Supplemental Digital Content is available in the text.
Keywords: cognitive dysfunction, coronavirus disease 2019, implementation, intensive care unit recovery, physical rehabilitation, postintensive care syndrome, posttraumatic stress disorder, safety
Abstract
OBJECTIVES:
Examine the safety and feasibility of a multimodal in-person or telehealth treatment program, administered in acute recovery phase for patients surviving critical coronavirus disease 2019.
DESIGN:
Pragmatic, pre-post, nonrandomized controlled trial with patients electing enrollment into one of the two recovery pathways.
SETTING:
ICU Recovery Clinic in an academic medical center.
PATIENTS:
Adult patients surviving acute respiratory failure due to critical coronavirus disease 2019.
INTERVENTIONS:
Patients participated in combined ICU Recovery clinic and 8 weeks of physical rehabilitation delivered: 1) in-person or 2) telehealth. Patients received medical care by an ICU Recovery Clinic interdisciplinary team and physical rehabilitation focused on aerobic, resistance, and respiratory muscle training.
MEASUREMENTS AND MAIN RESULTS:
Thirty-two patients enrolled with mean age 57 ± 12, 62% were male, and the median Sequential Organ Failure Assessment score was 9.5. There were no differences between the two groups except patients in telehealth pathway (n = 10) lived further from clinic than face-to-face patients (162 ± 60 vs 31 ± 47 kilometers, t = 6.06, p < 0.001). Four safety events occurred: one minor adverse event in the telehealth group, two minor adverse events, and one major adverse event in the in-person group. Three patients did not complete the study (two in-person and one telehealth). Six-minute walk distance increased to 101 ± 91 meters from pre to post (n = 29, t = 6.93, p < 0.0001), which was similar between the two groups (110 vs 80 meters, t = 1.34, p = 0.19). Self-reported levels of anxiety, depression, and distress were high in both groups with similar self-report quality of life.
CONCLUSIONS:
A multimodal treatment program combining care from an interdisciplinary team in an ICU Recovery Clinic with physical rehabilitation is safe and feasible in patients surviving the ICU for coronavirus disease 2019 acute respiratory failure.
Patients surviving critical illness may suffer long-term impairments in physical, emotional, and cognitive health (1). After hospital discharge, new or worsening health problems as a result of critical illness are collectively referred to as “postintensive care syndrome” (PICS) (2, 3). Patients surviving severe and critical illness due to coronavirus disease 2019 (COVID-19) disease who required a prolonged ICU admission are also at high risk of developing impairments associated with PICS (4, 5). Post-ICU disability leads to associations with reduced quality of life, inability to reintegrate into societal roles, lost wages, and increased risk of morbidity and mortality (6–8). Recently, the National Institute of Health announced research initiatives on “postacute sequelae of severe acute respiratory syndrome coronavirus 2 infection,” stressing the need to understand, treat, and prevent symptoms occurring post-COVID (9).
In the United States, following a hospitalization that required a medical ICU stay, patients historically would receive outpatient medical care through their primary care provider. More recently, patients may receive care in specialized ICU recovery or specific COVID recovery clinics (10–12). In addition, post-ICU survivors may participate or receive referrals to specific cognitive, psychologic, and physical rehabilitation treatments, but the exact number is unclear (13, 14). Thus, the current approach in ICU Recovery Clinics is to perform multidisciplinary assessments and refer patients in need to specific specialist. Therefore, ICU aftercare may be viewed as fragmented (15), that is, delivery of treatment through different “specialty-aligned silos,” but not centrally guided. Despite such expert recommendations (16), few data exist, which actually support effectiveness of such interventions. Furthermore, the Society of Critical Care Medicine recommends a “more coordinated approach to treatment and support during recovery after critical illness” (17).
For this study, we hypothesized that care coordinated by an ICU Recovery Clinic with an embedded outpatient rehabilitation team delivered to survivors of critical COVID-19 is feasible and could lead to improved patient outcomes. Thus, the primary purpose of this study is to examine the safety and feasibility of providing combined ICU Recovery Clinic appointments and 8 weeks of rehabilitation treatment initiated in the early recovery phase following acute respiratory failure due to COVID-19. In addition, this study explored whether the rehabilitation treatment could be delivered through telehealth. An exploratory purpose of this study is to understand whether treatment provided through telehealth is equivalent to in-person delivery.
MATERIALS AND METHODS
Ethical Considerations
The study was approved by the medical expedited Internal Review Board at the University of Kentucky (MEDXP No. 590957). Patients provided written informed consent to participate. The University of Kentucky ICU Recovery Clinic was established in 2012 and consists of an interdisciplinary team with expertise in critical illness recovery (14). Due to high potential for long-term impairments in COVID-19 survivors, the U.K. ICU Recovery Clinic team formulated a multimodal treatment program in the March of 2020 (18). Prior to February 2020, the ICU Recovery Clinic performed multidisciplinary assessments and referred patients in need to both rehabilitation specialist within our healthcare system and external providers.
Study Design
The study was a phase I feasibility study using a pragmatic, pre-post design with patients self-selecting enrollment into one of the two recovery pathways: in-person versus telehealth. The study was registered in ClinicalTrials.gov (NCT04412330), and the protocol was previously published (18).
Patients
Adult patients (greater than or equal to 18 yr old) surviving an ICU admission for critical COVID-19 (laboratory-confirmed) and attending the ICU Recovery Clinic for interdisciplinary follow-up were eligible. More detailed eligibility criteria are outlined in the protocol (18). Patients were recruited at hospital discharge or during the patients’ first ICU Recovery Clinic appointment (occurring ~2- to 4-wk posthospital discharge), where they underwent a full medical assessment to confirm appropriateness for physical rehabilitation. Exclusion criteria were recent myocardial infraction, acute myocarditis, angina, untreated ventricular or atrial arrhythmias, heart block, and acutely decompensated congestive heart failure. In addition, patients participating in home or community physical or occupational therapy through other delivery methods were encouraged to transition rehabilitation care to the study team; exceptions were made based on pragmatic design with one patient requiring ongoing outpatient occupational therapy due to a brachial plexus injury and three patients receiving home health nursing and physical therapy during the first 2 weeks of study timing. Patients selected their preferred rehabilitation treatment delivery pathway (in-person or telehealth).
Interventions
The interventions are described in brief below (18). The ICU Recovery Clinic and the outpatient rehabilitation center are housed in the same outpatient center and share common spaces.
ICU Recovery Clinic.
The clinic is to provide interdisciplinary care, supporting critical illness recovery and addressing symptoms related to PICS (14). In summary, patients surviving an ICU admission are eligible to attend up to five appointments in the first 12 months of recovery with an option for continued long-term care. The goal is to transition patients back to their primary care provider within 1-year post-ICU. Patients receive coordinated outpatient care centered on medical treatment, medication management, nutritional education, sleep hygiene education, mental health counseling, and education on physical activity and rehabilitation. Clinic providers may refer or recommend further treatment of specific issues, for example, referral to a sleep physician, mental health expert, or cognitive rehabilitation training.
Individualized Physical Rehabilitation Treatment.
Patients participated in 8 weeks of physical rehabilitation treatment initiated within 1 week of informed consent. Physical rehabilitation treatment was delivered via outpatient in-person pulmonary rehabilitation or teleconference. Both pathways were led by the same physical therapist and supported by a respiratory therapist. Telehealth subjects were required to have a caregiver in the home during rehabilitation interventions, and patients were provided home pulse oximeters and automated blood pressure monitors to ensure that delivery of interventions followed the same approach as the in-person treatment. Patients were scheduled for one or two supervised sessions per week (either in-person or supervised telehealth) and prescribed a supplemental unsupervised home program (3 or 4 d/wk). Treatment dosing was pragmatic and multifactorial including patients’ need and social determinant of health (transportation availability and access to caregiver). The treatment plan maintained the following major components:
Aerobic training: Patients participated in 15–30 minutes of aerobic training with a targeted range of 4–6 on the modified rating of perceived exertion (RPE). The initial aerobic intensity was calculated and defined previously (18–21). Aerobic intensities were progressively increased when patients rated an activity less than 4 on the RPE, and their heart rate did not exceed the established target rates.
Strength training: Strengthening exercises were prescribed based on RPE with an initial rating of 5–6 of 10 performing 10–15 repetitions as per guidelines for older and/or deconditioned patients (22, 23). Resistance exercises were progressed by increasing repetitions and load (free weights or elastic resistance bands) if a patient rated an exercise less than or equal to 4 of 10 on RPE and demonstrated ability to complete three sets of 15 repetitions.
Breathing and mindfulness techniques: Patients participated in respiratory muscle training with focus on controlled diaphragmatic breathing combined with mindfulness to reduce anxiety and improve overall mood (24, 25). Diaphragmatic breathing techniques were embedded into daily activities as well as paired with general core and trunk exercises in sitting, quadruped, or standing focused on thoracic chest wall expansion to enhance respiration (26, 27). Mindfulness techniques focused on components of mediation and guided imaginary (23). Breathing and mindfulness techniques were added to the program as a modification to original protocol during the first week of study enrollment; thus, all patients engaged in breathing and mindfulness techniques (18).
Supplemental home-exercise plan: Patients participated in a home-exercise plan (HEP) that included walking, strengthening exercises, and breathing techniques. Patients were instructed to walk at home for at least 30 minutes per day with RPE less than or equal to 4, to perform strengthening exercise (visual handouts provided) unsupervised 3–4 days per week, and to perform diaphragmatic breathing two to three times per day. Patients were instructed to log daily adherence to the HEP in an exercise diary.
Primary Outcomes
The primary outcomes are safety and feasibility.
Safety within the outpatient participation of rehabilitation was defined a priori as the number of adverse events occurring as a direct result of the protocol with sufficient safety defined as less than 5% of treatment sessions having an adverse event. Major adverse events included any event or physiologic abnormality that required escalation of care, for example, injurious fall, increased dosage of medication, emergency department visit, or hospital admission. Minor adverse events included noninjurious falls during or related to interventions, pain, or discomfort related to interventions, and any physiologic abnormality that required termination of rehabilitation interventions, thus warranting medical review before continuing the protocol. Adverse events were adjudicated at the midpoint and completion of the study by ICU Recovery Clinic team and the rehabilitation team; events were deemed related to protocol by consensus.
Feasibility of the multicomponent treatment program was defined a priori based on demand, implementation, and practicality (28); parameters defined a priori: 1) success of consent (greater than 75%) as a percentage of number agreeing to participate per the total number of patients approached, 2) attrition (lost to follow-up) of subjects, 3) attendance to ICU Recovery clinic in the first 6 months of recovery and attendance to the 8 weeks of physical rehabilitation sessions (greater than 75%), and 4) adherence to the home-exercise program.
Secondary Outcomes
Secondary outcomes were assessed at baseline and 3 months. All patients were assessed in-person for baseline assessment. Physical function was assessed using the short physical performance battery (29, 30) and Timed Up and Go Test (TUG) (31, 32). Muscle strength was assessed by the Medical Research Council sum score (MRC-ss) (33–35) and handgrip strength (35) and exercise capacity assessed using the 6-minute walk distance (6 MWD) and percentage achieved of predicted distance (36, 37) and the 2-minute step test for patients in telehealth pathway (38). Five patients in the telehealth pathway returned to the medical center at 3-month time point for other routine medical care (e.g., imaging and labs), and thus, measures were performed in person. The remaining four patients performed measures with supervision of the physical therapist on telehealth with a caregiver setting up the testing environment and providing guarding for safety.
Emotional health, cognitive function, and quality-of-life assessments were Hospital Anxiety and Depression Scale (HADS) (39, 40) for anxiety and depression, distress and posttraumatic stress disorder (PTSD) with the Impact of Events Scale-Revised (IES-R) (41), Visual Analog Scale (VAS) rating health-related quality of life (HRQOL) with EuroQol (EQ-5D) (42), and cognition with Montreal Cognitive Assessment (MOCA), whereas MOCA-blind for the telehealth pathway (43, 44). In addition, return to work for patients previously employed (45), unplanned hospital admissions or emergency department utilization, and mortality were assessed at 3 and 6 months.
Independent demographic and clinical variables were extracted from the electronic health record as described (18). In addition, sedative and agitation statuses were quantified with the Richmond Agitation Sedation Scale as the mean score in the first 72 hours of ICU admission.
Statistical Analysis
Data were initially assessed using descriptive statistics including mean and sd, or median and interquartile range. Safety and feasibility were reported as descriptive statistics. Normality was assessed with Shapiro-Wilk test. Independent t test with Welch correction was performed to assess differences between the two groups due to unequal sample sizes. Paired t test was performed to assess differences in outcomes from baseline to postevaluation for the entire cohort. Equivalence tests using pooled and Satterthwaite t test were performed with dependent variables of change in 6 MWD and self-reported quality of life on EQ-5D VAS to explore the secondary objective. Statistics were performed in SPSS-26 (IBM SPSS, Chicago, IL) and Prism 9 (GraphPad, San Diego, CA).
RESULTS
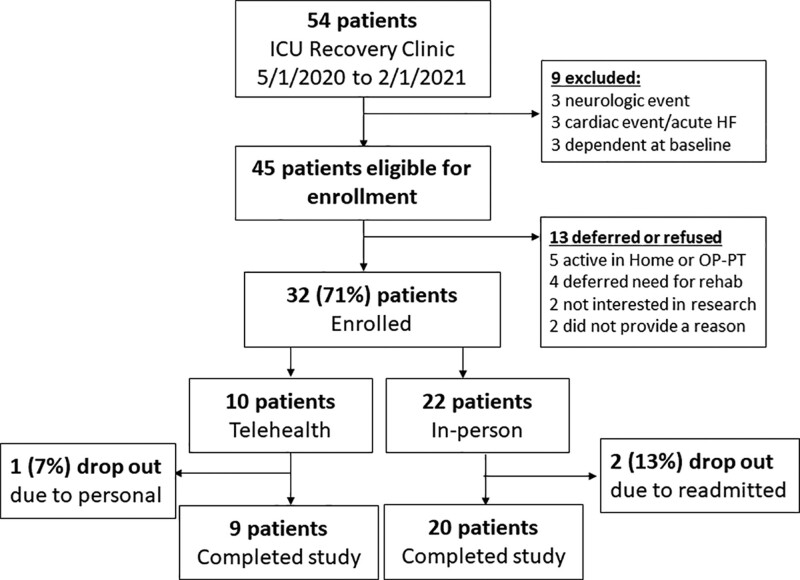
Thirty-two patients were enrolled in the study between May 1, 2020, and January 1, 2021 (Fig. 1). Patients’ mean age was 57 ± 12, 62% were male, and the median Sequential Organ Failure Assessment (SOFA) score was 9.5 (4–11) at ICU admission. Twenty-two patients (62%) selected to participate in the in-person pathway, and 10 elected to enroll in telehealth pathway (Fig. 1). Patients enrolling in the telehealth pathway lived further distances away from clinic (101 ± 37 vs 19.7 ± 29 miles, t = 6.06, p < 0.001) and were more likely to live in a rural-designated zip code (100% vs 23%, t = 8.4, p < 0.001). There were no differences in demographics or clinical data between the two groups, although patients in telehealth group were more likely to be identified as White (Table 1).
Figure 1.
Flow diagram for study recruitment, enrollment, and attrition. HF = heart failure, OP = outpatient, PT = physical therapy.
TABLE 1.
Demographics and Clinical Variables
| Parameter | Cohort (n = 32) | In-Person (n = 22) | Telehealth (n = 10) | Group Differencesa (p < 0.05) |
|---|---|---|---|---|
| Age, yr | 55.5 (52–65) | 56 (52–61) | 55 (44–66) | t = 0.97, p = 0.340 |
| Sex, male | 18 (56) | 13 (59) | 5 (50) | p = 0.712 |
| Race/ethnicity | ||||
| White/Anglo | 17 (53) | 9 (41) | 8 (80) | χ2 = 4.84, p = 0.089 |
| Black/African American | 10 (31) | 8 (36) | 2 (20) | |
| Hispanic/Latino | 5 (16) | 5 (23) | 0 (0) | |
| Body mass index, kg/m2 | 35 (31–37) | 35 (29–37) | 34 (32.5–38.9) | t = 0.76, p = 0.451 |
| Rural-designated residence, yes | 15 (47) | 5 (23) | 10 (100) | p < 0.001 |
| Distance of primary residence, miles | 11.5 (4–79) | 4 (3–23) | 110 (79–120) | t = 6.64, p < 0.001 |
| Charlson comorbidity index | 2 (1–4) | 2 (1–4) | 3 (1–4) | t = 0.04, p = 0.972 |
| Sequential Organ Failure Assessment | 9.5 (4–11) | 9 (4–11) | 9.5 (5–12) | t = 0.46, p = 0.653 |
| Acute Physiology And Chronic Health Evaluation II | 20 (12–24) | 19 (10–23) | 23 (15–24) | t = 0.85, p = 0.403 |
| High-flow nasal cannula, yes | 29 (91) | 20 (91) | 9 (90) | p = 0.735 |
| Mechanical ventilation, yes | 21 (65) | 14 (64) | 7 (70) | p = 0.999 |
| Mechanical ventilation duration, d | 11 (7–17) | 11.5 (6–19) | 10 (7–16.5) | t = 0.55, p = 0.585 |
| Tracheostomy, yes | 4 (13) | 4 (18) | 0 (0) | p = 0.283 |
| Steroids received, yes | 15 (47) | 17 (77) | 5 (50) | p = 0.217 |
| Vasopressor/inotrope received, yes | 22 (69) | 10 (45) | 5 (50) | p = 0.999 |
| Neuromuscular blocker received, yes | 10 (31) | 8 (36) | 2 (20) | p = 0.440 |
| Sedative status (mean Richmond Agitation Sedation Scale) | −2.1 (−3.95 to 0) | −2.5 (−3.8 to 0) | −1.9 (−4 to 0) | t = 0.43, p = 0.672 |
| Inpatient rehabilitation parameters | ||||
| Received PT treatment, yes | 31 (97) | 22 (100) | 9 (90) | p = 0.333 |
| Time to first PT treatment, d | 7.5 (5.5–11.8) | 6.7 (5–10) | 8.5 (6.7–16.9) | t = 1.61, p = 0.132 |
| Number of PT sessions, n | 4 (2.25–5.7) | 4 (2.7–6.2) | 5 (1.7–5) | t = 0.45, p = 0.654 |
| Received OT treatment, yes | 28 (88) | 20 (91) | 8 (80) | p = 0.103 |
| Time to first OT treatment, d | 7.6 (5.6–12.2) | 6.8 (5–10.3) | 11.5 (7.3–16.9) | t = 1.94, p = 0.078 |
| Number of OT sessions, n | 4 (2.2–5.8) | 4 (2.8–6.2) | 5 (1.7–5) | t = 0.317, p = 0.764 |
| ICU LOS, d | 11 (9–18) | 11.3 (7.4–17.9) | 10.6 (9.4–17.0) | t = 0.51, p = 0.612 |
| Hospital LOS, d | 19.5 (13–28) | 20.8 (12–28.3) | 18 (15.4–28.3) | t = 0.06, p = 0.950 |
| Discharge destination | ||||
| Secondary rehabilitation facility (acute or subacute) | 13 (41) | 9 (41) | 4 (40) | χ2 = 7.41, p = 0.595 |
| Home with home health rehabilitation | 10 (31) | 7 (32) | 3 (30) | |
| Home without order for services | 8 (25) | 6 (27) | 3 (30) | |
LOS = length of stay, OT = occupational therapy, PT = physical therapy.
aIndependent t test, χ2 test, or Fisher exact test comparing in-person and telehealth groups.
Safety
One major and three minor adverse events in four different patients occurred, which were deemed “directly” related to the protocol during 325 completed rehabilitation sessions (n = 4/325, 1.2%). A total of 18 potential adverse events occurred during the study (5.5%) (Table 2). The major adverse event occurred during the first week of a patient’s participation in the study with reports of increased dyspnea and exhaustion a few hours after in-person rehabilitation. The patient self-elected to visit the emergency department leading to a 48-hour observational stay and then requested to be removed from the study due to increasing oxygen requirements with minimal exertion. Three minor adverse events and 14 nonstudy-related events were reported and reviewed by the medical team (Supplemental Table 1, http://links.lww.com/CCX/A755).
TABLE 2.
Safety and Feasibility of Multimodal Treatment Program
| Parameter | n (%) or mean ± sd |
|---|---|
| Consent success | |
| Yes, n | 32 (71) |
| No, n | 13 (29)a |
| Selected in-person pathway | 22 (69) |
| Selected telehealth pathway | 10 (31) |
| Minor adverse events | 3 |
| Major adverse events | 1 |
| Rate of adverse event occurrence per total number of sessions | 4/325 (1.2) |
| Attrition | 3 (9.4)b |
| Attendance to ICU Recovery Clinic appointments | |
| Attended, mean ± sd | 2.69 ± 1.10 |
| Scheduled, mean ± sd | 2.93 ± 0.95 |
| Percent attendance | 88% ± 24% |
| Attendance to Physical Therapy appointments | |
| Attended, mean ± sd | 10.2 ± 4.7 |
| Scheduled, mean ± sd | 12.7 ± 3.4 |
| Percent attendance | 78.4% ± 26% |
| Frequency per wk | 1.34 ± 0.5 |
| Adherence to home-exercise plan | |
| Days completed, mean ± sd | 2.48 ± 1.1 |
| Percent completed of prescribed | 65% ± 28% |
| Adherence to walking/aerobic program | |
| Days completed, mean ± sd | 4.34 ± 1.6 |
| Percent completed of daily walking | 62% ± 23% |
aThirteen patients refused or deferred need to participate: five patients were already participating in home-based or outpatient physical therapy, four patients had returned to work or prehospital function and deferred the need for treatment, two patients were not interested in research, and two patients did not provide a reason.
bThree patients did not complete the study: one readmitted and did not reenter study posthospital discharge; eventually, the patient required a second readmission and expired due to acute or chronic respiratory failure with history of chronic obstructive pulmonary disease (in-person), one patient readmitted to hospital for pneumothorax and did not reenter study (in-person), and one patient asked to be removed due to personal reasons (telehealth).
Feasibility
The consent rate was 71% (32/45); 29 patients completed the full 8-week program combined with recovery clinic support. Patients agreed to participate in the study a mean 34.9 ± 17 days after hospital discharge and 25.7 ± 8.9 days from last institutional discharge accounting for patients attending posthospital secondary facilities. The group’s attrition rate was equal (10% telehealth and 9% in-person). The three patients who did not finish the study were as follows: 1) the aforementioned patient (in-person), 2) a patient developed a pneumothorax approximately 3 weeks after consent unrelated to study and required hospitalization with subsequent study withdrawal (in-person), and 3) one patient asked to be removed from telehealth group due to personal reasons. Patients attended 88% (range, 33–100%) of their ICU Recovery Clinic appointments and 77% of physical rehabilitation appointments (range, 13–100%) (Table 2). Attendance did not differ based on the rehabilitation pathway chosen by the patient. Patients participated in an average of 10.2 ± 4.7 supervised sessions lasting a mean 59.3 ± 7.6 minutes, which was not different between the groups. Patients self-reported in an exercise diary participating in 62% ± 23% and 65% ± 28% of their mobility program and HEP, respectively.
Muscle Strength and Physical Function
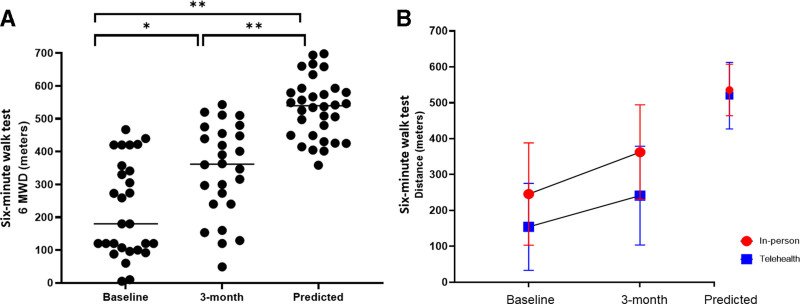
Seven patients (22%) met criteria for a diagnosis of ICU-acquired weakness at baseline testing (less than 48/60 on MRC-ss). The 6 MWD improved by mean 101 ± 93 meters from pre to post (n = 29, t = 5.97, p < 0.0001) (Fig. 2A). At postevaluation, distances on 6 MWD were a mean 61% ± 26% of the predicted distance, and distances achieved were not statistically different the in-person and telehealth cohorts (t = 1.34, p = 0.19) (Fig. 2B). The change from pre to post between the two groups was similar but not equivalent to the pooled t test using the lower and upper bounds of −30 to 30 meters (t = 0.01–1.62, p = 0.058–0.51) (Fig. 2B). Chair stand test was 15.7 ± 7.6 seconds at baseline with seven patients unable to complete due to deficits in lower extremity muscle strength and power. On average, patients improved their chair stand time by 4.9 ± 6.1 seconds (n = 28, t = 3.56, p = 0.001; Supplemental Table 2, http://links.lww.com/CCX/A756) with two patients unable to perform the test at postassessment. Habitual gait speed improved from pre to post by mean average 0.22 meters per second, and TUG improved by 3.2 seconds, respectively (Supplemental Table 2, http://links.lww.com/CCX/A756).
Figure 2.
Distance ambulated on the 6-mintue walk test in severe coronavirus disease 2019 survivors pre-post treatment delivery. A, Distance improved from 216 ± 142 meters at baseline to 326 ± 143 meters at 3-month assessment; significant change over time (* represents t = 7.43 and p < 0.0001), which is statistically lower than predicted distances (** represents t = 5.6 and p < 0.0001). At postevaluation, distances on 6-mintue walk distance (6 MWD) were a mean 61% ± 26% of the predicted distance. B, Distances improved for both groups from baseline to 3 months, but patients in the in-person group had a slightly higher change than that in the telehealth (110 vs 80 meters, mean difference of 30.5, p = 0.51 using Satterthwaite equivalence t test). Distances at postassessment were not statistically different between the in-person and telehealth cohorts with independent t test (t = 1.34, p = 0.19).
Emotional Health, Cognitive Function, and HrQOL
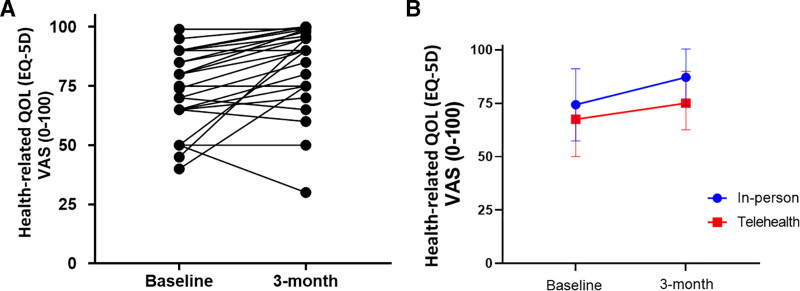
There was clinical improvement in scores across the domains of mental health, cognition, and quality of life (Supplemental Table 2, http://links.lww.com/CCX/A756). Twelve patients (40%) reported high anxiety (greater than 8 on HADS-anxiety), 27% had greater than 8 on HADS-depression, and 40% met criteria for provisional diagnosis of PTSD on IES-R at baseline. At postevaluation, 36% had anxiety, 25% were with depression, and 27% still met criteria for PTSD. HrQOL was higher in the in-person group at postevaluation but not statistically significant based on independent t test (Fig. 3A). The mean difference between the two groups in the change in VAS from pre to post was 0.09, which was approaching equivalence (Satterthwaite t = 1.4, p = 0.096) (Fig. 3B). Of the 25 patients working prior to hospitalization, eight (32%) and an additional five patients (n = 13, 52%) had returned to work at the 3- and 6-month time points, respectively.
Figure 3.
Patients surviving critical coronavirus disease 2019 (COVID-19) self-report health-related quality of life (HrQOL). A, HrQOL improved from mean 71 at baseline to 83 at postassessment in 29 survivors of critical COVID-19 (t = 3.85, p = 0.0005); seven patients reported no change or worse quality of life from baseline to 3 mo post. B, QOL improved in both cohorts (+11.2 for in-person and +11.3 in telehealth groups), which was approaching statistically significant for equivalence (mean difference = 0.095, t = 1.40, p = 0.096). EQ-5D = EuroQol, VAS = Visual Analog Scale.
Mortality, Readmissions, and Emergency Department Utilization
Two patients required three unplanned hospitalizations (Supplemental Table 2, http://links.lww.com/CCX/A756), and two patients required visits to emergency department before the 6-month time point. There was one death (3%) before the 6-month time point.
DISCUSSION
This study demonstrates that a multimodal treatment program in the acute recovery phase can be delivered safely to survivors of critical COVID-19. Of importance, a low adverse event rate and high attendance in both groups support the safety and feasibility of the program. The pragmatic nature of the study with patients self-selecting their delivery pathway may speak to increased participation and adherence among the participants. At the time of study inception (March 2020), our study and clinic team decided against enrolling a control group due to focus on pragmatic design and uncertainty surrounding developing COVID pandemic. Thus, the results should be interpreted with caution as the study design limits inferences regarding the efficacy of the program. Data from pre-post, however, may provide preliminary evidence to design further studies to test “embedded” and centrally coordinated delivery of post-ICU medical and rehabilitation care. Of interest, patients improved 6 MWD by 100 meters over the study duration, which is triple the suggested minimal detectable change (approximately 30 meters) within the ICU population (46, 47). Furthermore, the distance at postevaluation was similar to historical controls of previously published acute respiratory distress syndrome (ARDS) survivors assessed at the same time point (320 ± 138 [48, 49]; 281 [55–454] [50]) but not different to a combined meta-analysis in survivors of ICU with and without ARDS assessed 3 months after discharge (361 [321–401]) (51). The findings, however, provide the preliminary evidence, justifying that patients surviving critical illness can participate and may benefit from ICU aftercare provided through interdisciplinary programs.
The purpose of this study was to assess the safety and feasibility of an interdisciplinary ICU recovery program that included embedded outpatient physical rehabilitation. The goal of the program was to enhance the interconnectedness of medical and rehabilitation care in an attempt to reduce “fragmentation” of care. Previous models of ICU aftercare including our own clinic routinely perform multifaceted assessments and refer to other healthcare specialists when a need is identified (11, 12, 14, 52). Our pragmatic design was formulated to enhance communication and collaboration with clinical goal to optimize outcomes. Patients in this study and their outcomes were similar to previous cohorts on non-COVID ICU survivors including age (~50 to 55 yr old), index severity of illness (SOFA scores 9–10), and high occurrence of physical, emotional, and cognitive impairments (14, 52). Of clinical interest, our subjects surviving severe COVID-19 had similar rates of cognitive impairments (53), anxiety (54), depression (55), and PTSD (56) compared with previous findings in non-COVID survivors in systematic reviews and meta-analyses. Data are emerging that severe COVID-19 survivors are at risk of one or more impairments related to recovery (57, 58).
Physical, emotional, and cognitive outcomes all improved from baseline to postassessments, and there were no differences between the two pragmatic groups. Statistical analysis demonstrates that patients in both groups had similar changes from pre to post. These findings should be taken with caution since equivalence tests were not significant at the 0.05 level likely due to small sample sizes. Clinically, the telehealth pathway requires substantial planning and attention to safety in the patient’s home; the study does provide a framework for future studies. Furthermore, our pragmatic design included four occurrences when physical outcomes were performed through telehealth supported by a caregiver, which may reduce the validity and reliability of assessments and induce biases. There were two patients who lived a considerable distance from clinic but stated they did not prefer telehealth. Thus, telehealth is not appropriate for every patient but may be useful for patients living in areas with reduced access to treatment. The concept of delivery interventions via telehealth for ICU survivors is not new and may lead to improved care by “removing financial, geographic, and societal barriers to recovery” (59).
Very little is known about the optimal delivery of physical rehabilitation in ICU survivors and specifically patients surviving severe COVID-19. There is no consensus on the optimal aerobic intensity and dosing for persons with acute and chronic lung disease (23). Fatigue, muscle weakness, and anxiety or depression are common symptoms posthospital discharge in individuals surviving COVID-19 (8, 60–62). Patients recovering from COVID-19 may also be at risk of complications and new symptoms such as viral myocarditis (63), thromboembolic complications (64), postural orthostatic tachycardia syndrome, and chronic fatigue syndrome (65). Thus, individuals with or recovering from COVID-19 regardless of the initial severity should undergo medical or risk stratification screening before exercise or physical activity interventions (66). A few patients in this study experienced bouts of anxiety, racing heart rates, and one episode of atrial fibrillation but did not present with symptoms similar to postexertional malaise, potentially, due to the low training intensities with focus on functional recovery post-ICU. Breathing techniques and mindfulness training were added to our protocol to emphasize efficient respiratory muscle activation with previous findings demonstrating that core stabilization exercises with breathing techniques are more effective in improving pulmonary function (26, 27). Subjective reports from patients in both groups suggested that breathing and mindfulness techniques became habitual practice. The findings of this study suggest that combined aerobic, resistance, and breathing training is feasible for COVID-19-related acute respiratory failure and may lead to improvements in physical function.
This study’s primary limitation is the pragmatic design without a control, which prevents causative statements and limits the ability to conclude on efficacy. Furthermore, the multimodal design prevents interpretation of the benefit of specific interventions. The study is limited by the small patient numbers within groups but may provide preliminary evidence to develop and test similar interdisciplinary and embedded programs. Finally, the study did not include blinding that increases the risk of bias during outcome assessments. Due to the limitations in the study design, the study reports the pre-post outcomes as exploratory evidence and should be interpreted with caution.
CONCLUSIONS
A multimodal treatment plan emphasizing ICU aftercare with an embedded physical rehabilitation program is safe and feasible in patients surviving the ICU for COVID-19 acute respiratory failure, when administered either in-person or through telehealth.
ACKNOWLEDGMENT
We thank Stacey Slone, MS, Department of Statistics, University of Kentucky, for her assistance with the study analysis.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
Supported, in part, by the National Institute of Health National Center for Advancing Translational Sciences through grant number UL1TR001998.
The authors have disclosed that they do not have any potential conflicts of interest.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Health. The funders played no role in the design, conduct, or reporting of this study.
The study was registered on ClinicalTrials.gov on June 2, 2020 (NCT04412330).
REFERENCES
- 1.Desai SV, Law TJ, Needham DM. Long-term complications of critical care. Crit Care Med. 2011; 39:371–379 [DOI] [PubMed] [Google Scholar]
- 2.Colbenson GA, Johnson A, Wilson ME. Post-intensive care syndrome: Impact, prevention, and management. Breathe (Sheff). 2019; 15:98–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Needham DM, Davidson J, Cohen H, et al. Improving long-term outcomes after discharge from intensive care unit: Report from a stakeholders’ conference. Crit Care Med. 2012; 40:502–509 [DOI] [PubMed] [Google Scholar]
- 4.Jaffri A, Jaffri UA. Post-intensive care syndrome and COVID-19: Crisis after a crisis? Heart Lung. 2020; 49:883–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stam HJ, Stucki G, Bickenbach J; European Academy of Rehabilitation Medicine: Covid-19 and post intensive care syndrome: A call for action. J Rehabil Med. 2020; 52:jrm00044. [DOI] [PubMed] [Google Scholar]
- 6.Cuthbertson BH, Roughton S, Jenkinson D, et al. Quality of life in the five years after intensive care: A cohort study. Crit Care. 2010; 14:R6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Griffiths J, Hatch RA, Bishop J, et al. An exploration of social and economic outcome and associated health-related quality of life after critical illness in general intensive care unit survivors: A 12-month follow-up study. Crit Care. 2013; 17:R100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bellan M, Soddu D, Balbo PE, et al. Respiratory and psychophysical sequelae among patients with COVID-19 four months after hospital discharge. JAMA Netw Open. 2021; 4:e2036142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collins FS: NIH Launches New Initiative to Study “Long COVID”. 2021. The NIH Director. Available at: https://www.nih.gov/about-nih/who-we-are/nih-director/statements/nih-launches-new-initiative-study-long-covid. Accessed April 20, 2021 [Google Scholar]
- 10.Lutchmansingh DD, Knauert MP, Antin-Ozerkis DE, et al. A clinic blueprint for post-coronavirus disease 2019 RECOVERY: Learning from the past, looking to the future. Chest. 2021; 159:949–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Biehl M, Sese D. Post-intensive care syndrome and COVID-19 - implications post pandemic. Cleve Clin J Med. 2020August5. [online ahead of print] [DOI] [PubMed] [Google Scholar]
- 12.O’Brien H, Tracey MJ, Ottewill C, et al. An integrated multidisciplinary model of COVID-19 recovery care. Ir J Med Sci. 2021; 190:461–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Modrykamien AM. The ICU follow-up clinic: A new paradigm for intensivists. Respir Care. 2012; 57:764–772 [DOI] [PubMed] [Google Scholar]
- 14.Mayer KP, Boustany H, Cassity EP, et al. ICU recovery clinic attendance, attrition, and patient outcomes: The impact of severity of illness, gender, and rurality. Crit Care Explor. 2020; 2:e0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sevin CM, Boehm LM, Hibbert E, et al. Optimizing critical illness recovery: Perspectives and solutions from the caregivers of ICU survivors. Crit Care Explor. 2021; 3:e0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Major ME, Kwakman R, Kho ME, et al. Surviving critical illness: What is next? An expert consensus statement on physical rehabilitation after hospital discharge. Crit Care. 2016; 20:354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elliott D, Davidson JE, Harvey MA, et al. Exploring the scope of post-intensive care syndrome therapy and care: Engagement of non-critical care providers and survivors in a second stakeholders meeting. Crit Care Med. 2014; 42:2518–2526 [DOI] [PubMed] [Google Scholar]
- 18.Mayer KP, Steele AK, Joshi RR, et al. Optimizing outcomes with physical therapy treatment for individuALs surviving an intensive care units admission for COVID-19 (OPTImAL)—a protocol for a single center prospective study. Cardiopulm Phys Ther J. 2021; 32:S32–S39 [Google Scholar]
- 19.Holland AE, Hill K, Alison JA, et al. Estimating peak work rate during incremental cycle ergometry from the 6-minute walk distance: Differences between reference equations. Respiration. 2011; 81:124–128 [DOI] [PubMed] [Google Scholar]
- 20.ZuWallack R, Crouch R: American Association of Cardiovascular and Pulmonary Rehabilitation Guidelines for Pulmonary Rehabilitation Programs. Fourth Edition. Champaign, IL, Human Kinetics, 2011 [Google Scholar]
- 21.Balady GJ, Arena R, Sietsema K, et al. ; American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee of the Council on Clinical Cardiology; Council on Epidemiology and Prevention; Council on Peripheral Vascular Disease; Interdisciplinary Council on Quality of Care and Outcomes Research: Clinician’s Guide to cardiopulmonary exercise testing in adults: A scientific statement from the American Heart Association. Circulation. 2010; 122:191–225 [DOI] [PubMed] [Google Scholar]
- 22.Thompson W, Gordon E, Pescatello L: ACSM’s Guidelines for Exercise Testing and Prescription. Eighth Edition. Philadelphia,. Lippincott Williams and Wilkins, 2010 [Google Scholar]
- 23.Garvey C, Fullwood MD, Rigler J. Pulmonary rehabilitation exercise prescription in chronic obstructive lung disease: US survey and review of guidelines and clinical practices. J Cardiopulm Rehabil Prev. 2013; 33:314–322 [DOI] [PubMed] [Google Scholar]
- 24.Khusid MA, Vythilingam M. The emerging role of mindfulness meditation as effective self-management strategy, part 1: Clinical implications for depression, post-traumatic stress disorder, and anxiety. Mil Med. 2016; 181:961–968 [DOI] [PubMed] [Google Scholar]
- 25.Cahalin LP, Braga M, Matsuo Y, et al. Efficacy of diaphragmatic breathing in persons with chronic obstructive pulmonary disease: A review of the literature. J Cardiopulm Rehabil. 2002; 22:7–21 [DOI] [PubMed] [Google Scholar]
- 26.Bradley H, Esformes J. Breathing pattern disorders and functional movement. Int J Sports Phys Ther. 2014; 9:28–39 [PMC free article] [PubMed] [Google Scholar]
- 27.Cavaggioni L, Ongaro L, Zannin E, et al. Effects of different core exercises on respiratory parameters and abdominal strength. J Phys Ther Sci. 2015; 27:3249–3253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bowen DJ, Kreuter M, Spring B, et al. How we design feasibility studies. Am J Prev Med. 2009; 36:452–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chan KS, Aronson Friedman L, Dinglas VD, et al. Evaluating physical outcomes in acute respiratory distress syndrome survivors: Validity, responsiveness, and minimal important difference of 4-meter gait speed test. Crit Care Med. 2016; 44:859–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parry SM, Denehy L, Beach LJ, et al. Functional outcomes in ICU – what should we be using? – an observational study. Crit Care. 2015; 19:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muir-Hunter SW, Clark J, McLean S, et al. Identifying balance and fall risk in community-dwelling older women: The effect of executive function on postural control. Physiother Can. 2014; 66:179–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jackson JC, Ely EW, Morey MC, et al. Cognitive and physical rehabilitation of intensive care unit survivors: Results of the RETURN randomized controlled pilot investigation. Crit Care Med. 2012; 40:1088–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Connolly BA, Jones GD, Curtis AA, et al. Clinical predictive value of manual muscle strength testing during critical illness: An observational cohort study. Crit Care. 2013; 17:R229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Jonghe B, Sharshar T, Lefaucheur JP, et al. ; Groupe de Réflexion et d’Etude des Neuromyopathies en Réanimation: Paresis acquired in the intensive care unit: A prospective multicenter study. JAMA. 2002; 288:2859–2867 [DOI] [PubMed] [Google Scholar]
- 35.Parry SM, Berney S, Granger CL, et al. A new two-tier strength assessment approach to the diagnosis of weakness in intensive care: An observational study. Crit Care. 2015; 19:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories: ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002; 166:111–117 [DOI] [PubMed] [Google Scholar]
- 37.Enright PL, Sherrill DL. Reference equations for the six-minute walk in healthy adults. Am J Respir Crit Care Med. 1998; 158:1384–1387 [DOI] [PubMed] [Google Scholar]
- 38.Bohannon RW, Crouch RH. Two-minute step test of exercise capacity: Systematic review of procedures, performance, and clinimetric properties. J Geriatr Phys Ther. 2019; 42:105–112 [DOI] [PubMed] [Google Scholar]
- 39.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983; 67:361–370 [DOI] [PubMed] [Google Scholar]
- 40.Bjelland I, Dahl AA, Haug TT, et al. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. 2002; 52:69–77 [DOI] [PubMed] [Google Scholar]
- 41.Bienvenu OJ, Williams JB, Yang A, et al. Posttraumatic stress disorder in survivors of acute lung injury: Evaluating the Impact of Event Scale-Revised. Chest. 2013; 144:24–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rabin R, de Charro F. EQ-5D: A measure of health status from the EuroQol Group. Ann Med. 2001; 33:337–343 [DOI] [PubMed] [Google Scholar]
- 43.Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005; 53:695–699 [DOI] [PubMed] [Google Scholar]
- 44.Carson N, Leach L, Murphy KJ. A re-examination of Montreal Cognitive Assessment (MoCA) cutoff scores. Int J Geriatr Psychiatry. 2018; 33:379–388 [DOI] [PubMed] [Google Scholar]
- 45.Kamdar BB, Suri R, Suchyta MR, et al. Return to work after critical illness: A systematic review and meta-analysis. Thorax. 2020; 75:17–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bohannon RW, Crouch R. Minimal clinically important difference for change in 6-minute walk test distance of adults with pathology: A systematic review. J Eval Clin Pract. 2017; 23:377–381 [DOI] [PubMed] [Google Scholar]
- 47.Chan KS, Pfoh ER, Denehy L, et al. Construct validity and minimal important difference of 6-minute walk distance in survivors of acute respiratory failure. Chest. 2015; 147:1316–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pfoh ER, Wozniak AW, Colantuoni E, et al. Physical declines occurring after hospital discharge in ARDS survivors: A 5-year longitudinal study. Intensive Care Med. 2016; 42:1557–1566 [DOI] [PubMed] [Google Scholar]
- 49.Fan E, Dowdy DW, Colantuoni E, et al. Physical complications in acute lung injury survivors: A two-year longitudinal prospective study. Crit Care Med. 2014; 42:849–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Herridge MS, Cheung AM, Tansey CM, et al. ; Canadian Critical Care Trials Group: One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003; 348:683–693 [DOI] [PubMed] [Google Scholar]
- 51.Parry SM, Nalamalapu SR, Nunna K, et al. Six-minute walk distance after critical illness: A systematic review and meta-analysis. J Intensive Care Med. 2021; 36:343–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sevin CM, Bloom SL, Jackson JC, et al. Comprehensive care of ICU survivors: Development and implementation of an ICU recovery center. J Crit Care. 2018; 46:141–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wolters AE, Slooter AJ, van der Kooi AW, et al. Cognitive impairment after intensive care unit admission: A systematic review. Intensive Care Med. 2013; 39:376–386 [DOI] [PubMed] [Google Scholar]
- 54.Nikayin S, Rabiee A, Hashem MD, et al. Anxiety symptoms in survivors of critical illness: A systematic review and meta-analysis. Gen Hosp Psychiatry. 2016; 43:23–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rabiee A, Nikayin S, Hashem MD, et al. Depressive symptoms after critical illness: A systematic review and meta-analysis. Crit Care Med. 2016; 44:1744–1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Parker AM, Sricharoenchai T, Raparla S, et al. Posttraumatic stress disorder in critical illness survivors: A metaanalysis. Crit Care Med. 2015; 43:1121–1129 [DOI] [PubMed] [Google Scholar]
- 57.van Gassel RJJ, Bels J, Remij L, et al. Functional outcomes and their association with physical performance in mechanically ventilated coronavirus disease 2019 survivors at 3 months following hospital discharge: A cohort study. Crit Care Med. 2021May10. [online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martillo M, Dangayach N, Tabacof L, et al. Postintensive care syndrome in survivors of critical illness related to coronavirus disease 2019: Cohort study from a New York City Critical Care Recovery Clinic. Crit Care Med. 2021March16. [online ahead of print] [DOI] [PubMed] [Google Scholar]
- 59.Jalilian L, Cannesson M, Kamdar N. Post-ICU recovery clinics in the era of digital health and telehealth. Crit Care Med. 2019; 47:e796–e797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet. 2021; 397:220–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Paneroni M, Simonelli C, Saleri M, et al. Muscle strength and physical performance in patients without previous disabilities recovering from COVID-19 pneumonia. Am J Phys Med Rehabil. 2021; 100:105–109 [DOI] [PubMed] [Google Scholar]
- 62.Wiertz CMH, Vints WAJ, Maas GJCM, et al. COVID-19: Patient characteristics in the first phase of postintensive care rehabilitation. Arch Rehabil Res Clin Transl. 2021; 3:100108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Siripanthong B, Nazarian S, Muser D, et al. Recognizing COVID-19-related myocarditis: The possible pathophysiology and proposed guideline for diagnosis and management. Heart Rhythm. 2020; 17:1463–1471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020; 383:120–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Friedman KJ, Murovska M, Pheby DFH, et al. Our evolving understanding of ME/CFS. Medicina (Kaunas). 2021; 57:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Salman D, Vishnubala D, Le Feuvre P, et al. Returning to physical activity after covid-19. BMJ. 2021; 372:m4721. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.