Considering agroecosystem multifunctionality is essential for designing sustainable cropping systems.
Abstract
Ecosystems provide multiple services to humans. However, agricultural systems are usually evaluated on their productivity and economic performance, and a systematic and quantitative assessment of the multifunctionality of agroecosystems including environmental services is missing. Using a long-term farming system experiment, we evaluated and compared the agronomic, economic, and ecological performance of the most widespread arable cropping systems in Europe: organic, conservation, and conventional agriculture. We analyzed 43 agroecosystem properties and determined overall agroecosystem multifunctionality. We show that organic and conservation agriculture promoted ecosystem multifunctionality, especially by enhancing regulating and supporting services, including biodiversity preservation, soil and water quality, and climate mitigation. In contrast, conventional cropping showed reduced multifunctionality but delivered highest yield. Organic production resulted in higher economic performance, thanks to higher product prices and additional support payments. Our results demonstrate that different cropping systems provide opposing services, enforcing the productivity–environmental protection dilemma for agroecosystem functioning.
INTRODUCTION
Global food production has more than doubled in the past 60 years. This has been achieved through land use change and use of mineral fertilizers, pesticides, breeding of new crop varieties, and other technologies of the “Green Revolution” (1, 2). However, increased use of agrochemicals, land conversion, farm expansion, and farm specialization have a negative impact on the environment and have caused habitat and biodiversity loss, pollution, and eutrophication of water bodies, increasing greenhouse gases emissions and reduced soil quality (1, 3, 4). Thus, one of the main challenges for the future of agriculture is to produce sufficient amounts of food with minimal environmental impact (1). However, to date, there is lack of appropriate methods and tools to evaluate, design, and track the multifunctionality and sustainability of agricultural production.
For agronomists, the focus of agricultural systems is dedicated to productivity, while ecologists and environmental researchers focus on the environmental impact of agriculture. Ideally, agricultural systems should provide the desired balance of provisioning services (e.g., food production), regulating services (e.g., soil, water, and climate protection), and supporting services (e.g., biodiversity and soil quality conservation) within viable socioeconomic boundaries (e.g., ensured income and suitable working conditions). However, systemic evaluations of the diverse services and trade-offs provided by different agricultural practices are scarce, and this has been viewed as a major research gap (5, 6).
In the past 15 years, there have been considerable efforts to conceptualize ecosystem services (ESs), defining their contribution to human well-being and bring it into policy and planning. Examples such as the Millennium Ecosystem Assessment (MEA) (7), The Economics of Ecosystems and Biodiversity (8), or the Intergovernmental Platform on Biodiversity and Ecosystem Service (9) are global initiatives that are integrated in national monitoring programs such as the U.K. National Ecosystem Assessment (UKNEA) framework (10). Even if these concepts and framework are increasingly recognized, there is a lack of implementation in practice due to difficulties to appropriately measure and value ES and to institutionalize outcomes (11).
One of the key approaches to measure and appropriately manage agroecosystems is to gain a solid understanding of how farming practices influence a wide range of ecosystem functions and services and to summarize these effects in a meaningful way (12, 13). The “ability of ecosystems to simultaneously provide multiple functions and services” can be assessed by calculating ecosystem multifunctionality (EMF), an approach widely used in ecology (14, 15). Here, we define ecosystem functions as the biotic and abiotic processes that make up or contribute to ESs either directly or indirectly.
A range of studies has assessed how different drivers including biodiversity and land use intensity affect individual functions and EMF (16–19). However, this approach is still poorly developed for agroecosystems (15), where anthropogenic management plays a key role in determining ecosystem functioning (i.e., specific crop management practices like tillage intensity and chemical and organic input sources and amounts). Moreover, the number of ecosystem functions used to assess EMF varies greatly among studies, and there is often little explanation of why certain variables are included (15). Thus, a next frontier is to investigate how major cropping systems (e.g., conventional, organic, and conservation agriculture) influence different ecosystem functions and EMF and embed such analyses in a broader conceptual ES framework supporting producer and policy decisions.
The main objective of this study is to assess the overall performance of important cropping systems within an adapted ES framework using the EMF methodology applied in ecology. To do this, we use a 6-year dataset from the long-term FArming System and Tillage (FAST) experiment (fig. S1) where we compare the agronomical, ecological, and economic impacts of four arable cropping systems [conventional intensive tillage (C-IT), conventional no tillage (C-NT), organic intensive tillage (O-IT), and organic reduced tillage (O-RT); see Materials and Methods and tables S1 to S3 for detailed management description]. We focus on these specific management strategies since conservation and organic agriculture are two main alternatives to conventional management and are often promoted as more environmentally friendly practices. Organic agriculture prohibits the use of synthetic inputs (e.g., pesticides and fertilizers), and a range of studies show that organic farming enhances biodiversity and reduces environmental impacts but results in lower productivity (4, 5, 20, 21). Conservation agriculture, in turn, is based on three main pillars: minimum mechanical soil disturbance, permanent soil cover, and species diversification, which are applicable in many different farming contexts (22). Several studies indicate that conservation agriculture has positive effects on soil quality and protection, water regulation, energy use, and production costs (23), but productivity increases are minimal or even negative (24) and often dependent on herbicide use (25). In our study, C-NT and O-RT systems are considered to reflect conservation agriculture as the three defined pillars of conservation agriculture are largely fulfilled (minimum tillage, 6-year crop rotation, and permanent soil cover with crop residues and cover crops).
We assessed 43 variables in each cropping system, from which 38 were classified into nine agroecosystem goods and four ecosystem categories using an adapted ES framework and five were used as agronomic co-variables. We based our classification on the MEA and UKNEA frameworks (7, 10) and grouped our variables into proxies for ecosystem functions representing ecosystem processes and services and we finally valued them as ecosystem goods. Ecosystem functions, services, and goods were attributed to supporting, regulating, and provisioning ES categories. In addition, we added socioeconomic proxies and an economic category to the classical ES framework (Fig. 1 and tables S4 and S5). We did this because agroecosystems also have a socioeconomic dimension for producers and policy makers. The following nine final agroecosystem goods were used for multifunctionality assessments: biodiversity conservation, soil health preservation, erosion control, water and air pollution control, food production, income, work efficiency, and financial autonomy.
Fig. 1. Conceptual cascade model and framework for agroecosystem multifunctionality analyses.
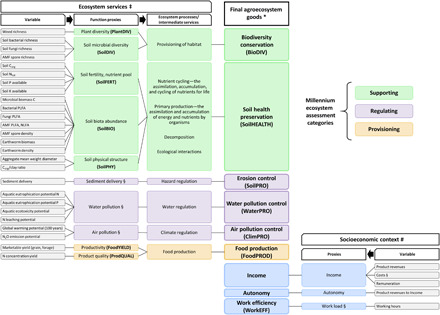
Variables on the left side are used, alone or bundled, as ecosystem function proxies representing ESs, which belong to the supporting (green), regulating (purple), and provisioning (yellow) MEA service categories (7). Variables on the right side represent socioeconomic proxies (blue), which, together with the supporting, regulating, and provisioning ESs, are translated into agroecosystem goods (10) relevant for producers, policy, and society. ‡: ES cascade model is based on the MEA and UKNEA frameworks (7, 10). #: Socioeconomic proxies and goods were added to the classical ES framework as agroecosystems also include a socioeconomic dimension for producers and policies. *: Goods refer to material and nonmaterial service valuation. §: Multiplied by −1 to maintain directional change with other ecosystem processes/services, such that an increase in value always represents a more desirable state.
We calculated agroecosystem multifunctionality using the averaging method, giving equal weight to each of the 13 proxies, the nine goods, and the four categories by simply averaging their score at the respective aggregation level (see Materials and Methods for calculation description). We additionally created an interactive online tool (https://apps.agroscope.info/sp/fast/emf) allowing individual weighting of goods and categories to evaluate how different cropping systems influence EMF using a wide range of scenarios. This is important, since a certain ES or good can be considered essential by one group of people but not valued by another group, particularly in an agricultural context. We also calculated diversity measures of function delivery for each cropping system, looking at the dissimilarities of ecosystem function supply among all cropping systems and the number of functions delivered over a wide range of thresholds (see Materials and Methods). This allowed us to investigate at which level (threshold) a diversity of functions can be maintained within each cropping system.
RESULTS
Productivity versus environmental protection
As expected, productivity, expressed as marketable yields, significantly decreased from conventional to organic systems with the highest yield in the conventional system with intensive tillage followed by the C-NT system (−6%), the organic system with intensive tillage (−22%), and the O-RT system (−34%) (figs. S2 and S3). Improved performance of the conventional systems can be explained by increased weed control and a better availability of applied nutrients (e.g., weed cover was six to nine times higher in the organic systems, while fertilizer utilization efficiency, especially N, was reduced in the organic systems; table S6 and fig. S4). Not all crops responded in the same way to the cropping systems. Reduced or no tillage had a negative impact on summer crops (maize and field beans), while the use of mineral fertilizers and herbicides in conventional systems resulted in higher yields for wheat and maize compared to organic systems. The differences between conventional and organic systems were less pronounced for legume crops (field beans and grass-clover ley) (fig. S2).
In contrast to productivity, both conservation agriculture (e.g., no tillage or reduced tillage) and organic farming positively influenced most soil quality variables. Organic farming and particularly reduced tillage intensity had a positive impact on aggregate stability, soil biodiversity, and the abundance of macro- and microbiota (table S6). Beneficial soil biota, such as earthworms and arbuscular mycorrhizal fungi, were promoted under organic management and conservation agriculture (table S6), supporting previous studies (3, 4, 26, 27). This confirms that improved soil management measures (e.g., crop diversity, omission of tillage, absence of synthetic pesticides, or application of organic amendments) have a positive impact on soil quality (6). Cropping system also affected the community composition of soil and root microbiomes within this experiment (28), with intensive tillage destabilizing the fungal community and increasing temporal variation in individual taxa (29).
Organic management and especially conservation tillage significantly reduced sediment delivery (−93% for C-NT, −79% for O-RT, and −46% for O-IT compared to C-IT) and contributed greatly to soil protection in this study (30). Thus, reduced tillage practices proved to be a major improvement also in organic farming. Increased soil cover, higher soil organic matter content, and improved aggregate stability explained this. Organic systems had also a reduced environmental impact as indicated by a 46 to 51% lower global warming potential and an 80 to 85% reduced aquatic ecotoxicity potential per hectare (table S6). These differences were less clear, when calculated per unit of product (table S6) (31, 32). We did not observe major changes in soil fertility (Corg, Ntot, available P, and K; table S6), which is not unexpected because the effects of management on these parameters are highly variable and often only become visible after long-term implementation of management (33–35).
Effects of different cropping systems on agroecosystem services and goods
In a next step, we analyzed the effects of the four cropping systems on different ecosystem functions, services, and goods (Fig. 2 and fig. S3). Overall, C-NT and both organic systems significantly improved supporting and regulating services (e.g. biodiversity, soil health, soil, water, and climate protection), while productivity (provisioning service) was highest in the conventional tillage system (Figs. 2 and 3). However, a loss of productivity in organic systems did not necessarily translate into reduced economic performance, as the highest income was obtained under organic production (Fig. 2 and table S6, remuneration). This results from higher product prices for organic products and additional support payments (36) but less financial autonomy. A reduction in tillage intensity also decreased costs and work load but only marginally affected general income (table S6).
Fig. 2. Standardized agroecosystem function and socioeconomic proxies for the four investigated cropping systems.
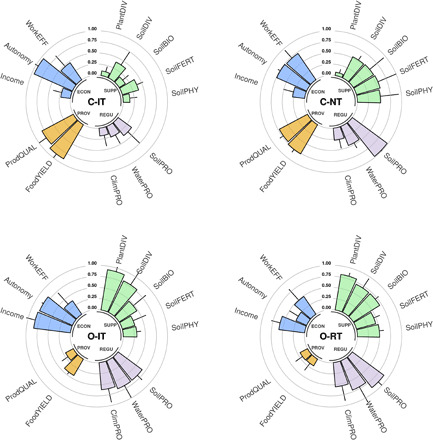
Mean + 90% confidence intervals, see table S4 for proxy descriptions. Proxies are grouped into SUPPorting (green), REGUlating (purple), PROVisioning (yellow), and ECONomic (blue) categories. The higher the bars, the better the function is performed.
Fig. 3. Agroecosystem multifunctionality of different cropping systems.
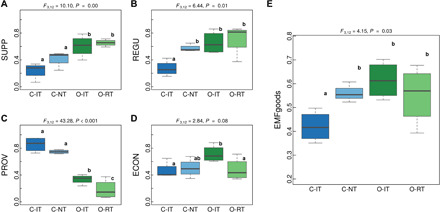
(A to D) Cropping system effects on (A) SUPPorting, (B) REGUlating, (C) PROVisioning, and (D) ECONomic agroecosystem good categories. (E) Cropping system effects on overall multifunctionality based on the average of the nine provided goods [(E) EMFgoods]. Different goods and service category weights can be calculated online at https://apps.agroscope.info/sp/fast. F values (df1, numerator degrees of freedom; df2, denominator degrees of freedom) for the corresponding linear mixed model are displayed above each plot, and different letters indicate significant differences between the four cropping systems [pairwise comparison with estimated marginal means (emmeans package), n = 4]. Boxplots display the median (horizontal line), the 25th and 75th percentiles (colored box), the minimum and maximum (whiskers), and outliers (points).
Synergies and trade-offs among agroecosystem goods and functions
The C-IT system and the O-RT system were fundamentally different in terms of function delivery and best displayed the trade-offs between different services (Fig. 2). In contrast, the O-IT system and the C-NT system showed a more balanced profile of function delivery (Fig. 2).
Overall, we observed a strong trade-off between the delivery of provisioning services and supporting and regulating services, respectively (Fig. 4). For instance, food productivity showed significant negative correlations with plant and soil diversity, soil biota abundance, and climate protection (figs. S4 to S6). In contrast, supporting and regulating services were positively correlated (Fig. 4) as evidenced by the positive correlation between soil biota and soil structure with soil protection. Plant diversity, among all supporting function’s proxies, was the only proxy with a significant positive correlation with water and climate protection (figs. S4 to S6). Our economic category was not linked with the other categories (Fig. 4). However, income, particularly driven by the amount of support payments, was negatively correlated with food production but positively with biodiversity, water, and climate protection, as well as more generally to supporting and regulating services (Fig. 4 and figs. S4 to S6).
Fig. 4. Correlation matrix illustrating the trade-offs and synergies between agroecosystem goods categories (PROVisioning, ECONomic, REGUlating, and SUPPorting).
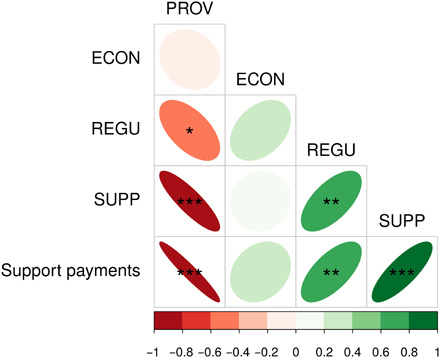
The variable support payments were also integrated to illustrate the impact of agroenvironmental policies on the delivery of services. Color legend and ellipses illustrate the rho correlation coefficient values. Stars indicate the significance level of the correlation (*P < 0.05, **P < 0.01, and ***P < 0.001). See fig. S6 for detailed correlation plots.
Organic and conservation agriculture promote EMF
We assessed the overall performance of the four cropping systems and determined EMF using different approaches and aggregation levels. Organic agriculture and conservation agriculture (no and reduced tillage) promoted agroecosystem multifunctionality when all six ESs and all nine agroecosystem goods (including economic goods) received equal weight (Fig. 3 and fig. S3). To provide researchers and policy-makers a tool to visualize the impact of management choice on ecosystem functions and services, we developed an interactive online tool that allows users to weight individual goods and categories (https://apps.agroscope.info/sp/fast) and to test how different scenarios affect the multifunctionality outcome of the different cropping systems. This tool also provides visualizations of the trade-offs between functions and between service categories and demonstrates that such trade-offs are often hidden when averaged into a single multifunctionality value. For instance, it shows that conventional systems performed best when provisioning services receive the most weight (50% or more to provisioning services) or that differences between cropping systems are not significant when EMF is calculated at the category level.
To further evaluate the performance of the different cropping systems, we applied a multiple thresholds approach and calculated how many agroecosystem goods were delivered above a specific threshold by the individual cropping systems. All three alternative systems (C-NT, O-IT, and O-RT) enhanced the delivery of more goods than C-IT over a wide range of thresholds (from ca. 25 to 75%) (Fig. 5, A to C). Moreover, C-NT and O-IT systems similarly supported the delivery of more diverse goods at moderate level in contrast to C-IT and O-RT systems, which both provided a limited number of goods at a higher level (Fig. 5D). Thus, conservation and organic agriculture are not providing a larger number of functions at high levels but instead a greater diversity of functions at more moderate levels. This observation highlights that each cropping system cannot simultaneously provide high multifunctionality and maximize all functions (37) and that there is a trade-off between the diversity and the level of function delivery. This is particularly true in an agricultural context where trade-offs between different services are pronounced and food provision is a main priority.
Fig. 5. Diversity measures for agroecosystem good delivery.
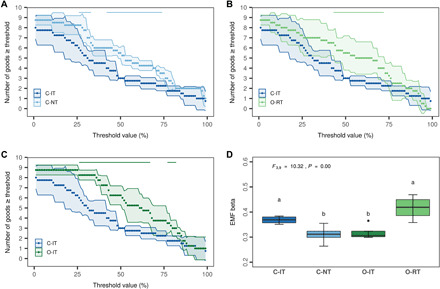
(A to C) Effect of cropping systems on the number of goods performed above a threshold [mean (points) ± 95% confidence intervals (shades), n = 4; horizontal lines indicate sections of significant differences to C-IT]. The continuous thresholds (%) are applied on the scaled good values (0 to 1). (D) Beta-multifunctionality, calculated as the average dissimilarities of goods supply between cropping systems. The higher the value, the more specialized is good delivery (few goods at higher level). F value (df1, numerator degrees of freedom; df2, denominator degrees of freedom) for the corresponding linear mixed model is displayed for the boxplot, and different letters indicate significant differences between the four cropping systems [pairwise comparison with estimated marginal means (emmeans package), n = 4]. Boxplot displays the median (horizontal line), the 25th and 75th percentiles (colored box), the minimum and maximum (whiskers), and outliers (points).
DISCUSSION
This study demonstrates that conservation agriculture and organic farming improve supporting and regulating services of arable cropping systems, resulting in the highest multifunctionality when all delivered goods are weighted equally. This implies that the agricultural practices implemented in these systems can improve multifunctionality and overall system performance at a satisfactory productivity level. Both conservation and organic agriculture have similar principles in terms of energy use and soil quality preservation. The use of permanent soil cover (especially in conservation agriculture, e.g., by use of cover crops), integrated plant protection, crop rotation, and the use of organic inputs (especially in organic agriculture) are beneficial and reduce the productivity–environmental protection dilemma (5, 38).
Our analysis further shows that, after all, an increase in environmental benefits tends to be coupled with a decrease in productivity. The observed higher productivity under conventional agriculture is comparable to values observed in earlier meta-analyses (+6% compared to conservation and +19 to +25% compared to organic agriculture) (20, 21, 24) but results in lower multifunctionality and higher environmental costs. To reduce the yield gap between conventional and organic agriculture and enhance EMF, future studies should focus on the main barriers limiting yield. That includes, here, lower N availability and higher weed pressure, particularly with the implementation of conservation tillage under organic management (33, 39). This points to the need to define which services and goods agriculture should deliver and to what extent—a goal also articulated for other ecosystem types (19). Note that pesticide and fertilizer use in conventional managed plots was relatively modest compared to levels applied in some countries, whereas crops were rotated in line with practices observed in various European countries (40). Thus, the observed differences between organic and conventional agriculture in this study may represent an under limit compared to countries where heavy fertilization, regular pesticide application, and monocropping are the rule in conventional agriculture.
The total areas of arable land globally devoted to organic and no tillage systems are currently 1.5 and 12.5%, respectively (22, 41). Thus, if environmental protection and an increase in the delivery of supporting and regulating services are a priority, then the total area for organic and no tillage cropping systems needs to be substantially expanded. For example, further acceptance of conservation tillage and incentives to promote conservation tillage are essential to reduce soil erosion (42, 43). The increased area that would be needed to meet productivity needs is strongly debated and depends on various socioeconomic developments. Recent studies show that an extension of organic production might be possible with reductions of food waste, a changed diet with reduced consumption of animal products, and an optimized use of water and nutrients (31, 44).
Our study also indicates that specific cropping practices that contribute to improved delivery of supporting and regulating services—such as increasing soil cover, crop diversification, reduced soil tillage, or organic amendments—could be integrated in conventional cropping systems to enhance their overall performance and multifunctionality (6, 40). This is particularly true in developed countries where actual yields are close to maximum attainable levels. However, our approach targeting assessment and improvement of multifunctional systems is also relevant for less developed countries where closing yield gap is a higher priority, especially if provisioning services (e.g., yield) in specific systems are positively affected by increases in supporting and regulating services (e.g., soil fertility, soil biodiversity, and water protection).
There is growing recognition that agriculture can provide ESs other than the provision of food and feed (45, 46), including biodiversity conservation, climate mitigation, and soil protection. However, it is still a challenge to monetize and integrate such costs into product prices. In addition, the negative environmental impacts of agricultural practices are costs that are typically unmeasured (1) and are usually externalized, being greater for society as a whole than for the farms on which they operate (47). Moreover, the integration of alternative environmental-economic evaluations, including measures of environmental costs, in key economic indicators is difficult and thus limits the potential of classic economic metrics to achieve real changes (48). In contrast, agroenvironmental policies play a major role in shaping agricultural practices. This is particularly true in Switzerland where a direct payment system was already introduced in the 1990s to improve the ecological performance of agriculture and also in other countries investing in “green payments” such as European Union countries or the United States (1). In our analysis, the amount of considered support payments correlated positively with the delivery of supporting and regulating services and negatively with provisioning services. This shows that agricultural policies can simultaneously support environmental protection and compensate farmers for yield reduction.
It is important to acknowledge that we did not specifically include “natural” or “theoretical” thresholds to assess multifunctionality (e.g., maximum attainable or targeted yield and prescriptive limits for greenhouse gas emissions) in this study. Moreover, we also did not define the multifunctionality level above which a system is said to sufficiently provide a wide range of services. Here, we use specific data from one experiment as a case study to demonstrate the possibility to assess agroecosystem multifunctionality. It is important to consider that within cropping systems, there is large variation in agricultural practices (e.g., crop rotation, cultivar choice, fertilization, plant protection, or soil management), and the investigated cropping systems are not necessarily representative for all forms of agriculture. The value of our analysis lies in the possibility that it offers to compare different cropping systems and to identify trade-offs and key leverage options. A further development of our methodological framework and interactive tool, e.g., by integrating broadly applicable indicators and associated standard or limit values, can facilitate researchers, farmers, and policy-makers to evaluate different management practices and to design policy instruments in different contexts. Our approach is not only limited at the field or farm level but could also be applied at a regional or national scale to evaluate more broadly the performance of agricultural production by combining the concept with spatial data on distribution of acreage and production systems. However, in each application case and level, the use of standards should clearly define (i) the terminology and context, (ii) data and methods used for services delivery, and (iii) how the service valuation is assessed (48).
We conclude that future cropping systems must be designed to optimize the delivery of multiple functions considering all available best practices. Our analysis demonstrates that, although manageable to a certain extent, trade-offs exist between high productivity and environmental protection. Thus, installing a balanced proportion between specialized cropping systems, providing a few functions at high levels (e.g., productivity), and cropping systems providing diverse functions at lower levels within a given area (farm, local, or regional scales) could be a strategy to simultaneously achieve a balance between satisfactory yields and environmental integrity. Improved monitoring and evaluation of agricultural practices based on impact assessment, as proposed here, would be a next frontier to cross in the development of a sustainable agriculture supported by appropriate policies.
MATERIALS AND METHODS
FAST experiment
This study is based on a long-term cropping system field trial entitled the “FAST experiment” located at the Swiss federal agricultural research station Agroscope, Reckenholz near Zürich (latitude 47°26′N, longitude 8°31′E). The FAST experiment compares different arable cropping systems, namely, conventional, organic, and two conservation tillage systems, and has a 6-year crop rotation. The four investigated cropping systems consist of conventional intensive tillage (C-IT), conventional no tillage (C-NT), organic intensive tillage (O-IT), and organic reduced tillage (O-RT). The four systems differ in the form of inputs (e.g., mineral versus organic fertilizers and herbicides versus mechanical weed control between conventional and organic management, respectively) and tillage intensity (intensive versus conservation tillage) (table S1).
FAST is composed of two equivalent experiments established on the same field beside each other. The first experiment started in the summer of 2009 (FAST I) and the second in the summer of 2010 (FAST II), following a staggered start design to capture yearly variation. Both comprise the following factors: cropping system and cover crop arranged in a split-plot design with four blocks per experiment. The factor cropping system was allocated to the main plots, which were each subdivided in four split-plots with the factor cover crop. The cover crop plots consisted of a legume, a nonlegume, a mixture, and a control (green fallow) treatment (39). The size of the main plots was 6 m by 30 m, allowing the use of standard farming equipment. The split-plot size was 3 m by 15 m (fig. S1).
The soil type at the experimental site is a calcareous Cambisol with a moderate plant available soil depth (ca. 70 cm). At the start of the experiment, soil cores from the upper soil layer (0 to 20 cm depth) of each experiment were randomly collected from the experimental area for FAST I and FAST II, and soil characteristics were assessed. The soil contained on average 1.4% Corg, 23% clay, 34% silt, 43% sand, and had a pH(H2O) of 7.3. The long-term (1981 to 2010) average annual precipitation was 1054 mm and the mean annual temperature 9.4°C.
Crop rotation
Before the start of each of the experiment, forage pea (Pisum sativum L. subsp. arvense) was cultivated after tillage with a moldboard plough. The first crop rotation (2009 to 2015 and 2010 to 2016, respectively) included the following main crops: winter wheat (Triticum aestivum L. cv. “Titlis”), maize (Zea mays L. cv. “Padrino”), field bean (Vicia faba L. cv. “Fuego”), winter wheat, and a 2-year grass-clover ley (“UFA330”). Cover crops were additionally planted, within the subplot, before the first winter wheat and before maize (39). For wheat and maize, coated seeds were sown in the conventional plots (wheat, Coral extra; maize, Mesurol) and untreated seeds in the organic plots. We used cultivars that are registered in both conventional and organic recommendation lists in Switzerland. All crop residues (cover crops, maize, and field bean) remained on the plots, except for winter wheat straw, which was removed from the field.
Soil tillage and seeding
The intensively tilled organic and conventional plots were tilled to a depth of 20 to 25 cm with a moldboard plough, followed by a seedbed preparation with a rotary harrow just before seeding. The conservation tillage treatment differed between the conventional and the organic systems. Under conventional management, no soil tillage operations were conducted during the entire experimental period corresponding to no tillage production (NT); the O-RT treatment consisted of noninversion tillage (RT) to a target depth of 5 cm operated with a disk harrow before wheat and thereafter with a rotary harrow at the same time as for the seedbed preparation in the IT tillage treatments. All crops were seeded directly either with a no-till cereal seeder or with a no-till single-grain seeder in the case of maize and field beans. The number, type, and date of tillage operations and seeding dates of the crops are listed in table S2.
Fertilization
Fertilization in the conventional plots was exclusively mineral, and no farmyard manure or slurry was applied. Fertilization (N, P, and K) was performed in accordance with the Swiss guidelines for fertilization (49). The organic plots were fertilized with cattle slurry at a target level of 1.4 livestock unit (LU) ha−1 (average LU for organic farms in Switzerland). The slurry was purchased from an organic farmer near the experimental site. The nutrient contents of the slurry varied between years. Consequently, the amount of nutrients applied to the crops varied slightly between experiments (table S2). On average, the conventional plots received 92 kg of Ntot, 67 kg of P2O5, and 135 kg of K2O per hectare and year. The organic plots received 121 kg of Ntot (of which 51 kg was in the form of plant-available N-NH4), 46 kg of P2O5, and 256 kg of K2O per hectare and year. Application dates and total amounts of applied nutrients for each crop are described in tables S2 and S3.
Weed control
Weed control in the conventional plots was achieved with the use of postemergence herbicides, whereas mechanical weed control was performed in the organic plots. In the C-NT treatment, glyphosate was applied before seeding of the main crops. In the organic systems, a harrow was used to control weeds in winter wheat, and a star cultivator was used for weed control in maize and field beans. Weed control operation dates, products, and machinery are described in table S2.
Plant protection
In all cropping systems, maize was treated with Trichogramma (Trichogramma brassicae) against the European corn borer (Ostrinia nubilalis). In field beans, Plenum WG (pymetrozine) against black bean aphid (Aphis fabae) was applied in the conventional systems. Beside herbicides, no further pesticides were applied, and the conventional systems thus represent more an integrated pest management system, which is broadly applied in Switzerland for the crops wheat, maize, and field beans.
Data collection
Agronomic variables
Main crop yields were assessed yearly at crop maturity by harvesting between 7.5 and 10.5 m2 within the inner 2 m by 10 m of each subplot with plot-sized combine harvesters. Grain and forage yields (grass-clover ley) were weighed, and a subsample was dried at 105°C for 30 hours to adjust yield to metric tons of dry matter per hectare. Another subsample was dried at 60°C for 30 hours and finely ground for nutrient analyses (N, P, and K).
Weed soil cover in the main crops was visually assessed on two 1-m2 frames per subplots at critical crop growth stages, a few weeks after the last weed control operations. The percentage ground cover for each weed species was estimated, and total weed richness was assessed (mean over all crops). In 2013, the weed seed bank was additionally determined in FAST I after wheat harvest by the seedling emergence method after Ter Heerdt et al. (50) and adapted by Mayor and Dessaint (51). Air-dried soil samples (0 to 20 cm depth) were sieved at 3.15 mm and then at 0.25 mm, and the remaining substrate was transferred to pots filled with steam-sterilized soil. The pots were then placed in a greenhouse with controlled light (15-hour daylight), water supply, and temperature (day/night, 25°/15°C). To promote maximum seed germination, relevant field conditions, such as reduced water supply or vernalization, were simulated in five phases for a total of 23 weeks of assessment. Weed germination was assessed on a weekly basis during every phase, with the exception of the vernalization period (2 weeks in cold room). Seedlings were identified, counted, and removed mostly at early leaf development stages (cotyledons). Roots were washed in the corresponding pots to ensure that other seeds attached to roots remained in the pots.
Fertilizer utilization efficiencies were calculated for the macronutrients N, P, and K as the ratio between the amount of harvested nutrient (nutrient concentration × yield) and total amount of nutrients applied as fertilizer. For N, atmospheric N fixation (Nfix) by legume crops (cover crops, field beans, and clover) was also accounted as N input. N fixation values were not directly measured but estimated on the basis of standard percentage Nfix (39, 52–54) and aboveground legume biomass.
Economic variables
A full cost analysis was performed to assess the economic performance of the four cropping systems. First, total costs were determined including direct (seed, fertilizer, and pesticide) costs and variable and indirect (land rent, machine, and labor) costs. Land rent was fixed at 659 Swiss francs (CHF) per hectare based on (55). Hourly rates for internal and external labor costs were fixed at 28 and 48 CHF, respectively (56). Machine costs were estimated on the basis of the report of Gazzarin (56), assuming standard machinery for Switzerland. Product revenues were calculated by multiplying marketable yield with product prices (2018). Total income was subsequently calculated by adding product revenues and direct payments (e.g., support payments) from the government. After deduction of the total costs to the total income, the net margin was obtained, which was divided by the calculated working hours and added to the assumed work costs (28 CHF) to obtain the labor remuneration. Last, the proportion of subsidies to total income was calculated as measure of financial autonomy.
Environmental variables
Soil sampling campaign
An intensive soil sampling campaign was conducted in the fourth year of FAST I and FAST II, in 2013 and 2014, respectively. Soil samples (0 to 20 cm soil depth) were taken early March in winter wheat before any fertilization took place. Ten cores per plots were pooled to a mix sample, which was sieved using 2-mm mesh after removal of large organic particles (e.g., crop residues and large roots). A fresh subsample was directly used for microbial biomass determination, and another subsample was frozen at −20°C for phospholipid-derived fatty acid (PLFA) analyses. A third subsample was air-dried and used for chemical (Corg, Ntot, P, and K) and texture analyses (clay, silt, and sand) according to the reference methods of the Swiss Federal Institutes of Agricultural Research. Soil organic carbon was determined by addition of potassium dichromate (K2Cr2O7), Ntot was assessed by elemental analysis, and the amounts of plant-available P and K were determined in CO2-saturated water (57).
Soil biota analyses
Earthworm abundance was evaluated in September 2013 and 2014, a few weeks after sowing the grass-clover ley. The soil from two quadrants of 0.5 m by 0.5 m per main plot was collected to a depth of 20 cm, and a combined hand picking and formalin (0.1%) extraction method was used to collect earthworms. Earthworms were stored in 4% formalin until counting and weighing.
Soil microbial biomass carbon was measured by chloroform fumigation extraction according to Vance et al. (58). Fresh soil samples (20 g of dry soil) were fumigated in duplicates with chloroform for 24 hours. Organic C content was measured by infrared spectrometry after combustion at 850°C (DIMATOC 2000, Dimatec, Essen, Germany). Microbial biomass C was calculated according to (59).
Bacterial, fungal, and arbuscular mycorrhiza fungal (AMF) abundance was estimated on the basis of fatty acid signatures of soil samples collected from FAST II (March 2014 samples). Lipids were extracted and analyzed as described by Hydbom et al. (60). Bacterial biomass was estimated by summarizing the concentration of 10 prokaryote-specific PLFAs: i15:0, i16:0, i17:0, a15:0, a17:0, cy17:0, cy19:0, 10Me16b, 10Me17:0, and 10Me18:0 (61). PLFA 18:2ω6,9 was used as an indication for fungal biomass (61). The concentrations of the neutral lipid fatty acid (NLFA) 16:1ω5 and PLFA 16:1ω5 were used to estimate the abundance of AMF (62, 63). PLFA 16:1ω5 is common also in some bacterial groups, and therefore, NLFA 16:1ω5 is a more specific indication of AM fungal abundance, which is necessary in systems where nonmycorrhizal controls are not included in the study design (62).
AMF spore density and richness were assessed on soil samples of FAST II (March 2014 samples). AMF spore extraction and identification were conducted as described in (64). In short, AMF spores were extracted from 25 g of soil samples by wet sieving and a sucrose density gradient centrifugation (65), passed to a petri dish, and their numbers were counted. Spores, spore clusters, and sporocarps were picked without preselection and mounted together on microscope slides. On average, 146 (minimum, 102; maximum, 209) spores per samples were examined systematically under a microscope up to 400-fold magnification to identify all morphologically distinct AMF spore types. Morphological AMF species identification was based on all existing species descriptions and two identification manuals (66, 67). Classification was based on the Glomeromycota system of Baltruschat et al. (68) and Wijayawardene et al. (69).
Bacterial and fungal diversity was assessed as described in (28): Soil samples were collected in June 2013 and 2014 for FAST I and FAST II, respectively. Five soil cores (at 10 to 20 cm depth) were collected in each plot between wheat rows, pooled, and immediately frozen at −80°C until DNA extraction. DNA was extracted from a 300-mg soil (dry weight) subsample using the NucleoSpin Soil DNA extraction kit (Machery-Nagel GmbH & Co. KG, Düren, Germany) according to the manufacturer’s instructions, except that each sample was extracted twice and the supernatants pooled to maximize DNA yield. The 16S ribosomal RNA gene amplicon library was generated using the polymerase chain reaction (PCR) primers 799F [72] and 1193R [73]. The ITS amplicon library was generated using the PCR primers fITS7 [74] and ITS4 [75]. Raw reads were processed using a custom-developed bioinformatic pipeline described in (28), and taxonomy assignment was done using the UNITE database (v7.0) with the RDP classifier in QIIME. In this study, we used soil bacterial and fungal richness as measure of microbiota diversity.
Aggregate and erosion risk assessment
Soil aggregation was assessed in samples collected at the end of the fourth growing season (August 2013 and 2014, after wheat harvest). Four intact soil cores (5.5 cm by 20 cm) were taken from each replicate plot using a Giddings hand sampler (Giddings Machinery Co, Windsor, Colorado, USA). Each 20-cm-length core was manually cut at 6 cm, separating the top 0 to 6 cm from the bottom 6 to 20 cm. Field-moist cores were sieved through an 8-mm sieve by manually crumbling along natural fracture lines to minimize aggregate disruption. The four cores from each plot were combined, and each composite sample was air-dried and stored at room temperature. Air-dried soil was wet-sieved following Elliott (70) to separate four aggregate size classes: large macroaggregates (>2000 μm), small macroaggregates (2000 to 250 μm), microaggregates (250 to 53 μm), and silt and clay (S + C; <53 μm) as described in (71). Mean weight diameter, used as a measure of aggregate stability, was calculated for the top soil samples (0 to 6 cm) using the proportional abundance of each aggregate fraction and the mean diameter of each size class.
In situ erosion assessments were performed once in both FAST experiments: (i) in August 2014 on fallow land 1 week after harvesting winter wheat in FAST II and (ii) in June 2017 in a maize stand (growth-stage BBCH 35 stem elongation) in FAST I. The determination of event-based sediment delivery was performed using the portable Tübingen rainfall simulator with microscale runoff plots (ROPs; 0.4 m by 0.4 m). The single nozzle simulator unit is generating a standardized rain spectrum under a protective tent and was calibrated with a laser disdrometer before and after measurement. A heavy rainfall event (60 mm hour−1) was simulated for 30 min on every ROP with a mean kinetic energy expenditure of 475 J m−2 hour−1. Runoff and sediment delivery were collected in 2-liter bottles and filtrated on fiberglass filters. Sediment was oven-dried (40°C) before weighing. For the full method description, see (30).
N leaching and N2O emission
In 2014, intact soil cores were excavated after wheat harvest from FAST II to assess potential N2O emissions and leaching losses. Soil cores were collected using high-density polyethylene tubes inserted into a steel sampler following Knacker et al. (72). Two intact soil cores (height, 40 cm; diameter, 17 cm) were extracted per mainplot and transferred into the greenhouse in a randomized block design. The cores were maintained in the greenhouse for 1 month, and all emerging seedlings were removed. Afterward, soil cores were adjusted to field capacity by successively adding water until leachate occurred at the bottom of the soil core. Leachates were collected, weighed, and stored at 4°C for later analyses of nutrient concentrations. NO3− and NH4+ in leachates were determined using a Dionex DX500 anion chromatograph (Dionex Corporation, Sunnyvale, CA, USA), and concentration values were used as N leaching potential.
Next, eight seedlings of Italian ryegrass (Lolium multiflorum, var. Morunga) and red clover (Trifolium pratense, var. Merula) were transplanted to the soil cores and regularly watered as required. After 6 weeks of plant growth, pots were watered close to field capacity and received 150 ml of a fertilizer solution containing 68.86 mM KNO3 and 5.19 mM KH2PO4, corresponding to an amount of 60 kg of N ha−1 and 10 kg of P ha−1. One day after fertilization, a heavy rainfall of 24 mm was simulated by adding 1000 ml of water using rainfall simulators, and excess water was allowed to drip out the bottom of the soil cores. Directly after the artificial rainfall, N2O emissions were measured using a TEI 46C automated infrared gas analyzer (Thermo Fisher Scientific, Waltham, MA, USA). Daily N2O fluxes were integrated to obtain the total amount of N2O emitted per soil core during the 5 days of measurement.
Life cycle assessment
To assess the environmental impact of the investigated cropping systems, a life cycle assessment (LCA) was performed over the 6-year crop rotation. The LCA was conducted by means of the Swiss Agriculture Life Cycle Assessment (SALCA) tool (73, 74), which includes the use of life cycle inventories from the ecoinvent database (75). SALCA comprises the SALCA database and models for estimating direct field emissions. A detailed description of the method can be found in (76). For the purpose of this study, we included the following impact categories: global warming potential (100 years; kg CO2 eq.; Intergovernmental Panel on Climate Change, 2007), aquatic eutrophication potential (kg N and P eq.; CML01), and aquatic ecotoxicity potential (1,4-dichlorobenzene eq.; CML01). For each impact category, we used two functional units, per product [cereal unit (CU)] and per area (hectare). These two units are linked to two foci: (i) products with low environmental impacts and (ii) land use with low environmental impacts. CU of a product expresses the nutrition value for pig fattening relative to 100 kg of barley, which is defined as the reference with a CU of 1. The borders of a field defined the spatial boundaries of the agricultural cropping system. Upstream emissions and resource use for the provision of infrastructure and the production of commodities (e.g., fertilizers) were also included.
ES and goods calculation
Along the first 6-year rotation, sampling intensity of the variables varied. Some were assessed yearly (e.g., yield, weed cover, and richness), while others were assessed in specific years, mostly in the fourth year of the rotation (e.g., erosion risk, soil aggregation, and N cycle variables) or modeled on the basis of management and yield information (e.g., LCA variables) (table S5). The data comprise two subdatasets: (i) variables assessed at the plot level where the blocks (n = 4 per experiment) are used as replicates and (ii) variables calculated on the basis of field management information where the crops (n = 4; wheat, maize, beans, and grass-clover ley) are used as replicates (costs, working hours, global warming potential per hectare and CU, aquatic eutrophication potential per hectare and CU, and aquatic ecotoxicity potential per hectare and CU). For these two datasets, mean values at the cropping system and replicate level (blocks and crops, respectively) were computed before merging both datasets. Subsequently, data were scaled using the z-transformation function (overall mean of 0 ± SD) to be able to combine different variables into composite variables.
Some variables were directly used as proxy for a function, while others were first bundled (averaged) in composite variables when contributing to the same function. This was done to avoid overweighting of certain aspects of ecosystem functioning and to avoid multifunctionality assessment bias due to overrepresentation of related variables (77). Data were multiplied by −1 (inverted around the 0 mean) to maintain directional change when proxies represented an undesirable environmental (e.g., sediment delivery and global warming potential) or economic (e.g., costs) perspective. As a consequence, an increase in value for specific services and goods always represents a more desirable state. Last, all agroecosystem proxies and goods were scaled between 0 and 1 to ease readability. Overall, 13 ecosystem function and socioeconomic proxies were derived out of the 41 variables assessed. The 10 ecosystem function proxies were further grouped into 6 ESs belonging to the categories supporting, regulating, and provisioning services (Fig. 1 and tables S4 and S5). We specifically limit our analyses to the classification of our variables into proxies for ecosystem functions, services, and goods and complement it with socioeconomic variables characterizing highly managed agroecosystem from a producer and policy perspective. However, we do not include a valuation of these goods, e.g., from a social, monetary, or health perspective, as this is out of the scope of the study. We also do not include the cultural category because we analyze field data at the plot level within a replicated field experiment.
Similarly to (78), we did not assume independence between functions, as functions in an ecosystem are often correlated. This is because we were also interested in single function performance, and the EMF analyses should include trade-offs and synergies between functions.
Multifunctionality assessments
To assess the overall performance of the investigated cropping systems, we calculated different EMF values. We first calculated EMF with the averaging method, giving equal weight to each of the 13 proxies and nine goods by simply averaging their score. We then calculated EMF in the same way but at the agroecosystem service level, weighting supporting, regulating, provisioning, and economic categories equally. This made it possible to remove bias due to uneven goods numbers between categories (77). We also calculated different diversity measures of goods delivery for each cropping system following the approach of Hölting et al. (79) including alpha- (diversity of ecosystem function delivery) and beta- (total abundance-based dissimilarities of ecosystem goods supply among all cropping systems) multifunctionality. We lastly performed a continuous threshold analysis on the alpha diversity measure to assess the stability of goods delivery over a wide range of thresholds following Byrnes et al. (78). This involves plotting the number of goods delivered across the full range of thresholds between 0 and 100% of good values.
Statistical analyses
Statistical analyses were performed in R version 3.6.3 (80) and the packages emmeans (81), vegan (82), betapart (83), and multifunc (84). All assessed variables were subjected to variance analyses in a linear mixed model. The term block nested in experiment was considered as random effect and cropping system as fixed effect. The factor cover crop (subplot level in the experiment) was not considered in this study, and mean values per main plots were used for the analyses. Similarly, the mean over all crops was used for variables that were assessed yearly, as intrinsic differences between crops are not the focus of this study. The effects of cropping system on the 13 calculated proxies, the nine agroecosystem goods, the four categories (supporting, regulating, provisioning, and economic), and the various multifunctionality indexes were analyzed in linear mixed models, with cropping system as fixed factor. Pairwise comparisons were tested on estimated marginal means among the four cropping systems for all variables (absolute variables and scaled variables).
Acknowledgments
We thank B. Dorn, W. Jossi, C. Scherrer, and all students and helpers for contribution within the FAST experiment. We thank P. Mouron, M. Lips, and A. Zorn for advices concerning the full cost analysis. We thank O. Benz (b-data GmbH) for maintaining the Agroscope RStudio-Server. Funding: The Swiss Federal Office of Agriculture and the following various grants financed this work: the Swiss National Research Foundation (grants 137136 and 143097), the Mercator Foundation through the ETH-Zürich World Food System Center, and the EU grant no. KBBE-245058-SOLIBAM. Author contributions: R.A.W.: Conceptualization, methodology, investigation, formal analysis, data curation, visualization, and writing—original draft. M.G.A.v.d.H.: Initiation, conceptualization, methodology, writing—review and editing, supervision, project administration, and funding acquisition. S.F.B.: Investigation (N soil core study), and review and editing. T.N.: Supervision (LCA study), review and editing, and funding acquisition. U.E.P.: LCA and emission modeling and review and editing. F.O.: Investigation (mycorrhizal fungi) and review and editing. R.A.A.L.: Investigation (mycorrhizal fungi). V.L.: Investigation (soil aggregate) and review and editing. J.S.: Supervision, funding acquisition, data interpretation, and review and editing. K.H. and K.S.: Investigation (soil microbiome) and review and editing. S.S. and T.S.: Methodology and investigation (soil erosion) and review and editing. S.H. and P.A.O.: Investigation (fatty acid signatures) and review and editing. O.P.: Review and editing. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. An online tool was developed along this manuscript and is available at https://apps.agroscope.info/sp/fast. Data are available upon request.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/7/34/eabg6995/DC1
REFERENCES AND NOTES
- 1.Tilman D., Cassman K. G., Matson P. A., Naylor R., Polasky S., Agricultural sustainability and intensive production practices. Nature 418, 671–677 (2002). [DOI] [PubMed] [Google Scholar]
- 2.Foley J. A., Defries R., Asner G. P., Barford C., Bonan G., Carpenter S. R., Chapin F. S., Coe M. T., Daily G. C., Gibbs H. K., Helkowski J. H., Holloway T., Howard E. A., Kucharik C. J., Monfreda C., Patz J. A., Prentice I. C., Ramankutty N., Snyder P. K., Global consequences of land use. Science 309, 570–574 (2005). [DOI] [PubMed] [Google Scholar]
- 3.Tsiafouli M. A., Thébault E., Sgardelis S. P., de Ruiter P. C., van der Putten W. H., Birkhofer K., Hemerik L., de Vries F. T., Bardgett R. D., Brady M. V., Bjornlund L., Jørgensen H. B., Christensen S., D’ Hertefeldt T., Hotes S., Gera Hol W. H., Frouz J., Liiri M., Mortimer S. R., Setälä H., Tzanopoulos J., Uteseny K., Pižl V., Stary J., Wolters V., Hedlund K., Intensive agriculture reduces soil biodiversity across Europe. Glob. Chang. Biol. 21, 973–985 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Mäder P., Fliessbach A., Dubois D., Gunst L., Fried P., Niggli U., Soil fertility and biodiversity in organic farming. Science 296, 1694–1697 (2002). [DOI] [PubMed] [Google Scholar]
- 5.Seufert V., Ramankutty N., Many shades of gray—The context-dependent performance of organic agriculture. Sci. Adv. 3, e1602638 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tamburini G., Bommarco R., Wanger T. C., Kremen C., van der Heijden M. G. A., Liebman M., Hallin S., Agricultural diversification promotes multiple ecosystem services without compromising yield. Sci. Adv. 6, eaba1715 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Millennium Ecosystems Assessment, Ecosystems and Human Well-being: Synthesis (Island Press, 2005). [Google Scholar]
- 8.The Economics of Ecosystems and Biodiversity, "Measuring what matters in agriculture and food systems: A synthesis of the results and recommendations of TEEB for Agriculture and Food’s Scientific and Economic Foundations report" (Geneva: UN Environment, 2018).
- 9.Díaz S., Pascual U., Stenseke M., Martín-López B., Watson R. T., Molnár Z., Hill R., Chan K. M. A., Baste I. A., Brauman K. A., Polasky S., Church A., Lonsdale M., Larigauderie A., Leadley P. W., van Oudenhoven A. P. E., van der Plaat F., Schröter M., Lavorel S., Aumeeruddy-Thomas Y., Bukvareva E., Davies K., Demissew S., Erpul G., Failler P., Guerra C. A., Hewitt C. L., Keune H., Lindley S., Shirayama Y., Assessing nature’s contributions to people. Science 359, 270–272 (2018). [DOI] [PubMed] [Google Scholar]
- 10.UK National Ecosystem Assessment, The UK National Ecosystem Assessment: Synthesis of the Key Findings (UNEP-WCMC, 2011).
- 11.Dick J., Turkelboom F., Woods H., Iniesta-Arandia I., Primmer E., Saarela S. R., Bezák P., Mederly P., Leone M., Verheyden W., Kelemen E., Hauck J., Andrews C., Antunes P., Aszalós R., Baró F., Barton D. N., Berry P., Bugter R., Carvalho L., Czúcz B., Dunford R., Garcia Blanco G., Geamănă N., Giucă R., Grizzetti B., Izakovičová Z., Kertész M., Kopperoinen L., Langemeyer J., Montenegro Lapola D., Liquete C., Luque S., Martínez Pastur G., Martin-Lopez B., Mukhopadhyay R., Niemela J., Odee D., Peri P. L., Pinho P., Patrício-Roberto G. B., Preda E., Priess J., Röckmann C., Santos R., Silaghi D., Smith R., Vădineanu A., van der Wal J. T., Arany I., Badea O., Bela G., Boros E., Bucur M., Blumentrath S., Calvache M., Carmen E., Clemente P., Fernandes J., Ferraz D., Fongar C., García-Llorente M., Gómez-Baggethun E., Gundersen V., Haavardsholm O., Kalóczkai Á., Khalalwe T., Kiss G., Köhler B., Lazányi O., Lellei-Kovács E., Lichungu R., Lindhjem H., Magare C., Mustajoki J., Ndege C., Nowell M., Nuss Girona S., Ochieng J., Often A., Palomo I., Pataki G., Reinvang R., Rusch G., Saarikoski H., Smith A., Soy Massoni E., Stange E., Vågnes Traaholt N., Vári Á., Verweij P., Vikström S., Yli-Pelkonen V., Zulian G., Stakeholders’ perspectives on the operationalisation of the ecosystem service concept: Results from 27 case studies. Ecosyst. Serv. 29, 552–565 (2018). [Google Scholar]
- 12.Lindenmayer D. B., Likens G. E., Effective monitoring of agriculture. J. Environ. Monit. 13, 1559–1563 (2011). [DOI] [PubMed] [Google Scholar]
- 13.Sachs J. D., Remans R., Smukler S. M., Winowiecki L., Andelman S. J., Cassman K. G., Castle D., DeFries R., Denning G., Fanzo J., Jackson L. E., Leemans R., Lehmann J., Milder J. C., Naeem S., Nziguheba G., Palm C. A., Pingali P. L., Reganold J. P., Richter D. D., Scherr S. J., Sircely J., Sullivan C., Tomich T. P., Sanchez P. A., Effective monitoring of agriculture: A response. J. Environ. Monit. 14, 738–742 (2012). [DOI] [PubMed] [Google Scholar]
- 14.Hector A., Bagchi R., Biodiversity and ecosystem multifunctionality. Nature 448, 188–190 (2007). [DOI] [PubMed] [Google Scholar]
- 15.Garland G., Banerjee S., Edlinger A., Oliveira E. M., Herzog C., Wittwer R., Philippot L., Maestre F. T., van der Heijden M. G. A., A closer look at the functions behind ecosystem multifunctionality: A review. J. Ecol. 109, 600–613 (2021). [Google Scholar]
- 16.Wagg C., Bender S. F., Widmer F., van der Heijden M. G., Soil biodiversity and soil community composition determine ecosystem multifunctionality. Proc. Natl. Acad. Sci. U.S.A. 111, 5266–5270 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maestre F. T., Quero J. L., Gotelli N. J., Escudero A., Ochoa V., Delgado-Baquerizo M., Garcia-Gomez M., Bowker M. A., Soliveres S., Escolar C., Garcia-Palacios P., Berdugo M., Valencia E., Gozalo B., Gallardo A., Aguilera L., Arredondo T., Blones J., Boeken B., Bran D., Conceicao A. A., Cabrera O., Chaieb M., Derak M., Eldridge D. J., Espinosa C. I., Florentino A., Gaitan J., Gatica M. G., Ghiloufi W., Gomez-Gonzalez S., Gutierrez J. R., Hernandez R. M., Huang X., Huber-Sannwald E., Jankju M., Miriti M., Monerris J., Mau R. L., Morici E., Naseri K., Ospina A., Polo V., Prina A., Pucheta E., Ramirez-Collantes D. A., Romao R., Tighe M., Torres-Diaz C., Val J., Veiga J. P., Wang D., Zaady E., Plant species richness and ecosystem multifunctionality in global drylands. Science 335, 214–218 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meyer S. T., Ptacnik R., Hillebrand H., Bessler H., Buchmann N., Ebeling A., Eisenhauer N., Engels C., Fischer M., Halle S., Klein A. M., Oelmann Y., Roscher C., Rottstock T., Scherber C., Scheu S., Schmid B., Schulze E. D., Temperton V. M., Tscharntke T., Voigt W., Weigelt A., Wilcke W., Weisser W. W., Biodiversity-multifunctionality relationships depend on identity and number of measured functions. Nat. Ecol. Evol. 2, 44–49 (2018). [DOI] [PubMed] [Google Scholar]
- 19.Allan E., Manning P., Alt F., Binkenstein J., Blaser S., Blüthgen N., Böhm S., Grassein F., Hölzel N., Klaus V. H., Kleinebecker T., Morris E. K., Oelmann Y., Prati D., Renner S. C., Rillig M. C., Schaefer M., Schloter M., Schmitt B., Schöning I., Schrumpf M., Solly E., Sorkau E., Steckel J., Steffen-Dewenter I., Stempfhuber B., Tschapka M., Weiner C. N., Weisser W. W., Werner M., Westphal C., Wilcke W., Fischer M., Land use intensification alters ecosystem multifunctionality via loss of biodiversity and changes to functional composition. Ecol. Lett. 18, 834–843 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seufert V., Ramankutty N., Foley J. A., Comparing the yields of organic and conventional agriculture. Nature 485, 229–232 (2012). [DOI] [PubMed] [Google Scholar]
- 21.Knapp S., van der Heijden M. G. A., A global meta-analysis of yield stability in organic and conservation agriculture. Nat. Commun. 9, 3632 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kassam A., Friedrich T., Derpsch R., Global spread of conservation agriculture. Int. J. Environ. Stud. 76, 29–51 (2018). [Google Scholar]
- 23.Holland J. M., The environmental consequences of adopting conservation tillage in Europe: Reviewing the evidence. Agr Ecosyst Environ 103, 1–25 (2004). [Google Scholar]
- 24.Pittelkow C. M., Liang X., Linquist B. A., van Groenigen K. J., Lee J., Lundy M. E., van Gestel N., Six J., Venterea R. T., van Kessel C., Productivity limits and potentials of the principles of conservation agriculture. Nature 517, 365–368 (2015). [DOI] [PubMed] [Google Scholar]
- 25.Triplett G. B. Jr., Dick W. A., No-tillage crop production: A revolution in agriculture! Agron. J. 100, S-153–S-165 (2008). [Google Scholar]
- 26.Lori M., Symnaczik S., Mader P., De Deyn G., Gattinger A., Organic farming enhances soil microbial abundance and activity—A meta-analysis and meta-regression. PLOS ONE 12, e0180442 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen H., Dai Z., Veach A. M., Zheng J., Xu J., Schadt C. W., Global meta-analyses show that conservation tillage practices promote soil fungal and bacterial biomass. Agric. Ecosyst. Environ. 293, 106841 (2020). [Google Scholar]
- 28.Hartman K., van der Heijden M. G. A., Wittwer R. A., Banerjee S., Walser J. C., Schlaeppi K., Cropping practices manipulate abundance patterns of root and soil microbiome members paving the way to smart farming. Microbiome 6, 14 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wagg C., Dudenhöffer J.-H., Widmer F., van der Heijden M. G. A., Linking diversity, synchrony and stability in soil microbial communities. Funct. Ecol. 32, 1280–1292 (2018). [Google Scholar]
- 30.Seitz S., Goebes P., Puerta V. L., Pereira E. I. P., Wittwer R., Six J., van der Heijden M. G. A., Scholten T., Conservation tillage and organic farming reduce soil erosion. Agron. Sustain. Dev. 39, 4 (2019). [Google Scholar]
- 31.Clark M., Tilman D., Comparative analysis of environmental impacts of agricultural production systems, agricultural input efficiency, and food choice. Environ. Res. Lett. 12, (2017). [Google Scholar]
- 32.Meier M. S., Stoessel F., Jungbluth N., Juraske R., Schader C., Stolze M., Environmental impacts of organic and conventional agricultural products–Are the differences captured by life cycle assessment? J. Environ. Manage. 149, 193–208 (2015). [DOI] [PubMed] [Google Scholar]
- 33.Cooper J., Baranski M., Stewart G., Lange M. N.-d., Bàrberi P., Fließbach A., Peigné J., Berner A., Brock C., Casagrande M., Crowley O., David C., De Vliegher A., Döring T. F., Dupont A., Entz M., Grosse M., Haase T., Halde C., Hammerl V., Huiting H., Leithold G., Messmer M., Schloter M., Sukkel W., van der Heijden M. G. A., Willekens K., Wittwer R., Mäder P., Shallow non-inversion tillage in organic farming maintains crop yields and increases soil C stocks: A meta-analysis. Agron. Sustain. Dev. 36, 22 (2016). [Google Scholar]
- 34.Leifeld J., Angers D. A., Chenu C., Fuhrer J., Kätterer T., Powlson D. S., Organic farming gives no climate change benefit through soil carbon sequestration. Proc. Natl. Acad. Sci. U.S.A. 110, E984 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Six J., Ogle S. M., Jay breidt F., Conant R. T., Mosier A. R., Paustian K., The potential to mitigate global warming with no-tillage management is only realized when practised in the long term. Glob. Chang. Biol. 10, 155–160 (2004). [Google Scholar]
- 36.G. Schwarz, H. Nieberg, J. Sanders, Organic Farming Support Payments in the EU (Johann Heinrich von Thünen-Institut, 2010).
- 37.Byrnes J., Lefcheck J. S., Gamfeldt L., Griffin J. N., Isbell F., Hector A., Multifunctionality does not imply that all functions are positively correlated. Proc. Natl. Acad. Sci. U.S.A. 111, E5490 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cavigelli M. A., Mirsky S. B., Teasdale J. R., Spargo J. T., Doran J., Organic grain cropping systems to enhance ecosystem services. Renew. Agric. Food Syst. 28, 145–159 (2013). [Google Scholar]
- 39.Wittwer R. A., Dorn B., Jossi W., Van Der Heijden M. G., Cover crops support ecological intensification of arable cropping systems. Sci. Rep. 7, 41911 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garland G., Edlinger A., Banerjee S., Degrune F., García-Palacios P., Pescador D. S., Herzog C., Romdhane S., Saghai A., Spor A., Wagg C., Hallin S., Maestre F. T., Philippot L., Rillig M. C., van der Heijden M. G. A., Crop cover is more important than rotational diversity for soil multifunctionality and cereal yields in European cropping systems. Nat. Food 2, 28–37 (2021). [DOI] [PubMed] [Google Scholar]
- 41.H. Willer, J. Lernoud, The World of Organic Agriculture. Statistics and Emerging Trends 2019 (Research Institute of Organic Agriculture FiBL and IFOAM Organics International, 2019).
- 42.Prasuhn V., Twenty years of soil erosion on-farm measurement: Annual variation, spatial distribution and the impact of conservation programmes for soil loss rates in Switzerland. Earth Surf. Process. Landf. 45, 1539–1554 (2020). [Google Scholar]
- 43.S. Seitz, V. Prasuhn, T. Scholten, in No-till Farming Systems for Sustainable Agriculture (Springer, 2020), pp. 195–211. [Google Scholar]
- 44.Muller A., Schader C., el-Hage Scialabba N., Brüggemann J., Isensee A., Erb K. H., Smith P., Klocke P., Leiber F., Stolze M., Niggli U., Strategies for feeding the world more sustainably with organic agriculture. Nat. Commun. 8, 1290 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Power A. G., Ecosystem services and agriculture: Tradeoffs and synergies. Philos. Trans R. Soc. Lond. B Biol. Sci. 365, 2959–2971 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Swinton S. M., Lupi F., Robertson G. P., Hamilton S. K., Ecosystem services and agriculture: Cultivating agricultural ecosystems for diverse benefits. Ecol. Econ. 64, 245–252 (2007). [Google Scholar]
- 47.Stoate C., Boatman N. D., Borralho R. J., Carvalho C. R., Snoo G. R., Eden P., Ecological impacts of arable intensification in Europe. J. Environ. Manage. 63, 337–365 (2001). [DOI] [PubMed] [Google Scholar]
- 48.Polasky S., Tallis H., Reyers B., Setting the bar: Standards for ecosystem services. Proc. Natl. Acad. Sci. U.S.A. 112, 7356–7361 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Flisch R., Sinaj S., Charles R., Richner W., Grundlagen für die Düngung im Acker-und Futterbau (GRUDAF). Agrarforschung 16, 1–101 (2009). [Google Scholar]
- 50.Ter Heerdt G. N. J., Verweij G. L., Bekker R. M., Bakker J. P., An improved method for seed-bank analysis: Seedling emergence after removing the soil by sieving. Funct. Ecol. 10, 144–151 (1996). [Google Scholar]
- 51.Mayor J. P., Dessaint F., Influence of weed management strategies on soil seedbank diversity. Weed Res. 38, 95–105 (1998). [Google Scholar]
- 52.Büchi L., Gebhard C.-A., Liebisch F., Sinaj S., Ramseier H., Charles R., Accumulation of biologically fixed nitrogen by legumes cultivated as cover crops in Switzerland. Plant and Soil 393, 163–175 (2015). [Google Scholar]
- 53.Anglade J., Billen G., Garnier J., Relationships for estimating N2 fixation in legumes: Incidence for N balance of legume-based cropping systems in Europe. Ecosphere 6, 1–24 (2015). [Google Scholar]
- 54.Oberson A., Frossard E., Bühlmann C., Mayer J., Mäder P., Lüscher A., Nitrogen fixation and transfer in grass-clover leys under organic and conventional cropping systems. Plant and Soil 371, 237–255 (2013). [Google Scholar]
- 55.Zorn A., Hoop D., Gazzarin C., Lips M., Produktionskosten der Betriebszweige des kombinierten Betriebstyps Verkehrsmilch/Ackerbau. Agroscope Sci., 1–46 (2015). [Google Scholar]
- 56.Gazzarin C., Maschinenkosten 2014. Agroscope Transfer , 1–52 (2014). [Google Scholar]
- 57.Eidgenössische Forschungsanstalten, Schweizerische Referenzmethoden der Eidgenössischen landwirtschaftlichen Forschungsanstalten. Band 1 (1996).
- 58.Vance E. D., Brookes P. C., Jenkinson D. S., An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 19, 703–707 (1987). [Google Scholar]
- 59.Joergensen R. G., The fumigation-extraction method to estimate soil microbial biomass: Calibration of the kEC value. Soil Biol. Biochem. 28, 25–31 (1996). [Google Scholar]
- 60.Hydbom S., Ernfors M., Birgander J., Hollander J., Jensen E. S., Olsson P. A., Reduced tillage stimulated symbiotic fungi and microbial saprotrophs, but did not lead to a shift in the saprotrophic microorganism community structure. Appl. Soil Ecol. 119, 104–114 (2017). [Google Scholar]
- 61.Frostegård A., Bååth E., The use of phospholipid fatty acid analysis to estimate bacterial and fungal biomass in soil. Biol. Fertil. Soils 22, 59–65 (1996). [Google Scholar]
- 62.Olsson P. A., Signature fatty acids provide tools for determination of the distribution and interactions of mycorrhizal fungi in soil. FEMS Microbiol. Ecol. 29, 303–310 (1999). [Google Scholar]
- 63.Olsson P. A., Johansen A., Lipid and fatty acid composition of hyphae and spores of arbuscular mycorrhizal fungi at different growth stages. Mycol. Res. 104, 429–434 (2000). [Google Scholar]
- 64.Säle V., Aguilera P., Laczko E., Mäder P., Berner A., Zihlmann U., van der Heijden M. G. A., Oehl F., Impact of conservation tillage and organic farming on the diversity of arbuscular mycorrhizal fungi. Soil Biol. Biochem. 84, 38–52 (2015). [Google Scholar]
- 65.E. Sieverding, J. Friedrichsen, W. Suden, Vesicular-arbuscular mycorrhiza management in tropical agrosystems. Sonderpublikation der GTZ (Germany) (1991).
- 66.J. Błaszkowski, Glomeromycota (W. Szafer Institute of Botany, Polish Academy of Sciences, 2012).
- 67.N. C. Schenck, Y. Perez, Manual for the Identification of VA Mycorrhizal Fungi (Synergistic Publications Gainesville, 1990), vol. 286. [Google Scholar]
- 68.Baltruschat H., Santos V. M., da Silva D. K. A., Schellenberg I., Deubel A., Sieverding E., Oehl F., Unexpectedly high diversity of arbuscular mycorrhizal fungi in fertile Chernozem croplands in Central Europe. Catena 182, 104135 (2019). [Google Scholar]
- 69.Wijayawardene N. N., Hyde K., Al-Ani L., Tedersoo L., Haelewaters D., Rajeshkumar K. C., Zhao R., Aptroot A., Leontyev D., Saxena R. K., Tokarev Y., Dai D., Letcher P. M., Stephenson S., Ertz D., Lumbsch H., Kukwa M., Issi I., Madrid H., Phillips A., Selbmann L., Pfliegler W. P., Horváth E., Bensch K., Kirk P., Kolaříková K., Raja H., Radek R., Papp V., Dima V., Ma J., Malosso E., Takamatsu S., Rambold G., Gannibal P., Triebel D., Gautam A., Avasthi S., Suetrong S., Timdal E., Fryar S., Delgado G., Réblová M., Doilom M., Dolatabadi S., Pawłowska J., Humber R., Kodsueb R., Sánchez-Castro I., Goto B., Silva D. K., Souza F. A., Oehl F., Silva G. A., Błaszkowski J., Jobim K., Maia L. C., Barbosa F. R., Fiuza P. O., Divakar P., Shenoy B. D., Castañeda-Ruíz R., Somrithipol S., Lateef A. A., Karunarathna S., Tibpromma S., Mortimer P., Wanasinghe D. N., Phookamsak R., Xu J., Wang Y., Tian F., Alvarado P., Li D. W., Kušan I., Matočec N., Mešić A., Tkalčec Z., Maharachchikumbura S., Papizadeh M., Heredia G., Wartchow F., Bakhshi M., Boehm E., Youssef N., Hustad V., Lawrey J., Santiago A. e. L. C. M. A., Bezerra J., Souza-Motta C., Firmino A. L., Tian Q., Houbraken J., Hongsanan S., Tanaka K., Dissanayake A., Monteiro J. S., Grossart H., Suija A., Weerakoon G., Etayo J., Tsurykau A., Vázquez V., Mungai P., Damm U., Li Q. R., Zhang H., Boonmee S., Lu Y., Becerra A., Kendrick B., Brearley F., Motiejūnaitė J., Sharma B., Khare R., Gaikwad S., Wijesundara D., Tang L. Z., He M., Flakus A., Rodriguez-Flakus P., Zhurbenko M., Mckenzie E., Stadler M., Bhat D., Liu J. K., Raza M., Jeewon R., Nassonova E., Prieto M., Jayalal R., Erdoğdu M., Yurkov A. M., Schnittler M., Shchepin O., Novozhilov Y., Silva-Filho A. G. S., Gentekaki E., Liu P., Cavender J. C., Kang Y., Mohammad S., Zhang L. F., Xu R., Li Y. M., Dayarathne M., Ekanayaka A. H., Wen T., Deng C., Pereira O. L., Navathe S., Hawksworth D., Fan X. L., Dissanayake L., Kuhnert E., Thines M., Outline of fungi and fungus-like taxa. Mycosphere 11, 1060–1456 (2020). [Google Scholar]
- 70.Elliott E., Aggregate structure and carbon, nitrogen, and phosphorus in native and cultivated soils. Soil Sci. Soc. Am. J. 50, 627–633 (1986). [Google Scholar]
- 71.Loaiza Puerta V., Pereira E. I. P., Wittwer R., van der Heijden M., Six J., Improvement of soil structure through organic crop management, conservation tillage and grass-clover ley. Soil Tillage Res. 180, 1–9 (2018). [Google Scholar]
- 72.Knacker T., van Gestel C. A. M., Jones S. E., Soares A. M. V. M., Schallnaß H. J., Förster B., Edwards C. A., Ring-testing and field-validation of a Terrestrial Model Ecosystem (TME)–an instrument for testing potentially harmful substances: Conceptual approach and study design. Ecotoxicology 13, 9–27 (2004). [DOI] [PubMed] [Google Scholar]
- 73.T. Nemecek, R. Freiermuth Knuchel, M. Alig, G. Gaillard, The advantages of generic LCA tools for agriculture: Examples SALCAcrop and SALCAfarm, in Proceeding of the 7th Int. Conference on Life Cycle Assessment in the Agri-Food Sector, Bari, Italy, 22 to 24 September 2010. [Google Scholar]
- 74.Nemecek T., Dubois D., Huguenin-Elie O., Gaillard G., Life cycle assessment of Swiss farming systems: I. Integrated and organic farming. Agric. Syst. 104, 217–232 (2011). [Google Scholar]
- 75.ecoinvent Centre (Swiss Centre for Life Cycle Inventories, Dübendorf, 2010).
- 76.Prechsl U. E., Wittwer R., van der Heijden M. G. A., Lüscher G., Jeanneret P., Nemecek T., Assessing the environmental impacts of cropping systems and cover crops: Life cycle assessment of FAST, a long-term arable farming field experiment. Agr. Syst. 157, 39–50 (2017). [Google Scholar]
- 77.Manning P., van der Plas F., Soliveres S., Allan E., Maestre F. T., Mace G., Whittingham M. J., Fischer M., Redefining ecosystem multifunctionality. Nat. Ecol.Evol. 2, 427–436 (2018). [DOI] [PubMed] [Google Scholar]
- 78.Byrnes J. E. K., Gamfeldt L., Isbell F., Lefcheck J. S., Griffin J. N., Hector A., Cardinale B. J., Hooper D. U., Dee L. E., Emmett Duffy J., Investigating the relationship between biodiversity and ecosystem multifunctionality: Challenges and solutions. Methods Ecol. Evol. 5, 111–124 (2014). [Google Scholar]
- 79.Hölting L., Jacobs S., Felipe-Lucia M. R., Maes J., Norström A. V., Plieninger T., Cord A. F., Measuring ecosystem multifunctionality across scales. Environ. Res. Lett. 14, 124083 (2019). [Google Scholar]
- 80.R Core Team, R Foundation for Statistical Computing, R: A language and environment for statistical computing (2020).
- 81.R. Lenth, H. Singmann, J. Love, Emmeans: Estimated marginal means, aka least-squares means, R package version 1.4.5 (2018).
- 82.J. Oksanen, F. G. Blanchet, M. Friendly, R. Kindt, P. Legendre, D. McGlinn, P. R. Minchin, R. B. O’Hara, G. L. Simpson, P. Solymos, M. H. H. Stevens, E. Szoecs, H. Wagner, Vegan: Community ecology package, R package version 2.5-6 (2013).
- 83.Baselga A., Orme C. D. L., betapart: An R package for the study of beta diversity. Methods Ecol. Evol. 3, 808–812 (2012). [Google Scholar]
- 84.J. Byrnes, multifunc: Analysis of Ecological Drivers on Ecosystem Multifunctionality (2017).
- 85.Johannes A., Matter A., Schulin R., Weisskopf P., Baveye P. C., Boivin P., Optimal organic carbon values for soil structure quality of arable soils. Does clay content matter? Geoderma 302, 14–21 (2017). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/7/34/eabg6995/DC1


