Abstract
Background
The outbreak of novel coronavirus pneumonia 2019 (COVID-19) has caused millions of deaths worldwide. It is well documented that troponin predicts the prognosis of patients. Myoglobin is not only an important marker of myocardial injury, but it indicates systemic muscle damage. However, its relationship with COVID-19 was rarely reported. The present study compared the predictive value of troponin and myoglobin on the final prognosis of COVID-19 patients by analyzing the clinical characteristics and serum levels of myoglobin and troponin in severe/critical COVID-19 patients.
Methods
We enrolled 499 consecutive eligible hospitalized patients with severe/critical COVID-19 from February 14 to March 24, 2020 at Leishenshan Hospital, Wuhan, China. Clinical characteristics and laboratory data were collected and compared between the patients who died and survived. We analyzed the receiver operating characteristic curves of myoglobin and troponin. Then, the patients were divided into myo+ group, myo− group, tro+ group, and tro− group, and survival curves were analyzed. The prognostic predictable values of myoglobin and troponin were further analyzed using Cox multifactorial analysis.
Results
Myoglobin and troponin were significantly elevated in the death group (134.4 [interquartile range (IQR) 24.80, 605] vs 38.02 [IQR 3.87, 11.73] ng/ml, p < 0.001), and troponin was also significantly elevated in the death group (0.01 [IQR 0.01, 0.01] vs 0.04 [IQR 0.02, 0.15] ng/ml, p < 0.001). The ROC curves demonstrated that the area under the curve when using myoglobin to predict patient death was 0.911, with a threshold of 1.17, which was equivalent to troponin. Kaplan–Meier survival analysis revealed a significantly lower survival curve in the myo+ group than the myo− group. Multifactor Cox survival analysis showed that troponin was no longer significant (HR = 0.98, 95% CI 0.92–1.03, p = 0.507), but elevated myoglobin was an independent predictor of death in COVID-19 patients (HR = 1.001, 95% CI 1.001–1.002, p < 0.001). The analysis of the Cox model for predicting patient death and plotting decision curves suggested that the single factor myoglobin model was superior to troponin, and the predictive value of the multifactor model was superior to the single-factor analyses.
Conclusions
In severe/critical COVID-19 patients, myoglobin and troponin were predictors of mortality and the probability of conversion to critical illness, and myoglobin may be superior to troponin for predictive value.
Keywords: Coronavirus disease 2019, Myoglobin, Troponin, Prognosis
Abstract
Antecedentes
El brote de la nueva neumonía por coronavirus 2019 (COVID-19) ha causado millones de muertes en todo el mundo. Está bien documentado que la troponina predice el pronóstico de los pacientes. La mioglobina no solo es un importante marcador de lesión miocárdica, sino que indica daño muscular sistémico. Sin embargo, su relación con la COVID-19 ha sido raramente comunicada. En el presente estudio se ha comparado el valor predictivo de la troponina y la mioglobina en el pronóstico final de los pacientes con COVID-19, analizando las características clínicas y los niveles séricos de mioglobina y troponina en pacientes con COVID-19 en estado grave o crítico.
Métodos
Se inscribió a 499 pacientes consecutivos elegibles hospitalizados con COVID-19 en estado grave o crítico del 14 de febrero al 24 de marzo de 2020 en el Hospital Leishenshan (Wuhan, China). Se recogieron las características clínicas y los datos de laboratorio y se compararon entre los pacientes que murieron y los que sobrevivieron. Se analizaron las curvas de características operativas del receptor de mioglobina y troponina. A continuación, se dividió a los pacientes en grupo myo+, grupo myo−, grupo tro+ y grupo tro−, y se analizaron las curvas de supervivencia. Los valores pronósticos de la mioglobina y la troponina se analizaron además mediante un análisis multifactorial de Cox.
Resultados
La mioglobina y la troponina estaban significativamente elevadas en el grupo de muerte (134,4; rango intercuartílico [RIQ: 24,80; 605] vs. 38,02; [RIQ: 3,87; 11,73] ng/ml; p < 0,001), y la troponina también estaba significativamente elevada en el grupo de muerte (0,01 [RIQ: 0,01; 0,01] vs. 0,04 [RIQ: 0,02; 0,15] ng/ml; p < 0,001). Las curvas ROC demostraron que el área bajo la curva al utilizar la mioglobina para predecir la muerte de los pacientes era de 0,911, con un umbral de 1,17, equivalente al de la troponina. El análisis de supervivencia de Kaplan-Meier reveló una curva de supervivencia significativamente menor en el grupo myo+ que en el grupo myo−. El análisis de supervivencia multifactorial de Cox mostró que la troponina ya no era significativa (HR = 0,98; IC 95%: 0,92-1,03; p = 0,507), pero la mioglobina elevada era un predictor independiente de muerte en los pacientes COVID-19 (HR = 1,001; IC 95%: 1,001-1,002; p < 0,001). El análisis del modelo de Cox para predecir la muerte de los pacientes y el trazado de las curvas de decisión indicaron que el modelo de mioglobina de un solo factor era superior al de la troponina y que el valor predictivo del modelo multifactorial era superior a los análisis de un solo factor.
Conclusiones
En los pacientes graves o críticos de COVID-19, la mioglobina y la troponina fueron predictores de la mortalidad y de la probabilidad de conversión a enfermedad crítica, y la mioglobina puede ser superior a la troponina en cuanto al valor predictivo.
Palabras clave: Enfermedad por coronavirus 2019, Mioglobina, Troponina, Pronóstico
Introduction
At the end of 2019, the coronavirus disease 2019 (COVID-19) became a pandemic. There were 7,823,289 cumulative confirmed cases of COVID-19 at 10:00 CEST on June 15, 2020 and 43,154 cumulative deaths worldwide according to real-time statistics from the World Health Organization.1 Of all COVID-19 patients, mild/average cases, severe cases and critical cases account for approximate 81.4%, 13.9%, and 4.7%, respectively. Several studies showed that some of the mild/average COVID-19 patients rapidly deteriorated in a short period of time.2, 3 Once they were transferred to critical illness, the mortality increased dramatically and reached 47% in the literature.4 Therefore, the identification and research of valuable factors that predict the progress of novel coronavirus pneumonia is important.
ACE2 receptors are the main target of the novel coronavirus, and cardiovascular system injury is increasingly important in COVID-19 patients, especially in patients who progress to critical illness or death. Twelve percent of patients with COVID-19 have comorbid myocardial injury because of viral infection, which results in elevated troponin and decreased ejection fraction.5 Recent studies showed that myocardial injury in patients with COVID-19 was ubiquitous and correlated with patient mortality.6, 7 Some articles also found a correlation between cardiac enzymes, such as LDH and CK, and endpoints, such as patient ICU admission and mechanical ventilation.8 Troponin predicted mortality in COVID-19 patients.2, 6, 7, 9
However, ACE2 receptors are found throughout the human body,10 and systemic muscle injury may be associated with COVID-19 progress. Myoglobin is an important biochemical marker of myocardial and systemic muscle injury, and it may be more valuable to predict mortality and the probability of conversion to critical illness in COVID-19 patients. The present research was a retrospective study of 499 patients with novel coronavirus pneumonia in a single center of Wuhan Leishenshan Hospital. The study investigated the value of posthospitalization myoglobin/troponin levels in predicting patient mortality and prognosis, analyzed the differences between these two markers, and discussed the causes of the differences.
Methods
Patient source and classification
We enrolled in-patients with severe/critical COVID-19 from February 14 to March 24, 2020 at Leishenshan Hospital, Wuhan, China. The data cutoff for the study was April 5, 2020. Leishenshan Hospital was the hospital designated to admit COVID-19 patients, primarily for the treatment of severe/critical COVID-19 patients. Inclusion criteria: (1) 2019-nCoV nucleic acid test-positive cases who met the diagnostic criteria for severe/critical cases and (2) complete clinical data with at least 1 test result of myoglobin and troponin within 2 days of hospital admission. Exclusion criteria:(1) non-2019-nCoV infection confirmed cases; (2) patients with incomplete clinical data; and (3) patients who did not meet the criteria of severe/critical cases.
The diagnostic criteria for COVID-19 referred to the latest WHO clinical guidelines for COVID-19,11 including epidemiological history, clinical presentation with fever and/or respiratory symptoms, and chest radiographs showing pneumonia. Real-time fluorescence PCR of the suspected patient's throat swab specimen was positive for COVID-19 nucleic acid (Bioperfectus, Taizhou, China).
Severe COVID-19 was defined as the meeting of any of the following criteria: (1) the presence of shortness of breath with ≥30 breaths/min; (2) finger oxygen saturation ≤93% at rest; or (3) arterial oxygen partial pressure (PaO2)/inspiratory oxygen concentration (FiO2) ≤300 mmHg (1 mmHg = 0.133 kPa). If the above criteria were not met but the patient's lung imaging showed significant lesion progression >50% within 24–48 h, the patient was also managed as severe.
Critical COVID-19 was defined as the meeting of any of the following criteria: (1) the presence of respiratory failure and the need for mechanical ventilation; (2) the presence of shock; or (3) the combination of other organ failure that required ICU monitoring.12
The ethics committee of Wuhan Leishenshan Hospital approved the study, which followed the principles of the Declaration of Helsinki.
Study design
All patients were divided into two groups, the survivor group and the death group, according to their final prognosis. Myoglobin, troponin, and other parameters were evaluated and compared between the two groups. According to the presence or absence of elevated myoglobin and troponin, the patients were divided into a myo+ group (myoglobin > 15.33 ng/ml, cut-off value), myo− group (myoglobin ≤15.33 ng/ml, cut-off value), tro+ group (troponin > 0.018 ng/ml, cut-off value) and tro− group (troponin ≤0.018 ng/ml, cut-off value). The clinical characteristics and prognosis were analyzed between groups. The study was based on the optimal thresholds of the ROC curves, which were set at abnormal values of myoglobin and troponin.
Data collection
Demographic data, clinical presentation, laboratory findings, imaging studies (chest CT scans), treatment, and prognosis were collected from all enrolled patients. Laboratory findings included myoglobin, CK-MB, high-sensitivity troponin I, white blood cell count, C-reactive protein (CRP), renal function, liver function, and B-type natriuretic peptide (BNP).
Blood samples were collected from all inpatients. Myoglobin and troponin were measured using the double-antibody sandwich method of chemiluminescence immunotechnology, and the diagnostic reagents were obtained from Beckman Coulter (Beckman Coulter, Suzhou, China). Other laboratory findings were obtained via routine blood examinations. Patient mortality was calculated. The treating physician determined the frequency of examinations.
Statistical analysis
Statistical analysis was performed using Rstudio (v1.2.1335, RStudio, Inc., Boston, MA, USA). Normally distributed statistics are expressed as the means ± standard deviation. Between-group comparisons were performed using the independent samples t-test. Continuous variables for partial distributions are expressed as medians (quartile interval), and between-group comparisons were performed using the Wilcoxon test. Frequency data are expressed as the number of cases (%), and the chi-squared test was used for comparisons between groups. A two-sided test was used, and p < 0.05 was considered a statistically significant difference. Receiver operating characteristic (ROC) curves were used to analyze the predictive value of myoglobin and troponin for death. Survival curves adjusted by age and sex were plotted and analyzed between groups. The correlation of hazard ratio (HR) with myoglobin and troponin was analyzed using the restricted cubic spline plot. The HR values of myoglobin and troponin were analyzed using the Cox survival model. Because there was co-collinearity between myoglobin and troponin, we performed variable selection using Lasso regression analysis. The variables selected for Lasso were entered into the Cox model, and line graphs were drawn based on the Cox model. We analyzed the value of the prediction of death from the decision curve of the Cox model. The predictive value of this multifactorial Cox model was compared with the univariate myoglobin model and the univariate troponin model for mortality.
Results
Clinical characteristics
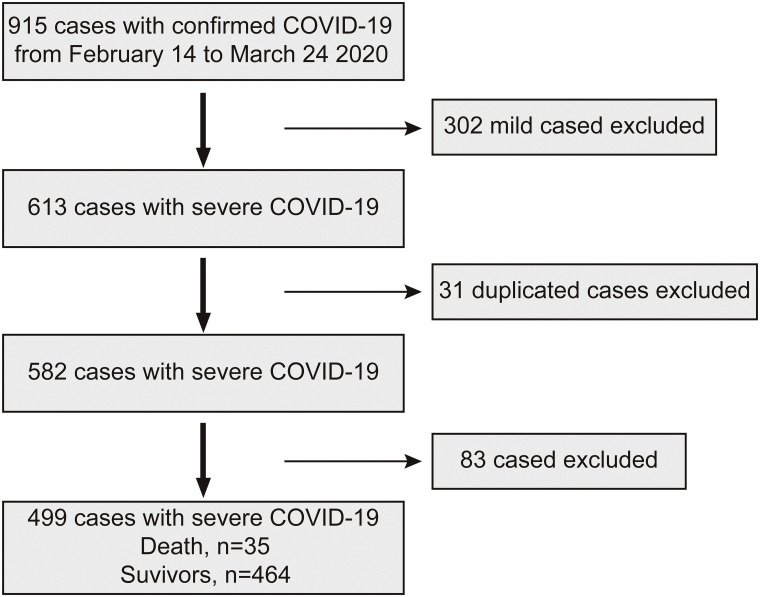
A total of 915 patients with COVID-19 were collected from Leishenshan Hospital in Wuhan, and 499 cases met the inclusion criteria. A total of 302 patients with common type COVID-19 were excluded, 31 patients were duplicated, and 83 patients had no measured myoglobin within 3 days (Fig. 1 ).
Fig. 1.
Flowchart of patient recruitment.
The mean age of the 499 patients was 59 ± 15 years, and other characteristics included 253 male patients (50.7%), 246 female patients (49.3%), 185 patients with hypertension (37.1%), 80 patients with diabetes (17.1%), 34 patients with coronary heart disease (6.8%), and 25 patients with chronic renal insufficiency (5%) (Table 1 ). A total of 464 patients (93%) were discharged from the hospital, and 35 patients (7%) died (Table 1).
Table 1.
Baseline characteristics and laboratory findings of 499 patients with COVID-19.
| Characteristics | All patients (n = 499) |
Survivors (n = 464) |
Death (n = 35) |
p-Value | |
|---|---|---|---|---|---|
| Age (mean (SD)) | 59 (15) | 58 (15) | 72 (11) | <0.001 | |
| Gender = m (%) | 253 (50) | 234 (50) | 19 (54) | 0.791 | |
| Comorbidities, n (%) | |||||
| Hypertension | 185 (37.1) | 168 (36.2) | 17 (48.6) | 0.201 | |
| Heart failure | 19 (3.8) | 17 (3.7) | 2 (5.7) | 0.878 | |
| Diabetes | 80 (16.0) | 73 (15.7) | 7 (20.0) | 0.671 | |
| Coronary heart disease | 34 (6.8) | 28 (6.0) | 6 (17.1) | 0.030 | |
| Chronic renal disease | 25 (5.0) | 19 (4.1) | 6 (17.1) | 0.003 | |
| Chronic liver disease | 14 (2.8) | 13 (2.8) | 1 (2.9) | 1.000 | |
| Laboratory findings | Normal range | ||||
| TNI, ng/ml (median [IQR]) | 0–0.04 | 0.01 [0.01, 0.01] | 0.01 [0.01, 0.01] | 0.04 [0.02, 0.15] | <0.001 |
| Creatinine, μmol/L (mean (SD)) | 64–104 | 84 (121) | 76 (86) | 182 (320) | <0.001 |
| MYO, ng/ml (median [IQR]) | 0–80 | 6 [3, 11] | 6 [3, 9] | 134 [24, 605] | <0.001 |
| CK-MB, ng/ml (median [IQR]) | 0–6.36 | 1.1 [0.8, 1.7] | 1.0 [0.7, 1.6] | 4.7 [2.3, 10.5] | <0.001 |
| D_DIMER, mg/L (median [IQR]) | 0–0.55 | 0.6 [0.2, 1.4] | 0.5 [0.2, 1.1] | 4.5 [1.80, 7.3] | <0.001 |
| Leukocyte counts, *109/L (mean (SD)) | 3.5–9.5 | 6.4 (3.0) | 6.1 (2.6) | 10.3 (4.7) | <0.001 |
| Hemoglobin, g/L (mean (SD)) | 130–175 | 121 (21) | 121 (20) | 112 (28) | 0.011 |
| Lymphocyte rate (mean (SD)) | 20–50% | 25% (11) | 26% (10) | 8% (6) | <0.001 |
| Lymphocyte count, *109/L (mean (SD)) | 1.1–3.2 | 1.4 (0.6) | 1.5 (0.6) | 0.7 (0.4) | <0.001 |
| Alanine aminotransferase, IU/L (median [IQR]) | 9–50 | 24 [15, 39] | 23 [15, 38] | 34 [19, 45] | 0.043 |
| Aspartate aminotransferase, IU/L (median [IQR]) | 15–40 | 21 [16, 30] | 20 [16, 28] | 38 [27, 66] | <0.001 |
| Albumin, g/L (mean (SD)) | 40–55 | 36 (5) | 36 (4) | 30 (6) | <0.001 |
| C-reactive protein, mg/L (median [IQR]) | 0–4 | 1.6 [0.5, 10.0] | 1.2 [0.5, 7.4] | 51.1 [12.2, 75.9] | <0.001 |
| BNP, pg/ml (median [IQR]) | 0–100 | 10 [10, 35] | 10 [10, 29] | 115 [44, 308] | <0.001 |
| Procalcitonin, ng/ml (median [IQR]) | 0–0.05 | 0.04 [0.03, 0.07] | 0.04 [0.03, 0.07] | 0.43 [0.15, 1.19] | <0.001 |
TNI, troponin; MYO, myoglobin; CK-MB, creatinine kinase-myocardial band; BNP, B-type natriuretic peptide; IQR, inter-quartile range; SD, standard deviation.
Laboratory finding comparisons between the survivor group and death group
All patients were divided into a survivor group (464 cases, 93%) and death group (35 cases, 7%). We found that myoglobin and troponin were more than 4 times higher in the death group than the survivor group (134.4 [IQR 24.80, 605] ng/ml vs 38.02 [IQR3.87, 11.73] ng/ml, p < 0.001 for myoglobin) and (0.04 [IQR 0.02, 0.15] vs. 0.01 [IQR 0.01, 0.01], p < 0.01 for troponin). Patients in death group were older (70.03 ± 15.52 vs 58.33 ± 11.03 years), had higher creatinine on admission (181.18 ± 320.16 μmol/l vs. 84.10 ± 121.46 μmol/l, p < 0.01), higher D-2 aggregates (4.51 [IQR 1.80, 7.37] mg/L vs 0.57 [IQR 0.25, 1.40] mg/L, p < 0.001), lower absolute lymphocytes (0.70 ± 0.38 * 109/L vs 1.43 ± 0.58 * 109/L, p < 0.001), and lower albumin (30.43 ± 6.85 g/L vs 36.72 ± 4.89 g/L, p < 0.001). However, gender, history of hypertensive disease, history of liver disease, and hemoglobin were not significantly different between the two groups (Table 1).
Myoglobin/troponin ROC curve analyses
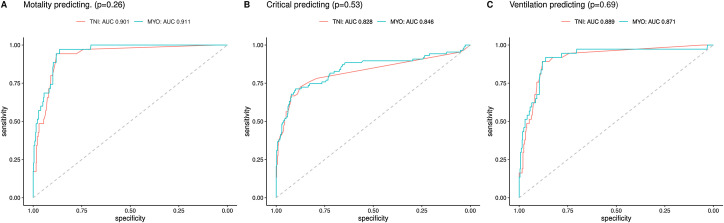
We studied myoglobin and troponin values to predict the mortality of patients and plotted ROC curves. The data showed that the AUC for myoglobin was 0.911, with a threshold of 20.5, and the AUC for troponin was 0.901, with a threshold of 1.17. The AUCs of myoglobin and troponin showed no significant difference (0.911, 95% CI 0.884–0.938 vs 0.901, 95% CI 0.879–0.923, p = 0.26)). When using the myoglobin value or troponin value to predict whether a patient would convert to critical illness and need to be admitted to the ICU, the myoglobin AUC was greater than troponin (0.846 (95% CI 0.790–0.902) vs 0.828 (95% CI 0.770–0.887), p = 0.53), but there was no significant difference. When using myoglobin/troponin values to predict the need for a ventilator, the myoglobin AUC was slightly smaller than the troponin AUC (0.871 (95% CI 0.818–0.924) vs 0.889 (95% CI 0.845–0.933), p = 0.69) (Fig. 2 ).
Fig. 2.
Myoglobin/troponin receiver operating characteristic analysis. (A) The area under the curve of MYO and TNI in predicting mortality was 0.911 and 0.901 respectively (p = 0.26). (B) The area under the curve of MYO and TNI in predicting to transfer to critical was 0.846 and 0.828 respectively (p = 0.53). (C) The area under the curve of MYO and TNI in predicting mechanism ventilation was 0.871 and 0.889 respectively (p = 0.69). MYO, myoglobin; TNI, troponin I.
Survival analysis
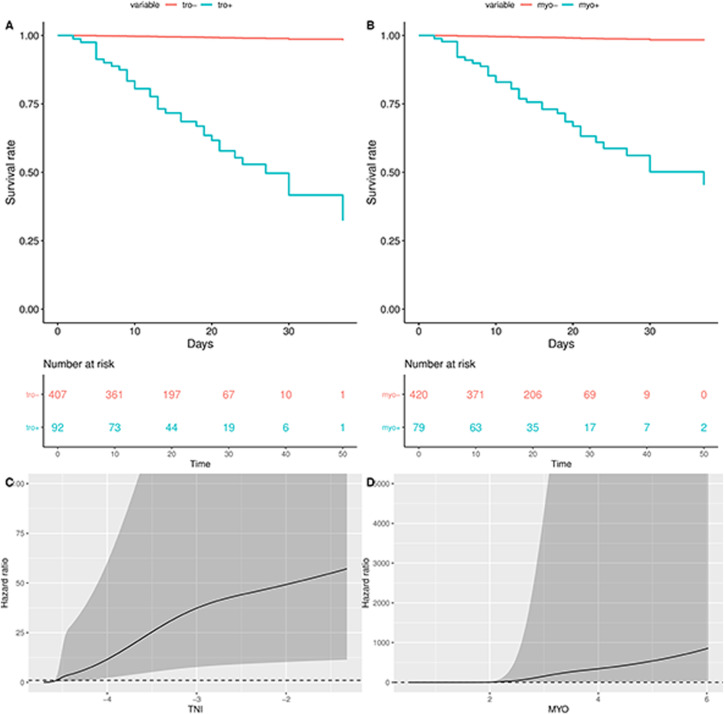
All patients were divided into myo+ group, myo− group, tro+ group, and tro− group, and survival analyses were performed using Kaplan–Meier analysis. After correcting for age and sex, the survival curves for the myo+ group and tro+ group were significantly lower than the myo− group and tro− group, respectively, and the restricted cubic spline analysis showed an upward trend in HR with increasing troponin and/or myoglobin (Fig. 3 ).
Fig. 3.
Kaplan–Meier plots and restricted cubic spline analysis of TNI, MYO in hospitalized patients with severe COVID-19. (A and B) Motality was significantly higher in of MYO+ and/or TNI+. (C and D) Hazard ratio increased in MYO+ and/or TNI+ group via restricted cubic spline analysis. MYO, myoglobin; TNI, troponin I.
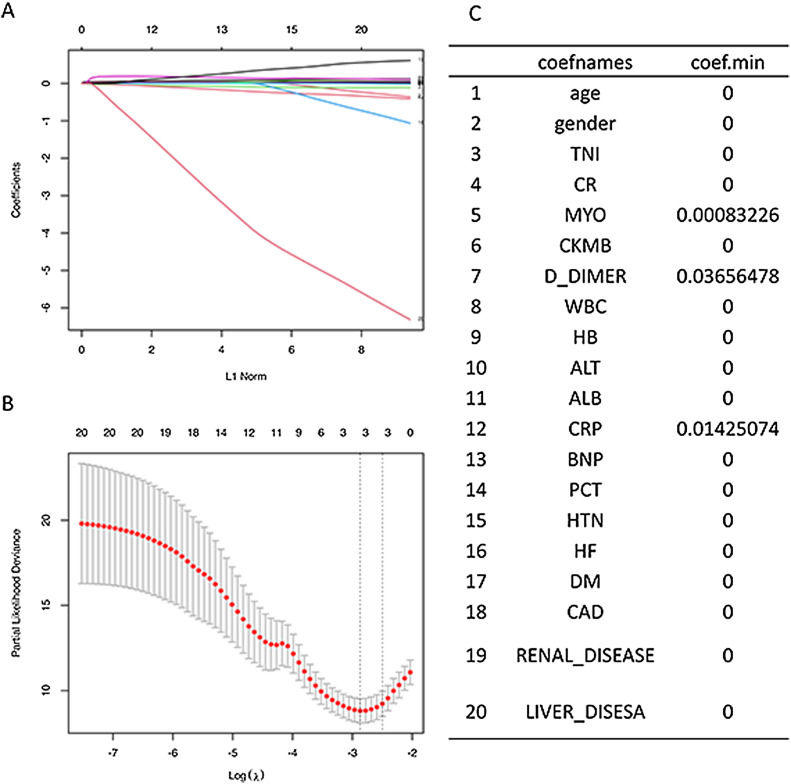
We performed further multivariate Cox survival analysis. Because the troponin and myoglobin may be co-collinear, we first performed Lasso regression analysis then variable penalty to eliminate collinearity. Lasso regression revealed that the minimum regression coefficient for myoglobin was not 0, and the minimum regression coefficient for troponin was close to 0 (Fig. 4 ).
Fig. 4.
Lasso regression of myoglobin and troponin. The minimum regression coefficient for myoglobin was not 0, the minimum regression coefficient for troponin was close to 0.
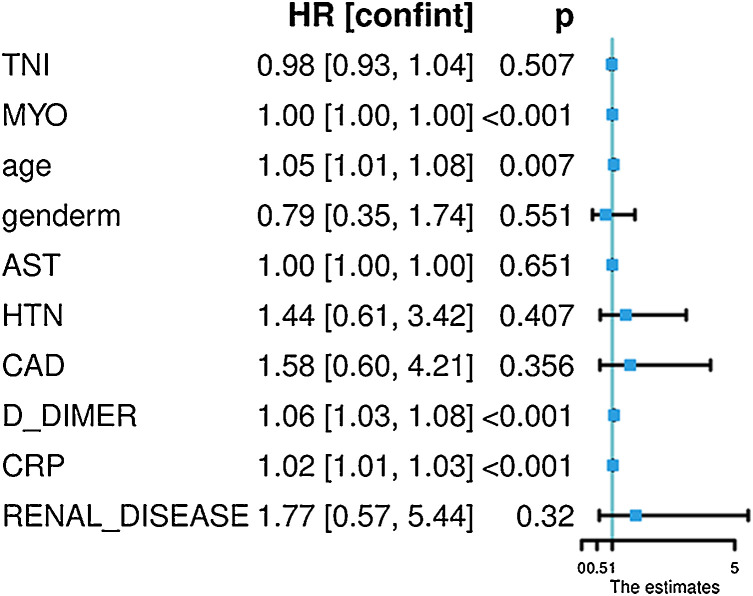
The variables for Cox regression included age, sex, troponin, myoglobin, AST, hypertension, coronary artery disease, D-DIMMER, CRP, and renal insufficiency, and the results indicated that elevated myoglobin was an independent predictor of death in COVID-19 patients (HR = 1.001, 95% CI 1.001–1.002, p < 0.001) (Fig. 5 ). However, troponin was no longer significant in this equation (HR = 0.98, 95% CI 0.92–1.03, p = 0.507) (Fig. 5).
Fig. 5.
Multivariate Cox regression analysis of clinical indicators of motality in hospitalized patient with severe COVID-19. Myoglobin was an independent predictor of death in COVID-19 patients (p < 0.001) and troponin was no longer significant in this equation (p = 0.507).
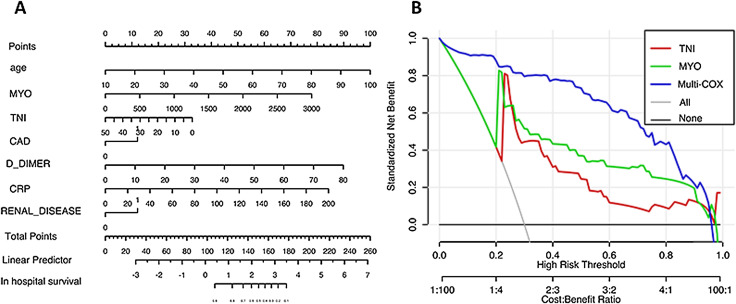
Nomogram/decision curve
Nomograms were drawn according to the Cox analysis and indicated that the coverage area of myoglobin was larger than troponin. We modeled and analyzed the Cox model to predict patient death and plotted the decision curve (blue curve is the multivariate Cox analysis model). The model showed that the univariate myoglobin model (green curve) was better than the troponin model (red curve), and the predictive value of the multivariate model (blue curve) was better than both univariate models (Fig. 6 ).
Fig. 6.
Nomogram/decision curve of myoglobin and troponin. The univariate myoglobin model (green curve) was better than the troponin model (red curve), and the predictive value of the multivariate model (blue curve) was better than both univariate models.
Discussion
The present study found that the presence of elevated myoglobin or troponin was not uncommon in patients with severe/critical COVID-19 in Wuhan, China. Comparison of the values of myoglobin and troponin between survivors and nonsurvivors and analysis of the ROC curves of myoglobin and troponin suggest that both parameters are predictors of mortality and the probability of conversion to critical illness. We found that myoglobin was an independent predictor of patient mortality using multivariate Cox analysis, and the predictive value of myoglobin may be superior to troponin according to our modeling analysis.
As the designated hospital for new coronavirus pneumonia in Wuhan, Leishenshan Hospital primarily treats patients with severe/critical illnesses. And increasing studies showed that myocardial injury was common in patients with COVID-19 and was closely related to patient mortality and prognosis.6, 7, 9 Troponin is the most commonly used marker of myocardial injury and was used to assess the status of myocardial injury in COVID-19 patients. Many articles confirmed that elevated troponin was associated with worse patient prognosis.6, 7, 8, 9, 13, 14 However, myoglobin is another important biomarker for myocardial injury, few articles elucidated the relationship between myoglobin and prognosis in patients with COVID-19. Zhong found that patients with a length of hospital stay fewer than 14 days had lower myoglobin values than patients with a hospital stay longer than 14 days.15 Seraji performed a meta-analysis and found that elevated myoglobin was strongly associated with patient mortality (WMD = 159.77 ng/mL, 95% CI = 99.54–220.01, p < 0.001).16 No additional studies examined the relationship between myoglobin and prognosis. Our results show that myoglobin was also an independent predictor of patient mortality, which stand for not only myocardial injury but systemic muscle injury. The predictive value of myoglobin may be superior to troponin because myoglobin tends to be more sensitive to systemic muscle and organ damage than troponin, such as heart, kidney, brain, and skeletal muscle.17, 18, 19 The prognosis of COVID-19 patients was strongly associated with systemic damage, because first, 2019-nCoV damage to alveolar cells and the reduced oxygenation function of the lung due to bind to alveolar surface ACE2 receptors.20, 21 However, ACE2 receptors are also expressed in other organs, such as the heart, kidney brain and blood vessels.22, 23, 24, 25 Therefore, COVID-19 patients tend to have multisystem and multiple organ injury. Second, the multiple organs, multisystem injury is likely due to the virus-induced inflammatory storm response, and previous studies confirmed that cytokine storms play a critical role in the pathogenesis of SARS-CoV and MERS-CoV.26, 27 Some studies of COVID-19 patients found that endothelial cell infection was present in many organs, including the cardiovascular system, kidneys and the brain, which results in myoglobin increase. Finally, viral infection causes the patient's oxygen saturation to decrease.28 Whereas hypoxemia causes multi-organ hypoxia, prolonged hypoxia causes nonspecific damage to multiple organs. This nonspecific damage also causes an increase in myoglobin. The greater the degree of hypoxia, the higher the myoglobin.
The Cox model suggests that myoglobin/troponin should be measured in patients with severe/critical COVID-19 as soon as possible. However, the patient's risk should be assessed in conjunction with the patient's myoglobin, troponin, age, creatinine, CRP, and D-dimer. Patients at higher risk should be monitored earlier, and more aggressive therapy should be used to interrupt the inflammatory storm. If necessary, earlier invasive ventilation to relieve hypoxia should be initiated.
The American College of Cardiology (ACC) recently published a bulletin on the cardiac impact of COVID-19.29 It is recommended that COVID-19 patients with concomitant cardiovascular disease should be treated aggressively. Life support therapy, such as extracorporeal membrane pulmonary oxygenation, should also be given as soon as signs of cardiac pump failure appear. However, there is no particularly good approach for the treatment of systemic muscle damage. We are convinced that aggressive treatment should first be directed at the 2019-nCoV infection to control the progression of the pneumonia-dominated disease. If the patient also has comorbid cardiovascular/renal/brain/systemic muscle disease, it should also be aggressively managed and treated.
Limitations
This study has the following limitations. First, this study was a single-center, retrospective study with a small sample size and a short observation period. Second, because Leishenshan Hospital is a new hospital, this study did not include cardiac ultrasound, electrocardiogram, or other related examinations. Third, this study did not include myoglobin/troponin from the patients other than the first time after admission because these factors were only tested on admission for most patients.
Conclusions
Myoglobin and troponin are predictors of mortality in severe/critical COVID-19 patients and the probability of conversion to critical illness. Myoglobin may be superior to troponin for predictive value.
Conflicts of interest
None declared.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.medcli.2021.01.013
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.Camm A.J., Turpie A.G.G., Hess S., Amarenco P., Lambelet M., Haas S., et al. Outcomes after catheter ablation and cardioversion in patients with non-valvular atrial fibrillation: results from the prospective, observational XANTUS study. Europace. 2018;20:e87–e95. doi: 10.1093/europace/eux127. [DOI] [PubMed] [Google Scholar]
- 2.Paules C.I., Marston H.D., Fauci A.S. Coronavirus infections – more than just the common cold. JAMA. 2020;3238 doi: 10.1001/jama.2020.0757. [DOI] [PubMed] [Google Scholar]
- 3.Hui D.S., Azhar E.I., Madani T.A., Ntoumi F., Kock R., Dar O., et al. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health – the latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis. 2020;91:264–266. doi: 10.1016/j.ijid.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 5.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo T., Fan Y., Chen M., Wu X., Zhang L., He T., et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:811–818. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi S., Qin M., Shen B., Cai Y., Liu T., Yang F., et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi S., Qin M., Cai Y., Liu T., Shen B., Yang F., et al. Characteristics and clinical significance of myocardial injury in patients with severe coronavirus disease 2019. Eur Heart J. 2020;41:2070–2079. doi: 10.1093/eurheartj/ehaa408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang J., Kaplan N., Wysocki J., Yang W., Lu K., Peng H., et al. The ACE2-deficient mouse: a model for a cytokine storm-driven inflammation. FASEB J. 2020;34 doi: 10.1096/fj.202001020R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Varghese G.M., John R., Manesh A., Karthik R., Abraham O.C. Clinical management of COVID-19. Indian J Med Res. 2020;151:401–410. doi: 10.4103/ijmr.IJMR_957_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization. Clinical management of severe acute respirator infection is suspected: interim guidance. https://apps.who.int/iris/handle/10665/331446.
- 13.Han H., Xie L., Liu R., Yang J., Liu F., Wu K., et al. Analysis of heart injury laboratory parameters in 273 COVID-19 patients in one hospital in Wuhan, China. J Med Virol. 2020;92:819–823. doi: 10.1002/jmv.25809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sandoval Y., Januzzi J.L., Jr., Jaffe A.S. Cardiac troponin for the diagnosis and risk-stratification of myocardial injury in COVID-19: JACC review topic of the week. J Am Coll Cardiol. 2020;76:1244–1258. doi: 10.1016/j.jacc.2020.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yiqun W., Bingbo H., Jielan L., Yingying C., Ping Z. Risk factors associated with long-term hospitalization in patients with COVID-19: a single-centered retrospective study. Front Med (Lausanne) 2020;7:315. doi: 10.3389/fmed.2020.00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parohan M., Yaghoubi, Seraji A. Cardiac injury is associated with severe outcome and death in patients with coronavirus disease 2019 (COVID-19) infection: a systematic review and meta-analysis of observational studies. Eur Heart J Acute Cardiovasc Care. 2020;9:665–677. doi: 10.1177/2048872620937165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mielgo-Ayuso J., Calleja-González J., Refoyo I., León-Guereño P., Cordova A., Del Coso J.J.N. Exercise-induced muscle damage and cardiac stress during a marathon could be associated with dietary intake during the week before the race. Nutrients. 2020;12:316. doi: 10.3390/nu12020316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okamoto K., Kubota K., Kawada E., Kurabayashi H., Shirakura T. Cerebral infarction and high serum levels of muscle-derived enzymes associated with abrupt increase in hematocrit in a patient with secondary erythrocytosis. Nihon Ronen Igakkai Zasshi. 1991;28:693–696. doi: 10.3143/geriatrics.28.693. [DOI] [PubMed] [Google Scholar]
- 19.Ferraro S., Boracchi P., Santagostino M., Marano G., Vendramin C., Rossi L., et al. Ultra-sensitive troponin I levels to exclude acute myocardial infarction from myocardial injury. Clin Chem Lab Med. 2011;50:159–166. doi: 10.1515/CCLM.2011.746. [DOI] [PubMed] [Google Scholar]
- 20.Wu L., Yao Y.A.N., Zheng L., Zhang K., Zhang S.H.U. Long-term follow-up of pure linear ablation for persistent atrial fibrillation without circumferential pulmonary vein isolation. J Cardiovasc Electrophysiol. 2014;25:471–476. doi: 10.1111/jce.12360. [DOI] [PubMed] [Google Scholar]
- 21.Lukassen S., Chua R.L., Trefzer T., Kahn N.C., Schneider M.A., Muley T., et al. SARS-CoV-2 receptor ACE2 and TMPRSS2 are primarily expressed in bronchial transient secretory cells. EMBO J. 2020;39:e105114. doi: 10.15252/embj.20105114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shao M., Li X., Liu F., Tian T., Luo J., Yang Y. Acute kidney injury is associated with severe infection and fatality in patients with COVID-19: a systematic review and meta-analysis of 40 studies and 25,278 patients. Pharmacol Res. 2020;161:105–107. doi: 10.1016/j.phrs.2020.105107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He Q., Mok T., Yun L., He C., Li J., Pan J., et al. Single-cell RNA sequencing analysis of human kidney reveals the presence of ACE2 receptor: a potential pathway of COVID-19 infection. Mol Genet Genomic Med. 2020:e1442. doi: 10.1002/mgg3.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han X., Xia N., Chen Z., Pan C., Huang X.J. Inpatients with brain damage, impaired airways and severely restricted daily activities have an increased infection rate during the COVID-19 pandemic: a single-center retrospective analysis from Wuhan. Am J Phys Med Rehabil. 2020;99:884–886. doi: 10.1097/PHM.0000000000001535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.South A.M., Diz D.I., Chappell M.C. COVID-19 ACE2, and the cardiovascular consequences. Am J Physiol Heart Circ Physiol. 2020;318:H90–H1084. doi: 10.1152/ajpheart.00217.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong C.K., Lam C.W., Wu A.K., Ip W.K., Lee N.L., Chan I.H., et al. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol. 2004;136:95–103. doi: 10.1111/j.1365-2249.2004.02415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Channappanavar R., Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. 2017;39:529–539. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu K., Cai H., Shen Y., Ni Q., Chen Y., Hu S., et al. Management of corona virus disease-19 (COVID-19): the Zhejiang experience. Med Sci. 2020;49:147–157. doi: 10.3785/j.issn.1008-9292.2020.02.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Susan J. ACC clinical bulletin focuses on cardiac implications of coronavirus (COVID-19) [EB/OL]. https://cardiology2.com/acc-clinical-bulletin-focuses-on-cardiac-implications-of-coronavirus-covid-19/.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.