Abstract
In December 2019, Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), a novel variant of coronavirus has emerged from Wuhan in China and has created havoc impulses across the world with a larger number of fatalities. At the same time, studies are on roll to discover potent vaccine against it or repurposing of approved drugs which are widely adopted are under trial to eradicate the SARS-CoV-2 causing COVID-19 pandemic. Reports have also shown that there are asymptomatic carriers of COVID-19 disease who can transmit the disease to others too. However, the first line defense of the viral attack is body's strong and well-coordinated immune response producing excessive inflammatory innate reaction, thus impaired adaptive host immune defense which lead to death upon the malfunctioning. Considerable works are going on to establish the relation between immune parameters and viral replication that, might alter both the innate and adaptive immune system of COVID-19 patient by up riding a massive cytokines and chemokines secretion. This review mainly gives an account on how SARS-CoV-2 interacts with our immune system and how does our immune system responds to it, along with that drugs which are being used or can be used in fighting COVID-19 disease. The curative therapies as treatment for it have also been addressed in the perspective of adaptive immunity of the patients.
Keywords: SARS-CoV-2, Immune response, COVID-19 therapy
Abbreviations: ACE, angiotensin converting enzyme; Agn, angiotensin; AMs, alveolar macrophages; APCs, antigen presenting cells; ARDS, acute respiratory distress syndrome; ASCs, antigen secreting cells; AT2, alveolar type 2; BAL, bronchoalveolar lavage; CD, cluster of differentiation; cDCs, conventional dendritic cells; CCL, C-C motif chemokine ligand; CDHR3, cadherin related family member 3; CLpro, chymotrypsin-like protease; Covid-19, coronavirus disease, 2019; CTL, cytotoxic T lymphocyte; CTLA, cytotoxic T lymphocyte associated antigen; CXCL, C-X-C motif chemokine ligand; CXCR, C-X-C motif chemokine receptor; DMVs, double membrane vesicles; DPP4, dipeptidyl peptidase 4; dsDNA, double stranded DNA; dsRNA, double stranded RNA; E, envelope; FGF, fibroblast growth factor; G-CSF, granulocyte colony stimulating factor; GM-CSF, granulocyte-monocyte colony stimulating factor; GZMA, granzyme; HCoV, human coronavirus; H, hydrogen; HLA, human leucocyte antigen; ICIs, immune checkpoint inhibitors; IFN, interferon; Ig, immunoglobulin; IL, interleukin; IMs, inflammatory monocytes; IMMs, inflammatory monocyte-macrophages; IP-10, inducible protein 10; IRF, interferon regulatory factor; ISG, interferon stimulated gene; LAG3, lymphocyte activation gene 3; M, membrane; mABs, monoclonal antibodies; MBL, mannose binding lectin; MCP1, membrane cofactor protein 1; MERS, middle east respiratory syndrome; MHC, major histocompatibility complex; MIP, macrophage inflammatory protein; moDCs, monocytes-derived dendritic cells; MSCs, mesenchymal stem cells; MYD88, myeloid differentiation primary response 88; N, nucleocapsid; nABs, neutralizing antibodies; NGS, of next generation sequencing; NK cells, natural killer cells; NKG2A, NK group 2 member 2A; Nsps, non-structural proteins; ORFs, open reading frames; PAMPs, pathogen associated molecular patterns; PCR, polymerase chain reaction; PD1, programmed cell death protein 1; PD-L1, programmed cell death protein ligand 1; PDGF, platelet derived growth factor; PLpro, papain like protease; Pp, polypeptides; PRRs, pattern recognition receptors; RA, rheumatoid arthritis; RBD, receptor binding domain; RBM, receptor binding motif; RLRs, RIG-I like receptors; RTC, replication transcription complex; S, spike; SARS-CoV-2, severe acute respiratory syndrome-coronavirus-2; scRNAseq, single cell RNA sequencing; SLE, systemic lupus erythematosus; ssRNA, single stranded RNA; STAT, signal transducer and activator of transcription; TIGIT, T cell immunoreceptor with Ig and ITIM domains; TIM 3, T cell immunoglobulin and mucin domain 3; TLRs, Toll like receptors; TMPRSS2, transmembrane protease serine 2; TNF, tumor necrosis factor; VEGF A, vascular endothelial growth factor A
Graphical ABSTRACT
1. Introduction
Evidences from the history focuses a spotlight on the incidences where coronavirus was found to be the reason for the outbreak of disease and recently a new strain of coronavirus, named Severe Acute Respiratory Syndrome-Coronavirus-2 (SARS-CoV-2), has been reported from Wuhan city, China. The disease is called Coronavirus disease, 2019 (COVID-19), named by WHO on Feb 11, 2020, that has first emerged in December 2019. As per the data, among the three outbreaks Middle East respiratory syndrome CoV (MERS-CoV) was the fatal most with a mortality rate of 34.77% while SARS-CoV stands out to be 10.87% of fatality and the SARS-CoV-2 has been reported to be 2.08%, although it is increasing with time. Meanwhile the mortality rate in SARS-CoV-2 infection is lower than the previously reported two pandemics but its transmission rate is quite higher in comparison to the earlier ones [1]. WHO reported that in September 4, 2020, there are 26,171,112 confirmed COVID-19 cases and death of 865,154 patients recorded [2]. Samples from the lower respiratory tract upon sequencing have depicted a novel coronavirus known to infect humans. The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is reported to be highly contagious [3]. The prime transmission route of COVID-19 is droplets formation of aerosols including all other possible modes of direct contact. SARS-CoV-2 incubation period is approximately 5–14 days or 24 days in some cases as per the retrospective pandemic report identified [4]. Most of the patients suspected for COVID-19 positive require supportive care, isolation to avoid the transmission chances and stronger immune power for recovery. Though the actual mechanism is still unclear, few anti-viral drugs such as remdesivir, lopinavir, ritonavir are being used for treatment [5]. The SARS-CoV-2 infection in lungs with adverse symptoms namely acute respiratory distress syndrome (ARDS) leads to severe lung injury mediated by host immune system [6]. The SARS-CoV-2 positive lung biopsy reports revealed that the content bilateral diffuse damage of alveoli, proliferation of fibroblasts and activated circulating CD4+/CD8+ lymphocytes [7]. Due to the rapid SARS-CoV-2 transmission globally, much more investigations are needed for the development of effective immunotherapy. In view of this context, addressing of the immunological aspects of SARS-CoV-2 spreading COVID-19 has become a major focus.
2. SARS-CoV background
According to WHO, SARS-CoV-2 is from the beta lineage of the coronavirus family of group 2B with 70% genetic similarities with SARS-CoV [8]. There are four genera classification of family namely Alphacoronavirus, Betacoronavirus, Deltacoronavirus and Gammacoronavirus [9]. Cryo-electron tomography and cryo-electron microscopy gives an idea about the morphology of SARS-CoV. HCoV-229E and HCoV-OC43 were the two human coronavirus that were responsible for causing mild respiratory dysfunctions in humans prior to the rise of SARS-CoV infection in 2002, thereafter emergence of two new human coronavirus, HCoV-NL63 in 2004 and HCoV-HKU1 in 2005 has occurred, where HCoV-229E and HCoV-NL63 are found in bats [10]. Genome sequencing of SARS-CoV-2 revealed more or less 79% similarity with SARS-CoV and 50% similarity MERS-CoV. According to genome sequencing, the strain of virus infecting the bat species underwent a series of genetic mutations and recombinations which enabled them to infect the human hosts [11]. In the present scenario, the life risk situation that is prevailing all over the world because of SARS-CoV-2, raises a question forward regarding the origin of the virus. The most probable origin that has been brought to light is the zoonotic transfer of the virus from the illegally imported Malayan pangolins (Manis javanica) as genetic and evolutionary evidences suggest that the SARS-CoV harbored by these pangolins is 91.02% similar to the SARS-CoV-2 [12]. While angiotensin converting enzyme 2 (ACE2) host receptor sequence in bats (Rhinolophus sinicus), pangolins and human were taken under consideration, it revealed that the ACE2 sequence similarity between human and bats was 80.60% which is less similar than between human and pangolins i.e. 84.76%, indicating that pangolins can be the original host or intermediate host of SARS-CoV-2 and therefore can promote transmission of the virus [13]. However, the genetic analysis of SARS-CoV-2 shows greater than 80% similarity compared with SARS-CoV and also more or less 50% similarity compared with MERS-CoV, both of them have a common origin i.e. bat [11]. According to phylogenetic analysis suggests that COVID-19, seventh member of the family of beta-coronavirus, is classified as a member of the ortho-coronavirinae subfamily and can be counted within the clade of the subgenus sarbecovirus [14]. Relating to the previous epidemiological investigations we can figure out that the emergence of the new coronavirus may be of zoonotic origin, keeping in mind the food habits of the Chinese people [15]. According to the present literatures, it is assumed that bats and pangolins are the original source of human SARS-CoV-2 and from them it is transmitted to humans but the actual intermediate host and the nature of emergence is yet to be explored [[16], [17], [18], [19]].
Being RNA virus, SARS-COV-2 has a high mutation rate that may involve in increasing virulence and pathogenicity of the infection in patients. Mutations in the surface proteins could change the tropism of the virus and increase its adaptability in new host with greater pathogenicity. Accumulation of mutations in SARS-CoV-2 may result in higher potency of pathogenicity. According to studies, high levels of mutations have been found in NSP and S proteins (Table 1 ). Current scenarios of COVID cases with 61.8 M (million) cases and over 1.4 M deaths globally, reported by WHO on 1st December 2020, shows a gradual increase in the COVID cases [20]. High level of mutations in S proteins may indicate a second wave of COVID-19 with greater severity if essential steps are not taken.
Table 1.
Various mutations found in SARS-CoV-2.
| Mutations | Features | Outcomes | Ref. |
|---|---|---|---|
| SpikeD614G | A missense mutation in S protein encoding gene, where an amino acid (aa) change from aspartate to glycine at 614 position was found. With this mutation this strain contains 3 other mutations as follow:
|
D614G substitution was a rare mutation at the beginning of the COVID-19 spread before March 2020, found as predominant in Europe, but later it occurred about 74% in all published sequences in June 2020 and spread worldwide. This mutation enhances the viral replication in the upper respiratory tract and also has higher susceptible to neutralization by monoclonal antibodies. | [21,22] |
| NSP2 and NSP3 | SARS-CoV-2 contains a polar aa instead of nonpolar aa unlike bat SARS at position 321 and glycine is replaced by serine in NSP3 at position of 543. | This may affect the mechanisms involved in viral entry and replication and increases the contagiousness of the virus. | [23] |
| SARS-CoV-2 VUI 202012/01 | This new variant of SARS-CoV-2 has 29 aa substitution from the original Wuhan strain with a mutation N501Y which is located in the receptor binding region. | It is first reported in UK. According to till now revealed reports this new strain possesses a high transmissible rate than the original strain. | [24] |
| Mutations in Rdrp | A mutation was found in the RNA dependent RNA polymerase at the position of 14,408. | It might result in drug resistant viral phenotype. | [25] |
| Mutations in ORF region | According to the present studies there are mutations in ORF region as follow:
|
Better studies needed to understand the role of this mutation in virulence of the virus. | [26] |
| ∆382 variant | This variant has 382 nucleotide deletion in ORF8. | This variant, seen during the early epidemic in Wuhan, is mild infectious with lower concentration of proinflammatory cytokines. | [27] |
| SARS-CoV-2 AZ-ASU2923 | This variant has a deletion of 81 nucleotide in the ORF7a region found in Arizona. | Pathogenic consequences are yet to be studied. | [28] |
3. Host pathogen interactions
The first five reported cases of COVID-19 in December, 2019 were hospitalized with ARDS out of which one deceased. Among all human associated CoVs, four patients were having mild respiratory symptoms, while two among them, with the infection of SARS-CoV and MERS-CoV were having severe respiratory diseases, [29] which mainly had been transmitted from animals to humans via an intermediate mammalian host [30]. The results of next generation sequencing (NGS) or Real-time polymerase chain reaction (RT-PCR) of patient's sputum targeted for the envelope gene of CoV confirmed the positive infection for COVID-19 [31] and SARS-CoV-19 shares almost 80% genome similarity with SARS-CoV [4]. Patients with positive infection of SARS-CoV-2, an enveloped single stranded RNA (ssRNA) virus with positive-sense RNA, show clinical manifestations [32]. In a nutshell, the pathogenesis of COVID-19 can be categorized as systemic disorders that include fever, dry cough, headache, fatigue, high sputum production, acute cardiac injury, dysponea, lymphopenia, cytokine storm and respiratory disorders that include sore throat, sneezing, rhinorrhoea, severe pneumonia, ground-glass opacities, RNAaemia and ARDS. As per improvised current clinical symptoms, loss of smell and taste has become a new and confirmatory symptom for COVID-19 along with the others. A very recent study has shown that a higher expression of ACE2 and Type 2 transmembrane serine protease (TMPRSS2) on olfactory cells are highly affected by SARS-CoV-2 resulting in the impairment of olfactory cells [33].
SARS-CoV-2, sourcing from symptomatic along with asymptomatic patients, after infecting a healthy person, has an incubation time of 4–14 days (average 3–7 days). Respiratory droplets from affected individual infect the healthy people to transmit the disease whereas it could also be transmitted through fecal-oral route because viral nucleic acid has been detected in the faeces and urine of COVID-19 patients [34,35]. Along with the disease-causing comorbidities (cardiovascular, cerebrovascular, diabetes) and people of age more than 55 has shown more susceptibility to the COVID-19 infection and also the cancer patients under chemotherapy and surgery treatment are more susceptible to SARS-CoV-2 [34] [36,37]. On contrary, the patients who are receiving immunotherapy using immune checkpoint inhibitors (ICIs) like anti-cytotoxic T lymphocyte associated antigen (CTLA) 4 or anti-PD-1/PD-L1 are comparatively less prone to the COVID-19 disease [38].
4. Molecular mechanisms of COVID-19 as pathogen
Based on the published literatures and the observations of the COVID-19 patients, the entry of the virus occurs via nasal and larynx mucosal membranes and reaches to the lungs via respiratory tract. S (spike) protein imparts virulence by binding to the host cell ACE2 receptor followed by their entry through clathrin-mediated endocytosis [39]. Different strains of coronavirus can recognize different host cell receptors e.g. the receptor for SARS-CoV is ACE2 which affects the pneumatocytes (Type II) and ciliated bronchial epithelial cells [40,41], the receptor for HCoV-229E is aminopeptidase N or CD13, the receptors for MERS-CoV is DPP4 (dipeptidyl peptidase4) or CD26 [41]. Based on the genetic sequence analysis, difference lies between SARS-CoV-2 and SARS-CoV-1 and thus emerged as an absolute new betacorona virus of the novel coronavirus i.e. nCOVID-19. Overall structural analysis of S protein between the two SARS-CoVs showed similarity of approximately 50–53% for the RBM (receptor binding motif), around 75% for the receptor binding domain (RBD) along with 76 to 78% whole protein [42]. Assumption of using same receptors for binding comes from amino acid sequence analysis that revealed a high similarity in binding domain of ACE2 receptor in SARS-CoV [42,43]. In addition, ACE2 is an integral member of glycoprotein which is highly expressed in the lung, kidney, heart and epithelial cells as well as endothelial cells of small intestine. [44]. The main function of ACE2 is the degradation of angiotensin (Ang)-II into Ang 1–7 [44]. Pulmonary ACE2 maintains the balance between the circulating AngII /Ang1–7 levels. AngII, in response to hypoxia, induces pulmonary vasoconstriction and hunts lung injury in victims and thus pneumonia is prevented [45].
In ARDS, ACE causes disease prognosis by increasing AngII levels but ACE2 protects lungs from failure by degrading AngII. Experimental evidences show that mice model where ACE2 is knockdown, drastic symptoms of ARDS is more prominent than wildtype while overexpression seems to be protective [46]. An increase of CD14+HLA−DRlow inflammatory monocytes (IMs) and Ficolin-1+ monocyte-derived macrophages has been detected by single cell RNA sequencing (scRNAseq) of pulmonary tissues of COVID-19 patients. In addition, the activation of interferon (IFN) signaling and monocytes recruitment decreases the alveolar potency and aids ARDS progression [47]. S protein on SARS-CoV-2 binds with greater affinity to host ACE2 receptors in comparison to SARS-CoV-1 [48]. Apart from ACE2+ cells, another study has focused on TMPRSS2, a cellular protease, which is required by the virus for entering into the cell as it helps the S protein on the virus surface to bind to the host ACE2 receptor, specially to alveolar type-2 cells (AT2 cells) which express TMPRSS2 in large amounts [49,50]. Whereas, the affinity towards the cadherin related family member 3 (CDHR3) which serve as a receptor for a rhinovirus-C of ciliated epithelial cells in the upper airway, is still not clear [51,52]. COVID-19 infection results into the inflammation in the lung tissues due to less frequent exchange between oxygen and carbondioxide upon decrease in haemoglobin. This occurs due to the role of open reading frames (ORF1ab, ORF3a, ORF10), which breaks the 1-beta chain of haemoglobin into porphyrin where surface glycoproteins attach. This mechanism can be treated with drugs like chloroquine, favipiravir [53]. Transcriptomic study revealed that the genome of the virus is highly complicated and undergoes innumerable transcription events that in turn contribute to the production of unknown ORFs harboring mutations and undergoes recombination events. Rapid evolution of the virus, aids the virus to be drug resistant along with frequently altered host specificity, thereby contributing to the virulence of the virus [54].
5. COVID-19 affecting immune system
5.1. Antigen presentation
Whenever a pathogen enters into our body, it is recognized as antigen and presented through antigen presenting cells (APCs) via major histocompatibility complex (MHC) molecules present on their surface. The exact mechanism of presentation of coronavirus is not fully known. According to the researches on SARS-CoV, MHC I molecule mediates the antigen presentation of the virus [55] and sometimes MHC II also participates in the process [56]. Human leukocyte antigen (HLA) gene plays an important role in viral antigen presentation pathway and is also associated with viral susceptibility and disease severity. While HLA-DR of MHC II is involved in the antigen presentation to Th (T helper cells) whereas HLA-A, -B and –C of MHC I molecule present antigen to Tc (cytotoxic T cells) [57,58]. The polymorphism in HLA gene is associated with differential antigen presentation which leads to differential disease susceptibility in case of SARS-CoV-2 infection [59]. HLA genes present small pathogen derived peptides to T cells and the polymorphism in HLA influences the binding strength with pathogen peptides. According to the studies, the HLA allele frequencies of healthy ones did not match with the COVID-19 infected patients, clearly depicting a link between the HLA polymorphism and viral susceptibility. Evidences have shown that various HLA polymorphisms are related to strong susceptibility of SARS-CoV-2 infection whereas some are protective against the infection. According to various data, a variety of polymorphisms such as human HLA-DR, B1*1202, HLA-B*4601, HLA-B*0703a and HLA-Cw*0801 correlates to the infection susceptibility of SARS virus [56,60]. Especially, HLA-B*4601 has the lowest binding with peptides of SARS-CoV-2. Patients with this polymorphism are highly vulnerable to SARS-CoV-2 infection or have correlated with severe condition during COVID-19 infection [61]. On the other hand, HLA-B*1503 has the ability to present highly conserved SARS-CoV-2 peptides [61]. According to the study, HLA-Cw1502 and HLA-DR0301 may be involved in playing a protective role in SARS-CoV infection by facilitating the viral antigen presentation and thereby enhancing the functional activity of CD4+ T cells, CD8+ T cells and NK cells [62]. Polymorphisms like HLA-DRB1*11:01 and HLA-DQB1*02:0 in MHC II molecules elevate the risk for developing MERS-CoV infection. Moreover, mannose binding lectin (MBL) also presents SARS-CoV to the immune cells. According to the present literatures, other than HLA, alleles associated with low MBL production are a susceptible-factors for SARS infection and also those who are associated with greater production of CCL2 protein are more susceptible to SARS infection. The correlation of MBL and CCL2 with SARS-CoV-2 infection susceptibility is yet to be studied. [63].
5.2. Immune evasion
In SARS-CoV-2, pattern recognition receptors (PRRs) activate the innate immune responses via extracellular and endosomal Toll- like receptors (TLRs) in concert with cytosolic RIG-I like receptors (RLRs) [64]. Following the activation of PRRs, downstream signaling cascades stimulate the cytokine production like Type I/III IFNs as defense against virus, tumor necrosis factor alpha (TNF-α), interleukins (IL-1, IL-6, IL-18) and other proinflammatory cytokines [65]. The complex signaling pathways involving myeloid differentiation primary response 88 (MYD88) produce Type I IFNs and activate the transcription factor NF-κB which in turn induces the transcription and production of pro-inflammatory cytokines [66]. Type-I IFNs activate the downstream signal transducer and activator of transcription (STAT) proteins that catalyze generation of interferon stimulated genes (ISGs) coded antiviral proteins like IFN-induced protein with tetratricopeptide repeats-1. This phenomenon retards the replication of the virus in both neighboring and infected cells by activating an immune response against the virus. So, how does COVID-19 cause severe infection in patients? What are the immune escape strategies that are being adapted by the deadly virus?
Evidences suggest that, not only SARS-CoV but MERS-CoV too produces double-membrane vesicles (DMVs) and avoid detection of their double stranded RNA (dsRNA) by host [67]. The nonspecific proteins 1 (Nsp1) of SARS-CoV represses the activation of IFN regulatory factor 3 (IRF3) and IRF7 and together with nsp3, nsp1 also inhibit activation of IFN-β promoter which is viral dependent [68]. The induction of IFN is blocked by accessory protein 4a of MERS-CoV upon interaction with double stranded DNA (dsDNA) directly [69]. Furthermore, studies have shown that in MERS-CoV infection ORF4a, ORF4b, ORF5 and membrane (M) proteins block the transport of IRF3 into the nucleus and also activate IFNβ promoter [70]. The gene expressions for antigen presentation are also downregulated after MERs-CoV infection. Thus, SARS-CoV and MERS-CoV has modified themselves to escape from host immune surveillance. Efficient data is not available to support the theory that SARS-CoV-2 also uses the same mechanism to avoid immune surveillance or not. Table 2 provides a comparative study on the immunological functions played by structural proteins of the virus.
Table 2.
The structural proteins involved in immunopathology.
| Name of the structural protein | Structure | Function on immunological aspects |
||
|---|---|---|---|---|
| SARS-CoV-1 | MERS | SARS-CoV-2 | ||
| Nonstructural protein (Nsp)1 [[71], [72], [73]] | It is a leader protein, cleaved chain of ORF1b. | Antagonizes IFN-β production by decreasing the phosphorylation level of STAT1. | Helps in viral replication. | Detailed functions are still not known. |
| Nsp 15 [[74], [75], [76], [77], [78]] | Nidoviral RNA uridylate specific endoribonuclease (NendoU) that belongs to EndoU family. 34 KDa, around 345 amino acids, with three domains: N- terminal, middle and C-terminal domain. |
Cleaves polyuridine (polyU) sequences from PUN RNAs and limits the formation of a PAMP and thus impedes the ability of activation the innate immune response to infection by MDA5. | Prevents activation of dsRNA sensors in host cell for evading immune system. | Inhibits the nuclear localization of IFR3 and antagonize the production of IFN and also target RNF41 (also known as NRDP1) to regulate innate immune system. |
| Nsp 9 and Nsp 10 [[78], [79], [80]] | The crystalline structure of Nsp9 of SARS-CoV revealed that the molecule forms two distinct types of dimers where the core of the protein is an open 6-stranded β-barrel that in turn comprises of two antiparallel β sheets packed orthogon. | Nsp10 regulates the activity of the 2′-O- Methyltransferase (2′-O- MTase) that prevents virus detection by cell innate immunity mechanisms and viral translation inhibition by the interferon-stimulated IFIT-1 protein. | According to available data Nsp9 helps in viral replication. | Interacts with NF-κB repressor, NKRF and activates IL-8/IL-6 mediated chemotaxis of neutrophils that results in inflammatory response in patients. |
| Nsp 13 [77,78,81] | It is a helicase of superfamily 1 and helps in viral RNA replication via unwinding of duplex RNA and DNA leaving a 5′ single-stranded tail in a 5′ to 3′ direction. | It acts as a helicase and helps in unfold the RNA-DNA hybrid. | Nsp13 attenuates the viral replication. | Targets TBK1 and TBKBP1 to inhibit interferon pathway to regulate innate immune response in host cell. |
| N (Nucleocapsid) protein [82] | N protein of SARS-CoV-2 is 29.9 kb in length, similar to 27.9 kb SARS-CoV and 30.1 kb MERS-CoV genome. | Generation of IFN is retarded upon crosstalk between the SPRY domain of TRIM25 and C terminus of the N protein as it blocks RIG-I ubiquitination by TRIM25. | Interacts with TRIM25 and interfere the IFN production in host cell. | Detailed functions are not clearly known. |
| ORF (open reading frame) 9b [83,84] | ORF-9b possesses a long hydrophobic lipid binding tunnel formed due to interwined dimer with an amphipathic outer surface | ORF-9b manipulates host cell mitochondrial function by disable MAVS signaling that results in reducing NLRP3 inflammasome activity, thus evading innate immune system. | Function in immune system is not known. | ORF9b in association with Tom70 interacts with a signaling adaptor MAVS indirectly |
| ORF6 [76,77] | SARS-CoV-1 and SARS-CoV-2 share only 69% amino acid similarity | It prevents primary production of interferon. | Helps in viral assembly and viral release and can act as a potential B cell epitope. | It prevents interferon production by various signaling molecules MDA5, MAVS, TBK1 and IRF3-5D, which is a phospho-mimic of the activated form of IRF3. |
| ORF3 [[85], [86], [87]] | Accessory protein formed by the cleavage of ORF1 and ORF1b. | ORF3a is responsible for activation of the NLRP3 inflammasome by secreting IL-1β. | Prevents interferon production and prevents inflammation. | The hypothesis is that ORF3a of SARS-CoV-2 may be less efficient in inflammasome activation. |
IFIT, interferon-induced protein with tetratricopeptide repeats; IFN, interferon; IRF, interferon regulatory factor; MTase, methyltransferase; N, nucleocapsid; NendoU, nidoviral RNA uridylate specific endoribonuclease; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; NLRP3, NLR family pyrin domain containing 3; NRDP1, neuregulin receptor degradation protein 1; Nsps, non-structural proteins; ORFs, open reading frames; PAMP, pathogen associated molecular patterns; PolyU, poluuridine; RNF41, ring finger protein 41; SPRY, sprouty RTK signaling antagonist; STAT, signal transducer and activator of transcription; TBK1, TANK binding kinase 1; TBKBP1, TANK binding kinase-1, binding protein 1; TRIM25, tripartite motif containing 25.
5.3. Innate immune system and SARS-CoV-2
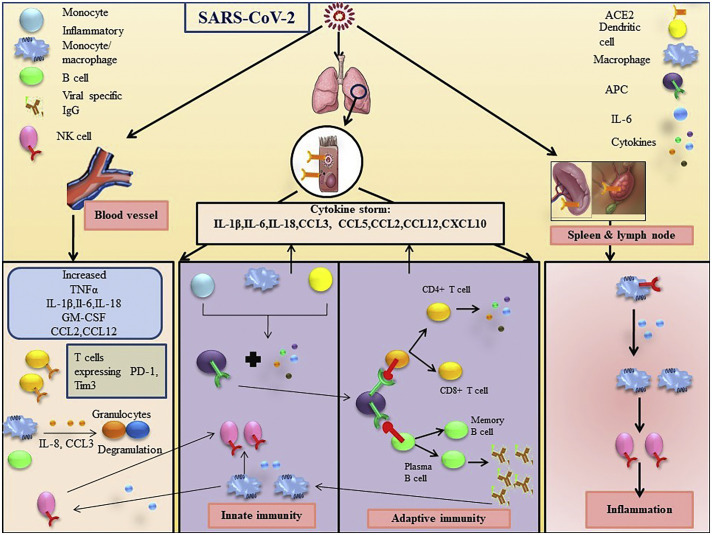
First line of defense comes from the cells of innate immune system that include residential macrophages, conventional dendritic cells (cDCs), monocytes-derived dendritic cells (moDCs), granulocytes and natural killer cells [88]. In any viral infection, the innate immune system relies on Type I interferon (IFN) responses whose downstream cascade regulate the viral replication and induce adaptive immune responses. However, nCOVID-19 may dampen the IFN Type-1 response to terminate the anti-viral response. According to studies, SARS-CoV directly affect macrophages and T cells [89]. Recent research has shown that SARS-CoV-2 induces CD169+ tissue-resident macrophages to produce IL-6 which results into lymphocyte apoptosis via upregulation of Fas in human spleen and lymph nodes [90]. According to the data of scRNAseq of COVID patients, there was expansion of CD14+IL-1β+ monocytes [91], IMs, Ficolin-1+ monocyte-derived macrophages and tissue-resident reparative alveolar macrophages (AMs) and elevated level of IL-β associated inflammation in peripheral blood of COVID-19 patients with severe condition [92,93]. In severe infection, lung macrophages express high levels of IL-1β, IL-6, TNF-α and chemokines like C-C motif chemokine ligand 2 (CCL2), CCL3, CCL4 and CCL7, C-X-C motif chemokine ligand (CXCL) 9, CXCL10, CXCL11 but CXCL16, whose binding receptor C-X-C motif chemokine receptor (CXCR) 6 was more highly expressed in patients with moderate infection [93]. Moreover, lung macrophages in patients with severe COVID-19 infection may recruit IMs and neutrophils though CCR1 and CXCR2 [93]. According to earlier data, SARS-CoV-1 infection resulted in an diverging phenotype of AM phenotype which limits the trafficking of DCs and activation of T cells [94] and YM1+ FIZZ1+ alternatively activated macrophages increased hypersensitivity in airway, thus worsening the fibrosis caused by SARS-infection [95]. These mechanisms, in SARS-CoV-2, need more research focus. Recent research revealed that ACE2 and SARS-CoV-2 N protein is also present on CD169+ macrophages of spleen and lymph node of SARS-CoV-2 patients that are involved in production of IL-6 [96]. As mentioned earlier, SARS-CoV-2 undergoes the process of causing infection via ACE2 receptors but very low macrophages percentage in lungs express ACE2 receptors. So, the question arises is that whether there any other receptor present through which SARS-CoV-2 is infecting the immune cells? Evidences revealed reduced number of natural killer (NK) cells, in peripheral blood are positively correlated with COVID severity [[97], [98], [99]]. In influenza infection, CXCR3 mediated NK cells infiltration [100]. In vitro study has shown there is increased levels of CXCR3 ligand (CXCL9–11) in SARS-CoV-2 infected tissues of human lungs along with expanded monocytes level stimulated by CXCR3 ligands in SARS-CoV-2 infection [47]. These studies suggest that the CXCR3 pathway recruits NK cells in SARS-CoV-2 infected patients towards the lungs from the peripheral blood. Recent studies have shown that peripheral blood NK cells of SARS-CoV-2 patients have deceased expression of enzymes such as granzyme B, granulysin and also reduced surface markers CD107a, Ksp37 along with impaired chemokine production of TNF-α and IFN-ɣ that suggest an impaired cytotoxicity [98,101]. Moreover, SARS-CoV-2 infection has shown less number of CD16+KIR+ peripheral blood NK cells [102]. The expression of immune checkpoint NK group 2 member 2A (NKG2A) is increased with the upregulation of genes encoding inhibitory receptors lymphocyte activation gene 3 (LAG3) and T cells immunoglobulin and mucin domain 3 (TIM3) on NK cells of COVID patients [98,101]. Thus SARS-CoV-2 impairs the activity of NK cells. The impaired immune response stimulated by SARS-CoV-2 has been summarized in Fig. 1 .
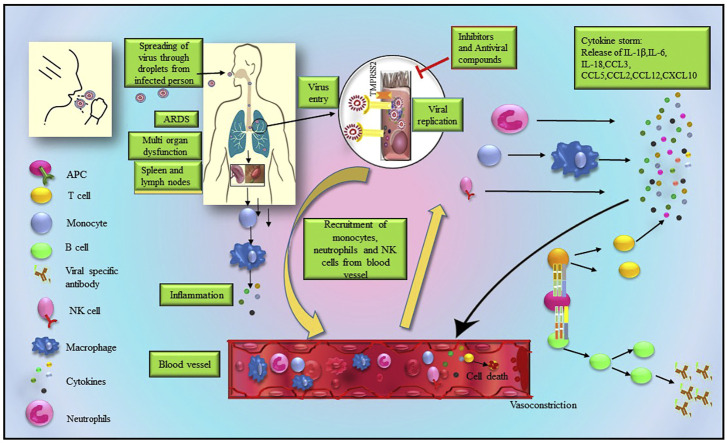
Fig. 1.
Immune response following SARS-CoV-2 infection. SARS-CoV-2 mostly affects the lungs because of higher expression of angiotensin converting enzyme 2 (ACE2) receptor. Upon binding with ACE2 receptor, SARS-CoV-2 enters into cells and elicits immune responses. Dendritic cells, monocytes, macrophages act as antigen presenting cells (APCs) that interact with CD4+ and CD8+ T cells to induce the proliferation of virus specific T cells and facilitate secretion of various cytokines in the lungs. APCs also induce the release of viral specific antibodies, which recruit natural killer (NK) cells from peripheral blood to the lungs, B cells in the lungs and ultimately results in cytokine storm by increasing the secretions of various interleukins (ILs) including IL-6, IL-6, IL-β, TNF-α, C-C motif chemokine ligand (CCL)3, CCL5, CCL2, CCL12, C-X-C motif chemokine ligand (CXCL)10, granulocyte-monocyte colony stimulating factor (GM-CSF). On the other hand, in peripheral blood, in response to virus entry in concert with the high expression of exhaustion markers such as T cell immunoglobulin and mucin domain 3 (Tim3), programmed death-1 (PD-1) in T cells, the expressions of IL-1β, IL-6, IL-18, TNF-α, GM-CSF, CCL2, CCL12 are also elevated. Inflammatory monocytes (IMs) induce the degranulation of granulocytes by secreting CCL3 and IL-8. Due to the expression of ACE2 receptor, SARS-CoV-2 affects spleen along with lymph nodes to the same extent. Virus entry also induces IMs to secret IL-6 and recruits NK cells in tissue microenvironment.
5.4. Adaptive immune system and SARS-CoV-2
Antigen presentation by APCs to other immune cells subsequently activates pathogen (virus) specific B cells and T cells. Similarly in SARS-CoV, viral infection a typical pattern of IgG and IgM has been observed where the IgM antibodies disappear in the 12th week but the IgGs which are viral S-specific and N-specific, last for a longer period [103]. The near-universal presence of IgGs, IgM, IgAs and neutralizing IgGs antibodies (nABs) in COVID patients indicates a humoral immune response mediated by increased B cells. COVID-19 patients show higher levels of antigen secreting cells (ASCs) derived from precursor naïve B cells. These B cells are regarded as double negative2 (DN2) as they lack naïve IgD, memory CD27 markers, CXCR5 and CD21 markers. The ASCs express high levels of CD11c and T-bet molecular markers and respond to TLR7 [104]. Patients in early stages with high levels of ACEs provide a protective function against eradication of virus whereas in later stages high levels ACE show poor outcomes. The circulating ACE2 enzymes protect virus-induced lung injury in influenza (H7N9) infection [105]. In COVID-19 infection, the higher level of circulating ACE2 might provide a protective function towards severe lung injury. According to studies, estrogen facilitates the upregulation of ACE2 which might explain the protectiveness of female vs male and also increases protection in children as they have higher level of circulating ACE2 than adults [106]. SARS-CoV-2 specific IgG of S protein was found in the serum of patients even after 60 days of symptoms onset, which decreased within 8 weeks of onset of post symptoms period [65]. Further studies are needed on the existence of viral specific IgG+ memory cells in recovered COVID-19 patients.
Latest data has shown that in COVID-19 patients the peripheral count of CD4+ and CD8+ T cells have been greatly reduced but they were hyperactive in functional status as evidenced by i) a higher proportion of HLA-DR (CD4 3·47%) and CD38 (CD8 39·4%) double-positive fractions, ii) a hike in highly proinflammatory CCR4+CCR6+ CD4+ T cells (Th17 cells) producing IL-17 and granulysin expressing Tc cells were observed in patients with severe immune injury and iii) the cytotoxic Tc cells (CD8+ T cells) harbor greater concentration of cytotoxic granules e.g. 31.6% cells were perforin positive, 64.2% cells were granulysin positive and 30.5% cells were double positive for both perforin and granulysin [107]. These results implicate that the hyperactive function of Th17 and CD8+ T cells are responsible for severe immune inflammation in patients and produce low IFN-γ and TNF-α in CD4+ T cells and high granzyme B and perforin in CD8+ T cells in COVID-19 infected patients [108]. It has been reported that CD8+ T cells, developed during SARS-CoV infection, are specifically produced for the antigen S, M, E and N proteins. In SARS-CoV infection, CD8+ T cells have been observed to differentiate into CD45ROˉCCR7ˉCD62Lˉ effector memory cells while CD4+ T cells express CD45+CCR7+CD62Lˉ central memory T cells [109]. Th1 cells which were hyperactivated release granulocyte-monocyte colony stimulating factors (GM-CSF) and IFNγ. This recruited increased numbers of CD14+CD16+ monocytes that are inflammatory, stimulated by IL-6 [110]. In moderately infected lung, macrophages produce increased chemokines, that will attract T cells, via the engagement of CXCR3 and CXCR6 [47]. So, innate and adaptive immune cells interact with each other and are involved in a positive loopback in expressing higher inflammation in COVID-19 infection. CD8+ T cells expressing high level cytotoxic genes such as granzyme K, A, B (GZMK, GZMA, GZMB) and XCL1 along with KLRC1 which remained high in mild symptoms, have been detected in bronchoalveolar lavage (BAL) of COVID patients [111]. Moreover, experimental analysis suggests that these memory cells lasts for 3–4 years after the infection has been cured and slowly diminishes in the absence of antigen after 4 years [109]. Moreover, all the subtypes of T cells found in SARS-CoV-2 infection, show higher expression of negative immune checkpoint markers and exhaustion markers that is correlated with severe immune pathogenicity. The study of 10 patients group revealed increased levels of PD-1 in CD8+ T cells and Tim-3 in CD4+ T cells were observed in three patients of both prodromal and symptomatic stages of SARS-CoV-2 infection [112]. Furthermore, several other investigations reported increase in the expression of both co-stimulatory and inhibitory molecules such as OX-40 and CD137 [110], CTLA-4 and T cell immunoreceptor with Ig and ITIM domains (TIGIT) [108] and NKG2A [98], were found in T cells which suppressed the cytotoxic activity. Till now there is no potent evidence of any memory cells developed in cured COVID-19 patients against SARS-CoV-2.
5.5. Cytokine storm and SARS-CoV-2
Till now according to the reports, the main cause of death due to COVID-19 is severe pneumonia and ARDS. The key cause behind the occurrence of ARDS is the severe “cytokine storm” in infected patients that resulted in pneumonia, respiratory failure and other organs failure. A high cytokine storm occurring in COVID-19 patients include IL1-β, IL-17RA, IL-7, IL-9, IL-10, basic-fibroblast growth factor 2 (FGF2), granulocyte colony stimulating factor (G-CSF), GM-CSF, IFNγ, inducible protein (IP)10, membrane cofactor protein 1 (MCP1), macrophage inflammatory protein 1α (MIP1α), MIP1-β, platelet derived growth factor B (PDGF-B), TNFα, and vascular endothelial growth factor A (VEGFA) [31,113]. The ICU patients show high levels of pro-inflammatory cytokines such as IL-2, IL-7, IL-10, G-CSF, IP-10, MCP-1, MIP-1α, and TNFα, which are positively correlated with disease severity [31]. In a report from Wuhan where 99 cases had been studied, an increase in total neutrophils, decrease in total lymphocytes and increased in serum IL-6 has been observed. A delayed IFN-I signaling was observed which accumulate inflammatory monocyte-macrophages (IMMs). This resulted in high levels of cytokines and chemokines in lungs, vascular leakage and impaired the response of viral-specific T cells [114]. In SARS infected patients, an elevated level of IL-6, IL-8 and IP-10 has been found in lung tissues [115]. Increased level of pro-inflammatory cytokines is mainly responsible for severe lung injury, leading to demise of COVID-19 victims [107]. High levels of IP-10 has been related with immune mediated severe lung injury and apoptosis of lymphocytes in SARS [115]. Together with the cytokines, certain chemokines such as CXCL10, IP10, CCL2, CCL3, CCL5, CXCL8, CXCL9 support the impaired systemic inflammatory response in SARS-CoV-2 [116]. In comparison with SARS- CoV, SARS-CoV-2 upregulated five chemokines namely CXCL1, CXCL5, CXCL10, CCL2 and IL-6 [117]. SARS-CoV-2 patients with more severe pneumonia and pulmonary syndrome showed correlated higher expression of GM-CSF+ and IL-6+CD4+ T cells, higher co-expression of IFN-γ and GM-CSF in pathogenic Th1 cells, much higher expression of CD14+CD16+ monocytes [110]. In a nutshell, high infiltration of all types of immune cells such as T cells, monocytes, macrophages, NK cells, DCs and secretion of their proinflammatory cytokines into lungs cause severe ARDS leading to death of the patients.
6. Ongoing therapies
Currently there are not potent antiviral vaccine for the treating SARS-CoV-2. All patients are treated with supportive treatment strategies targeted to culminate the patients' symptoms (like pneumonia, fever, breathing problems) and often supported with combination of drugs. However, these strategies cannot be implemented for a long time. Being RNA virus, SARS-CoV-2 can be inhibited by repurposing drugs used for other RNA viruses such as the Human Immunodeficiency Virus (HIV). Clinical trials are currently undergoing with combinational drugs mainly ritonavir and lopinavir. Several other drugs are under clinical trials such as Kevzara, a rheumatoid arthritis (RA) drug that decreases lung complications. Kevzara has been successfully tested in COVID-19 patients. As per data, there are 11 phase 4, 36 phase 2 and 4 phase 1 trails [118]. Table 3 encloses a list of commonly used combinational drugs for the treatment of COVID-19.
Table 3.
Drugs and their combinations are currently used in the treatment.
| Name of drug | Description | Function |
|---|---|---|
| Chloroquine and hydroxyl chloroquine [[119], [120], [121]] | These drugs are basically used in malaria treatment and some extent to Systemic Lupus Erythematosous (SLE) and rheumatoid arthritis (RA) treatment. | Chloroquine and hydroxychloroquine inhibits viral entry into cells. The glycosylation of host receptors, endosomal acidification and proteolytic processing are inhibited. These agents also affect immunopathology via inhibition of cytokines production for lysosomal activity and autophagy of immune cells. |
| Lopinavir/ritonavir [122] | Lopinavir and ritonavir are approved by US Food and Drug Administration (FDA) and in treatment of HIV. | No published data are available but invitro studies show that they act by inhibiting 3-chymotrypsin-like protease. |
| Remdesivir [123] | Remdesivir, also called GS-5734, is a monophosphate prodrug that forms an active C-adenosine nucleoside triphosphate analogue undergoing metabolism. | The drug was designed against microbes with activity also against RNA viruses. Remdesivir targets the RNA dependent RNA polymerase and hamper the replication cycle of RNA viruses. Remdesivir first used for the treatment of Ebola. |
| Umifenovir [124] | Umifenovir or Arbid, an antiviral drug. | It inhibits S protein/ACE2 interaction via blocking the fusion of membrane with the viral envelope. Arbid is used for the treatment of influenza in Russia and China and is recently in the interest for treating COVID-19. |
ACE2, angiotensin converting enzyme 2; HIV, human immunodeficiency virus; RA, rheumatoid arthritis; S, spike; SLE, systemic lupus erythematosus; FDA, food and drug administration.
As it has been an absolute outbreak and pandemic disease declared by WHO, a specific cure has to be found out to cure the disease completely. According to the genomic and structural analysis of SARS-CoV-2, there are a number of therapeutic targets which are under clinical trials in different laboratories across the whole world.
6.1. Viral targets
The Washington Department of Health Administration has first introduced remdesivir which inhibit RNA dependent RNA polymerase activity intravenously and found that it has a potential to protect from SARS-CoV-2 infection. The combination of remdesivir and choloroquine has shown to prevent SARS-CoV-2 infection invitro. Therefore, other nucleotide analogue such as favipiravir, ribavirin can also be administered as potential inhibitors. There are certain proteases such as 3 chymotrypsin-like protease (CLpro) along with papain like protease (PLpro) that cleaves viral polyproteins, can be the noble drug targets for the treatment. These also affect the replication of virus and antagonize IFN, IL-6. As SARS-CoV-2 binds with the ACE2 receptors of host cells, therefore targeting the S protein on the surface of the virus or the binding of the S protein and ACE2 can be a potential therapeutic target to combat COVID-19 infection. Fig. 2 points out the role of various drugs in distinctive stages of SARS-CoV-2 replication process.
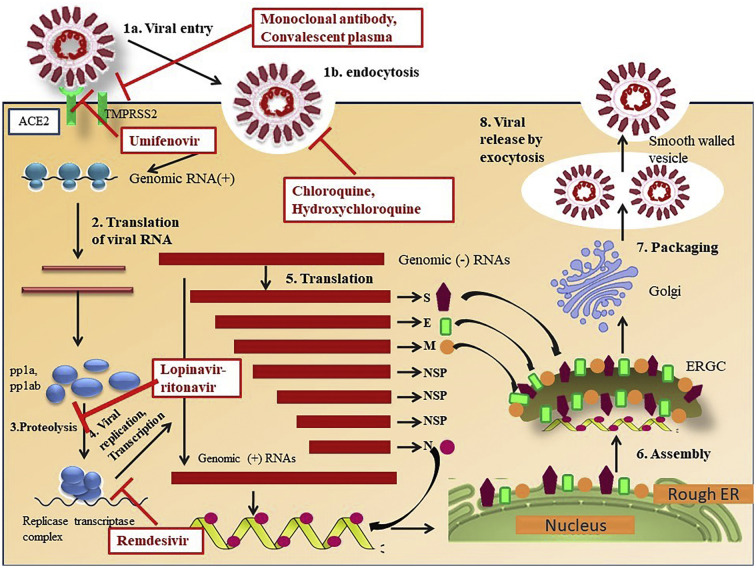
Fig. 2.
Molecular mechanism of relevant drugs and therapies for the infection of SARS-CoV-2. SARS-CoV-2 enters into the host cell via angiotensin converting enzyme 2(ACE2) receptor by endocytosis with the release of its RNA contents into the recipient cell to replicate. Monoclonal antibody or convalescent plasma therapy inhibits the ACE2 receptor or interferes with transmembrane serine protease 2 (TMPRSS2) function to affect the viral binding with the host cell. Chloroquine and hydroxychloroquine interfere with the entry of virus via endocytosis. Umifenovir targets spike (S) protein/ACE2 interaction and inhibits membrane fusion of the viral envelope. After the shedding of viral RNA, it translates into polypeptide (pp)1b, pp1ab leading to the formation of double membrane vesicles (DMVs) and establishment of the replication-transcription complex (RTC). This stage is followed by generation of intermediate negative strand RNA from which more numbers of positive strand RNA and mRNAs are generated. Lopinavir/ritonavir inhibits protease to prevent proteolysis, a mechanism that establishes RTC. Remdesivir targets the RNA dependent RNA polymerase and hamper the replication cycle of RNA viruses. Structural nucleocapsid (N) proteins are generated from the translation of nucleocapsid (N) mRNA, which in turn encapsulates the newly generated positive RNA strands. However, other structural proteins, envelope (E) proteins and membrane (M) proteins are formed via the translation in endoplasmic reticulum (ER) and gather in endoplasmic reticulum golgi intermediate complex (ERGIC) and cis-Golgi. In addition to the structural proteins some non-structural proteins (Nsps) are also generated. The assembly of the viral components begins when the accumulated proteins exit from the golgi apparatus and eventually fuse with the cell membrane, resulting in the release of new virus particles.
6.2. Antibody and plasma therapy
According to studies the development of recombinant monoclonal antibody (mAb) can be a noble way to neutralize SARS-CoV-2. For example, CR3022, a SARS specific human mAb, can bind with the RBD of SARS-CoV-2 and can be used as candidate vaccine for SARS-CoV-2. Other mAbs, such as m396, CR3014, can be an alternative against SARS-CoV-2. Recently, a recombinant mAb named tocilizumab has come into application that can bind to IL-6 receptors, thereby terminating its signal transduction but its efficiency is still under study [38]. In addition to it, virus neutralizing antibody isolated from convalescent serum of COVID-19 patients, who has recovered from the infection, has also been administered in susceptible individuals as it had proved to be promising treatment approach during the previous corona outbreaks. It can impose immediate immune response in the unaffected susceptible individuals [125]. The generation of antibodies against the S proteins of the virus is being followed by Moderna Inc., MA, USA. There is also hope for development of new mAbs, which might take less time to be available to the doctors due to their speedy trials and their high specificity.
6.3. Development of vaccine
In this pandemic situation, approved vaccines against SARS-CoV-2 are essentially required as soon as possible for decreasing disease severity together with reduced shedding and transmission of virus. In the world full of darkness, a keen ray of light has been illuminated by the recent development of a vaccine mRNA1273 by The National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health (NIH). Various vaccination platforms are now being considered to eradicate SARS-CoV-2 by developing passive immunization namely live attenuated vaccine (whole virus), sub-unit vaccine, viral-vector based vaccine, RNA vaccines, DNA vaccines. Although each of them has their own discrete benefits and disadvantages [126]. NVX-CoV2373 (Novavax, Inc.| Emergent BioSolutions), Triple Antigen Vaccine (Premas Biotech, India), Coroflu (University of Wisconsin-Madison | FluGen | Bharat Biotech), Ad5-nCoV (CanSino Biologics Inc. | Beijing Institute of Biotechnology), BNT162b1 (BioNTech| FosunPharma| Pfizer), DelNS1-SARS-CoV-2-RBD (University of Hong Kong) etc. are some of the vaccines that are being developed under the vaccination platforms [126]. Despite of the immense struggle of the researchers towards development of the vaccines, the mutant strains that are developing these days has compelled the research platforms to rethink and implement budding strategies for developing new vaccination formulas. A number of vaccines have been developed and some others are under development. Among them Covaxin and Covishield has shown promising ways in terms of immunization, especially in India as most of the populations are vaccinated with either of them. Although the approved vaccines that are currently available in the market have given promising effect, but their roles against the new mutant strains need to be studied.
6.4. Epitope mapping
Significant studies have been focused on identifying various target epitopes mapping on SARS-CoV-2 for development of targeted vaccines. Apart from antibodies targeting RBD of S proteins, studies have been undertaken to identify additional viral fragments (epitopes). This investigation has utilized the data on genetic differences and similarities among the three strains of coronavirus by utilizing bioinformatics analysis [127,128]. By implementing immune informatics scientists discovered five cytotoxic T lymphocytes (CTLs) epitopes and eight B-cell epitopes in the viral surface glycoproteins. Among the B cell epitopes three are sequential and rest five are discontinuous. Furthermore, CTL epitopes which are activated as judged by molecular dynamicity that the interaction between the CTL epitopes and HLA chains of MHC-I complexes are mediated by hydrogen (H) bonds and salt bridges, indicating their efficacy to confer immune responses [128]. Another study has identified five linear and two conformational B cell epitopes of SARS-CoV-2 surface proteins [129].
6.5. Stem cell therapy
This noble therapeutic approach was not undertaken into study until two distinct studies conducted by China and detected that stem cell therapy can be a new aspect in the treatment of COVID-19. Intravenous infusion of mesenchymal stem cells (MSCs) can play a vital role in curing the dysfunctions of the lung i.e. complications like pulmonary edema, dysfunction of air-exchange, ARDS, acute cardiac injury which are the results increased inflammation due to the account of the virally triggered cytokine storm caused damage to lung tissues [50,130]. These MSCs, depending on their properties of modulating the immune system and regeneration or differentiating capability can counteract the increased release of cytokines and their repairing capacity thus restored the damaged tissues, followed by curing the disease. Moreover, RNA-sequencing of transplanted MSCs has revealed the presence of undifferentiated transfused MSCs and remained to ACE2 negative thus there exist no chance for the virus to affect these cells [130].
7. Ongoing interventional studies
A landmark initiative to put forward the development of vaccines and their potential is undertaken across the world on observing the severity of COVID-19. From the increasing number of fatalities and affected individuals reported across the world, it is clear that COVID-19 is escaping the treatment strategies undertaken to eradicate it. Due to the immense capability of SARS-CoV-2 to mutate very rapidly, a challenge has been thrown to the scientists to develop a potent vaccine that can destroy it all. Keeping pace with the above-mentioned treatment strategies, several drugs which were used for treating other diseases, are now under clinical trials to find a solution to the menace of COVID-19. A list of interventional drugs and vaccines under trial has been illustrated in the Table 4 .
Table 4.
List of drugs and vaccines that are on clinical trials.
| Candidate drug | Mode of action and dose | Existing disease approval | Trial sponsor | Location | Expected result | Phase trial |
|---|---|---|---|---|---|---|
| Hydroxycholoroquine (ID: NCT04329611) [131] | Hydroxychloroquine inhibits acidification of endosomes, deglycosylates receptors of recipient cells, prevents proteolytic processing thus retards entry of virus. Inhibition of cytokine production modulates the host immune system. It also inhibits in host autophagy and lysosomal functionality Dose: Hydroxychloroquine dose of 400 mg po bid on day 1 followed by 200 mg po which will be given twice daily for 4 days |
Malaria, rheumatoid arthritis (RA), lupus | Dr. Michael Hill | University of Calgary | Preliminary trials indicated that it is a potential and safe drug against COVID-19 pneumonia and shorten the disease course about 50%. Later it was found that both these drugs have side effects like allergic reactions, hypoglycemia, cardiomyopathy. On April, 2020 in Brazil 11 patients died due to irregular heart rates. | Phase III |
| Remdesivir (ID:NCT04292899 and NCT04292730) [[132], [133], [134]] | Antiviral Dose: RDV 200 mg on first Day followed by RDV 100 mg for next 4 days together with standard therapy. |
– | Gilead, WHO, INSERM | China, japan | According to US NIAID, remdesivir shows faster recover from COVID-19 in 11 days compared to other drugs. A clinical trial in china, reported on 29thApril several adverse effect of remdesivir in treated patients. | In April 2020, there was 9 phase III clinical trials across the world. |
| Duvelisib (ID:NCT04372602) [135] | Target PI3K and control hyperactivation of innate immune system by affecting macrophage polarization, reducing inflammation in pulmonary and limit the persistence of viral load. Dose: 25 mg twice daily for 10 days, orally |
– | Washington University School of Medicine. | Washington University School of Medicine, Saint Luis, Missouri, United Sates | Current primary outcome reported on 30th April overall survival | Phase II |
| Deferoxamine (ID: NCT04333550) [136,137] | It is a natural product which is isolated from Streptomyces pilosus. It helps in the formation of iron complexes and its mesylate form perform as chelating agent, | – | Kermanshah University of Medical Science | Regenerative Medicine Research Center, Kermanshah University of Medical Science, Iran, kemanshah. | Trial ongoing | Phase I Phase II |
| Favipiravir (ID:NCT04336904) [138] | It targets RNA-dependent RNA polymerase (RdRp) enzymes, which are necessary for the transcription and replication of viral genomes. Dose: Day 1:1800 mg, BID is given at 1800 mg on day 1 and day 2 followed by TID dose at 600 mg for 14 days. Thereafter: 600 mg, TID, for a maximum of 14 days. |
Used before against Ebola virus and lassa virus. | Giuliano Rizzardini | Asst Fatebenefratelli Sacco, Milan Ilaty | the normalization of pyrexia, normal respiratory rate and relief from cough is maintained for at least 72 h. | Phase III |
| Tocilizumab (ID:NCT04345445) [139] | Human monoclonal antibody against IL-6 receptor. Dose: Intravenously administered with a concentration of 8 mg/kg (body weight) once, within 60 min. |
This drug has been used against immune suppression and in RA. | Genentech-hoffmann La Roche | Multiple countries | As per 8-point WHO scale, improvement of more than 2 point is observed. | Phase II |
| Sarilumab (ID:NCT04327388) [140] | Human monoclonal antibody against IL-6 receptor. Dose: Sarilumab Dose 1 given intravenously one time on Day 1 |
RA | Regeneron-Sanofi | Multiple countries | Patients improvement in oxygenation. | Phase II/ Phase III |
| Dapagliflozin (ID:NCT04350593) [141,142] | It acts as a sodium glucose cotransporter inhibitor. Dose: Dapagliflozin 10 mg daily |
Hypoglycemia | Saint Luke's Mid America Heart Institute, Astrazeneca | Multiple countries | No detoriation in functionality of organs are observed in hospitalized patients at 30th day. | Phase III |
| Recombinant human angiotensin-converting enzyme 2 (rhACE2) (ID:NCT04287686) [143] | It is a monocarboxy-peptidase that metabolizes several peptides, including the degradation of angiotensin II, and contributes to cardiovascular effect. Dose: Together with standard treatment0.4 mg/kg IV BID given for 7 days. |
– | Hospital of Guangzhou Medical University | Guangdong, China | 24–48 h of Pulmonary imaging showed that progression of the lesions are more than 50% and the patients were managed as severe | Phase II |
| Clevudine with combination hydroxychloroquine (ID:NCT04347915) [144] | Clevudine is an antiviral drug used against hepatitis B. Dose: Clevudine 120 mg once daily for 14 days (Hydroxychloroquine 200 mg twice daily for 14 days. |
Hepatitis B | Bukwang Pharmaceutical | Trial ongoing | Phase II | |
| Drug: FT516 (ID:NCT04363346) [145] | FT516 is a cryopreserved NK cell product of an iPSC that was transduced with ADAM17 non-cleavable CD16 (Fc receptor). Dose: Firstly, FT516 is administered at 9 x107 cells/dose in low concentration Secondly, FT516 is first given at low dose (9 x107 cells/dose) additionally at Day 4 it is provided in medium dose at (3 x 108 cells/dose) Thirdly, along with the low and medium doses, a higher dose of drug is given at day 7 (9 x 108 cells/dose) |
Cancer | Masonic Cancer Center, University of Minnesota | Minneapolis, Minnesota, United States. | Trial ongoing | Phase I |
| DAS181 (ID: NCT04324489) [146] | Enzymatically cleaves viral receptors on host cells Dose: From day 1 to 10, once or twice a day, for 10 consecutive days, a total of 9 mg (7 mL) |
Parainfluenza and other flu viruses including resistant strains | Renmin Hospital of Wuhan University | China | Better clinical outcomes | Completed |
| Losartan (ID: NCT04335123) [147] | – Dose: 25 mg for first 3 days folllowed by 50 mg QD till study completion |
– | University of Kansas Medical Center | Kansas City, United States. | Trial ongoing | Phase I |
| Ivermectin with Nitazoxanide (ID:NCT04360356) [[148], [149], [150]] | Antiviral drug that affects the viral RNA and DNA replication in a broad spectrum. Dose: Ivermectin 200 mcg/kg once orally on empty stomach plus Nitazoxanide 500 mg twice daily orally with meal for 6 days |
– | Tanta University | – | Trial ongoing | Phase II |
| Transfusion of SARS-CoV-2 Convalescent Plasma. (ID:NCT04372979) [151,152] | Convalescent plasma contains antibody against SARS-CoV-2. Dose: Intravenous |
– | Direction Centrale du Service de Santé des Armées | France | Trial ongoing | Phase III |
| Isotretinoin (ID:NCT04361422) [153] | Inhibitors of PLpro, a protein encoded by SARS-CoV-2 Dose: Orally |
Used to decrease virema in HIV+ patients | Tanta University | – | Clinical clearance Change in COVID-19 virus load |
Phase III |
| Colchicine (ID:NCT04375202) [154] | Non-selective inhibition of NLRP3, a pathophysiologic component of SARS-CoV Dose: 0.5 mg every 8 h for 30 days, orally (Tablet) |
Cardiovascular disease | University of Perugia | Italy | Trial ongoing | Phase II |
| Ruxolitinib (ID:NCT04355793,NCT04338958) [[155], [156], [157]] | Treat the cytokine storm and hyperinflammation in COVID-19 patients Dose: 5 mg orally twice daily |
Treat bone marrow disorders like myelofibrosis | Incyte corporation University of Jena |
USA | Reduce 25% hyperinflammation caused due to the cytokine storm | Phase II |
| Sildenafil (ID:NCT04304313) [158] | Relaxes the muscles of the lungs by increasing the potency of nitric oxide gas to widen the blood vessels resulting in more oxygen inhalation Dose: 0.1 g daily for 14 days, orally |
Erectile dysfunction | Tongji hospital | China | Respiratory symptom remission Decrease in fever C-reactive protein recovery |
Phase III |
| Sirolimus (ID: NCT04341675) [159] | mTOR inhibitor, immune suppressor Dose: 6 mg on first Day then 2 mg daily for next 13 days. |
Used for preventing organ transplant rejection and lymphangioleiomyomatosis (LAM) | University of Cincinnati | USA | Trail ongoing | Phase II |
| Peginterferon Lambda-1a (ID:NCT04331899) [160] | Reduces viral shedding of SARS-CoV-2 Dose: One subcutaneous injection of 180 ug |
Hepatitis B Virus infection Hepatitis C virus infection |
Stanford University | USA | Trial ongoing | Phase II |
| Rintatolimod and IFN Alpha-2b (ID:NCT04379518) [161] | Rintatolimod is a dsRNA designed to mimic viral infection by activating immune pathways and IFN Alpha-2b activate immune responses and both participate in limiting viral replication and shedding Dose: IV rintatolimod for 2.5–3 h together with IV of recombinant interferon alpha-2b over 20 min on days 1, 3, 5, and 8 if there will be the disease progression or no unacceptable toxicity treatment will be followed up at 14th day and 28th day |
Viral infections | Roswell Park Center Cancer Institute | USA | Trial ongoing | Phase I/ IIa |
| l-ascorbic acid (ID: NCT04357782) [162] | Reduce inflammation, ARDS, reduce supplement oxygenation, reduce risk respiratory failure which intubation Dose: 50 mg/kg IV given every 6 h for 4 days (16 total doses), |
Sepsis | Hunter Holmes Mcguire Veteran Affairs Medical Center | Virginia, USA | Trial ongoing | Phase I/ II |
| mRNA1273 (ID:NCT04283461) [163,164] | A lipid nanoparticle (LNP) encapsulated with mRNA encoding full length S protein of SARS-CoV-2. Dose: 10/25/50/100/250 mcg mRNA1273 with .05 mL intramuscular injection in deltoid muscle. |
– | National Institute of Allergy and Infectious Disease (NIAID) | – | Trial ongoing | Phase I |
| INO-4880 (ID:NCT04336410) [165] | It is a DNA vaccine against whole- length S protein of SARS-CoV-2. Dose: intradermal injection of 1.0 mg of INO-4800 |
– | Inovio Pharmaceuticals | – | Trial ongoing | Phase I |
| ChAdOx1 nCoV-19 COVID-19 (ID:NCT04324606) [166,167] | Adenovirus encoding full-length S protein Dose: One dose of 5 x 10^10vp |
– | University of Oxford | UK | Trial ongoing | Phase II |
| COVID-19 LV- SMENP-DC (ID:NCT04276896) [168] | Lentivirus infected dendritic cells with SMENP minigenes that express COVID-19 antigens and activated CTLs. | – | Shenzhen Geno-Immune Medical Institute | China | Trial ongoing | Phase II |
| SARS-Cov-2 (ID:NCT04368988) [169] | Nanoparticle vaccine of spike (S) protein of SARS-CoV-2 | – | Novavax | – | Trial ongoing | Phase I |
| BNT162a1, b1, b2, c2 (ID:NCT04368728) [170] | It is a LNP encapsulated mRNA vaccines with mRNA targets for both larger S sequence and RBD. Dose:0.5 mL intramuscular injection. |
– | BioNTech SE and Pfizer, Inc. | – | Trial ongoing | Phase I |
| Recombinant Novel Coronavirus Vaccine (Adenovirus Type 5 Vector) (ID:NCT04313127) [72] | Adenovirus type 5 encoded with full length S protein | – | CanSino Biologics, Inc. | China | The vaccine is tolerable and immunogenic at 28 days post-vaccination in healthy adults, and rapid specific T-cell responses were noted from day 14 post-vaccination. | Phase I |
| bacTRL-Spike-1 (ID:NCT04334980) [171] | Live Bifidobacteriumlongum, engineered for the delivery of plasmids containing synthetic DNA encoding spike protein from SARS-CoV-2. | – | Symvivo Corporation | Trail ongoing | Phase I | |
| Mrna-1273 vaccine (ID: NCT04470427) [164] | Neutralizing activity against recombinant vesicular stomatitis virus (rVSV)–based SARS-CoV-2 (a pseudovirus-based model) in serum samples of new variants of the virus. Dose: Participants will receive 1 intramuscular (IM) injection of 100 microgram (ug) mRNA-1273 on Day 1 and on Day 29. |
– | ModernaTX, Inc. | Efficacy, safety, and immunogenicity of mRNA-1273 to prevent COVID-19 for up to 2 years after the second dose of mRNA-1273. | Phase III | |
| Decitabine for Coronavirus (COVID-19) Pneumonia - Acute Respiratory Distress Syndrome (ARDS) Treatment (ID: NCT04482621) [172] | Works as hypomethylating agents, affecting replication Dose: 10 mg/m^2/day IV day x 5 days (1 cycle only) intravenously |
Myelodysplastic syndrome (MDS) | Johns Hopkins University | USA | Recruiting | Phase II |
| Xuanfei Baidu granules (XFBD), Traditional Chinese medicine (TCM) (ID: NCT04810689) [173] | Acts as anti-coagulant agent which dissolves blood clots, relieves shortness of breath by relaxing the tracheal muscles. Dose: Orally twice daily for 14 days, 1 h after food in the morning and at night with at least 8 h in between doses |
Used for fever recovery and suppress of cough by clearing phlegm | Darcy Spicer, University of Southern California | USA | Recruiting | Phase II |
| STI-5656 (Abivertinib Maleate) (ID: NCT04528667) [174] | Third-generation EGFR tyrosine kinase inhibitor and BTK inhibitor. Dose: Subjects receive either 100 mg of STI-5656 or placebo daily for 7 days |
Advanced non-small cell lung carcinoma | Sorrento Therapeutics, Inc. | Brazil | Recruiting | Phase II |
| Desferal (ID: NCT04333550) [136,175] | Acts as chelating agent Dose: Intravenous injection |
Patients with acute or chronic iron and aluminium toxicity | Kermanshah University of Medical Sciences | Iran | Recruiting | Phase I/II |
| Pulmozyme/Recombinant human deoxyribonuclease (rhDNase) (ID: NCT04445285) [176] | Reduces sputum viscoelasticity. Dose: 2.5 mg Pulmozyme/ Recombinant human deoxyribonuclease (rh-DNase) aerosolized treatment once every 24 h for five (5) consecutive days; a total of five (5) doses |
Cystic fibrosis | Jon Simmons, University of South Alabama | USA | Recruiting | Phase II |
| COVID-19 vaccine (COVAXIN) (BBV152) (ID: NCT04641481 [177] | The vaccine is used along with immune stimulants, commonly known as vaccine adjuvants (Alhydroxiquim-II), to improve immune response and longer-lasting immunity Dose: 0.5 mL per dose |
– | Bharat Biotech International Limited | India | Active, not recruiting | Phase III |
| Dornase Alpha (Pulmozyme) (ID: NCT04432987) [178] | It helps in clearing the NETs created by neutrophils in the lungs Dose: Drug will be administered at a dose of 2.5 mg/2 times per day for 7 days |
Cystic fibrosis | Acibadem University | Istanbul, Tutkey | Recruiting | Phase II |
| AZD7442 (A combination of AZD8895 and AZD1061) (ID: NCT04723394) [179] | mABs affects the RBD of viral S protein. Amino acid substitutions have been introduced into the antibodies to both extend their half-lives, which can prolong their potential prophylactic benefit, and minimize Fc effector function. Dose: Single dose (× 2 separate IM injections) of 600 mg of AZD7442. |
– | AstraZeneca | England, UK | Recruiting | Phase III |
| Emricasan (ID:NCT04803227) [180] | Peripheral blood lymphocytes of COVID-19 patients overexpress caspase-1. Caspase-1 plays a role in a form of cell death called pyroptosis. EMR inhibits pyroptosis, thus it serves as a pan caspase inhibitor Dose: 25 mg BID (days 1–14). Oral (capsule) administration. |
– | Histogen | SUNY Downstate Health Sciences University Brooklyn, New York, United States |
Recruiting | Phase I |
| Sirolimus (ID: NCT04461340) [181] | Inhibits IL-2 and other cytokine receptor-dependent signal transduction mechanisms Dose: Oral dose of 6 mg on day1 followed by 2 mg daily for 9 days |
Head and Neck Lymphatic Malformations | Alexandria University | Egypt | Recruiting | Phase II |
| Sitagliptin (ID: NCT04382794) [182] | Inhibits the action of dipeptilpeptidase-4 expressed at parenchyma and lung interstitium level | Type -II diabetes | University of Milan | Italy | Lessens the activity of proinflammatory cytokines, growth factors and vasoactive peptides | Completed |
| Rivaroxaba (ID:NCT04757857) [183] | Acts as anticoagulants Dose: 1st to the 14th day, a dose of 10 mg of rivaroxaban - OA (Oral Administration). |
Thrombosis and pulmonary embolism | Alemão Oswaldo Cruz | Brazil | Recruiting | Phase IV |
| Dendritic Cell Vaccine (ID: NCT04685603) [184] | – | Cancer | Indonesia-MoH | Indonesia | Recruiting | Phase I |
| AZD1222 (ID:NCT04516746) [185] | Recombinant replication-defective chimpanzee adenovirus expressing the SARS-CoV-2-5 surface glycoprotein. Dose: 0.5 mL of 2 dose on day 1 and day 29. |
– | AstraZeneca | United States | Active, not recruiting | Phase III |
| SII-ChAdOx1 nCoV-19 [186] | Recombinant replication-defective chimpanzee adenovirus expressing the SARS-CoV-2- Spike protein. Dose: 0.5 ml of 2 dose on day 1 and day 29. |
– | Serum Institute of India | India | – | Phase III |
ADAM 17, a disintegrin and metalloproteinase 17; CTLs, cytotoxic T lymphocytes; dsRNA, double stranded RNA; EGFR, epidermal growth factor receptor; IFN, interferon; LAM, lymphangioleiomyomatosis; LNP, lipid nanoparticle; mTOR, mammalian target of rapamycin; NETs, neutrophils extracellular traps; NK, natural killer; NLRP 3, NLR family pyrin domain containing 3; PI3K, phosphatidylinositol 3 kinase; RBD, receptor binding domain; RA, rheumatoid arthritis; RdRp, RNA dependent RNA polymerase; S, spike; SARS-CoV-2, severe acute respiratory syndrome coronavirus.
Another concept of controlling the rapid spread of the virus is to develop herd immunity which is defined as decrease in population of susceptible individuals below the threshold value required for transmission. The contagious state of SARS-CoV-2 (R0) varies between 2%–3%. So, for acquiring herd immunity the threshold value is 67% for this virus.
8. Conclusion
The current scenario of rapidly spreading and unpredicted infectious nature of SARS-CoV-2 demands an urgency to focus on basic science and clinical research. Though there are a few resemblances of immunopathogenesis of SARS-CoV-2 with SARS-CoV-1 and MERS but the differences are prominent enough to focus on new therapeutic targets for developing vaccines. Within short time there is significant knowledge about the immunology of SARS-CoV-2 infection which can aid in potent vaccine development. The emerging cases of asymptomatic situations is demanding a better and deep evaluation about the mechanisms of immune response following SARS-CoV-2 infection to develop a promising therapeutic approach. Recently new variants of COVID-19 virus are being reported across the world and targeting these mutated forms of virus has thrown a challenge to the researchers these days. Furthermore, food habits of the communities need to be checked and considered by food authorities to avoid the inevitable menace caused by the zoonotic origin of the virus. The SARS-CoV-2 pandemic is just another example of the emergence of new virus types due to a straight forward link between humans and animals via food chain. In this background, this review comprises of some recent literatures that interrogate the viral entry, invasion, immune escape and immune mechanisms, the dysfunctions of various immune cells like T cells, NK cells, monocytes lineages with a brief view on the memory cells. We have also addressed monoclonal antibody therapy and plasma therapy as well as vaccine development against SARS-CoV-2. Further studies are needed to explain the immune response varying in victims encompassing both symptomatic and asymptomatic ones. Existing clinical trials of SARS-CoV-2 may give an appropriate idea to accomplish the unmet needs. From the reported evidences, it is clear that the immune system is highly affected by this infection and it is critically important to discover efficient drugs to reduce the mortality rate and gain the normal situation as before. Furthermore, some vaccines like Covaxin, Covishield have already undergone the trial phase and is now commercially available for implementation but the question rises on the efficiency of the vaccines against the new evolving strains of the virus. Finally, concluding remarks can be made on the initiatives taken by the respective Government authorities to combat the spread. Personal hygiene, maintaining proper nutritional value, hydration has to be kept in mind to avoid being affected by the virus and for boosting the immune system to fight the virus until a proper and effective treatment strategy towards all the deadly strains of the SARS-CoV-2.
Author contributions
A.D and S.R wrote the manuscript. S.S. and N.C. reviewed and corrected the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
Authors do not have any conflict of interest.
Acknowledgments
Acknowledgement
We are thankful to our Director, CNCI, Kolkata for his continuous support and encouragement to complete this scientific input.
References
- 1.Meo S.A., Alhowikan A.M., Al-Khlaiwi T., Meo I.M., Halepoto D.M., Iqbal M., Usmani A.M., Hajjar W., Ahmed N. Novel coronavirus 2019-nCoV: prevalence, biological and clinical characteristics comparison with SARS-CoV and MERS-CoV. Eur. Rev. Med. Pharmacol. Sci. 2020;24:2012–2019. doi: 10.26355/eurrev_202002_20379. [DOI] [PubMed] [Google Scholar]
- 2.WHO Coronavirus Disease (COVID-19) Dashboard . 2021. WHO Coronavirus Disease (COVID-19) Dashboard. [Google Scholar]
- 3.Gorbalenya A.E., Baker S.C., Baric R.S., de Groot R.J., Drosten C., Gulyaeva A.A., Haagmans B.L., Lauber C., Leontovich A.M., Neuman B.W., Penzar D., Perlman S., Poon L.L.M., Samborskiy D.V., Sidorov I.A., Sola I., Ziebuhr J. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N., Bi Y., Ma X., Zhan F., Wang L., Hu T., Zhou H., Hu Z., Zhou W., Zhao L., Chen J., Meng Y., Wang J., Lin Y., Yuan J., Xie Z., Ma J., Liu W.J., Wang D., Xu W., Holmes E.C., Gao G.F., Wu G., Chen W., Shi W., Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pillaiyar T., Meenakshisundaram S., Manickam M. Recent discovery and development of inhibitors targeting coronaviruses. Drug Discov. Today. 2020;25:668–688. doi: 10.1016/j.drudis.2020.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou M., Zhang X., Qu J. Coronavirus disease 2019 (COVID-19): a clinical update. Front. Med. 2020;14:126–135. doi: 10.1007/s11684-020-0767-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tian S., Xiong Y., Liu H., Niu L., Guo J., Liao M., Xiao S.-Y. Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Mod. Pathol. 2020;33:1007–1014. doi: 10.1038/s41379-020-0536-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hui D.S., Azhar E.I., Madani T.A., Ntoumi F., Kock R., Dar O., Ippolito G., Mchugh T.D., Memish Z.A., Drosten C., Zumla A., Petersen E. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health — The latest 2019 novel coronavirus outbreak in Wuhan, China. Int. J. Infect. Dis. 2020;91:264–266. doi: 10.1016/j.ijid.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perlman S., Netland J. Coronaviruses post-SARS: Update on replication and pathogenesis. Nat. Rev. Microbiol. 2009;7:439–450. doi: 10.1038/nrmicro2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fung T.S., Liu D.X. Human Coronavirus: host-pathogen interaction. Annu. Rev. Microbiol. 2019;73:529–557. doi: 10.1146/annurev-micro-020518-115759. [DOI] [PubMed] [Google Scholar]
- 11.Cui J., Li F., Shi Z.L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019;17:181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang T., Wu Q., Zhang Z. Probable pangolin origin of SARS-CoV-2 associated with the COVID-19 outbreak. Curr. Biol. 2020;30:1346–1351. doi: 10.1016/j.cub.2020.03.022. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guzzi P.H., Mercatelli D., Ceraolo C., Giorgi F.M. Master regulator analysis of the SARS-CoV-2/human interactome. J. Clin. Med. 2020;9:982. doi: 10.3390/jcm9040982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ye Z.W., Yuan S., Yuen K.S., Fung S.Y., Chan C.P., Jin D.Y. Zoonotic origins of human coronaviruses. Int. J. Biol. Sci. 2020;16:1686–1697. doi: 10.7150/ijbs.45472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leitner T., Kumar S. Where did SARS-CoV-2 come from? Mol. Biol. Evol. 2020;37:2463–2464. doi: 10.1093/molbev/msaa162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Banerjee A., Doxey A.C., Mossman K., Irving A.T. Unraveling the zoonotic origin and transmission of SARS-CoV-2. Trends Ecol. Evol. 2020;36:180–184. doi: 10.1016/j.tree.2020.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tiwari R., Dhama K., Sharun K., Iqbal Yatoo M., Malik Y.S., Singh R., Michalak I., Sah R., Bonilla-Aldana D.K., Rodriguez-Morales A.J. COVID-19: animals, veterinary and zoonotic links. Vet. Q. 2020;40:169–182. doi: 10.1080/01652176.2020.1766725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoo H.S., Yoo D. COVID-19 and veterinarians for one health, zoonotic- and reverse-zoonotic transmissions. J. Vet. Sci. 2020;21 doi: 10.4142/jvs.2020.21.e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organisaton . 2020. COVID-19 Weekly Epidemiological Update Global summary; pp. 1–22. [Google Scholar]
- 21.Plante J.A., Liu Y., Liu J., Xia H., Johnson B.A., Lokugamage K.G., Zhang X., Muruato A.E., Zou J., Fontes-Garfias C.R., Mirchandani D., Scharton D., Bilello J.P., Ku Z., An Z., Kalveram B., Freiberg A.N., Menachery V.D., Xie X., Plante K.S., Weaver S.C., Shi P.Y. Spike mutation D614G alters SARS-CoV-2 fitness. Nature. 2020 doi: 10.1038/s41586-020-2895-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Isabel S., Graña-Miraglia L., Gutierrez J.M., Bundalovic-Torma C., Groves H.E., Isabel M.R., Eshaghi A.R., Patel S.N., Gubbay J.B., Poutanen T., Guttman D.S., Poutanen S.M. Evolutionary and structural analyses of SARS-CoV-2 D614G spike protein mutation now documented worldwide. Sci. Rep. 2020;10:1–9. doi: 10.1038/s41598-020-70827-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Angeletti S., Benvenuto D., Bianchi M., Giovanetti M., Pascarella S., Ciccozzi M. COVID-2019: the role of the nsp2 and nsp3 in its pathogenesis. J. Med. Virol. 2020;92:584–588. doi: 10.1002/jmv.25719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.European Centre for Disease Prevention and Control Rapid increase of a SARS-CoV-2 variant with multiple spike protein mutations observed in the United Kingdom. 2020. http://covid19-country-overviews.ecdc.europa.eu/#34_United_Kingdom
- 25.Pachetti M., Marini B., Benedetti F., Giudici F., Mauro E., Storici P., Masciovecchio C., Angeletti S., Ciccozzi M., Gallo R.C., Zella D., Ippodrino R. Emerging SARS-CoV-2 mutation hot spots include a novel RNA-dependent-RNA polymerase variant. J. Transl. Med. 2020;18:1–9. doi: 10.1186/s12967-020-02344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.R.A. Khailany, M. Safdar, M. Ozaslan, Since January 2020 Elsevier has created a COVID-19 resource centre with free information in English and Mandarin on the novel coronavirus COVID- 19. The COVID-19 resource centre is hosted on Elsevier Connect, the company’ s public news and information, Gene Reports. 19 (2020) 1–6.
- 27.Young B.E., Fong S.W., Chan Y.H., Mak T.M., Ang L.W., Anderson D.E., Lee C.Y.P., Amrun S.N., Lee B., Goh Y.S., Su Y.C.F., Wei W.E., Kalimuddin S., Chai L.Y.A., Pada S., Tan S.Y., Sun L., Parthasarathy P., Chen Y.Y.C., Barkham T., Lin R.T.P., Maurer-Stroh S., Leo Y.S., Wang L.F., Renia L., Lee V.J., Smith G.J.D., Lye D.C., Ng L.F.P. Effects of a major deletion in the SARS-CoV-2 genome on the severity of infection and the inflammatory response: an observational cohort study. Lancet. 2020;396:603–611. doi: 10.1016/S0140-6736(20)31757-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holland L.A., Kaelin E.A., Maqsood R., Estifanos B., Wu L.I., Varsani A., Halden R.U., Hogue B.G., Scotch M., Lim E.S. An 81-nucleotide deletion in SARS-CoV-2 ORF7a identified from sentinel surveillance in Arizona (January to March 2020) J. Virol. 2020;94 doi: 10.1128/jvi.00711-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Corman V.M., Lienau J., Witzenrath M. Coronaviren als Ursache respiratorischer Infektionen. Internist (Berl). 2019;60:1136–1145. doi: 10.1007/s00108-019-00671-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sheahan T.P., Sims A.C., Graham R.L., Menachery V.D., Gralinski L.E., Case J.B., Leist S.R., Pyrc K., Feng J.Y., Trantcheva I., Bannister R., Park Y., Babusis D., Clarke M.O., Mackman R.L., Spahn J.E., Palmiotti C.A., Siegel D., Ray A.S., Cihlar T., Jordan R., Denison M.R., Baric R.S. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci. Transl. Med. 2017;9 doi: 10.1126/scitranslmed.aal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020;102433 doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bilinska K., Jakubowska P., Von Bartheld C.S., Butowt R. Expression of the SARS-CoV-2 entry proteins, ACE2 and TMPRSS2, in cells of the olfactory epithelium: identification of cell types and trends with age. ACS Chem. Neurosci. 2020;11:1555–1562. doi: 10.1021/acschemneuro.0c00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guo M., Tao W., Flavell R.A., Zhu S. Potential intestinal infection and faecal–oral transmission of SARS-CoV-2. Nat. Rev. Gastroenterol. Hepatol. 2021;18 doi: 10.1038/s41575-021-00416-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arslan M., Xu B., El-din M.G. 2020. Since January 2020 Elsevier has created a COVID-19 resource centre with free information in English and Mandarin on the novel coronavirus COVID- 19. The COVID-19 resource centre is hosted on Elsevier Connect, the company’s public news and information. [Google Scholar]
- 36.Sanyaolu A., Okorie C., Marinkovic A., Patidar R., Younis K., Desai P., Hosein Z., Padda I., Mangat J., Altaf M. Comorbidity and its impact on patients with COVID-19. SN Compr. Clin. Med. 2020;2:1069–1076. doi: 10.1007/s42399-020-00363-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Erener S. Diabetes, infection risk and COVID-19. Mol. Metab. 2020;39:101044. doi: 10.1016/j.molmet.2020.101044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bersanelli M. Controversies about COVID-19 and anticancer treatment with immune checkpoint inhibitors. Immunotherapy. 2020;12:269–273. doi: 10.2217/imt-2020-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang H., Yang P., Liu K., Guo F., Zhang Y., Zhang G., Jiang C. SARS coronavirus entry into host cells through a novel clathrin- and caveolae-independent endocytic pathway. Cell Res. 2008;18:290–301. doi: 10.1038/cr.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li W., Moore M.J., Vasilieva N., Sui J. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu G., Hu Y., Wang Q., Qi J., Gao F., Li Y., Zhang Y., Zhang W., Yuan Y., Bao J., Zhang B., Shi Y., Yan J., Gao G.F. Molecular basis of binding between novel human coronavirus MERS-CoV and its receptor CD26. Nature. 2013;500:227–231. doi: 10.1038/nature12328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor recognition by the novel Coronavirus from Wuhan: an analysis based on decade-long structural studies of sARS Coronavirus. J. Virol. 2020;94:e00127-20. doi: 10.1128/jvi.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou P., Lou Yang X., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., Chen H.D., Chen J., Luo Y., Guo H., Di Jiang R., Liu M.Q., Chen Y., Shen X.R., Wang X., Zheng X.S., Zhao K., Chen Q.J., Deng F., Liu L.L., Yan B., Zhan F.X., Wang Y.Y., Xiao G.F., Shi Z.L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tikellis C., Thomas M.C. Angiotensin-converting enzyme 2 (ACE2) is a key modulator of the renin angiotensin system in health and disease. Int. J. Pept. 2012;2012:256294. doi: 10.1155/2012/256294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kiely D.G., Cargill R.I., Wheeldon N.M., Coutie W.J., Lipworth B.J. Haemodynamic and endocrine effects of type 1 angiotensin II receptor blockade in patients with hypoxaemic cor pulmonale. Cardiovasc. Res. 1997;33:201–208. doi: 10.1016/s0008-6363(96)00180-0. [DOI] [PubMed] [Google Scholar]
- 46.Imai Y., Kuba K., Rao S., Huan Y., Guo F., Guan B., Yang P., Sarao R., Wada T., Leong-poi H., Crackower M.A., Fukamizu A., Hui C., Hein L., Uhlig S., Slutsky A.S., Jiang C., Penninger J.M. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liao M., Liu Y., Yuan J., Wen Y., Xu G., Zhao J., Cheng L., Li J., Wang X., Wang F., Liu L., Amit I., Zhang S., Zhang Z. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat. Med. 2020;26:842–844. doi: 10.1038/s41591-020-0901-9. [DOI] [PubMed] [Google Scholar]
- 48.Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B., Huan Y., Yang P., Zhang Y., Deng W., Bao L., Zhang B., Liu G., Wang Z., Chappell M., Liu Y., Zheng D., Leibbrandt A., Wada T., Slutsky A.S., Liu D., Qin C., Jiang C., Penninger J.M. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus – induced lung injury. Nat. Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mousavizadeh L., Ghasemi S. Genotype and phenotype of COVID-19: their roles in pathogenesis. J. Microbiol. Immunol. Infect. 2020 doi: 10.1016/j.jmii.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ali Golchin A.A., Seyedjafari E. Mesenchymal stem cell therapy for COVID-19: present or future. Stem Cell Rev. Rep. 2020;16:427–433. doi: 10.1007/s12015-020-09973-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Griggs T.F., Bochkov Y.A., Basnet S., Pasic T.R., Brockman-Schneider R.A., Palmenberg A.C., Gern J.E. Rhinovirus C targets ciliated airway epithelial cells. Respir. Res. 2017;18:84. doi: 10.1186/s12931-017-0567-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Everman J.L., Sajuthi S., Saef B., Rios C., Stoner A.M., Numata M., Hu D., Eng C., Oh S., Rodriguez-Santana J., Vladar E.K., Voelker D.R., Burchard E.G., Seibold M.A. Functional genomics of CDHR3 confirms its role in HRV-C infection and childhood asthma exacerbations. J. Allergy Clin. Immunol. 2019;144:962–971. doi: 10.1016/j.jaci.2019.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu W., Li H. COVID-19: attacks the 1-beta chain of hemoglobin and captures the porphyrin to inhibit human heme metabolism. ChemRxiv. Preprint. 2020 doi: 10.26434/chemrxiv.11938173.v5. [DOI] [Google Scholar]
- 54.Kim D., Lee J., Yang J., Kim J.W., Kim V.N., Chang H. 2020. The architecture of SARS-CoV-2 transcriptome; pp. 1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu J., Wu P., Gao F., Qi J., Kawana-tachikawa A., Xie J., Vavricka C.J., Iwamoto A., Li T., Gao G.F. Novel immunodominant peptide presentation strategy: a featured HLA-A * 2402-restricted cytotoxic T-lymphocyte epitope stabilized by intrachain hydrogen bonds from severe acute respiratory syndrome coronavirus nucleocapsid protein. J. Virol. 2010;84:11849–11857. doi: 10.1128/JVI.01464-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Keicho N., Itoyama S., Kashiwase K., Chi N., Thuy H. Association of human leukocyte antigen class II alleles with severe acute respiratory syndrome in the Vietnamese population. HIM. 2009;70:527–531. doi: 10.1016/j.humimm.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jendro M., Goronzy J.J., Weyand C.M. Structural and functional characterization of hla-dr molecules circulating in the serum. Autoimmunity. 1991;8:289–296. doi: 10.3109/08916939109007636. [DOI] [PubMed] [Google Scholar]
- 58.Levy A.R.R.A., Rojas-villarraga A., Levy R.A. Cancer and Autoimmunity. 2000 doi: 10.1016/b978-0-444-50331-2.x5000-0. [DOI] [Google Scholar]
- 59.Ng M.H.L., Lau K.M., Li L., Cheng S.H., Chan W.Y., Hui P.K., Zee B., Leung C.B., Sung J.J.Y. Association of human-leukocyte-antigen class I (B*0703) and class II (DRB1*0301) genotypes with susceptibility and resistance to the development of severe acute respiratory syndrome. J. Infect. Dis. 2004;190:515–518. doi: 10.1086/421523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen Y.M., Liang S.Y., Shih Y.P., Chen C.Y., Lee Y.M., Chang L., Jung S.Y., Ho M.S., Liang K.Y., Chen H.Y., Chan Y.J., Chu D.C. Epidemiological and genetic correlates of severe acute respiratory syndrome coronavirus infection in the hospital with the highest nosocomial infection rate in Taiwan in 2003. J. Clin. Microbiol. 2006;44:359–365. doi: 10.1128/JCM.44.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nguyen A., David J.K., Maden S.K., Wood M.A., Weeder B.R., Nellore A., Thompson R.F. Human leukocyte antigen susceptibility map for SARS-CoV-2. MedRxiv. 2020;94:1–12. doi: 10.1101/2020.03.22.20040600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Infection S.S., Wang S., Chen K., Chen M., Li W., Chen Y. Human-leukocyte antigen class I Cw 1502 and class II DR 0301 genotypes are associated with resistance to severe acute respiratory. Viral Immunol. 2011;24:421–426. doi: 10.1089/vim.2011.0024. [DOI] [PubMed] [Google Scholar]
- 63.Tu X., Po W., Zhai Y. Functional polymorphisms of the CCL2 and MBL genes cumulatively increase susceptibility to severe acute respiratory syndrome coronavirus infection. J. Inf. Secur. 2015;71:101–109. doi: 10.1016/j.jinf.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kikkert M. Innate immune evasion by human respiratory RNA viruses. J. Innate Immun. 2020;12:4–20. doi: 10.1159/000503030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vabret N., Britton G.J., Gruber C., Hegde S., Kim J., Kuksin M., Levantovsky R., Malle L., Moreira A., Park M.D., Pia L., Risson E., Saffern M., Salomé B., Selvan M. Esai, Spindler M.P., Tan J., van der Heide V., Gregory J.K., Alexandropoulos K., Bhardwaj N., Brown B.D., Greenbaum B., Gümüş Z.H., Homann D., Horowitz A., Kamphorst A.O., de Lafaille M.A. Curotto, Mehandru S., Merad M., Samstein R.M., Agrawal M., Aleynick M., Belabed M., Brown M., Casanova-Acebes M., Catalan J., Centa M., Charap A., Chan A., Chen S.T., Chung J., Bozkus C.C., Cody E., Cossarini F., Dalla E., Fernandez N., Grout J., Ruan D.F., Hamon P., Humblin E., Jha D., Kodysh J., Leader A., Lin M., Lindblad K., Lozano-Ojalvo D., Lubitz G., Magen A., Mahmood Z., Martinez-Delgado G., Mateus-Tique J., Meritt E., Moon C., Noel J., O'Donnell T., Ota M., Plitt T., Pothula V., Redes J., Torres I. Reyes, Roberto M., Sanchez-Paulete A.R., Shang J., Schanoski A.S., Suprun M., Tran M., Vaninov N., Wilk C.M., Aguirre-Ghiso J., Bogunovic D., Cho J., Faith J., Grasset E., Heeger P., Kenigsberg E., Krammer F., Laserson U. Immunology of COVID-19: current state of the science. Immunity. 2020;52:910–941. doi: 10.1016/j.immuni.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.De Wit E., Van Doremalen N., Falzarano D., Munster V.J. SARS and MERS: recent insights into emerging coronaviruses. Nat. Rev. Microbiol. 2016;14:523–534. doi: 10.1038/nrmicro.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Snijder E.J., van der Meer Y., Zevenhoven-Dobbe J., Onderwater J.J.M., van der Meulen J., Koerten H.K., Mommaas A.M. Ultrastructure and origin of membrane vesicles associated with the severe acute respiratory syndrome coronavirus replication complex. J. Virol. 2006;80:5927–5940. doi: 10.1128/jvi.02501-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wathelet M.G., Orr M., Frieman M.B., Baric R.S. Severe acute respiratory syndrome coronavirus evades antiviral signaling: role of nsp1 and rational design of an attenuated strain. J. Virol. 2007;81:11620–11633. doi: 10.1128/jvi.00702-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Niemeyer D., Zillinger T., Muth D., Zielecki F., Horvath G., Suliman T., Barchet W., Weber F., Drosten C., Muller M.A. Middle east respiratory syndrome coronavirus accessory protein 4a is a type I interferon antagonist. J. Virol. 2013;87:12489–12495. doi: 10.1128/jvi.01845-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang Y., Zhang L., Geng H., Deng Y., Huang B., Guo Y., Zhao Z., Tan W. The structural and accessory proteins M, ORF 4a, ORF 4b, and ORF 5 of Middle East respiratory syndrome coronavirus (MERS-CoV) are potent interferon antagonists. Protein Cell. 2013;4:951–961. doi: 10.1007/s13238-013-3096-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dong S., Sun J., Mao Z., Wang L., Lu Y.L., Li J. A guideline for homology modeling of the proteins from newly discovered betacoronavirus, 2019 novel coronavirus (2019-nCoV) J. Med. Virol. 2020 doi: 10.1002/jmv.25768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhu F.C., Li Y.H., Guan X.H., Hou L.H., Wang W.J., Li J.X., Wu S.P., Sen Wang B., Wang Z., Wang L., Jia S.Y., Jiang H.D., Wang L., Jiang T., Hu Y., Gou J.B., Xu S.B., Xu J.J., Wang X.W., Wang W., Chen W. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Lancet. 2020 doi: 10.1016/S0140-6736(20)31208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang R., Li Y., Cowley T.J., Steinbrenner A.D., Phillips J.M., Yount B.L., Baric R.S., Weiss S.R. The nsp1, nsp13, and M proteins contribute to the hepatotropism of murine coronavirus JHM.WU. J. Virol. 2015;89:3598–3609. doi: 10.1128/jvi.03535-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hackbart M., Deng X., Baker S.C. Coronavirus endoribonuclease targets viral polyuridine sequences to evade activating host sensors. Proc. Natl. Acad. Sci. U. S. A. 2020;117:8094–8103. doi: 10.1073/pnas.1921485117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Young M.N.I. Kim, Robert J. Crystal structure of Nsp15 endoribonuclease NendoU from SARS-CoV-2. Protein Sci. Publ. Protein Soc. 2019;29:1596–1605. doi: 10.1002/pro.3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yuen C.K., Lam J.Y., Wong W.M., Mak L.F., Wang X., Chu H., Cai J.P., Jin D.Y., K.K.W. To, Chan J.F.W., Yuen K.Y., Kok K.H. SARS-CoV-2 nsp13, nsp14, nsp15 and orf6 function as potent interferon antagonists. Emerg. Microbes Infect. 2020;9:1418–1428. doi: 10.1080/22221751.2020.1780953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li Y.H., Hu C.Y., Wu N.P., Yao H.P., Li L.J. Molecular characteristics, functions, and related pathogenicity of MERS-CoV proteins. Engineering. 2019;5:940–947. doi: 10.1016/j.eng.2018.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liang Q., Li J., Guo M., Tian X., Liu C., Wang X., Yang X., Wu P., Xiao Z., Qu Y., Yin Y., Fu J., Zhu Z., Liu Z., Peng C., Zhu T. Virus-host interactome and proteomic survey of PMBCs from COVID-19 patients reveal potential virulence factors influencing SARS-CoV-2 pathogenesis. BioRxiv. 2020 doi: 10.1101/2020.03.31.019216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Aouadi W., Blanjoie A., Vasseur J., Canard B., Decroly E. Vol. 91. 2017. Crossm binding of the methyl donor; pp. 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sutton G., Fry E., Carter L., Sainsbury S., Walter T., Nettleship J., Berrow N., Owens R., Gilbert R., Davidson A., Siddell S., Poon L.L.M., Diprose J., Alderton D., Walsh M., Grimes J.M., Stuart D.I. The nsp9 replicase protein of SARS-coronavirus, structure and functional insights. Structure. 2004;12:341–353. doi: 10.1016/j.str.2004.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jang K.J., Jeong S., Kang D.Y., Sp N., Yang Y.M., Kim D.E. A high ATP concentration enhances the cooperative translocation of the SARS coronavirus helicase nsP13 in the unwinding of duplex RNA. Sci. Rep. 2020;10:1–13. doi: 10.1038/s41598-020-61432-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kang S., Yang M., Hong Z., Zhang L., Huang Z., Chen X., He S., Zhou Z., Zhou Z., Chen Q., Yan Y., Zhang C., Shan H., Chen S. Crystal structure of SARS-CoV-2 nucleocapsid protein RNA binding domain reveals potential unique drug targeting sites. Acta Pharm. Sin. B. 2020 doi: 10.1016/j.apsb.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shi C.-S., Qi H.-Y., Boularan C., Huang N.-N., Abu-Asab M., Shelhamer J.H., Kehrl J.H. SARS-coronavirus open reading frame-9b suppresses innate immunity by targeting mitochondria and the MAVS/TRAF3/TRAF6 signalosome. J. Immunol. 2014;193:3080–3089. doi: 10.4049/jimmunol.1303196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gordon D.E., Jang G.M., Bouhaddou M., Xu J., Obernier K., White K.M., O'Meara M.J., Rezelj V.V., Guo J.Z., Swaney D.L., Tummino T.A., Huettenhain R., Kaake R.M., Richards A.L., Tutuncuoglu B., Foussard H., Batra J., Haas K., Modak M., Kim M., Haas P., Polacco B.J., Braberg H., Fabius J.M., Eckhardt M., Soucheray M., Bennett M.J., Cakir M., McGregor M.J., Li Q., Meyer B., Roesch F., Vallet T., Kain A. Mac, Miorin L., Moreno E., Naing Z.Z.C., Zhou Y., Peng S., Shi Y., Zhang Z., Shen W., Kirby I.T., Melnyk J.E., Chorba J.S., Lou K., Dai S.A., Barrio-Hernandez I., Memon D., Hernandez-Armenta C., Lyu J., Mathy C.J.P., Perica T., Pilla K.B., Ganesan S.J., Saltzberg D.J., Rakesh R., Liu X., Rosenthal S.B., Calviello L., Venkataramanan S., Liboy-Lugo J., Lin Y., Huang X.P., Liu Y.F., Wankowicz S.A., Bohn M., Safari M., Ugur F.S., Koh C., Savar N.S., Tran Q.D., Shengjuler D., Fletcher S.J., O'Neal M.C., Cai Y., Chang J.C.J., Broadhurst D.J., Klippsten S., Sharp P.P., Wenzell N.A., Kuzuoglu D., Wang H.Y., Trenker R., Young J.M., Cavero D.A., Hiatt J., Roth T.L., Rathore U., Subramanian A., Noack J., Hubert M., Stroud R.M., Frankel A.D., Rosenberg O.S., Verba K.A., Agard D.A., Ott M., Emerman M., Jura N., von Zastrow M., Verdin E., Ashworth A., Schwartz O., d'Enfert C., Mukherjee S., Jacobson M., Malik H.S., Fujimori D.G., Ideker T., Craik C.S., Floor S.N., Fraser J.S., Gross J.D., Sali A., Roth B.L., Ruggero D., Taunton J., Kortemme T., Beltrao P., Vignuzzi M., García-Sastre A., Shokat K.M., Shoichet B.K., Krogan N.J. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020;583 doi: 10.1038/s41586-020-2286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yuen K.S., Ye Z.W., Fung S.Y., Chan C.P., Jin D.Y. SARS-CoV-2 and COVID-19: the most important research questions. Cell Biosci. 2020;10:1–5. doi: 10.1186/s13578-020-00404-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Menachery V.D., Mitchell H.D., Cockrell A.S., Gralinski L.E., Yount B.L., Graham R.L., McAnarney E.T., Douglas M.G., Scobey T., Beall A., Dinnon K., Kocher J.F., Hale A.E., Stratton K.G., Waters K.M., Baric R.S. MERS-CoV accessory orfs play key role for infection and pathogenesis. MBio. 2017;8:1–14. doi: 10.1128/mBio.00665-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen I.Y., Moriyama M., Chang M.F., Ichinohe T. Severe acute respiratory syndrome coronavirus viroporin 3a activates the NLRP3 inflammasome. Front. Microbiol. 2019;10:1–9. doi: 10.3389/fmicb.2019.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Guilliams M., Lambrecht B.N., Hammad H. Division of labor between lung dendritic cells and macrophages in the defense against pulmonary infections. Mucosal Immunol. 2013;6:464–473. doi: 10.1038/mi.2013.14. [DOI] [PubMed] [Google Scholar]
- 89.Perlman S., Dandekar A.A. Immunopathogenesis of coronavirus infections: implications for SARS. Nat. Rev. Immunol. 2005;5:917–927. doi: 10.1038/nri1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Diao B., Feng Z., Wang C., Wang H., Liu L., Wang C., Wang R., Liu Y., Liu Y., Wang G., Yuan Z., Wu Y., Chen Y. Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. MedRxiv. 2020;2 doi: 10.1101/2020.03.04.20031120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wen W., Su W., Tang H., Le W., Zhang X., Zheng Y., Liu X., Xie L., Li J., Ye J., Dong L., Cui X., Miao Y., Wang D., Dong J., Xiao C., Chen W., Wang H. Immune cell pro fi ling of COVID-19 patients in the recovery stage by single-cell sequencing. Cell Discov. 2020;6:31. doi: 10.1038/s41421-020-0168-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ong E.Z., Fu Y., Chan Z., Leong W.Y., Bertoletti A., Ooi E.E., Ong E.Z., Fu Y., Chan Z., Leong W.Y., Mei N., Lee Y., Kalimuddin S. Brief report. A dynamic immune response shapes COVID-19 progression. Cell Host Microbe. 2020;27:879–882. doi: 10.1016/j.chom.2020.03.021. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liao M., Liu Y., Yuan J., Wen Y., Xu G., Zhao J., Chen L., Li J., Wang X., Wang F., Liu L., Zhang S., Zhang Z. The landscape of lung bronchoalveolar immune cells in COVID-19 revealed by single-cell RNA sequencing. MedRxiv. 2020 doi: 10.1101/2020.02.23.20026690. 2020.02.23.20026690. [DOI] [Google Scholar]
- 94.Zhao J., Zhao J., Van Rooijen N., Perlman S. Evasion by stealth: inefficient immune activation underlies poor T cell response and severe disease in SARS-CoV-infected mice. PLoS Pathog. 2009;5 doi: 10.1371/journal.ppat.1000636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Page C., Goicochea L., Matthews K., Zhang Y., Klover P., Holtzman M.J., Hennighausen L., Frieman M. Induction of alternatively activated macrophages enhances pathogenesis during severe acute respiratory syndrome coronavirus. J. Virol. 2012;86:13334–13349. doi: 10.1128/JVI.01689-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Feng Z., Diao B., Wang R., Wang G., Wang C., Tan Y., Wang C., Liu Y., Liu Y., Yuan Z., Ren L., Wu Y. The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) directly decimates human spleens and lymph nodes running title: SARS-CoV-2 infects human spleens and lymph nodes. MedRxiv. 2020;2:1–18. [Google Scholar]
- 97.Wenjun J.H., Xiaoqing L., Sipei W., Puyi L., Liyan H., Yimin L., Linling C., Sibei C., Lingbo N., Yongping L. The definition and risks of cytokine release syndrome-like in 11 COVID-19-infected pneumonia critically ill patients: disease characteristics and retrospective analysis. MedRxiv. 2020 doi: 10.1101/2020.02.26.20026989. [DOI] [Google Scholar]
- 98.Zheng M., Gao Y., Wang G., Song G., Liu S., Sun D., Xu Y., Tian Z. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell. Mol. Immunol. 2020;17:533–535. doi: 10.1038/s41423-020-0402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Song C.-Y., Xu J., He J.-Q., Lu Y.-Q. COVID-19 early warning score: a multi-parameter screening tool to identify highly suspected patients. MedRxiv. 2020 doi: 10.1101/2020.03.05.20031906. [DOI] [Google Scholar]
- 100.Carlin L.E., Hemann E.A., Zacharias Z.R., Heusel J.W., Legge K.L. Natural killer cell recruitment to the lung during influenza A virus infection is dependent on CXCR3, CCR5, and virus exposure dose. Front. Immunol. 2018;9:781. doi: 10.3389/fimmu.2018.00781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liao M., Liu Y., Yuan J., Wen Y., Xu G., Zhao J., Cheng L., Li J., Wang X., Wang F., Liu L., Amit I., Zhang S., Zhang Z. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat. Med. 2020;26:842–844. doi: 10.1038/s41591-020-0901-9. [DOI] [PubMed] [Google Scholar]
- 102.Wang F., Nie J., Wang H., Zhao Q., Xiong Y., Deng L., Song S., Ma Z., Mo P., Zhang Y. Characteristics of peripheral lymphocyte subset alteration in COVID-19 pneumonia. J. Infect. Dis. 2020;221:1762–1769. doi: 10.1093/infdis/jiaa150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li G., Chen X., Xu A. Profile of specific antibodies to the SARS-associated coronavirus. N. Engl. J. Med. 2003;349:508–509. doi: 10.1056/NEJM200307313490520. [DOI] [PubMed] [Google Scholar]
- 104.Thomas D.L. 2020. COVID 19 progression linked to B cell activation. [Google Scholar]
- 105.Yang P., Gu H., Zhao Z., Wang W., Cao B., Lai C., Yang X., Zhang L.Y., Duan Y., Zhang S., Chen W., Zhen W., Cai M., Penninger J.M., Jiang C., Wang X. Angiotensin-converting enzyme 2 (ACE2) mediates influenza H7N9 virus-induced acute lung injury. Sci. Rep. 2014;4:7027. doi: 10.1038/srep07027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ciaglia E., Vecchione C., Puca A.A. COVID-19 infection and circulating ACE2 levels: protective role in women and children. Front. Pediatr. 2020;8:11–13. doi: 10.3389/fped.2020.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., Zhao P., Liu H., Zhu L., Tai Y., Bai C., Gao T., Song J., Xia P., Dong J., Zhao J., Wang F.S. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zheng H., Zhang M., Yang C., Zhang N., Wang X., Yang X., Dong X., Zheng Y. Elevated exhaustion levels and reduced functional diversity of T cells in peripheral blood may predict severe progression in COVID-19 patients. Cell. Mol. Immunol. 2020;17:541–543. doi: 10.1038/s41423-020-0401-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fan Y.Y., Huang Z.T., Li L., Wu M.H., Yu T., Koup R.A., Bailer R.T., Wu C.Y. Characterization of SARS-CoV-specific memory T cells from recovered individuals 4 years after infection. Arch. Virol. 2009;154:1093–1099. doi: 10.1007/s00705-009-0409-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhou Y., Fu B., Zheng X., Wang D., Zhao C., Qi Y., Sun R., Tian Z., Xu X., Wei H. Pathogenic T-cells and inflammatory monocytes incite inflammatory storms in severe COVID-19 patients. Natl. Sci. Rev. 2020;7:998–1002. doi: 10.1093/nsr/nwaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liao M., Liu Y., Yuan J., Wen Y., Xu G., Zhao J., Chen L., Li J., Wang X., Wang F., Liu L., Zhang S., Zhang Z. The landscape of lung bronchoalveolar immune cells in COVID-19 revealed by single-cell RNA sequencing. Br. Med. J. 2020;26:842–844. doi: 10.1101/2020.02.23.20026690. [DOI] [PubMed] [Google Scholar]
- 112.Diao B., Wang C., Tan Y., Chen X., Liu Y., Ning L., Chen L., Li M., Liu Y., Wang G., Yuan Z., Feng Z., Zhang Y., Wu Y., Chen Y. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19) Front. Immunol. 2020;11:827. doi: 10.3389/fimmu.2020.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wong C.K., Lam C.W.K., Wu A.K.L., Ip W.K., Lee N.L.S., Chan I.H.S., Lit L.C.W., Hui D.S.C., Chan M.H.M., Chung S.S.C., Sung J.J.Y. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin. Exp. Immunol. 2004;136:95–103. doi: 10.1111/j.1365-2249.2004.02415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Channappanavar R., Fehr A.R., Vijay R., Mack M., Zhao J., Meyerholz D.K., Perlman S. Dysregulated type I interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoV-infected mice. Cell Host Microbe. 2016;19:181–193. doi: 10.1016/j.chom.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jiang Y., Xu J., Zhou C., Wu Z., Zhong S., Liu J., Luo W., Chen T., Qin Q., Deng P. Characterization of cytokine/chemokine profiles of seven acute respiratory syndrome. Am. J. Respir. Crit. Care Med. 2005;171:850–857. doi: 10.1164/rccm.200407-857OC. [DOI] [PubMed] [Google Scholar]
- 116.Coperchini F., Chiovato L., Croce L., Magri F., Rotondi M. The cytokine storm in COVID-19: an overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020;53:25–32. doi: 10.1016/j.cytogfr.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li X., Geng M., Peng Y., Meng L., Lu S. Molecular immune pathogenesis and diagnosis of COVID-19. J. Pharm. Anal. 2020;10:102–108. doi: 10.1016/j.jpha.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sanders J.M., Monogue M.L., Jodlowski T.Z., Cutrell J.B. Pharmacologic treatments for coronavirus disease 2019 (COVID-19). A review. JAMA. 2020;323:1824–1836. doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- 119.Devaux C.A., Rolain J.-M., Colson P., Raoult D. New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19? Int. J. Antimicrob. Agents. 2020;105938 doi: 10.1016/j.ijantimicag.2020.105938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Colson P., Rolain J.M., Lagier J.C., Brouqui P., Raoult D. Chloroquine and hydroxychloroquine as available weapons to fight COVID-19. Int. J. Antimicrob. Agents. 2020;55:105932. doi: 10.1016/j.ijantimicag.2020.105932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhou D., Dai S.-M., Tong Q. COVID-19: a recommendation to examine the effect of hydroxychloroquine in preventing infection and progression. J. Antimicrob. Chemother. 2020;75:1667–1670. doi: 10.1093/jac/dkaa114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G., Ruan L., Song B., Cai Y., Wei M., Li X., Xia J., Chen N., Xiang J., Yu T., Bai T., Xie X., Zhang L., Li C., Yuan Y., Chen H., Li H., Huang H., Tu S., Gong F., Liu Y., Wei Y., Dong C., Zhou F., Gu X., Xu J., Liu Z., Zhang Y., Li H., Shang L., Wang K., Li K., Zhou X., Dong X., Qu Z., Lu S., Hu X., Ruan S., Luo S., Wu J., Peng L., Cheng F., Pan L., Zou J., Jia C., Wang J., Liu X., Wang S., Wu X., Ge Q., He J., Zhan H., Qiu F., Guo L., Huang C., Jaki T., Hayden F.G., Horby P.W., Zhang D., Wang C. A trial of lopinavir-ritonavir in adults hospitalized with severe covid-19. N. Engl. J. Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Al-Tawfiq J.A., Al-Homoud A.H., Memish Z.A. Remdesivir as a possible therapeutic option for the COVID-19. Travel Med. Infect. Dis. 2020;34:101615. doi: 10.1016/j.tmaid.2020.101615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kadam R.U., Wilson I.A. Structural basis of influenza virus fusion inhibition by the antiviral drug Arbidol. Proc. Natl. Acad. Sci. U. S. A. 2017;114:206–214. doi: 10.1073/pnas.1617020114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Casadevall A., Pirofski L. The convalescent sera option for containing COVID-19. J. Clin. Invest. 2020;130:1545–1548. doi: 10.1172/jci138003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kaur S.P., Gupta V. COVID-19 vaccine: a comprehensive status report. Virus Res. 2020;288:198114. doi: 10.1016/j.virusres.2020.198114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yuan M., Wu N.C., Zhu X., Lee C.C.D., So R.T.Y., Lv H., Mok C.K.P., Wilson I.A. A highly conserved cryptic epitope in the receptor binding domains of SARS-CoV-2 and SARS-CoV. Science (80-.) 2020;368:630–633. doi: 10.1126/science.abb7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Baruah V., Bose S. Immunoinformatics-aided identification of T cell and B cell epitopes in the surface glycoprotein of 2019-nCoV. J. Med. Virol. 2020;92:495–500. doi: 10.1002/jmv.25698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lon J.R., Bai Y., Zhong B., Cai F., Du H. Prediction and evolution of B cell epitopes of surface protein in SARS-CoV-2. BioRxiv. 2020 doi: 10.1101/2020.04.03.022723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Metcalfe S.M. Mesenchymal stem cells and management of COVID-19 pneumonia. Med. Drug Discov. 2020;5:100019. doi: 10.1016/j.medidd.2020.100019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.ALBERTA HOPE COVID-19 for the Prevention of Severe COVID19 Disease - Full Text View. 2021. ClinicalTrials.gov
- 132.Study to Evaluate the Safety and Antiviral Activity of Remdesivir (GS-5734™) in Participants With Severe Coronavirus Disease (COVID-19) - Full Text View. 2021. ClinicalTrials.gov
- 133.Goldman J.D., Lye D.C.B., Hui D.S., Marks K.M., Bruno R., Montejano R., Spinner C.D., Galli M., Ahn M.-Y., Nahass R.G., Chen Y.-S., SenGupta D., Hyland R.H., Osinusi A.O., Cao H., Blair C., Wei X., Gaggar A., Brainard D.M., Towner W.J., Muñoz J., Mullane K.M., Marty F.M., Tashima K.T., Diaz G., Subramanian A. Remdesivir for 5 or 10 Days in Patients with Severe Covid-19. N. Engl. J. Med. 2020 doi: 10.1056/nejmoa2015301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Spinner C.D., Gottlieb R.L., Criner G.J., López J.R. Arribas, Cattelan A.M., Viladomiu A. Soriano, Ogbuagu O., Malhotra P., Mullane K.M., Castagna A., Chai L.Y.A., Roestenberg M., Tsang O.T.Y., Bernasconi E., Le Turnier P., Chang S.-C., SenGupta D., Hyland R.H., Osinusi A.O., Cao H., Blair C., Wang H., Gaggar A., Brainard D.M., McPhail M.J., Bhagani S., Ahn M.Y., Sanyal A.J., Huhn G., Marty F.M. Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19. JAMA. 2020 doi: 10.1001/jama.2020.16349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Duvelisib to Combat COVID-19 - Full Text View. 2021. ClinicalTrials.gov
- 136.Application of Desferal to Treat COVID-19 - Full Text View. 2021. ClinicalTrials.gov
- 137.Wang H., Li Z., Niu J., Xu Y., Ma L., Lu A., Wang X., Qian Z., Huang Z., Jin X., Leng Q., Wang J., Zhong J., Sun B., Meng G. Antiviral effects of ferric ammonium citrate. Cell Discov. 2018;4:14. doi: 10.1038/s41421-018-0013-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Clinical Study To Evaluate The Performance And Safety Of Favipiravir in COVID-19 - Full Text View. 2021. ClinicalTrials.gov
- 139.Study to Evaluate the Efficacy and Safety of Tocilizumab Versus Corticosteroids in Hospitalised COVID-19 Patients With High Risk of Progression - Full Text View. 2021. ClinicalTrials.gov
- 140.Sarilumab COVID-19 - Full Text View. 2021. ClinicalTrials.gov
- 141.McMurray J.J.V., Solomon S.D., Inzucchi S.E., Kober L., Kosiborod M.N., Martinez F.A., Ponikowski P., Sabatine M.S., Anand I.S., Lohlavek J.B., Bohm M., Chiang C.E., Chopra V.K., De Boer R.A., Desai A.S., Diez M., Drozdz J., Dukat A., Ge J., Howlett J.G., Katova T., Kitakaze M., Ljungman C.E.A., Merkely B., Nicolau J.C., O'Meara E., Petrie M.C., Vinh P.N., Schou M., Tereshchenko S., Verma S., Held C., DeMets D.L., Docherty K.F., Jhund P.S., Bengtsson O., Sjostrand M., Langkilde A.M. Dapagliflozin in patients with heart failure and reduced ejection fraction. N. Engl. J. Med. 2019;381:1995–2008. doi: 10.1056/NEJMoa1911303. [DOI] [PubMed] [Google Scholar]
- 142.Dapagliflozin in Respiratory Failure in Patients With COVID-19 - Full Text View. 2021. ClinicalTrials.gov
- 143.Recombinant Human Angiotensin-converting Enzyme 2 (rhACE2) as a Treatment for Patients With COVID-19 - Full Text View. 2021. ClinicalTrials.gov
- 144.The Phase 2 Study to Evaluate the Safety and Efficacy of Clevudine in Patients With Moderate COVID-19 - Full Text View. 2021. ClinicalTrials.gov
- 145.Study of FT516 for the Treatment of COVID-19 in Hospitalized Patients With Hypoxia - Full Text View. 2021. ClinicalTrials.gov
- 146.DAS181 for Severe COVID-19: Compassionate Use - Full Text View. 2021. ClinicalTrials.gov
- 147.Study of Open Label Losartan in COVID-19 - Full Text View. 2021. ClinicalTrials.gov
- 148.Lv C., Liu W., Wang B., Dang R., Qiu L., Ren J., Yan C., Yang Z., Wang X. Ivermectin inhibits DNA polymerase UL42 of pseudorabies virus entrance into the nucleus and proliferation of the virus in vitro and vivo. Antivir. Res. 2018;159:55–62. doi: 10.1016/j.antiviral.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 149.Hong S.K., Kim H.J., Song C.S., Choi I.S., Lee J.B., Park S.Y. Nitazoxanide suppresses IL-6 production in LPS-stimulated mouse macrophages and TG-injected mice. Int. Immunopharmacol. 2012;13:23–27. doi: 10.1016/j.intimp.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 150.Ivermectin and Nitazoxanide Combination Therapy for COVID-19 - Full Text View. 2021. ClinicalTrials.gov
- 151.Shen C., Wang Z., Zhao F., Yang Y., Li J., Yuan J., Wang F., Li D., Yang M., Xing L., Wei J., Xiao H., Yang Y., Qu J., Qing L., Chen L., Xu Z., Peng L., Li Y., Zheng H., Chen F., Huang K., Jiang Y., Liu D., Zhang Z., Liu Y., Liu L. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA - J. Am. Med. Assoc. 2020;323:1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Efficacy of Convalescent Plasma Therapy in the Early Care of COVID-19 Patients. - Full Text View. 2021. ClinicalTrials.gov
- 153.Isotretinoin in Treatment of COVID-19 - Full Text View. 2021. ClinicalTrials.gov
- 154.Colchicine in COVID-19: a Pilot Study - Full Text View. 2021. ClinicalTrials.gov
- 155.Ruxolitinib in Covid-19 Patients With Defined Hyperinflammation - Full Text View. 2021. ClinicalTrials.gov
- 156.Expanded Access Program of Ruxolitinib for the Emergency Treatment of Cytokine Storm From COVID-19 Infection - Full Text View. 2021. ClinicalTrials.gov
- 157.La Rosée F., Bremer H.C., Gehrke I., Kehr A., Hochhaus A., Birndt S., Fellhauer M., Henkes M., Kumle B., Russo S.G., La Rosée P. The Janus kinase 1/2 inhibitor ruxolitinib in COVID-19 with severe systemic hyperinflammation. Leukemia. 2020;34:1805–1815. doi: 10.1038/s41375-020-0891-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.A Pilot Study of Sildenafil in COVID-19 - Full Text View. 2021. ClinicalTrials.gov
- 159.Sirolimus Treatment in Hospitalized Patients With COVID-19 Pneumonia - Full Text View. 2021. ClinicalTrials.gov
- 160.Single-Blind Study of a Single Dose of Peginterferon Lambda-1a Compared With Placebo in Outpatients With Mild COVID-19 - Full Text View. 2021. ClinicalTrials.gov
- 161.Rintatolimod and IFN Alpha-2b for the Treatment of Mild or Moderate COVID-19 Infection in Cancer Patients - Full Text View. 2021. ClinicalTrials.gov
- 162.Administration of Intravenous Vitamin C in Novel Coronavirus Infection (COVID-19) and Decreased Oxygenation - Full Text View. 2021. ClinicalTrials.gov
- 163.Safety and Immunogenicity Study of 2019-nCoV Vaccine (mRNA-1273) for Prophylaxis of SARS-CoV-2 Infection (COVID-19) - Full Text View. 2021. ClinicalTrials.gov
- 164.Jackson L.A., Anderson E.J., Rouphael N.G., Roberts P.C., Makhene M., Coler R.N., McCullough M.P., Chappell J.D., Denison M.R., Stevens L.J., Pruijssers A.J., McDermott A., Flach B., Doria-Rose N.A., Corbett K.S., Morabito K.M., O'Dell S., Schmidt S.D., Swanson P.A., Padilla M., Mascola J.R., Neuzil K.M., Bennett H., Sun W., Peters E., Makowski M., Albert J., Cross K., Buchanan W., Pikaart-Tautges R., Ledgerwood J.E., Graham B.S., Beigel J.H. An mRNA vaccine against SARS-CoV-2 — Preliminary report. N. Engl. J. Med. 2020 doi: 10.1056/nejmoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Safety, Tolerability and Immunogenicity of INO-4800 for COVID-19 in Healthy Volunteers - Full Text View. 2021. ClinicalTrials.gov
- 166.Folegatti P.M., Ewer K.J., Aley P.K., Angus B., Becker S., Belij-Rammerstorfer S., Bellamy D., Bibi S., Bittaye M., Clutterbuck E.A., Dold C., Faust S.N., Finn A., Flaxman A.L., Hallis B., Heath P., Jenkin D., Lazarus R., Makinson R., Minassian A.M., Pollock K.M., Ramasamy M., Robinson H., Snape M., Tarrant R., Voysey M., Green C., Douglas A.D., Hill A.V.S., Lambe T., Gilbert S.C., Pollard A.J., Aboagye J., Adams K., Ali A., Allen E., Allison J.L., Anslow R., Arbe-Barnes E.H., Babbage G., Baillie K., Baker M., Baker N., Baker P., Baleanu I., Ballaminut J., Barnes E., Barrett J., Bates L., Batten A., Beadon K., Beckley R., Berrie E., Berry L., Beveridge A., Bewley K.R., Bijker E.M., Bingham T., Blackwell L., Blundell C.L., Bolam E., Boland E., Borthwick N., Bower T., Boyd A., Brenner T., Bright P.D., Brown-O'Sullivan C., Brunt E., Burbage J., Burge S., Buttigieg K.R., Byard N., Puig I. Cabera, Calvert A., Camara S., Cao M., Cappuccini F., Carr M., Carroll M.W., Carter V., Cathie K., Challis R.J., Charlton S., Chelysheva I., Cho J.S., Cicconi P., Cifuentes L., Clark H., Clark E., Cole T., Colin-Jones R., Conlon C.P., Cook A., Coombes N.S., Cooper R., Cosgrove C.A., Coy K., Crocker W.E.M., Cunningham C.J., Damratoski B.E., Dando L., Datoo M.S., Davies H., De Graaf H., Demissie T., Di Maso C., Dietrich I., Dong T., Donnellan F.R., Douglas N., Downing C., Drake J., Drake-Brockman R., Drury R.E., Dunachie S.J., Edwards N.J., Edwards F.D.L., Edwards C.J., Elias S.C., Elmore M.J., Emary K.R.W., English M.R., Fagerbrink S., Felle S., Feng S., Field S., Fixmer C., Fletcher C., Ford K.J., Fowler J., Fox P., Francis E., Frater J., Furze J., Fuskova M., Galiza E., Gbesemete D., Gilbride C., Godwin K., Gorini G., Goulston L., Grabau C., Gracie L., Gray Z., Guthrie L.B., Hackett M., Halwe S., Hamilton E., Hamlyn J., Hanumunthadu B., Harding I., Harris S.A., Harris A., Harrison D., Harrison C., Hart T.C., Haskell L., Hawkins S., Head I., Henry J.A., Hill J., Hodgson S.H.C., Hou M.M., Howe E., Howell N., Hutlin C., Ikram S., Isitt C., Iveson P., Jackson S., Jackson F., James S.W., Jenkins M., Jones E., Jones K., Jones C.E., Jones B., Kailath R., Karampatsas K., Keen J., Kelly S., Kelly D., Kerr D., Kerridge S., Khan L., Khan U., Killen A., Kinch J., King T.B., King L., King J., Kingham-Page L., Klenerman P., Knapper F., Knight J.C., Knott D., Koleva S., Kupke A., Larkworthy C.W., Larwood J.P.J., Laskey A., Lawrie A.M., Lee A., Lee K.Y. Ngan, Lees E.A., Legge H., Lelliott A., Lemm N.M., Lias A.M., Linder A., Lipworth S., Liu X., Liu S., Ramon R. Lopez, Lwin M., Mabesa F., Madhavan M., Mallett G., Mansatta K., Marcal I., Marinou S., Marlow E., Marshall J.L., Martin J., McEwan J., McInroy L., Meddaugh G., Mentzer A.J., Mirtorabi N., Moore M., Moran E., Morey E., Morgan V., Morris S.J., Morrison H., Morshead G., Morter R., Mujadidi Y.F., Muller J., Munera-Huertas T., Munro C., Munro A., Murphy S., Munster V.J., Mweu P., Noé A., Nugent F.L., Nuthall E., O'Brien K., O'Connor D., Oguti B., Oliver J.L., Oliveira C., O'Reilly P.J., Osborn M., Osborne P., Owen C., Owens D., Owino N., Pacurar M., Parker K., Parracho H., Patrick-Smith M., Payne V., Pearce J., Peng Y., Peralta Alvarez M.P., Perring J., Pfafferott K., Pipini D., Plested E., Pluess-Hall H., Pollock K., Poulton I., Presland L., Provstgaard-Morys S., Pulido D., Radia K., Lopez F. Ramos, Rand J., Ratcliffe H., Rawlinson T., Rhead S., Riddell A., Ritchie A.J., Roberts H., Robson J., Roche S., Rohde C., Rollier C.S., Romani R., Rudiansyah I., Saich S., Sajjad S., Salvador S., Sanchez Riera L., Sanders H., Sanders K., Sapaun S., Sayce C., Schofield E., Screaton G., Selby B., Semple C., Sharpe H.R., Shaik I., Shea A., Shelton H., Silk S., Silva-Reyes L., Skelly D.T., Smee H., Smith C.C., Smith D.J., Song R., Spencer A.J., Stafford E., Steele A., Stefanova E., Stockdale L., Szigeti A., Tahiri-Alaoui A., Tait M., Talbot H., Tanner R., Taylor I.J., Taylor V., Te Water Naude R., Thakur N., Themistocleous Y., Themistocleous A., Thomas M., Thomas T.M., Thompson A., Thomson-Hill S., Tomlins J., Tonks S., Towner J., Tran N., Tree J.A., Truby A., Turkentine K., Turner C., Turner N., Turner S., Tuthill T., Ulaszewska M., Varughese R., Van Doremalen N., Veighey K., Verheul M.K., Vichos I., Vitale E., Walker L., Watson M.E.E., Welham B., Wheat J., White C., White R., Worth A.T., Wright D., Wright S., Yao X.L., Yau Y. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396:467–478. doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.A Study of a Candidate COVID-19 Vaccine (COV001) - Full Text View. 2021. ClinicalTrials.gov
- 168.Immunity and Safety of Covid-19 Synthetic Minigene Vaccine - Full Text View. 2021. ClinicalTrials.gov
- 169.Evaluation of the Safety and Immunogenicity of a SARS-CoV-2 rS (COVID-19) Nanoparticle Vaccine With/Without Matrix-M Adjuvant - Full Text View. 2021. ClinicalTrials.gov
- 170.Study to Describe the Safety, Tolerability, Immunogenicity, and Potential Efficacy of RNA Vaccine Candidates Against COVID-19 in Healthy Adults - Full Text View. 2021. ClinicalTrials.gov
- 171.Evaluating the Safety, Tolerability and Immunogenicity of bacTRL-Spike Vaccine for Prevention of COVID-19 - Full Text View. 2021. ClinicalTrials.gov
- 172. NCT04482621 Decitabine for Coronavirus (COVID-19) Pneumonia- Acute Respiratory Distress Syndrome (ARDS) Treatment: DART Trial. 2021. https://Clinicaltrials.Gov/Show/NCT04482621
- 173.Pilot Trial of XFBD, a TCM, in Persons With COVID-19 - Full Text View. 2021. ClinicalTrials.gov
- 174.Study of the Safety and Efficacy of STI-5656 (Abivertinib Maleate) in Subjects Hospitalized Due to COVID-19 - Full Text View. 2021. ClinicalTrials.gov
- 175.Deferoxamine - StatPearls - NCBI Bookshelf. 2021. [Google Scholar]
- 176.Phase 2 Trial Using rhDNase to Reduce Mortality in COVID-19 Patients With Respiratory Failure - Full Text View. 2021. ClinicalTrials.gov
- 177.An Efficacy and Safety Clinical Trial of an Investigational COVID-19 Vaccine (BBV152) in Adult Volunteers - Full Text View. 2021. ClinicalTrials.gov
- 178.Dornase Alpha for the Treatment of COVID-19 - Full Text View. 2021. ClinicalTrials.gov
- 179. NCT04723394, Phase III Study of AZD7442 for Treatment of COVID-19 in Outpatient Adults. 2021. https://Clinicaltrials.Gov/Show/NCT04723394
- 180.Safety and Tolerability of Etanercept in Alzheimer's Disease - Full Text View. 2021. ClinicalTrials.gov
- 181.Efficacy and Safety of Sirolimus in COVID-19 Infection - Full Text View. 2021. ClinicalTrials.gov
- 182.Sitagliptin Treatment in Diabetic COVID-19 Positive Patients - Full Text View. 2021. ClinicalTrials.gov
- 183.COVID-19 Antithrombotic Rivaroxaban Evaluation - Full Text View. 2021. ClinicalTrials.gov
- 184.Dendritic Cell Vaccine to Prevent COVID-19 - Full Text View. 2021. ClinicalTrials.gov
- 185.Nct Phase III Double-blind, Placebo-controlled Study of AZD1222 for the Prevention of COVID-19 in Adults. 2020. https://Clinicaltrials.Gov/Show/NCT04516746
- 186.AstraZeneca . 2021. ChAdOx1-S/nCoV-19 [recombinant], COVID-19 vaccine. [Google Scholar]