Highlights
-
•
Ribosome display used for recombinant antibody selection and scFv generation targeting CCK-BR.
-
•
Engineered scFv have stronger affinity and increased tissue penetrability due to smaller size.
-
•
Single dose scFv reduces hypersensitivity, anxiety- and depression-like behavior in two models.
-
•
Reduced neuronal firing frequency in TG primary neuronal cultures treated in vitro.
-
•
CCK-BR scFv is ideal for neuropathic pain with both nociceptive and emotional components.
Keywords: Chronic pain, Anxiety, Depression, Eukaryotic ribosome display, scFv
Abbreviations: ANOVA, analysis of variance; ARM, antibody ribosome mRNA; BBB, blood–brain barrier; CCK-8, cholecystokinin octapeptide; CCK-BR, cholecystokinin B receptor; CPP, conditioned place preference; DRG, dorsal root ganglia; FRICT-ION, foramen rotundum inflammatory compression trigeminal infraorbital nerve model; GPCR, G-protein-coupled receptor; IACUC, Institutional Animal Care and Use Committee; ION, infraorbital nerve; ms, milliseconds; MΩ, megaOhms; pA, picoAmps; PBS, phosphate buffered saline; scFv, single-chain Fragment variable antibody; SEM, standard error of the mean; TG, trigeminal ganglia
Abstract
The cholecystokinin B receptor and its neuropeptide ligand are upregulated in chronic neuropathic pain models. Single-chain Fragment variable antibodies were generated as preferred non-opioid targeting therapy blocking the cholecystokinin B receptor to inhibit chronic neuropathic pain models in vivo and in vitro. Engineered antibodies of this type feature binding activity similar to monoclonal antibodies but with stronger affinity and increased tissue penetrability due to their smaller size. More importantly, single-chain Fragment variable antibodies have promising biotherapeutic applications for both nervous and immune systems, now recognized as interactive in chronic pain. A mouse single-chain Fragment variable antibody library recognizing a fifteen amino acid extracellular peptide fragment of the cholecystokinin B receptor was generated from immunized spleens. Ribosome display, a powerful cell-free technology, was applied for recombinant antibody selection. Antibodies with higher affinity, stability, solubility, and binding specificity for cholecystokinin B not A receptor were selected and optimized for in vivo and in vitro efficacy. A single dose of the lead candidate reduced mechanical and cold hypersensitivity in two rodent models of neuropathic pain for at least seven weeks. Continuing efficacy was evident with either intraperitoneal or intranasal dosing. Likewise, the lead single-chain Fragment variable antibody totally prevented development of anxiety- and depression-like behaviors and cognitive deficits typical in the models. Reduction of neuronal firing frequency was evident in trigeminal ganglia primary neuronal cultures treated in vitro with the cholecystokinin B receptor antibody. Immunofluorescent staining intensity in the trigeminal neuron primary cultures was significantly reduced incrementally after overnight binding with increasingly higher dilutions of the single-chain Fragment variable antibody. While it is reported that single-chain Fragment variable antibodies are removed systemically within 2–6 h, Western blot evidence indicates the His-tag marker remained after 7 weeks in the trigeminal ganglia and in the dorsolateral medulla, providing evidence of brain and ganglia penetrance known to be compromised in overactivated states. This project showcases the in vivo efficacy of our lead single-chain Fragment variable antibody indicating its potential for development as a non-opioid, non-addictive therapeutic intervention for chronic pain. Importantly, studies by others have indicated treatments with cholecystokinin B receptor antagonists suppress maintenance and reactivation of morphine dependence in place preference tests while lowering tolerance and dose requirements. Our future studies remain to address these potential benefits that may accompany the cholecystokinin B receptor biological therapy. Both chronic sciatic and orofacial pain can be unrelenting and excruciating, reducing quality of life as well as diminishing physical and mental function. An effective non-opiate, non-addictive therapy with potential to significantly reduce chronic neuropathic pain long term is greatly needed.
Introduction
Single-chain Fragment variable antibodies (scFvs) are opening a new era of therapeutics, pharmacology, and pathophysiology research for trigeminal nerve syndromes (Neal et al., 2016, Ayoub et al., 2017). These technologies have overcome previous challenges of providing therapeutic applications for G-protein-coupled receptors (GPCRs) (Ayoub et al., 2017). More importantly, these small antibodies (~27 kDa) are brain penetrant and praised as having promising biotherapeutic applications for the nervous and immune systems, now recognized as interactive in chronic pain. Several scFvs are being investigated as therapeutics for arthritis, Creutzfeldt-Jakob, and Huntington’s disease due to their solubility, small size, and ability to cross the blood–brain barrier (BBB) compared to monoclonal antibodies (Robert et al., 2009, Butler et al., 2012, Škrlj et al., 2013, Angelini et al., 2018, Dodick et al., 2019, Raffaelli et al., 2019, Ossipov et al., 2020, Dreier and Plückthun, 2018). More typically monoclonal antibodies and small-molecule receptor antagonists are available for migraine (Galcanezumab, Erenumab) (Raffaelli et al., 2019, Ossipov et al., 2020, Dreier and Plückthun, 2018). Despite the popularity of scFvs generated by ribosome display for chemotherapy, obtaining high-affinity scFvs from ribosome display libraries has remained a challenging task (Dreier and Plückthun, 2018, Ahmad et al., 2012). Addressing this gap we have engineered scFvs directed to a fifteen amino acid (15-a.a.) extracellular peptide of the receptor for neuropeptide cholecystokinin B (CCK-B) using a more robust recombinant cell-free platform technology and affinity maturation. The CCK-B receptor scFv lead is effective for reduction of pain related behaviors, anxiety, and depression with the ultimate goal of reducing/eliminating use of continuing opioid analgesics for chronic pain treatment for patients.
Rationale for targeting CCK-B receptors for chronic pain
Rationale for targeting the CCK-B receptors is clearly supported in the literature. Activating CCK-B receptors with agonist CCK-8 increases pain scores in placebo-controlled patients with spontaneous pain more so than in controls (Roberts-Thomson et al., 1992). Accompanying increases in nausea in the study were restricted to patients with irritable bowel syndrome and patients with pain after cholecystectomy.
CCK-B is also involved in several different aspects of the human pain experience that are particularly prominent in females including anxiety (Adams et al., 1995, Rehfeld, 2000, Keppel Hesselink, 2020, Mercer et al., 1996). In placebo-controlled trials a CCK-B antagonist relieved generalized anxiety (Adams et al., 1995, Rehfeld, 2000, Keppel Hesselink, 2020). In addition to anxiety, CCK is also highly involved in stress, reward/addiction, and cognition, evoking dose dependent anxiety/panic attacks in healthy subjects (Kramer et al., 1995, Bradwejn et al., 1991, Bradwejn and Koszycki, 2001, Daugé and Léna, 1998). More importantly, selective CCK-B receptor antagonists enhance morphine analgesia and prevent / reverse tolerance without worsening respiratory depression in non-human primates and without side effects other than orthostatic dizziness in placebo-controlled clinical trials (Kramer et al., 1995, Agnes et al., 2006).
In experimental animals, CCK-B receptor, its neuropeptide ligand, CCK, and RNA are widely expressed in sensory ganglia, spinal cord, glia, and the brain pain and limbic circuitry involved in nociception, stress, anxiety, reward/addiction, and cognition (Mercer et al., 1996, Agnes et al., 2006, Andre et al., 2005, Liang et al., 2020, Ghilardi et al., 1992, Wiesenfeld-Hallin et al., 2002, Kayser et al., 1998, Wiesenfeld-Hallin et al., 1997, Xu et al., 1996, Bras et al., 1999, Kovelowski et al., 2000, Vialou et al., 2014, Manning et al., 2017, Jiang et al., 2019). Sites with high levels of CCK-B receptor expression overlap with pain circuitry in the trigeminal ganglia, mPFC, and rostroventral medulla (RVM), a brainstem serotoninergic descending pain modulation site (Heinricher et al., 2001). As an example, injecting CCK-B receptor blockers/antagonists into the RVM of rodents reverses mechanical hypersensitivity (Kovelowski et al., 2000, Xie et al., 2005, Vanegas and Schaible, 2004). Axotomy results in CCK upregulation in sensory neurons (30%) after 14 days (Wiesenfeld-Hallin et al., 1997, Bras et al., 1999, Xu et al., 1993). In fact, CCK-B receptor expression changes over time are contributory to chronic pain in a variety of animal models (Gutierrez‐Mecinas et al., 2019, Bangash et al., 2018, Korczeniewska et al., 2018).
Naïve CCK-B receptor deficient mice (CCK-BR KO) are mechanically hyposensitive after sciatic nerve chronic constriction injury model (CCI) (Kõks et al., 2008). Sciatic nerve section is associated with a marked ipsilateral increase in both CCK-B receptor mRNA levels in these ganglia (+70%) at 2 weeks in rats (Bras et al., 1999). Our recent microarray gene chip expression profile data identified > 4-fold upregulation (p < 0.05) of Cckbr in whole trigeminal ganglia (TG) on day 3 and 2.7-fold on day 21, compared to naïves in our chronic trigeminal neuropathic pain model (Danaher et al., 2018). Previous work has also demonstrated 4.7-fold upregulation in dorsal root ganglia (DRG) in a mouse sciatic nerve injury model after 2 weeks (Bangash et al., 2018).
CCK exerts a direct anti-opioid action in both midbrain and medullary structures interacting with CCK 2 (CCK-B) receptor (Kovelowski et al., 2000, Heinricher et al., 2001, Friedrich and Gebhart, 2003). In fact, upregulation of CCK in primary sensory neurons in the experimental sciatic nerve axotomy model antagonizes the antinociceptive effects of opioids, but CKK receptors have no effect on tonic nociceptive responses (Xu et al., 1993). Treatments that specifically block CCK-B receptors suppress maintenance and reactivation of morphine dependence in place preference tests (Mitchell et al., 2006). CCK-B receptor mRNA expression is upregulated in a hindpaw burn injury model in mouse, and while morphine had little efficacy, proglumide—a clinically used non-specific blocker for both CCK-A and CCK-B receptors—reduces hypersensitivity (Yin et al., 2016). Proglumide potentiates morphine and endogenous opiates while reducing tolerance (Watkins et al., 1984). Thus, CCK-B receptor is an ideal candidate to impact both nociceptive and limbic components of chronic pain without an impact on normal nociception, while it can increase effectiveness and reduce tolerance of morphine. These previous studies and others have identified CCK-BR as an important therapeutic target that as yet has no beneficial therapy available.
To address this need, a robust platform technology, i.e. ribosome display in combination with directed molecular evolution (DME) was utilized to develop, characterize, and validate single chain Fragment variable antibody variants as pain therapy directed at the CCK-B receptor.
Methods
Cholecystokinin B (CCK-BR) scFv generation
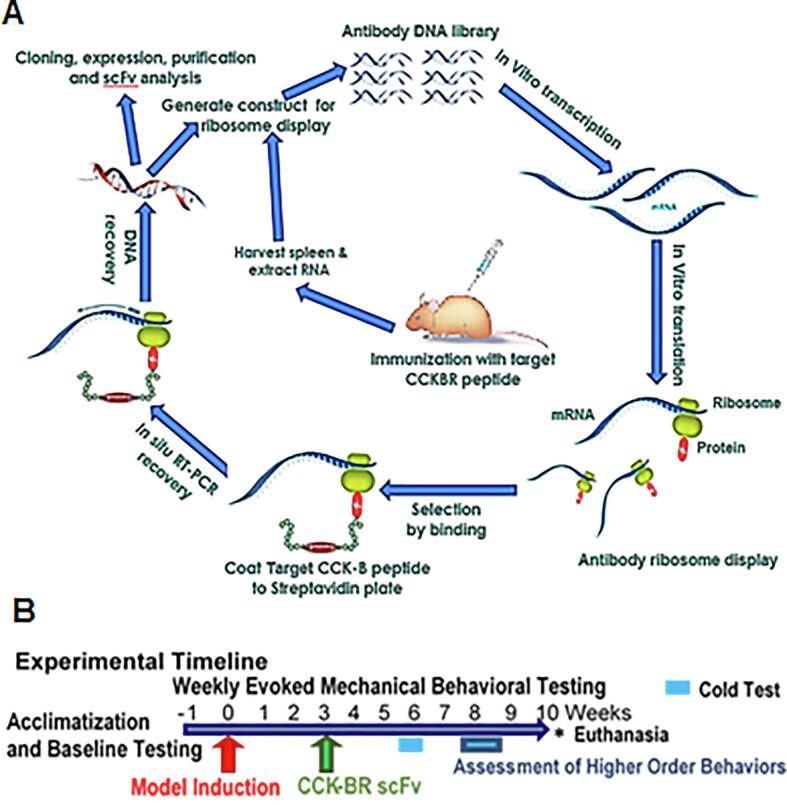
Fig. 1A provides a schematic overview of the method for generating scFv antibodies that bind to a target peptide. The methods of the immunization of mice, panning combinatorial antibody library against CCK-B peptide antigen using in vitro ribosome display, construction of antibody libraries, pull-down and selection, expression, purification, and characterization of antibodies are found in the Supplementary material.
Fig. 1.
Experimental overview. A. Eukaryotic ribosome display selection and CCK-BR scFv antibody generation. The scFv’s were generated using cell-free ribosome display from mice spleens immunized against a human CCK-BR peptide fragment selected at an extracellular binding region, followed by eukaryotic ribosome display selection. B. Timeline for in vivo model induction, behavioral and downstream tissue analysis. After acclimatization the FRICT-ION or SNI neuropathic pain model was induced in anesthetized mice. Three weeks later when hypersensitivity was stable, the CCK-BR scFv 77-2 was administered intraperitoneally. Hypersensitivity testing with von Frey fibers continued weekly. The cold hypersensitivity was assessed in week 6, and anxiety-, depression-, and cognitive-like behaviors were assessed in Weeks 8–9. Tissues were collected at 10 weeks post-injury for immunohistochemical, Western blot, and RNAseq analysis or 3–4 weeks post-injury electrophysiological analysis.
In vivo characterization of CCK-BR scFv efficacy
Fig. 1B illustrates the generalized scheme for the timing of the chronic pain model induction, treatment with CCK-BR scFv 3 weeks after induction of chronic neuropathic pain models, and behavioral testing characterizing the in vivo effects in the mice. After one week acclimatization baseline mechanical hypersensitivity was assessed. Trigeminal or sciatic nerve injury model of chronic neuropathic pain was induced. Three weeks later the single CCK-BR scFv treatment was given (4 mg/kg i.p. injection) to test in vivo efficacy. Nociceptive and anxiety-like behaviors were assessed through the subsequent 7 weeks. Mice were 4.5 months old at experiment end.
FRICT-ION trigeminal nerve injury chronic pain model
The Foramen Rotundum Inflammatory Constriction Trigeminal Infraorbital Nerve injury (FRICT-ION) chronic neuropathic pain model combines chemical irritation with compression of the trigeminal nerve provided by chromic gut suture, simulating wound debris or blunt force traumatic injury (Montera and Westlund, 2020). The FRICT-ION model is easier, quicker (5–10 min/mouse), 100% reliable, and leaves no external indication of a surgery for study blinding. In this study the intraoral FRICT-ION model was induced in both male and female BALB/cAnNHsd mice (at age 9–10 weeks, Envigo Harlan) anesthetized with isoflurane (2–3%), surgical incision was made with a small scalpel puncture at the bucchal cheek crease. A piece of chromic gut suture (3 mm, 4–0) was placed parallel to the infraorbital nerve (ION) and pushed into the tight space to follow the trigeminal maxillary nerve branch (V2) as it passes into the foramen rotundum of the skull. Neuropathic pain in FRICT-ION mice is likely due to continuing mechanical irritation of the nerve during chewing. Mice with sham surgery received anesthesia and the surgical procedure, but no chromic gut suture was inserted. The testing and treatment scheme is shown in Fig. 1B.
Spared nerve injury (SNI) sciatic chronic neuropathic pain model
Efficacy of CCK-BR scFv in some of the tests was determined in male and female BALB/cAnNHsd mice (Envigo Harlan) subjected to the SNI chronic neuropathic pain model (Decosterd and Woolf, 2000, Shields et al., 2003). The tibial and common peroneal nerves were ligated with 5–0 silk and cut distal to the ligation, but the sural nerve was left intact. In sham operations, nerves were exposed but were neither ligated nor cut. The surgery and treatment scheme proceeded as shown in Fig. 1B, similar to the FRICT-ION mice. Surgery was done in mice at 2 months of age. While the primary SNI study dosed the mice at 3 weeks post surgery, a secondary study was performed with dosing at 7 weeks post surgery, with similar efficacy.
In vivo testing of CCK-BR scFv and behavioral characterization of effects
Mechanical sensitivity was tested at baseline prior to surgery, at least weekly, and more frequently after scFv to observe the early effects. Thermal assessments were conducted in week 6 post-treatment. Mechanical and thermal hypersensitivity develop reliably in all FRICT-ION or SNI mice within a week on the snout or hindpaw, respectively. Hypersensitivity remains steadily and persistently through > 10 weeks in both chronic models in the untreated mice. Weight gain equivalent to naïve and sham controls was maintained throughout the studies in both models.
Higher order non-evoked measures were also tested in post-surgical weeks 8–9 in the female and some of the male mice. Anxiety-like behavioral assessments were done using the light/dark box. Depression-related assessments used the sucrose splash test. Novel object recognition test assessed long-term memory and learning. Conditioned place preference assessment of addictive potential was done in naïve male mice comparing morphine and the CCK-BR scFv treatments.
Data for the male mice is shown for many of the assessments. However, data is shown for female mice tested in von Frey, as well as in anxiety- and depression-related spontaneous behavioral assessments relevant to the human condition. All non-reflexive assays were digitally recorded for post hoc computer analysis. Mean experimental results were compared among groups. All studies were performed by an experienced laboratory team blinded to experimental groups. These procedures common in the field have been used to provide ready proof-of-concept, dose-ranging, and efficacy testing for many potential pain therapeutics.
Supplementary material is available to detail all behavioral assay methods.
Isolated TG primary cell cultures
Male mice were euthanized 3–4 weeks after inducing the FRICT-ION model. The TG were dissected, minced, and dissociated in an enzymatic combination containing papain, dispase II and collegenase. Primary cultures were established in 5% CO2 with DMEM culture medium supplemented with 10% fetal bovine serum, 1% antibiotic - antimycotic solution (Sigma). Cells were plated at a density of 1,270 cells/mm (Ayoub et al., 2017) on poly-d-lysine-coated glass coverslips. Supplementary material is available at Brain online.
Whole cell patch-clamp electrophysiology
Neurons with a diameter of < 30 µm were identified by infrared differential interference contrast (IR-DIC) imaging with a microscope connected to an Olympus digital camera. Current clamp recordings were performed using a Molecular Devices Multiclamp 700B (Scientifica, UK). Signals are filtered at 5 KHz, acquired at 50 KHz using a Molecular Devices 1550B converter (Scientifica, UK) and recorded using Clampex 11 software (Molecular Devices, Scientifica, UK). Electrodes are pulled with a Zeitz puller (Werner Zeitz, Martinsreid, Germany) from borosilicate thick glass (GC150F, Sutter Instruments). Electrode resistance was 5–8 MΩ. Bridge balance was applied to all recordings. Intracellular solution contained (in mM) 125 K-gluconate, 6 KCl, 10 HEPES, 0.1 EGTA, 2 Mg-ATP, pH 7.3 with KOH, and osmolarity of 290–310 mOsm. Artificial cerebrospinal fluid (aCSF) contains (in mM) 113 NaCl, 3 KCl, 25 NaHCO3, 1 NaH2PO4, 2 CaCl2, 2 MgCl2, and 11 glucose. For whole-cell current clamp recordings, to evaluate the basic input–output action potential frequency response to hyperpolarization and depolarization, DC current was injected from 0 pA to + 250 pA in 10-pA increments for a duration of 1,000 ms at the cell’s intrinsic resting membrane potential. Data acquisition was sampled at 20 kHz and filtered at 2.4 kHz. Recordings with a series resistance >20 MΩ were discarded, and series resistance was compensated to 70%. Electrophysiological recordings were performed 16–24 hrs after plating cells and after providing direct treatment of the CCK-BR scFv 77-2 (10 µg/ml) applied to culture media for 1–2 hrs prior to recording to the neurons already primed in vivo. In a separate stimulation paradigm, treatment of the cultures was done with CCK8 (100 nM) agonist, with and without treatment with CCK-BR scFv 77-2 (10 µg/mL).
Binding/preadsorption block of trigeminal ganglia neurons for semi-quantitative intensity assessment
Naïve male mice (n = 4) were euthanized and the TG dissected, minced, and dissociated with enzymes (2 mg mL−1 dispase and 1 mg mL−1 collagenase IV (Worthington Biochemical, Lakewood, NJ, USA) at 37 °C). Primary cultures were established in 5% CO2 with D-MEM culture medium supplemented with 10% fetal bovine serum, 1% penicillin/streptomycin and 625 μmol L−1 glutamine, to stop the enzymatic reaction. Immunohistological localization of CCK-BR on the TG primary cultures was done using a commercially available CCK-BR primary antibody (Cat# MBS421039, BioSource, Inc., San Diego, CA) and fluorescent-tagged secondary antibody using conventional methods. Quantitative immunohistology of over 120 cells per condition (30–50 cells per coverslip, 4–6 coverslips) was done using computer assisted fluorescence microscopy analysis was done in three rounds with a minimum of four regions/ coverslip/ animal to obtain mean staining intensities ± standard error of the mean (SEM) for group comparisons.
Western blot
Dorsal root ganglia and brain were collected at necropsy in week 10 and rapidly frozen for storage at minus 80 °C degrees until tested. Protein content was assessed with Western blots using standard methods. Briefly, the sample protein was denatured, followed by gel electrophoresis to separate proteins by molecular weight. An anti-His-tag monoclonal antibody (C-terminal A01857, GenScript) that binds to the His-tag conjugated to the CCK-BR scFv or anti-β-actin antibody (ab8226, Abcam) was applied to the electrophoresis membrane. After washing off the antibody, specific secondary antibodies were added which recognized and bound to the primary antibodies. The secondary antibody was visualized through immunofluorescence probe attached to the secondary antibody, allowing indirect detection, validation, and semi-quantitative assessment of the specific target protein.
Statistical analysisss
Study comparison groups included naïve, surgical sham, and nerve injured male and female mice with and without (w/wo) scFv. The power analysis predicted sufficient power is provided with group size n = 3, based on the pilot von Frey behavior data and mean in our previous assessments (Raffaelli et al., 2019, Danaher et al., 2018). However, studies were repeated twice to produce behavioral data and fresh tissue samples (n = 6–8). All data passed normality tests (Shapiro-Wilk at minimum, alpha = 0.05). Two-way ANOVA (Dunnett’s multiple comparisons test) was performed on all von Frey behavioral data. One-way ANOVA (Dunnett’s multiple comparisons tests) was used to compare expression changes to controls in Western blot and histochemical analyses, as well as in comparisons of the anxiety- and depression-like behavior, conditioned place preference (CPP), and novel object recognition tests. In all cases α = 0.05 was accepted for significant differences and data expressed as means +/− SEM for independent male and female group analyses. Curve fitting was performed in Graphpad Prism 8.1.2 using a nonlinear regression model. Significance and post-hoc analyses are shown in each study figure.
Results
CCK-BR scFvs Generated from Cell-free Ribosomal Display
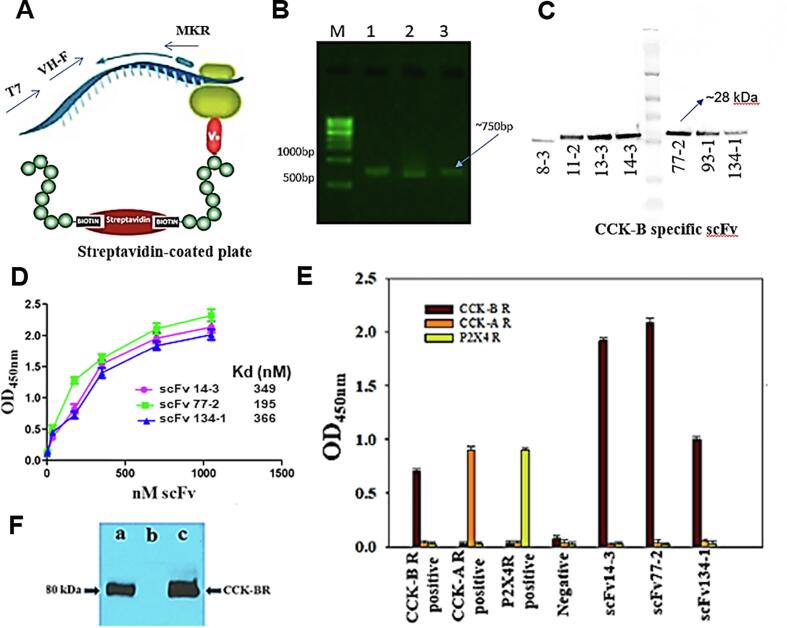
Fig. 2 illustrates the generalized scheme utilized for the generation of the CCK-BR scFv antibodies. Seven scFv antibodies bound to CCK-B receptor peptide in a concentration dependent manner and showed high apparent affinity (Fig. 2A). The scFv were generated from CCK-BR peptide immunized male BALB/cANHsd mice. Fig. 2B provides ELISA detection and quantification of CCK-BR reactivity from blood drawn in the 1st, 2nd, and 3rd immunization cycles preceding spleen RT-PCR recovery of VH/K cDNA. The seven CCK-BR recombinant scFvs were subcloned into a pET32a expression vector, expressed and purified from E. coli Rosetta-gami cytoplasm (Fig. 2C), as done previously to generate scFvs against Zika virus and filovirus glycoproteins (Kunamneni et al., 2018, Kunamneni et al., 2019).
Fig. 2.
Isolation and efficacy of CCK-BR scFv antibodies. A. Schematic of stalled ARM complex and position of primers used for RT-PCR recovery in the 1st, 2nd, and 3rd cycles of ribosome display. B. Analysis of RT-PCR recovery of VH/K cDNA from CCK-B receptor immunized spleen in the 1st, 2nd, and 3rd cycles. C. Western blot of seven purified unique CCK-BR scFvs generated by the cell-free ribosome display platform. D. Plot of ELISA data showing binding affinity of increasing concentrations of the three scFv antibodies with the best binding. E. Binding specificity and cross-reactivity of 3 CCK-BR scFvs to CCK-BR, but not CCK-AR or P2X4R. F. Immunoblot of recombinant cholecystokinin-B receptor (CCK-BR) antigen by soluble single-chain variable fragment (scFv) antibody fragments. a. Purified scFvs. b. Unrelated scFv as a negative control. c. A goat anti-CCK-BR polyclonal antibody as a positive control.
Binding, specificity, and affinity of scFvs to the target peptide was determined by ELISA and immunoblot analyses (Fig. 2D–F). In the case of scFv antibodies generated against the CCK-BR peptide, this was done to select for CCK-BR-specific antibodies. Of the seven unique CCK-BR scFvs demonstrating differential CCK-B receptor binding capability and target specificity, the CCK-BR scFvs 77-2, 14-3, and 134-1 had the highest, second and third highest affinity, respectively, reflecting efficient panning and selection. The scFv clones showing high reactivity were analyzed further for cross-reactivity against proteins related to the target peptide. In Fig. 2D, the scFv clones showing high reactivity were analyzed further for dose cross-reactivity against the target CCK-BR peptide.
Fig. 2E shows that three CCK-BR scFvs were bound specifically to CCK-B receptor, but did not bind to CCK-A or P2X4 receptors. As an scFv negative control, anti-Zika scFv7-2 antibody did not react with the CCK-BR scFv. As shown in Fig. 2F, Western blot specificity analysis revealed CCK-BR scFv77-2 recognized a defined protein band of 80 kDa corresponding to the expected molecular weight of recombinant CCK-BR.
In Vivo testing
Reduction of mechanical and cold hypersensitivity induced by FRICT-ION and SNI
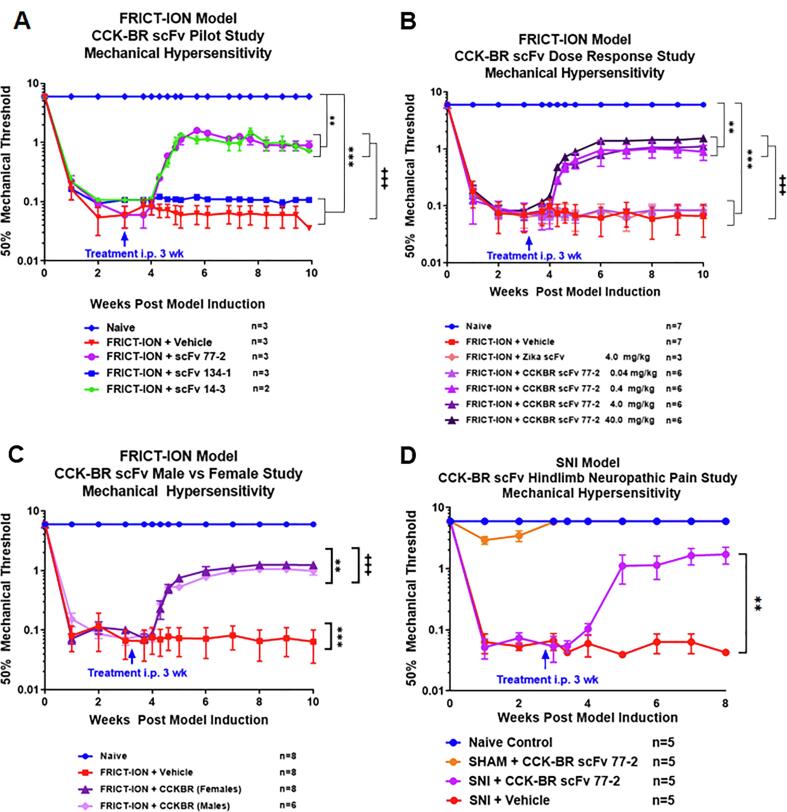
The three lead CCK-BR scFvs with the highest binding affinity were tested in male mice with the FRICT-ION chronic neuropathic pain model (Fig. 3A). A single i.p. dose of two of the scFvs (scFv 77-2 and scFv 14-3, 100 μg) effectively reversed the induced orofacial hypersensitivity by 75% through the subsequent 7 weeks, indicated by a rise in the mechanical threshold. The scFv 134-1 and PBS vehicle were ineffective. There can be numerous explanations. One, the binding affinity is lower. Secondly, the 3 dimensional configuration of this antibody likely did not allow binding. Attenuation by lead scFv 77-2 was validated for doses 0.4–40 mg/kg15-fold in male mice, while low dose (0.04 mg/kg) scFv 77-2 and a control Zika scFv were ineffective (Fig. 3B). A curve with scFv77‐2 dose on the xaxis and % reversal mechanical allodynia on the y axis was plotted. This graph helped visualize the % reversal mechanical allodynia values for each condition. The curve was then fit with a sigmoidal equation to derive an IC50 value of approximately 1.739 mg/kg for IP injection of the scFv in reversing mechanical pain hypersensitivity in the FRICT‐ION model.
Fig. 3.
Selection of lead CCK-BR scFv 77-2 dose that alleviates neuropathic pain in 2 rodent models and time points. A. Single 4 mg/kg i.p. injection of 77-2, 14-3, and 134-1 scFvs in week 3 reversed orofacial pain shown for male mice [F(72, 175) = 76.05]. B. Dose response effect of scFv 77-2 for reversal of ipsilateral trigeminal nerve mechanical hypersensitivity (0.04, 0.4, 4 and 40 mg/kg). Zika and low dose CCK-BR (0.04 mg/kg) scFvs were ineffective, shown for male mice [F(78, 474) = 171.80] C. scFv 77-2 was equally effective in males and females. [F(39, 361) = 212.80] D. Single dose (4 mg/kg) CCK-BR scFv was effective in alleviating the SNI model as well, as shown for male mice. Data was identical when CCK-BR scFv 77-2 was given at a later time point (7 weeks). [F(18, 130) = 20.76] ** indicates a p-value of < 0.01, *** indicates a p-value of < 0.001]. In post-hoc analyses, Bonferroni adjustment to all p-values for week-by-week comparisons of FRICT-ION versus naïve control yields p-values < 0.001 for all.
The lead CCK-BR candidate scFv 77-2 was equally effective in both sexes as shown here for male mice with FRICT-ION (Fig. 3C). The single dose of CCK-BR scFv 77-2 (4 mg/kg) plotted separately for male mice in Fig. 4D, provided an equally significant reduction of mechanical hypersensitivity in the chronic sciatic SNI model. The effectiveness of the single i.p. dose given 3 weeks post nerve injury persisted through to the experiment end at 10 weeks. A secondary study was performed in which CCK-BR scFv given at 7 weeks, and was equivalently effective in the SNI model.
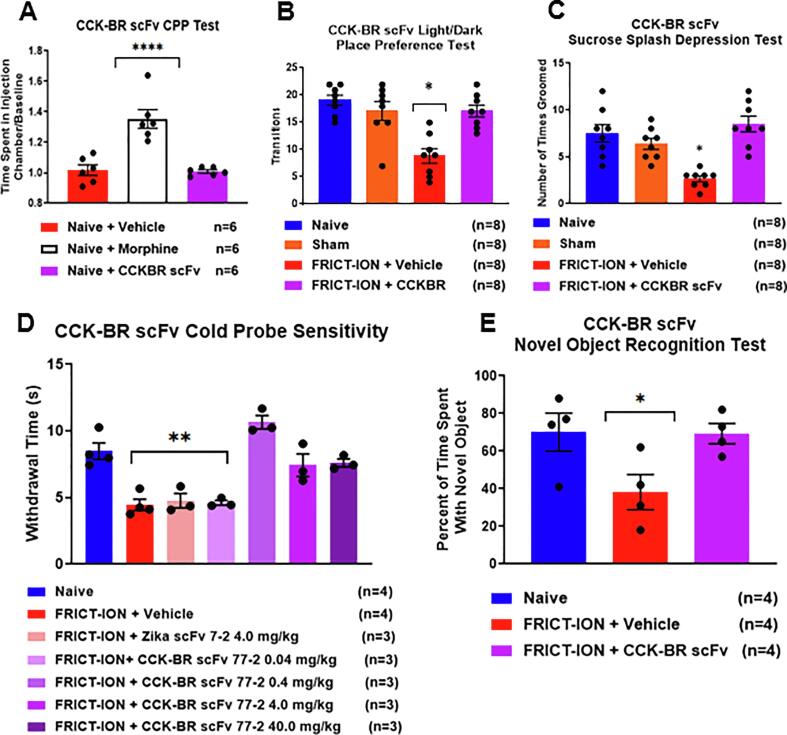
Fig. 4.
Lead CCK-BR scFv 77-2 in female mice with FRICT-ION does not predict abuse potential, but reduces anxiety- and depression-like behaviors, cold hypersensitivity, and prevents higher order behavioral effects in vivo. A. The condition place preference (CPP) test found no difference between the vehicle and CCK-BR scFv77-2 treated groups (4 mg/kg, p = 0.41), compared to increased time spent in the morphine (5 mg/kg) administration box demonstrating its addictive potential. B. The FRICT-ION mice with chronic neuropathic pain had significantly fewer light /dark transitions indicating anxiety. There were no differences from naïve and sham controls for the mice receiving CCK-BR scFv 77-2. C. Sucrose splash depression-like behavior was significant only among the mice with FRICT-ION. Mice treated with CCK-BR 77-2 groomed normally equivalent to the naïve group mice. D. Cold hypersensitivity was increased by CCK-BR scFv 77-2 (0.4, 4 and 40 mg/kg). The PBS vehicle, Zika scFv, and low dose CCK-BR scFv 77-2 (0.04 mg/kg) were ineffective. E. In the novel object cognitive measure mice with FRICT-ION were significantly affected, while those receiving scFv 77-2 (4 mg/kg, n = 4) had results similar to naïve mice. *p < 0.05, **p < 0.01, ****p < 0.0001 vs naïve; +++p < 0.001 vs FRICT-ION + Vehicle, One-way ANOVA.
Conditioned place preference
While abuse liability was demonstrated for morphine (5 mg/kg) with conditioned place preference (CPP) testing, the CCK-BR scFv 77-2 showed no abuse potential in the FRICT-ION mice (Fig. 4A). There was no difference between the vehicle and CCK-BR scFv77-2 treated groups (4 mg/kg, p = 0.41), compared to increased time spent in the morphine administration box demonstrating its addictive potential.
Reduction of anxiety- and depression-like behaviors
Since females with chronic pain are more susceptible to development of anxiety and depression, the results are shown for female mice tested with the higher order non-evoked anxiety- and depression-related spontaneous behavioral assessments (Fig. 4). Cognitive dependent tests were used to assess anxiety- and depression-like behaviors and decreased cognitive ability at 6–9 weeks post FRICT-ION injury with light/dark box place preference and sucrose splash testing, respectively (Fig. 4B, C). These tests were given only once to avoid confounds in repeat testing.
Light/dark box place preference. The lead CCK-BR candidate scFv 77-2 prevented development of anxiety (Fig. 4B). Anxiety-like behavior is significantly greater in FRICT-ION mice that did not receive the parent CCK-BR scFv (n = 6, *p < 0.05 ANOVA). The FRICT-ION model increased anxiety measures indicated by decreased occupancy on the lighted side of the test chamber, i.e. more time in the dark chamber, compared to the naïve controls.
Sucrose Splash Depression Test. Depression-like behaviors were measurable at 6–8 weeks. Mice with FRICT-ION failed to groom, while control mice readily groom the sweet 10% sucrose splash solution. Mice with FRICT-ION nerve injury treated with CCK-BR scFv 77-2 displayed less depression-like behavior. Treated mice spent increased time grooming and licking the sucrose splashed on their rump and had a shorter latency to begin licking and grooming than mice treated with other scFvs or PBS. The results for female mice are shown (Fig. 4C). Grooming time after sucrose splash test was increased significantly after scFvs 12 and 95 in an initial test (n = 6, *p < 0.05 ANOVA).
Cold hypersensitivity test. The 0.4–40 mg/kg doses of CCK-BR scFv 77-2 returned response to cold hypersensitivity to baseline, while mice with FRICT-ION treated with the neutral phosphate buffered saline vehicle (PBS) or treated with the control Zika scFv displayed significant cold hypersensitivity (Fig. 4D). The effectiveness of the single i.p. dose given 3 weeks post nerve injury persisted through to the experiment end at 10 weeks. A second time point of single dose scFv administration was tested. CCK-BR scFv given at 7 weeks was also effective in the SNI model.
CCK-BR scFv binding to trigeminal ganglia in vitro
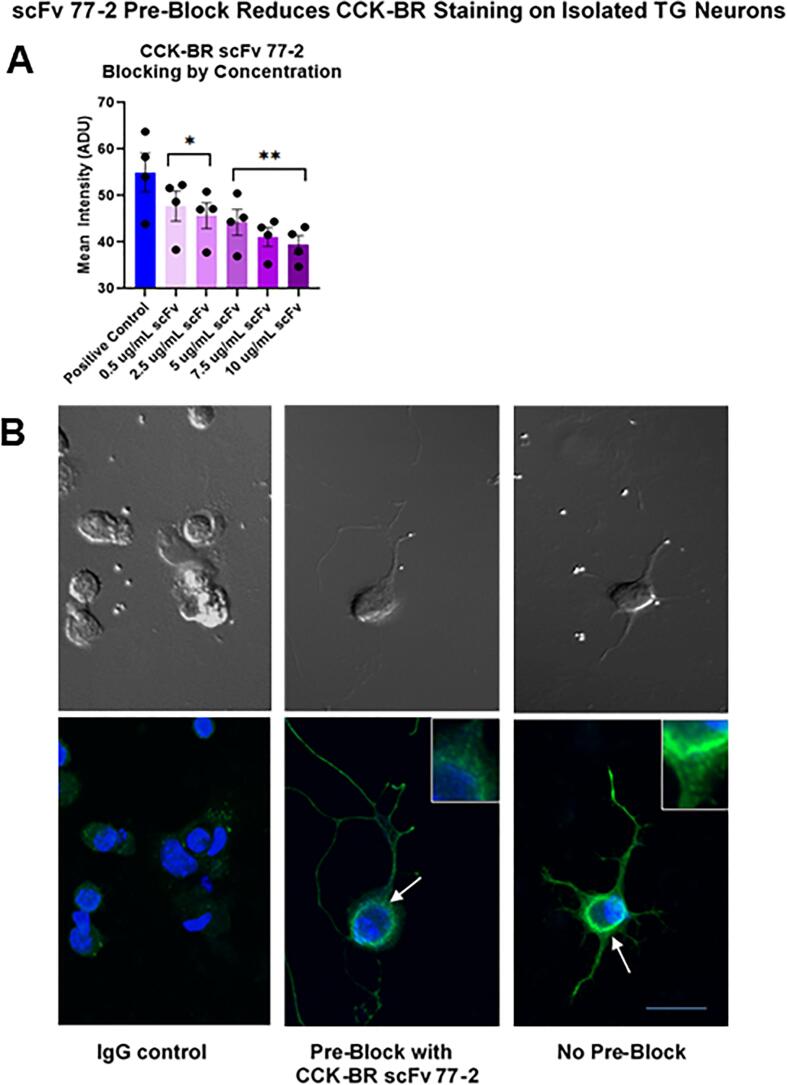
The ability of the anti-CCK-BR scFv to bind specifically to the CCK-B receptor on trigeminal ganglia neuron primary cultures harvested from naïve male mice was tested by using incremental increases of the CCK-BR scFv 77-2 to pre-block the site prior to immunostaining (Fig. 5). The CCK-BR scFv 77-2 concentrations were added overnight to the Day 2 cultures as the pre-block. The pre-block of trigeminal ganglia (TG) by incrementally increasing concentrations of CCK-BR scFv 77-2 (0.5–10 µg/ml) incrementally diminished the subsequent immunostaining intensity with a commercial CCK-BR polyclonal antibody. The immunostaining intensity of CCK-BR after pre-block with the various concentrations of scFv was quantified as shown in the bar graph (Fig. 5A). Confocal images of TG neurons are shown in Fig. 5B. The negative control shown in the left panel was processed with secondary IgG only (i.e. no primary CCK-BR antibody was applied). The middle panel illustrates the effect of pre-block of the TG cultures with the CCK-BR scFv 77-2 (10 µg/ml). Without the pre-block CCK-BR staining is more clearly evident on the soma and terminal growth cones in the TG neuron shown in the right hand panel. The insets show the staining intensity differences at higher power.
Fig. 5.
In vitro pre-block of CCK-BR with scFv 77-2 in TG primary cultures isolated from naïve male mice. A. Incremental increase of scFv 77-2 added to TG primary cultures 24 h prior as a pre-block significantly diminished CCK-BR immunocytochemical staining intensity with a polyclonal commercial antibody. n = 4. * p < 0.05, ** p < 0.01 versus the positive control (no pre-block), One-way ANOVA B. Confocal images of IgG negative staining control omitting primary CCK-BR antibody, pre-block with scFv 77-2 prior to CCK-BR staining, and CCK-BR staining. The arrows indicate the sites shown at higher power in the insets. The upper panels show phase contrast images of the same cells. Bar = 50 µm.
Electrophysiological effects of CCK-BR scFv treatment on neuronal excitability
To determine how the direct effect of CCK-BR scFv 77-2 on isolated TG neurons in vitro might be related to in vivo behavioral effects, patch-clamp electrophysiological studies were performed. The TG neuronal excitability was compared between CCK-BR scFv-treated vs untreated TG neurons isolated from male mice already primed in vivo by the FRICT-ION model (3–4 weeks post-injury). Current clamp recordings were performed and neurons were subjected to a series of 10-picoAmps (pA) depolarizing current steps to elicit neuronal firing as shown in Fig. 6A.
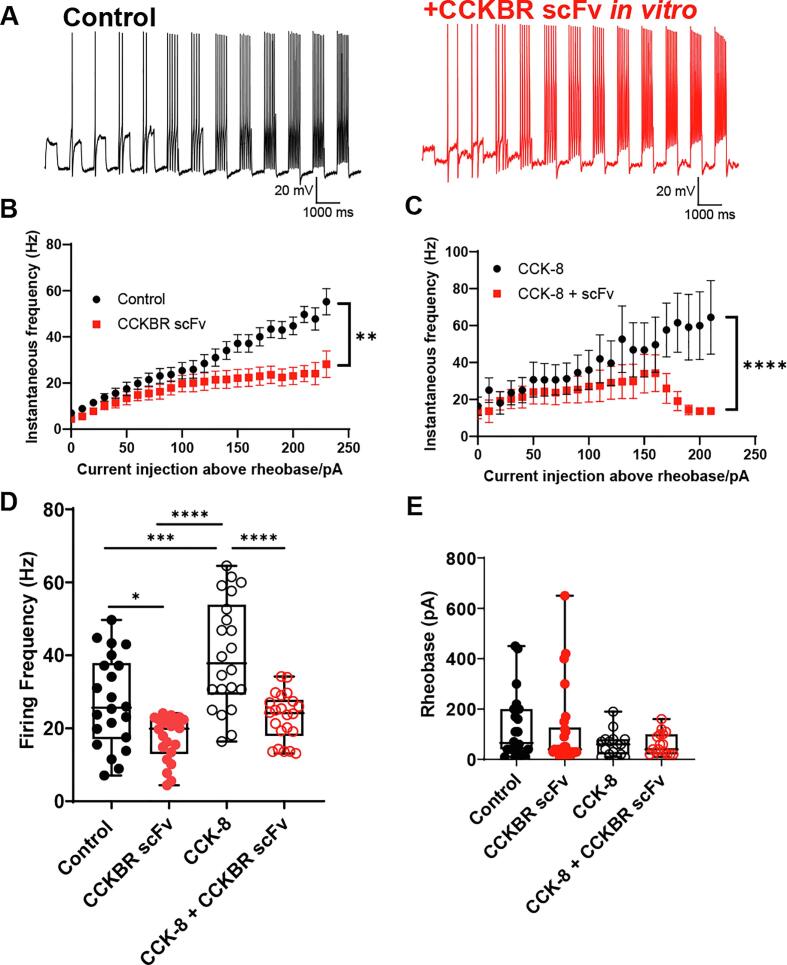
Fig. 6.
CCK-BR scFv 77-2 reduces instantaneous firing frequency of TG neurons from FRICT-ION mice. A. Representative current clamp recordings of control and CCK-BR scFv 77-2 treated TG neurons from FRICT-ION mice. Current injections are shown starting from 10 pA sub-rheobase with subsequent 10 pA stepwise current injection. B. Frequency-current (f-I) relationship of control and CCK-BR scFv-treated TG neurons from FRICT-ION mice. This analysis was restricted to neurons that fired 2 or more times with up to 250 pA injections above rheobase. n = 8–9 neurons per condition. CCK-BR scFv-treated neurons displayed significantly reduced firing frequency (p = 0.0024, Mann-Whitney test). CCK-BR scFv (10 ug/ml) was applied to culture media for 1–2 hrs prior to recording. C. Frequency-current (f-I) relationship of CCK-8 (100 nM)-treated TG neurons from FRICT-ION mice in the presence and absence of CCK-BR scFv (10 ug/ml). CCK-8 was applied in vitro at the same time as scFv 77-2 prior to recording. n = 6–11 neurons per condition. The effect of scFv in reducing firing frequency was more significant in the presence of CCK-8 (p < 0.0001, Mann-Whitney test). D. Firing frequency comparison for all conditions based on data in B-C. *p < 0.05, ***p < 0.001, ****p < 0.0001, ANOVA with Tukey’s multiple comparisons test. E. Rheobase was not significantly changed between control and CCK-BR scFv-treated, CCK-8-treated or CCK-8 + CCK-BR scFV-treated (p > 0.05, ANOVA). n = 16–28 neurons per condition. All neurons recorded from were < 30 µm in diameter.
A frequency-current (f-I) relationship for ‘multiple firing’ neurons was plotted as shown in Fig. 6B, C. This analysis was restricted to neurons that fired two or more times with up to 250 pA injections above rheobase (current required to elicit firing, n = 8–9 neurons per condition). CCK-BR scFv treatment (10 ug/ml) was applied to culture media for 1–2 hrs prior to recording from the trigeminal ganglia isolated from the FRICT-ION mice sensitized in vivo for 10 weeks prior. Significant reduction of instantaneous firing frequency of TG neurons was observed in neurons treated with CCK-BR scFv 77-2 compared to untreated control TG neurons from FRICT-ION mice (Fig. 6B, p = 0.0024, Mann Whitney test). In order to determine whether effects of CCK-BR scFv could be potentiated in vitro, we treated TG cultures from FRICT-ION mice with an agonist of CCK-BR, CCK-8 (100 nM), in the presence and absence of CCK-BR scFv. CCK-8 (100 nM) and CCK-BR scFv (10 ug/ml) were applied at the same time to the TG cultured neurons prior to electrophysiological recording. The in vitro dose was chosen based on the affinity binding and the IHC studies. As shown in Fig. 6C, we observed a reduction in firing frequency in CCK-BR scFv-treated neurons compared to untreated neurons in the presence of CCK-8 (p < 0.0001, Mann-Whitney test). This concentration of CCK-8 used has previously been shown to sensitize sensory neurons in culture using electrophysiology assays (Yu et al., 2019).
We further analyzed the data from Fig. 6B–C as shown in Fig. 6D using an ANOVA with Tukey’s multiple comparisons test. Our data indicate that CCK-8 was effective in sensitizing cultures as we observed a significant 39% increase in median firing frequency compared to control untreated cultures (p < 0.001, ANOVA with Tukey’s multiple comparisons test). We observed that CCK-BR scFv reduced median firing frequency by 25% compared to control untreated cultures (p < 0.05, ANOVA with Tukey’s multiple comparisons test) and that CCK-BR scFv reduced firing frequency 36% compared to CCK-8 sensitized cultures (p < 0.0001, ANOVA with Tukey’s multiple comparisons test) (Fig. 6D). Therefore, the effect of CCK-BR scFv was more significant in the presence of CCK-8. We did not observe significant effects on rheobase among any of the conditions used (Fig. 6E, p > 0.05, Mann Whitney test).
Brain penetrance demonstration with western blot
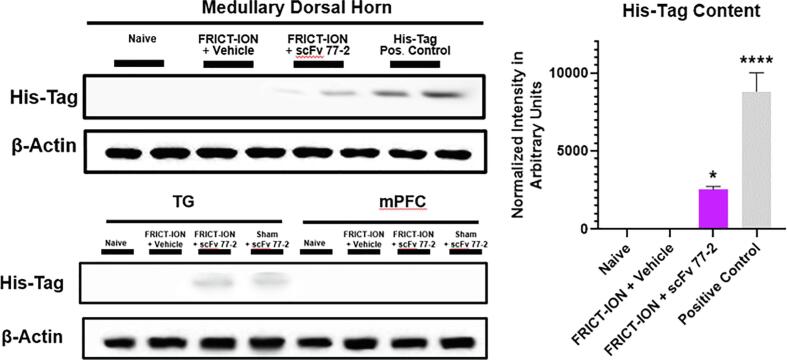
In Western blots of whole medullary lysate dissected at week 10 experiment end were loaded at 1/40 dilution. This was 7 weeks after the single i.p. injection of 100 ug of His-Tag labeled CCK-BR scFv. The His-Tag content remained evident in the trigeminal ganglia and the medullary spinal nucleus of the trigeminal, the brain termination site of the trigeminal primary afferent nerve fibers (Fig. 7). Since 100 ug of His-Tag labeled CCK-BR scFv was injected in each mouse (slight variance depending on weight), it was estimated that 3.2 ug is ending up in the medulla based on the positive control lane loaded with 100 ng of His-Tag labeled scFv. The His-tag staining intensity in FRICT-ION mice treated with CCK-BR scFv antibody was significant compared to vehicle treated FRICT-ION mice (p < 0.001). No His-Tag was evident in mPFC samples utilized.
Fig. 7.
CCK-BR scFv 77-2 His-tag biomarker brain penetrance. Western blot of the scFv His-tag remaining in the brainstem medullary dorsal horn, trigeminal ganglia (TG), and medial prefrontal cortex (mPFC), 7 weeks after the single i.p. dose was given in male mice with FRICT-ION. The bar graph indicates its brain penetrance with normalized intensity in arbitrary units. n = 4, *p < 0.05; ****p < 0.0001, One-way ANOVA.
Transcriptomic analyses of whole TG tissue from scFv-treated mice using RNAseq
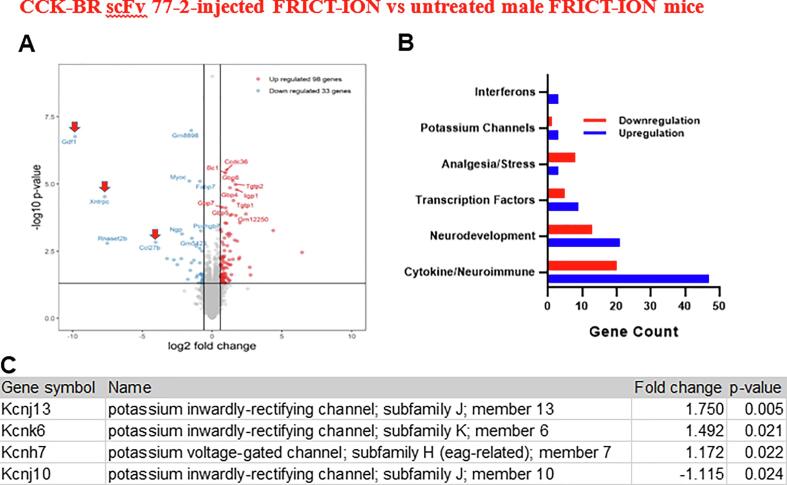
In order to determine whether our scFv was capable of inducing long-lasting changes in gene expression, we performed RNAseq of whole TG tissue obtained from scFv-injected versus untreated FRICT-ION mice. In spite of the rapid clearance of the scFv, we reveal changes in several genes at 10 weeks post-treatment. We observed upregulation of pro-inflammatory chemokine Ccl27b (~17 fold, p < 0.01), growth factor Gdf1 (~904-fold, p < 0.0001) and a gene encoding a TRPC channel called Xntrpc (208-fold, p < 0.0001) at 10 weeks post-injury (Fig. 8A). A broad analysis provided in Fig. 8B is sorted with gene ontology enriched designation by function.
Fig. 8.
RNA Profile for CCK-BR scFv 77-2-injected FRICT-ION vs untreated male mice with FRICT-ION (7 wks post). A. Differential expression of genes most up and down regulated comparing treated and untreated TG shown as an RNAseq volcano plot (*p < 0.02). Arrows indicate genes for growth factor, a TRPC channel, and a chemokine. B. Number of genes in categories pertinent to pain and immune response. C. Changes in K + gene family-related genes.
Fold changes and p values of transcripts per million are from TG samples harvested from BALBc mice in week 10 post model induction which was 7 weeks after scFv treatment. Compared are genes expressed in TG of FRICT-ION mice treated with CCK-BR scfv 77-2 versus FRICT-ION mice without treatment. The highest fold changes were noted for upregulation of Growth Differentiation Factor 1, Xndc1-transient receptor potential cation channel; subfamily C, member 2 readthrough; and Chemokine (C–C motif) ligand 27b. Of particular note, numerous potassium channel genes (Kcnj13, Slc9a2, Wnk4, Kcnk6, Kcnh7, and Slac12a7) were elevated (Fig. 8C). Other genes significantly increased or decreased (p < 0.05) could be sorted into categories shown as number of genes significantly increased or decreased involved with pain and stress behaviors, brain and neuron development, cytokine and immune regulation, transcription factors, and interferons (Fig. 8B). The 1.5 fold increase in claudin 11 (p < 0.035) may be related to axon ensheathment repair. The significant 6.69 fold decrease in POMC (p < 0.007), prohormone for corticotropin stimulator of stress hormone cortisol, is also notable.
Discussion
Generation and effective use of CCK-BR scFv 77-2 as our lead non-addictive, non-opioid biological is shown here from among the numerous scFvs generated via ribosome display to target a 15-a.a. CCK-BR extracellular peptide sequence. We performed the affinity maturation required to preserve epitope specificity and functional activity as well as boost antibody potency to the highest level. Our lead scFv 77-2 with the highest affinity for CCK-BR (Kd 195 nM, 750 bp) is ~ 1/6 the size of a MAb and thus we showed can access the central nervous system (CNS). There was no cross-reactivity with CCK-A receptor in the binding assays, and immunoblot experiments confirmed the presence of a single band for CCK-BR. Effectiveness of lead CCK-BR scFv 77-2 as a potential therapy for chronic neuropathic pain was demonstrated in in vivo and in vitro analyses and is discussed below in the context of the literature.
Analgesic efficacy of the lead CCK-BR scFv 77-2
The lead biologic CCK-BR scFv 77-2 effectively inhibited both mechanical and cold hypersensitivity in chronic trigeminal and sciatic neuropathic pain syndrome models. These findings support previous reports that CCK is a pro-nociceptive peptide (Xie et al., 2005). Given as a single dose post-treatment, equivalent decrease in mechanical hypersensitivity (75%) was shown in males and females with the FRICT-ION chronic trigeminal neuropathic pain model. Administration at two time points (3 or 7 weeks) was shown in male mice with SCI chronic neuropathic pain. Results were similar among the variables tested in the two nerve injury models. Others have shown that naïve CCK-BR deficient mice (CCK-BR KO) are mechanically hyposensitive after sciatic nerve constriction injury (Kurrikoff et al., 2004). Our in vivo behavioral results show that CCK-BR scFv behaves as a potent inhibitor of CCK-BR’s actions with enduring effects reversing the cascade of chronic pain- and anxiety-like effects.
After the single dose i.p. treatment in week 3, the mechanical hypersensitivity reversal over the subsequent week was reminiscent of natural healing while hypersensitivity persists indefinitely in untreated mice with FRICT-ION. The RNA profile indicated upregulation of specific genes indicative of ongoing neuroplastic events and decreasing immune response persisting in TG samples 7 weeks after CCK-BR scFv 77-2 administration. Pain-related behavior did not reappear in the subsequent 7 weeks of testing. The diminished cold hypersensitivity in week 6 in treated mice demonstrated the persisting effectiveness in this measure as well. Mice with FRICT-ION nerve injury treated with the CCK-BR scFv 77-2 tested in week 6 post model induction had no evidence of the anxiety- or depression-like behavior which was evident in the vehicle treated mice with chronic neuropathic pain. This further indicates lack of ongoing pain. Likewise, higher order novel object memory testing indicated scFv 77-2 treated mice were spared effects seen in untreated mice with FRICT-ION. After hindlimb peripheral nerve injury, rodents also show deficits in hippocampal-dependent memory extinction tasks and information processing abnormalities (Mutso et al., 2014).
BBB penetration of CCK-BR scFv 77-2: Potential sites of action
Western blot found the His-tag bound to the CCK-BR scFv in trigeminal ganglia and brainstem medullary dorsal horn 7 weeks after the single intraperitoneal dose indicating penetrance into the primary afferent nerves and the medullary brainstem. This provides an indication that the scFv potentially acted in the trigeminal ganglia and medullary dorsal horn. This supports the ability of the scFv to cross the BBB and blood-ganglia barrier compromised by the persisting pain state. The reduced firing frequency observed when the lead CCK-BR scFv 77-2 was applied directly to the neuronal cultures also indicated the potential to directly affect peripheral nerve activity. The increased firing frequency in response to CCK-BR agonist CCK8 that may be due to the in vivo priming in the FRICT-ION model and was blocked by the CCK-BR scFv 77-2.
The Western blots did not provide evidence of CCK-BR scFv 77-2 in mPFC after 7 weeks despite the ability of the scFv to prevent anxiety- and depression-like behavior. This may be due to the limited sensitivity of the method to detect the scFv at that site in mice 7 weeks post treatment. Alternatively, binding after the single dose may not have occurred in this region. Lack of his-tag in mPFC may be dependent on a volume dilution effect of the sample taken or even less brain-barrier compromise compared to the highly activated peripheral nerve and medullary brainstem dorsal horn regions.
In any event, the cascade of effects throughout the brain that occur in the chronic model due to continual neuronal activation and higher brain pain-related progression response to chronic pain did not occur after the CCK-BR scFv treatment blocked hypersensitivity. The block of activation at the nerve was sufficient to prevent the development of the anxiety- and depression-like behavior, and cognitive deficit. Further study is warranted.
Several patient studies and social stress rodent models have used CCK to chemically induce experimental anxiety and panic attacks specifically to test novel pharmacological interventions (Bradwejn et al., 1991, Bradwejn and Koszycki, 2001, Vialou et al., 2014, de Montigny, 1989, Zwanzger and Rupprecht, 2005). Conversely, blocking centrally active CCK-B receptors prevents development of anxiety- and fear-like behaviors as well as inhibits CCK release in the mPFC. These higher order pain related behaviors are apparent in the untreated FRICT-ION mice here and as reported previously for our FRICT-ION model (Montera and Westlund, 2020).
Direct effects of CCK-BR scFv 77-2 on TG neurons from FRICT-ION mice
Peripheral nociceptor sensitization and hyperexcitability are common features of several different chronic pain states (Gold and Gebhart, 2010, Alles et al., 2018). We have shown that neuronal firing frequency in small diameter peripheral TG neurons obtained from FRICT-ION injured mice is significantly reduced by in vitro CCK-BR scFv treatment. The pre-adsorption block of TG neurons with incrementally increasing concentrations of CCK-BR scFv 77-2 that significantly reduced immunostaining intensity for the CCK-B receptor was an indication of direct binding specificity.
Previous work demonstrates that application of cholecystokinin, an endogenous agonist of CCK-B receptors, increases firing frequency of spinal dorsal root ganglia neurons through a decrease in A-type K + channel current (IA) and caused mechanical and thermal hypersensitivity (Yu et al., 2019). Indeed, our RNAseq studies indicate changes in several K + channel-related genes that would explain the effects of the scFv on nociceptor excitability, but requires further study.
The effects of CCK-BR scFv are potentiated in the presence of CCK-B agonist, CCK-8. In addition, there is no significant effect of CCK-BR scFv on the rheobase of neurons regardless of condition. These results show that the primary mechanism of action of our scFv on injured sensory neurons appears to be to reducing firing frequency. It is also noticeable that we only observe a clear effect of CCK-BR scFv when injecting current up to 250 pA above rheobase. Importantly, our scFv does not significantly effect rheobase. Our data show that CCK-BR scFv has an effect on spike frequency adaptation (SFA). Several ion channels are involved in mechanisms of SFA including calcium-activated K + channels such as BKCa and SKCa (Ha and Cheong, 2017). However, most previous work on SFA is on central neurons of the brain and not peripheral sensory neurons under conditions primed by nerve injury and chronic pain. Therefore, much further study is required to elucidate specifically which voltage-gated ion channels are most important for the mechanism of action of our scFv, not to mention the role of SFA in peripheral neurons in chronic pain. Our results nonetheless show that CCK-BR scFv behaves as a potent inhibitor of CCK-BR’s actions during chronic pain and in the TG neurons primed in the FRICT-ION nerve injury model. These direct effects correlate well with the in vivo anti-hypersensitivity actions of CCK-BR scFv reported here.
CCK-BR scFv antibody generation promoting effectiveness
The scFv antibodies were generated from mice immunized with a model target, in this case, an extracellular 15-a.a. peptide fragment of the CCK-B receptor (mouse sequence CETPRIRGTGTRELE). UniProtKB 2021_03 web based BLAST search lists four CCK-BR sequence homologies with this peptide sequence (3 human, 1 gorilla). The ribosome display method utilized has produced repertoires of high-affinity scFv antibodies against the target peptide. We generated seven scFvs using a ribosome display library to target the small CCK-B receptor extracellular peptide fragment. Sequencing analyses revealed selected antibody fragments as seven unique scFvs antibodies. This diverse panel of full-length scFv antibody fragments implies that they may recognize distinct epitopes and/or bind to the same epitopes, probably with different affinities. Additional validation studies remain to clarify the exact target recognized by the lead scFv since several CCK-BR isoform are described in nervous tissue. However, DRG data from patients with neuropathic pain include CCK-BR in a very long list of affected genes in RNAseq analysis (North et al., 2019), although differential gene expression analysis from patients with trigeminal pain is unavailable. Relevant citations from the literature are available linking CCK-BR to relief of pain and anxiety in patients, including treatment with CCK-8, ceruletide, and other CCK-BR antagonists in IBS patients, after acupuncture, as reducers of gastric and pancreatic tumors, and in many other conditions, as reviewed in Boyce et al. (2016).
As ribosome display can avoid library limitation and is capable of screening much larger libraries, it is increasingly popular in selecting high affinity, specific antibodies from combinatorial libraries. This scFv antibody technology is well known for its potential to efficiently provide therapeutic antibodies with high specificity, persisting effects, and small size (~25 kD) for better tissue penetration compared to whole IgG (~150 kD). The modified cell-free ribosome display scFv antibody technology was favored over time intensive hybridoma monoclonal antibody development.
Although production of monoclonal antibodies against some specified GPCRs has been successful, a generic reproducible selection platform applicable to different GPCR members has not been achieved yet. The intrinsic properties of GPCR proteins are the major obstacle for successful selection. Ribosome display technology is aptly suitable for generating properly-folded full-length functional GPCRs, which can then be used for generating anti-GPCR antibodies through conventional hybridoma technology (Rothe et al., 2006). This technology is also useful as a drug screening tool as it allows selection of a larger repertoire of high-affinity GPCR agonists and antagonists owing to its huge library size (Rothe et al., 2006). Ravn et al. used ribosome display technique to generate antibodies against glucose-dependent insulinotropic polypeptide receptor (GIPr), a class B GPCR that binds to GIP on pancreatic beta cells and stimulates the production of insulin (Ravn et al., 2013). Tohidkia et al. (Tohidkia et al., 2013) reported the establishment of a diverse panel of scFv antibody fragments via phage display that are specific to the native conformation of CCK-BR (Tohidkia et al., 2013). Based on these results, they suggested the selected scFv antibody fragments as potential agents for diagnosis, imaging, targeting, and/or immunotherapy of cancers that overexpress CCK-BR.
Potential for reduced addiction liability
In the CPP test CCK-BR scFv 77-2 did not display addictive potential. Additional study is warranted, but since antibody therapies are typically given at multiple week/monthly intervals, addictive potential may not be a key issue. Sites with high levels of CCK-B receptor expression overlap with pain circuitry in the TG, mPFC, and rostroventral medulla (RVM), a brainstem serotoninergic pain modulation site (Rotzinger and Vaccarino, 2003). Studies have demonstrated CCK-B receptors play a crucial role in homeostasis of the supraspinal opioid system. Treatments that specifically block CCK-B receptors suppress maintenance and reactivation of morphine dependence in place preference tests (Mitchell et al., 2006). Injections of CCK-B receptor blockers/antagonists into the RVM reverse anxiety measures induced by opioid withdrawal in rats (Jiang et al., 2019, Xie et al., 2005, Vera-Portocarrero et al., 2011). In those studies treatments specifically blocking CCK-B receptors suppressed maintenance and reactivation of morphine dependence in place preference tests. Pharmacological ablation of CCK-B receptor expressing cells in mouse RVM inhibits descending pain facilitation and reverses somatic chronic constriction injury (CCI) induced hypersensitivity (Zhang et al., 2009). CCK-B receptor mRNA expression is upregulated in a mouse hindpaw burn injury model and while morphine had little efficacy, proglumide—a clinically used non-specific blocker for both CCK-A and CCK-B receptors—reduced hypersensitivity (Yin et al., 2016). Proglumide potentiates morphine and endogenous opiates while reducing tolerance (Watkins et al., 1984). Naïve CCK-B receptor deficient mice (CCK-BR KO) are mechanically hyposensitive after sciatic nerve CCI. Thus, treatment with anti-CCK-BR scFvs in future studies might likewise be effective for treatment of opiate or alcohol withdrawal, acute pain, and chronic pain in other body regions.
Potential as a safe clinical therapy
No side effects were evident in the mice treated with the CCK-BR scFv. Weight gain matched naïve control mice and necropsy analysis were negative for changes in organ appearance and weight. A limitation of this study, however, is that it is unknown how long the scFv circulates and if it is available to bind afferent endings. It should be noted that typically soluble scFvs are cleared within hours unlike full length antibodies that can remain for weeks or months (Schmitz et al., 2001). However, the duration of efficacy various antibodies is variable in the literature. Pharmacokinetic analysis of an Epidermal Growth Factor Receptor (EGFR) scFv in a mouse tumor model indicated it was only evident in the blood up to 6 h, but its specificity of bind to the target and effectiveness for solid tumor volume reduction was highly significant (Kim et al., 2014). In clinical trials adenoviral vector therapy given i.p. are measurable at 56 days in the peritoneal fluid but come with side effects (Alvarez et al., 2000). Bound scFvs are reported to be visible by IVIS for 8–14 days after a single treatment (Angelini et al., 2018, Ahmad et al., 2012, Krishnaswamy et al., 2014).
Gene expression profiling by others found elements of the immune response are not activated in CCK-B receptor KO mice in a somatic CCI model (Kõks et al., 2008). It is also well known that immune cells have abundant CCK-BR (Liang et al., 2020, Schmitz et al., 2001), and that they are often found closely associated with highly activated nerve endings. The upregulation of Tlr4 and IL-1β expression in wild type mice is absent in CCK-B receptor KO mice in that study, suggesting CCK-BR has a role in regulation of innate immunity. Another potential source of increased behavioral hypersensitivity is the M2 macrophages that are transported into the nervous system during chronic pain. This area of study remains for the future.
The diminished cytokines/chemokines in the RNAseq profile and increased neuronal repair/remyelination genes 10 weeks after giving CCK-BR scFv 77-2 in the present study are reflected in the reduced hypersensitivity, absence of anxiety- and depression-like behaviors, reduced firing frequency of TG neurons in culture, and potentially indicate a reversal of nerve damage and activation. Giving a single dose of scFv antibody, three or seven weeks after the nerve injury models were well established, acknowledges the relevance of the scFv’s potential clinical usefulness to treat these behaviors observed with persistent or chronic pain. These features strongly suggest our lead scFv 77-2 targeting human CCK-BR has tremendous potential to provide safe and efficacious treatment for both chronic neuropathic pain and related anxiety.
Conclusion
A known serious consequence of nerve injury pain, or “neuropathic pain” persisting after tissue healing, is the transition to chronic pain. Chronic pain remains a significant clinical challenge with a treatment response rate of only 11% (Haviv et al., 2014, Baad-Hansen and Benoliel, 2017). While decades of research have been devoted to acute “nociceptive” mechanisms, it is clear that complex, multifactorial mechanisms are also responsible for maintaining neuropathic pain long term. Current understanding is that pain chronification involves neural, physiological, molecular, epigenetic, and brain circuitry changes (Lee et al., 2021, Hashmi et al., 2013). In patients, altered connectivity with amygdala, medial prefrontal cortex/anterior cingulate gyrus (mPFC/ACC), and hippocampus evident with MRI has been correlated with pain level and duration of chronic pain (Mutso et al., 2014, Apkarian et al., 2004, Baliki et al., 2012, Vachon-Presseau et al., 2016, Tsai et al., 2018). Persisting dysfunctional pain and neuroplasticity occurring in the brain after damage to peripheral nerves exert powerful influences on the pain experience, mediated particularly by the brain’s limbic system. In particular, the amygdala processes aversive and emotional aspects of pain (Basbaum et al., 2009). After months of suffering, the limbic system can produce secondary dysfunctional affective/emotional responses symptoms including anxiety, depression, and altered cognitive function via the mPFC/ACC and hippocampus. Similar long term effects which can be seen in our FRICT-ION chronic pain model were prevented with CCK-BR scFv 77-2 treatment.
The present studies indicate the CCK-B receptor is an ideal target for therapeutics that can likewise affect nociceptive and/or aversive emotional components of chronic pain in multiple neuropathic pain syndromes. Improved pain management using optimized CCK-BR scFvs as adjuvants to conventional pain management predicts improved effectiveness of low dose opioids since the scFv therapeutics described improve physiological analgesia for treating neuropathic pain. These studies address the great need for better non-opioid chronic pain management, a major current knowledge gap that has been a major contributor to the current opioid crisis.
Credit author statement
This study was conceived and designed by KNW, RD and AK. The target peptide selection and parent IgG molecule generation in the spleen was done by KNW. The scFv antibody was designed, generated and characterized by AK. The behavioral studies were performed by MM and AEG. Electrophysiological studies were designed by SRAA and performed by AEG. Western blots and in vitro TG culture studies were performed by MA-B and RB, respectively. KNW, AK, and SRAA wrote the manuscript. All authors have read and contributed to the final form of the manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
The authors thank Michael Paffett, PhD, Technical Director of the University of New Mexico Fluorescence Microscopy & Cell Imaging Shared Resource, for all his assistance with training, fluorescent photography, and image analysis assistance. Dr. Sabrina McIlwrath is acknowledged for her assistance with the phase and confocal blocking images. Kathrine Gott is acknowledged for the initial mouse immunization injections, cheek bleeds, and spleen harvesting.
Funding information
The authors wish to acknowledge the support of NIH grant R21 DE028096 (KNW, AK), the University of New Mexico Department of Anesthesiology & Critical Care Medicine endowment funds (KNW, SA), DoD CPMRP CP190116 (KNW, SA, AK), and salary support from VA Merit grant 7I01BX002695-02 (KNW). This communication does not necessarily reflect the views of the Department of Veterans Affairs or the U.S. government.
Disclosures
The authors declare no conflicts of interest.
The CCK-BR scFvs used in this study are protected under U.S. Provisional Patent Applications.
Provisional Patent Application No. 62/755,054 “THERAPEUTIC ANTIBODY FRAGMENTS, METHODS OF MAKING, AND METHODS OF USE”, Karin Westlund High, Ravi Durvasula, Adinarayana Kunamneni, Provisional U.S. Application File 310.01390160, filed November 2, 2018.
Provisional U.S. Application No. 62/890,879 Patent File 0310.000152US60 “NON-OPIOID COMPOSITIONS AND THERAPIES FOR PAIN MANAGEMENT”, Karin Westlund High, Ravi Durvasula, Adinarayana Kunamneni, filed August 23, 2019.
Non-Provisional U.S. Application, STC.UNM Ref.: 2019-037-03; MRG ref.: 0310.000139WO01 “THERAPEUTIC ANTIBODY FRAGMENTS, METHODS OF MAKING, AND METHODS OF USE”, Karin Westlund High, Ravi Durvasula, Adinarayana Kunamneni, filed October 30, 2019.
This work was supported by NIH NIDCR R21 DE028096, VA Merit grant BX002695, DoD CPMRP CP190116, and the Research Endowment of the Department of Anesthesiology & Critical Care Medicine, University of New Mexico Health Sciences Center, Albuquerque, NM.
References
- Adams J.B., Pyke R.E., Costa J., Cutler N.R., Schweizer E., Wilcox C.S., Wisselink P.G., Greiner M., Pierce M.W., Pande A.C. A double-blind, placebo-controlled study of a CCK-B receptor antagonist, CI-988, in patients with generalized anxiety disorder. J. Clin. Psychopharmacol. 1995;15(6):428–434. doi: 10.1097/00004714-199512000-00007. [DOI] [PubMed] [Google Scholar]
- Agnes R.S., Lee Y.S., Davis P., Ma S.-w., Badghisi H., Porreca F., Lai J., Hruby V.J. Structure-activity relationships of bifunctional peptides based on overlapping pharmacophores at opioid and cholecystokinin receptors. J. Med. Chem. 2006;49(10):2868–2875. doi: 10.1021/jm050921q10.1021/jm050921q.s001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad Z.A., Yeap S.K., Ali A.M., Ho W.Y., Alitheen N.B.M., Hamid M. scFv antibody: principles and clinical application. Clin Dev Immunol. 2012;2012:1–15. doi: 10.1155/2012/980250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alles S.R.A., Smith P.A., Isom L.L. Etiology and Pharmacology of Neuropathic Pain. Pharmacol. Rev. 2018;70(2):315–347. doi: 10.1124/pr.117.014399. [DOI] [PubMed] [Google Scholar]
- Alvarez R.D., Barnes M.N., Gomez-Navarro J. A cancer gene therapy approach utilizing an anti-erbB-2 single-chain antibody-encoding adenovirus (AD21): a phase I trial. Clin. Cancer Res. 2000;6(8):3081–3087. [PubMed] [Google Scholar]
- Andre J, Zeau B, Pohl M, Cesselin F, Benoliel JJ, Becker C. Involvement of cholecystokininergic systems in anxiety-induced hyperalgesia in male rats: behavioral and biochemical studies. J Neurosci. 2005;31;25(35):789–76904. doi:10.1523/JNEUROSCI.0743-05.2005. [DOI] [PMC free article] [PubMed]
- Angelini A., Miyabe Y., Newsted D., Kwan B.H., Miyabe C., Kelly R.L., Jamy M.N., Luster A.D., Wittrup K.D. Directed evolution of broadly crossreactive chemokine-blocking antibodies efficacious in arthritis. Nat. Commun. 2018;9(1) doi: 10.1038/s41467-018-03687-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antunes Bras J.M., Laporte A.M., Benoliel J.J. Effects of peripheral axotomy on cholecystokinin neurotransmission in the rat spinal cord. J. Neurochem. 1999;72(2):858–867. doi: 10.1046/j.1471-4159.1999.720858.x. [DOI] [PubMed] [Google Scholar]
- Apkarian A.V., Sosa Y., Sonty S. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci Off J Soc Neurosci. 2004;24(46):10410–10415. doi: 10.1523/JNEUROSCI.2541-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayoub M.A., Crépieux P., Koglin M., Parmentier M., Pin J.-P., Poupon A., Reiter E., Smit M., Steyaert J., Watier H., Wilkinson T. Antibodies targeting G protein-coupled receptors: Recent advances and therapeutic challenges. mAbs. 2017;9(5):735–741. doi: 10.1080/19420862.2017.1325052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baad-Hansen L., Benoliel R. Neuropathic orofacial pain: Facts and fiction. Cephalalgia Int J Headache. 2017;37(7):670–679. doi: 10.1177/0333102417706310. [DOI] [PubMed] [Google Scholar]
- Baliki M.N., Petre B., Torbey S., Herrmann K.M., Huang L., Schnitzer T.J., Fields H.L., Apkarian A.V. Corticostriatal functional connectivity predicts transition to chronic back pain. Nat. Neurosci. 2012;15(8):1117–1119. doi: 10.1038/nn.3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangash M.A., Alles S.R.A., Santana-Varela S., Millet Q., Sikandar S., de Clauser L., ter Heegde F., Habib A.M., Pereira V., Sexton J.E., Emery E.C., Li S., Luiz A.P., Erdos J., Gossage S.J., Zhao J., Cox J.J., Wood J.N. Distinct transcriptional responses of mouse sensory neurons in models of human chronic pain conditions. Wellcome Open Res. 2018;3:78. doi: 10.12688/wellcomeopenres10.12688/wellcomeopenres.14641.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basbaum A.I., Bautista D.M., Scherrer G., Julius D. Cellular and Molecular Mechanisms of Pain. Cell. 2009;139(2):267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce M., Lloyd K.A., Pritchard D.M. Potential clinical indications for a CCK2 receptor antagonist. Curr. Opin. Pharmacol. 2016 Dec;31:68–75. doi: 10.1016/j.coph.2016.09.002. [DOI] [PubMed] [Google Scholar]
- Bradwejn J., Koszycki D., Shriqui C. Enhanced sensitivity to cholecystokinin tetrapeptide in panic disorder. Clinical and behavioral findings. Arch. Gen. Psychiatry. 1991;48(7):603–610. doi: 10.1001/archpsyc.1991.01810310021005. [DOI] [PubMed] [Google Scholar]
- Bradwejn J., Koszycki D. Cholecystokinin and panic disorder: past and future clinical research strategies. Scand J. Clin. Lab. Investig. Suppl. 2001;61(7):19–27. [PubMed] [Google Scholar]
- Butler D.C., McLear J.A., Messer A. Engineered antibody therapies to counteract mutant huntingtin and related toxic intracellular proteins. Prog. Neurobiol. 2012;97(2):190–204. doi: 10.1016/j.pneurobio.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danaher RJ, Zhang L, Donley CJ, et al. Histone deacetylase inhibitors prevent persistent hypersensitivity in an orofacial neuropathic pain model. Mol Pain. 2018;14:1744806918796763. doi:10.1177/1744806918796763. [DOI] [PMC free article] [PubMed]
- Daugé V., Léna I. CCK in Anxiety and Cognitive Processes. Neurosci. Biobehav. Rev. 1998;22(6):815–825. doi: 10.1016/s0149-7634(98)00011-6. [DOI] [PubMed] [Google Scholar]
- de Montigny C. Cholecystokinin tetrapeptide induces panic-like attacks in healthy volunteers. Preliminary findings. Arch Gen Psychiatry. 1989;46(6):511–517. doi: 10.1001/archpsyc.1989.01810060031006. [DOI] [PubMed] [Google Scholar]
- Decosterd I, Woolf CJ. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain. 2000;Aug;87(2):149-58. PMID:10924808. [DOI] [PubMed]
- Dodick D.W., Lipton R.B., Ailani J., Lu K., Finnegan M., Trugman J.M., Szegedi A. Ubrogepant for the Treatment of Migraine. N. Engl. J. Med. 2019;381(23):2230–2241. doi: 10.1056/NEJMoa1813049. [DOI] [PubMed] [Google Scholar]
- Dreier B., Plückthun A. Rapid selection of high-affinity antibody scFv fragments using ribosome display. Methods Mol. Biol. 2018;1827:235–268. doi: 10.1007/978-1-4939-8648-4_13. [DOI] [PubMed] [Google Scholar]
- Friedrich AE, Gebhart GF. Modulation of visceral hyperalgesia by morphine and cholecystokinin from the rat rostroventral medial medulla. Pain. 2003;Jul;104(1-2):93-101. doi:10.1016/s0304-3959(02)00469-4. [DOI] [PubMed]
- Ghilardi J.R., Allen C.J., Vigna S.R., McVey D.C., Mantyh P.W. Trigeminal and dorsal root ganglion neurons express CCK receptor binding sites in the rat, rabbit, and monkey: possible site of opiate-CCK analgesic interactions. J. Neurosci. 1992;12(12):4854–4866. doi: 10.1523/JNEUROSCI.12-12-04854.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold M.S., Gebhart G.F. Nociceptor sensitization in pain pathogenesis. Nat. Med. 2010;16(11):1248–1257. doi: 10.1038/nm.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez-Mecinas M, Bell AM, Shepherd F, et al. Expression of cholecystokinin by neurons in mouse spinal dorsal horn. J Comp Neurol. Published online August 1, 2019. [DOI] [PMC free article] [PubMed]
- Ha G.E., Cheong E. Spike Frequency Adaptation in Neurons of the Central Nervous System. Exp Neurobiol. 2017;26(4):179–185. doi: 10.5607/en.2017.26.4.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashmi JA, Baliki MN, Huang L, et al. Shape shifting pain: chronification of back pain shifts brain representation from nociceptive to emotional circuits. Brain J Neurol. 2013;136(Pt 9):2751-2768. doi:10.1093/brain/awt211. [DOI] [PMC free article] [PubMed]
- Haviv Y., Zadik Y., Sharav Y., Benoliel R. Painful traumatic trigeminal neuropathy: an open study on the pharmacotherapeutic response to stepped treatment. J Oral Facial Pain Headache. 2014;28(1):52–60. doi: 10.11607/jop.1154. [DOI] [PubMed] [Google Scholar]
- Heinricher M.M., McGaraughty S., Tortorici V. Circuitry underlying antiopioid actions of cholecystokinin within the rostral ventromedial medulla. J. Neurophysiol. 2001;85(1):280–286. doi: 10.1152/jn.2001.85.1.280. [DOI] [PubMed] [Google Scholar]
- Jiang M., Bo J., Lei Y. Anxiety-induced hyperalgesia in female rats is mediated by cholecystokinin 2 receptor in rostral ventromedial medulla and spinal 5-hydroxytryptamine 2B receptor. J. Pain Res. 2019;12:2009–2026. doi: 10.2147/JPR.S187715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser V., Idänpään-Hekkilä J.J., Christensen D., Guilbaud G. The selective cholecystokininB receptor antagonist L-365,260 diminishes the expression of naloxone-induced morphine withdrawal symptoms in normal and neuropathic rats. Life Sci. 1998;62(10):947–952. doi: 10.1016/s0024-3205(98)00012-5. [DOI] [PubMed] [Google Scholar]
- Keppel Hesselink J.M. Rediscovery of ceruletide, a CCK agonist, as an analgesic drug. J. Pain Res. 2020;13:123–130. doi: 10.2147/JPR.S232714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YP, Park D, Kim JJ, et al. Effective therapeutic approach for head and neck cancer by an engineered minibody targeting the EGFR receptor. PLoS One. 2014;1;9(12):e113442. doi:10.1371/journal.pone.0113442. [DOI] [PMC free article] [PubMed]
- Kõks S., Fernandes C., Kurrikoff K., Vasar E., Schalkwyk L.C. Gene expression profiling reveals upregulation of Tlr4 receptors in Cckb receptor deficient mice. Behav. Brain Res. 2008;188(1):62–70. doi: 10.1016/j.bbr.2007.10.020. [DOI] [PubMed] [Google Scholar]
- Korczeniewska O.A., Husain S., Khan J., Eliav E., Soteropoulos P., Benoliel R. Differential gene expression in trigeminal ganglia of male and female rats following chronic constriction of the infraorbital nerve. Eur. J. Pain Lond. Engl. 2018;22(5):875–888. doi: 10.1002/ejp.2018.22.issue-510.1002/ejp.1174. [DOI] [PubMed] [Google Scholar]
- Kovelowski C.J., Ossipov M.H., Sun H., Lai J., Malan T.P., Porreca F. Supraspinal cholecystokinin may drive tonic descending facilitation mechanisms to maintain neuropathic pain in the rat. Pain. 2000;87(3):265–273. doi: 10.1016/S0304-3959(00)00290-6. [DOI] [PubMed] [Google Scholar]
- Kramer MS, Cutler NR, Ballenger JC, et al. A placebo-controlled trial of L-365,260, a CCKB antagonist, in panic disorder. Biol Psychiatry. 1995;1;37(7):462-466. doi:10 1016 0006-3223 94 00190-. [DOI] [PubMed]
- Krishnaswamy S., Lin Y., Rajamohamedsait W.J., Rajamohamedsait H.B., Krishnamurthy P., Sigurdsson E.M. Antibody-derived in vivo imaging of tau pathology. J Neurosci Dec. 2014;34(50):16835–16850. doi: 10.1523/JNEUROSCI.2755-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunamneni A., Ye C., Bradfute S.B., Durvasula R., Goldman E.R. Ribosome display for the rapid generation of high-affinity Zika-neutralizing single-chain antibodies. PLoS ONE. 2018;13(11):e0205743. doi: 10.1371/journal.pone.0205743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunamneni A., Clarke E.C., Ye C., Bradfute S.B., Durvasula R. Generation and Selection of a Panel of Pan-Filovirus Single-Chain Antibodies using Cell-Free Ribosome Display. Am. J. Trop. Med. Hyg. 2019;101(1):198–206. doi: 10.4269/ajtmh.18-0658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurrikoff K., Koks S., Matsui T., Bourin M., Arend A., Aunapuu M., Vasar E. Deletion of the CCK2 receptor gene reduces mechanical sensitivity and abolishes the development of hyperalgesia in mononeuropathic mice. Eur. J. Neurosci. 2004;20(6):1577–1586. doi: 10.1111/ejn.2004.20.issue-610.1111/j.1460-9568.2004.03619.x. [DOI] [PubMed] [Google Scholar]
- Lee J.-J., Kim H.J., Čeko M., Park B.-Y., Lee S.A., Park H., Roy M., Kim S.-G., Wager T.D., Woo C.-W. A neuroimaging biomarker for sustained experimental and clinical pain. Nat. Med. 2021;27(1):174–182. doi: 10.1038/s41591-020-1142-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Z., Hore Z., Harley P., Uchenna Stanley F., Michrowska A., Dahiya M., La Russa F., Jager S.E., Villa-Hernandez S., Denk F. A transcriptional toolbox for exploring peripheral neuroimmune interactions. Pain. 2020;161(9):2089–2106. doi: 10.1097/j.pain.0000000000001914. [DOI] [PubMed] [Google Scholar]
- Manning C.E., Williams E.S., Robison A.J. Reward network immediate early gene expression in mood disorders. Front. Behav. Neurosci. 2017;11(77):28503137. doi: 10.3389/fnbeh.2017.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer L., Beart P., Horne M., Finkelstein D., Carrive P., Paxinos G. On the distribution of cholecystokinin B receptors in monkey brain. Brain Res. 1996;738(2):313–318. doi: 10.1016/S0006-8993(96)00477-5. [DOI] [PubMed] [Google Scholar]
- Mitchell J.M., Bergren L.J., Chen K.S., Fields H.L. Cholecystokinin is necessary for the expression of morphine conditioned place preference. Pharmacol. Biochem. Behav. 2006;85(4):787–795. doi: 10.1016/j.pbb.2006.11.014. [DOI] [PubMed] [Google Scholar]
- Montera MA, Westlund KN. Minimally Invasive Oral Surgery Induction of the FRICT-ION Chronic Neuropathic Pain Model. Bio-Protoc. 2020;10(8):e3591-e3591. [DOI] [PMC free article] [PubMed]
- Mutso A.A., Petre B., Huang L., Baliki M.N., Torbey S., Herrmann K.M., Schnitzer T.J., Apkarian A.V. Reorganization of hippocampal functional connectivity with transition to chronic back pain. J. Neurophysiol. 2014;111(5):1065–1076. doi: 10.1152/jn.00611.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal F., Arnold J., Rossant C.J., Podichetty S., Lowne D., Dobson C., Wilkinson T., Colley C., Howes R., Vaughan T.J. Isolation of potent CGRP neutralizing antibodies using four simple assays. J. Biomol. Screen. 2016;21(1):24–34. doi: 10.1177/1087057115610070. [DOI] [PubMed] [Google Scholar]
- North RY, Li Y, Ray P, et al. Electrophysiological and transcriptomic correlates of neuropathic pain in human dorsal root ganglion neurons. Brain. 2019;142(5):1215-1226. doi: 10.1093/brain/awz063. [DOI] [PMC free article] [PubMed]
- Ossipov M.H., Raffa R.B., Pergolizzi J.,.J.V. Galcanezumab: a humanized monoclonal antibody for the prevention of migraine and cluster headache. Drugs Today Barc. 2020;56(1):5. doi: 10.1358/dot.2020.56.1.3069863. [DOI] [PubMed] [Google Scholar]
- Raffaelli B., Neeb L., Reuter U. Monoclonal antibodies for the prevention of migraine. Expert Opin. Biol. Ther. 2019;19(12):1307–1317. doi: 10.1080/14712598.2019.1671350. [DOI] [PubMed] [Google Scholar]
- Ravn P., Madhurantakam C., Kunze S., Matthews E., Priest C., O'Brien S., Collinson A., Papworth M., Fritsch-Fredin M., Jermutus L., Benthem L., Gruetter M., Jackson R.H. Structural and pharmacological characterization of novel potent and selective monoclonal antibody antagonists of glucose-dependent insulinotropic polypeptide receptor. J. Biol. Chem. 2013;288(27):19760–19772. doi: 10.1074/jbc.M112.426288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehfeld, J.F., 2000. Cholecystokinin and panic disorder–three unsettled questions. Regul. Pept. 93(1–3). 10. 1016 0167-0115 00 00179-8. [DOI] [PubMed]
- Robert R., Dolezal O., Waddington L. Engineered antibody intervention strategies for Alzheimer’s disease and related dementias by targeting amyloid and toxic oligomers. Protein Eng. Sel. 2009 Mar;22(3):199–208. doi: 10.1093/protein/gzn052. [DOI] [PubMed] [Google Scholar]
- Roberts-Thomson I.C., Fettman M.J., Jonsson J.R., Frewin D.B. Responses to cholecystokinin octapeptide in patients with functional abdominal pain syndromes. J. Gastroenterol. Hepatol. 1992;7(3):293–297. doi: 10.1111/j.1440-1746.1992.tb00983.x. [DOI] [PubMed] [Google Scholar]
- Rothe A., Hosse R.J., Power B.E. Ribosome display for improved biotherapeutic molecules. Expert Opin. Biol. Ther. 2006;6(2):177–187. doi: 10.1517/14712598.6.2.177. [DOI] [PubMed] [Google Scholar]
- Rotzinger S., Vaccarino F.J. Cholecystokinin receptor subtypes: role in the modulation of anxiety-related and reward-related behaviours in animal models. J. Psychiatry Neurosci. 2003;28(171–181):12790157. [PMC free article] [PubMed] [Google Scholar]
- Schmitz F., Schrader H., Otte J.-M., Schmitz H., Stüber E., Herzig K.-H., Schmidt W.E. Identification of CCK-B/gastrin receptor splice variants in human peripheral blood mononuclear cells. Regul. Pept. 2001;101(1-3):25–33. doi: 10.1016/S0167-0115(01)00281-6. [DOI] [PubMed] [Google Scholar]
- Shields S.D., Eckert W.A., Basbaum A.I. Spared nerve injury model of neuropathic pain in the mouse: a behavioral and anatomic analysis. J. Pain. Off. J. Am. Pain Soc. 2003;4(8):465–470. doi: 10.1067/S1526-5900(03)00781-8. [DOI] [PubMed] [Google Scholar]
- Škrlj N., Drevenšek G., Hudoklin S., Romih R., Čurin Šerbec V., Dolinar M. Recombinant single-chain antibody with the Trojan peptide penetratin positioned in the linker region enables cargo transfer across the blood-brain barrier. Appl. Biochem. Biotechnol. 2013;169(1):159–169. doi: 10.1007/s12010-012-9962-7. [DOI] [PubMed] [Google Scholar]
- Tohidkia M.R., Asadi F., Barar J., Omidi Y. Selection of potential therapeutic human single-chain Fv antibodies against cholecystokinin-B/gastrin receptor by phage display technology. BioDrugs. 2013;27(1):55–67. doi: 10.1007/s40259-012-0007-0. [DOI] [PubMed] [Google Scholar]
- Tsai Y.-H., Yuan R., Patel D., Chandrasekaran S., Weng H.-H., Yang J.-T., Lin C.-P., Biswal B.B. Altered structure and functional connection in patients with classical trigeminal neuralgia. Hum. Brain Mapp. 2018;39(2):609–621. doi: 10.1002/hbm.v39.210.1002/hbm.23696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vachon-Presseau E., Tétreault P., Petre B., Huang L., Berger S.E., Torbey S., Baria A.T., Mansour A.R., Hashmi J.A., Griffith J.W., Comasco E., Schnitzer T.J., Baliki M.N., Apkarian A.V. Corticolimbic anatomical characteristics predetermine risk for chronic pain. Brain J Neurol. 2016;139(7):1958–1970. doi: 10.1093/brain/aww100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanegas H., Schaible H.-G. Descending control of persistent pain: inhibitory or facilitatory? Brain Res. Brain Res. Rev. 2004;46(3):295–309. doi: 10.1016/j.brainresrev.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Vera-Portocarrero L.P., Ossipov M.H., Lai J., King T., Porreca F. Descending facilitatory pathways from the rostroventromedial medulla mediate naloxone-precipitated withdrawal in morphine-dependent rats. J. Pain. 2011;12(6):667–676. doi: 10.1016/j.jpain.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vialou V., Bagot R.C., Cahill M.E. Prefrontal cortical circuit for depression- and anxiety-related behaviors mediated by cholecystokinin: role of ΔFosB. J. Neurosci. Off. J. Soc. Neurosci. 2014;34(11):3878–3887. doi: 10.1523/JNEUROSCI.1787-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins L.R., Kinscheck I.B., Mayer D.J. Potentiation of opiate analgesia and apparent reversal of morphine tolerance by proglumide. Science. 1984;224(4647):395–396. doi: 10.1126/science.6546809. [DOI] [PubMed] [Google Scholar]
- Wiesenfeld-Hallin Z., Aldskogius H., Grant G., Hao J.-X., Hökfelt T., Xu X.-J. Central inhibitory dysfunctions: mechanisms and clinical implications. Behav Brain Sci. 1997;20(3):420–425. doi: 10.1017/S0140525X97261480. [DOI] [PubMed] [Google Scholar]
- Wiesenfeld-Hallin Z., Xu X.-J., Hökfelt T. The role of spinal cholecystokinin in chronic pain states. Pharmacol. Toxicol. 2002;91(6):398–403. doi: 10.1034/j.1600-0773.2002.910619.x. [DOI] [PubMed] [Google Scholar]
- Xie JY, Herman DS, Stiller CO, et al. Cholecystokinin in the rostral ventromedial medulla mediates opioid-induced hyperalgesia and antinociceptive tolerance. J Neurosci. 2005;12;25(2):409-16. doi:10.1523/JNEUROSCI.4054-04.2005. [DOI] [PMC free article] [PubMed]
- Xu XJ, Puke MJ, Verge VM, Wiesenfeld-Hallin Z, Hughes J, Hökfelt T. Up-regulation of cholecystokinin in primary sensory neurons is associated with morphine insensitivity in experimental neuropathic pain in the rat. Neurosci Lett. 1993;2;152(1-2):129-32. doi:10 1016 0304-3940 93 90500-. [DOI] [PubMed]
- Xu X.J., Hoffmann O., Wiesenfeld-Hallin Z. L-740,093, a new antagonist of the CCK-B receptor, potentiates the antinociceptive effect of morphine: electrophysiological and behavioural studies. Neuropeptides. 1996;30(2):203–206. doi: 10.1016/s0143-4179(96)90088-8. [DOI] [PubMed] [Google Scholar]
- Yin K, Deuis L JR, RJ V, I. Transcriptomic and behavioural characterisation of a mouse model of burn pain identify the cholecystokinin 2 receptor as an analgesic target. Mol Pain. 2016;12(1744806916665366). doi:10.1177/1744806916665366. [DOI] [PMC free article] [PubMed]
- Yu S., Zhang Y., Zhao X., Chang Z., Wei Y., Sun Y., Jiang D., Jiang X., Tao J. Cholecystokinin type B receptor-mediated inhibition of A-type K+ channels enhances sensory neuronal excitability through the phosphatidylinositol 3-kinase and c-Src-dependent JNK pathway. Cell Commun Signal. 2019;17(1) doi: 10.1186/s12964-019-0385-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Gardell S, Zhang D, et al. Neuropathic pain is maintained by brainstem neurons co-expressing opioid and cholecystokinin receptors. Brain J Neurol. 2009;132(Pt 3):778-787. doi:10.1093/brain/awn330. [DOI] [PMC free article] [PubMed]
- Zwanzger P., Rupprecht R. Selective GABAergic treatment for panic? Investigations in experimental panic induction and panic disorder. J. Psychiatry Neurosci. 2005;30(3):15944741. [PMC free article] [PubMed] [Google Scholar]