Abstract
Background
Awake prone positioning has been reported to improve oxygenation for patients with COVID-19 in retrospective and observational studies, but whether it improves patient-centred outcomes is unknown. We aimed to evaluate the efficacy of awake prone positioning to prevent intubation or death in patients with severe COVID-19 in a large-scale randomised trial.
Methods
In this prospective, a priori set up and defined, collaborative meta-trial of six randomised controlled open-label superiority trials, adults who required respiratory support with high-flow nasal cannula for acute hypoxaemic respiratory failure due to COVID-19 were randomly assigned to awake prone positioning or standard care. Hospitals from six countries were involved: Canada, France, Ireland, Mexico, USA, Spain. Patients or their care providers were not masked to allocated treatment. The primary composite outcome was treatment failure, defined as the proportion of patients intubated or dying within 28 days of enrolment. The six trials are registered with ClinicalTrials.gov, NCT04325906, NCT04347941, NCT04358939, NCT04395144, NCT04391140, and NCT04477655.
Findings
Between April 2, 2020 and Jan 26, 2021, 1126 patients were enrolled and randomly assigned to awake prone positioning (n=567) or standard care (n=559). 1121 patients (excluding five who withdrew from the study) were included in the intention-to-treat analysis. Treatment failure occurred in 223 (40%) of 564 patients assigned to awake prone positioning and in 257 (46%) of 557 patients assigned to standard care (relative risk 0·86 [95% CI 0·75−0·98]). The hazard ratio (HR) for intubation was 0·75 (0·62−0·91), and the HR for mortality was 0·87 (0·68−1·11) with awake prone positioning compared with standard care within 28 days of enrolment. The incidence of prespecified adverse events was low and similar in both groups.
Interpretation
Awake prone positioning of patients with hypoxaemic respiratory failure due to COVID-19 reduces the incidence of treatment failure and the need for intubation without any signal of harm. These results support routine awake prone positioning of patients with COVID-19 who require support with high-flow nasal cannula.
Funding
Open AI inc, Rice Foundation, Projet Hospitalier de Recherche Clinique Interrégional, Appel d'Offre 2020, Groupement Interrégional de Recherche Clinique et d'Innovation Grand Ouest, Association pour la Promotion à Tours de la Réanimation Médicale, Fond de dotation du CHRU de Tours, Fisher & Paykel Healthcare Ltd.
Introduction
Severe illness characterised by progressive hypoxaemic respiratory failure develops in a large number of patients with COVID-19, resulting in the need for invasive mechanical ventilation.1, 2, 3 In patients who are intubated and have moderate to severe acute respiratory distress syndrome, prone positioning is an effective intervention to improve oxygenation and reduce mortality.4, 5, 6, 7 Awake prone positioning has been associated with improved oxygenation in observational studies of non-intubated patients with acute respiratory distress syndrome8 and, more recently, in patients with severe COVID-19.9, 10, 11 Two small (n=30 and n=60) pilot trials studied the feasibility of awake prone positioning in non-intubated patients but did not have the power to show improvement in oxygenation, escalation of respiratory support, or mortality.12, 13 Despite the paucity of large scale randomised controlled evidence evaluating patient-centred outcomes, awake prone positioning generated great interest in the clinical and scientific communities, and it has been incorporated into clinical guidelines14 and expert consensus statements.15, 16 Awake prone positioning has been identified as a research priority by the Surviving Sepsis Research Committee.17
We aimed to determine whether awake prone positioning reduces the rate of treatment failure at 28 days, defined as either death or intubation, in patients with severe COVID-19 acute hypoxaemic respiratory failure who require respiratory support with high-flow nasal cannula. We prospectively designed a collaborative meta-trial, a novel multicentre trial design consisting of a prospective, a priori set up and defined, individual participant data meta-analysis of six randomised controlled open-label superiority trials.
Research in context.
Evidence before this study
Awake prone positioning has been associated with improved oxygenation in observational studies of non-intubated patients with acute respiratory distress syndrome and, more recently, in patients with severe COVID-19. Whether these improvements translate into a reduced need for intubation or reduced mortality remains unknown, and observational studies have shown conflicting results. Moreover, there is concern that awake prone positioning might prove harmful if transient improvement of oxygenation leads to false reassurance and delayed intubation.
We searched MEDLINE, EMBASE, and PubMed, Web of Science, Scopus, medRxiv, bioRxiv, and ClinicalTrials.gov for studies published from Jan 1, 2020, to April 26, 2021, for completed randomised controlled trials published in any language evaluating the effect of awake prone positioning to treat patients with COVID-19. The keywords (“prone position*” OR “pron*”) AND (“COVID-19” OR “SARS” OR “coronavirus”) AND (“awake” OR “non-intubated” OR “conscious”) were used to search the databases. We identified two small pilot trials (n=60 and n=30) that studied the feasibility of awake prone positioning comparing it with usual care among non-intubated patients with COVID-19. Neither of these studies reported significant differences in oxygenation improvement, escalation of respiratory support, or mortality. We did not identify any randomised controlled trial designed to determine whether awake prone positioning reduces the combined incidence of intubation or death in patients with severe COVID-19.
Added value of this study
In this prospectively designed, multicentre, international, randomised, open-label meta-trial, with a large sample size (1121 patients), we found that awake prone positioning reduced the incidence of treatment failure within 28 days of enrolment (the primary composite outcome of intubation or death) in patients with acute severe hypoxaemic respiratory failure due to COVID-19 supported with high-flow nasal cannula. Adverse effects were mild, infrequent, and occurred at similar rates between awake prone positioning and standard care groups.
Implications of all the available evidence
Awake prone positioning is a safe intervention that reduces the risk of treatment failure in hypoxaemic patients with COVID-19 who require advanced respiratory support with high-flow nasal cannula oxygen. Our findings support routine implementation of awake prone positioning in those patients.
Methods
Study design
On April 29, 2020, the lead investigators of five national randomised, controlled, open-label trials of awake prone positioning (NCT04325906, NCT04347941, NCT04358939, NCT04395144, NCT04391140) agreed to participate in this meta-trial. In each trial, awake prone positioning was compared with standard care in patients with acute hypoxaemic respiratory failure due to COVID-19 and undergoing high-flow nasal cannula support. A meta-trial protocol incorporating a collaborative prospective meta-analysis of individual patient data from each randomised controlled trial was agreed. Inclusion and exclusion criteria and the planned intervention were harmonised across all five trials. Investigators identified a common set of core data that could be extracted from each trial, primary and secondary outcomes of the meta-trial which were agreed a priori and recorded identically across trials, planned collaborative interim and final statistical analyses at the meta-trial level, and agreed to report the findings jointly as a unified group of investigators, before any reporting of individual trial results.18 A priori, the investigators also agreed that once the meta-trial result was known, provided it gave a clear answer, the individual studies still recruiting (due to slower recruitment given geographical and unpredictable variations in pandemic waves) would be terminated for loss of equipoise. A sixth group conducting a trial with a similar and compatible research design (NCT04477655) joined the consortium shortly thereafter (on Aug 26, 2020).19 In total, hospitals from six countries were involved: Canada, France, Ireland, Mexico, USA, Spain (appendix 1 pp 3–4). This innovative meta-trial approach combined the benefits of a prospective design, and the high power of a large multinational trial with the convenience of faster setup times of individual national trials, an important advantage during a pandemic.20 Its statistical underpinnings have been previously reported.21
Each individual national trial was approved by each participating centre's ethics committee. The meta-trial was supervised by a steering committee formed by principal investigators of each national trial, assisted by two independent advisors. The meta-trial protocol has been published18 and is available along with each individual trial protocol in appendix 2.
Patients
All adults (>18 years old) with acute hypoxaemic respiratory failure due to proven (or highly clinically suspected, pending microbiological confirmation) COVID-19 pneumonia were eligible for enrolment at participating hospitals. Acute hypoxaemic respiratory failure was defined as a requirement of respiratory support with high-flow nasal cannula and a ratio of peripheral arterial oxygen saturation (SpO2) to the fraction of inspired oxygen (FiO2) [SpO2:FiO2] of 315 or less (which is equivalent to a ratio of partial pressure of arterial oxygen [PaO2] to FiO2 [PaO2:FiO2] ≤300 mmHg).22 We excluded patients who were unable or refused to provide informed consent, were haemodynamically unstable, were severely obese with a body-mass index higher than 40 kg/m2, were pregnant, or had a contraindication to awake prone positioning (trial inclusion and exclusion criteria by country are presented in appendix 1 pp 5–6). Written informed consent was obtained for all patients according to national regulations.
Randomisation and masking
A statistician not involved in patient recruitment generated the allocation sequence for each individual trial. Patients were assigned to either the intervention (awake prone positioning group) or standard care (control group) using a 1:1 computer-generated variable block size sequence. Allocation concealment at randomisation was ensured by an online randomisation system or with on-site opaque sealed envelopes, depending on the trial (the research protocols for each trial are available in appendix 2). By the very nature of the intervention and design, trial participants, care providers, outcome assessors, and data analysts could not be blinded to the intervention.
Procedures
Patients in the awake prone positioning group were instructed and assisted to lie in the prone position for as long and as frequently as possible each day. The duration of each proning session was recorded by bedside nurses. High-flow nasal cannula was initiated at maximally tolerated flow setting, and the FiO2 was titrated to maintain SpO2 between 90% and 95%. The use of non-invasive ventilation was not included in the trial protocol but was recorded prospectively. Study endpoints at which awake prone positioning was ceased were weaning of high-flow nasal cannula (based on improved oxygenation defined in each individual trial; appendix 1 p 7), discharge from hospital, intubation, or death. Patients in the standard care group received standard care with high-flow nasal cannula. The use of awake prone positioning as a so-called rescue intervention was discouraged in the standard care group and recorded as a protocol violation. To harmonise triggers for intubation, initially slightly different across individual trials, predefined criteria for tracheal intubation were provided in both groups at the meta-trial level and disseminated across participating centres, including worsening respiratory failure (respiratory rate above 40 breaths per min, respiratory muscle fatigue, respiratory acidosis with a pH below 7·25, copious tracheal secretions, severe hypoxaemia with SpO2 below 90% despite an FiO2 of ≥0·8), haemodynamic instability, or deteriorating mental status.18 Among intubated patients, the subsequent management (including prone positioning) was left at the treating physician's discretion.
Outcomes
The primary outcome was treatment failure within 28 days of enrolment, defined as intubation or death. The reason we combined a non-fatal outcome (intubation) with death is that they are competing and causally related outcomes. Main secondary outcomes (all censored at 28 days after enrolment) were: intubation; mortality; use of non-invasive ventilation; length of hospital stay; time to high-flow nasal cannula weaning in patients with treatment success (defined as the patient being alive and not having required intubation within 28 days of enrolment); time to treatment failure; time to intubation; time to death; duration of invasive mechanical ventilation in intubated patients surviving to day 28; mortality in invasively mechanically ventilated patients; predefined safety outcomes as prospectively recorded by investigators; and physiological response to awake prone positioning, including the ratio of SpO2:FiO2 to respiratory rate, known as the ROX index.23
Statistical analysis
All analyses were done at the individual patient level. Treatment failure was analysed in an intention-to-treat population comprising all patients recruited across all six trials and a prespecified, strictly defined per-protocol population (appendix 1 p 20). Individual trial analysis was planned to be done secondarily, after analysis of the complete meta-trial population and will be published later.
Relative risks were estimated for the primary outcome and all binary outcomes, in a mixed effect log-binomial model with a random effect on the individual trial. All time to event outcomes were compared using survival analyses with a frailty term on the individual trials. The proportional hazards assumption was checked by a visual inspection of the Kaplan-Meier curves, using a graphical diagnostic based on the scaled Schoenfeld residuals. The primary outcome and mortality were analysed in a Cox proportional hazard model. Intubation was analysed with death as a competing event using a Fine and Gray with proportional hazards model, and weaning of high-flow nasal cannula was analysed with escalation to non-invasive ventilation or treatment failure as competing events. Mean difference was estimated for duration of hospital stay and was analysed in a mixed-effect linear regression. All estimates were reported with the two-sided corresponding 95% CI.
Interim analyses at the meta-trial level of aggregated data for the individual trials were planned, a priori, after each 200 patients were enrolled. Prespecified multiplicity-adjustment methods were used to control the overall one-sided type 1 error rate at 0·025. Based on previous reports,24, 25 we estimated the incidence of the primary outcome to be between 60% and 70% in the standard care group. The meta-trial was designed to show superiority of awake prone positioning over standard care with 90% power and a one-sided type 1 error rate of 0·025. For an asymmetric two-sided group sequential analysis with five interim analyses (including the last analysis), the sample size was 1000. We determined continuous stopping boundaries using the Kim-DeMets alpha-spending approach,26 with a Pocock superiority bound for efficacy and O'Brien-Fleming bound for futility.17 Both bounds were binding, in the sense that recruitment was to stop once they were crossed, and was designed to allow for the best chance to stop early in case of shown superiority (aggressive upper bound), therefore enabling all subsequent patients to benefit from the intervention, and to minimise the risk of premature interruption of the meta-trial without reaching good evidence of futility (conservative lower bound).
All outcomes were further analysed in subgroups determined a priori of severe (SpO2:FiO2 <190, equivalent to PaO2:FiO2 <150 mmHg22 at enrolment) versus less severe (SpO2:FiO2 ≥190, equivalent to PaO2:FiO2 ≥150 mmHg22 at enrolment) hypoxaemia. To test the difference of treatment effect between the two subgroups, we added an interaction term in the primary outcome model. Statistical heterogeneity between individual trials was assessed by calculating the I 2 statistic, using the DerSimonian and Laird method. Analyses were done with R, version 3.6.3.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
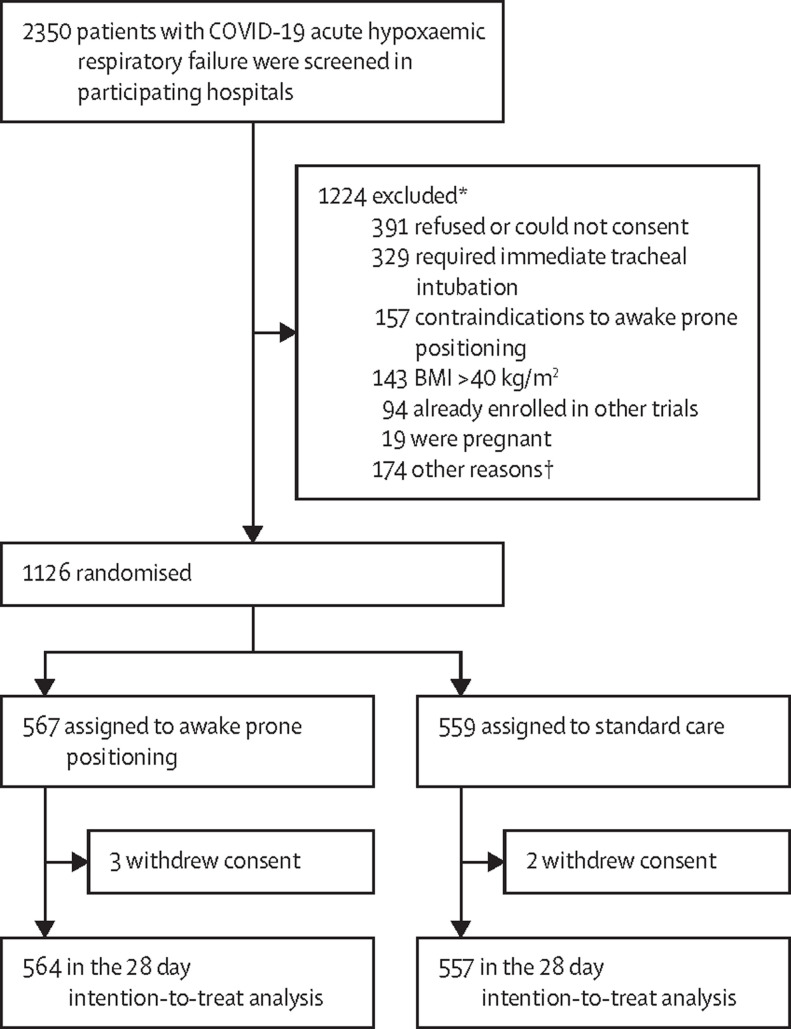
The first randomised controlled trial began screening patients on April 2, 2020, and enrolment for all trials was terminated on Jan 26, 2021, after the third interim analysis on 928 patients who had been followed-up for at least 28 days, showed that the predefined statistical criteria for efficacy were met (appendix 1 pp 8–9). A total of 2350 patients were assessed for eligibility, of whom 1126 underwent randomisation, five withdrew consent after randomisation, and 564 patients assigned to the awake prone positioning group and 557 to the standard care group were included in the intention-to-treat analysis of the primary outcome (figure 1 ). The median time from hospital admission to enrolment was 1·0 day (IQR 0·4 to 1·9) in the awake prone positioning group and 1·0 day (0·4 to 1·5) in the standard care group.
Figure 1.
Screening, enrolment, randomisation, and follow-up of trial participants
BMI=body-mass index. *Could have more than one reason. †Other trial specific reasons for exclusion were: initiation of awake prone positioning on treating physicians' orders before inclusion in the trial, physician decision not to include the patient, respiratory support with high-flow nasal cannula for more than 48 h before enrolment, no insurance coverage.
Most patients were recruited in Mexico (n=430, 38%), France (n=402, 36%), and the USA (n=222, 20%); patients were also recruited from Spain (n=30, 3%), Ireland (n=24, 2%), and Canada (n=13, 1%). Baseline demographic and disease characteristics were well balanced between the two groups of the meta-trial (table 1 ) and between the two groups of each individual randomised controlled trial (appendix 1 pp 10–15). In total 986 (88%) of 1121 patients received glucocorticoids. At enrolment, the mean SpO2:FiO2 was 147·9 (SD 43·9) for the awake prone positioning group versus 148·6 (43·1) for the standard care group with similar high-flow nasal cannula settings (median flow rate set at 50·0 L/min [IQR 40·0–55·0] versus 50·0 [40·0–50·0], and median FiO2 set at 0·6 [0·5–0·8] in both groups).
Table 1.
Characteristics of patients at enrolment
| Awake prone positioning group (n=564) | Standard care group (n=557) | ||
|---|---|---|---|
| Age, years | 61·5 (13·3) | 60·7 (14·0) | |
| Female sex | 184 (33%) | 191 (34%) | |
| Male sex | 380 (67%) | 366 (66%) | |
| Body-mass index, kg/m2 | 29·7 (4·6) | 29·7 (4·6) | |
| Clinical parameters at enrolment | |||
| Respiratory rate, breaths/min | 24·7 (5·1) | 24·9 (5·6) | |
| Mean arterial pressure, mmHg | 88·2 (12·1) | 87·4 (11·4) | |
| SpO2:FiO2 | 147·9 (43·9) | 148·6 (43·1) | |
| Recruitment of individual trials | |||
| Mexico | 216 (38%) | 214 (38%) | |
| France | 200 (35%) | 202 (36%) | |
| USA | 112 (20%) | 110 (20%) | |
| Spain | 17 (3%) | 13 (2%) | |
| Ireland | 12 (2%) | 12 (2%) | |
| Canada | 7 (1%) | 6 (1%) | |
| Coexisting illness | |||
| Chronic heart disease* | 120 (21%) | 127 (23%) | |
| Chronic lung disease† | 63 (11%) | 64 (12%) | |
| Chronic kidney disease‡ | 45 (8%) | 35 (6%) | |
| Severe liver disease§ | 8 (1%) | 6 (1%) | |
| Diabetes (type 1 and 2) | 176 (31%) | 173 (3%) | |
| Obesity¶ | 221 (39%) | 231 (42%) | |
| Active malignancy | 45 (8%) | 31 (6%) | |
| Confirmed COVID-19 | 557 (99%) | 552 (99%) | |
| Use of glucocorticoids for treatment of COVID-19 | 494 (88%) | 492 (88%) | |
| Do-not-intubate order | 44 (8%) | 44 (8%) | |
| Location at enrolment | |||
| Intensive care unit | 336 (60%) | 339 (61%) | |
| Intermediate care unit | 197 (35%) | 189 (34%) | |
| Emergency department | 5 (1%) | 5 (1%) | |
| General ward | 26 (5%) | 24 (4%) | |
Data are mean (SD), or n (%). SpO2=peripheral blood oxygen saturation. FiO2=fraction of inspired oxygen.
Heart failure or coronary artery disease or hypertension.
Obstructive or restrictive lung disease.
Estimated glomerular filtration rate <60 mL/min per 1·73 m2 before hospital admission.
Cirrhosis or portal hypertension with history of variceal bleeding, or liver disease with Child-Pugh score ≥10.
Data for obesity were missing for two patients.
In the intervention group, the median daily duration of awake prone positioning (recorded until day 14) was 5·0 h (IQR 1·6–8·8), with variations among individual trials, from a median daily awake prone positioning duration of 1·6 h in Spain to 8·6 h in Mexico (appendix 1 p 16).
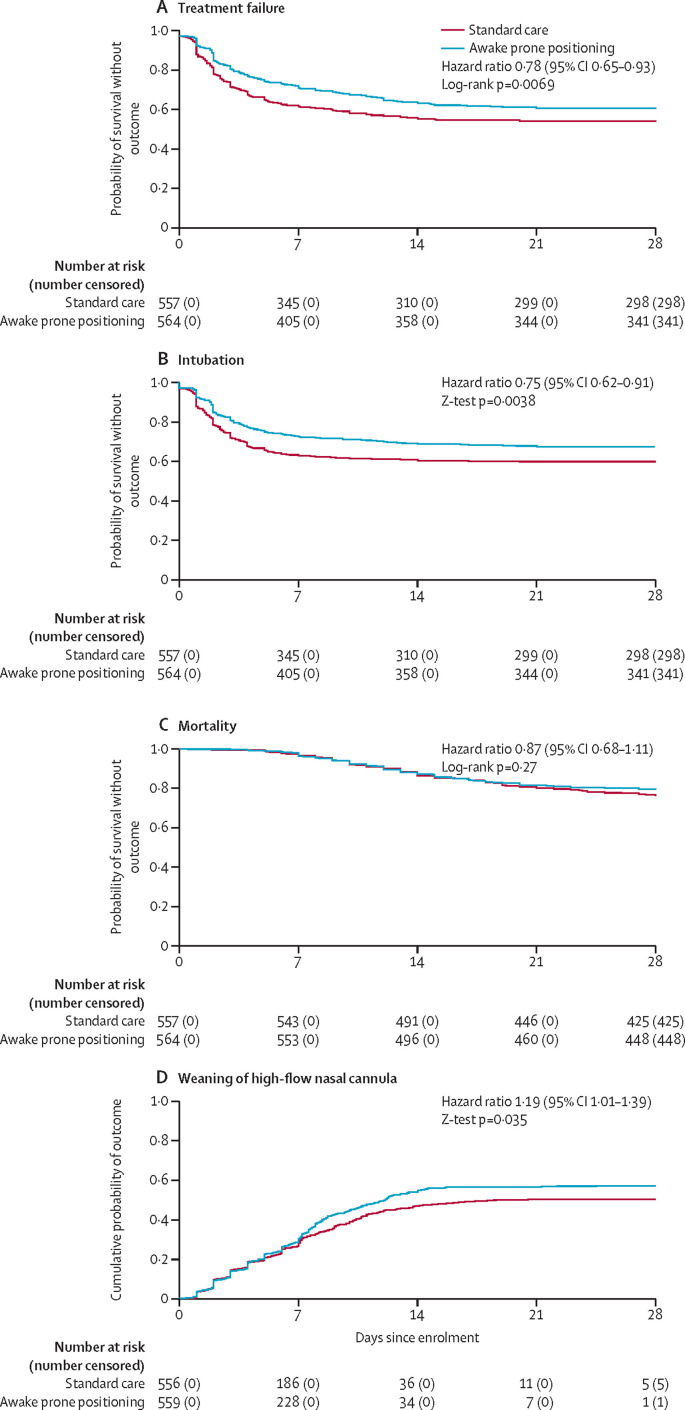
In the intention-to-treat population, the primary endpoint of treatment failure (intubation or death) within 28 days of enrolment occurred in 223 (40%) of 564 patients randomly assigned to awake prone positioning and in 257 (46%) of 557 patients randomly assigned to standard care (relative risk 0·86 [95% CI 0·75–0·98], p=0·02; figure 2A ; table 2 ). No statistical heterogeneity was detected between individual trials estimates (I 2=0%, 95% CI 0–69; appendix 1 p 17). The number needed to treat to avoid one treatment failure was 15 (95% CI 8–156).
Figure 2.
Kaplan-Meier probabilities estimates in the intention-to-treat population over 28 days after enrolment
(A) Probability of treatment failure (intubation or death). (B) Probability of intubation. (C) Probability of survival. (D) Probability of successful weaning of high-flow nasal cannula, with death, intubation, and non-invasive ventilation as competing events. The criteria for weaning were protocolised in each individual trial and are described in the appendix 1 p 7.
Table 2.
Primary and secondary outcomes
| Awake prone positioning group (n=564) | Standard care group (n=557) | RR (95% CI), HR (95% CI), or mean difference (95% CI) | ||
|---|---|---|---|---|
| Primary outcome | ||||
| Treatment failure at day 28 (intubation or death) | 223/564 (40%) | 257/557 (46%) | RR 0·86 (0·75 to 0·98) | |
| Secondary outcomes | ||||
| Intubation rate at day 28 | 185/564 (33%) | 223/557 (40%) | .. | |
| Mortality at day 28 | ||||
| All patients | 117/564 (21%) | 132/557 (24%) | RR 0·87 (0·71 to 1·07) | |
| Invasively mechanically ventilated patients | 79/185 (43%) | 98/223 (44%) | .. | |
| Time to event analysis, median days* | ||||
| Treatment failure (intubation or death) | 2·0 (1·0 to 4·3) | 2·0 (1·0 to 3·8) | HR 0·78 (0·65 to 0·93) | |
| Intubation | 2·3 (1·3 to 5·0) | 2·0 (1·0 to 3·8) | HR 0·75 (0·62 to 0·91) | |
| Death | 12·0 (9·0 to 17·0) | 14·0 (9·8 to 19·0) | HR 0·87 (0·68 to 1·11) | |
| Non-invasive ventilation, intubation or death | 3·0 (1·0 to 7·4) | 2·3 (1·0 to 5·0) | HR 0·79 (0·67 to 0·94) | |
| Weaning of high-flow nasal cannula | 6·9 (3·3 to 9·2) | 6·0 (3·0 to 9·8) | HR 1·19 (1·01 to 1·39) | |
| Mean duration, days | ||||
| Hospital length of stay | 16·4 (10·5) | 16·5 (9·7) | Mean difference −0·2 (−1·3 to 1·0) | |
| Mechanical ventilation among intubated patients who survived until day 28 | 12·4 (9·0) | 12·4 (8·4) | Mean difference 0·2 (−1·9 to 2·3) | |
| Safety outcomes | ||||
| Skin breakdown | 8 (1%) | 10 (2%) | .. | |
| Vomiting | 15 (3%) | 18 (3%) | .. | |
| Central or arterial line dislodgement | 26 (5%) | 17 (3%) | .. | |
| Cardiac arrest at any time† | 3 (1%) | 1 (0%) | .. | |
Data are n (%), mean (SD), or median (IQR). HR=hazard ratio. RR=relative risk. All outcomes were censored at 28 days.
The median time to event is reported for patients who experienced the reported event in each group, while the corresponding HRs are computed from the whole groups and reflect the difference in the incidence of those outcomes over time.
No cardiac arrest occurred in prone position, nor during manoeuvres to place patients prone or supine.
We did not measure a statistically significant interaction between the SpO2:FiO2 at enrolment and the intervention effect with regards to the primary outcome, within the limits of the trial not being powered for this purpose (p=0·62; appendix 1 p 19).
The cumulative incidence of intubation at day 28 was lower in the awake prone positioning group than in the standard care group (figure 2B, table 2). The number needed to treat to avoid one intubation was 14 (95% CI 8–69). The 28 day mortality was not different between the awake prone positioning group versus the standard care group (figure 2C, table 2). Among patients who were invasively mechanically ventilated, the 28 day mortality was similar between groups (table 2). Mean duration of invasive mechanical ventilation was also similar between groups among patients who were intubated and survived until day 28 (table 2). Non-invasive ventilation was used in 94 (17%) patients in the awake prone positioning group and 110 (20%) patients in the standard care group, in whom 77 (81%) of patients in the awake prone positioning group and 92 (84%) of patients in the standard care group were intubated or died within 28 days. Patients in the awake prone positioning group were more likely to be weaned from high-flow nasal cannula up to day 28 (figure 2D, table 2). The SpO2:FiO2, respiratory rate, and ROX index were all significantly improved during the first awake prone positioning session, which lasted a median of 3·0 h (IQR 1·2–4·0), and this improvement persisted after returning to the supine position (figure 3 ). Other secondary outcomes are reported in table 2.
Figure 3.
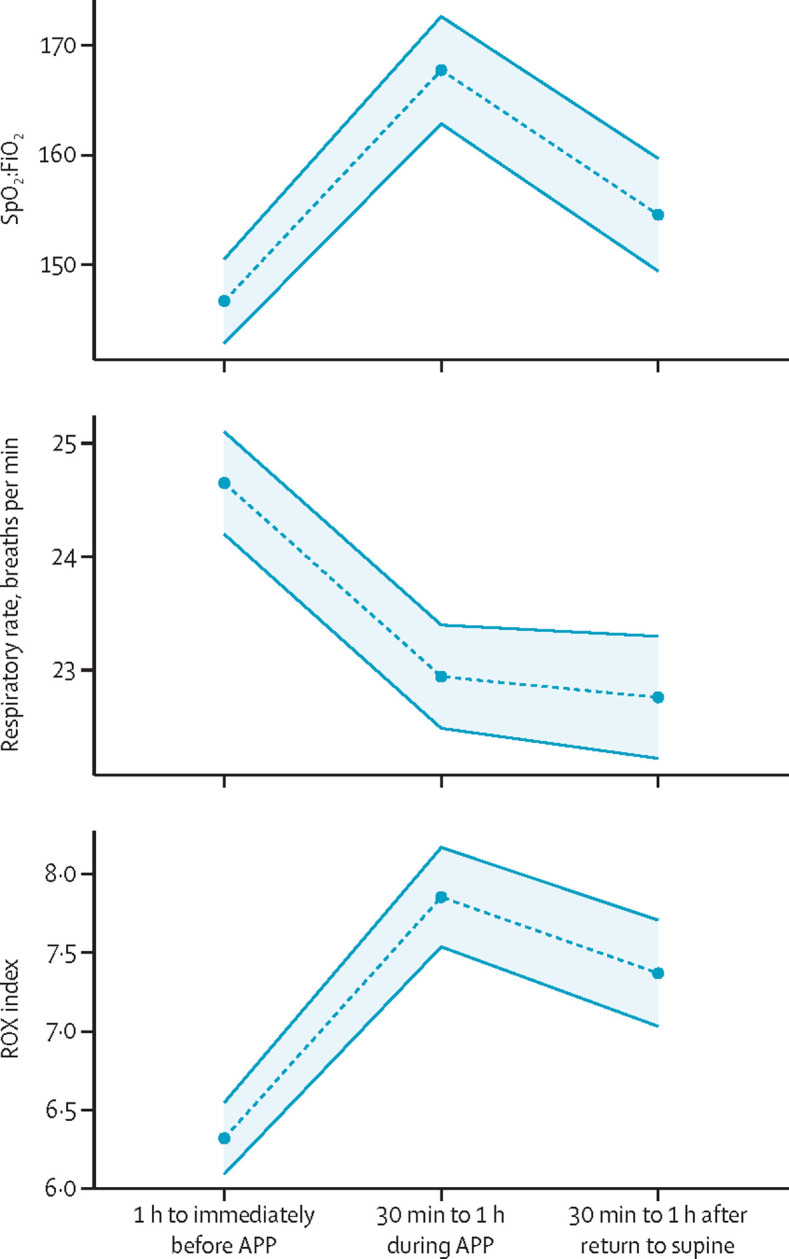
Physiological effects of awake prone positioning
Means are indicated by points, with standard deviation indicated by the shaded area. (A) Ratio of peripheral arterial oxygen saturation to the fraction of inspired oxygen (SpO2:FiO2). (B) Respiratory rate in breaths per minute. (C) The ROX index is equal to SpO2:FiO2 divided by the respiratory rate. Lower values indicate more severe respiratory compromise. Values were recorded 1 h to immediately before the first awake prone positioning session, during the session 30 min to 1 h after the patient was placed into prone position and 30 min to 1 h after the patient had returned into the supine position.
Longer mean daily duration of awake prone positioning was reported more frequently in patients that ultimately had treatment success at day 28 (figure 4 ). Treatment failure occurred in 25 (17%) of 151 patients who remained in awake prone positioning for at least 8 h daily on average while on high-flow nasal cannula, compared with 198 (48%) of 413 patients who remained in awake prone positioning less than 8 h daily on average while on high-flow nasal cannula.
Figure 4.
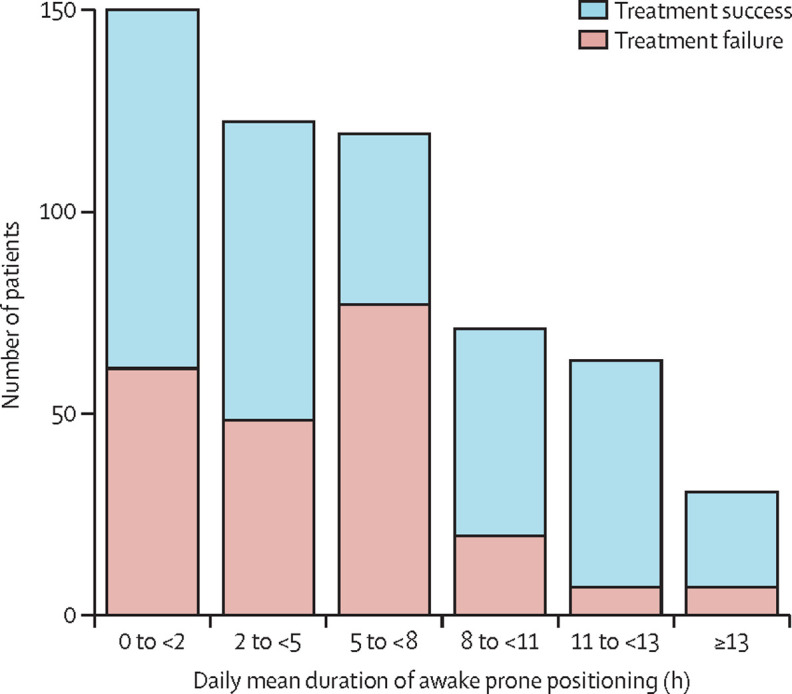
Daily mean duration of prone positioning and outcomes in patients allocated to awake prone positioning
Each bar represents the total number of patients having received a mean daily duration of awake prone positioning indicated on the horizontal axis in the population of patients with treatment success (patient was alive and did not require intubation after 28 days) and treatment failure (intubation or death by day 28).
64 (11%) of 557 patients of the standard care group received at least one episode of awake prone positioning. Intubation or death occurred in 36 (56%) of those patients within 28 days of enrolment. Among the 32 patients intubated after undergoing awake prone positioning in the standard care group, 19 (59%) patients died by day 28, and the median duration of invasive mechanical ventilation was 7·1 days (IQR 6·0–14·0) among survivors.
The incidence of prespecified adverse events, including skin breakdown, vomiting, and central or arterial line dislodgement, was low and similar in both groups (table 2). No patient had a cardiac arrest during awake prone positioning or in relation to proning. Additional results (patients' characteristics in individual trials, awake prone positioning durations, and per-protocol and subgroup analyses) are provided in appendix 1.
Discussion
In this multicentre, international, randomised, open-label meta-trial, awake prone positioning decreased the incidence of treatment failure (the primary composite outcome of intubation or death) in patients with acute severe hypoxaemic respiratory failure due to COVID-19 supported with high-flow nasal cannula. Adverse effects were mild, infrequent, and occurred at similar rates between the awake prone positioning and standard care groups.
At 28 days, the incidence of intubation was significantly reduced with awake prone positioning compared with standard care. 14 patients needed to be treated with awake prone positioning to avoid one intubation. Mortality and duration of invasive mechanical ventilation were similar between groups among intubated patients, suggesting no signal for harm from awake prone positioning. Beyond individual benefits, reduced intubation might relieve pressure on ventilator requirements and use of intensive care unit resources, whereas the hospital length of stay was not affected by awake prone positioning. Several physiological mechanisms might underpin those favourable clinical outcomes. As observed during invasive mechanical ventilation, prone positioning might induce a more homogenous distribution of pleural pressure throughout lung regions, resulting in reduced regional lung stress and strain.8, 9 Awake prone positioning improves oxygenation, probably through reducing ventilation to perfusion mismatch and alveolar shunt. Furthermore, the reduced respiratory rate also observed during awake prone positioning might be indicative of reduced respiratory drive and might result in reduced transpulmonary pressure swings leading to reduced patient self-inflicted lung injury.27, 28 Physiological studies are required to investigate those potential mechanisms.
In contrast to proning of patients who are intubated and sedated or even paralysed, effective awake prone positioning implementation requires patients' cooperation. Important variations in the duration of awake prone positioning reflecting individual characteristics such as age, body stature, tolerance, health-care team support, and availability of escalation options existed between patients and studies. The effect size of awake prone positioning on the primary outcome was greatest in the trial from Mexico, which also had the longest mean daily duration of awake prone positioning (appendix 1 p 16). Longer awake prone positioning sessions were associated with greater treatment success. This study was not designed to evaluate the effect of awake prone positioning duration, and patients' baseline severity and the response to awake prone positioning might influence commitment to the awake prone positioning procedure, thus awake prone positioning duration data are to be considered primarily as hypothesis generating. Future studies are needed to explore the dose-response effect. Given that longer durations of awake prone positioning were associated with a lower risk of treatment failure, patients should be encouraged to remain prone for as long as they can tolerate. Further trials could investigate modifiable factors to promote awake prone positioning.
The major strengths of this meta-trial are the large sample size and international scope, which allows generalisation to a variety of clinical settings. In addition, the meta-trial implemented a harmonised research protocol to include a well-defined population of patients suffering from severe COVID-19 induced acute hypoxaemic respiratory failure, all undergoing high-flow nasal cannula with the majority of patients receiving glucocorticoid therapy.
The meta-trial concept, prospectively defined by the investigators of this project18, 20 presents advantages in the pandemic setting, beyond faster setup and lower cost compared with a centralised international trial. Because of the cumulative sample size of several trials, it provides adequate power for most effect sizes that are difficult to estimate early in a pandemic. The cumulative sample size also enables a coordinated prospective interim analysis plan to be set up (appendix 1 pp 8–9). This represents the key feature of the meta-trial concept and leads to reducing the time to reach a conclusion compared with individually conducted trials. In contrast to alternative designs, such as platform trials,29 the meta-trial enables the equal and concurrent enrolment of the control group, exactly as in a conventional randomised controlled trial. The meta-trial concept has recently been adopted by other groups of investigators30 while others took similar approaches under the multiplatform trial denomination.31
The present work has several limitations. First, the very nature of the intervention precluded blinding, and we cannot exclude that at least part of the effect of awake prone positioning was mediated by influencing the decision-making of treating physicians. Despite the provision of clear criteria for intubation,18 clinicians could have refrained from intubation on the basis of transient improvements of respiratory parameters during awake prone positioning or conversely have a lower intubation threshold for patients in the standard care group. However, that awake prone positioning was not associated either with longer duration of mechanical ventilation or with higher mortality in intubated patients would suggest that the physicians were influenced in the right direction, correctly identifying patients who did not require intubation. Similarly, a bias towards excessive intubation in the control group is unlikely. Overall, these considerations should not distract from the pragmatic finding that awake prone positioning reduced intubation, regardless of the underlying mechanism of this effect. Second, in the standard care group, one patient out of ten underwent awake prone positioning. These protocol violations could have led to an underestimation of the efficacy of awake prone positioning in the intention-to-treat population. Last, the meta-trial design has some disadvantages compared with a multisite trial following a common protocol at all sites, such as slightly different inclusion criteria between trials or the complexity of tracking the global inclusion rate across trials in real-time, which could contribute to overshoot planned interim analysis or trial sample size in the case of efficient recruitment as observed in the present trial. These limits are outweighed by the benefit of setting up very quickly an international randomised study generating high-level evidence in a short period of time.
In conclusion, in this meta-trial of patients with acute hypoxaemic respiratory failure due to COVID-19 treated with high-flow nasal cannula, awake prone positioning appeared safe and had a favourable effect on the primary composite outcome of intubation or death within 28 days of enrolment.
Data sharing
The research protocols for the meta-trial and each individual trial are available in the appendix 2. De-identified data will be available from 9 months to 36 months after article publication to researchers who provide a methodologically sound and ethically approved proposal, for any purpose of analysis.
Declaration of interests
SE discloses consultancies from Aerogen Ltd, research support from Aerogen Ltd, Fisher & Paykel Healthcare Ltd, Hamilton medical, travel reimbursements from Aerogen Ltd and Fisher & Paykel Healthcare Ltd. JL discloses research funding from Fisher & Paykel Healthcare Ltd, Aerogen Ltd, and Rice Foundation, and speaker fees from AARC and Fisher & Paykel Healthcare Ltd. IP discloses a research grant and speaker fees from Fisher & Paykel Healthcare Ltd. YP discloses research support from Fisher & Paykel Healthcare Ltd. OR discloses a research grant from Hamilton Medical and speaker fees from Hamilton Medical, Ambu and Aerogen Ltd, and non-financial research support from Timpel and Masimo Corporation. His institution received fees for consultancy from Hamilton Medical. DV discloses research funding from Teleflex Medical, Inc and Rice Foundation, and speaker fees from Theravance Biopharma. MWT discloses consulting fees from Fisher and Paykel. JRM discloses research support from Fisher & Paykel, and speaker fees from Fisher & Paykel, Gilead, Dextro, and Linet. JGL discloses consulting fees from Baxter Healthcare and Glaxosmithkline. All other authors have no competing interests to disclose.
Acknowledgments
Acknowledgments
This meta-trial was funded by Open AI inc, Rice Foundation, Projet Hospitalier de Recherche Clinique Interrégional, Appel d'Offre 2020, Groupement Interrégional de Recherche Clinique et d'Innovation Grand Ouest, Association Pour la Promotion à Tours de la Réanimation Médicale, Fond de Dotation du CHRU de Tours, and Fisher & Paykel Healthcare Ltd. The meta-trial was carried out with the support of the CRICS-TriggerSEP, a FCRIN endorsed research network, REVA network, and the Irish Critical Care Clinical Trials Network. We thank Richard Kallet (University of California, San Francisco at San Francisco General Hospital and Trauma Center, San Francisco, CA, USA) and Paolo Biselli (University Hospital, University of São Paulo, São Paulo, Brazil) for acting as an independent advisory board of the meta-trial steering committee. We thank the members of the Awake Prone Positioning Meta-Trial Group (see appendix 1 for a complete list) for their contributions in conducting the meta-trial, and the patients and relatives for their participation.
Contributors
SE, JL, and ET designed the meta-trial project. MIE, YP, SE, JL, DV, SM, BM, JGL, DC, IP, and OR designed and conducted the individual trials. All authors significantly contributed to the conduct of the meta-trial, attending monthly web meetings. ET conducted data analysis. IP, BM, JL, YP, SE, ET, MIE, and OR had full access to the data, verified the data, and drafted the manuscript. All authors vouch for the accuracy and completeness of data and for adherence to the protocol. All authors reviewed the manuscript for important intellectual content and approved the final manuscript. SE, JL, MIE, YP, IP, BM, and OR equally contributed to the overall project described in this article. SE and JL were responsible for the decision to submit the manuscript.
Contributor Information
Awake Prone Positioning Meta-Trial Group:
Jie Li, Sara Mirza, David Vines, Ahmad A Elshafei, Brady J Scott, Tyler Weiss, Ramandeep Kaur, Lauren J Harnois, Amanda Miller, Flor Cerda, Andrew Klein, Jacob R Burd, Kathleen Posa-Kearney, Matthew Trump, Julie Jackson, Trevor Oetting, Mark Greenwood, Lindsay Hazel, Lisa Kingery, Idrees Mogri, Lindsey Morris, Joon Yong Moon, Julianne Garnett, Shijing Jia, Kristine Nelson, Bairbre McNicholas, David Cosgrave, Camilla Giacomini, John Laffey, Aoife Brennan, Conor Judge, Maeve Kernan, Claire Kelly, Ritika Ranjan, Siobhan Casey, Kevin O'Connell, Evelyn Newell, David Gallagher, Alistair Nichol, Ger Curley, Miguel Ibarra Estrada, Roxana García-Salcido, Alexandra Vargas-Obieta, Guadalupe Aguirre-Avalos, Sara A Aguirre-Díaz, Luz Alcántar-Vallín, Montserrat Alvarado-Padilla, Quetzalcóatl Chávez-Peña, José A López-Pulgarín, Julio C Mijangos-Méndez, Miguel Marín-Rosales, Jorge E García-Alvarado, Oscar G Baltazar-González, Maura C González-Guerrero, Paola G Gutiérrez Ramírez, Ivan Pavlov, Sean Gilman, Patrice Plamondon, Rachel Roy, Dev Jayaraman, Jason Shahin, Raham Ragoshai, Aasmine Kaur, Josie Campisi, Joseph Dahine, Stefanie Perron, Slimane Achouri, Ronald Racette, Anne Kulenkamp, Oriol Roca, Andrés Pacheco, Marina García-de-Acilu, Joan R Masclans, Irene Dot, Yonatan Perez, Laetitia Bodet-Contentin, Denis Garot, Stephan Ehrmann, Emmanuelle Mercier, Charlotte Salmon Gandonnière, Marlène Morisseau, Youenn Jouan, Walid Darwiche, Annick Legras, Antoine Guillon, Elsa Tavernier, Pierre-François Dequin, Anne-Charlotte Tellier, Jean Reignier, Jean-Baptiste Lascarrou, Amélie Seguin, Luc Desmedt, Emmanuel Canet, Christophe Guitton, Rémy Marnai, Jean-Christophe Callahan, Mickaël Landais, Nicolas Chudeau, Cédric Darreau, Patrice Tirot, Marjorie Saint Martin, Charlene Le Moal, Mai-Anh Nay, Grégoire Muller, Sophie Jacquier, Gwenaël Prat, Pierre Bailly, Nicola Ferrière, Arnaud W Thille, Jean-Pierre Frat, Jean Dellamonica, Clément Saccheri, Matthieu Buscot, Gaëtan Plantefève, Damien Contou, Damien Roux, Jean-Damien Ricard, Laura Federici, Noémie Zucman, Santiago Freita Ramos, Marc Amouretti, Sébastien Besset, Coralie Gernez, Agathe Delbove, Guillaume Voiriot, Alexandre Elabbadi, Muriel Fartoukh, Saad Nseir, Sébastien Préau, Raphaël Favory, Alexandre Pierre, Arnaud Sement, Nicolas Terzi, Florian Sigaud, Clara Candille, Emanuele Turbil, Julien Maizel, Clément Brault, Yoan Zerbib, Aurélie Joret, Cédric Daubin, Laurent Lefebvre, Alais Giraud, Adrien Auvet, Christophe Vinsonneau, Mehdi Marzouk, Jean-Pierre Quenot, Pascal Andreu, Marie Labruyère, Jean-Baptiste Roudaut, François Aptel, Alexandre Boyer, Philippe Boyer, Jean-Claude Lacherade, Hugo Hille, Marie Bouteloup, Matthieu Jeannot, Marc Feller, Guillaume Grillet, Bruno Levy, and Antoine Kimmoun
Supplementary Materials
References
- 1.Intensive care national audit and research centre ICNARC report on COVID-19 in critical care: England, Wales and Northern Ireland. 2021. https://www.icnarc.org/DataServices/Attachments/Download/2d288f8e-728e-eb11-912f-00505601089b
- 2.Berlin DA, Gulick RM, Martinez FJ. Severe COVID-19. N Engl J Med. 2020;383:2451–2460. doi: 10.1056/NEJMcp2009575. [DOI] [PubMed] [Google Scholar]
- 3.COVID-ICU Group on behalf of the REVA Network and the COVID-ICU Investigators Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: a prospective cohort study. Intensive Care Med. 2021;47:60–73. doi: 10.1007/s00134-020-06294-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guérin C, Reignier J, Richard J-C, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368:2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 5.Scholten EL, Beitler JR, Prisk GK, et al. Treatment of ARDS with prone positioning. Chest. 2017;151:215–224. doi: 10.1016/j.chest.2016.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kallet RH. A comprehensive review of prone position in ARDS. Respir Care. 2015;60:1660–1687. doi: 10.4187/respcare.04271. [DOI] [PubMed] [Google Scholar]
- 7.Guérin C, Albert RK, Beitler J, et al. Prone position in ARDS patients: why, when, how and for whom. Intensive Care Med. 2020;46:2385–2396. doi: 10.1007/s00134-020-06306-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scaravilli V, Grasselli G, Castagna L, et al. Prone positioning improves oxygenation in spontaneously breathing nonintubated patients with hypoxemic acute respiratory failure: a retrospective study. J Crit Care. 2015;30:1390–1394. doi: 10.1016/j.jcrc.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 9.Coppo A, Bellani G, Winterton D, et al. Feasibility and physiological effects of prone positioning in non-intubated patients with acute respiratory failure due to COVID-19 (PRON-COVID): a prospective cohort study. Lancet Respir Med. 2020;8:765–774. doi: 10.1016/S2213-2600(20)30268-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prud'homme E, Trigui Y, Elharrar X, et al. Effect of prone positioning on the respiratory support of nonintubated patients with coronavirus disease 2019 and acute hypoxemic respiratory failure: a retrospective matching cohort study. Chest. 2021;160:85–88. doi: 10.1016/j.chest.2021.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pavlov I, He H, McNicholas B, et al. Awake prone positioning in non-intubated patients with acute hypoxemic respiratory failure due to COVID-19: a systematic review of proportional outcomes comparing observational studies with and without awake prone positioning in the setting of COVID-19. Respir Care. 2021 doi: 10.4187/respcare.09191. published online July 7. [DOI] [Google Scholar]
- 12.Johnson SA, Horton DJ, Fuller MJ, et al. Patient-directed prone positioning in awake patients with COVID-19 requiring hospitalization (PAPR) Ann Am Thorac Soc. 2021;18:1424–1426. doi: 10.1513/AnnalsATS.202011-1466RL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jayakumar D, Ramachandran P, Rabindrarajan E, et al. Standard care versus awake prone position in adult non-intubated patients with acute hypoxemic respiratory failure secondary to COVID-19 infection – a multicentre feasibility randomized controlled trial. J Intensive Care Med. 2021;36:918–924. doi: 10.1177/08850666211014480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chalmers JD, Crichton ML, Goeminne PC, et al. Management of hospitalised adults with coronavirus disease-19 (COVID-19): a European Respiratory Society living guideline. Eur Respir J. 2021;57 doi: 10.1183/13993003.00048-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nasa P, Azoulay E, Khanna AK, et al. Expert consensus statements for the management of COVID-19-related acute respiratory failure using a Delphi method. Crit Care. 2021;25:106. doi: 10.1186/s13054-021-03491-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neto AS, Checkley W, Sivakorn C, et al. Pragmatic recommendations for the management of acute respiratory failure and mechanical ventilation in patients with COVID-19 in low- and middle-income countries. Am J Trop Med Hyg. 2021;104:60–71. doi: 10.4269/ajtmh.20-0796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coopersmith CM, Antonelli M, Bauer SR, et al. The surviving sepsis campaign: research priorities for coronavirus disease 2019 in critical illness. Crit Care Med. 2021;49:598–622. doi: 10.1097/CCM.0000000000004895. [DOI] [PubMed] [Google Scholar]
- 18.Tavernier E, McNicholas B, Pavlov I, et al. Awake prone positioning of hypoxaemic patients with COVID-19: Protocol for a randomised controlled open-label superiority meta-trial. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2020-041520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ibarra-Estrada MÁ, Marín-Rosales M, García-Salcido R, et al. Prone positioning in non-intubated patients with COVID-19 associated acute respiratory failure, the PRO-CARF trial: a structured summary of a study protocol for a randomised controlled trial. Trials. 2020;21:940. doi: 10.1186/s13063-020-04882-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li J, Pavlov I, Laffey JG, et al. Meta-trial of awake prone positioning with nasal high flow therapy: Invitation to join a pandemic collaborative research effort. J Crit Care. 2020;60:140–142. doi: 10.1016/j.jcrc.2020.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tavernier E, Trinquart L, Giraudeau B. Finding alternatives to the dogma of power based sample size calculation: is a fixed sample size prospective meta-experiment a potential alternative? PLoS One. 2016;11 doi: 10.1371/journal.pone.0158604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rice TW, Wheeler AP, Bernard GR, et al. Comparison of the SpO2/FIO2 ratio and the PaO2/FIO2 ratio in patients with acute lung injury or ARDS. Chest. 2007;132:410–417. doi: 10.1378/chest.07-0617. [DOI] [PubMed] [Google Scholar]
- 23.Roca O, Caralt B, Messika J, et al. An index combining respiratory rate and oxygenation to predict outcome of nasal high-flow therapy. Am J Respir Crit Care Med. 2019;199:1368–1376. doi: 10.1164/rccm.201803-0589OC. [DOI] [PubMed] [Google Scholar]
- 24.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim K, Demets DL. Design and analysis of group sequential tests based on the type i error spending rate function. Biometrika. 1987;74:149–154. [Google Scholar]
- 27.Cruces P, Retamal J, Hurtado DE, et al. A physiological approach to understand the role of respiratory effort in the progression of lung injury in SARS-CoV-2 infection. Crit Care. 2020;24:494. doi: 10.1186/s13054-020-03197-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brochard L, Slutsky A, Pesenti A. Mechanical ventilation to minimize progression of lung injury in acute respiratory failure. Am J Respir Crit Care Med. 2017;195:438–442. doi: 10.1164/rccm.201605-1081CP. [DOI] [PubMed] [Google Scholar]
- 29.Dodd LE, Freidlin B, Korn E. Platform trials—beware the noncomparable control group. N Engl J Med. 2021;384:1572–1573. doi: 10.1056/NEJMc2102446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Haren FMP, Richardson A, Yoon H-J, et al. INHALEd nebulised unfractionated HEParin for the treatment of hospitalised patients with COVID-19 (INHALE-HEP): protocol and statistical analysis plan for an investigator-initiated international metatrial of randomised studies. Br J Clin Pharmacol. 2021;87:3075–3091. doi: 10.1111/bcp.14714. [DOI] [PubMed] [Google Scholar]
- 31.The REMAP-CAP, ACTIV-4a, ATTACC Investigators. Zarychanski R. Therapeutic anticoagulation with heparin in critically ill patients with Covid-19. N Engl J Med. 2021 doi: 10.1056/NEJMoa2103417. published online Aug 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The research protocols for the meta-trial and each individual trial are available in the appendix 2. De-identified data will be available from 9 months to 36 months after article publication to researchers who provide a methodologically sound and ethically approved proposal, for any purpose of analysis.