102437. doi: 10.1016/j.bcmd.2020.102437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen G, Wu D, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019 [J]. J Clin Invest, 2020,130(5):2620–2629. doi: 10.1172/JCI137244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yan W, Chen D, Bigambo FM, et al. Differences of blood cells, lymphocyte subsets and cytokines in COVID-19 patients with different clinical stages: a network meta-analysis [J]. BMC Infect Dis, 2021,21(1):156. doi: 10.1186/s12879-021-05847-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fara A, Mitrev Z, Rosalia RA, et al. Cytokine storm and COVID-19: a chronicle of pro-inflammatory cytokines [J]. Open Biol,2020,10(9):200160. doi: 10.1098/rsob.200160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ragab D, Salah Eldin H, Taeimah M, et al. The COVID-19 cytokine storm; what we know so far [J]. Front Immunol, 2020,11:1446. doi: 10.3389/fimmu.2020.01446 [DOI] [PMC free article] [PubMed] [Google Scholar]